Abstract
Microsecretory adenocarcinoma (MSA) is a recently described salivary gland tumor with a characteristic histologic and immunophenotypic profile and recurrent MEF2C-SS18 fusions. Because only six cases of MSA have been published, its complete clinicopathologic spectrum is unclear, and its biologic behavior has not been documented. Here, we present an updated and expanded experience of 24 MSA cases. All cases of MSA were obtained from the authors’ files. Immunohistochemistry for S100, SOX10, p63, p40, SMA, calponin, and mammaglobin was performed. Molecular analysis was performed by targeted RNA sequencing, SS18 break apart fluorescence in situ hybridization, and/or reverse transcriptase polymerase chain reaction for MEF2C-SS18 fusion. Clinical follow-up was obtained from medical records. A total of 24 MSA cases were collected, from 13 women and 11 men, ranging from 17 to 83 years (mean 49.5 years). The vast majority (23 of 24) arose in the oral cavity, with the palate (n = 14) and buccal mucosa (n = 6) as the most frequent subsites. Tumors showed consistent histologic features including: (1) microcystic tubules, (2) flattened intercalated duct-like cells, (3) monotonous oval hyperchromatic nuclei, (4) abundant basophilic luminal secretions, (5) fibromyxoid stroma, and (6) circumscribed borders with subtle infiltration. The tumors were very consistently positive for S100 (24 of 24), p63 (24 of 24), and SOX10 (14 of 14) and negative for p40 (0 of 21), calponin (0 of 12) and mammaglobin (0 of 16), while SMA (4 of 20) was variable. MEF2C-SS18 fusion was demonstrated in 21 of 24 cases; in the remaining 3 cases with insufficient RNA, SS18 break apart FISH was positive. Treatment information was available in 17 cases, all of which were managed with surgery only. In 14 cases with follow-up (1–216 months, mean 30), no cases recurred or metastasized. MSA is a distinct salivary gland neoplasm with remarkably consistent clinical, histologic, immunophenotypic, and genetic features that generally behaves in an indolent manner following surgery alone. These observations solidify MSA as a unique, low-grade salivary gland carcinoma that warrants inclusion in the next version of the WHO classification of head and neck tumors.
Keywords: Salivary gland neoplasms, Adenocarcinoma not otherwise specified, Microsecretory adenocarcinoma, MEF2C-SS18
Introduction
Salivary gland tumor classification has been revolutionized in the past 10 years by the discovery of characteristic gene fusions which in some cases have resulted in the recognition of novel tumor entities [1]. For example, microsecretory adenocarcinoma (MSA) was proposed in 2019 to be a unique salivary gland neoplasm with distinctive histologic and immunophenotypic features as well as a novel MEF2C-SS18 fusion [2]. As the original series included only 5 cases, and only 1 additional case has been subsequently reported, much has remained unknown about MSA [3]. Identifying and describing additional cases would be helpful to complete our understanding of its clinicopathologic spectrum. Most important, although MSA is presumed to be a low-grade carcinoma, the original series contained no follow-up data and its behavior remains unknown. Herein, we expand the description of MSA to (1) solidify this neoplasm as a genuine tumor entity, (2) provide a more complete description of its morphologic spectrum, (3) define its clinical behavior, and (4) characterize it at the genetic level.
Methods
Case Selection
Cases of MSA were retrieved from the authors’ surgical pathology archives and consultation files. Six cases had been previously published [2, 3]. All cases were reviewed centrally by the primary author, and various histologic features were tabulated. Any available clinical and follow-up information was collected for each case from the electronic medical record.
Immunohistochemistry
Immunohistochemistry was performed on all cases. Using standard automated protocols, staining was performed on a Ventana BenchmarkXT autostainer (Ventana Medical Systems. Tucson, AZ) using antibodies for S100 (Ventana Medical Systems, Inc. Tucson, AZ), p40 (Biocare), SOX10 (Ventana), smooth muscle actin (SMA) (Ventana), calponin (Dako, Carpinteria, CA), and mammaglobin (Dako). All immunohistochemical signals were visualized using the Ultra view polymer detection kit (Ventana).
Fluorescence In Situ Hybridization (FISH)
Break apart FISH was performed on a subset of cases using a standard dual color break-apart probe (centromeric 3ʹ-side green, telomeric 5ʹ-side orange) for SS18 following manufacturer’s protocol (Abbott Molecular, Des Plains, IL). The sections were deparaffinized, pre-treated for 25 min at 80 °C, treated with protease for 38 min at 37 °C, probe and target co-denatured at 80 °C for 15 min, hybridized overnight at 37 °C, and finally washed at 74 °C for 2 min. Slides were then stained with DAPI and evaluated using epifluorescence microscope and ASI software (Applied Spectral Imaging, Chicago, IL); 100 nuclei were evaluated from each tumor. Tumors with split signals in > 12% of cells were regarded as positive.
RNA Sequencing
Targeted RNA sequencing was attempted on 22 of 24 cases using different TruSight RNA Fusion panels or modified Pan-Cancer kits (Illumina, San Diego, CA) as previously described [2, 4, 5]. Briefly, whole-slide tissue sections were cut at 5 μm, and Qiagen AllPrep kits (Qiagen, Germantown, USA) were utilized for RNA isolation. A sequencing library was made using a modified TruSight RNA Pan-Cancer kit (Illumina, San Diego, USA) with 1425 genes. Sequencing was performed on the NextSeq 550 (Illumina) with a minimum of 6,000,000 mapped reads. Fusions were called using the Star-Fusion algorithm [6]. All fusions were manually reviewed via the Integrated Genomics Viewer (Broad Institute, Cambridge, USA).
Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
For four cases, RT-PCR was performed to identify the MEF2C-SS18 fusion. In three cases, this was done to confirm the RNA sequencing result as previously described [2], while in the remaining case, it was done prospectively to confirm the tumor diagnosis. Briefly, three sections at 8 microns each were collected in a tube, then RNA extraction was performed with the Maxwell RSC RNA FFPE-KIT (Promega, Madison, USA). Next, reverse transcription was performed with Superscript III (Invitrogen, Carlsbad, USA), followed by PCR amplification with primers as published by Bishop et al. [2] Lastly, Sanger sequencing was performed to identify the fusion product.
Results
Twenty-four cases of MSA were identified, and they are summarized in Table 1. Most cases (23 of 24) arose in the oral cavity, with the palate (hard, soft, junction, or not specified) being the most frequent oral subsite (n = 14), followed by the buccal mucosa (n = 6), retromolar trigone (n = 2), and angle of mandible (n = 1). The single non-oral MSA arose in the parotid gland. The MSAs were seen in 13 women and 11 men, ranging from 17 to 83 years (mean, 49.5 years). The tumors were generally small, with an average dimension of 1.1 cm (range, 0.6 to 3.0 cm), (Fig. 1) and where known, patients presented uniformly with a painless mass. Treatment information was available in 16 cases, all of which were managed with surgery only. In 14 cases with some follow-up (mean 30 months, range 1–216 months), none of the MSAs recurred or metastasized.
Table 1.
Clinical and demographic findings of microsecretory adenocarcinoma
| Case | Original/submitted diagnosis | Age | Sex | Tumor location | Oral subsite | Tumor size (cm) | Treatment | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|
| 1 | Adenocarcinoma NOS | 21 | M | Oral cavity | Buccal | 0.8 | Unknown | None |
| 2 | Adenocarcinoma NOS with features of PAC | 80 | F | Oral cavity | Hard palate | 0.8 | Unknown | None |
| 3 | Adenocarcinoma NOS | 48 | F | Oral cavity | Buccal | 2.2 | Unknown | None |
| 4 | PAC | 32 | M | Oral cavity | Hard palate | 1.5 | Surgery | NED (24) |
| 5 | Adenocarcinoma NOS | 51 | F | Parotid gland | N/A | 0.8 | Unknown | None |
| 6 | Adenocarcinoma NOS with mucinous features | 62 | F | Oral cavity | Palate, NOS | 0.96 | Surgery | NED (33) |
| 7 | Secretory myoepithelial carcinoma | 46 | M | Oral cavity | Angle of mandible | 1.2 | Surgery | None |
| 8 | Adenocarcinoma NOS | 35 | F | Oral cavity | Hard palate | 0.7 | Surgery | NED (11) |
| 9 | Adenocarcinoma NOS | 69 | M | Oral cavity | Soft palate | 0.6 | Surgery | NED (10) |
| 10 | Mucin-producing adenocarcinoma | 75 | M | Oral cavity | Buccal | 0.9 | Surgery | NED (216) |
| 11 | PAC | 79 | F | Oral cavity | Hard palate | 0.8 | Surgery | NED (21) |
| 12 | MSA | 26 | F | Oral cavity | Junction of hard/soft palate | 0.7 | Surgery | NED (15) |
| 13 | Atypical glandular proliferation | 61 | M | Oral cavity | Palate, NOS | 0.6 | Unknown | None |
| 14 | Adenocarcinoma NOS | 58 | F | Oral cavity | Palate, NOS | 1.0 | Unknown | None |
| 15 | MSA | 37 | F | Oral cavity | Hard palate | 1.5 | Surgery | NED (14) |
| 16 | Adenocarcinoma NOS | 56 | M | Oral cavity | Hard palate | 0.8 | Surgery | NED (12) |
| 17 | MSA | 38 | M | Oral cavity | Hard palate | 0.9 | Surgery | NED (5) |
| 18 | MSA | 45 | F | Oral cavity | Buccal | 2.3 | Surgery | NED (2) |
| 19 | MSA | 75 | F | Oral cavity | Retromolar trigone | 0.8 | Surgery | NED (3) |
| 20 | MSA | 17 | F | Oral cavity | Hard palate | 0.6 | Surgery | None |
| 21 | Adenoid cystic carcinoma | 83 | M | Oral cavity | Palate, NOS | 1.0 | Surgery | None |
| 22 | PAC | 20 | F | Oral cavity | Buccal | 1.0 | Surgery | NED (36) |
| 23 | MSA | 46 | M | Oral cavity | Retromolar trigone | 2.0 | Surgery | NED (1) |
| 24 | Salivary gland tumor NOS | 28 | M | Oral cavity | Buccal | 3.0 | Unknown | None |
NOS not otherwise specified, PAC polymorphous adenocarcinoma, NED no evidence of disease, MSA microsecretory adenocarcinoma
Fig. 1.
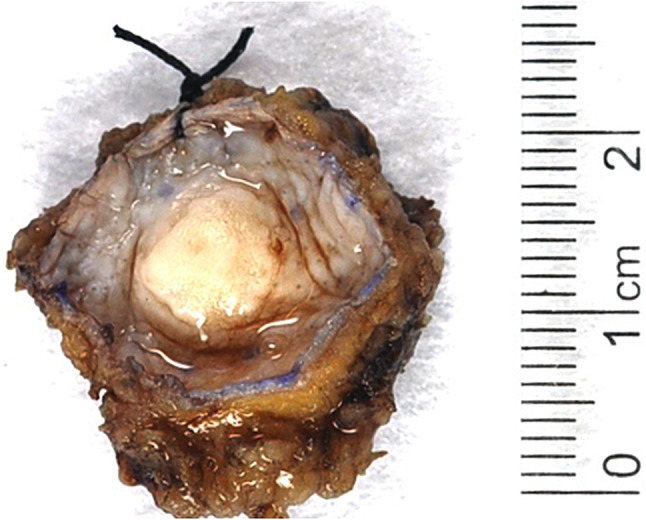
Grossly, the microsecretory adenocarcinomas usually consisted of well circumscribed, tan-white masses covered by an intact epithelium in the oral mucosa
The MSAs received a variable range of original or presenting diagnoses. The most common diagnosis was adenocarcinoma not otherwise specified (NOS) (9 of 24), some of which were specified as having features of mucinous adenocarcinoma or polymorphous adenocarcinoma (1 case each). Seven of the 17 cases encountered after the initial description of this entity were correctly identified as MSA. Other submitted/original diagnoses included polymorphous adenocarcinoma (n = 3), mucin-producing adenocarcinoma (n = 1), secretory myoepithelial carcinoma (n = 1), adenoid cystic carcinoma (n = 1), salivary gland tumor NOS (n = 1) or atypical glandular proliferation (n = 1).
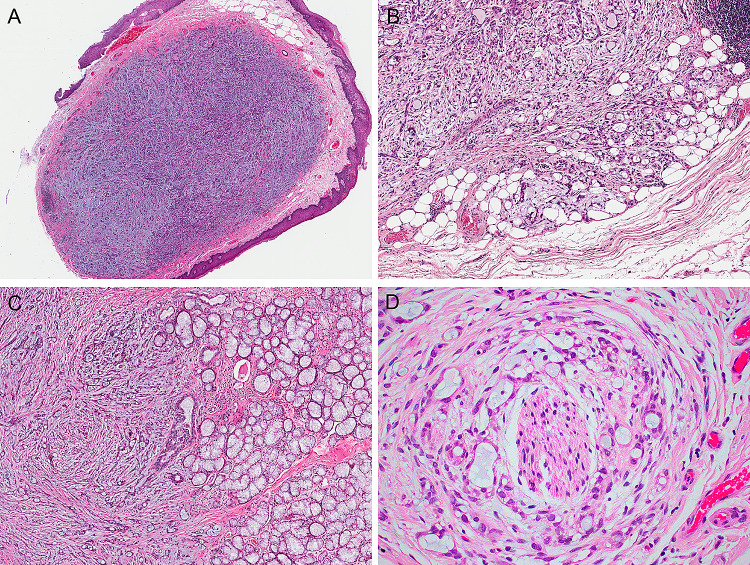
The MSAs displayed a remarkably similar histologic appearance. They consisted of well-circumscribed but unencapsulated epithelial proliferations (Fig. 2a) that, on close inspection, showed subtle infiltration of nearby skeletal muscle, adipose tissue, or native seromucinous glands (Fig. 2b, c). Perineural invasion was seen in only 1 of 24 cases (Fig. 2d), and lymphovascular invasion was not identified. The tumors were arranged as microcysts that occasionally fused into loose cribriform structures or were compressed into cords or rare single cells, and were lined by a single layer of flattened cells with modest amounts of eosinophilic to clear cytoplasm with monotonous oval hyperchromatic nuclei with indistinct nucleoli. Amphophilic to basophilic, mucicarmine-positive secretory material was universally present within the microcysts. Also uniformly present was a cellular fibromyxoid stroma. The caliber of the microcysts and the cellularity of the stroma varied slightly from case to case (Fig. 3). Uncommon features included pseudoepitheliomatous hyperplasia of the overlying oral squamous epithelium (n = 5) (Fig. 4a), tumor-associated lymphoid proliferation around the tumor (n = 4) (Fig. 4b), metaplastic bone (n = 2), hyalinizing fibrosis (n = 2), and psammomatoid calcifications (n = 1). Only one MSA drastically departed from this typical morphologic appearance (Fig. 5). Case 23 was a 2.0 cm tumor that arose within the retromolar trigone of a 46-year-old man. While it focally had features of classic MSA, it was dominated by solid growth with spindle cell morphology, rosette-like structures and clear cell change. The mitotic rates were low in each tumor, and necrosis was absent.
Fig. 2.
The microsecretory adenocarcinomas appeared well circumscribed at low power (a), yet on closer inspection infiltrated nearby tissues such as adipose tissue (b) or nearby seromucinous glands (c). Perineural invasion was only seen in 1 of 24 cases (d)
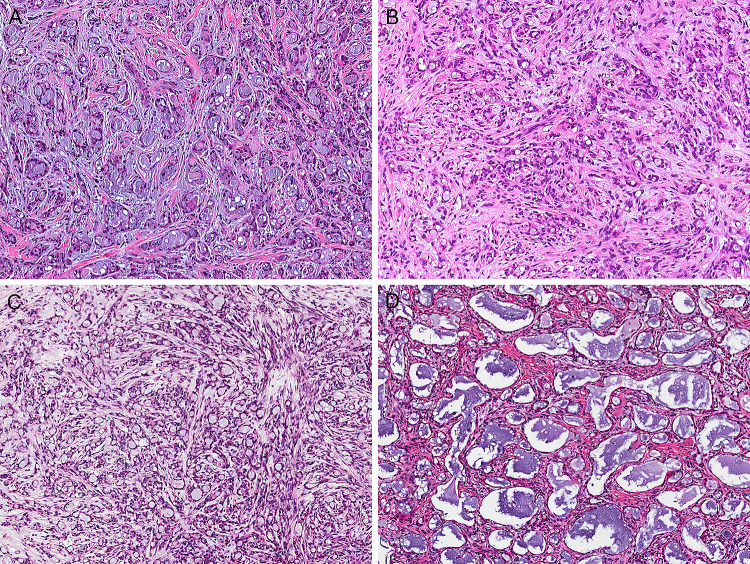
Fig. 3.
Microsecretory adenocarcinoma consistently grows as microcysts with bluish intraluminal secretions, set in a fibromyxoid stroma. However, the size of the tubules and prominence/cellularity of the stroma varied from case to case (all images at the same power, X100)
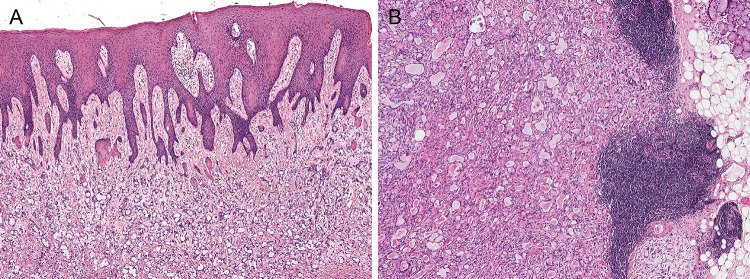
Fig. 4.
Infrequent features for microsecretory adenocarcinoma included pseudoepitheliomatous hyperplasia of the overlying oral squamous epithelium (5 of 24 cases) (a) and tumor-associated lymphoid proliferation (4 of 24 cases) (b)
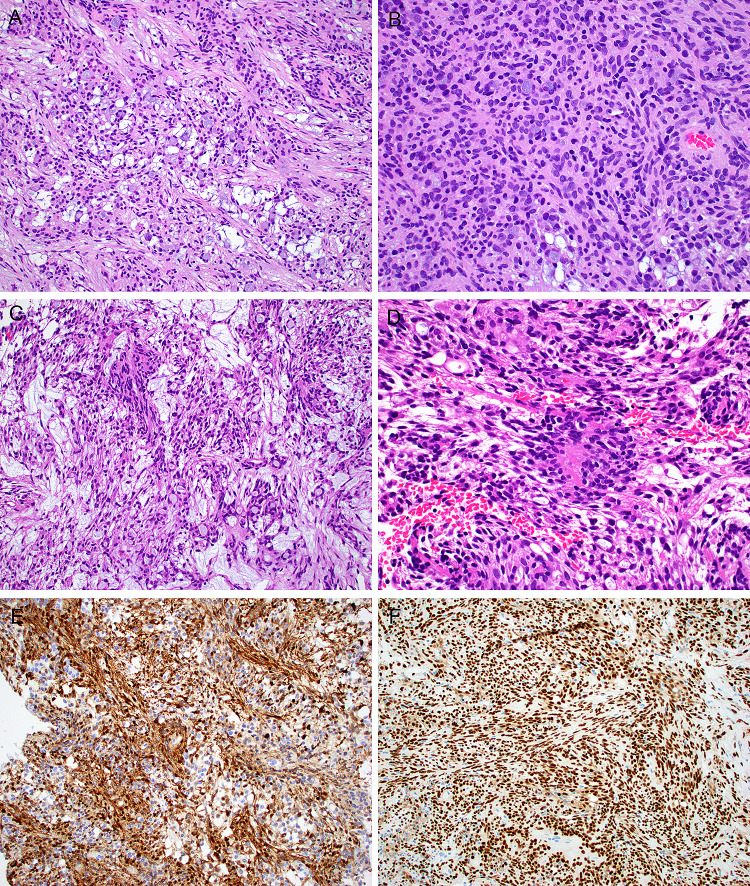
Fig. 5.
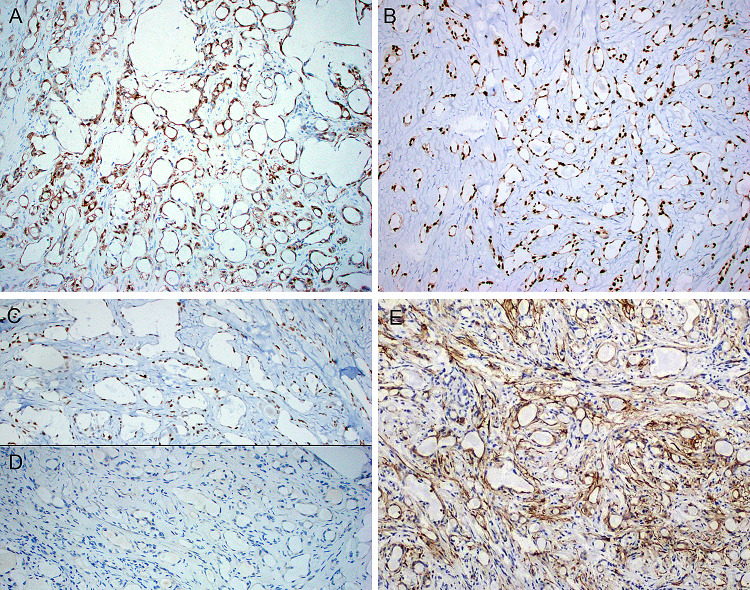
One microsecretory adenocarcinoma had an unusual morphologic appearance. Although in foci it exhibited typical microcystic growth (a), it was dominated by solid growth (b) with tumor cell spindling (c) and peculiar rosette-like structures (d). This tumor had the typical immunoprofile including diffuse staining with S100 (e) and SOX10 (f), and harbored the classic MEF2C-SS18 fusion
Immunohistochemical and molecular findings are summarized in Table 2. By immunohistochemistry, the MSAs were consistently positive for S100 (24 of 24) (Fig. 6a), although 3 cases showed only focal or weak staining. SOX10 was diffuse and strongly positive in all cases tested (14 of 14) (Fig. 6b), including 2 of the cases that were only weakly positive for S100. The MSAs were also consistently positive for p63 (24 of 24) (Fig. 6c), either diffusely (n = 16) or focally (n = 5), but every case tested was negative for p40 (0 of 21) (Fig. 6d). Mammaglobin (0 of 16) and calponin (0 of 12) were also consistently negative, but SMA was variable, with 4 of 20 cases showing focal positivity in a luminal distribution (Fig. 6e).
Table 2.
Immunohistochemical and molecular testing for microsecretory adenocarcinomas
| Case | S100 | SOX10 | p63 | p40 | SMA | Calp | MGB | SS18 FISH | RNA-Seq or PCR |
|---|---|---|---|---|---|---|---|---|---|
| 1 | + | + | + | − | − | − | − | + | MEF2C-SS18 |
| 2 | + | + | + | − | − | − | − | + | MEF2C-SS18 |
| 3 | + | ND | + | − | − | − | − | ND | MEF2C-SS18 |
| 4 | + | + | + | − | − | − | − | + | MEF2C-SS18 |
| 5 | + | ND | + | − | − | − | − | + | MEF2C-SS18 |
| 6 | W+ | + | + | − | ND | ND | − | ND | MEF2C-SS18 |
| 7 | + | ND | + | ND | F+ | ND | ND | − | MEF2C-SS18 |
| 8 | + | + | + | − | − | − | ND | ND | MEF2C-SS18 |
| 9 | + | + | + | − | − | ND | − | ND | MEF2C-SS18 |
| 10 | + | ND | + | − | − | ND | − | + | Failed |
| 11 | + | + | + | − | − | − | − | + | MEF2C-SS18 |
| 12 | + | ND | F+ | − | − | ND | − | + | MEF2C-SS18 |
| 13 | + | ND | F+ | − | − | ND | − | + | MEF2C-SS18 |
| 14 | F+ | ND | F+ | − | F+ | ND | − | + | MEF2C-SS18 |
| 15 | + | + | + | − | − | − | ND | ND | MEF2C-SS18 |
| 16 | + | + | + | − | − | ND | − | ND | MEF2C-SS18 |
| 17 | W+ | + | + | − | − | − | − | ND | MEF2C-SS18 |
| 18 | + | + | + | − | ND | ND | ND | + | MEF2C-SS18 |
| 19 | + | ND | + | − | ND | ND | ND | ND | MEF2C-SS18 |
| 20 | + | ND | + | − | − | − | − | ND | MEF2C-SS18 |
| 21 | + | + | + | ND | F+ | ND | ND | ND | MEF2C-SS18 |
| 22 | + | + | F+ | − | − | − | ND | + | Failed |
| 23 | + | + | + | − | F+ | − | − | + | MEF2C-SS18 |
| 24 | + | ND | F+ | ND | ND | ND | ND | + | ND |
SMA smooth muscle actin, Calp calponin, MGB mammaglobin, FISH fluorescence in situ hybridization, RNA-seq RNA sequencing, PCR polymerase chain reaction, ND not done, F focal, W weak
Fig. 6.
The microsecretory adenocarcinomas were very consistently positive for S100 (a), SOX10 (b) and p63 (c), but always negative for p40 (d). SMA was usually negative, but a few cases had focally positive staining within the tumor epithelium (e)
Targeted RNA sequencing was attempted on 21 cases, and was successful in 19. In the remaining two cases which failed, there was insufficient RNA to pass quality assurance. For each case with successful RNA sequencing, MEF2C-SS18 fusion was found. The breakpoints for each identified fusion were identical: exon 7 of the MEF2C gene (NCBI Reference Sequence: NM_002397.4) and exon 4 of the SS18 gene (NM_001007559.2). The four cases for which RT-PCR and Sanger sequencing was done also successfully demonstrated the MEF2C-SS18 fusion product. Break apart FISH for SS18 was done on 14 cases, including on the two cases where RNA sequencing failed. It was positive in 13 of 14 cases; the single negative case was positive for MEF2C-SS18 fusion by RNA sequencing. Taken together, all 24 MSAs were demonstrated by molecular analysis to have an alteration of SS18, and MEF2C was identified as the fusion partner in 21 of 24 cases.
Discussion
Salivary gland tumor classification has drastically evolved over the last decade, with emerging molecular findings helping to refine the histologic boundaries of many well-known salivary gland tumors [7]. In some cases, the identification of a novel fusion has actually allowed for the recognition of entirely new tumor types. Secretory carcinoma, a tumor which characteristically harbors ETV6-NTRK3 fusion, is the best-known example of this phenomenon [8]. More recently, a purportedly distinct tumor called microsecretory adenocarcinoma (MSA) was described with seemingly unique histologic, immunohistochemical, and molecular features including a novel MEF2C-SS18 fusion [2]. However, the initial report of MSA only included five cases, none of which had clinical follow-up available. Since that report, only one additional case of MSA has been published [3]. This series was designed to collect a more comprehensive experience with MSA that permits a clearer understanding of its biologic behavior and a more complete description of its histologic and immunophenotypic features. In doing so, it aims to solidify MSA as a bona fide tumor entity worthy of inclusion in future classification schemes such as the WHO classification of head and neck tumors.
First, this expanded series underscores the remarkably consistent and distinctive pathological profile of MSA. Virtually all MSA demonstrate the recurrent and recognizable histological features described in the original characterization of this tumor including: (1) a microcystic-predominant growth pattern, (2) uniform intercalated duct-like cells with attenuated eosinophilic to clear cytoplasm, (3) monotonous oval hyperchromatic nuclei with indistinct nucleoli, (4) abundant basophilic luminal secretions, (5) a cellular fibromyxoid stroma, and (6) rounded borders with subtle infiltrative growth. Only one case looked dramatically different with a prominent component of spindled epithelial cells. Even this case, however, had focal areas with classic MSA histologic features. The immunoprofile of MSA was also extremely consistent in this larger series with consistent lesional expression of S100, SOX10, and p63 while p40, calponin and mammaglobin were negative in all tested cases. Unexpectedly, a handful of cases did show focal SMA positivity in the tumor ducts. This finding should not be taken as evidence of true myoepithelial differentiation but rather part of the established tendency of tumors with intercalated-duct like phenotypes to show occasional focal reactivity with myoepithelial markers, as also observed in polymorphous adenocarcinoma [9, 10]. Overall, this highly consistent histologic appearance and immunophenotype support classification as a distinctive entity.
This expanded experience also highlights that the characteristic MEF2C-SS18 fusion reported in the original description of MSA is a highly consistent feature of tumors that show this distinctive morphologic appearance and immunohistochemical profile. All tumors in this series demonstrated evidence of this fusion by at least one modality, including RNA sequencing, SS18 FISH, or RT-PCR. All cases evaluated with RNA sequencing even displayed identical breakpoints at exon 7 of the MEF2C gene and exon 4 of the SS18 gene. Interestingly, one case with MEF2C-SS18 had a false-negative break apart SS18 FISH result, suggesting that this assay is not entirely sensitive for MSA as previously reported in small numbers [11]. False negative FISH is a well-described phenomenon for many fusion-driven tumors [12], and FISH still demonstrates excellent sensitivity for MEF2C-SS18 fusions and provides a widely-available means of confirming this diagnosis. Of course, MSAs with variant molecular profiles despite classic histologic and immunohistochemical features may well be discovered later, as may additional tumors such as the previously-reported carcinoma with SS18-ZBTB7A fusion and overlapping but distinctive morphology from MSA [2], the significance of which remains unclear. However, it appears that the genetic underpinnings of MSA are overall quite uniform, similar to the overwhelming predominance of ETV6-NTRK3 in secretory carcinoma or EWSR1-ATF1 in clear cell carcinoma and in contrast to the heterogeneous amalgamation of fusions seen in adenoid cystic carcinoma or myoepithelial carcinoma [8, 13–17]. Indeed, the histologic, immunohistochemical, and genetic profile of MSA is so characteristic that it can reasonably be asked whether molecular testing will always be indicated to classify this entity when the classic morphology and staining pattern is present. Right now, molecular confirmation of the diagnosis is certainly still desirable as pathologists gain experience with this novel entity and will likely remain necessary in unusual cases like the partially spindled tumor in this series. However, it would not be surprising if a diagnosis of MSA, like most other common or rare salivary tumor types, can soon be routinely made based on its recognizable histologic pattern and immunoprofile alone.
Not only have the increased numbers of MSA cases confirmed the characteristic clinical presentation of this tumor, but they also provided concrete insight into its actual behavior. As observed in the original series, the vast majority of MSAs occur as small tumors in intraoral minor salivary glands of adults with a slight female predominance. Specifically, MSA seems to have a proclivity for the palate, with almost 60% of cases arising in this subsite; another 25% of tumors involved the buccal mucosa. The single parotid tumor also included in the original study remains the only instance of MSA in a non-oral site to date. More importantly, these findings confirm the expected indolent behavior of this tumor type. MSA was initially presumed to be a low-grade carcinoma on the basis of bland cytomorphology and an often subtly infiltrative growth pattern, but no follow-up was available in the initial report to confirm this impression. In this expanded series, all 24 cases did still display low-grade histologic features. Furthermore, treatment and follow-up information was available in 17 and 14 cases, respectively, and no cases metastasized or recurred following surgery alone, including one case with 18 years of follow-up. This data also strongly supports the notion that MSA is a low-grade neoplasm with indolent behavior. Although no cases metastasized, the presence of clear, destructive invasion of surrounding tissues nevertheless warrants its classification as a carcinoma.
Finally, the submitting or original diagnoses for this wider spectrum of MSA cases expand the differential diagnosis of these tumors. As expected, most cases were originally classified as adenocarcinoma, NOS, and reclassification as MSA further highlights the diminishing nature of that category [18]. Another significant subset were called polymorphous adenocarcinoma, reflecting the overlapping intercalated duct-like morphology and immunophenotypes of these two groups [19]. However, additional diagnostic considerations recognized in this series included mucinous adenocarcinoma and secretory myoepithelial carcinoma. Mucinous adenocarcinoma is a historically ambiguous and histologically heterogeneous category that has only recently has become better defined as a unified group based on its seemingly tumor-defining AKT1 mutations [20, 21]. Not only were two cases in this series flagged as having mucinous features, but several cases historically reported as signet ring carcinomas bear a notable morphologic resemblance to MSA [22]. While bluish mucinous secretions are a striking feature of MSA and attenuated tubules that confer a signet ring appearance can be focally seen, the vast majority of mucinous adenocarcinomas have macrocystic, papillary, and colloid architecture or sheets of discohesive signet ring cells that are quite distinct from the uniform and predominant microcysts seen in MSA. Moreover, mucinous adenocarcinoma is consistently negative for S100, SOX10, and p63. Secretory myoepithelial carcinoma is a relatively recently-described variant of myoepithelial carcinoma characterized by tumor cells that have intracellular or extracellular mucin production but a myoepithelial phenotype [23, 24]. Although MSA expresses S100, SOX10, and p63, which are myoepithelial markers, they are not specific indicators of myoepithelial lineage and are also frequently positive in tumors which recapitulate intercalated ducts. Again, while a subset of MSA did express SMA, which is a more specific marker of myoepithelial differentiation, it is also known to show occasional nonspecific reactivity in intercalated duct-like tumors [9, 10]. Recognition that MSA is a duct-forming tumor allows it to be distinguished in most cases from myoepithelial carcinoma which grows as cords, solid nests, and trabeculae but not well-formed ducts. Furthermore, myoepithelial tumors will show concordant p63/p40 staining patterns (either both positive or negative), in contrast to the consistent discordant pattern of MSA. Ultimately, demonstration of an SS18 fusion, either by SS18 FISH or by RNA sequencing or PCR, resolves the differential diagnosis since it has not been encountered in any other salivary gland tumor to date [11].
In summary, MSA is a distinctive, low-grade salivary gland malignancy with remarkably consistent clinical, histologic, immunophenotypic, and genetic characteristics. As a unique, recently-described neoplasm, MSA should be considered for inclusion in the next version of the WHO classification of head and neck tumors.
Funding
This study was funded by the Jane B. and Edwin P. Jenevein M.D Endowment for Pathology at UT Southwestern Medical Center. No external funding was obtained for this study.
Declarations
Conflict of interest
All authors declare that he/she has no conflict of interest as it relates to this research project.
Ethical Approval
All procedures performed in this retrospective data analysis involving human participants were in accordance with the ethical standards of the institutional review board (IRB 112017–073), which did not require informed consent.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Skalova A, Stenman G, Simpson RHW, et al. The role of molecular testing in the differential diagnosis of salivary gland carcinomas. Am J Surg Pathol. 2018;42:e11–e27. doi: 10.1097/PAS.0000000000000980. [DOI] [PubMed] [Google Scholar]
- 2.Bishop JA, Weinreb I, Swanson D, et al. Microsecretory adenocarcinoma: a novel salivary gland tumor characterized by a recurrent MEF2C-SS18 fusion. Am J Surg Pathol. 2019;43:1023–1032. doi: 10.1097/PAS.0000000000001273. [DOI] [PubMed] [Google Scholar]
- 3.Kawakami F, Nagao T, Honda Y, et al. Microsecretory adenocarcinoma of the hard palate: a case report of a recently described entity. Pathol Int. 2020;70:781–785. doi: 10.1111/pin.12987. [DOI] [PubMed] [Google Scholar]
- 4.Bishop JA, Gagan J, Baumhoer D, et al. Sclerosing polycystic "Adenosis" of salivary glands: a neoplasm characterized by PI3K pathway alterations more correctly named sclerosing polycystic adenoma. Head Neck Pathol. 2019. [DOI] [PMC free article] [PubMed]
- 5.Agaimy A, Togel L, Haller F, et al. YAP1-NUTM1 gene fusion in porocarcinoma of the external auditory canal. Head Neck Pathol. 2020;14:982–990. doi: 10.1007/s12105-020-01173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haas BJ, Dobin A, Li B, et al. Accuracy assessment of fusion transcript detection via read-mapping and de novo fusion transcript assembly-based methods. Genome Biol. 2019;20:213. doi: 10.1186/s13059-019-1842-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skalova A, Vanecek T, Simpson RHW, et al. Molecular advances in salivary gland pathology and their practical application. Diagn Histopathol. 2012;18:388–396. doi: 10.1016/j.mpdhp.2012.08.002. [DOI] [Google Scholar]
- 8.Skalova A, Vanecek T, Sima R, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34:599–608. doi: 10.1097/PAS.0b013e3181d9efcc. [DOI] [PubMed] [Google Scholar]
- 9.Epivatianos A, Poulopoulos A, Dimitrakopoulos I, et al. Application of α-smooth muscle actin and c-kit in the differential diagnosis of adenoid cystic carcinoma from polymorphous low-grade adenocarcinoma. Oral Oncol. 2007;43:67–76. doi: 10.1016/j.oraloncology.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Beltran D, Faquin WC, Gallagher G, et al. Selective immunohistochemical comparison of polymorphous low-grade adenocarcinoma and adenoid cystic carcinoma. J Oral Maxillofacial Surg. 2006;64:415–423. doi: 10.1016/j.joms.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 11.Bishop JA, Koduru P, Veremis BM, et al. SS18 Break-apart fluorescence in situ hybridization is a practical and effective method for diagnosing microsecretory adenocarcinoma of salivary glands. Head Neck Pathol. 2021. [DOI] [PMC free article] [PubMed]
- 12.Rashidi A, Fisher SI. FISH-negative, cytogenetically cryptic acute promyelocytic leukemia. Blood Cancer J. 2015;5:e320. doi: 10.1038/bcj.2015.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antonescu CR, Katabi N, Zhang L, et al. EWSR1-ATF1 fusion is a novel and consistent finding in hyalinizing clear-cell carcinoma of salivary gland. Genes Chromosomes Cancer. 2011;50:559–570. doi: 10.1002/gcc.20881. [DOI] [PubMed] [Google Scholar]
- 14.Brayer KJ, Frerich CA, Kang H, et al. Recurrent fusions in MYB and MYBL1 define a common, transcription factor-driven oncogenic pathway in salivary gland adenoid cystic carcinoma. Cancer Discov. 2016;6:176–187. doi: 10.1158/2159-8290.CD-15-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalin MG, Desrichard A, Katabi N, et al. Comprehensive molecular characterization of salivary duct carcinoma reveals actionable targets and similarity to apocrine breast cancer. Clin Cancer Res. 2016;22:4623–4633. doi: 10.1158/1078-0432.CCR-16-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitani Y, Liu B, Rao PH, et al. Novel MYBL1 gene rearrangements with recurrent MYBL1-NFIB fusions in salivary adenoid cystic carcinomas lacking t(6;9) translocations. Clin Cancer Res. 2016;22:725–733. doi: 10.1158/1078-0432.CCR-15-2867-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rettig EM, Talbot CC, Jr, Sausen M, et al. Whole-genome sequencing of salivary gland adenoid cystic carcinoma. Cancer Prev Res. 2016;9:265–274. doi: 10.1158/1940-6207.CAPR-15-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rooper LM, Mansour M, Yonescu R, et al. The decline of salivary adenocarcinoma not otherwise specified as a tumor entity: reclassification using contemporary immunohistochemical profiling and diagnostic criteria. Am J Surg Pathol. 2020. [DOI] [PubMed]
- 19.Rooper L, Sharma R, Bishop JA. Polymorphous low grade adenocarcinoma has a consistent p63+/p40- immunophenotype that helps distinguish it from adenoid cystic carcinoma and cellular pleomorphic adenoma. Head Neck Pathol. 2015;9:79–84. doi: 10.1007/s12105-014-0554-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agaimy A, Mueller SK, Bumm K, et al. Intraductal papillary mucinous neoplasms of minor salivary glands with AKT1 p.Glu17Lys mutation. Am J Surg Pathol. 2018;42:1076–1082. doi: 10.1097/PAS.0000000000001080. [DOI] [PubMed] [Google Scholar]
- 21.Rooper LM, Argyris PP, Thompson LDR, et al. Salivary mucinous adenocarcinoma is a histologically diverse single entity with recurrent AKT1 E17K mutations: clinicopathologic and molecular characterization with proposal for a unified classification. Am J Surg Pathol. 2021. [DOI] [PubMed]
- 22.Ghannoum JE, Freedman PD. Signet-ring cell (mucin-producing) adenocarcinomas of minor salivary glands. Am J Surg Pathol. 2004;28:89–93. doi: 10.1097/00000478-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Bastaki JM, Purgina BM, Dacic S, et al. Secretory myoepithelial carcinoma: a histologic and molecular survey and a proposed nomenclature for mucin producing signet ring tumors. Head Neck Pathol. 2014;8:250–260. doi: 10.1007/s12105-014-0518-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gnepp DR. Mucinous myoepithelioma, a recently described new myoepithelioma variant. Head Neck Pathol. 2013;7(Suppl 1):S85–89. doi: 10.1007/s12105-013-0464-x. [DOI] [PMC free article] [PubMed] [Google Scholar]