Abstract
Clear Cell odontogenic Carcinomas (CCOC) are rare, aggressive malignant odontogenic tumours which are often misdiagnosed as benign odontogenic tumours due to the non-specific histologic appearance, and benign early clinical presentation. However, due to their propensity to metastasize, the best outcomes are experienced with they are diagnosed early and treated aggressively. In this paper, we present a case of a CCOC misdiagnosed as a clear cell calcifying epithelial odontogenic tumour which was only found to be a CCOC after cervical node metastasis. The original diagnosis was questioned and confirmed to be a CCOC by identification of the chromosomal translocation EWSR1 on fluorescence in situ hybridization. This has recently been described in CCOC and a wide variety of other mesenchymal and epithelial neoplasms. Previous reports have demonstrated EWSR1–ATF1 and EWSR1–CREB1 fusions in CCOC. Next generation sequencing of this case demonstrated the EWSR1–CREM fusion gene which has not been previously reported for CCOC. CREM fusion proteins have only recently been found in several tumour types including the closely associated hyalinizing clear cell carcinoma of salivary glands. This is discussed in this paper, and the role of the discovery of the CREM fusion protein in CCOC adds to your understating of the role of CREM in oncogenesis, and the possible link between CCOCs and hyalinizing clear cell carcinomas.
Keywords: Clear cell odontogenic carcinoma, Calcifying epithelial odontogenic tumour, Odontogenic tumours, EWSR1–ATF1, EWSR1–CREB, EWSR1–CREM
Introduction
Clear Cell Odontogenic Carcinomas (CCOCs) are rare, aggressive malignant odontogenic tumours with a propensity to metastasize [1, 2]. They were first described by Hansen in 1985, and originally recognized by the World Health Organization (WHO) as “clear cell odontogenic tumour” [3]. It was reclassified as a malignancy in 2005, due to its locally aggressive nature, high recurrence rates, and tendency to metastasize to distant sites [4]. CCOCs often have an inconspicuous clinical and radiological presentation. Patients develop non-specific symptoms such as expansion, pain and loosening of teeth, and the radiological appearance is characterised by ill-defined radiolucencies in the maxillofacial skeleton with potential for dental resorption similar to any other odontogenic tumour.
CCOCs are also difficult to diagnose and differentiate from other odontogenic lesions on histopathological examination. They are exceedingly rare, with just over 100 reported cases in the literature [5], and are difficult to distinguish from the clear cell variants of other odontogenic tumours, such as calcifying epithelial odontogenic tumours (CEOT). Three different histological patterns have been described: biphasic, ameloblastomatous and monomorphic [6]. These patterns are often not specific enough to be pathognomonic, can closely resemble other odontogenic tumours, and may co-occur within a single lesion. Additionally, CCOCs histologically resemble other clear cell tumours arising from non-odontogenic tissues, including salivary gland hyalinizing clear cell carcinomas, renal carcinomas and some sarcomas. These similarities may in fact be causative rather than coincidental: recent findings suggest that CCOC may be the bony counterpart of the hyalinizing clear cell carcinoma of the salivary gland [7], based on evidence of chromosomal translocation involving EWSR1 (Ewing Sarcoma RNA Binding Protein 1) in both lesions.
In this paper we present a case of a CCOC which was misdiagnosed for many years as a CEOT, until it metastasized to the cervical lymph nodes. This case is also the first recorded case demonstrating a novel fusion gene in clear cell odontogenic carcinoma, EWSR1–CREM, using next generation sequencing (NGS).
Case Report
A 63 year old male was referred in 2008 to the Oral and Maxillofacial Surgery unit with an incidental finding of a radiolucent lesion in the left posterior maxilla. He had been diagnosed with an odontogenic tumour in the left maxilla 15 years earlier at another unit, and had undergone surgical excision at that time. The original pathology was reported as an odontogenic tumour, and due to the non-specific nature of the findings, it was reviewed by several expert head and neck pathologists who concluded that it was an unusual clear cell variant of a calcifying epithelial odontogenic tumour. The features were unusual because of the lack of certain pathognomonic features of CEOT including amyloid deposition and concentric calcifications known as Liesegang rings.
He had a past medical history of cerebrovascular disease and hypertension. His regular medications included amlodipine, aspirin and clopidogrel. He was allergic to penicillin. He was a non-smoker and drank alcohol in moderation. He has a family history of ischaemic heart disease, but no known family history of cancer.
On presentation to our department (in 2008), he underwent an enucleation and curettage of the lesion. At this time, histopathologic features were consistent with the previous pathology. Over the following 7 years (2008–2015), the tumour recurred several times, and he underwent 5 resections of the tumour, culminating in a left posterior maxillectomy and wide excision of the tumour, with a lip-split mandibulotomy for access. Of note, progressive pathological examinations demonstrated increasing clusters of clear cells amongst the epidermoid cells within the tumour, but no significant numbers of mitoses or features of malignancy. The clear cells were negative for mucin following diastase-PAS, and mucicarmine staining. The clear cells were faintly positive for PAS. Hyalinised material was present, and negative on Congo Red staining.
Two years later (2017), 25 years after his initial diagnosis, the patient attended clinic complaining of visual disturbance and left sided headaches. An MRI showed recurrence with infiltration into the infratemporal fossa and base of skull (Fig. 1). He underwent endoscopic debulking of the tumour and 5-fluorouracil packing with some symptomatic relief. The histopathology showed the same odontogenic tumour as in previous excisions. with some increased clear cell differentiation and hyalinized material, but no malignant change (Fig. 4). In November 2018, on clinical review, a palpable cervical node was noted in the left neck. MRI and CT of the region (Fig. 2) showed significant base of skull persistent disease with slow progression of the left infratemporal fossa tumour, and stable perineural tumour spread at the ipsilateral foramen ovale. These scans (Figs. 2 and 3) also demonstrated an enlarged left cervical level II cervical lymph node of 19 mm diameter, with pathological features (Fig. 4).
Fig. 1.
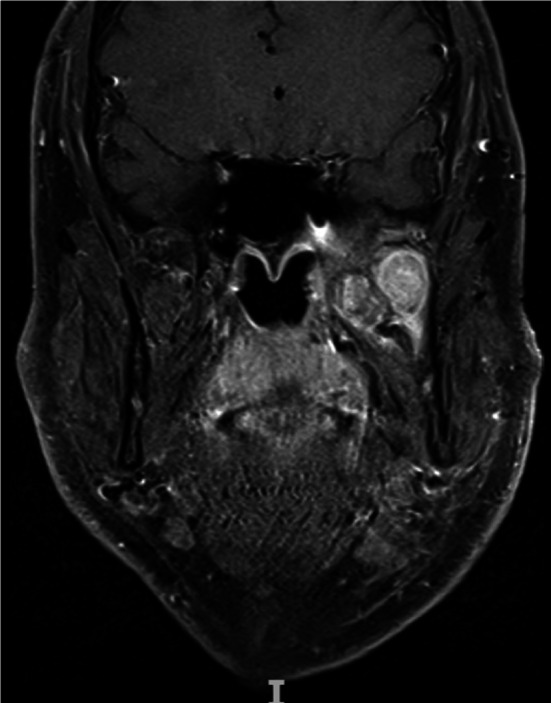
MRI T1 weighted coronal view shows the recurrence within the infratemporal fossa. Laterally the lesion abuts the coronoid process of the mandible and abuts the skull base extending into the orbital apex
Fig. 4.
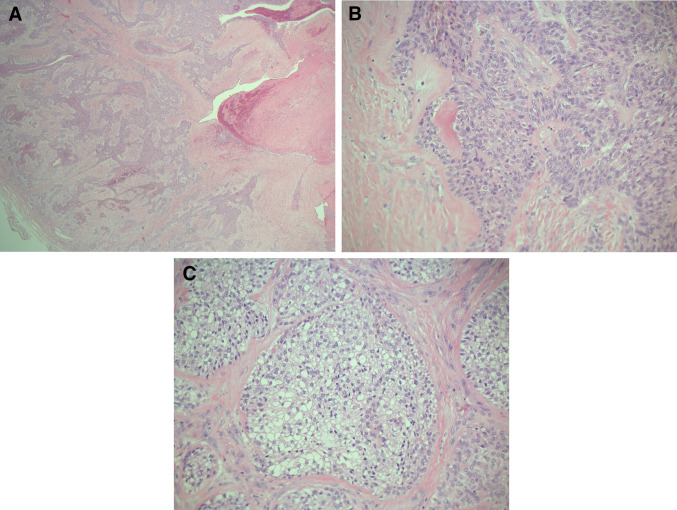
Histopathology of primary recurrent tumour. a H&E stain, ×25 magnification. The tumour comprises of islands, cords, trabeculae, and sheet of neoplastic epithelial cells. b H&E stain, ×200 magnification, the lesional cells are polyhedral with abundant well-defined cytoplasm. No calcifications or amyloid are clearly noted. c H&E stain, ×200 magnification. Clear cell change in the epithelial cells (glycogen accumulation)
Fig. 2.
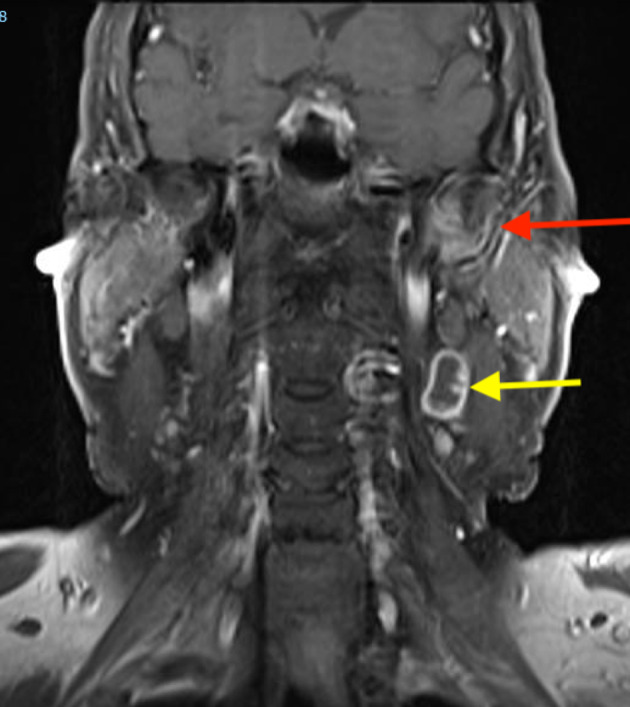
T1 weighted coronal view showing the skull base recurrence (red arrow) and enlarged left level II lymph node with a necrotic core (yellow arrow)
Fig. 3.
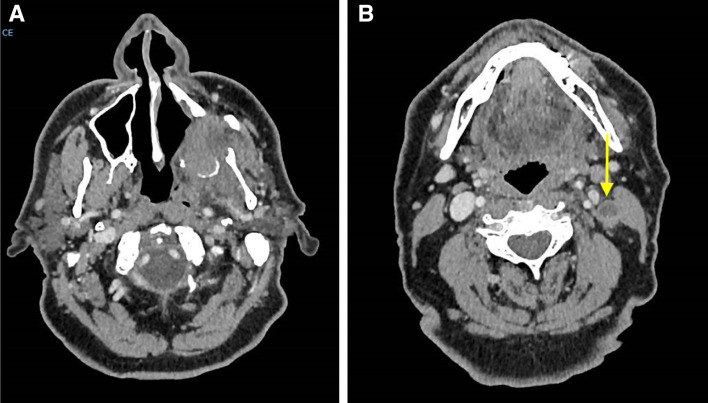
CT showing posterior maxillary and skull base recurrence (a) and pathologic necrotic level II lymph node (marked by yellow arrow) (b)
Core biopsy of the lymph node showed a metastatic odontogenic tumour. Initially, it was thought that this was a malignant clear cell variant of CEOT, however, the features of the lymph node biopsy were most in keeping with a clear cell odontogenic carcinoma, showing lymphoid tissue focally infiltrated by patchy cohesive sheets of atypical cells with intermediate sized nuclei, and inconspicuous nucleoli with abundant vacuolated cytoplasm (Fig. 5). There was evidence of increased mitoses, apoptotic bodies and focal coagulative type tumour necrosis.
Fig. 5.
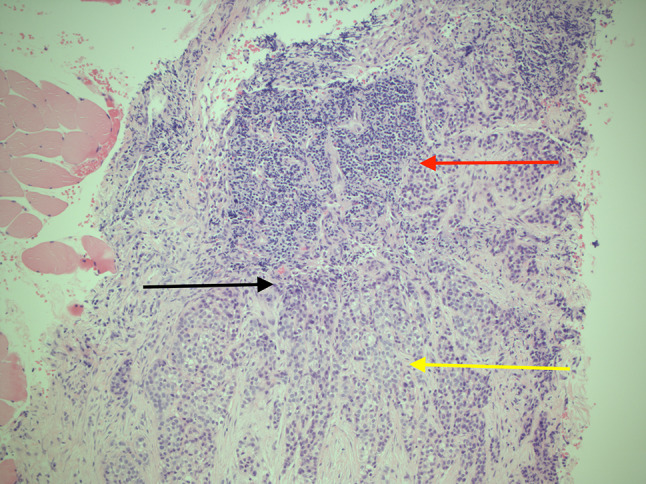
Histopathologic slides showing the lymph node core biopsy. H&E stain, ×100 magnification. Demonstrates sheets of polyhedral epithelial cells adjacent to lymphoid tissue of the lymph node. Red arrow shows lymphoid tissue. Yellow arrow shows clusters of clear cells with pleomorphism and hyperchromatic nuclei. Black arrow shows epithelial cells with pleomorphism and hyperchromatic nuclei adjacent to the lymphoid tissue. Note also the small blood vessel within the area with malignant cells surrounding the vessel
The findings were discussed at the head and neck multidisciplinary team. The skull base involvement was considered too advanced for further resection, and so the patient was referred to oncology for consideration of radiotherapy to the primary site and left neck, which the patient initially declined.
The inconsistency between the original diagnosis of CEOT and the metastatic lesion led to a series of further tests to determine if this was a case of malignant transformation of calcifying epithelial odontogenic tumour, or a clear cell odontogenic carcinoma.
Further immunohistochemistry staining was performed to exclude the node being a metastatic lesion of other tumours with clear cell changes such as a melanoma or a renal cell carcinoma. Tumour suppressor p53 expression was low (< 10%) in the tumour and the cervical node, and Ki-67 expression was found to be substantially higher in the cervical lymph node than in the tumour (50% vs 3%), suggesting an increased proliferative phenotype. Increased Ki67 expression has been described in cases of malignant transformation of CEOT, however it is a nonspecific marker of proliferation.
To exclude clear cell odontogenic carcinoma, fluorescence in situ hybridization (FISH) was then performed on the formalin fixed paraffin wax embedded tissue. 51 cells were examined over a minimum of 4 lesional areas with Vysis EWSR1 (22q12) dual colour break apart rearrangement FISH probe (Abbot laboratories, Illinois, USA) (Fig. 6). EWSR1 gene rearrangement was found in 46/51 cells (90%). Reverse transcriptase-PCR was then performed using Qiagen One-step rt-PCR (Qiagen, Vialo, The Netherlands) to analyze for the partner fusion genes known to be associated with CCOC: EWSR1–ATF1 and EWSR1–CREB1. Neither ATF1 nor CREB1 were detected.
Fig. 6.
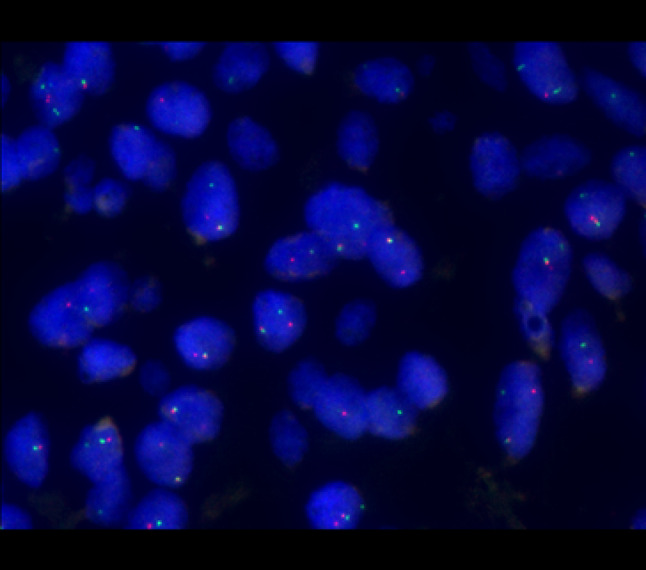
The Vysis LSI EWSR1 (22q12) Dual Colour, Break Apart Rearrangement Probe consists of a mixture of two FISH DNA probes. The first probe is labelled in SpectrumOrange (~ 500 kb probe), flanks the 5′ side of the EWSR1 gene and extends into intron 4. The second probe is labelled SpectrumGreen (~ 1100 kb probe), flanks the 3′ side of the EWSR1 gene. Between the two probes, there is a 7 kb gap. The known break points within the EWSR1 gene are restricted to introns 7 through 10. The majority of cells in the images show a signal pattern of one fusion, one orange and one green signal. This is indicative of a rearrangement of one copy of the EWSR1 gene region
Next generation sequencing was then performed using the pan cancer TruSight Tumour 170 kit (Illumina, San Diego, CA) to investigate tumour DNA and RNA. Data analysis was performed using custom bioinformatics pipeline: SMP2v3 v1.1.1851, specifically analysing for EWSR1 (exons 1–19) and all reported EWSR1 rearrangements [8]. This analysis detected a rearrangement in the EWSR1 gene: EWSR1 (exon 9)-CREM (exon 4) [9]. Although this partner gene has never been described in clear cell odontogenic carcinoma, the finding of EWSR1 gene rearrangement confirms that this lesion was a CCOC rather than malignant transformation of CEOT.
Eight months post-diagnosis of the neck node, he developed a painful lesion in the left nasolabial fold which was excised and confirmed to be a further metastasis of the tumour. This appeared to develop by spread through the infraorbital canal. Imaging showed some progression of the primary tumour and cervical node. After excision of the further metastasis, he was offered radiotherapy again to the primary lesion including the nasolabial region and the left neck. He agreed to proceed with radiotherapy which was completed 1 year after diagnosis of the metastatic neck node. Further imaging after completing radiotherapy demonstrated multiple bony metastases in the vertebrae and the pelvis, and the patient was deemed palliative. The patient died of the disease 6 months after radiotherapy. (Note: Patient consent was acquired to publish his findings and imaging).
Discussion
Clear cell odontogenic carcinoma is a rare malignant tumour. Although generally considered a low grade malignancy, CCOCs have the potential to be locally destructive and aggressive, with a high rate of local recurrence and propensity to metastasize to regional lymph nodes and distant sites [10]. They can also progress in a very indolent manner almost behaving like benign tumours, with symptoms lasting many years before metastasis, as seen in the current case [5]. Microscopically, they are characterized by proliferation of neoplastic epithelial cells with predominantly clear cytoplasm, arranged in islands and strands, often with a surrounding fibrotic stroma. Clear cells make diagnosis difficult because they can be found in several lesions that can present in a similar manner clinically (Table 1) [11].
Table 1.
The odontogenic tumours in this list are the most difficult to differentiate from CCOC due to the similar histopathological patterns, similar clinical and radiographical appearance, and potential for malignancy
| Lesions presenting with similar histology to Clear Cell Odontogenic Carcinoma |
| Clear cell variant of Calcifying Epithelial Odontogenic Tumour (CEOT) |
| Mucoepidermoid Carcinoma |
| Primary Clear Cell Sarcoma |
| Melanoma |
| Metastatic Renal Cell Carcinoma |
| Hyalinising Clear Cell Carcinoma (HCCC) |
Many odontogenic tumours straddle a fine line between benign and malignant. This can be seen in their expression of oncogenic signalling pathways also found in malignant tumours [12]. Even more elusive, in large part due to their rarity, are benign odontogenic tumours, such as ameloblastoma, that demonstrate metastatic behaviour. Metastasising ameloblastoma is characterized by a cytologically benign ameloblastoma that metastasises but maintains the characteristic benign cytological characteristics of the parent tumour. The molecular signature of these metastasizing ameloblastomas remains unclear [13], but further highlights the unusual benign/malignant phenotype of odontogenic tumours.
In the presented case, the initial diagnosis was thought to be a clear cell variant of calcifying epithelial odontogenic tumour (CEOT). Although missing certain important histologic characteristics including amyloid deposition and Leisegang rings, several experts felt the features were most in keeping with a CEOT. After it metastasised, it was thought to be a case of malignant transformation of CEOT, which has been previously reported in 7 cases [14–20]. Of note, all of these cases demonstrated clear features of CEOT including presence of amyloid. Clear cell variant is considered by some authors as a more aggressive form of CEOT, however a recent systematic review demonstrated that this was not the case [21]. It is unclear if these cases are more likely to transform into the malignant variant. In one case of malignant transformation, it was clearly reported that the lesion was a clear cell variant of CEOT that transformed [22]. This was considered in our case, however, the metastatic node demonstrated a malignant clear cell tumour, most in keeping with a clear cell odontogenic carcinoma. This prompted a review of the original pathology and FISH was performed to assess for EWSR1 gene rearrangement.
A review of the literature demonstrates several other reported cases of CCOC misdiagnosed as a CEOT [7, 23]. Bilodeau et al., reclassified 2 cases of presumed CCOC as clear cell variant of CEOT after testing negative for EWSR1 [7]. In addition to the case presented in this paper, these cases cement the role of FISH to look for EWSR1 gene rearrangement to differentiate between CCOC and other odontogenic tumours.
Recurrent chromosomal translocations and fusion events can be uniquely diagnostic as oncologic drivers in certain tumours. Their role has been well established in sarcomas [24], but recently they have become central in the diagnosis and characterization of certain carcinomas, including the hyalinizing clear cell carcinoma (HCC) of salivary glands. HCC is a low grade malignant tumour of the salivary glands, mainly arising in submucosal minor salivary glands. The pathologic features of this tumour are hard to distinguish from other salivary gland tumours, or from other clear cell tumours. Immunophenotypically, they also resemble a squamous cell carcinoma or mucoepidermoid carcinoma. The EWSR1-ATF1 fusion oncogene has recently been demonstrated in over 80% of cases of hyalinizing clear cell carcinoma, and this is now considered pathognomonic for diagnosis of this tumour [25, 26]. In 2013, Bilodeau et al. demonstrated that 5 out of 6 cases of CCOC were positive for the EWSR1–ATF1 rearrangement and fusion protein [7]. This demonstrated a potential biologic link between HCC, and importantly provided an additional test to confirm the diagnosis of CCOC. Several case reports have since demonstrated the EWSR1–ATF1 gene fusion in CCOC, with one case demonstrating a different fusion protein EWSR1–CREB1 [27–30].
EWSR1 gene rearrangement is an important diagnostic translocation in several tumour types. It encodes the RNA-binding protein EWSR1, a member of the TET family of transcription factors, and increasing evidence is demonstrating rearrangement of this gene in a variety of mesenchymal and epithelial neoplasms [31]. It does not appear to affect one particular lineage of differentiation, and most tumours that demonstrate EWSR1 rearrangement are of uncertain lineage (Table 2).
Table 2.
A list of tumours known to be associated with ESWR1 translocation. Note that these tumours arise from both mesenchymal and epithelial tissues
| Tumours demonstrating ESWR1 rearrangement |
| Ewing family of tumours |
| Angiomatoid Fibrous Histocytoma |
| Clear Cell Sarcoma |
| Clear Cell Sarcoma-like tumour of the gastrointestinal tract |
| Primary pulmonary myxoid sarcoma |
| Soft tissue myoepithelial tumour |
| Hyalinising Clear Cell (HCC) carcinoma of salivary glands |
EWSR1 is considered ‘promiscuous’, with documented evidence of fusion with a host of different genes. The fusion of EWSR1 with the CREB family of transcription factors is particularly remarkable. Cyclic AMP (cAMP)-responsive element-binding protein (CREB) belongs to the family of basic leucine zipper (bZIP) transcription factors, which are expressed in a variety of tissues. The family constitutes CREB, activating transcription factor-1 (ATF-1) and cAMP response element modulator (CREM) [31, 32]. These 3 members have a high degree of homology in the C-terminal bZIP domain, which is responsible for DNA binding and dimerization. The bZIP domain binds to its target cAMP response element (CRE) which has been found in many target genes, and is believed to be involved in a variety of functions including neuronal development, synaptic plasticity, glucose homeostasis, and cytokine regulation [33]. The presence of these genes in a wide array of functions partially explains why they may have a role in different tumours of different differentiation lines. However, this may not be true where the histological overlap raises the possibility of a single pathologic entity characterized by morphologic diversity and shared genetics, rather than distinct tumour types with overlapping gene fusions [32].
To date, EWSR1–ATF1 [7] and EWSR1–CREB1 [28] have been detected in cases of CCOC. The case presented in this paper represents the first recorded case with evidence of EWSR1–CREM detected in CCOC. Much less is known about the CREM fusions and the phenotypic correlations than the ATF1 and CREB1 fusions [9]. However, it is becoming increasingly recognised in a variety of tumour types, demonstrating that CREM fusions also have a wide array of phenotypic expressions. CREM is mainly expressed in neuroendocrine tissue, compared with CREB and ATF1 which are expressed more ubiquitously. This finding was supported by the expression of EWSR1-CREM in 2 myxoid mesenchymal tumours in young adults that preferentially involved intracranial locations [32]. However, the discovery of the EWSR1–CREM fusion in our case, and in multiple extracranial tumours (including extracranial myxoid mesenchymal tumours) without neuronal differentiation or preference suggests a more ubiquitous expression similar to ATF1 and CREB [9, 34].
The possible link between HCC and CCOC was first suggested by Bilodeau et al., 2013 after the discovery of EWSR1-ATF1 fusion in CCOC. Due to the histological similarities between the two entities, the authors felt that at least a subset of CCOC cases (Those with EWSR1 rearrangement) may be a central form of HCC rather than an odontogenic tumour [7]. Although HCC and CCOC are believed to originate from different differentiation lines (salivary vs odontogenic respectively), immunohistochemical analysis is, for the most part, not helpful in differentiating them. The discovery of EWSR1 gene rearrangement in both may further cement the link between them. Although initially hyalinizing clear cell carcinoma was found to express only the EWSR1–ATF1 fusion, a recent paper demonstrated the expression of EWSR1–CREM in 3 cases of HCC [34]. The discovery of expression of EWSR1–CREM in both HCC and CCOC further adds evidence of shared genetics, and a possible relationship between the two tumours. Discovering markers of odontogenesis to determine if there are in CCOC is in fact an odontogenic tumour, or whether those that express EWSR1 rearrangements are of a different lineage may be another research path to determine the link. LEF1 expression seemed a promising immunohistochemical marker of differentiation between salivary and odontogenic differentiation, however, the variable expression in odontogenic tumours especially limits its diagnostic utility [35]. Larger studies evaluating the expression of these mutations in a large number of each tumour type will be needed to confirm these findings, however these studies will be unlikely due to the rarity of both tumours.
Although the differentiation between HCC and CCOC is of academic importance, clinically, it does not affect management, as they are both malignancies and are treated in a similar manner. Conversely, differentiating between CEOT and CCOC significantly affects management, and it is prudent to highlight that in the case reported in this paper, appropriately diagnosing this lesion as a CCOC would have prompted early, more aggressive surgical resection and likely adjuvant radiotherapy. This may have prevented the eventual metastasis and ultimately, the premature death of the patient.
This case highlights the importance of FISH and NGS in looking for known gene rearrangements and fusion proteins to accurately diagnose clear cell lesions of the head and neck to confirm the pathology, and guide appropriate treatment.
Funding
No financial assistance was sought or provided for this manuscript.
Compliance with Ethical Standards
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical Approval
Patient consent to publish was provided by the patient. Ethics approval to publish this case report was provided by the Queen Elizabeth Hospital Ethics Committee (Approval number CARMS-00093).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hadj Saïd M, Ordioni U, Benat G, Gomez-Brouchet A, Chossegros C, Catherine JH. Clear cell odontogenic carcinoma. A review. J Stomatol Oral Maxillofac Surg. 2017;118:363–370. doi: 10.1016/j.jormas.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Bilodeau EA, Hoschar AP, Barnes EL, Hunt JL, Seethala RR. Clear cell carcinoma and clear cell odontogenic carcinoma: a comparative clinicopathologic and immunohistochemical study. Head Neck Pathol. 2011;5:101–107. doi: 10.1007/s12105-011-0244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansen LS, Eversole LR, Green TL, Powell NB. Clear cell odontogenic tumor—a new histologic variant with aggressive potential. Head Neck Surg. 1985;8:115–123. doi: 10.1002/hed.2890080208. [DOI] [PubMed] [Google Scholar]
- 4.Wright JM, Vered M. Update from the 4th Edition of the World Health Organization Classification of head and neck tumours: odontogenic and maxillofacial bone tumors. Head Neck Pathol. 2017;11:68–77. doi: 10.1007/s12105-017-0794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guastaldi FPS, Faquin WC, Gootkind F, Hashemi S, August M, Iafrate AJ, et al. Clear cell odontogenic carcinoma: a rare jaw tumor. A summary of 107 reported cases. Int J Oral Maxillofac Surg. 2019;48:1405–1410. doi: 10.1016/j.ijom.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loyola AM, Cardoso SV, de Faria PR, Servato JPS, de Paulo LF, Eisenberg ALA, et al. Clear cell odontogenic carcinoma: report of 7 new cases and systematic review of the current knowledge. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:483–496. doi: 10.1016/j.oooo.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Bilodeau EA, Weinreb I, Antonescu CR, Zhang L, Dacic S, Muller S, et al. Clear cell odontogenic carcinomas show EWSR1 rearrangements. Am J Surg Pathol. 2013;37:1001–1005. doi: 10.1097/PAS.0b013e31828a6727. [DOI] [PubMed] [Google Scholar]
- 8.Zhu G, Benayed R, Ho C, Mullaney K, Sukhadia P, Rios K, et al. Diagnosis of known sarcoma fusions and novel fusion partners by targeted RNA sequencing with identification of a recurrent ACTB-FOSB fusion in pseudomyogenic hemangioendothelioma. Mod Pathol. 2019;32:609–620. doi: 10.1038/s41379-018-0175-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshida A, Wakai S, Ryo E, Miyata K, Miyazawa M, Yoshida KI, et al. Expanding the phenotypic spectrum of mesenchymal tumors harboring the EWSR1-CREM fusion. Am J Surg Pathol. 2019;43:1622–1630. doi: 10.1097/PAS.0000000000001331. [DOI] [PubMed] [Google Scholar]
- 10.August M, Faquin W, Troulis M, Kaban L. Clear cell odontogenic carcinoma: evaluation of reported cases. J Oral Maxillofac Surg. 2003;61:580–586. doi: 10.1053/joms.2003.50114. [DOI] [PubMed] [Google Scholar]
- 11.Jain A, Shetty DC, Juneja S, Narwal N. Molecular characterization of clear cell lesions of head and neck. J Clin Diagn Res. 2016;10:18–23. doi: 10.7860/JCDR/2016/14394.7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diniz MG, Gomes CC, de Sousa SF, Xavier GM, Gomez RS. Oncogenic signalling pathways in benign odontogenic cysts and tumours. Oral Oncol. 2017;72:165–173. doi: 10.1016/j.oraloncology.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 13.Nodit L, Barnes L, Childers E, Finkelstein S, Swalsky P, Hunt J. Allelic loss of tumor suppressor genes in ameloblastic tumors. Mod Pathol. 2004;17(9):1062–1067. doi: 10.1038/modpathol.3800147. [DOI] [PubMed] [Google Scholar]
- 14.Basu MK, Matthews JB, Sear AJ, Bkowne RM. Calcifying epithelial odontogenic tumour: a case showing features of malignancy. J Oral Pathol Med. 1984;13:310–319. doi: 10.1111/j.1600-0714.1984.tb01429.x. [DOI] [PubMed] [Google Scholar]
- 15.Veness MJ, Morgan G, Collins AP, Walker DM. Calcifying epithelial odontogenic (Pindborg) tumor with malignant transformation and metastatic spread. Head Neck. 2001;23:692–696. doi: 10.1002/hed.1097. [DOI] [PubMed] [Google Scholar]
- 16.Cheng YSL, Wright JM, Walstad WR, Finn MD. Calcifying epithelial odontogenic tumor showing microscopic features of potential malignant behavior. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:287–295. doi: 10.1067/moe.2002.121991. [DOI] [PubMed] [Google Scholar]
- 17.Goldenberg D, Sciubba J, Koch W, Tufano RP. Malignant odontogenic tumors: a 22-year experience. Laryngoscope. 2004;114:1770–1774. doi: 10.1097/00005537-200410000-00018. [DOI] [PubMed] [Google Scholar]
- 18.Kawano K, Ono K, Yada N, Takahashi Y, Kashima K, Yokoyama S, et al. Malignant calcifying epithelial odontogenic tumor of the mandible: report of a case with pulmonary metastasis showing remarkable response to platinum derivatives. Oral Surge Oral Med Oral Pathol Oral Radiol Endodontol. 2007;104:76–81. doi: 10.1016/j.tripleo.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 19.Demian N, Harris RJ, Abramovitch K, Wilson JW, Vigneswaran N. Malignant transformation of calcifying epithelial odontogenic tumor is associated with the loss of p53 transcriptional activity: a case report with review of the literature. J Oral Maxillofac Surg. 2010;68:1964–1973. doi: 10.1016/j.joms.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong Y, Wang L, Li T, Chen XM. Calcifying epithelial odontogenic tumour showing malignant transformation: a case report and review of the literature. Chin J Dent Res. 2010;13:157–162. [PubMed] [Google Scholar]
- 21.Chrcanovic BR, Gomez RS. Calcifying epithelial odontogenic tumor: an updated analysis of 339 cases reported in the literature. J Cranio-Maxillofacial Surg. 2017;45:1117–1123. doi: 10.1016/j.jcms.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Cheng Y-SL, Wright JM, Walstad WR, Finn MD. Calcifying epithelial odontogenic tumor showing microscopic features of potential malignant behavior. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2002;93:287–295. doi: 10.1067/moe.2002.121991. [DOI] [PubMed] [Google Scholar]
- 23.Kumar M, Fasanmade A, William Barrett A, Mack G, Newman L, Hyde NC. Metastasising clear cell odontogenic carcinoma: a case report and review of the literature. Oral Oncol. 2003;39:190–194. doi: 10.1016/S1368-8375(02)00012-X. [DOI] [PubMed] [Google Scholar]
- 24.Taylor BS, Barretina J, Maki RG, Antonescu CR, Singer S, Ladanyi M. Advances in sarcoma genomics and new therapeutic targets. Nat Rev Cancer. 2011;11:541–557. doi: 10.1038/nrc3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antonescu CR, Katabi N, Zhang L, Sung YS, Seethala RR, Jordan RC, et al. EWSR1-ATF1 fusion is a novel and consistent finding in hyalinizing clear-cell carcinoma of salivary gland. Genes Chromosom Cancer. 2011;50:559–570. doi: 10.1002/gcc.20881. [DOI] [PubMed] [Google Scholar]
- 26.Shah AA, LeGallo RD, van Zante A, Frierson HF, Mills SE, Berean KW, et al. EWSR1 genetic rearrangements in salivary gland tumors. Am J Surg Pathol. 2013;37:571–578. doi: 10.1097/PAS.0b013e3182772a15. [DOI] [PubMed] [Google Scholar]
- 27.Yancoskie AE, Sreekantaiah C, Jacob J, Rosenberg A, Edelman M, Antonescu CR, et al. EWSR1 and ATF1 rearrangements in clear cell odontogenic carcinoma: presentation of a case. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118:e115–e118. doi: 10.1016/j.oooo.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Vogels R, Baumhoer D, van Gorp J, Eijkelenboom A, Verdijk M, van Cleef P, et al. Clear cell odontogenic carcinoma: occurrence of EWSR1-CREB1 as alternative fusion gene to EWSR1-ATF1. Head Neck Pathol. 2019;13:225–230. doi: 10.1007/s12105-018-0953-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santana T, de Andrade FL, de Sousa Melo MC, da Rocha GBL, Trierveiler M. Clear cell odontogenic carcinoma harboring the EWSR1–ATF1 fusion gene: report of a rare case. Head Neck Pathol. 2020;14:847–851. doi: 10.1007/s12105-019-01103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ginat DT, Villaflor V, Cipriani NA. Oral cavity clear cell odontogenic carcinoma. Head Neck Pathol. 2016;10:217–220. doi: 10.1007/s12105-015-0635-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thway K, Fisher C. Tumors with EWSR1-CREB1 and EWSR1-ATF1 fusions. Am J Surg Pathol. 2012;36:e1–e11. doi: 10.1097/PAS.0b013e31825485c5. [DOI] [PubMed] [Google Scholar]
- 32.Kao YC, Sung YS, Zhang L, Chen CL, Vaiyapuri S, Rosenblum MK, et al. EWSR1 fusions with CREB family transcription factors define a novel myxoid mesenchymal tumor with predilection for intracranial location. Am J Surg Pathol. 2017;41(4):482. doi: 10.1097/PAS.0000000000000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68(1):821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- 34.Chapman E, Skalova A, Ptakova N, Martinek P, Goytain A, Tucker T, et al. Molecular profiling of hyalinizing clear cell carcinomas revealed a subset of tumors harboring a Novel EWSR1-CREM fusion: Report of 3 Cases. Am J Surg Pathol. 2018;42:1182–1189. doi: 10.1097/PAS.0000000000001114. [DOI] [PubMed] [Google Scholar]
- 35.Bilodeau EA, Acquafondata M, Barnes EL, Seethala RR. A comparative analysis of LEF-1 in odontogenic and salivary tumors. Hum Pathol. 2015;46:255–259. doi: 10.1016/j.humpath.2014.10.018. [DOI] [PubMed] [Google Scholar]