Abstract
Hepatocellular carcinoma (HCC) is the sixth most common cancer with a high mortality rate. Early diagnosis and treatment before tumor progression into an advanced stage is ideal. The current diagnosis of HCC is mainly based on imaging modalities such as ultrasound, computed tomography, and magnetic resonance imaging. These methods have some limitations including diagnosis in the case of very small tumors with atypical imaging patterns. Extracellular vesicles (EVs) are nanosized vesicles which have been shown to act as an important vector for cell‐to‐cell communication. In the past decade, EVs have been investigated with regard to their roles in HCC formation. Since these EVs contain biomolecular cargo such as nucleic acid, lipids, and proteins, it has been proposed that they could be a potential source of tumor biomarkers and a vector for therapeutic cargo. In this review, reports on the roles of HCC‐derived EVs in tumorigenesis, and clinical investigations using circulating EVs as a biomarker for HCC and their potential diagnostic roles have been comprehensively summarized and discussed. In addition, findings from in vitro and in vivo reports investigating the potential roles of EVs as therapeutic interventions are also presented. These findings regarding the potential benefits of EVs will encourage further investigations and may allow us to devise novel strategies using EVs in the early diagnosis as well as for treatment of HCC in the future.
Keywords: Biomarker, Diagnosis, Exosome, Extracellular vesicle, HCC, miRNA
The roles of hepatocellular carcinoma (HCC)‐derived extracellular vesicles (EVs) in tumorigenesis, and clinical investigations using circulating EVs as a biomarker for HCC and their potential diagnostic roles have been comprehensively reviewed. The potential benefits of EVs from this review will encourage further investigations for the benefits of HCC patients.
1. INTRODUCTION
Liver cancer is the sixth most common cancer found in cancer patients worldwide 1 and 90% of liver cancer is hepatocellular carcinoma (HCC). 2 Chronic liver injury from infection (hepatitis B or C viruses), chronic alcoholic intake, and fatty liver are well‐known risk factors for hepatocarcinogenesis. 3 The treatments for HCC are dependent on tumor size, tumor location, and liver function. These interventions include tumor resection, local ablation, transarterial chemoembolization, and liver transplantation which are shown to be effective for HCC treatment. 4 , 5 However, some patients cannot be treated by these methods due to an advanced tumor stage and poor liver function. 6 Currently, the overall 5‐year survival rate for HCC patients is still low despite the availability of multiple treatment modalities. 7
Because most HCC patients are asymptomatic in the early stages, the early detection of small HCC before it progresses to the later stages is very important. Several pieces of evidence show that early detection of small HCC by screening with a liver ultrasound can significantly improve survival time. 8 However, data from a meta‐analysis report showed that the sensitivity of ultrasound screening for detection of an early HCC is only 45%, and the sensitivity of combined ultrasound screening with serum alpha‐fetoprotein (AFP) for early HCC detection is only 63%. 9 Currently, imaging such as CT and MRI are primary tools for the diagnosis of HCC in most guidelines. 10 , 11 With typical arterial enhancement and contrast washout in the delayed phase, HCC can be diagnosed without the need for tissue diagnosis. 12 However, since approximately 40% of HCC patients showed atypical imaging features, this group of patients still need a liver biopsy for a definite diagnosis. 13 All these findings indicate the need for improved tools to detect HCC earlier with increased sensitivity and specificity while being less invasive.
Liquid biopsy is one of the noninvasive methods for cancer diagnosis. Detection of circulating tumor DNA, 14 circulating tumor cell, 15 or extracellular vesicle (EV) in patients’ blood has been shown to be useful for cancer diagnosis. However, as the amount of circulating tumor cells and DNA are usually low in number with their short survival time in circulation, these factors, therefore, limit the use of these methods. 16 The extracellular vesicle (EV) is a nano‐sized vesicle which can be produced and secreted by all cells. 17 EVs can deliver molecules such as protein, mRNA, microRNA (miRNA), circularRNA (circRNA), long noncoding RNA (lncRNA), and DNA and play an important role in cell‐to‐cell communication. 17 These vesicles can be found in almost all body fluid types such as blood, urine, ascites, effusion, and breast milk. 18 There is increasing evidence to demonstrate that certain tumor cells can secrete EVs to modify the microenvironment of tumors and have a major role in tumor progression. 19 , 20
A previous study demonstrated that some liver‐derived transcripts can be found in the circulation of an HCC patient. 21 The lipid raft domain structure of EVs can protect these transcripts against degradation by circulating RNase, thus allowing the EV‐RNA to be stable in the blood. 22 As a result, these EVs have been proposed as a potential diagnostic biomarker. 22 , 23 , 24 Furthermore, it has been shown that EVs could be used to stimulate an immune response as well as to carry the tumor suppressor gene, all of which could have therapeutic potential in cancer treatment. 25 , 26 In this review, the biologic roles of HCC‐derived EVs obtained from in vitro and in vivo reports are comprehensively summarized. Reports on their potential roles as diagnostic biomarkers and possible therapeutic interventions are also presented and discussed.
Literature was searched on the PubMed database from its inception until August 30, 2021 using the following search terms: hepatocellular carcinoma, extracellular vesicle, and exosome. In vitro, in vivo, and clinical studies were included in this review.
2. BIOLOGIC FUNCTION OF HCC CELL‐DERIVED EVS: REPORTS FROM IN VITRO STUDIES
Tumor microenvironments play an essential role in HCC development and progression. 27 , 28 Recent evidence showed the dysregulation of multiple signaling pathways and cell‐to‐cell communication in HCC. 29 HCC‐derived EVs are one of the major mediators of cell‐to‐cell communication. 30 They were found to contain multiple miRNAs, lncRNA, and circRNA which regulate tumor cell proliferation, 31 , 32 , 33 , 34 , 35 , 36 , 37 migration, 33 and decrease tumor cell apoptosis and chemoresistance. 32 , 36 , 37 Several RNAs can regulate the tumor microenvironment by increasing angiogenesis and decreasing cell adhesion. 31 , 33 , 34 In addition, serum‐free miRNA in cancer patients has been shown to correlate with tumor cell proliferation, metabolism, invasion, and metastasis. 38 Recent evidence showed that other noncoding RNA (lncRNA and circRNA) are also related to tumorigenesis. CircRNA has been demonstrated to act as an miRNA sponge, 39 whereas the dysregulation of lncRNA was associated with cancer. 40 High metastatic HCC cell (LM3) had increased expression of EV‐circ‐PTGR1 which has been shown to increase cell migration and apoptosis. 41 HepG2 and HuH7 cell lines also had increased EV‐lnc 544, 239, 959, 171, and 85 and were shown to increase cell proliferation. 42 As these molecules in HCC‐derived EVs promoted the survival of the tumor cells, they could be a target for HCC treatment. A summary of these reports from in vitro studies is shown in Table 1.
TABLE 1.
Biologic functions of HCC cell‐derived EVs: Reports from in vitro studies
| HCC cell line | EV extraction method | EV molecule expression | Major findings (tumor cells) | Interpretation | References | ||||
|---|---|---|---|---|---|---|---|---|---|
| Proliferation | Apoptosis | Migration | Chemoresistance | Microenvironment | |||||
| Hep3B | DC, UF | ↑ miR−584‐5p | ↑ | ↑ | ↑ Angiogenesis | miR−584‐5p increased HCC cell proliferation, migration, and angiogenesis | 33 | ||
| Hep3B, HuH7 | DC, Exoquick | ↓ circ−0051443 | ↑ | ↓ Bak1 | HCC cell showed decreased circ−0051443 which acts as a tumor suppressor gene | 32 | |||
| Hep3B, HepG2, PLC/PRF/5 | DC, DGC | ↑ TUC−339 | ↑ | ↓ HCC cell adhesion | TUC−339 increased HCC cell proliferation and might increase invasion/metastasis | 34 | |||
| Hep3B, SNU18, SNU38, Li7, and MHCC97H | DC | ↑ ANGPT−2 | ↑ | ↑ Angiogenesis | ANGPT−2 increased HCC cell proliferation and angiogenesis | 31 | |||
| MHCC−97L, MHCC−97H | DC, Exoquick | ↑ miR−665 | ↑ | miR−665 increased HCC cell proliferation | 35 | ||||
| HUH7 | DC, UF | ↑ miR−122 | ↓ | ↑ cleaved PARP, Caspase 9 | ↓ (Doxorubicin) | HUH7 cell (less‐aggressive cell line) had high miR−122 which decreased HCC cell proliferation, increased apoptosis, and decreased chemoresistance of tumor cell | 88 | ||
| HepG2 | UC | ↑ linc‐VLDLR | ↑ | ↑ (Sorafenib, Doxorubicin) | linc‐VLDLR increased HCC cell proliferation and chemoresistance | 36 | |||
| HepG2, PLC‐PRF5 | DC | ↑ linc‐ROR | ↑ | ↓ (Caspase 3/7) | ↑ (Sorafenib, Doxorubicin, Camptothecin) | linc‐ROR increased HCC cell proliferation, chemoresistance and decreased HCC cell apoptosis | 37 | ||
| HepG2, HuH7 | Ribo exosome isolation reagent | ↑lnc 544 | ↑ | ↓ | ↔ |
Lnc−85, −171, −959, −239, −554 increased HCC cell proliferation Lnc−85, −171, −544 decreased HCC cell apoptosis Lnc−85, −959, −239 increased HCC cell migration |
42 | ||
| ↑lnc 239 | ↑ | ↔ | ↑ | ||||||
| ↑lnc 959 | ↑ | ↔ | ↑ | ||||||
| ↑lnc 171 | ↑ | ↓↓ | ↔ | ||||||
| ↑lnc 85 | ↑↑ | ↓↓ | ↑ | ||||||
| 97hm, Huhm | UC | ↑ miR−92a−3p | ↑ | ↑ Metastasis | miR−92a−3p increased HCC cell migration and metastasis | 89 | |||
| LM3 | Exoquick | ↑ circ‐PTGR1 | ↓ | ↑ | High metastatic HCC cell (LM3) had increased EV‐circ‐PTGR1 and was associated with increased cell migration and decreased apoptosis | 41 | |||
Abbreviations: ABC, ATP‐binding cassette; ANGPT, angiopoietin; circ‐RNA, circular RNA; DC, differential centrifugation; DGC, density‐gradient separation; IGF, insulin growth factor; linc‐ROR, long intergenic noncoding RNA; lnc‐RNA, long noncoding RNA; omiR, oncogenic microRNA; tsmiR, tumor suppressor miRNATUC siRNA, ultraconserved long noncoding siRNA; TUC, tumor ultraconserved RNA; UF, ultrafiltration.
3. CIRCULATING EV MIRNA IN HCC PATIENTS: REPORTS FROM CLINICAL STUDIES
In the last decades, multiple studies have reported an association between serum circulating miRNA and HCC including miR‐15b, miR‐16, miR‐19a, miR‐21, miR‐27b, miR‐92a, miR‐107p, miR‐122, miR‐130b, miR‐183, miR‐192, miR‐195, miR‐221, miR‐222, miR‐223, miR‐224, and ETC. 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 Recent evidence has also shown the superiority of EV‐miRNA over serum biomarkers in accurate diagnosis. 55 , 56 , 57 Since EVs selectively pack and carry specific cargo (i.e., protein, DNA, RNA, and tumor‐specific transcripts) from their cell of origin, it is proposed that the cargos in EVs are potential diagnostic biomarkers. 58 , 59 , 60
EVs can be secreted by all cell types including HCC cells 61 and can be detected in bodily fluids, especially in the blood. 18 Unlike RNA in the serum, RNA inside the EV can be protected from RNase and other adverse conditions in circulation making EV‐RNA more stable in the blood. EV‐RNA can also be used as a diagnostic biomarker for tumors. 23 Moreover, EV‐RNA can also be quantitated by qRT‐PCR, thus making their analysis comparable with conventional proteomic methods. 62 Currently, there are multiple methods to extract EVs from plasma by using their physical properties including ultracentrifugation, filtration, size exclusion chromatography, and precipitation. Ultracentrifugation (UC) is the commonly used method; however, it is burdensome and is unlikely to be used in clinical practice. 63 The filtration method uses less time compared with the UC, however, some EVs may be lost due to the jamming of EVs on the filter. 64 Size exclusion chromatography has been shown to effectively isolate protein contaminants from EVs; however, it has relatively low throughput. 65 Precipitation with UC is a time‐saver and can be used to test multiple concurrent samples simultaneously. 66 However, some non‐EV contaminants will also be obtained. Recently, Sun et al. had developed the novel EV purification system, EV Click chips. 67 This method combined covalent chemistry‐mediated EV capture/release, multimarker antibody cocktails, nanostructured substrates, and microfluidic chaotic mixers to selectively extract tumor‐derived EV from total circulating EV. This method has been shown to overcome the limitation of prior methods for EV extraction and was proposed to increase the diagnostic power of circulating EV. 67
In the majority of reports included in this review, a combination of a UC method and other methods were frequently used. However, despite almost all studies used the qRT‐PCR technique to measure the EV content (Table 2), one used the TLN biochip combined with TIRF microscopy to detect small fragments of mRNA. 55 This method might be more effective than qRT‐PCR which can detect only intact large fragments of mRNA.
TABLE 2.
Circulating EV miRNA in HCC patients
| Subjects (N) | EV source | EV extraction method | Molecule detection method | EV biomarker expression | Clinical relevance | Interpretation | Reference |
|---|---|---|---|---|---|---|---|
|
HCC (48) LC (38) Healthy controls (20) |
Plasma (5 ml) | TEIp kit (DC, PEG) | qRT‐PCR |
HCC ↑ miR−21‐5p ↓ miR−92a−3p |
HCC patients had increased exosomal miR−21‐5p and decreased miR−92a−3p in comparison with the cirrhotic patient. | 56 | |
|
LC ↔ miR−21‐5p ↔ miR−92a−3p | |||||||
|
HCC (50) CHB (40) LC (40) Healthy controls (64) |
Serum | DC, PEG | miRNeasy mini kit +qRT‐PCR |
HCC, CHB ↑ miR−122, −148a, −1246 |
HCC and CHB patients had increased exosomal miR−122, 148a, 1246 None of these biomarkers could be used to differentiate HCC from CH |
57 | |
|
LC ↔ miR−122, −148a, −1246 | |||||||
|
HCC (86) CCA (38) LC (54) Healthy controls (202) |
Serum (7.5 ml) | DC | FACS |
HCC & CCA ↑↑ AnnexinV+ EpCAM+ MV ↑↑ AnnexinV+ EpCAM+ ASGPR1+ MV |
AnnexinV+ EpCAM+ MV correlates with tumor size |
Circulating AnnexinV+ EpCAM+ and AnnexinV+ EpCAM+ ASGPR1+ MV increased in HCC patients | 74 |
|
LC ↑ AnnexinV+ EpCAM+ MV ↔ AnnexinV+ EpCAM+ ASGPR1+MV | |||||||
|
HCC (71) Healthy controls (32) |
Plasma | N/A | qRT‐PCR | ↑ TST1 | ↓ TST1 after curative surgery | Presence of TST1 correlated with HCC | 60 |
|
HCC (40) Healthy controls (38) |
Plasma (6 ml) | DC, Exoquick | qRT‐PCR vs TLN biochip +TIRF microscopy |
↑ AFP mRNA (AFP−174, MB−1096, MB−1171) ↑ GPC3 mRNA (GPC3 MB) |
Increased exosomal AFP mRNA and GPC3 mRNA in HCC patients | 55 | |
|
HCC (30) Healthy controls (10) |
Serum | DC, Exoquick | qRT‐PCR |
HCC ↑ miR−665 |
↑ miR−665 (>5 fold) correlate with higher stage, larger tumor size (>5 cm), and metastasis | HCC patient had significantly increased serum exosomal miR−665 and it can be a prognostic marker | 35 |
|
HCC (30) CHB (30) Healthy controls (30) |
Serum exosome | DC, Isolation agent (Invitrogen) | qRT‐PCR |
HCC (serum exosome) ↑↑↑ miR−21 HCC (whole serum) ↑↑ miR−21 |
↑ miR−21 in HCC patient correlate with tumor stage |
Exosomes increased sensitivity of miRNA detection in serum Exosomal miR−21 is increased in HCC and CHB patients |
68 |
|
CHB (serum exosome) ↑↑ miR−21 CHB (serum exosome) ↑ miR−21 | |||||||
| HCC (59) | Serum | DC, UF | qRT‐PCR |
↑ miR−1246 ↓ miR−718 (tumor >3 cm) |
↓ miR−718 correlate with tumor size (>3 cm) and number of tumor | In HCC patients, exosomal miR−1246 was increased and miR−718 was decreased | 62 |
|
HCC (20) LC (20) CHB control (20) |
Serum exosome (0.5 ml) | Exoquick | qRT‐PCR |
HCC (serum exosome) ↑ miR−18a, −221, −222, −224 ↔ miR−21, −93 ↓ miR−101, −106b, −122, −195 HCC (whole serum) ↔ miR−21, −101, −195, −221, −222, −224 |
Serum exosomal microRNAs was more effective in distinguishing HCC from CHB and LC compared with whole serum circulating microRNAs | 69 | |
|
LC (serum exosome) ↔ miR−18a, −21, −93, −101, −106b, −122, −195, −221, −222, −224 | |||||||
|
HCC (90) CHB (28) LC (35) Normal (29) |
Serum (5 ml) | Exoquick | qRT‐PCR |
HCC ↑ lncRNAs (LINC00853, SFTA1P, HOTTIP, HAGLROS, LINC01419, HAGLR, CRNDE) |
LINC00853 elevated in AFP‐negative HCC Patients with high EV‐LINC00853 had a lower survival rate |
HCC patients had increased EV‐lncRNAs. LINC00853 might be used for HCC diagnosis, especially in AFP‐negative HCC. It was also correlated with patients’ prognosis |
70 |
|
LC, CHB, Normal ↔ lncRNAs (LINC00853, SFTA1P, HOTTIP, HAGLROS, LINC01419, HAGLR, CRNDE) | |||||||
|
HCC (46) CHB (25) LC (26) Normal (23) Liver metastasis (12) Other primary cancer (26) |
Plasma | EV click chips | RT‐ddPCR |
HCC ↑ GPC3, AFP, AHSG, TF ↑↑ ALB, APOH, FABP1, FGB, FGG, RBP4 |
FGG, FGB, and RBP4 showed increased expression in HCC‐BCLC stage B‐C compared with stage 0‐A | EV click chips could selectively purify EV from HCC cells. These 10 EV‐mRNA were increased in HCC patients, compared with CHB, LC, liver metastasis, other primary cancer, and normal control | 67 |
|
CHB ↔ GPC3, AFP, FABP1 ↑ AHSG, APOH, FGB, FGG, RBP4 ↑↑ ALB | |||||||
|
LC, Normal, Liver metastasis, Other cancer ↔ GPC3. AFP, AHSG, APOH, FABP1, FGB, FGG, RBP4, TF ↑ ALB | |||||||
|
HCC (38) Chronic hepatitis (35) Liver cirrhosis (25) Normal (11) |
Plasma | ExoEnrich | qRT‐PCR |
HCC ↑↑ miR21‐5p ↑ miR10b−5p, miR221‐3p, mir223‐3p |
miR 21‐5p showed significantly increased expression in HCC compared with chronic hepatitis and LC patients | 71 | |
|
Chronic hepatitis and LC ↑ miR10b−5p, miR221‐3p, mir223‐3p, mir21‐5p |
|||||||
|
HCC (71) Normal (40) |
Plasma | UC | qRT‐PCR |
HCC ↑ hsa‐circ−0004001, 0004123, 0075792 |
Hsa‐circ−0004001 and 0075792 were significantly associated with TNM tumor staging while hsa‐circ−0004001 and 0004123 were associated with tumor size. | EV‐hsa‐circ−0004001, 0004123, 0075792 were increased in HCC patients | 73 |
|
Early stage HCC (50) Normal (100) |
Plasma | ExoRNeasy | qRT‐PCR |
HCC ↑ LDHC‐mRNA |
LDHC‐mRNA was associated with survival outcome | EV‐LDHC‐mRNA expression was increased in early stage HCC and was associated with survival outcome | 72 |
Abbreviations: AUC, area under the curve; BCLC, Barcelona clinic liver cancer; CCA, cholangiocarcinoma; CHB, chronic hepatitis B; circ, circular RNA; DC, differential centrifugation; FACS, fluorescence‐activated cell scanning; HCC, hepatocellular carcinoma; LC, liver cirrhosis; lnc‐RNA, long noncoding RNA; MV, microvesicle; PEG, polyethylene glycol precipitation; TEIp, total exosome isolation; TIRF, total internal reflective fluorescence; TLN, tethered lipoplex nanoparticles; TST, tumor‐specific transcript; UF, ultrafiltration.
Many clinical studies have shown alterations in circulating EV‐RNA in HCC patients. Wang et al. and Sohn et al. investigated both EV‐miRNA and serum‐free miRNA. They proved that miRNA detection in EVs can result in increased sensitivity compared with serum‐free miRNA. 68 , 69 Seven reports found a significant difference in circulating EV‐RNA in HCC patients compared with LC or CHB patients which represent real‐life situations. 56 , 67 , 68 , 69 , 70 , 71 , 72 Moreover, multiple EV‐mRNA, miRNA, lncRNA, and circRNA have been shown to correlate with tumor burden. 35 , 62 , 67 , 68 , 72 , 73 In addition, EV surface antigens (Annexin V, EpCAM, and ASGPR1) were also detected in the serum of HCC and CCA patients. 74 Reports on these findings are summarized in Table 2.
4. EV AS A DIAGNOSTIC TEST: EVIDENCE FROM CLINICAL REPORTS
In the past decade, there are several studies which used a combination of multiple circulating EV‐RNA or EV‐surface antigens to develop diagnostic tests for HCC. There are five reports of high diagnostic performance model scores for diagnosing HCC in liver cirrhosis patients. 42 , 56 , 57 , 67 , 70 Combined Z‐score of 10 EV‐mRNA (ALB, GPC3, AFP, AHSG, APOH, FABP1, FGB, FGG, RBP4, and TF) showed high diagnostic performance for HCC diagnosis from at‐risk patients (sensitivity 93.8%, specificity 74.5%, AUC 0.87) and other primary cancer (sensitivity 95.7%, specificity 89.5%, AUC 0.95). 67 High ability for early HCC detection from liver cirrhosis patients was also demonstrated (sensitivity 94.4%, specificity 88.5%, AUC 0.93). 67 One report used a combination of serum EV‐miR‐21‐5p, EV‐miR‐92a‐3p, and AFP which showed a sensitivity of 95% with a specificity of 50%, and an AUC of 0.85. 56 , 57 The other report used a combination of serum EV‐miR‐122, EV‐miR‐148a, and AFP which resulted in a sensitivity of 86%, specificity of 87.5%, and AUC of 0.93. 56 , 57 A better diagnostic performance using a combination of model scores was demonstrated compared with conventional biomarkers (AFP and GPC3) in HCC patients. 55 , 56
Recently, two reports using the EV‐lncRNA method have demonstrated that it could provide higher diagnostic performance than serum AFP. 42 , 70 EV‐LINC00853 demonstrated the sensitivity of 93.75% with a specificity of 89.77% and AUC of 0.97 for the diagnosis of early HCC in patients at‐risk group (liver cirrhosis and hepatitis B). 70 In addition, EV‐lnc85 showed a high diagnostic performance for differentiating both AFP‐positive (AUC 0.90) and AFP‐negative HCC (AUC 0.88) from liver cirrhosis patients. 42
In cirrhosis patients, in addition to serum EV‐RNA, the EV surface antigen has been investigated for its diagnostic potential of HCC and CCA. 74 Although an increase in serum EV surface antigens was found in HCC and CCA patients, 74 the results indicated that EV surface antigens could not be used to differentiate HCC from CCA patients. 74 In HCC patients, another report using tumor‐specific transcript‐1 (TST‐1) demonstrated its very high specificity (100%) for HCC detection but low sensitivity (28%). 60 These reports are summarized in Table 3.
TABLE 3.
EV as a potential diagnostic marker: Reports from clinical studies
| Subjects (N) | EV source | EV extraction method | Molecule Detection method | Index test | Aim of test | Diagnostic indices | Interpretation | References | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | AUC | ||||||||
|
HCC (48) LC (38) Control (20) |
Plasma (5 ml) | TEIp kit (DC, PEG) | qRT‐PCR | Model score of miR−21‐5p + miR−92a−3p + AFP | Dx HCC from cirrhosis | 95 | 50 | 0.85 | Model score of miR−21‐5p + miR−92a−3p + AFP had high accuracy for diagnosis of HCC in cirrhosis | 56 |
|
HCC (50) CHB (40) LC (40) Control (64) |
Serum | DC, PEG | miRNeasy mini kit +qRT‐PCR | Model score of miR−122 + 148a + AFP | Dx HCC from cirrhosis | 86 | 87.5 | 0.93 | This test could be used in LC patients without hepatitis B infection | 57 |
|
HCC (86) CCA (38) LC (54) Control (202) |
Serum (7.5 ml) | DC | FACS | AnnexinV+ EpCAM+ ASGPR1+ taMP (4.2 fold rising) | Dx liver tumor from cirrhosis | 75 | 47 | 0.7 | Index test could not distinguish between HCC and CCA | 74 |
|
HCC (71) Control (32) |
Plasma | N/A | qRT‐PCR | TST1 | Dx HCC vs. healthy controls | 28 | 100 | – | Presence of TST1 had very high specificity for HCC diagnosis | 60 |
|
HCC (40) Control (38) |
Plasma (6 ml) | DC, Exoquick | qRT‐PCR vs. TLN biochip +TIRF microscopy | Combined AFP mRNA +GPC3 mRNA | Dx HCC vs. healthy controls | 95 | 100 | 0.99 | Combined AFP mRNA +GPC3 mRNA had high accuracy for diagnosis of HCC | 55 |
|
HCC (90) CHB (28) LC (35) Normal (29) |
Serum (5 ml) | Exoquick | qRT‐PCR | EV‐LINC00853 | Dx early HCC from cirrhosis and chronic hepatitis B | 93.75 | 89.77 | 0.97 | Ev‐LINC00853 level had high accuracy for HCC diagnosis in cirrhosis | 70 |
|
HCC (112) LC (43) Normal (52) |
Plasma | Ribo exosome isolation reagent | qRT‐PCR | EV‐lnc85 | Dx HCC from cirrhosis and normal | 80.0 | 74.5 | 0.87 | EV‐lnc85 level had high accuracy for HCC diagnosis in both AFP‐negative and AFP‐positive HCC | 42 |
| Dx HCC from cirrhosis | 80.0 | 74.4 | 0.89 | |||||||
| Dx AFP‐positive HCC from cirrhosis | 80.5 | 76.7 | 0.90 | |||||||
| Dx AFP‐negative HCC from cirrhosis | 80.0 | 76.7 | 0.88 | |||||||
|
HCC (46) CHB (25) LC (26) Normal (23) Liver metastasis (12) Other primary cancer (26) |
Plasma | EV‐click chips | RT‐ddPCR |
Combined Z‐score of 10 mRNA (ALB,GPC3. AFP, AHSG, APOH, FABP1, FGB, FGG, RBP4, TF) |
Dx HCC from noncancer (CHB, LC) | 93.8 | 74.5 | 0.87 | Combined Z‐score of mRNA had high accuracy for differentiating HCC from noncancer, other primary cancer and liver cirrhosis patients | 67 |
| Dx HCC from other primary cancer | 95.7 | 89.5 | 0.95 | |||||||
| Dx early HCC from liver cirrhosis | 94.4 | 88.5 | 0.93 | |||||||
|
HCC (38) CHB or CHC (35) Liver cirrhosis (25) Normal (11) |
Plasma | ExoEnrich | qRT‐PCR | Model score of 4 miRNA (miR10b−5p, miR221‐3p, mir223‐3p, mir21‐5p) | Dx HCC from LC and hepatitis | 58.0 | 95.0 | 0.80 | Model score of four miRNA showed high specificity for HCC diagnosis, but its sensitivity was limited | 71 |
| HCC (71) Normal (40) | Plasma | UC | qRT‐PCR | Model score of 3 circRNAs (hsa_circ_0004001, 0004123, 0075792) | Dx HCC from normal control | 90.5 | 78.1 | 0.89 | Model score of three circRNAs had high diagnostic accuracy for HCC diagnosis from normal control | 73 |
| Early stage HCC (50) Normal (100) | Plasma | ExoRNeasy | qRT‐PCR | EV‐LDHC‐mRNA | Dx early stage HCC from normal control | 88.2 | 93.3 | 0.95 | EV‐LDHC‐mRNA had high diagnostic accuracy for early HCC diagnosis from normal control | 72 |
Abbreviations: AUC, area under the curve; CCA, cholangiocarcinoma; CHB, chronic hepatitis B; CHC, chronic hepatitis C; DC, differential centrifugation; HCC, hepatocellular carcinoma; LC, liver cirrhosis; MV, Microvesicle; PEG, polyethylene glycol precipitation; TEIp, total exosome isolationTIRF, total internal reflective fluorescence; TLN, tethered lipoplex nanoparticles; TST, tumor‐specific transcript.
5. EV AS A THERAPEUTIC INTERVENTION FOR HCC: EVIDENCE FROM IN VITRO REPORTS
EVs can be loaded with therapeutic cargo such as miRNA enabling transference into the target tumor cell. EVs may therefore be used as a personalized cancer treatment. 75 There are several in vitro studies using EVs for HCC cell treatment. The sources of EVs vary, examples being hepatic stellate cells, stem cells, HCC cells, hepatocytes, and bovine fat‐free milk. The majority of studies used an endogenous loading method to load miRNA or other therapeutic molecules into EVs. The endogenous loading method (preloading) is to modify the target donor cells before EV shedding. 76 , 77 After loading therapeutic molecules to the donor cells, the donor and recipient HCC cells are cocultured enabling EV transference into the recipient HCC cell. All studies have shown that their therapeutic cargo can be transferred to target tumor cells and increase cell apoptosis, 78 decrease chemoresistance, 79 reduce cell proliferation, 78 , 80 , 81 , 82 and reduce cell migration. 80
In another report, sodium iodide symporter (NIS) genes were transfected to donor HCC cells and cocultured with recipient HCC cells. 83 The NIS protein increased the I‐131 toxicity in recipient HCC cells, thus allowing the HCC cells to be susceptible to I‐131 ablation. 83 Most studies use EVs as a therapeutic cargo. 78 , 79 , 80 , 81 In one study, EVs from tumor cells were used to activate bone marrow stem cells. 82 Then, these activated bone marrow stem cells were cocultured with tumor cells. The results showed that tumor proliferation was significantly decreased. This method is known as the “Exosome‐based vaccine”. 82 All these in vitro reports are summarized in Table 4.
TABLE 4.
EV as a potential therapeutic intervention for HCC: Evidence from in vitro reports
| Molecule | Donor cell | Recipient cell | EV extraction method | TEV | TEV using method | Major Findings (Tumor cell) | Interpretation | References | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Proliferation | Apoptosis | Migration | Chemoresistance | ||||||||
| miR335‐5p | HSC cell (LX2) | HCC cell (MHCC97H, MHCC97L, HepG2, Huh7) | DC | HSC‐EV‐miR335‐5p (EL) | Coculture | ↓ | ↓ | HSC‐EV‐ miR335‐5p (EL) decreased tumor cell proliferation and migration | 80 | ||
| miR125b | ASC cell | HCC cell (HuH7, HepG2) | Exoquick | ASC‐EV‐miR125b (EL) | Coculture | ↓ | Human adipose cell might be useful as a source of therapeutic EVs | 81 | |||
| miR451, 223, 24, 125b, and 31 | HLSC | HCC cell (HepG2) | DC | HLSC‐MV (contained miR451, 223, 24, 125b, and 31) | Coculture | ↓ | ↑ (TUNEL assay) | HLSC‐MV decreased tumor cell proliferation and increased apoptosis | 78 | ||
| BMSCTEX,IFN− γ (exosome based tumor vaccine) | Mouse HCC cell (H22) | Murine BMSC | DC, UF | TEX from H22 cell | Coculture HCC cell with TEX or unactivated BMSC or IFN‐ γ | ↔ | Exosome‐based vaccine was a potential way to treat HCC | 82 | |||
| Use TEX +IFN‐γ to activate BMSC then coculture it with H22 cell | ↓ | ||||||||||
| miR−122 | AMSC | HCC cell (HepG2, HuH7) | Exoquick | AMSC‐EV‐miR−122 (EL) | Coculture | ↔ | ↔ (Annexin V, PI) | AMSC‐EV‐miR−122 (EL) treatment alone had no effect on HCC cell but increased the effect of chemotherapy | 79 | ||
| Give sorafenib or 5‐FU | ↓ | ↑ (Annexin V, PI) | |||||||||
| Coculture and give sorafenib or 5‐FU | ↓↓ | ↑↑ (Annexin V, PI) | ↓ | ||||||||
| NIS protein | HCC cell (HuH7) | HCC cell (HuH7) | DC | HCC‐EV ‐NIS gene (EL) | Coculture and give I−131 | NIS protein transferred by EV increased cytotoxicity and DNA damage of I−131 to HCC cell | 83 | ||||
| miR150‐3p | Normal fibroblast (NF) | HCC cell (HuH7, Hep3B) | Total exosome isolation reagent | NF‐EV‐miR150‐3p (EL) | Coculture | ↓ | NF‐EV‐ miR150‐3p (EL) decreased tumor cell migration | 90 | |||
Abbreviations: ANGPT, angiopoietin; AMSC, adipose mesenchymal stem cell; ASC, human adipose stem cell; BMSC, bone marrow stem cell; CAF, cancer‐associated fibroblast; DC, differential centrifugation; EL, endogenous loaded; HCC, hepatocellular carcinoma; HLSC, human liver stem cell; HSC, hepatocyte stellate cell; HUVEC, human umbilical vein endothelial cell; I‐131, iodine isotope 131; IFN, interferon; Linc, long intergenic noncoding RNA; NIS, sodium iodide symporter; TDEV, tumor‐derived EV; TEV, therapeutic EV; TEX, tumor‐derived exosome; UF, ultrafiltration.
6. EV AS A THERAPEUTIC INTERVENTION FOR HCC: EVIDENCE FROM IN VIVO REPORTS
In this context, most in vivo studies were done in an immunocompromised mouse model such as NOD SCID mice or nude mice in which an HCC xenograft tumor was subcutaneously implanted to these mice (Table 5). Therapeutic molecules which had antitumoral effects from in vitro studies were endogenously loaded into EVs. The route for EVs treatment introduction was mainly either intratumoral injections or intravenous injections via tail veins. The therapeutic EVs resulted in decreased tumor sizes, 32 , 78 , 79 , 80 , 84 , 85 , 86 , 87 increased tumor cell apoptosis, 32 , 78 , 79 , 80 and increased chemoresistance. 79 , 84
TABLE 5.
EV as a therapeutic intervention for HCC: Evidence from In Vivo Reports
| Model (N) | Xenograft cell line | Donor cell | Molecule | TEV | Route/Dose/Duration | Major findings | Interpretation | References | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Tumor volume | Apoptosis | Chemoresistance | ||||||||
| Female NOD SCID gamma mice | HCC cell (MHCC97H) | HSC cell (LX2) | miR335‐5p | LX2‐EV‐miR−335 | IT/50 μg exosome two times/week/4 weeks | ↓ | ↑ (Caspase 3) | IT LX2‐EV‐miR−335 decreased tumor volume and increased apoptosis of xenograft HCC | 80 | |
| Male nude mice | HCC cell (HuH7) | Hepatocyte (HL7702) | Circ−0051443 | HL7702‐EV‐circ−0051443 | IT/10 μg exosome OD/15 days | ↓ | ↑ (BAK1 expression) | IT HL7702‐EV‐circ−0051443 decreased tumor volume and increased apoptosis of xenograft HCC | 32 | |
| Friend virus B mice (21) | Induce HCC by coactivation of cMET and β‐catenin mutation | Bovine fat‐free milk | β‐catenin siRNA | MNV‐loaded siRNA β‐catenin | IV/TEV 2 x 1012 particles/body every 3 days/5 doses | ↓ | Fat‐free milk can be used as a source of EV MNV loaded siRNA β‐catenin decreased tumor size and chemoresistance (anti‐PD−1) | 84 | ||
| IP/250 μg anti‐PD−1 three times/week/2 weeks | ↓↓ | |||||||||
| Combined IV TEV and IP anti‐PD−1/2 weeks | ↓↓↓ | ↓ | ||||||||
| Athymic nude mice | HCC cell (LCSC, Hep3B) | Bovine fat‐free milk | β‐catenin siRNA | ET‐tMNV‐loaded siRNA β‐catenin | IV/tMNV 5 × 1010 particles/body every 2 days/5 doses | ↓ | ET‐tMNV targeted EpCAM‐expression cells and decreased xenograft HCC growth | 85 | ||
| Male C57L/J mice (40) | Mouse HCC cell (Hepa 1–6) | Mouse macrophage cell line (raw 264.7) | miR−142‐3p | TAM‐EV‐miR−142‐3p (stimulated by propofol) | IP/propofol 20 or 50 mg/kg every day/3 weeks | ↓ | Propofol decreased HCC growth by activating TAM to produce EV‐miR−142‐3p | 86 | ||
| C57BL/6 wild‐type mice (10), BALB/C nude mice (20) | Mouse HCC cell (Hepa 1–6) | Mouse HCC cell (Hepa 1–6) | DCCTEX (exosome‐based tumor vaccine) | Use TEX to activated DCC (DCCTEX) | IV/DCCTEX 2 × 106 one dose every 2 weeks/three doses | ↓ | Tumor exosome activated DCC. The activated DCCTEX decreased tumor volume | 87 | ||
| Nude mice (10) | HCC cell (HepG2) | AMSC | miR−122 | AMSC‐EV‐miR−122 | Single‐dose IT/AMSC‐EV‐miR−122 50 μg with IP sorafenib (5 mg/kg) five doses/week/5 weeks | ↓ | ↑ (Caspase 3, Bax) | ↓ | AMSC‐EV‐miR−122 alone had no effect on xenograft HCC, but it decreased chemoresistance | 79 |
| Single‐dose IT/AMSC‐EV‐miR−122 50 μg | ↔ | ↔ | ||||||||
| Male SCID mice | HCC cell (HepG2) | HLSC | miR451, 223, 24, 125b, and 31 | HLSC‐MV | IT/HLSC‐MV 100 μg/20 μl weekly/3 weeks | ↓ | ↑ (TUNEL assay) | HLSC‐MV decreased HCC growth and increased apoptosis | 78 | |
Abbreviations: AMSC, adipose mesenchymal stem cell; CAF, cancer‐associated fibroblast; circ, circular RNA; cMET, c‐tyrosine‐protein kinase MET; DC, differential centrifugation; DCC, dendritic cell; EpCAM, epithelium cell adhesion molecule; ET, EpCAM targeted; HCC, hepatocellular carcinoma; HLSC, human liver stem cell; HSC, hepatocyte stellate cell; IP, intraperitoneal injection; IT, intratumoral injection; IV, intravenous injection; LCSC, liver cancer stem cell; MNV, milk‐derived nanovesicle; SC, subcutaneous injection; TAM, tumor‐associated macrophage; TDEV, tumor‐derived EV; TEV, therapeutic EV; TEX, tumor‐derived exosome; tMNV, therapeutic milk‐derived nanovesicle; UC, ultra centrifugation.
In addition to using EVs as a therapeutic cargo, indirect use regarding the efficacy of EVs for HCC treatment has been reported. Intraperitoneal injection of propofol was shown to stimulate tumor‐associated macrophages to produce miR‐142‐3p EVs, which was associated with decreased tumor growth. 86 In one study, an exosome‐based tumor vaccine (dendritic cells stimulated by tumor EVs) was used to treat xenograft HCC in mice. 87 The result showed that intravenous injection of exosome‐based tumor vaccine effectively decreased xenograft tumor volume. 87 All these in vivo studies demonstrated the benefit of EVs as a potential tool for HCC treatment including the use of EVs as a therapeutic cargo or a tumor vaccine since EV could transfer therapeutic molecules into the xenograft HCC, leading to decreased tumor proliferation. These reports are summarized in Table 5.
7. CONCLUSION AND FUTURE PROSPECTIVE
HCC is common cancer with a high mortality rate. Clinical outcomes of HCC patients have been improved in the past decade due to early tumor detection and the availability of multiple treatment modalities. Currently, although HCC surveillance using ultrasound and serum AFP, imaging‐based diagnosis (CT, MRI), and the new therapeutic methods such as local ablation and transarterial chemoembolization have been shown to be beneficial in increasing survival time, recent data show that the sensitivity of HCC surveillance is still low (about 60%) and that some HCC patients had impaired liver function contradictory to undergoing TACE. 11 Accumulating evidence shows that HCC cells can use EVs for cell‐to‐cell communication to promote their growth. Clinical studies have already demonstrated that HCC derived‐EV containing RNA cargo can be potential serum biomarkers which may help in the diagnosis of HCC. Some of this EV‐RNA is also associated with tumor burden, indicating that it might be used as a prognostic indicator. In addition, reports from in vitro and in vivo studies indicated that EVs could be used to decrease tumor growth and tumor size. A summary of potential roles of EVs is shown in Figure 1.
FIGURE 1.
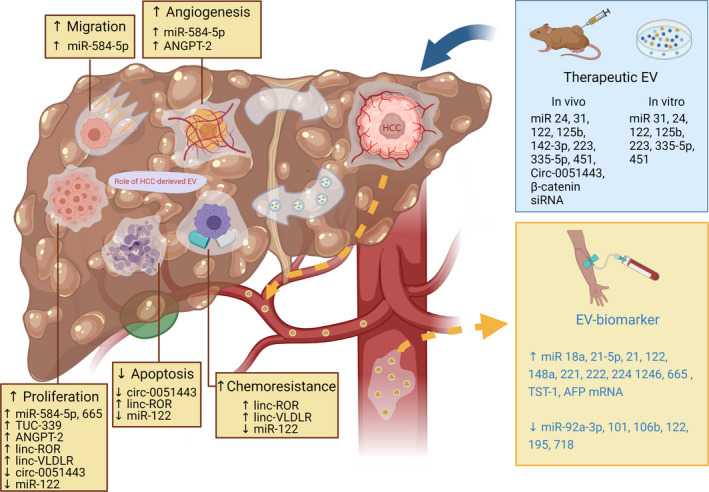
The roles of HCC‐derived EVs, HCC‐derived EVs as a biomarker, and the evidence of therapeutic EVs from currently available reports. HCC cells secrete EVs that can lead to increased tumor cell proliferation, migration, chemoresistance, and decreased tumor cell apoptosis. They can also affect tumor microenvironments such as increased angiogenesis. Some of these HCC‐derived EVs can be detected in circulation, making them available for use as a diagnostic biomarker. Moreover, it is possible for EVs to be used as a therapeutic cargo to transfer therapeutic molecules into tumor cells. Several in vitro and in vivo reports have demonstrated antitumoral effects using this method. miR: microRNA; Circ: circular RNA; siRNA: signal interference RNA; ANGPT2: angiopoietin2; linc: long interceding/intergenic noncoding RNA; TUC: tumor ultraconserved RNA.
This increasing evidence suggests the potential translational use of EVs in HCC patients in the near future. In the case of diagnostic roles, circulating EVs might be used as a serum biomarker for HCC or as an added value for the diagnosis of liver mass with uncertain imaging. Correlating the EVs with tumor staging, tumor size, and metastasis may help to determine tumor burden and prognosis. However, as regards the therapeutic role, there are no clinical reports available at this time. Further studies are needed to discover the best way to use EV for HCC patient care. Investigations aiming to deliver therapeutic EVs via endovascular methods such as using transarterial chemoembolization (TACE) will also provide important information and warrants further clinical investigation.
ETHICS STATEMENT
Not applicable.
CONFLICT OF INTEREST
All authors declare that they have no conflict of interest to disclose.
ACKNOWLEDGMENTS
This work was supported by an NSTDA Research Chair grant from the National Science and Technology Development Agency Thailand (NC), the Senior Research Scholar grant from the National Research Council of Thailand (SCC), and the Chiang Mai University Center of Excellence Award (NC).
Nimitrungtawee N, Inmutto N, Chattipakorn SC, Chattipakorn N. Extracellular vesicles as a new hope for diagnosis and therapeutic intervention for hepatocellular carcinoma. Cancer Med. 2021;10:8253–8271. doi: 10.1002/cam4.4370
DATA AVAILABILITY STATEMENT
All information is provided in the manuscript.
REFERENCES
- 1. Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability‐adjusted life‐years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3(4):524‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. [DOI] [PubMed] [Google Scholar]
- 3. Mittal S, El‐Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47(Supplement 1):S2‐S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lancet. Global, regional, and national life expectancy, all‐cause mortality, and cause‐specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. 2016;388(10053):1459‐1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marrero JA. Current treatment approaches in HCC. Clin Adv Hematol Oncol. 2013;11(Suppl 5):15‐18. [PubMed] [Google Scholar]
- 6. Blum HE. Hepatocellular carcinoma: therapy and prevention. World J Gastroenterol. 2005;11(47):7391‐7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang J, He XD, Yao N, Liang WJ, Zhang YC. A meta‐analysis of adjuvant therapy after potentially curative treatment for hepatocellular carcinoma. Can J Gastroenterol. 2013;27(6):351‐363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chan ACY, Poon RTP, Ng KKC, Lo CM, Fan ST, Wong J. Changing paradigm in the management of hepatocellular carcinoma improves the survival benefit of early detection by screening. Ann Surg. 2008;247(4):666‐673. [DOI] [PubMed] [Google Scholar]
- 9. Tzartzeva K, Obi J, Rich NE, et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta‐analysis. Gastroenterology. 2018;154(6):1706‐18.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liver EAFTSOT. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182‐236. [DOI] [PubMed] [Google Scholar]
- 11. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358‐380. [DOI] [PubMed] [Google Scholar]
- 12. Finn RS. The role of liver biopsy in hepatocellular carcinoma. Gastroenterol Hepatol (N Y). 2016;12(10):628‐630. [PMC free article] [PubMed] [Google Scholar]
- 13. Kim JH, Joo I, Lee JM. Atypical appearance of hepatocellular carcinoma and its mimickers: how to solve challenging cases using gadoxetic acid‐enhanced liver magnetic resonance imaging. Korean J Radiol. 2019;20(7):1019‐1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rossi G, Ignatiadis M. Promises and pitfalls of using liquid biopsy for precision medicine. Cancer Res. 2019;79(11):2798‐2804. [DOI] [PubMed] [Google Scholar]
- 15. Dong J, Chen J‐F, Smalley M, et al. Nanostructured substrates for detection and characterization of circulating rare cells: from materials research to clinical applications. J Adv Mater. 2020;32(1):1903663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crowley E, Di Nicolantonio F, Loupakis F, Bardelli A. Liquid biopsy: monitoring cancer‐genetics in the blood. Nat Rev Clin Oncol. 2013;10(8):472‐484. [DOI] [PubMed] [Google Scholar]
- 17. Gangadaran P, Hong CM, Ahn BC. Current perspectives on in vivo noninvasive tracking of extracellular vesicles with molecular imaging. Biomed Res Int. 2017;2017:9158319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lässer C. Exosomal RNA as biomarkers and the therapeutic potential of exosome vectors. Expert Opin Biol Ther. 2012;12(Suppl 1):S189‐S197. [DOI] [PubMed] [Google Scholar]
- 19. Kogure T, Lin WL, Yan IK, Braconi C, Patel T. Intercellular nanovesicle‐mediated microRNA transfer: a mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatology. 2011;54(4):1237‐1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Peinado H, Alečković M, Lavotshkin S, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro‐metastatic phenotype through MET. Nat Med. 2012;18(6):883‐891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sayeed A, Dalvano BE, Kaplan DE, et al. Profiling the circulating mRNA transcriptome in human liver disease. Oncotarget. 2020;11(23):2216‐2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhou J, Li XL, Chen ZR, Chng WJ. Tumor‐derived exosomes in colorectal cancer progression and their clinical applications. Oncotarget. 2017;8(59):100781‐100790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Witwer KW. Circulating microRNA biomarker studies: pitfalls and potential solutions. Clin Chem. 2015;61(1):56‐63. [DOI] [PubMed] [Google Scholar]
- 24. Katsuda T, Kosaka N, Ochiya T. The roles of extracellular vesicles in cancer biology: toward the development of novel cancer biomarkers. Proteomics. 2014;14(4–5):412‐425. [DOI] [PubMed] [Google Scholar]
- 25. Zitvogel L, Regnault A, Lozier A, et al. Eradication of established murine tumors using a novel cell‐free vaccine: dendritic cell derived exosomes. Nat Med. 1998;4(5):594‐600. [DOI] [PubMed] [Google Scholar]
- 26. Rocco GD, Baldari S, Toietta G. Exosomes and other extracellular vesicles‐mediated microRNA delivery for cancer therapy. Transl Cancer Res. 2017;S1321‐S1330. [Google Scholar]
- 27. Katoh M. FGFR inhibitors: effects on cancer cells, tumor microenvironment and whole‐body homeostasis (review). Int J Mol Med. 2016;38(1):3‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Son B, Lee S, Youn H, Kim E, Kim W, Youn B. The role of tumor microenvironment in therapeutic resistance. Oncotarget. 2017;8(3):3933‐3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Whittaker S, Marais R, Zhu AX. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010;29(36):4989‐5005. [DOI] [PubMed] [Google Scholar]
- 30. Kourembanas S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol. 2015;77:13‐27. [DOI] [PubMed] [Google Scholar]
- 31. Xie J‐Y, Wei J‐X, Lv L‐H, et al. Angiopoietin‐2 induces angiogenesis via exosomes in human hepatocellular carcinoma. J Cell Commun Signal. 2020;18(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen W, Quan Y, Fan S, et al. Exosome‐transmitted circular RNA hsa_circ_0051443 suppresses hepatocellular carcinoma progression. Cancer Lett. 2020;475:119‐128. [DOI] [PubMed] [Google Scholar]
- 33. Shao Z, Pan Q, Zhang Y. Hepatocellular carcinoma cell‐derived extracellular vesicles encapsulated microRNA‐584‐5p facilitates angiogenesis through PCK1‐mediated nuclear factor E2‐related factor 2 signaling pathway. Int J Biochem Cell Biol. 2020;125:105789. [DOI] [PubMed] [Google Scholar]
- 34. Kogure T, Yan IK, Lin WL, Patel T. Extracellular Vesicle‐Mediated Transfer of a Novel Long Noncoding RNA TUC339: A Mechanism of Intercellular Signaling in Human Hepatocellular Cancer. Genes Cancer. 2013;4(7–8):261‐272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Qu Z, Wu J, Wu J, et al. Exosomal miR‐665 as a novel minimally invasive biomarker for hepatocellular carcinoma diagnosis and prognosis. Oncotarget. 2017;8(46):80666‐80678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Takahashi K, Yan IK, Wood J, Haga H, Patel T. Involvement of extracellular vesicle long noncoding RNA (linc‐VLDLR) in tumor cell responses to chemotherapy. Mol Cancer Res. 2014;12(10):1377‐1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Takahashi K, Yan IK, Kogure T, Haga H, Patel T. Extracellular vesicle‐mediated transfer of long non‐coding RNA ROR modulates chemosensitivity in human hepatocellular cancer. FEBS Open Bio. 2014;4:458‐467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ruan K, Fang X, Ouyang G. MicroRNAs: novel regulators in the hallmarks of human cancer. Cancer Lett. 2009;285(2):116‐126. [DOI] [PubMed] [Google Scholar]
- 39. Huang Y, Zhang C, Xiong J, Ren H. Emerging important roles of circRNAs in human cancer and other diseases. Genes Dis. 2021;8(4):412‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fang Y, Fullwood MJ. Roles, functions, and mechanisms of long non‐coding RNAs in cancer. GPB. 2016;14(1):42‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang G, Liu W, Zou Y, et al. Three isoforms of exosomal circPTGR1 promote hepatocellular carcinoma metastasis via the miR449a‐MET pathway. EBioMedicine. 2019;40:432‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huang X, Sun L, Wen S, et al. RNA sequencing of plasma exosomes revealed novel functional long noncoding RNAs in hepatocellular carcinoma. Cancer Sci. 2020;111(9):3338‐3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu AM, Yao TJ, Wang W, et al. Circulating miR‐15b and miR‐130b in serum as potential markers for detecting hepatocellular carcinoma: a retrospective cohort study. BMJ Open. 2012;2(2):e000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Qu KZ, Zhang K, Li H, Afdhal NH, Albitar M. Circulating microRNAs as biomarkers for hepatocellular carcinoma. J Clin Gastroenterol. 2011;45(4):355‐360. [DOI] [PubMed] [Google Scholar]
- 45. Motawi TK, Shaker OG, El‐Maraghy SA, Senousy MA. Serum MicroRNAs as potential biomarkers for early diagnosis of hepatitis C virus‐related hepatocellular carcinoma in Egyptian patients. PLoS One. 2015;10(9):e0137706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tomimaru Y, Eguchi H, Nagano H, et al. Circulating microRNA‐21 as a novel biomarker for hepatocellular carcinoma. J Hepatol. 2012;56(1):167‐175. [DOI] [PubMed] [Google Scholar]
- 47. Xu J, Wu C, Che X, et al. Circulating microRNAs, miR‐21, miR‐122, and miR‐223, in patients with hepatocellular carcinoma or chronic hepatitis. Mol Carcinog. 2011;50(2):136‐142. [DOI] [PubMed] [Google Scholar]
- 48. Li J, Wang Y, Yu W, Chen J, Luo J. Expression of serum miR‐221 in human hepatocellular carcinoma and its prognostic significance. Biochem Biophys Res Commun. 2011;406(1):70‐73. [DOI] [PubMed] [Google Scholar]
- 49. Meng F, Henson R, Wehbe‐Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA‐21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133(2):647‐658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhou J, Yu L, Gao X, et al. Plasma microRNA panel to diagnose hepatitis B virus‐related hepatocellular carcinoma. J Clin Oncol. 2011;29(36):4781‐4788. [DOI] [PubMed] [Google Scholar]
- 51. Zhu HT, Liu RB, Liang YY, et al. Serum microRNA profiles as diagnostic biomarkers for HBV‐positive hepatocellular carcinoma. Liver Int. 2017;37(6):888‐896. [DOI] [PubMed] [Google Scholar]
- 52. Zhang Y, Li T, Qiu Y, et al. Serum microRNA panel for early diagnosis of the onset of hepatocellular carcinoma. Medicine (Baltimore). 2017;96(2):e5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Coulouarn C, Factor VM, Andersen JB, Durkin ME, Thorgeirsson SS. Loss of miR‐122 expression in liver cancer correlates with suppression of the hepatic phenotype and gain of metastatic properties. Oncogene. 2009;28(40):3526‐3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Qi P, Cheng SQ, Wang H, Li N, Chen YF, Gao CF. Serum microRNAs as biomarkers for hepatocellular carcinoma in Chinese patients with chronic hepatitis B virus infection. PLoS One. 2011;6(12):e28486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang X, Kwak KJ, Yang Z, et al. Extracellular mRNA detected by molecular beacons in tethered lipoplex nanoparticles for diagnosis of human hepatocellular carcinoma. PLoS One. 2018;13(6):e0198552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sorop A, Iacob R, Iacob S, et al. Plasma small extracellular vesicles derived miR‐21‐5p and miR‐92a‐3p as potential biomarkers for hepatocellular carcinoma screening. Front Genet. 2020;11:712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang Y, Zhang C, Zhang P, et al. Serum exosomal microRNAs combined with alpha‐fetoprotein as diagnostic markers of hepatocellular carcinoma. Cancer Med. 2018;7(5):1670‐1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Melo SA, Luecke LB, Kahlert C, et al. Glypican‐1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523(7559):177‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wei JX, Lv LH, Wan YL, et al. Vps4A functions as a tumor suppressor by regulating the secretion and uptake of exosomal microRNAs in human hepatoma cells. Hepatology. 2015;61(4):1284‐1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zheng Q, Zhao J, Yu H, et al. Tumor‐specific transcripts are frequently expressed in hepatocellular carcinoma with clinical implication and potential function. Hepatology. 2020;71(1):259‐274. [DOI] [PubMed] [Google Scholar]
- 61. Hornick NI, Huan J, Doron B, et al. Serum exosome MicroRNA as a minimally‐invasive early biomarker of AML. Sci Rep. 2015;5:11295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sugimachi K, Matsumura T, Hirata H, et al. Identification of a bona fide microRNA biomarker in serum exosomes that predicts hepatocellular carcinoma recurrence after liver transplantation. Br J Cancer. 2015;112(3):532‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Witwer KW, Buzás EI, Bemis LT, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2(1):20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zeringer E, Barta T, Li M, Vlassov AV. Strategies for isolation of exosomes. Cold Spring Harb Protoc. 2015;2015(4):319‐323. [DOI] [PubMed] [Google Scholar]
- 65. Welton J, Webber J, Botos L‐A, Jones M, Clayton A. Ready‐made chromatography columns for extracellular vesicle isolation from plasma. J Extracell Vesicles. 2015;4:27269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rider MA, Hurwitz SN, Meckes DG. ExtraPEG: a polyethylene glycol‐based method for enrichment of extracellular vesicles. Sci Rep. 2016;6(1):23978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sun N, Lee Y‐T, Zhang RY, et al. Purification of HCC‐specific extracellular vesicles on nanosubstrates for early HCC detection by digital scoring. Nat Commun. 2020;11(1):4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang H, Hou L, Li A, Duan Y, Gao H, Song X. Expression of serum exosomal microRNA‐21 in human hepatocellular carcinoma. Biomed Res Int. 2014;2014:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sohn W, Kim J, Kang SH, et al. Serum exosomal microRNAs as novel biomarkers for hepatocellular carcinoma. Exp Mol Med. 2015;47(9):e184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kim SS, Baek GO, Ahn HR, et al. Serum small extracellular vesicle‐derived LINC00853 as a novel diagnostic marker for early hepatocellular carcinoma. Mol Oncol. 2020;14(10):2646‐2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ghosh S, Bhowmik S, Majumdar S, et al. The exosome encapsulated microRNAs as circulating diagnostic marker for hepatocellular carcinoma with low alpha‐fetoprotein. Int J Cancer. 2020;147(10):2934‐2947. [DOI] [PubMed] [Google Scholar]
- 72. Cui Z, Li Y, Gao Y, Kong L, Lin Y, Chen Y. Cancer‐testis antigen lactate dehydrogenase C4 in hepatocellular carcinoma: a promising biomarker for early diagnosis, efficacy evaluation and prognosis prediction. Aging (Albany NY). 2020;12(19):19455‐19467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sun X‐H, Wang Y‐T, Li G‐F, Zhang N, Fan L. Serum‐derived three‐circRNA signature as a diagnostic biomarker for hepatocellular carcinoma. Cancer Cell Int. 2020;20(1):226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Julich‐Haertel H, Urban SK, Krawczyk M, et al. Cancer‐associated circulating large extracellular vesicles in cholangiocarcinoma and hepatocellular carcinoma. J Hepatol. 2017;67(2):282‐292. [DOI] [PubMed] [Google Scholar]
- 75. Katakowski M, Buller B, Zheng X, et al. Exosomes from marrow stromal cells expressing miR‐146b inhibit glioma growth. Cancer Lett. 2013;335(1):201‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Di Rocco G, Baldari S, Toietta G. Exosomes and other extracellular vesicles‐mediated microRNA delivery for cancer therapy. Transl Cancer Res. 2017;6:S1321‐S1330. [Google Scholar]
- 77. Villa F, Quarto R, Tasso R. Extracellular vesicles as natural, safe and efficient drug delivery systems. Pharmaceutics. 2019;11(11):557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fonsato V, Collino F, Herrera MB, et al. Human liver stem cell‐derived microvesicles inhibit hepatoma growth in SCID mice by delivering antitumor microRNAs. Stem Cells. 2012;30(9):1985‐1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lou G, Song X, Yang F, et al. Exosomes derived from miR‐122‐modified adipose tissue‐derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol. 2015;8:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang F, Li L, Piontek K, Sakaguchi M, Selaru FM. Exosome miR‐335 as a novel therapeutic strategy in hepatocellular carcinoma. Hepatology. 2018;67(3):940‐954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Baldari S, Di Rocco G, Magenta A, Picozza M, Toietta G. Extracellular vesicles‐encapsulated MicroRNA‐125b produced in genetically modified mesenchymal stromal cells inhibits hepatocellular carcinoma cell proliferation. Cells. 2019;8(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ma B, Jiang H, Jia J, et al. Murine bone marrow stromal cells pulsed with homologous tumor‐derived exosomes inhibit proliferation of liver cancer cells. Clin Transl Oncol. 2012;14(10):764‐773. [DOI] [PubMed] [Google Scholar]
- 83. Son SH, Gangadaran P, Ahn B‐C. A novel strategy of transferring NIS protein to cells using extracellular vesicles leads to increase in iodine uptake and cytotoxicity. Int J Nanomedicine. 2019;14:1779‐1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Matsuda A, Ishiguro K, Yan IK, Patel T. Extracellular vesicle‐based therapeutic targeting of β‐catenin to modulate anticancer immune responses in hepatocellular cancer. Hepatol Commun. 2019;3(4):525‐541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ishiguro K, Yan IK, Lewis‐Tuffin L, Patel T. Targeting liver cancer stem cells using engineered biological nanoparticles for the treatment of hepatocellular cancer. Hepatol Commun. 2020;4(2):298‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhang J, Shan WF, Jin TT, et al. Propofol exerts anti‐hepatocellular carcinoma by microvesicle‐mediated transfer of miR‐142‐3p from macrophage to cancer cells. J Transl Med. 2014;12(1):279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rao Q, Zuo B, Lu Z, et al. Tumor‐derived exosomes elicit tumor suppression in murine hepatocellular carcinoma models and humans in vitro. Hepatology. 2016;64(2):456‐472. [DOI] [PubMed] [Google Scholar]
- 88. Basu S, Bhattacharyya SN. Insulin‐like growth factor‐1 prevents miR‐122 production in neighbouring cells to curtail its intercellular transfer to ensure proliferation of human hepatoma cells. Nucleic Acids Res. 2014;42(11):7170‐7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yang B, Feng X, Liu H, et al. High‐metastatic cancer cells derived exosomal miR92a‐3p promotes epithelial‐mesenchymal transition and metastasis of low‐metastatic cancer cells by regulating PTEN/Akt pathway in hepatocellular carcinoma. Oncogene. 2020;39(42):6529‐6543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yugawa K, Yoshizumi T, Mano Y, et al. Cancer‐associated fibroblasts promote hepatocellular carcinoma progression through downregulation of exosomal miR‐150‐3p. Eur J Surg Oncol. 2021;47(2):384‐393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All information is provided in the manuscript.


