Abstract
Idiopathic pulmonary fibrosis (IPF) is an aging‐associated disease with a poor prognosis. Emerging evidence has revealed that targeting senescent cells may be a potential treatment for IPF. In this study, we aimed to explore whether roxithromycin (RXM) can improve lung fibrosis by targeting senescent cells. First, we confirmed the ability of RXM to selectively kill senescent cells by inducing apoptosis and inhibiting the expression of senescence‐associated secretory phenotype (SASP) factors, suggesting the potential role of RXM as a “senolytic” and “senomorphic” drug. Next, we observed that TGF-β- and senescent cell-induced lung fibroblast activation was inhibited by RXM treatment, which prompted us to further investigate its effect in vivo. In a mouse model of bleomycin (BLM)-induced pulmonary fibrosis, RXM was shown to attenuate lung injury, inflammation, and fibrosis. Furthermore, the senescent phenotype of lung tissues induced by BLM was significantly diminished after RXM administration, indicating the potential of RXM as an antifibrotic and antisenescent agent. Interestingly, NADPH oxidase 4 (NOX4), implicated in lung fibrosis and cell senescence, was shown to be inhibited by RXM treatments. The antifibroblast activation and antisenescent effects of RXM were abolished in NOX4 knockdown cells, demonstrating that RXM may ameliorate BLM-induced pulmonary fibrosis by targeting senescent cells mediated by the NOX4 pathway. Collectively, these data demonstrated that RXM may be a potential clinical agent for IPF and further supported the notion that targeting cellular senescence is a promising treatment for progressive age-related disease.
Keywords: idiopathic pulmonary fibrosis, cellular senescence, roxithromycin, NOX4, senescence-associated secretory phenotype (SASP)
Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive, irreversible, and ultimately fatal age-related disease that is classified as the most common and severe type of idiopathic interstitial pneumonia [1–3]. IPF is characterized by the excessive deposition of collagen, enlarged interstitial spaces between alveoli, thickened alveolar walls and inflammatory cell infiltration, leading to the destruction of the lung structure and even lung failure. Previous studies have shown that the incidence and prevalence of IPF increases with age (mean age at diagnosis is ~66 years old), and the estimated survival is 3–4 years [1, 4–6]. Although two drugs, nintedanib (OFEV) and pirfenidone, were approved for the treatment of IPF mainly based on their ability to increase forced vital capacity and slow disease progression for most patients, it is still unclear whether these drugs improve symptoms and survival [7, 8]. Therefore, therapeutic interventions for IPF are in urgent demand.
The potential risk factors for IPF are complex and include genetic susceptibility, environmental factors, and aging-associated processes, such as cellular senescence [9]. A growing body of evidence has shown that senescent cells play a critical role in the pathogenesis of IPF [10, 11]. Cellular senescence is an evolutionarily conserved state, exhibiting irreversible cell replicative arrest, apoptotic resistance and acquisition of a senescence-associated secretory phenotype (SASP). The SASP is characterized by the secretion of multiple active substances, including inflammatory cytokines, chemokines, and matrix proteases, that promote abnormal and persistent fibroblast activation [12–14].
In the case of IPF, lung epithelial injury caused by multiple unknown risk factors is characterized by the senescence of alveolar epithelial type 2 cells (AEC2s), which secrete SASP and profibrotic factors, especially TGF-β1 [15]. These factors further induce myofibroblast differentiation, present a stressed and senescent phenotype, and exacerbate the deposition of extracellular matrix (ECM) components, thus causing irreversible damage and fibrosis [16, 17]. Collectively, this evidence indicates that senescent AEC2s and myofibroblasts play a pivotal role in accelerating the aberrant injury-remodeling process and fibrosis.
Therefore, targeting and eliminating senescent cells have emerged as promising therapies for IPF [13, 18–20]. Recently, emerging studies have shown two main therapeutic strategies to address the presence of senescent cells. One is the compounds termed “senolytics”, which can selectively kill senescent cells [21, 22]. The other relates to the potential to neutralize the deleterious effects of SASP on senescent cells by drugs named “senomorphics” [23]. Moreover, the classic senolytic drug cocktail of dasatinib plus quercetin (DQ) was reported to improve pulmonary function and physical health, which provided proof-of-concept evidence for targeting senescent cells as a novel pharmacological approach for the treatment of IPF.
Roxithromycin (RXM), a macrolide antibiotic, was identified as a senolytic drug, as it was shown to eliminate senescent MRC-5 cells [24]. Additionally, several studies have reported that RXM reduced airway inflammation injury in asthmatic mice and bleomycin-induced acute lung injury [25–27]. Furthermore, low-dose, long-term RXM could ameliorate remodeling of dilated bronchial walls in patients with noncystic fibrosis bronchiectasis [28], which prompted us to explore whether RXM can improve lung fibrosis via its ability to selectively kill senescent cells. It is also unknown whether RXM can suppress SASP factors beyond eliminating senescent cells. In this paper, we not only confirmed the ability of RXM to selectively eliminate senescent lung fibroblasts but also found that RXM inhibited the expression of SASP factors to prevent senescent cells from deteriorating. Furthermore, RXM was demonstrated to inhibit senescent cell-induced fibroblast activation in vitro and attenuate bleomycin (BLM)-induced pulmonary fibrosis as well as the senescent phenotype during pathogenesis in vivo. The potential mechanism of RXM was associated with the downregulation of NADPH oxidase 4 (NOX4) expression, which is implicated in lung fibrosis and cell senescence [18, 29]. The antifibroblast activation and antisenescent effects of RXM were abolished in NOX4 knockdown cells, indicating that RXM may ameliorate BLM-induced pulmonary fibrosis by targeting senescent cells mediated by the NOX4 pathway.
Materials and methods
Mouse model
For the experiments, we used male C57BL/6 mice (8 weeks), weighing ~20–25 g, obtained from Shanghai SLAC and maintained in a 12 h light/dark cycle with free access to food and water. All experimental methods were carried out in accordance with the guidelines of the IACUC of Shanghai and the National Research Council Guide for the Care and Use of Laboratory Animals. All animal experiments were performed according to procedures approved by the Animal Care and Use Committee of the Shanghai Institute of Materia Medica. C57BL/6 male mice wre randomly divided into six groups: the control (WT) group (n ≥ 6), vehicle group (n ≥ 6), OFEV group (60 mg/kg, n ≥ 6), and RXM groups (40, 80, and 160 mg/kg, n ≥ 6). For the experiments, all mice were anesthetized with 1% pentobarbital sodium dissolved in saline by intraperitoneal injection at 50 mg/kg body weight and then fixed on the surgery board. The animals received a single dose of BLM at 0.85 U/kg or saline by intratracheal instillation with a MicroSprayer® aerosolizer (BJ-PW-R, BioJane, Shanghai, China). After 5 days of injection, the control and vehicle mice were subjected to 5% sodium carboxymethyl cellulose (CMC-Na, dissolved in saline) by oral gavage at a volume of 10 μL/g per body weight every day, and the vehicle-treated mice received 60 mg/kg OFEV solution (dissolved in 5% CMC-Na) by oral gavage every day. The mice from the remaining three groups were administered RXM at 40, 80, and 160 mg/kg by oral gavage every day. The body weight was recorded every other day and the day the mice were killed. All mice were killed 3 weeks post-intratracheal injection.
The reagents used were as follows: OFEV (HY-50904, MedChemExpress, NJ, USA), BLM (BD01259671, Bidepharma, Shanghai, China), RXM (MB1621, Meilun BioTech, Dalian, China).
Sample and tissue extraction
Bronchoalveolar lavage was performed with 6 mL of ice-cold PBS, and 90% bronchoalveolar lavage fluid (BALF) was retrieved. The left lobes of the lungs were fixed in 4 mL of 4% polyoxymethylene solution and then sectioned for histological analysis. The right lobes of the lungs were weighed and kept in liquid nitrogen and then transferred to −80 °C for storage.
LDH and cell number evaluation
The remaining BALF was centrifuged (800 × g, 10 min), and 2 mL of supernatant was collected and stored at −80 °C for LDH measurement. Determination of LDH concentrations was performed with an automatic biochemical analyzer (AU480, Beckman Coulter, Pasadena, CA, USA). The precipitate was resuspended in ice-cold PBS up to 500 μL, and the cell numbers were counted using CountStar (IC1000, Countstar BioTech, Shanghai, China).
Histological staining and semiquantitative analysis
The tissues were fixed in 4% paraformaldehyde and embedded in paraffin. Hematoxylin–eosin (H&E) staining and Masson’s trichome staining were performed, and semiquantitative analysis was performed according to the Ashcroft scoring criteria.
Immunohistochemistry (IHC) staining
The tissues were fixed in 4% paraformaldehyde and embedded in paraffin. For immunohistochemical detection of α-SMA and p21 expression, sections were deparaffinized and rehydrated, and then, endogenous peroxidase was quenched with 3% aqueous hydrogen peroxide for 15 min. Then, the sections were blocked with 5% BSA (B2064-10G, Sigma-Aldrich, St. Louis, MO, USA) in Tris-buffered saline for 1 h and incubated with the respective primary antibodies overnight at 4 °C. After PBS washes, the secondary antibody (abs20002A, Absin Bioscience, Shanghai, China) was used. After incubation for 30 min, 3,3′-diaminobenzidine tetrahydrochloride was added for 10 min at room temperature. Finally, the slides were stained with hematoxylin and examined under a microscope. The primary antibodies included α-SMA (ab7817, Abcam, Cambridge, UK) and p21 (56062T, Cell Signaling Technology, Trask Lane Danvers, MA, USA).
Immunofluorescence costaining
The tissues were fixed in 4% paraformaldehyde and embedded in paraffin. Sections were deparaffinized and rehydrated and then boiled in EDTA Antigen Retrieval Solution (P0085, Beyotime, Haimen, China) for antigen retrieval. Slides were preblocked with 5% BSA in PBS and then incubated with primary antibodies against p21 (56062T, Cell Signaling Technology, Trask Lane Danvers, MA, USA) at 4 °C overnight. The slides were then washed with PBS and incubated with secondary antibodies at 37 °C for 1 h. TUNEL staining was performed as described (C1090, Beyotime, Haimen, China). Nuclei were stained with Hoechst (C1017, Beyotime, Haimen, China). Finally, the slides were observed and photographed using a fluorescence microscope (1013688, PE Vectra3, Perkin Elmer, Waltham, MA, USA).
Detection of lung hydroxyproline (HYP) content
The hydroxyproline (HYP) assay was performed as follows. Briefly, 20 mg of tissue from the right lobes of the lungs was homogenized in 5 mL of 50% HCl and then placed at 120 °C for 22 h. If liquid was lost, the samples were filled with up to 5 mL of 50% HCl. The samples were filtered with a 0.45 μm microfiltration membrane, and 100 μL of the samples was dried at 40 °C for 3 days. Each sample was added to 1200 μL of 50% isopropanol and 200 μL of chloramine T working solution (1.8% trisodium citrate dihydrate, 0.3% citric acid, 2.8% sodium acetate, 19.2% isopropanol, 0.6% chloramine T) at room temperature for 10 min. One milliliter of Ehrlich working solution (13.6% dimethylaminobenzaldehyde, 14.9% perchloric acid, 73% isopropanol) was added to each sample and then incubated in a 50 °C water bath for 1.5 h. The OD value was measured at 558 nm using a spectrophotometer (SpectraMax M5, Molecular Devices, Santa Clara, CA, USA) and compared to a HYP standard curve.
RNA extraction and quantitative real-time polymerase chain reaction
Total RNA was extracted using ~200 μL of TRIzol reagent (9019, TaKaRa, Japan). One microgram of the total RNA was transcribed into cDNA in 20 μL reactions using the PrimeScript RT Master Mix (RR036, TaKaRa, Japan) and then diluted to 300 μL, which was used in subsequent real-time qPCR reactions. Real-time qPCR was performed using the SYBR Green qPCR Kit (B21702, Bimake, Houston, TX, USA) on an Agilent Mx3000P system (Agilent Technologies, Santa Clara, CA, USA) as specified by the manufacturer. All reactions were performed in triplicate.
Table 1 shows the primer sequences (5′–3′). The data were averaged and normalized to the expression level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Table 1.
Primer information of qPCR experiments.
| Gene names | Sense (5′–3′) | Anti-sense (5′–3′) |
|---|---|---|
| H-GAPDH | CATTTCCTGGTATGACAACGA | GTCTACATGGCAACTGTGAG |
| H-p21 | CCAGCATGACAGATTTCTACC | CAGGGTATGTACATGAGGAGG |
| H-p53 | AGACCTATGGAAACTACTTCCTG | GGGAAGGGACAGAAGATGAC |
| H-p16 | CTCGTGCTGATGCTACTGAGGA | GGTCGGCGCAGTTGGGCTCC |
| H-IL-6 | AGACAGCCACTCACCTCTTCAG | TTCTGCCAGTGCCTCTTTGCTG |
| H-IL-8 | GAGAGTGATTGAGAGTGGACCAC | CACAACCCTCTGCACCCAGTTT |
| H-TNFα | CTCTTCTGCCTGCTGCACTTTG | ATGGGCTACAGGCTTGTCACTC |
| H-MMP-9 | GCCACTACTGTGCCTTTGAGTC | CCCTCAGAGAATCGCCAGTACT |
| H-Fibronectin | ACAACACCGAGGTGACTGAGAC | GGACACAACGATGCTTCCTGAG |
| H-α-SMA | CTATGCCTCTGGACGCACAACT | CAGATCCAGACGCATGATGGCA |
| H-Collagen I | GATTCCCTGGACCTAAAGGTGC | AGCCTCTCCATCTTTGCCAGCA |
| M-GAPDH | ATGGTGAAGGTCGGTGTGAAC | GCCGTGAGTGGAGTCATACTG |
| M-IL-6 | TACCACTTCACAAGTCGGAGGC | CTGCAAGTGCATCATCGTTGTTC |
| M-p53 | GTTATGTGCACGTACTCTCC | ACTCGGGATACAAATTTCCT |
| M-Fibronectin | CCCTATCTCTGATACCGTTGTCC | TGCCGCAACTACTGTGATTCGG |
| M-α-SMA | TGCTGACAGAGGCACCACTGAA | CAGTTGTACGTCCAGAGGCATAG |
| M-Collagen I | CCTCAGGGTATTGCTGGACAAC | CAGAAGGACCTTGTTTGCCAGG |
| M-TNFα | TGATACGCCTGAGTGGCTGTCT | CACAAGAGCAGTGAGCGCTGAA |
| M-CXCL15 | GGTGATATTCGAGACCATTTACTG | GCCAACAGTAGCCTTCACCCAT |
Cell culture
MRC-5 and WI-38 cells were purchased from ATCC and maintained in EMEM (30-2003, Gibco, Waltham, MA, USA) supplemented with 10% FBS (10091-148, Gibco, Waltham, MA, USA) and 1% penicillin-streptomycin-glutamine (0242,0382, VWR Life Science, PA, USA). A549 cells were purchased from ATCC and maintained in EMEM (30-2003, Gibco, Waltham, MA, USA) supplemented with 10% FBS (10091-148, Gibco, Waltham, MA, USA), 0.1% penicillin-streptomycin-glutamine (0242,0382, VWR Life Science, PA, USA), and 1% MEM nonessential amino acids (2176640, VWR Life Science, PA, USA). The cells were all incubated in a humidified atmosphere of 5% CO2 and 95% air at 37 °C and split when they reached 95% confluency.
For the fibroblast activation experiment, MRC-5 cells were cultured in a 24-well plate. After 12 h, the original medium was removed, and MRC-5 cells were starved with EMEM (30-2003, Gibco, Waltham, MA, USA) supplemented with 0.5% FBS (10091-148, Gibco, Waltham, MA, USA). After another 12 h, the cells were treated with OFEV (0.5 μM, HY-50904, MedChemExpress, NJ, USA), TGF-β (5 ng/mL, 10804-HNAC, Sino Biological Inc, Beijing, China) + RXM (10, 20, 40, and 80 μM, MB1621, Meilun BioTech, Dalian, China) or TGF-β (5 ng/mL, 10804-HNAC, Sino Biological Inc, Beijing, China) alone for 24 h before Western blot analysis. For the NOX4 dependency experiment, 24-well plate-cultured MRC-5 cells were transfected with 1 μL of siNOX4 using Lipofectamine® RNAiMAX (2044225, Invitrogen, Carlsbad, CA, USA) for 24 h before treatment with 5 ng/mL TGF-β (10804-HNAC, Sino Biological Inc, Beijing, China) alone or 40 μM RXM (MB1621, Meilun BioTech, Dalian, China) for another 24 h. The knockdown efficiency was confirmed at the protein level. Further detection of fibrotic markers was performed via Western blot analysis.
NOX4 siRNA was purchased from GenePharma, Shanghai, China.
Sense (5′–3′): UCUGUAUCCCAUCUGUUUGAC
Anti-Sense (5′–3′): CAAACAGAUGGGAUACAGAAG
Senescent cell models
WI-38 cells were exposed to 15 Gy of irradiation using a biological X-ray irradiator (RS2000, Rad Source Technologies, Buford, GA, USA) or treated with BLM (25 μg/mL) to obtain the senescent phenotype after 7 days. Senescence was confirmed by expression profiling (p21, 53, and IL-8) and senescence-associated β-galactosidase (SA-β-gal) staining. For the MTS assay, proliferating and senescent cells were cultured in 96-well plates for 24 h before the cells were treated with 20, 40, 60, 80, or 100 μM RXM for another 60–72 h. The MTS assay was performed to evaluate cell viability.
For induction of cell cycle arrest (short-term senescence), A549 cells were exposed to 25 μg/mL BLM (BD01259671, Bidepharma, Shanghai, China) with 40 or 80 μM RXM (MB1621, Meilun BioTech, Dalian, China) for 24 h, and fresh medium was added for another 24 h before the detection of p53, p21, and p16 expression via Western blots and qPCR analysis. For cellular senescence, A549 cells were simultaneously treated with 5 μg/mL BLM (BD01259671, Bidepharma, Shanghai, China) and 40 or 80 μM RXM (MB1621, Meilun BioTech, Dalian, China) for 6 days or treated with 5 μg/mL BLM for 6 days followed by RXM exposure for another 3 days before Western blot or qPCR analysis. For SASP factor-induced activation of lung fibroblasts, A549 cells were treated with 10 μg/mL BLM (BD01259671, Bidepharma, Shanghai, China) alone or with 40 or 80 μM RXM (MB1621, Meilun BioTech, Dalian, China) for 3 days. After fresh medium was added and the cells were cultured for another 3 days, the supernatant was obtained to culture MRC-5 cells for 3 days. Then, the MRC-5 cells were harvested for the evaluation of α-SMA, fibronectin, and collagen I expression. For the NOX4 dependency experiment, 24-well-cultured A549 cells were transfected with 1 μL of siNOX4 using Lipofectamine® RNAiMAX (2044225, Invitrogen, Carlsbad, CA, USA) for 24 h before treatment with 15 μg/mL of BLM (BD01259671, Bidepharma, Shanghai, China) alone or with 40 μM RXM (MB1621, Meilun BioTech, Dalian, China) for 3 days. The knockdown efficiency was confirmed at the mRNA level. Further detection of the senescent marker MMP-9 was performed via qPCR analysis.
MTS assay
The MTS assay was performed using a CellTiter96® AQueous Non-Radioactive Cell Proliferation Assay (MTS) kit (G111, Promega, Madison, WI, USA) according to the manufacturer’s instructions. Briefly, the MTS solution and PMS solution (20:1) were mixed and added to each well of a 96-well plate at a volume of 10 μL and incubated for 4 h. The absorbance was read at 490 nm with a spectrophotometer (SpectraMax M5, Molecular Devices, Santa Clara, CA, USA).
Senescence-associated β-galactosidase (SA-β-gal) staining
SA-β-gal staining was performed using a Senescence β-Galactosidase Staining Kit (C0602, Beyotime, Haimen, China) according to the manufacturer’s instructions. Cell samples were fixed with the fixation solution from the kit for 15 min. After three rinses with PBS for 5 min, the cells were incubated with freshly prepared SA‐β‐Gal staining solution overnight in a 37 °C humidified chamber. The next day, the cells were washed three times in PBS for 5 min at room temperature and observed with a microscope.
Flow cytometric assay
WI-38 cells were exposed to 25 μg/mL BLM (BD01259671, Bidepharma, Shanghai, China) for 7 days and treated with 40, 80, or 120 μM RXM (MB1621, Meilun BioTech, Dalian, China) for another 3 days. Then, the cells were harvested for Annexin V-FITC staining (KGA108, KeyGEN BioTech, Nanjing, China) according to the manufacturer’s instructions. Briefly, the cells were trypsinized (EDTA free) and centrifuged at 2000 r/min for 5 min three times. Then, the supernatant was discarded, and (1–5) × 105 cell pellets were resuspended in 500 μL of binding buffer. Annexin V (5 μL) was added to the cell suspension in the dark. The suspension was then detected by flow cytometry within 15 min. The apoptosis rate was determined by flow cytometry.
Human IL-8 ELISA measurement
The Human IL-8/CXCL15 ValkineTM ELISA kit (VAL103) was purchased from Novus Biologicals (Littleton, CO, USA) and the measurement was conducted according to the manufacturer’s instructions. Briefly, the supernatant was collected and added to the well of a human IL-8 microplate for 2 h. After the samples were washed with wash solution, 100 μL of human IL-8 conjugate was added to each well for 2 h. The washing process was repeated, and 200 μL of Substrate Solution was added and incubated for 30 min. Then, 50 μL of Stop Solution was added to each well, and the optical density was determined within 30 min using a microplate reader (SpectraMax M5, Molecular Devices, Santa Clara, CA, USA) set to 450 nm.
Western blot analysis
Protein samples were processed with SDS loading buffer and boiled to denature the proteins. Then, the denatured samples were separated based on size using 10%–15% SDS-polyacrylamide gel electrophoresis, followed by transferring the proteins of interest to a membrane. The membrane was blocked with 5% milk or BSA solution for 1 h to prevent nonspecific antibody binding. Then, the membrane was incubated with the appropriate primary antibodies at 4 °C overnight, followed by incubation with a secondary antibody at room temperature for 1 h. Finally, the membrane was stained with ECL detection buffer (1705061, Bio-Rad, Hercules, CA, USA) and imaged by a ChemiDoc system. Primary antibodies included GAPDH (ab8245, Abcam, Cambridge, UK), α-SMA (ab7817, Abcam, Cambridge, UK), fibronectin (ab2413, Abcam, Cambridge, UK), collagen Iα1 (ab34710, Abcam, Cambridge, UK), and NOX4 (A11274, ABclonal, Wuhan, China), and a senescence marker antibody sampler kit (56062T, Cell Signaling Technology, Trask Lane Danvers, MA, USA) containing p21, p16, IL-6, MMP-3, TNFα, HMGB1, and p53 (48818S, Cell Signaling Technology, Trask Lane Danvers, MA, USA) was used.
Statistical analysis
All values are expressed as the mean ± SEM, and comparisons between two groups were performed using two-tailed unpaired Student’s t test or one-way ANOVA. Statistical analyses were performed using GraphPad Prism software. Statistical significance was determined at P < 0.05 (*); P < 0.01 (**); and P < 0.001 (***).
Results
Roxithromycin selectively killed senescent cells
To determine whether RXM selectively kills senescent cells, we devised an in vitro assay with human fetal lung diploid fibroblasts (WI-38). WI-38 cells were exposed to 15 Gy irradiation (IR) to induce observable senescence after 7 days. The senescent phenotype was confirmed by SA-β-gal staining and mRNA and protein expression of senescent factors (p21 and p53) and SASP factors (IL-8 and IL-6) (Fig. 1a–f). Subsequently, cell viability was measured by MTS assays, which showed that RXM, even at a maximum concentration, had almost no effect on the viability of normal cells but selectively killed senescent cells in a concentration-dependent manner (Fig. 1g). We further confirmed its potential role as a senolytic drug in BLM-induced WI-38 cell senescence (Fig. 1h). The results also revealed that RXM exhibited a weaker senolytic effect in BLM-induced senescence than IR-induced senescence, as a higher concentration was required to elicit similar efficacy. It was reported that BLM is more potent in inducing whole-chromosome loss than X-rays [30], so we next confirmed the differences in the degree of senescence between BLM and X-irradiation stimulation. WI-38 cells were treated with 25 μg/mL BLM or 15 Gy X-irradiation for 7 days, and then, qPCR and Western blot experiments were performed. As shown in Supplementary Fig. S1, compared to irradiation, BLM treatment led to a higher expression of SASP factors, such as IL-6, IL-8, and p21, which indicated that this dose of BLM induced more severe senescence in WI-38 cells. Considering that senescent cells are resistant to cell death, we assume that this senescent status requires a higher concentration of senolytics to kill senescent cells [23]. According to previous studies, RXM could induce apoptosis in neutrophils and airway smooth muscle cells [31, 32], so we also performed flow cytometry to determine the type of cell death in senescent WI-38 cells. As shown in Fig. 1i, j, RXM significantly increased the proportion of apoptotic cells, indicating that RXM induced apoptosis to eliminate senescent cells. Cumulatively, these data indicated that RXM selectively targeted and cleared senescent lung fibroblasts.
Fig. 1. Roxithromycin selectively killed senescent cells.
a, b Representative images of SA-β-gal staining of proliferating and 15 Gy irradiation-induced senescent WI-38 cells. Senescent cell numbers were counted by ImageJ after 7 days of irradiation. c–f The mRNA levels of p21, p53, and IL-8 determined by qPCR and the protein levels of p21, p53, and IL-6 determined by Western blots in proliferating and senescent WI-38 cells were analyzed 7 days after irradiation. g After 7 days of irradiation, proliferating and senescent WI-38 cells were subjected to RXM treatments at different concentrations, and cell viability was tested by MTS assays. h WI-38 cells were treated with 25 μg/mL BLM for 7 days, and then, the supernatant was changed with RXM at the indicated concentration for another 3 days. Cell viability was evaluated by MTS assays. i, j The apoptotic rate of WI-38 cells from the indicated group of (h) was measured by flow cytometric analysis. The results are presented as the mean ± SEM from three independent experiments. Statistical significance, *P < 0.05; **P < 0.01; ****P < 0.0001.
Roxithromycin inhibited the expression of SASP factors
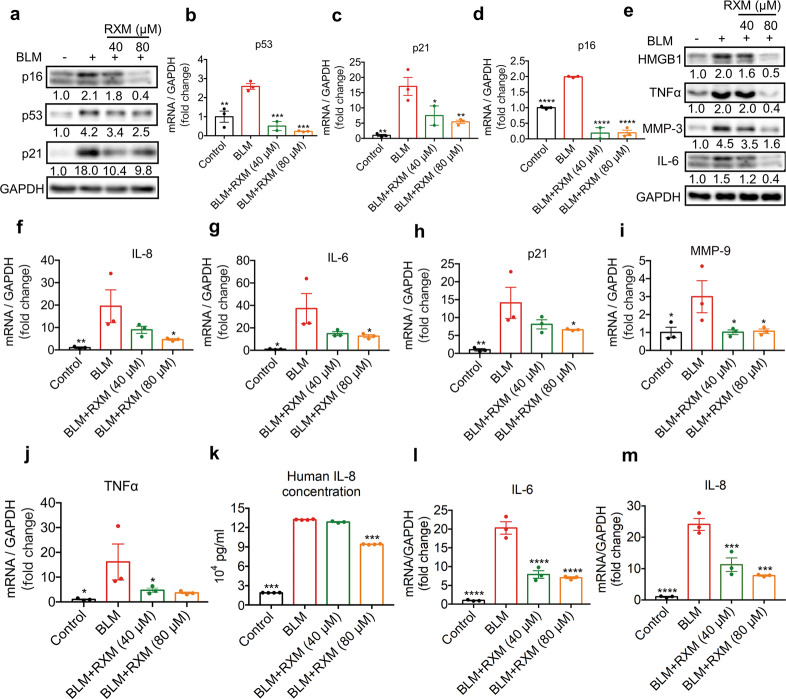
Next, we sought to determine whether RXM could suppress SASP factors to prevent extensive local and systemic dysfunction. A549, a cell line usually used as a replacement for primary AECs, was exposed to BLM to induce a senescent phenotype and treated with RXM at the indicated concentrations and time points. As shown in Fig. 2a–d, 25 μg/mL BLM treatment for 24 h led to cell cycle arrest, resulting in increased expression of p53, p21, and p16 at the protein and mRNA levels, which were significantly suppressed by RXM treatment. In addition, the expression of SASP factors, including IL-6, IL-8, MMP-9, and TNFα, was markedly elevated after 6 days of 5 μg/mL BLM treatment, and simultaneous RXM treatment dramatically decreased their expression levels in a dose-dependent manner (Fig. 2e–j). We also collected the supernatant of A549 cells from the indicated group in Fig. 2e to conduct an IL-8 ELISA. As shown in Fig. 2k, the secretion of IL-8 was significantly reduced by RXM. To better address the senomorphic potential of RXM to reverse SASP factor expression, we exposed A549 cells to 5 μg/mL BLM for 6 days followed by RXM treatment for another 3 days. The transcriptional expression of the SASP factors IL-6 and IL-8 was dramatically reversed by RXM in a concentration-dependent manner. These results suggested that RXM not only attenuated BLM-induced cell cycle arrest and prevented SASP factors secretion but also reversed SASP factor expression.
Fig. 2. Roxithromycin inhibited the expression of SASP factors.
a–d A549 cells were exposed to 25 μg/mL BLM alone or with RXM at the indicated concentrations for 24 h and replaced with fresh medium for another 24 h. The expression of p53, p21, and p16 was determined with (a) Western blots and (b–d) qPCR analysis. A549 cells were treated with 5 μg/mL BLM alone or with RXM at the indicated concentrations for 6 days before (e) Western blots, (f–j) qPCR analysis of IL-8, IL-6, p21, MMP-9, and TNFα, and (k) human IL-8 secretion measurement by ELISAs. l, m A549 cells were exposed to 5 μg/mL BLM for 6 days followed by RXM treatment for another 3 days. mRNA expression of SASP factors, including IL-6 and IL-8, was evaluated. The results are presented as the mean ± SEM from three independent experiments. Statistical significance, *P < 0.05; **P < 0.01; ***P < 0.001, ****P < 0.0001.
Roxithromycin inhibited senescent cell-induced fibroblast activation
During the pathogenesis of IPF, senescent AEC2-derived TGF-β1 is probably the strongest profibrotic mediator that promotes the differentiation of fibroblasts into myofibroblasts [33]. Therefore, we first investigated the effect of RXM on fibroblast activation stimulated by TGF-β1 in MRC-5 cells. With OFEV as the positive control, immunoblot and RNA expression analysis revealed that the expression of fibronectin, collagen I and α-SMA was dose-dependently inhibited in MRC-5 cells by RXM treatment compared with that of the cells subjected to TGF-β treatment alone (Fig. 3a–d).
Fig. 3. Roxithromycin inhibited senescent cell-induced fibroblast activation.
MRC-5 cells were treated with OFEV (0.5 μM), TGF-β (5 ng/mL) + RXM (10, 20, 40, and 80 μM) or TGF-β (5 ng/mL) alone for 24 h before (a) Western blots and (b–d) qPCR analysis of fibronectin, collagen I and α-SMA. e–h A549 cells were treated with 10 μg/mL BLM alone or with 40 or 80 μM RXM for 3 days, followed by replacement with fresh medium and culture for another 3 days. Then, the cell medium was collected to culture MRC-5 cells for 3 days again. e Schematic map of the in vitro assay. f–h MRC-5 cells were harvested for the evaluation of α-SMA, fibronectin, and collagen I expression via RT-qPCR analysis. The results are presented as the mean ± SEM from three independent experiments. Statistical significance, *P < 0.05; **P < 0.01; ***P < 0.001.
To better mimic the effect of senescent AEC2s in fibroblast activation in vivo, we devised an in vitro assay to test the effect of RXM on SASP factor-induced activation in lung fibroblasts. A549 cells were treated with 10 μg/mL BLM alone or with RXM for 3 days to generate cell senescence. After changing with the fresh medium and cultured for another 3 days, the cell medium containing SASP factors (SASP-CM) was obtained to culture MRC-5 cells for another 3 days (Fig. 3e). Then, fibrotic markers, including α-SMA, fibronectin, and collagen I, in MRC-5 cells were analyzed. As shown in Fig. 3f–h, compared with that in the control conditioned medium group, the expression of ECM proteins was increased in the SASP-CM group and was significantly decreased in the medium from the RXM-treated groups. These results indicated that RXM was effective in preventing fibroblast activation by inhibiting profibrotic SASP factors, which play a crucial role in the progression of IPF.
Roxithromycin attenuated bleomycin-induced lung injury and inflammation
Next, we further substantiated the efficacy of RXM on IPF in an in vivo mouse model of BLM-induced pulmonary fibrosis, which is a classic model and widely used in IPF studies [34]. To show the possible clinical relevance of RXM in the treatment of IPF, we performed intragastric administration of RXM at different concentrations on day 6 following intratracheal BLM treatment, with the nintedanib (OFEV) group as a positive control (Fig. 4a). Compared with the WT group, the vehicle group showed significantly diminished body weight upon BLM treatment, an important indicator of pathological severity following BLM challenge, while 80 mg/kg RXM and OFEV largely reversed this effect (Fig. 4b) [35]. After sacrifice on day 22, the 80 mg/kg RXM-treated mice displayed a significantly lower lung/body weight ratio than the vehicle-treated mice (Fig. 4c). As the BLM-exposed mice were initially characterized by lung injury and proinflammatory cytokines, the total number of inflammatory cells, lactate dehydrogenase (LDH) concentration, and histological structure of the lungs were investigated to evaluate the effect of RXM. We found that the inflammatory cell numbers presented a decreasing trend in the BALF of the 80 mg/kg RXM- and OFEV-treated mice compared with that of the vehicle group (Fig. 4d). LDH, a useful indicator of lung tissue damage and inflammation in BALF, was significantly reduced by 80 mg/kg RXM compared with vehicle treatment (Fig. 4e) [36]. Histologically, H&E staining showed severe dysfunction in the structure of the lung sections from the vehicle-treated mice, which had thickened alveolar walls and inflammatory cell infiltration, thus resulting in an enlarged alveolar space (Fig. 4f, g). The RXM- and OFEV-treated mice showed decreased dysfunction, presenting much more intact alveoli and thin, clear alveolar walls. These results collectively demonstrated that RXM attenuated BLM-induced lung injuries and inflammation.
Fig. 4. Roxithromycin attenuated bleomycin-induced lung injury and inflammation.
C57BL/6 male mice were randomly divided into six groups: the control (WT) group (n ≥ 6), vehicle group (n ≥ 6), OFEV group (60 mg/kg, n ≥ 6), and RXM groups (40 and 80 mg/kg, n ≥ 6). The animals received a single dose of 0.85 U/kg BLM or saline by intratracheal instillation. After 5 days of injection, the control and vehicle-treated mice were subjected to 5% CMC-Na in a volume of 10 μL/g per body weight by oral gavage every day, and the vehicle-treated mice received 60 mg/kg OFEV solution by oral gavage every day. The mice from the remaining three groups were administered RXM at 40, 80, and 160 mg/kg by oral gavage every day. All mice were sacrificed 3 weeks post-intratracheal injection. a Schematic picture of the experiment. b Body weight change of mice from the groups recorded every other day after BLM administration. c Lung/body ratio 21 days after BLM administration. d, e Bronchoalveolar lavage fluid (BALF) was harvested on the day the mice were sacrificed. d Inflammatory cell numbers (n = 4) and e LDH concentrations in the BALF (n ≥ 6) were measured. Histological images of lung sections by (g) hematoxylin and eosin (H&E) staining and (f) Ashcroft fibrosis scoring. Scale bar = 200 μm. The results are presented as the mean ± SEM. Statistical significance, *P < 0.05; **P < 0.01; ***P < 0.001.
Roxithromycin ameliorated bleomycin-induced pulmonary fibrosis
Based on these anti-inflammatory effects of RXM, we further investigated its efficacy on fibrosis induced by BLM. Masson’s trichome staining demonstrated that the 80 mg/kg RXM treatment ameliorated the collagen deposition induced by BLM in a dose-dependent manner (Fig. 5a, b). Consistently, HYP, the main component of collagen, was also significantly inhibited by RXM compared with that in the vehicle group, and RXM treatment was much more efficient than OFEV treatment (Fig. 5c). We further evaluated ECM proteins and TGF-β expression, which are biomarkers for fibrotic levels, in the indicated groups. As shown in Fig. 5d–f, the transcriptional levels of TGF-β, fibronectin, and collagen I were significantly decreased in both the RXM- and OFEV-treated mice compared with the vehicle-treated mice. Similarly, immunohistochemistry (IHC) analysis also revealed downregulated α-SMA expression in the RXM-treated groups (Fig. 5g). Collectively, these data indicated that RXM, especially at a dose of 80 mg/kg, protected against bleomycin-induced pulmonary fibrosis.
Fig. 5. Roxithromycin ameliorated bleomycin-induced pulmonary fibrosis.
a, b Histopathological images of lung sections by Masson’s trichrome staining and Masson score. Scale bar = 200 μm. c Hydroxyproline (HYP) content was measured in lung tissues according to the assay protocol. d–f Relative mRNA expression of TGF-β, fibronectin, and collagen I in lung tissues was evaluated by qPCR analysis. g Representative images of immunohistochemistry (IHC) staining for α-SMA in the lungs. The results are presented as the mean ± SEM from three independent experiments. Statistical significance, *P < 0.05; **P < 0.01; ***P < 0.001.
The bleomycin-induced cellular senescent phenotype was diminished by roxithromycin
Senescent cells are the source of activation of multiple signaling pathways that mediate persistent fibrotic activation in lung fibrosis [13]. Recent studies have revealed that BLM-induced cellular senescence contributes to the progression of lung fibrosis, making it a target for improving lung function and physical health [13]. To determine whether RXM can ameliorate the senescent phenotype of lung fibrosis, we investigated senescent biomarkers such as p53, IL-6, TNFα, and CXCL15 [1, 37]. As shown in Fig. 6a–d, RXM treatment significantly blunted the increased transcriptional levels of p53, IL-6, TNFα, and CXCL15 stimulated by BLM. Consistently, IHC analysis of p21 showed a marked decrease in the lungs of the RXM-treated mice compared with those of the vehicle group (Fig. 6e). Next, to further verify the senolytic effect of RXM in vivo, we performed immunofluorescence costaining of p21 and TUNEL on the tissue sections. The costaining results demonstrated that RXM induced apoptosis of senescent cells marked by p21 (Fig. 6f). Taken together, these murine data strongly indicate that RXM treatment may suppress the senescent phenotype in BLM-induced pulmonary fibrosis.
Fig. 6. The bleomycin-induced cellular senescent phenotype was diminished by roxithromycin.
a–d Relative mRNA levels of IL-6, p53, TNFα, and CXCL15 in lung tissues were detected by qPCR analysis. e Representative images of immunohistochemistry (IHC) staining for p21 in the lungs. f Representative images of immunofluorescence costaining of p21 and TUNEL. The results are presented as the mean ± SEM from three independent experiments. Statistical significance, *P < 0.05; **P < 0.01; ***P < 0.001, ****P < 0.0001.
Roxithromycin downregulated the expression of NOX4 in lung fibrosis
It has been reported that cellular senescence aggravates lung fibrosis through a mechanism that involves NOX4-mediated ROS production, which is implicated in regulating TGF-β-mediated cell signaling and myofibroblast differentiation [18, 29]. Furthermore, many kinds of macrolides, including RXM, have been reported to inhibit NOX4 expression and myofibroblast differentiation during TGF-β stimulation of nasal polyp-derived fibroblasts [38]. Based on these findings, we aimed to evaluate whether RXM mediated antifibrotic and antisenescent effects in IPF by regulating NOX4 expression. As shown in Fig. 7a, b, we observed that RXM dose-dependently reduced NOX4 expression in both the lung tissues from the BLM-treated mice and the TGF-β-activated MRC-5 cells. We then designed NOX4 siRNA for gene silencing to explore the dependence of RXM on NOX4. The knockdown efficiency of siRNA in MRC-5 and A549 cells was confirmed by RNA expression and immunoblot analysis, as shown in Fig. 7c, d. MRC-5 cells were transfected with NC or siNOX4 and then stimulated with TGF-β alone or together with RXM treatment. We found that NOX4 deficiency in MRC-5 cells reversed TGF-β-induced fibronectin and α-SMA upregulation compared with that in the NC group (Fig. 7e). More importantly, NOX4 deficiency abolished the antifibrotic effect of RXM (Fig. 7e). In the parallel senescent cell model, similar results showed that the increase in the SASP factor MMP-9 due to BLM treatment was no longer downregulated by RXM when NOX4 was knocked down, which suggested the dependency of RXM on NOX4 (Fig. 7f). Taken together, these results supported the notion that RXM mediated the antifibrotic and antisenescent effects mainly through downregulation of NOX4.
Fig. 7. Roxithromycin downregulated the expression of NOX4 in lung fibrosis.
a The mRNA level of NOX4 in the lungs of the mice from the indicated groups was evaluated with qPCR analysis. b The mRNA level of NOX4 in the MRC-5 cells stimulated with TGF-β or RXM was determined by qPCR analysis. c–f Twenty-four-well-cultured MRC-5 and A549 cells were transfected with 1 μL of siNOX4 for 24 h. MRC-5 cells were then treated with 5 ng/mL TGF-β alone or with 40 μM RXM for another 24 h. A549 cells were treated with 15 μg/mL BLM alone or with 40 μM RXM for another 3 days. The cells were harvested for further analysis. c, d The knockdown efficiency of NOX4 in MRC-5 and A549 cells was confirmed by qPCR and Western blot analysis, respectively. e Detection of fibrotic markers in MRC-5 cells was performed by Western blot analysis. f Evaluation of MMP-9 in A549 cells was performed with qPCR analysis. The results are presented as the mean ± SEM from three independent experiments. Statistical significance, *P < 0.05; **P < 0.01; ***P < 0.001, ****P < 0.0001.
Discussion
In the present study, we used ionizing irradiation and BLM-induced senescent cell models and found that RXM could selectively eliminate senescent cells and inhibit SASP factors. Further, RXM was demonstrated to inhibit senescent cell-induced fibroblast activation in vitro. Then, the anti-inflammatory and antifibrotic effects of RXM were confirmed in mouse model of a BLM-induced lung fibrosis. To determine whether RXM inhibited the senescent phenotype in vivo, we assessed SASP markers and apoptosis of lung tissues, which showed that cellular senescence was diminished while apoptosis of senescent cells was enhanced in the RXM group. NOX4 has been implicated in TGF-β-induced myofibroblast differentiation and the senescent phenotype in IPF, the inhibition of which was shown to reduce senescent markers of lung fibroblasts according to previous studies. Azithromycin, another kind of macrolide antibiotic, was also reported to attenuate the progression of pulmonary fibrosis through proteasomal degradation of NOX4 [39, 40]. Additionally, previous studies revealed that azithromycin and RXM inhibited α-SMA and collagen expression in TGF-β-stimulated nasal polyp-derived fibroblasts, potentially through their antioxidant effect on NOX4 [4, 38]. Based on these findings, we next evaluated the effect of RXM on NOX4 in our model and found that RXM suppressed its expression in both TGF-β-stimulated MRC-5 cells and a mouse model of BLM-induced lung fibrosis. The dependence of RXM on NOX4 was further elucidated using siRNA targeting NOX4 in MRC-5 and A549 cells. Considering that there are indeed many other pathways that induce NOX4 expression other than TGF-β, such as PKC and TxNIP, it is reasonable that RXM did not reverse NOX4 expression to baseline [23]. However, the specific mechanism by which RXM downregulates NOX4 expression and one of the downstream pathways involved in mediating cellular senescence is unclear, and further studies will be employed to demonstrate this issue.
Emerging evidence has shown that IPF is an aging-related disease involving accumulation of cell senescence, fibrosis, matrix remodeling, inflammation, DNA damage, telomere attrition, and alveolar epithelial oxidation [41]. Two therapeutic approaches involving drugs termed “senolytics” or “senomorphics” are the main strategies to address age-related diseases [23]. “Senolytic” refers to the effect of selective elimination of senescent cells. Recently, it was reported that senolytic drugs could relieve or reverse the development of aging-related diseases, such as cardiac dysregulation, diabetes mellitus, and pulmonary fibrosis [42]. Studies have revealed that treatment with dasatinib (D) and quercetin (Q) in combination (DQ) in large randomized, controlled trials for IPF is feasible [41]. Notably, senescent cells also play an important role in tissue homeostasis under physiological conditions, making senolytics a cautious choice. An alternative application is the drugs named “senomorphics”, which have the ability to neutralize the detrimental effect of SASP factors. Currently, major efforts have been made to identify novel antisenescent compounds for IPF therapy. Previous studies have defined azithromycin and RXM, both macrolide antibiotics that act as senolytic drugs that target senescent lung fibroblasts, but their parent macrolide compound erythromycin shows no senolytic properties [24]. In addition, it has been reported that RXM and clarithromycin inhibit BLM-induced acute lung injury in mice, whereas azithromycin showed a weaker anti-inflammatory effect in this model [27]. Previous findings, together with our work, highlight the problem of whether the difference in this effect is due to a specific molecular structure. Could this chemical structure be employed for further modifications to obtain compounds with even better senolytic and antifibrotic activities? These questions deserve further consideration and research. Despite this, systematic and comprehensive studies of RXM in lung fibrosis and its relationship with cell senescence remain elusive. Our work not only supported the previous discoveries but also provided extended evidence that RXM is not only a senolytic but also a senomorphic. In addition, RXM was able to ameliorate both BLM-induced pulmonary fibrosis and the senescent phenotype in a NOX4-dependent manner, suggesting the potential of RXM as a therapy for IPF.
In the mouse model of BLM-induced pulmonary fibrosis, we started RXM intervention 5 days after BLM challenge when the inflammatory and fibrotic status of the lung tissues was significantly enhanced, similar to the status at day 7 after BLM exposure (data not shown). Among the studies on pulmonary fibrosis, drug intervention usually starts at 0 or 7 days after BLM administration in vivo, including preclinical pharmacological studies of the FDA-approved drug nintedanib, which might be attributed to the self-resolution of BLM-induced lung fibrosis in the late stage [43]. Therefore, it is currently difficult to start drug interventions in the late stage, indicating that animal models that can completely simulate human pulmonary fibrosis are in demand. Moreover, IPF patients are usually in the end stage of fibrosis when they present for clinical treatment; thus, it would be logical to test drugs in a more appropriate model of fibrosis, which may help replace the existing lung transplantation treatment of end-stage patients in the future.
Supplementary information
Acknowledgements
This work is supported by the National Science and Technology Major Project “Key New Drug Creation and Manufacturing Program” (2019ZX09201001-004-010, 2019ZX09201001-003-010), the National Natural Science Foundation of China (Grant U1703235, 31871414), the National Science Fund for Distinguished Young Scholars (Grant 81125023), and the K. C. Wong Education Foundation.
Author contributions
XZ and YD designed and performed the experiments, analyzed the data, and wrote the paper; WCL and BXT contributed to the performance of experiments; JL and YZ initiated the project, planned and analyzed experiments, and supervised the research.
Competing interests
The authors declear no competing interests.
Footnotes
These authors contributed equally: Xuan Zhang, Ying Dong
Contributor Information
Jia Li, Email: Jli@simm.ac.cn.
Yi Zang, Email: Yzang@simm.ac.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-021-00618-3.
References
- 1.Ley B, Collard HR, King TE. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respiratory. Crit Care Med. 2011;183:431–40. doi: 10.1164/rccm.201006-0894CI. [DOI] [PubMed] [Google Scholar]
- 2.Wong AW, Ryerson CJ, Guler SA. Progression of fibrosing interstitial lung disease. Respir Res. 2020;21:32. doi: 10.1186/s12931-020-1296-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King TE, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949–61. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 4.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci USA. 2006;103:13180–5. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noble PW, Barkauskas CE, Jiang D. Pulmonary fibrosis: patterns and perpetrators. J Clin Invest. 2012;122:2756–62. doi: 10.1172/JCI60323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh SLF, Inoue Y, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med. 2019;381:1718–27. doi: 10.1056/NEJMoa1908681. [DOI] [PubMed] [Google Scholar]
- 8.Vancheri C, Kreuter M, Richeldi L, Ryerson CJ, Valeyre D, Grutters JC, et al. Nintedanib with add-on pirfenidone in idiopathic pulmonary fibrosis. Results of the INJOURNEY trial. Am J Respir Crit Care Med. 2018;197:356–63. doi: 10.1164/rccm.201706-1301OC. [DOI] [PubMed] [Google Scholar]
- 9.Faner R, Rojas M, Macnee W, Agusti A. Abnormal lung aging in chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;186:306–13. doi: 10.1164/rccm.201202-0282PP. [DOI] [PubMed] [Google Scholar]
- 10.Naikawadi RP, Disayabutr S, Mallavia B, Donne ML, Green G, La JL, et al. Telomere dysfunction in alveolar epithelial cells causes lung remodeling and fibrosis. JCI Insight. 2016;1:e86704. doi: 10.1172/jci.insight.86704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mora AL, Rojas M, Pardo A, Selman M. Emerging therapies for idiopathic pulmonary fibrosis, a progressive age-related disease. Nat Rev Drug Discov. 2017;16:755–72. doi: 10.1038/nrd.2017.170. [DOI] [PubMed] [Google Scholar]
- 12.LeBrasseur NK, Tchkonia T, Kirkland JL. Cellular senescence and the biology of aging, disease, and frailty. Nestle Nutr Inst Workshop Ser. 2015;83:11–8. doi: 10.1159/000382054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, et al. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun. 2017;8:14532. doi: 10.1038/ncomms14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abbadie C, Pluquet O, Pourtier A. Epithelial cell senescence: an adaptive response to pre-carcinogenic stresses? Cell Mol Life Sci. 2017;74:4471–509. doi: 10.1007/s00018-017-2587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parimon T, Yao C, Stripp BR, Noble PW, Chen P. Alveolar epithelial type II cells as drivers of lung fibrosis in idiopathic pulmonary fibrosis. Int J Mol Sci. 2020;21:2269. doi: 10.3390/ijms21072269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chanda D, Otoupalova E, Smith SR, Volckaert T, De Langhe SP, Thannickal VJ. Developmental pathways in the pathogenesis of lung fibrosis. Mol Asp Med. 2019;65:56–69. doi: 10.1016/j.mam.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, et al. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem. 2003;278:12384–9. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- 18.Hecker LLN, Kurundkar D, Kurundkar A, Bernard K, Hock T, Meldrum E, et al. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci Transl Med. 2014;6:231ra47. doi: 10.1126/scitranslmed.3008182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehmann M, Korfei M, Mutze K, Klee S, Skronska-Wasek W, Alsafadi HN, et al. Senolytic drugs target alveolar epithelial cell function and attenuate experimental lung fibrosis ex vivo. Eur Respir J. 2017;50:1602367. doi: 10.1183/13993003.02367-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sellarés J, Rojas M. Quercetin in idiopathic pulmonary fibrosis- another brick in the senolytic wall. Am J Respir Cell Mol Biol. 2019;60:3–4. doi: 10.1165/rcmb.2018-0267ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB, et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell. 2016;15:428–35. doi: 10.1111/acel.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14:644–58. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fafian-Labora JA, O’Loghlen A. Classical and nonclassical intercellular communication in senescence and ageing. Trends Cell Biol. 2020;30:628–39. doi: 10.1016/j.tcb.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Ozsvari B, Nuttall JR, Sotgia F, Lisanti MP. Azithromycin and roxithromycin define a new family of senolytic drugs that target senescent human fibroblasts. Aging (Albany NY) 2018;10:3294–307. doi: 10.18632/aging.101633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pei QM, Jiang P, Yang M, Qian XJ, Liu JB, Kim SH. Roxithromycin inhibits VEGF-induced human airway smooth muscle cell proliferation: opportunities for the treatment of asthma. Exp Cell Res. 2016;347:378–84. doi: 10.1016/j.yexcr.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 26.Xia M, Xu H, Dai W, Zhu C, Wu L, Yan S, et al. The role of HDAC2 in cigarette smoke–induced airway inflammation in a murine model of asthma and the effect of intervention with roxithromycin. J Asthma. 2017;55:337–44. doi: 10.1080/02770903.2017.1337788. [DOI] [PubMed] [Google Scholar]
- 27.Kawashima M, Yatsunami J, Fukuno Y, Nagata M, Tominaga M, Hayashi S. Inhibitory effects of 14-membered ring macrolide antibiotics on bleomycin-induced acute lung injury. Lung. 2001;179:257. doi: 10.1007/pl00021246. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Zhong X, He Z. Effect of low-dose, long-term roxithromycin on airway inflammation and remodeling of stable noncystic fibrosis bronchiectasis. Mediators Inflamm. 2014;2014:708608. doi: 10.1155/2014/708608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amara N, Goven D, Prost F, Muloway R, Crestani B, Boczkowski J. NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFbeta1-induced fibroblast differentiation into myofibroblasts. Thorax. 2010;65:733–8. doi: 10.1136/thx.2009.113456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Odagiri Y, Dempsey J, Morley A. Damage to lymphocytes by X-ray and bleomycin measured with the cytokinesis-block micronucleus technique. Mutat Res. 1990;237:147–52. doi: 10.1016/0921-8734(90)90020-r. [DOI] [PubMed] [Google Scholar]
- 31.Inamura K, Ohta N, Fukase S, Kasajima N, Aoyagi M. Effects of erythromycin on human peripheral neutrophil apoptosis. Rhinology. 2000;38:124–9. [PubMed] [Google Scholar]
- 32.Dai YR, Wu HY, Wu LQ, Xu H, Yin J, Yan SS, et al. Roxithromycin reduces the viability of cultured airway smooth muscle cells from a rat model of asthma. Eur Rev Med Pharmacol Sci. 2014;18:3564–72. [PubMed] [Google Scholar]
- 33.Xu YD, Hua J, Mui A, O’Connor R, Grotendorst G, Khalil N. Release of biologically active TGF-beta1 by alveolar epithelial cells results in pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2003;285:L527–39. doi: 10.1152/ajplung.00298.2002. [DOI] [PubMed] [Google Scholar]
- 34.Moeller A, Ask K, Warburton D, Gauldie J, Kolb M. The bleomycin animal model: A useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int J Biochem Cell Biol. 2008;40:362–82. doi: 10.1016/j.biocel.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sikic BI, Young DM, Mimnaugh EG, Gram TE. Quantification of bleomycin pulmonary toxicity in mice by changes in lung hydroxyproline content and morphometric histopathology. Cancer Res. 1978;38:787–92. [PubMed] [Google Scholar]
- 36.Drent M, Cobben NA, Henderson RF, Wouters EF, van Dieijen-Visser M. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur Respir J. 1996;9:1736–42. doi: 10.1183/09031936.96.09081736. [DOI] [PubMed] [Google Scholar]
- 37.Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–67. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park HH, Park IH, Cho JS, Lee YM, Lee HM. The effect of macrolides on myofibroblast differentiation and collagen production in nasal polyp-derived fibroblasts. Am J Rhinol Allergy. 2010;24:348–53. doi: 10.2500/ajra.2010.24.3520. [DOI] [PubMed] [Google Scholar]
- 39.Tian Y, Li H, Qiu T, Dai J, Zhang Y, Chen J, et al. Loss of PTEN induces lung fibrosis via alveolar epithelial cell senescence depending on NF-kappaB activation. Aging Cell. 2019;18:e12858. doi: 10.1111/acel.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsubouchi K, Araya J, Minagawa S, Hara H, Ichikawa A, Saito N, et al. Azithromycin attenuates myofibroblast differentiation and lung fibrosis development through proteasomal degradation of NOX4. Autophagy. 2017;13:1420–34. doi: 10.1080/15548627.2017.1328348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Justice JN, Nambiar AM, Tchkonia T, LeBrasseur NK, Pascual R, Hashmi SK, et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine. 2019;40:554–63. doi: 10.1016/j.ebiom.2018.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirkland JL, Tchkonia T, Zhu Y, Niedernhofer LJ, Robbins PD. The clinical potential of senolytic drugs. J Am Geriatr Soc. 2017;65:2297–301. doi: 10.1111/jgs.14969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cabrera S, Selman M, Lonzano-Bolanos A, Konishi K, Richards TJ, Kaminski N, et al. Gene expression profiles reveal molecular mechanisms involved in the progression and resolution of bleomycin-induced lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2013;304:L593–601. doi: 10.1152/ajplung.00320.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.