Abstract
Cisplatin is a clinically advanced and highly effective anticancer drug used in the treatment of a wide variety of malignancies, such as head and neck, lung, testis, ovary, breast cancer, etc. However, it has only a limited use in clinical practice due to its severe adverse effects, particularly nephrotoxicity; 20%–35% of patients develop acute kidney injury (AKI) after cisplatin administration. The nephrotoxic effect of cisplatin is cumulative and dose dependent and often necessitates dose reduction or withdrawal. Recurrent episodes of AKI result in impaired renal tubular function and acute renal failure, chronic kidney disease, uremia, and hypertensive nephropathy. The pathophysiology of cisplatin-induced AKI involves proximal tubular injury, apoptosis, oxidative stress, inflammation, and vascular injury in the kidneys. At present, there are no effective drugs or methods for cisplatin-induced kidney injury. Recent in vitro and in vivo studies show that numerous natural products (flavonoids, saponins, alkaloids, polysaccharide, phenylpropanoids, etc.) have specific antioxidant, anti-inflammatory, and anti-apoptotic properties that regulate the pathways associated with cisplatin-induced kidney damage. In this review we describe the molecular mechanisms of cisplatin-induced nephrotoxicity and summarize recent findings in the field of natural products that undermine these mechanisms to protect against cisplatin-induced kidney damage and provide potential strategies for AKI treatment.
Keywords: natural products, cisplatin, acute kidney injury, nephrotoxicity, antioxidant, anti-inflammation, anti-apoptosis, nephroprotection
Introduction
Cisplatin is a clinically advanced and highly effective anticancer drug that is used for the treatment of various solid tumors, such as lung cancer, stomach cancer, and ovarian cancer [1]. However, nephrotoxicity is the major side effect of cisplatin administration. Clinically, the risk of nephrotoxicity in patients taking cisplatin is between 20% and 35% and leads to death in acute kidney injury (AKI) patients [2, 3]. In addition, pediatric patients also develop nephrotoxicity when using cisplatin [4]. Patients with AKI are clinically characterized by impaired renal tubular function, acute renal failure, a reduction in whole blood cells, anemia, physical tremors, weight loss, gastrointestinal dysfunction, lethargy, and orbital tightening, which limit the antitumor use of cisplatin [5]. Cisplatin mediates nephrotoxicity via a number of different cytotoxic mechanisms. In addition to DNA damage, cisplatin also causes cytoplasmic organelle dysfunction, particularly in the endoplasmic reticulum and mitochondria, activates apoptotic pathways, and inflicts cellular damage via oxidative stress and inflammation [6].
Presently, there is no clinically effective drug to prevent or treat cisplatin-induced nephrotoxicity. Many high-efficacy and low-toxicity drugs from natural products have been developed to protect against cisplatin-induced AKI. For example, ginseng, curcumin, and pomegranate can act as antioxidants and anti-inflammatory agents and possibly protect against oxidative stress by restoring the levels of antioxidant enzymes [7]. In addition, pretreatment with vitamin supplements, such as vitamin E and riboflavin (vitamin B), significantly reduces serum urea and increases the expression levels of antioxidant enzymes in children with steroid-responsive nephrotic syndrome [8]. These natural products have potential antioxidant and anti-inflammatory properties and can be used as supplements to alleviate cisplatin-induced nephrotoxicity.
In this review, we first introduce the pathological manifestations of cisplatin-induced nephrotoxicity and clarify the molecular events of the underlying mechanisms. Finally, we summarize the roles of various kinds of natural products in protecting against cisplatin-induced AKI. This review focuses on the different mechanisms and protective effects of natural products, providing a comprehensive understanding of the prevention of cisplatin-induced nephrotoxicity and potential implications for drug combinations or natural supplements for AKI patients.
Pathological manifestations of cisplatin-induced nephrotoxicity
Clinically, different doses of cisplatin may lead to different degrees of nephrotoxicity. Patients who receive a single dose of cisplatin may suffer from reversible kidney injury, while large doses or multiple courses of treatment may cause irreversible renal failure [9]. Pharmacokinetic studies also show that nephrotoxicity is mainly due to the high volume of cisplatin distribution and long-term accumulation of cisplatin in the kidney [10]. In general, the pathological mechanisms of cisplatin-induced nephrotoxicity mainly manifest as decreases in renal blood flow and glomerular filtration rate [11] and ischemia or necrosis of proximal renal tubular epithelial cells [12].
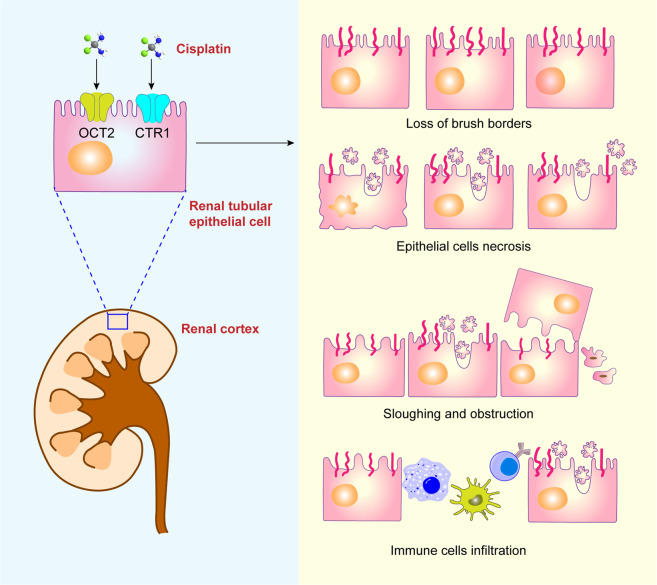
Histopathological changes in cisplatin-induced nephrotoxicity are positively correlated with the dose of cisplatin. First, cisplatin is passively absorbed into renal tubular cells via organic cation transporter 2 (OCT2) and forms hydrates with water molecules, leading to continuous accumulation in renal cells [13]. The formation of cisplatin hydrate is a reversible process, and cisplatin hydrate can be dissociated into cisplatin and water molecules and discharged from the cells [13]. Thus, the accumulation and retention of cisplatin in renal cells leads to DNA damage, oxidative stress, apoptosis, and autophagy (Fig. 1).
Fig. 1. Schematic illustration of pathological manifestations of cisplatin-induced nephrotoxicity.
The normal epithelium is damaged by cisplatin, as characterized by the loss of brush borders, epithelial cell necrosis, sloughing and obstruction, and immune cell infiltration.
Cisplatin first causes shedding of the brush shape of renal tubular epithelial cells. With increasing cisplatin accumulation, epithelial cells undergo necrosis and are gradually shed, accompanied by the formation of proteinaceous casts [14]. Moreover, the proximal tubule basement membrane becomes thickened, and tubules become dilated [15]. Electron microscopy observation of epithelial cell ultrastructure shows swollen and vacuolated mitochondria, endoplasmic reticulum expansion, and increased numbers of lysosomes [16]. Taken together, these organelle malfunctions result in the destruction and sloughing of epithelial cells, as well as the formation of intratubular obstructions.
Damaged renal tubular epithelial cells recruit many immune cells, such as macrophages, dendritic cells, and T cells, which release a variety of inflammatory factors [17]. Moreover, cisplatin can cause reduced medullary blood flow and exacerbate tubular cell injury, leading to acute ischemic injury in the kidneys [18]. Instead of the typical self-regulatory renal vasodilation in ischemic kidneys, evident vasoconstriction occurs in cisplatin-induced AKI, leading to hypoxic injury and vascular injury in severe cases [19]. Some studies have shown that cisplatin forms a complex with reduced glutathione in the liver and then enters the kidney. Cisplatin is decomposed into a nephrotoxic metabolite due to the action of glutamyltransferase in the brush edge of the renal proximal tubule, causing renal cell apoptosis or necrosis [20].
Mechanisms of cisplatin-induced nephrotoxicity
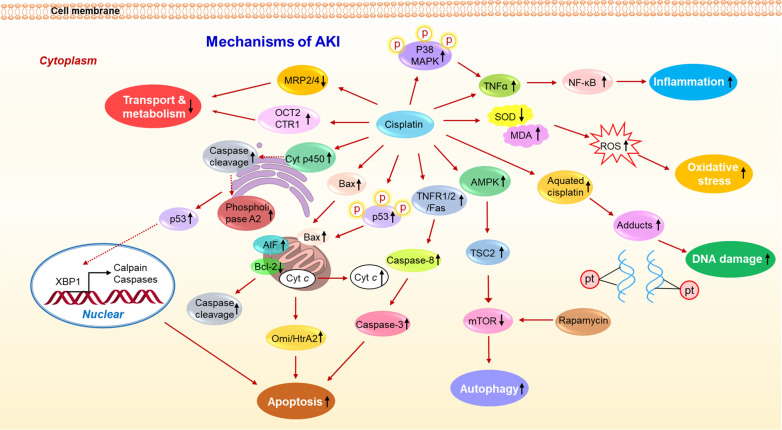
The application of cisplatin chemotherapy is often limited by severe adverse effects, including nephrotoxicity, ototoxicity, neurotoxicity, and vomiting. Nephrotoxicity, which is the major limiting factor of cisplatin use, involves various mechanisms, such as oxidative stress, apoptosis, inflammation, and autophagy (Fig. 2). Understanding the underlying mechanism is important for investigating intervention strategies for nephrotoxicity.
Fig. 2. The mechanism summary of cisplatin-induced nephrotoxicity.
The mechanisms mainly include the transport and metabolism of cisplatin, apoptosis, autophagy, DNA damage, oxidative stress, and inflammation, which work together to aggravate AKI induced by cisplatin.
Cellular uptake and transport
Cisplatin is mainly excreted through the kidneys. It becomes concentrated during excretion, and the concentration in renal tubular epithelial cells is much higher than that in the blood. In the kidney, cisplatin is absorbed by renal cells via passive diffusion. During excretion, cisplatin and its metabolites are secreted and reabsorbed in the renal tubules during glomerular filtration, leading to a high concentration of cisplatin in the kidneys.
Recent studies have shown that cisplatin is taken up by renal tubular cells via OCT2, copper ion transporter 1 (CTR1), and solute carrier family 22 member 2 [21]. In addition, cisplatin is secreted into the lumen by solute carrier family 47 member 1 and multidrug and toxin extrusion 1 [22]. Knockdown of the Oct2 gene can significantly reduce cisplatin-induced nephrotoxicity [23]. Consistently, patients with Oct2 mutations show low OCT2 expression and reduced cisplatin transport into renal tubular cells, resulting in decreased nephrotoxicity [24]. In addition, when CTR1 expression is downregulated, cisplatin uptake and the subsequent cytotoxicity decrease significantly [25]. Moreover, peroxiredoxin I (Prx I)-deficient mice have higher resistance to cisplatin-induced nephrotoxicity than wild-type mice due to increased cisplatin excretion via the high expression of the renal efflux transporters multidrug resistance-related protein 2 (MRP2) and MRP4 in Prx I-deficient mice [26].
DNA damage
Cisplatin mediates its cytotoxic effects by binding DNA to form adducts that cause DNA damage [27]. In an aqueous environment, the chloride ligand of cisplatin is replaced by water molecules to form a positively charged hydrated complex ion, which is transferred to the nucleus by DNA electrostatic attraction. Then, this complex binds to DNA to form an adduct, resulting in DNA cross-linking and preventing DNA synthesis and replication in rapidly proliferating cells [28]. This phenomenon is pronounced in cells with defective DNA repair.
However, cisplatin binds nonspecifically to nuclear DNA, and less than 1% of platinum binds to nuclear DNA [29]. Interestingly, mitochondrial DNA is more sensitive than nuclear DNA to cisplatin-mediated cytotoxicity [30]. The positively charged metabolites produced by the hydrolysis of cisplatin preferentially accumulate in mitochondria, which are negatively charged. Therefore, the sensitivity of cells to cisplatin depends on mitochondrial density and the mitochondrial membrane potential in cells [31]. Given that the renal proximal tubule contains sites of quite high mitochondrial density, it is the most highly sensitive site in the kidney to cisplatin [32].
Apoptosis
It has been reported that a low concentration (8 μM) of cisplatin causes renal tubular epithelial apoptosis, while a high concentration (800 μM) of cisplatin induces necrosis [33]. Cisplatin-induced apoptosis in renal tubular cells is primarily associated with mitochondria-mediated endogenous pathways, death receptor-mediated exogenous pathways, and endoplasmic reticulum stress (ERS) pathways.
Mitochondria-mediated endogenous pathways
Cisplatin-induced mitochondria-mediated apoptotic pathways mainly include caspase-dependent and -independent pathways. When cisplatin enters renal tubular epithelial cells, BAX translocates to mitochondria and activates caspase-2, resulting in the release of cytochrome c, second mitochondria-derived activator of caspase/direct inhibitor of apoptosis proteins binding protein with low Pi (isoelectric point) (SMAC/DIABLO), high temperature requirement A2 (HtrA2/Omi), and apoptosis-inducing factor (AIF) from mitochondria [34]. Then, caspase-9 is activated, which eventually leads to apoptosis [35]. Apart from the caspase-dependent pathway, cytoplasmic Omi/HtrA2 also promotes caspase-independent apoptosis by binding and cleaving inhibitors of apoptotic proteins after cisplatin-induced apoptotic stimulation [36].
AIF is an apoptosis-related protein located on the mitochondrial membrane, and poly (ADP-ribose) polymerase-1 (PARP-1) is a nuclear factor that participates in DNA repair and protein modification. Once cellular DNA is severely damaged by cisplatin, nuclear PARP-1 activity is increased, causing AIF activation and nuclear translocation, which induces apoptosis [37]. PARP-1 activation is a primary signal in the process of cisplatin-induced nephrotoxicity. Moreover, PARP-1 inhibition or deletion protects the kidneys from nephrotoxicity, providing a therapeutic strategy for cisplatin-induced nephrotoxicity [38].
The role of p53 in cisplatin-induced cytotoxicity mainly involves activation of the mitochondrial pathway. Upon exposure to cisplatin-induced cellular DNA damage, p53 is phosphorylated, and the proapoptotic protein BAX undergoes structural modifications and alters mitochondrial membrane integrity, causing the activation of p53 upregulated modulator of apoptosis-α and Ca2+-independent phospholipase A2. Then, the antiapoptotic proteins BCL-2 and BCL-XL are downregulated, triggering the mitochondrial apoptotic pathway [39].
Death receptor-mediated exogenous pathways
In the exogenous apoptotic pathways, cisplatin binds to death receptors such as tumor necrosis factor receptor 1 (TNFR1), TNFR2, and FAS on the cell membrane to activate caspase-8, which further activates caspase-3, ultimately leading to apoptosis [40]. Cisplatin upregulates the expression of tumor necrosis factor-α (TNF-α), promoting the interaction of TNF-α and TNF receptors, including TNFR1 and TNFR2. TNFR1 has a death domain and is able to directly trigger exogenous apoptosis. However, TNFR2 mainly regulates the inflammatory response to induce apoptosis because it has no death domain [41]. In addition, cisplatin can also activate the FAS/FAS-L system [42], and the FAS-associated death domain further interacts with FAS or TNFR1 to trigger apoptosis, but the detailed mechanisms have not been elucidated.
Endoplasmic reticulum stress pathways
Cisplatin can also activate the apoptotic pathway that is mediated by ERS. After cisplatin enters cells, it acts on the cytochrome P450 (CYP450) enzymatic system on the endoplasmic reticulum membrane to induce oxidative stress and activate caspase-12, which leads to apoptosis [43]. As expected, cisplatin-induced apoptosis is significantly reduced in cytochrome P450, family 2, subfamily E, polypeptide 1 (Cyp2e1)-knockout mice [44]. Similarly, another study showed that the expression of the ERS marker X-box-binding protein 1 was increased, and calpain and caspase-12 cleavage products were observed in rat kidneys after cisplatin treatment [45]. Furthermore, transfection with an anti-caspase-12 antibody significantly attenuated cisplatin-induced apoptosis in porcine kidney LLC-PK1 cells [46]. The ERS pathway is also involved in the activation of endoplasmic reticulum phospholipase A2, which limits downstream p53 and activates upstream caspase-3. The endoplasmic reticulum may be a link between p53 and caspase-3 in the absence of mitochondrial dysfunction [47].
Oxidative stress
In recent years, studies have shown that oxidative stress and nitrosative stress play vital roles in cisplatin-induced nephrotoxicity, which is characterized by increased malondialdehyde (MDA), 4-hydroxy, 8-hydroxydeoxyguanosine, and 3-nitrotyrosine, and decreased superoxide dismutase (SOD) and catalase (CAT) after cisplatin treatment. Thus, reactive oxygen species (ROS) scavengers and antioxidants show robust protective effects against nephrotoxicity [48].
After entering renal tubular cells, cisplatin can rapidly react with the thiol-containing antioxidants glutathione and metallothionein to degrade or inactivate them. Moreover, some antioxidant enzymes, such as glutathione peroxidase, SOD, and glutathione reductase, are also inhibited, leading to increased ROS levels [49]. ROS affect the activity of mitochondrial complex enzymes I–IV, thereby inhibiting the normal transmission of the oxidative respiratory chain and leading to adenosine triphosphate depletion [40]. Then, increased ROS results in lipid peroxidation, changing membrane structure and permeability, which further affect cellular function [50]. Finally, ROS impair amino acids, proteins, and carbohydrates, thus promoting DNA damage and apoptosis. In addition, increased ROS can induce increased expression of FAS-L, FAS, TNFR1, and TNF-α, eventually resulting in apoptosis [45].
Inflammation
Cisplatin-induced nephrotoxicity is associated with the inflammatory response. Renal TNF-α expression is increased in a cisplatin-induced nephrotoxic mouse, and cisplatin-induced renal insufficiency and injury can be significantly alleviated by TNF-α inhibition or knockout, indicating that increased TNF-α expression plays an important role in cisplatin-induced nephrotoxicity [51]. Interestingly, after cisplatin administration, TNF-α in the circulation and urine may be derived from renal epithelial cells rather than immune cells. Moreover, TNF-α induces the production of ROS, further activating the transcription factor, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), which in turn induces the production of proinflammatory cytokines such as TNF-α [52]. The inhibition of NF-κB transcriptional activity by JSH-23 (a kind of NF-κB inhibitor) improves kidney function in mice [53].
TNF-α activates proinflammatory cytokines and chemokines to trigger oxidative stress, ultimately exacerbating kidney damage. Hydroxyl free radicals produced by cisplatin are involved in the phosphorylation of p38 mitogen-activated protein kinase (p38 MAPK) and the regulation of TNF-α synthesis, ultimately inducing the activation of NF-κB. Therefore, the hydroxyl radical scavenger dimethyl thiourea inhibits p38 MAPK activation and TNF-α mRNA expression in murine kidneys. The inhibition of p38 MAPK reduces the production of TNF-α, thereby effectively protecting against cisplatin-induced kidney damage [54]. Other cytokines, such as transforming growth factor-β, monocyte chemoattractant protein-1 (MCP-1), intercellular adhesion molecule, and heme oxygenase-1 (HO-1), are also associated with cisplatin-induced nephrotoxicity [55]. N-Acetylcysteine (NAC), an antioxidative agent, effectively inhibits inflammation and activation of the complement system to exert renal protection [56]. Mitochondrial dysfunction leads to the formation of O2–, while the inflammatory response induced by cisplatin involves the upregulation of TNF-α, nicotinamide adenine dinucleotide phosphate oxidase, and inducible nitric oxide synthase (iNOS), which directly leads to NO– formation. NO– and O2– produce ONOO–, which has strong oxidation and nitration properties, further inducing apoptosis and necrosis [57].
Autophagy
Autophagy plays an important role in maintaining cellular homeostasis and surviving cisplatin-induced nephrotoxicity. In NRK-52E cells treated with cisplatin, the increases in autophagy and apoptosis were both inhibited after beclin-1 knockdown, indicating that autophagy mediates cell damage [58]. However, another study showed that autophagy inhibition accelerated apoptosis, demonstrating the protective effect of autophagy in cisplatin-induced kidney injury [59]. Moreover, autophagy can prevent AKI and proximal tubule apoptosis caused by cisplatin [60].
Studies have reported that the suppression of mammalian target of the rapamycin (mTOR) activity alleviates the inhibitory phosphorylation of Unc-51-like autophagy activating kinase 1, which leads to the activation of autophagy [61]. Pretreatment with rapamycin, an mTOR inhibitor, induces autophagy to improve renal function in rats with ischemia/reperfusion [62]. Interestingly, NAD(P)H quinone dehydrogenase 1 deletion (an oxidative stress barrier) enhances the effect of rapamycin and leads to increased tuberous sclerosis complex 2 phosphorylation, indicating that autophagy may be activated to counter the increased stress and protect against AKI [63].
Current treatment of cisplatin-induced nephrotoxicity
Various treatments have been applied to address the different mechanisms of cisplatin-induced nephrotoxicity (Table 1). For example, cimetidine acts as an OCT2 inhibitor that inhibits the transportation of cisplatin in the kidney to protect against AKI [64], carvedilol works as an antioxidant against the oxidative stress process [65], cilastatin inhibits the apoptotic pathway [66], and rosiglitazone reduces inflammation [67].
Table 1.
Current treatments for cisplatin-induced nephrotoxicity.
| Strategies | Mechanisms | Advantages | Limitations |
|---|---|---|---|
| Cimetidine | OCT2 inhibitor [64] | Treat gastric ulcer and gastrointestinal bleeding | May induce mental confusion, hematologic depression, cardiac depression, and hypersensitivity-type hepatitis in elderly patients, patients with nephropathy or liver disease [159] |
| Carvedilol | Antioxidant, inhibit oxidative stress process [65] | Treat hypertension | Cause liver damage and peripheral vascular disease |
| Cilastatin | Anti-apoptosis [67] | Protect cyclosporin A-induced nephrotoxicity in clinic [160], septicemia, peritonitis infection, etc. | Cause gastrointestinal adverse reactions, skin allergic reactions, hepatorenal toxicity |
| Rosiglitazone | Anti-inflammation [66] | Treat diabetes and complications, improve blood lipid level | In treatment of tumors, only for ovarian cancer, limited in other tumors |
| Amifostine | Cytoprotective agent [70] | Reduce nephrotoxicity and neurotoxicity; the only available therapy that can ameliorate the cumulative nephrotoxic effects of cisplatin without reducing antitumor efficacy [70]. | Blood pressure drops and hypocalcemia |
| Hydration and diuresis, with magnesium or mannitol supplementation | Enhance cisplatin excretion and reduce renal exposure | Safe and feasible, convenient to patients [161] | A large amount of hydration, long cycle period |
| Chemoprevention (e.g., sodium thiosulfate) | Chemical action (reduction reaction) | Detoxication is quite effective | Osmotic pressure change |
| Hemodialysis | Principle of material exchange | Removes metabolites and regulates electrolyte and acid-base balance | Hypophosphatemia and heart rate disorder, high cost |
At present, although several kinds of drugs are applied clinically in response to kidney damage caused by cisplatin, these drugs exhibit different degrees of inadequacy. For example, hydration and diuresis in the clinic enhance cisplatin excretion and reduce renal exposure [68]. However, the disadvantage is that a large amount of hydration is required before and after cisplatin administration [69]. Moreover, adverse reactions such as osmotic pressure changes may occur during chemoprevention. In addition, metabolic waste in the body can be excreted through hemodialysis, which is often accompanied by hypophosphatemia and heart rate disorders. Amifostine is a broad-spectrum cytoprotective agent approved by the FDA as a kidney protectant for cisplatin chemotherapy in patients with advanced ovarian cancer; however, its application in other tumors is limited due to blood pressure drops and hypocalcemia [70].
Protective effects of natural products that prevent cisplatin-induced nephrotoxicity
Traditional and complementary medicines, including a variety of natural products, such as herbs, vitamins, minerals, trace elements, and nutritional supplements, have been widely used in most countries [71]. Adopting natural products in healthcare can improve the physical fitness of patients. To better understand the roles of natural products in AKI, we summarized the protective effects of various classes of natural products on cisplatin-induced nephrotoxicity (Fig. 3 and Tables 2 and 3).
Fig. 3. The summary of natural products to protect against cisplatin-induced nephrotoxicity.
Potential natural product treatments for cisplatin-induced nephrotoxicity classified by chemical structures.
Table 2.
Natural products in the treatments for cisplatin-induced nephrotoxicity classified by chemical structures.
| Types | Natural products | Mechanisms/targets | Drug-drug interaction |
|---|---|---|---|
| 1. Flavonoids | Naringin | Regulates redox balance, inhibits inflammatory, NF-κB activation, iNOS pathways, p53 activation, and apoptosis response [162] | Naringin, trimetazidine, or their combination could attenuate renal IR injury through inhibition of lipid peroxidase and enhancement of antioxidant activity [163] |
| Icariin | Reduces oxidative stress, NF-κB activation, and inflammation cascade and apoptosis [75] | – | |
| Breviscapine | Inhibits oxidative stress: increases SOD and decreases MDA [76] | Icariin combined with breviscapine has synergistic effects on erectile function of spontaneously hypertensive rat [76] | |
| Epicatechin and epicatechin gallate | Inhibits oxidative stress, inflammation, NF-κB, NRF2/HO-1 signaling, reduces ERK activity, MAPK pathway [164] | Combined treatment of epigallocatechin gallate and Coenzyme Q10 attenuates cisplatin-induced nephrotoxicity via suppression of oxidative/nitrosative stress, inflammation, and cellular damage [165] | |
| Sappanone A | Reduces oxidative stress, upregulates NRF2 and HO-1, inhibits MPO, MDA, TNF-α, IL-1β, inhibits NF-κB activation [79] | – | |
| Morin and morin hydrate | Suppresses oxidative stress, inflammation and apoptosis, MAPK, PARP-1 regulation, inhibits autophagy stimulation [166] | – | |
| Quercetin | Inhibits oxidative stress, inflammatory and apoptosis response, MAPK signaling, inhibits M1, and upregulates M2 macrophage activities [81] | Quercetin-rich guava (Psidium guajava) juice in combination with trehalose reduces kidney injury of type II diabetic rats [167]; quercetin and allopurinol ameliorate kidney injury in STZ-treated rats [168]; combination of resveratrol and quercetin suppresses acetaminophen-induced AKI [169]; quercetin in combination with vitamins (C and E) improves renal injury in cadmium intoxicated rats [170] | |
| Silymarin | Selectively protects renal cells with no interfering effect on cancer cells [82] | Palmitoylethanolamide and silymarin combination attenuates the degree of renal inflammation in kidney ischemia and reperfusion model [171] | |
| Daidzein | Blocks inflammation, oxidative stress, and cell death, inhibits MAPK signaling pathway [83] | – | |
| Xanthohumol | Inhibits NF-κB, activates NRF2 signaling pathway [84] | – | |
| Wogonin | Inhibits RIPK1-mediated necrosis and attenuates WNT/β-catenin pathway, inhibits inflammation and apoptosis [172] | Baicalein, wogonin and oroxylin A combination contributes to anti-inflammatory effect [173] | |
| Baicalein | Upregulates antioxidant defense mechanisms and downregulates MAPKs and NF-κB signaling pathways [174] | – | |
| Apigenin | Suppresses oxidative stress and inflammation [86] | Apigenin enhances other antitumor drugs’ efficacy or reduces their toxicity in cancer treatments [175] | |
| Scutellaria baicalensis Georgi | Enhances tumor therapeutic efficacy and attenuates AKI [87] | Acacia catechu Willd and Scutellaria baicalensis Georgi combination suppress LPS-induced proinflammatory response [94] | |
| Glycyrrhizic acid and 18β-glycyrrhetinic acid | Inhibits NF-κB phosphorylation and HMGB1 cytoplasmic translocation, upregulates NRF2 and HO-1 [88] | – | |
| Hyperin | Inhibits NF-κB and upregulates NRF2 and HO-1 [89] | – | |
| Eriodictyol | Inhibits oxidative stress and inflammation, upregulates NRF2/HO-1 [90] | – | |
| D-pinitol | Inhibits inflammation, oxidative stress, MAPK pathway [176] | – | |
| Mangiferin | Upregulates NRF2 and activates PI3K, modulates MAPK pathway [177] | Mangiferin and morin combination attenuates oxidative stress [94] | |
| Hesperidin | Decreases oxidative stress, inflammation and DNA damage [178] | Taurine and hesperidin rescues carbon tetrachloride-triggered kidney damage in rats [178] | |
| Hesperetin | Inhibits oxidative stress, lipid peroxidation, inflammation and apoptosis, activates NRF2, inhibits MAPK signaling pathway [179] | Administration of naringenin and hesperetin combination downregulates FAK and p38 signaling pathways [131] | |
| Galangin | Inhibits ERK and NF-κB signaling, RIP1/RIP3-dependent necroptosis, oxidative stress, inflammation [180] | Quercetin and galangin combination enhances anti-inflammatory effect [181] | |
| Biochanin A | Inhibits inflammatory response and p53 apoptosis [182] | Formononetin and biochanin A modulate NF-κB/p-AKT signaling molecules [183] | |
| Luteolin | Decreases platinum accumulation and suppresses oxidative/nitrosative stress, inflammation and apoptosis [92] | – | |
| Genistein | Inhibits NF-κB and p53 activation [93] | – | |
| Scutellarin | Inhibits inflammation and apoptosis, activates autophagy [94] | Edaravone and scutellarin combination activates anti-inflammatory effect in ischemia injury [184] | |
| Anthocyanin | Inhibits TNF-α, IL-1β and increases BCL-2, antioxidant, antiapoptotic and anti-inflammatory responses [185] | – | |
| Puerarin | Inhibits TLR4/NF-κB signaling, promotes antitumor activity in COLO205 and HeLa [73] | The combination of tanshinone IIA and puerarin inhibits the immersion of inflammatory cells [186] | |
| 2. Saponins | Saikosaponin D | Reduces apoptosis, inhibits TNF-α, IL-1β and IL-6, and NO, reduces nitriding stress, and inhibits the activation of the NF-κB-P38-JNK-MAPK signaling cascades [97] | Antitumor effect is enhanced in combination saikosaponin D with SP600125 [187] |
| Ginsenoside 20(S)-Rg3 | Inhibits autophagy, blocks cell apoptosis, inhibits JNK-P53-caspase-3 signaling cascades [101] | Both studies of acute toxicity and seven-day repeated dose toxicity indicated the safety of the salvianolic acid B and ginsenoside Rg1 combination [188] | |
| Ginsenoside Rb3 | Inhibits AMPK-/mTOR-mediated autophagy and apoptosis [102] | ||
| Ginsenoside Rd/Rg5/Re/Rh2, red ginseng, Pseudoginsengenin DQ, Platycodon grandiflorum saponins | Reduces oxidative stress, inflammation and apoptosis, reduces COX-2 and iNOS expression, sirt1/NF-κB and caspase signaling pathway, PI3K/AKT/Apoptosis signaling pathways [98, 99, 189–193] | ||
| Panax notoginseng saponins | Increases autophagy, BCL-2, reduces mitochondria-mediated endogenous apoptosis, HIF-1α/mitochondria/ROS pathway [194] | Panax notoginseng saponins could increase the gastrointestinal tract absorption of aspirin and salicylic acid [195] | |
| Saponins from Terminalia arjuna | Reduces oxidative stress, downregulates TGF-β, NF-κB and KIM-1 [100] | – | |
| Leaves of panax quinquefolius | Suppresses oxidative stress, inflammation and apoptosis, regulates PI3K/AKT/apoptosis | – | |
| American ginseng berry extract | Suppresses MAPK and NF-κB signaling pathways [196] | – | |
| Dioscin | Targets miR-34a/sirtuin 1 signaling pathway [103] | Dioscin reverses adriamycin-induced multidrug resistance by inhibition of the NF-κB signaling pathway [197] | |
| Peroxidized ergosterol | Reduces apoptosis, blocks MAPK-caspase-3 signaling cascade [104] | – | |
| Saponins extracted from the fruit of hibiscus | Blocks MAPKs signaling cascade [105] | – | |
| 3. Alkaloids | Ligustrazine | Inhibits oxidative stress, apoptosis, neutrophils infiltration and the overexpression of TNF-α and ICAM-1 [109] | Toxic study revealed ligustrazine was low toxic, LD50 was larger than 5 g/kg, both the level of ALT and AST and histopathology in the liver and kidney exhibited no distinctions between the tetramethylpyrazine, resveratrol, and curcumin (TRC) combination [198] |
| Tetramethylpyrazine | Inhibits HMGB1/TLR4/NF-κB and activates NRF2 and PPAR-γ signaling pathways [110] | The effect of herbal compounds identified by network pharmacology approaches to reduce the toxicity of methotrexate was assessed by methotrexate-induced rat toxicity model [199] | |
| Berberine | Inhibits oxidative stress, inflammation, autophagy, and apoptosis [112] | Combination of berberine with pentoxifylline leads to more significant renoprotective effects than either berberine or pentoxifylline when used alone on diclofenac-induced AKI [200]; combination of berberine with doxorubicin is a novel strategy that has the potential for protecting against doxorubicin‑induced hepatorenal toxicity in clinical practice [201] | |
| Betaine | Alleviates inflammatory and apoptotic mediators, improves antioxidant abilities [113] | Caffeic acid phenethyl ester and betaine attenuate abamectin-induced hepatotoxicity and nephrotoxicity [181] | |
| 4. Polysaccharides | Lentinan | Activates NRF2-ARE, decreases ROS [117] | Lentinan combines with gemcitabine chemotherapy significantly inhibits UBC cell proliferation [202] |
| Ganoderma lucidum polysaccharides/Lycium barbarum polysaccharides | Increases antioxidant enzymes, reduces oxidative stress and lipid peroxidation [118] | Lycium barbarum polysaccharides (LBP) and scopolamine combination prevents these SCO-induced reductions in cell proliferation and neuroblast differentiation [203] | |
| Lycium europaeum Linn | Antioxidant activities [119] | – | |
| 5. Phenylpropanoids | Schizandrin B | Inhibits oxidative stress, inflammatory and apoptosis response, β-catenin pathway, activates ERK/NF‑κB signaling [204] | Schizandrin B and lapatinib combination enhances the suppression on cell migration and invasion [205] |
| Nordihydroguaiaretic acid | Inhibits oxidative stress, inflammatory and apoptosis response [121] | Erythropoietin (EPO) and nordihydroguaiaretic acid accelerate renal function recovery by stimulating tubular epithelial cell regeneration [206] | |
| Schisandra sphenanthera extract | NRF2 nuclear accumulation, inhibits ROS and increases GSH [122] | Schisandra sphenanthera extract could enhance the bioavailability of tacrolimus primarily through the inhibition of P-gp-mediated efflux and CYP3A-mediated metabolism in the intestine [207] | |
| 6. Others | Ficus religiosa latex extract | Antioxidant, increases GSH, SOD, CAT levels [111] | Ficus religiosa latex and constituents (glycoside, alkaloids, tannins, flavonoids, and amino acids) have excellent nephroprotective and curative activities [111] |
| Astragaloside IV | Activates NRF2 and HO-1, inhibits NF-κB, induces autophagy, and limits NLRP3 expression [208] | – | |
| Z-ligustilide and E-ligustilide isolated from Angelica sinensis | Inhibits oxidative stress, suppresses β-catenin pathway [209] | – | |
| Wedge leaf tea extract | Inhibits oxidative stress, ROS, reduces apoptosis [140] | – | |
| Carvacrol | Suppresses oxidative stress, apoptosis, inflammation, suppresses ERK and PI3K/AKT pathways [124] | Combination of carvacrol and thymol upregulates the antimicrobial activity and antioxidant activity [210] | |
| Ginkgo biloba extract | Antioxidant activities, inhibits MDA, NO, MPO [211] | – | |
| Pomegranate rind extract | Inhibits oxidative stress, apoptosis, inflammation [143] | – | |
| Eisenia foetida extract | Prevents oxidative stress and inhibits lipid peroxidation [144] | – | |
| Pine bark extract | Increases antioxidant enzyme activities, inhibits lipid peroxidation [212] | – | |
| Mallow extract | Reduces MDA levels and inhibits inflammation [141] | – | |
| Stevia rebaudiana/stevioside | Inhibits ERK1/2, STAT3, and NF-κB [213] | – | |
| Total coumarins | Inhibits ERK1/2 and STAT3 signaling pathway, suppresses inflammation and apoptosis [134] | – | |
| Oleuropein | Inhibits ERK signaling, restores antioxidant system [214] | Oleuropein and 2-methoxyestradiol combination upregulates anticancer potential [215] | |
| Sinapic acid | Inhibits NF-κB and upregulates NRF2 and HO-1 [137] | – | |
| Vanillin | Inhibits NF-κB and decreases MDA, inhibits oxidative/nitrosative stress, inflammation and apoptosis [216] | Ortho-vanillin exacerbate the anti-arthritic effects of methotrexate in adjuvant-induced arthritis [217] | |
| Daphnetin | Inhibits NF-κB and upregulates NRF2 and HO-1 [138] | – | |
| Zingerone | Inhibits oxidative stress, apoptosis and inflammation [139] | Zingerone and dihydroartemisinin combination presents synergistic antimalarial activity [218] | |
| Asiatic acid | Suppresses IL-1β, TNF-α, MCP-1, and caspase-1 [135] | Combination of carnosine and asiatic acid enhances anti-inflammation activity [219] | |
| Celastrol | Inhibits NF-κB and improves mitochondrial function [136] | Combination of Lapatinib and celastrol downregulates subcellular distribution of HER2 [220] | |
| Eleutheroside B | Activates IGF pathway and reduces IGFBP-7 [125] | – | |
| Chlorogenic acid | Suppresses p53, activates caspase-3 and LC3-II expression, inhibits apoptosis and autophagy [126] | Combination of lapatinib with chlorogenic acid inhibits breast cancer metastasis by suppressing macrophage M2 polarization [221] | |
| Protocatechuic aldehyde | Suppresses NOX-mediated oxidative stress and inflammation [127] | – | |
| Oleanolic acid | Inhibits ERK, STAT3 and NF-κB, promotes sensitivity of Hela to cisplatin [222] | Combination of rho iso-alpha acids from hops, rosemary, and oleanolic acid decreased pain by 50% in patients with osteoarthritis [223] | |
| Green tea | Restores antioxidant defense system [224] | – | |
| Carnosic acid | Enhances SOD, CAT, GR, and GST activities, inhibits apoptosis [225] | Carnosic acid and fisetin combination therapy enhances inhibition of lung cancer through apoptosis induction [226] | |
| Emodin | Increases antioxidant enzyme activities, modulates AMPK/mTOR signaling pathways, activates autophagy [227] | Emodin combined with cytarabine induces apoptosis [228] | |
| Ethanolic extract of Trigonella foenum-graecum | Inhibits oxidative stress, apoptosis, inflammation [229] | Dietary fenugreek (Trigonella foenum-graecum) seeds and onion (Allium cepa) attenuate diabetic nephropathy [230] | |
| Geraniin | Inhibits NF-κB and upregulates NRF2 and HO-1 [231] | Geraniin combines with morphine or diclofenac to enhance anti-nociceptive effect [232] | |
| Apodytes dimidiata | Scavenges ROS, increases GSH, GPx, SOD, and catalase [233] | – | |
| Lycopene | Useful therapy for nephrotoxicity [234] | The combination therapy of rosmarinic acid and lycopene shows better protective effects than the corresponding monotherapy [235] | |
| Genipin | Inhibits oxidative stress, apoptosis, inflammation [236] | Combination of genipin and oxaliplatin enhances the therapeutic effects by upregulating BIM in colorectal cancer [237] | |
| Schisandra chinensis bee pollen extract | Inhibits oxidative stress, apoptosis, inflammation [238] | – | |
| Schisandra chinensis stems | Inhibits oxidative stress, apoptosis, inflammation [239] | – | |
| Filipendula ulmaria extract | Inhibits oxidative stress [240] | – | |
| Nigella sativa extract | Inhibits oxidative stress [241] | Mixed hydroalcoholic extracts of Nigella sativa and Curcuma longa improve adriamycin-induced renal injury in rat [242] | |
| Nigella sativa oil | Decreases BBM enzymes activities, inhibits oxidative stress [243] | Fish oil/Nigella sative volatile oil emulsion is the most promising hepato-regenerative and renoprotective formula [244] | |
| Danshen | Modulates NRF2 signaling pathway, inhibits oxidative stress [245] | The combination of rhein (RH) and danshensu (DSS) conferred a protective effect, as shown by a significant improvement in the chronic renal function [246] | |
| Plantago major extract | Inhibits oxidative stress [247] | Plantago major (300, 600, and 1200 mg/kg) and vitamin E significantly attenuated kidney tissue damage [248] | |
| Ethanolic fruit extract of Citrullus colocynthis | Inhibits oxidative stress [249] | – | |
| Ethanol leaf extract of Andrographis paniculata | Modulates NRF2/KIM-1 signaling pathway [250] | – | |
| Lycium europaeum methanol extract | Enhances antioxidant activities [251] | – | |
| Porphyra yezoensis | Inhibits MAPK/NF-κB pathways, inhibits inflammation [252] | – | |
| Whortleberry | Inhibits oxidative stress, caspase-3 level [128] | – | |
| Tangeretin | Inhibits oxidative stress and inflammation, regulates NF-κB-TNF-α/iNOS signaling pathway [253] | Combination of luteolin and tangeretin enhances anti-inflammatory activities [254] | |
| Cynaroside | Inhibits caspase-3/MST-1 signal pathway [129] | – | |
| Resveratrol | Inhibits oxidative stress, apoptosis and inflammation, activates ERK pathway, decreases cisplatin concentration, and lowers its accumulation [255] | Combination of hUCMSCs and resveratrol can better protect renal podocyte function, reduction of blood glucose and renal injury for diabetic nephropathy [256]; resveratrol (RES) and quercetin (QUR) can protect against APAP-induced nephrotoxicity [257] | |
| Dendropanax morbifera | Inhibits oxidative stress and inflammation [131] | – | |
| Troxerutin | Inhibits oxidative stress, inhibits MDA, increases SOD and GPx [132] | Troxerutin potentiates 5-fluorouracil treatment of human gastric cancer through suppressing STAT3/NF-κB and BCL-2 signaling pathways [258] | |
| Meclofenamic acid | Inhibits fat mass and obesity-associated protein (FTO)-mediated m6A abrogation [133] | Simvastatin in combination with meclofenamic acid inhibits the proliferation and migration of human prostate cancer PC-3 cells [259] | |
| QiShenYiQi (QSYQ) pills | Inhibits caspase-3, inhibits oxidative stress and apoptosis [146] | – | |
| Huaiqihuang (HQH) extractum | Reduces nuclear-cytoplasmic translocation of HMGB1 and inactivates TLR4 and NF-κB signaling pathway [145] | – | |
| Curcumin | Inhibits oxidative stress, apoptosis, inflammation, increases NAMPT and SIRT levels, inhibits M1 macrophage, and increases M2 macrophage polarization [260–262] | Curcumin and dexmedetomidine was effective in reducing oxidative stress and renal histopathologic injury in I/R rat model [261]; combination of curcumin and metformin might be functional to treat or inhibit nephrotoxicity [262] |
Table 3.
Natural products in the treatments for cisplatin-induced nephrotoxicity classified by mechanisms.
| Mechanisms | Natural products types | Representative natural products |
|---|---|---|
| 1. Cellular uptake and transport | Flavonoids | Formononetin |
| 2. DNA damage | Flavonoids | Hesperidin |
| 3. Apoptosis | Flavonoids | Naringin, Icariin, Morin and morin hydrate, Quercetin, Wogonin, Hesperetin, Luteolin, Scutellarin, Anthocyanin |
| Saponins | Saikosaponin D, Ginsenoside 20(S)-Rg3, Ginsenoside Rb3, Ginsenoside Rd/Rg5/Re/Rh2, red ginseng, Pseudoginsengenin DQ, Platycodon grandiflorum saponins, Leaves of panax quinquefolius, Peroxidized ergosterol | |
| Alkaloids | Ligustrazine, Berberine, Betaine | |
| Phenylpropanoids | Schizandrin B, Nordihydroguaiaretic acid | |
| Others | Wedge leaf tea extract, Carvacrol, Pomegranate rind extract, Total coumarins, Vanillin, Zingerone, Chlorogenic acid, Carnosic acid, Genipin | |
| 4. Oxidative stress | Flavonoids | Icariin, Breviscapine, Epicatechin and epicatechin gallate, Sappanone A, Morin and morin hydrate, Quercetin, Daidzein, Baicalein, Apigenin, Eriodictyol, D-pinitol, Mangiferin, Hesperidin, Hesperetin, Galangin, Luteolin, Anthocyanin, etc. |
| Saponins | Ginsenoside Rd/Rg5/Re/Rh2, red ginseng, Pseudoginsengenin DQ, Platycodon grandiflorum saponins, Saponins from Terminalia arjuna, leaves of panax quinquefolius, Dioscin, Saponins extracted from the fruit of hibiscus | |
| Alkaloids | Ligustrazine, Tetramethylpyrazine, Ficus religiosa latex extract, Berberine, Betaine | |
| Polysaccharides | Lentinan, Ganoderma lucidum polysaccharides /Lycium barbarum polysaccharides, Lycium europaeum Linn | |
| Phenylpropanoids | Schizandrin B, Nordihydroguaiaretic acid, Schisandra sphenanthera extract | |
| Others | Astragaloside IV, Z-ligustilide and E-ligustilide isolated from Angelica sinensis, Wedge leaf tea extract, Carvacrol, Ginkgo biloba extract, Pomegranate rind extract, Eisenia foetida extract, Pine bark extract, Mallow extract, Oleuropein, Sinapic acid, Vanillin, Zingerone, Protocatechuic aldehyde, Carnosic acid, Emodin, Apodytes dimidiate, Genipin | |
| 5. Inflammation | Flavonoids | Naringin, Icariin, Epicatechin and epicatechin gallate, Sappanone A, Morin and morin hydrate, Quercetin, Daidzein, Xanthohumol, Wogonin, Apigenin, Eriodictyol, D-pinitol, Hesperidin, Hesperetin, Galangin, Biochanin A, Luteolin, Genistein, Naringin, Scutellarin, Anthocyanin, Puerarin, etc. |
| Saponins | Saikosaponin D, Ginsenoside Rd/Rg5/Re/Rh2, red ginseng, Pseudoginsengenin DQ, Platycodon grandiflorum saponins, Leaves of panax quinquefolius, American ginseng berry extract | |
| Alkaloids | Ligustrazine, Tetramethylpyrazine, Berberine, Betaine | |
| Phenylpropanoids | Schizandrin B, Nordihydroguaiaretic acid | |
| Others | Carvacrol, Pomegranate rind extract, Mallow extract, Stevia rebaudiana/stevioside, Total coumarins, Sinapic acid, Vanillin, Daphnetin, Zingerone, Asiatic acid, Celastrol, Protocatechuic aldehyde, Genipin | |
| 6. Autophagy | Flavonoids | Morin and morin hydrate, Scutellarin, Berberine |
| Saponins | Ginsenoside 20(S)-Rg3, Ginsenoside Rb3, Panax notoginseng saponins | |
| Alkaloids | Berberine | |
| Others | Astragaloside IV, Chlorogenic acid, Emodin |
Flavonoids
Studies have shown that formononetin can effectively reduce OCT2 expression and increase MRP expression, resulting in decreased accumulation of cisplatin in renal tubular cells [72]. Similarly, puerarin protects against cisplatin-induced nephrotoxicity and promotes the antitumor activity of cisplatin in COLO205 and HeLa tumor cells in a dose-dependent manner [73]. Interestingly, naringin can alleviate cisplatin-induced renal dysfunction by inhibiting the inflammatory response and reducing apoptosis [74]. Flavonoids with multiple activities, such as icariin, breviscapine, epicatechin and epicatechin gallate, sappanone A, morin and its hydrate, quercetin, silymarin, daidzein, and xanthohumol, can reduce cisplatin-induced oxidative and nitrosative stress and decrease creatinine (Cre) and blood urea nitrogen (BUN) levels to improve renal function, thereby alleviating cisplatin-induced nephrotoxicity [75–84]. In addition, wogonin markedly inhibits receptor-interacting protein kinase 1-mediated necrosis and the canonical WNT pathway (WNT/β-catenin pathway) to protect against cisplatin-induced nephrotoxicity [85]. Further studies demonstrated that baicalein and apigenin ameliorated cisplatin-induced renal damage through the upregulation of antioxidant pathways and downregulation of the MAPK and NF-κB signaling pathways [86].
Interestingly, Scutellaria baicalensis Georgi not only enhances the therapeutic efficacy of cisplatin but also attenuates chemotherapy-induced AKI [87]. Glycyrrhizic acid, 18β-glycyrrhetinic acid, hypericin, and eriodictyol reduce AKI by inhibiting the cisplatin-induced phosphorylation of NF-κB and upregulating the expression of nuclear factor erythroid 2 (NFE2)-related factor 2 (NRF2) and HO-1 [88–90]. D-Pinitol and mangiferin attenuate inflammatory infiltration, DNA damage, and renal dysfunction in rats by modulating the MAPK pathway [91]. Furthermore, cisplatin-induced oxidative stress is mitigated by hesperidin and hesperetin by reducing MDA/Myeloperoxidase (MPO) levels and increasing SOD/Glutathione (GSH) levels. Galangin and the isoflavonoid biochanin A exhibit renoprotective effects in mice by targeting the inflammatory response and p53-mediated apoptosis. Importantly, luteolin significantly reduces histological and biochemical changes induced by cisplatin by blocking platinum accumulation and inflammation [92]. Genistein and naringin inhibit the NF-κB and iNOS pathways and p53 activation to improve HK-2 cell viability and kidney morphology in the presence of cisplatin and have become a potential effective treatment strategy for AKI [93]. A recent study demonstrated that scutellarin and anthocyanin from the fruits of Panax ginseng attenuate cisplatin-induced nephrotoxicity by inhibiting TNF-α [94]. In summary, flavonoids exhibit great potential as dietary supplements to ameliorate cisplatin-induced nephrotoxicity.
It is worth noting that the flavonoid phloretin is a robust toxicant (LC50 = 362 μM) that potentiates H2O2-induced toxicity, which is consistent with the previously noted cytotoxicity of phloretin and other hydroxychalcones. This toxicity is due to the oxidative activities of these polyphenols and the possible induction of mitochondrial toxicity [95].
Many flavonoids show strong protective effects against cisplatin-induced AKI. To date, researchers have found that many kinds of flavonoids activate NRF2/HO-1 signaling and inhibit NF-κB activity to alleviate kidney injury. More interestingly, some flavonoids not only protect against cisplatin-induced kidney injury but also synergistically inhibit the growth of tumors, enhancing the efficacy of cisplatin in tumor-bearing mice [74]. These results suggest that flavonoids may be used in the comprehensive treatment of cancer patients. Although flavonoids exhibit strong protection against kidney injury, there are some challenges in the clinical application of flavonoids. For example, monomers of flavonoid compounds are difficult to extract and have poor lipid solubility and low bioavailability, limiting their clinical applications [96]. If researchers can overcome these challenges, flavonoids will become promising drugs for AKI treatment.
Saponins
Oxidative stress and inflammation are important mechanisms involved in the pathogenesis of AKI. Some studies have shown that saikosaponin D can increase the survival rate of HK-2 cells and maintain the normal morphology of the nucleus. Saikosaponin D can inhibit the activation of the NF-κB-P38-JNK-MAPK signaling cascade, thereby reducing cisplatin-induced apoptosis [97]. Red ginseng, ginsenoside Rg5, and Platycodon grandiflorum saponins can inhibit inflammation by reducing the expression of cyclooxygenase-2 and iNOS to inhibit acute tubular necrosis and apoptosis [98, 99]. Renal oxidative stress, as evidenced by increased MDA levels and declines in GSH and SOD activities, is significantly reduced by saponins from Terminalia arjuna [100].
In addition, some saponin components mainly regulate autophagy and apoptosis to exert protective effects against kidney injury. Ginsenoside 20(S)-Rg3 and ginsenoside Rb3 can inhibit autophagy to improve renal injury by blocking the JNK-P53-caspase-3 signaling cascade [101, 102]. Panax notoginseng saponins can improve cisplatin-induced damage to mitochondria, reduce mitochondria-mediated endogenous apoptosis, and enhance autophagy in renal cells, thus reducing cisplatin-induced nephrotoxicity. Dual luciferase reporter assays and molecular docking assays demonstrated that dioscin could target the miR-34a/sirtuin 1 signaling pathway to alter cisplatin-induced nephrotoxicity [103]. Peat moss sphagnum palustre can prevent colon cancer and has antibacterial effects, and peroxidized ergosterol can reduce cisplatin-induced apoptosis and improve cisplatin-induced kidney injury [104]. Other researchers have found that saponins extracted from Hibiscus fruit have protective effects on cisplatin-induced cytotoxicity in LLC-PK1 kidney cells [105].
Compared with other saponin components, ginsenoside 20(S)-Rg3 and Rb3 inhibit autophagy to block apoptosis [101, 102]. Further studies are needed to examine whether all saponin components can play important roles in regulating autophagy to protect against AKI. On the other hand, some studies show that saponin components play protective roles in alleviating kidney injury by regulating the NF-κB signaling pathway to reduce inflammation. However, whether saponin components affect the recruitment of immune cells and which type of immune cell is the main regulator are unclear. More studies need to be conducted to elucidate the role of immune cells in saponin component-mediated inhibition of the inflammatory response to protect against cisplatin-induced nephrotoxicity.
In summary, these findings clearly suggest that saponin components can exert protective effects against cisplatin-induced nephrotoxicity, mainly due to the regulation of autophagy and inhibition of oxidative stress, inflammation, and apoptosis. However, both in vitro and in vivo studies have demonstrated that M. charantia may also exert toxic or adverse effects under different conditions and can decrease plasma progesterone and estrogen levels in a dose-dependent manner [106]. This plant causes acute symptoms such as changes in respiratory and heart rates and may induce termination of early pregnancy and cause abortion [107]. In addition, it has been reported that M. charantia fruit causes abdominal pain and diarrhea in individuals with diabetes [108].
Alkaloids
Studies have shown that ligustrazine can reduce the levels of urinary protein, as well as serum Cre and BUN, and enhance the antioxidant capacity, thus exerting a certain protective effect against nephrotoxicity [109]. Tetramethylpyrazine inhibits HMGB1/TLR4/NF-κB and activates the NRF2 and PPAR-γ signaling pathways to achieve nephroprotective effects [110]. Ficus religiosa latex extract has glycoside, alkaloid, and amino acid constituents and shows excellent nephroprotective and curative effects in rats [111]. A study indicated that berberine exerted a nephroprotective effect via the inhibition of oxidative stress, inflammation, autophagy, and apoptosis in cisplatin-induced AKI [112]. Betaine exerts renoprotective effects by alleviating inflammatory and apoptotic mediators and improving antioxidant capacity in rats and may be a beneficial dietary supplement to attenuate cisplatin-induced nephrotoxicity [113].
In contrast, alkaloids found in aconitum species are highly toxic cardiotoxins and neurotoxins [114]. Moreover, further investigations are necessary to determine the exact toxicological mechanisms because the coadministration of alkaloids with drugs that are substrates of DMEs and/or ETs may cause herb-drug interactions [115]. In addition, dehydropyrrolizidine alkaloid (DHPA) can induce chronic disease, which may accumulate over a long period of time and develop slowly until liver failure. The incidence of tumors in rodents increased even in response to very low DHPA doses for a short period of time [116]. These concerns have limited animal or human exposure to alkaloids.
Taken together, these studies demonstrated that alkaloids could ameliorate cisplatin-induced AKI in mice and rats. Alkaloid treatment regulates immune cell infiltration, inhibits oxidative stress, and suppresses apoptosis in the kidney to protect against cisplatin-induced AKI. Mechanistically, some alkaloids efficiently reverse the cisplatin-induced activation of the TLR4/NF-κB pathway and ameliorate renal oxidative stress by increasing GSH, SOD, and CAT levels. Further investigations aimed at delineating the signaling pathways involved in the beneficial effects of alkaloids on cisplatin-induced AKI are needed.
Polysaccharides
Lentinan can alleviate cisplatin-induced apoptosis of HK-2 human kidney proximal tubular cells and disrupt renal function in mice. The mechanism is related to the activation of the NRF2-ARE signaling pathway and decrease in intracellular ROS. Moreover, lentinan also inhibits the proliferation of HeLa and A549 cells [117]. In addition, Ganoderma lucidum polysaccharides and Lycium barbarum polysaccharides can increase the activities of antioxidant enzymes and reduce the levels of oxidative stress and lipid peroxidation, thereby protecting against cisplatin-induced nephrotoxicity [118]. Lycium europaeum Linn is a well-known medicinal plant and is a source of polysaccharides with antioxidant activities in vivo and in vitro [119].
No adverse reactions to polysaccharides have been reported so far, but there can be slight gastrointestinal reactions, which can be alleviated after 1 week of administration. In addition, astragal polysaccharides may cause dry mouth, chest distension, easy excitation, and other adverse reactions. No recommendations on the clinical use of polysaccharides are currently available. In summary, polysaccharide components generally show obvious antioxidant activities by decreasing ROS levels and increasing antioxidative enzymes. However, it is still unknown how polysaccharide components exhibit oxidative effects to protect against kidney injury.
Phenylpropanoids
Schizandrin B and nordihydroguaiaretic acid have inhibitory effects against cisplatin-induced nephrotoxicity, and their renoprotective mechanisms are associated with oxidative stress, inflammatory response, and apoptosis [120, 121]. It is believed that Schisandra sphenanthera extract facilitates the nuclear accumulation of the transcription factor NRF2 to mitigate cisplatin-induced nephrotoxicity, which is important for therapeutic approaches to AKI [122]. In some cases of illness, star anise tea can cause severe neurological and gastrointestinal toxicity, which is characterized by convulsions, diarrhea, and vomiting [123]. No recommendations on the clinical use of Schisandra sphenanthera extract are currently available.
Overall, phenylpropanoids inhibit oxidative stress, inflammation, and apoptosis to alleviate AKI by increasing GSH levels and NF-κB signaling. Phenylpropanoids mainly play roles of preventing or protecting against cisplatin-induced kidney injury through these three mechanisms, but the interactions between these mechanisms are still unclear.
Others
Current evidence suggests that carvacrol attenuates AKI by suppressing oxidative stress, apoptosis, and inflammation by modulating the extracellular-regulated protein kinases (ERK) and PI3K/AKT pathways [124]. In addition, eleutheroside B activates the insulin-like growth factor pathway and reduces the expression of insulin-like growth factor binding protein 7, thereby increasing HK-2 cell viability against cisplatin-induced damage [125]. Chlorogenic acid significantly suppresses the expression of p53, active caspase-3 and light chain 3-II, suggesting the inhibition of both apoptosis and autophagy [126]. Notably, protocatechuic aldehyde blocks cisplatin-induced AKI by suppressing Nox-mediated oxidative stress and inflammation without affecting the antitumor activity of cisplatin [127]. In addition, whortleberry, tangeretin, cynaroside, resveratrol, Dendropanax morbifera, troxerutin, and meclofenamic acid also have certain inhibitory effects on nephrotoxicity [128–133].
A variety of other natural products show renoprotective effects mainly by regulating the NF-κB pathway. For example, astragaloside IV effectively protects against cisplatin-induced nephrotoxicity by activating NRF2 and HO-1 and inhibiting the NF-κB pathway. The natural sweetener Stevia rebaudiana and its constituent stevioside protect against cisplatin-induced nephrotoxicity by inhibiting ERK1/2, STAT3, and NF-κB activation, as do total coumarins from Hydrangea paniculata [134]. Increasing evidence suggests that asiatic acid, a terpene, suppresses the increased mRNA expression of the proinflammatory cytokines interleukin-1β, NF-κB, and MCP-1 in the kidneys [135]. Moreover, celastrol can ameliorate cisplatin-induced nephrotoxicity by inhibiting NF-κB and improving mitochondrial function [136]. Oleuropein, sinapic acid, vanillin, daphnetin, and zingerone can inhibit NF-κB activation and upregulate the expression of NRF2 and HO-1 to prevent cisplatin-induced nephrotoxicity [137–139].
In addition, extracts from various natural plants can play protective roles in cisplatin-induced nephrotoxicity. Wedge leaf tea extract is used in Mongolian medicine to treat urinary system-related diseases by inhibiting renal oxidative stress and reducing apoptosis caused by cisplatin [140]. Mallow extract can reduce MDA levels and inhibit the release of inflammatory factors, thus improving the understanding of kidney protection [141]. Moreover, Ginkgo biloba extract, pomegranate rind extract, and Eisenia foetida extract are rich in flavonoids and terpenoid lactones, which can remove excess free radicals and inhibit lipid peroxidation, thus resisting cisplatin-induced nephrotoxicity [142–144]. A recent study clarifies that Huaiqihuang extract, a kind of Chinese herbal complex, reduces the nuclear-cytoplasmic translocation of HMGB1 and inactivates the TLR4 and NF-κB signaling pathways, exerting robust renoprotective effects [145].
By replenishing spirits and activating blood circulation, some traditional Chinese medicines and compound preparations can attenuate cisplatin-induced nephrotoxicity. For example, both artificial Cordyceps sinensis-Bailing capsules and QiShenYiQi pills, which are compound Chinese medicines, can inhibit the expression of caspase-3 in kidney tissue, thereby protecting the kidneys [146]. In addition, Astragalus injection can prevent kidney morphological and functional damage caused by cisplatin without reducing its antitumor activity. Astragalus contains saponins, polysaccharides, flavonoids, trace elements, and amino acids, which can scavenge free radicals to regulate immune functions [147].
Our review suggests that numerous natural products that possess potent medicinal properties, such as flavonoids with antioxidant and anti-inflammatory properties, are capable of protecting against kidney injury based on various promising laboratory findings. The factors can be applied as supplementary regimens or combinations against cisplatin-induced nephrotoxicity. Many active compounds have attracted much attention from chemists. Through structural modifications and optimization, the synthesis of compounds with improved activity and biosafety is the ultimate goal of every study. However, at present, many monomers with good activity are obtained from complicated sources, and the isolation and extraction processes have not been perfected [148]. Therefore, it is difficult to thoroughly isolate these compounds, the purity cannot be guaranteed, and the compounds have a variety of functions. In addition, some compounds may have multiple targets; thus, their effects may be multifaceted, including protective effects and side effects.
In addition to focusing on compounds in natural plants, some active ingredients in the ocean have been less well studied. The ocean is not only a huge treasure trove of materials but also a source of natural medicines with great potential. Some marine drugs have strong antioxidant effects, pharmacological effects, and clinical applications, such as seaweed, laminaria, oyster, cuttlefish bones, and wakame [149]. The overfishing of marine resources and other reasons are the reasons that natural resources cannot meet increasing needs. Therefore, we should focus our attention on the development and utilization of traditional Chinese patent medicines and traditional Chinese medicinal materials. With marine medicine sources, the scientific formula should be strengthened to make new doses and forms easy to use. For example, a soft capsule of Huoxiangzhengqi liquid mixed with a marine medicine source is a very successful example.
Future perspectives
Worldwide, AKI is a serious health problem, and the number of cases is increasing because of the side effects of medications or the complications of other diseases. In addition, the onset of AKI leads to chronic kidney disease, uremia, and hypertensive nephropathy [150–152]. The pathogenesis of cisplatin-induced nephrotoxicity is complex, early diagnosis is difficult, and effective treatment options are lacking. The urgency of developing a renoprotective strategy has pushed researchers to look at active natural products with few side effects. There are broad development prospects in the treatment of cisplatin-induced nephrotoxicity compared to the existing relief pathways. For example, curcumin has been used in traditional medicine because of its efficacy against kidney damage [153–156]. Recent reports show that pretreatment with curcumin can ameliorate cisplatin-induced kidney damage by suppressing inflammation and apoptosis [157].
The mechanisms of cisplatin-induced kidney damage involve various pathways, such as inflammatory mediators, oxidative stress, necrosis and apoptosis, and autophagy. To date, researchers have not found that these mechanisms are involved in cisplatin-induced nephrotoxicity, starting with excess ROS generation, which leads to oxidative stress, triggering inflammatory and autophagy pathways that damage DNA and induce apoptosis in the kidney. It is still unclear how the various pathways integrate and ultimately lead to kidney damage. In recent years, many natural products have been discovered by different mechanisms. A natural compound may have multiple active targets rather than only one unique target. Therefore, a natural product may play multiple roles and exhibit wide use and may have increased potential toxicity or side effects. Since some pathways of cisplatin-induced kidney injury are also involved in the antitumor effects of cisplatin, natural products may also affect cisplatin-mediated antitumor effects. While most compounds have anti-inflammatory and antioxidant properties, NAC and vitamin E have been reported to act as antioxidants and contribute to the development of lung cancer [158]. Therefore, it is unclear whether natural compounds with antioxidant activity interfere with the development of tumors while protecting against kidney injury. In this case, in addition to check the protective role against AKI, it is necessary to further study if natural products have effects on tumor growth, which may help to break through the limited use of cisplatin in clinic.
It is worth noting that natural products that have robust therapeutic effects on cisplatin-induced AKI also alleviate kidney diseases caused by other factors. Further research is needed to verify the beneficial effects of certain products on humans and other animals with kidney diseases to elucidate the detailed mechanisms of the renoprotective effects. To achieve the desired protective effect against nephrotoxicity, researchers should take all aspects of the relevant mechanisms into account and consider comprehensive measures or combinations of drugs. In addition, although certain natural products are excellent in protecting against kidney damage in vitro and in vivo, it is necessary to study the optimal dose for protecting against different tumors and different cisplatin strengths.
Furthermore, the development of molecular biology technology has led to the research of targeted therapy using cisplatin and natural products or derivatives that are highly selective for the kidney or tumor as carriers, and chemically coupling these factors into biological treatments. Direct delivery of cisplatin to the tumor site rather than the kidney can not only reduce the amount of cisplatin needed but also improve the efficacy and reduce adverse reactions. This opens up new ideas for the study of protective measures against cisplatin-induced nephrotoxicity.
Acknowledgements
This work was supported by the State Key Program of National Natural Science Foundation of China (No. 81872878) and a grant from Zhejiang Provincial Natural Science Foundation (No. LGF20H030001).
Competing interests
The authors declare no competing interests.
Footnotes
The original version of the article was unfortunately missing the Open Access License text and had an issue with the copyrightholder name.
These authors contributed equally: Chun-yan Fang, Da-yong Lou
Change history
10/19/2022
A Correction to this paper has been published: 10.1038/s41401-022-01012-3
Contributor Information
Jia-jia Wang, Email: wangjiajia3301@zju.edu.cn.
Qin-jie Weng, Email: wengqinjie@zju.edu.cn.
References
- 1.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–78. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzales-Vitale JC, Hayes DM, Cvitkovic E, Sternberg SS. The renal pathology in clinical trials of cis-platinum (II) diamminedichloride. Cancer. 1977;39:1362–71. doi: 10.1002/1097-0142(197704)39:4<1362::AID-CNCR2820390403>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 3.Pierson-Marchandise M, Gras V, Moragny J, Micallef J, Gaboriau L, Picard S, et al. The drugs that mostly frequently induce acute kidney injury: a case-noncase study of a pharmacovigilance database. Br J Clin Pharmacol. 2017;83:1341–9. doi: 10.1111/bcp.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barton CD, Pizer B, Jones C, Oni L, Pirmohamed M, Hawcutt DB. Identifying cisplatin-induced kidney damage in paediatric oncology patients. Pediatr Nephrol. 2018;33:1467–74. doi: 10.1007/s00467-017-3765-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Effects and side-effects of cisplatin. Lancet. 1982;1:682. [PubMed]
- 6.Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 7.Ridzuan NRA, Rashid NA, Othman F, Budin SB, Hussan F, Teoh SL. Protective role of natural products in cisplatin-induced nephrotoxicity. Mini Rev Med Chem. 2019;19:1134–43. doi: 10.2174/1389557519666190320124438. [DOI] [PubMed] [Google Scholar]
- 8.Mathew JL, Kabi BC, Rath B. Anti-oxidant vitamins and steroid responsive nephrotic syndrome in Indian children. J Paediatr Child Health. 2002;38:450–37. doi: 10.1046/j.1440-1754.2002.00016.x. [DOI] [PubMed] [Google Scholar]
- 9.Cornelison TL, Reed E. Nephrotoxicity and hydration management for cisplatin, carboplatin, and ormaplatin. Gynecol Oncol. 1993;50:147–58. doi: 10.1006/gyno.1993.1184. [DOI] [PubMed] [Google Scholar]
- 10.Ibrahim ME, Chang C, Hu Y, Hogan SL, Mercke N, Gomez M, et al. Pharmacokinetic determinants of cisplatin-induced subclinical kidney injury in oncology patients. Eur J Clin Pharmacol. 2019;75:51–7. doi: 10.1007/s00228-018-2552-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Q, Bowmer CJ, Yates MS. The protective effect of glycine in cisplatin nephrotoxicity: inhibition with NG-nitro-L-arginine methyl ester. J Pharm Pharmacol. 1994;46:346–51. doi: 10.1111/j.2042-7158.1994.tb03810.x. [DOI] [PubMed] [Google Scholar]
- 12.Safirstein R, Winston J, Moel D, Dikman S, Guttenplan J. Cisplatin nephrotoxicity: insights into mechanism. Int J Androl. 1987;10:325–46. doi: 10.1111/j.1365-2605.1987.tb00200.x. [DOI] [PubMed] [Google Scholar]
- 13.Eljack ND, Ma HY, Drucker J, Shen C, Hambley TW, New EJ, et al. Mechanisms of cell uptake and toxicity of the anticancer drug cisplatin. Metallomics. 2014;6:2126–33. doi: 10.1039/C4MT00238E. [DOI] [PubMed] [Google Scholar]
- 14.Ozkok A, Edelstein CL. Pathophysiology of cisplatin-induced acute kidney injury. Biomed Res Int. 2014;2014:967826. doi: 10.1155/2014/967826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Y, Yu X, Zhang Y, Ding G, Zhu C, Huang S, et al. Hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat (FG-4592) protects against cisplatin-induced acute kidney injury. Clin Sci (Lond) 2018;132:825–38. doi: 10.1042/CS20171625. [DOI] [PubMed] [Google Scholar]
- 16.Prasad SB, Rosangkima G, Kharbangar A. Structural and biochemical changes in mitochondria after cisplatin treatment of Dalton’s lymphoma-bearing mice. Mitochondrion. 2010;10:38–45. doi: 10.1016/j.mito.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Salei N, Rambichler S, Salvermoser J, Papaioannou NE, Schuchert R, Pakalniskyte D, et al. The kidney contains ontogenetically distinct dendritic cell and macrophage subtypes throughout development that differ in their inflammatory properties. J Am Soc Nephrol. 2020;31:257–78. doi: 10.1681/ASN.2019040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winston JA, Safirstein R. Reduced renal blood flow in early cisplatin-induced acute renal failure in the rat. Am J Physiol. 1985;249:F490–6. doi: 10.1152/ajprenal.1985.249.4.F490. [DOI] [PubMed] [Google Scholar]
- 19.Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int. 2004;66:480–5. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- 20.Wainford RD, Weaver RJ, Stewart KN, Brown P, Hawksworth GM. Cisplatin nephrotoxicity is mediated by gamma glutamyltranspeptidase, not via a C-S lyase governed biotransformation pathway. Toxicology. 2008;249:184–93. doi: 10.1016/j.tox.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Yonezawa A, Masuda S, Nishihara K, Yano I, Katsura T, Inui K. Association between tubular toxicity of cisplatin and expression of organic cation transporter rOCT2 (Slc22a2) in the rat. Biochem Pharmacol. 2005;70:1823–31. doi: 10.1016/j.bcp.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 22.Iwata K, Aizawa K, Kamitsu S, Jingami S, Fukunaga E, Yoshida M, et al. Effects of genetic variants in SLC22A2 organic cation transporter 2 and SLC47A1 multidrug and toxin extrusion 1 transporter on cisplatin-induced adverse events. Clin Exp Nephrol. 2012;16:843–51. doi: 10.1007/s10157-012-0638-y. [DOI] [PubMed] [Google Scholar]
- 23.Filipski KK, Mathijssen RH, Mikkelsen TS, Schinkel AH, Sparreboom A. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin Pharmacol Ther. 2009;86:396–402. doi: 10.1038/clpt.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ciarimboli G. Membrane transporters as mediators of cisplatin side-effects. Anticancer Res. 2014;34:547–50. [PubMed] [Google Scholar]
- 25.Pabla N, Murphy RF, Liu K, Dong Z. The copper transporter Ctr1 contributes to cisplatin uptake by renal tubular cells during cisplatin nephrotoxicity. Am J Physiol Ren Physiol. 2009;296:F505–11. doi: 10.1152/ajprenal.90545.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okada K, Ma D, Warabi E, Morito N, Akiyama K, Murata Y, et al. Amelioration of cisplatin-induced nephrotoxicity in peroxiredoxin I-deficient mice. Cancer Chemother Pharmacol. 2013;71:503–9. doi: 10.1007/s00280-012-2046-0. [DOI] [PubMed] [Google Scholar]
- 27.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307–20. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 28.Fujikawa Y, Kawanishi M, Kuraoka I, Yagi T. Frequencies of mutagenic translesion DNA synthesis over cisplatin-guanine intra-strand crosslinks in lacZ plasmids propagated in human cells. Mutat Res Genet Toxicol Environ Mutagen. 2014;770:23–8. doi: 10.1016/j.mrgentox.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of cisplatin nephrotoxicity. Toxins (Basel) 2010;2:2490–518. doi: 10.3390/toxins2112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yimit A, Adebali O, Sancar A, Jiang Y. Differential damage and repair of DNA-adducts induced by anti-cancer drug cisplatin across mouse organs. Nat Commun. 2019;10:309. doi: 10.1038/s41467-019-08290-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirama M, Isonishi S, Yasuda M, Ishikawa H. Characterization of mitochondria in cisplatin-resistant human ovarian carcinoma cells. Oncol Rep. 2006;16:997–1002. [PubMed] [Google Scholar]
- 32.Brady HR, Kone BC, Stromski ME, Zeidel ML, Giebisch G, Gullans SR. Mitochondrial injury: an early event in cisplatin toxicity to renal proximal tubules. Am J Physiol. 1990;258:F1181–7. doi: 10.1152/ajprenal.1990.258.5.F1181. [DOI] [PubMed] [Google Scholar]
- 33.Chirino YI, Pedraza-Chaverri J. Role of oxidative and nitrosative stress in cisplatin-induced nephrotoxicity. Exp Toxicol Pathol. 2009;61:223–42. doi: 10.1016/j.etp.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–12. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 35.Guerrero-Beltran CE, Calderon-Oliver M, Martinez-Abundis E, Tapia E, Zarco-Marquez G, Zazueta C, et al. Protective effect of sulforaphane against cisplatin-induced mitochondrial alterations and impairment in the activity of NAD(P)H: quinone oxidoreductase 1 and gamma glutamyl cysteine ligase: studies in mitochondria isolated from rat kidney and in LLC-PK1 cells. Toxicol Lett. 2010;199:80–92. doi: 10.1016/j.toxlet.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 36.Lau AH. Apoptosis induced by cisplatin nephrotoxic injury. Kidney Int. 1999;56:1295–8. doi: 10.1046/j.1523-1755.1999.00687.x. [DOI] [PubMed] [Google Scholar]
- 37.Rodrigues MA, Rodrigues JL, Martins NM, Barbosa F, Curti C, Santos NA, et al. Carvedilol protects against cisplatin-induced oxidative stress, redox state unbalance and apoptosis in rat kidney mitochondria. Chem Biol Interact. 2011;189:45–51. doi: 10.1016/j.cbi.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 38.Chirino YI, Trujillo J, Sanchez-Gonzalez DJ, Martinez-Martinez CM, Cruz C, Bobadilla NA, et al. Selective iNOS inhibition reduces renal damage induced by cisplatin. Toxicol Lett. 2008;176:48–57. doi: 10.1016/j.toxlet.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 39.Servais H, Ortiz A, Devuyst O, Denamur S, Tulkens PM, Mingeot-Leclercq MP. Renal cell apoptosis induced by nephrotoxic drugs: cellular and molecular mechanisms and potential approaches to modulation. Apoptosis. 2008;13:11–32. doi: 10.1007/s10495-007-0151-z. [DOI] [PubMed] [Google Scholar]
- 40.Tsuruya K, Ninomiya T, Tokumoto M, Hirakawa M, Masutani K, Taniguchi M, et al. Direct involvement of the receptor-mediated apoptotic pathways in cisplatin-induced renal tubular cell death. Kidney Int. 2003;63:72–82. doi: 10.1046/j.1523-1755.2003.00709.x. [DOI] [PubMed] [Google Scholar]
- 41.Hong SJ, Dawson TM, Dawson VL. Nuclear and mitochondrial conversations in cell death: PARP-1 and AIF signaling. Trends Pharmacol Sci. 2004;25:259–64. doi: 10.1016/j.tips.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 42.Razzaque MS, Koji T, Kumatori A, Taguchi T. Cisplatin-induced apoptosis in human proximal tubular epithelial cells is associated with the activation of the Fas Fas ligand system. Histochem Cell Biol. 1999;111:359–65. doi: 10.1007/s004180050368. [DOI] [PubMed] [Google Scholar]
- 43.Boyce M, Yuan J. Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ. 2006;13:363–73. doi: 10.1038/sj.cdd.4401817. [DOI] [PubMed] [Google Scholar]
- 44.Liu H, Baliga R. Cytochrome P450 2E1 null mice provide novel protection against cisplatin-induced nephrotoxicity and apoptosis. Kidney Int. 2003;63:1687–96. doi: 10.1046/j.1523-1755.2003.00908.x. [DOI] [PubMed] [Google Scholar]
- 45.Peyrou M, Hanna PE, Cribb AE. Cisplatin, gentamicin, and p-aminophenol induce markers of endoplasmic reticulum stress in the rat kidneys. Toxicol Sci. 2007;99:346–53. doi: 10.1093/toxsci/kfm152. [DOI] [PubMed] [Google Scholar]
- 46.Liu H, Baliga R. Endoplasmic reticulum stress-associated caspase 12 mediates cisplatin-induced LLC-PK1 cell apoptosis. J Am Soc Nephrol. 2005;16:1985–92. doi: 10.1681/ASN.2004090768. [DOI] [PubMed] [Google Scholar]
- 47.Cilenti L, Kyriazis GA, Soundarapandian MM, Stratico V, Yerkes A, Park KM, et al. Omi/HtrA2 protease mediates cisplatin-induced cell death in renal cells. Am J Physiol Ren Physiol. 2005;288:F371–9. doi: 10.1152/ajprenal.00154.2004. [DOI] [PubMed] [Google Scholar]
- 48.Jiang M, Wang CY, Huang S, Yang T, Dong Z. Cisplatin-induced apoptosis in p53-deficient renal cells via the intrinsic mitochondrial pathway. Am J Physiol Ren Physiol. 2009;296:F983–93. doi: 10.1152/ajprenal.90579.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jesse CR, Bortolatto CF, Wilhelm EA, Roman SS, Prigol M, Nogueira CW. The peroxisome proliferator-activated receptor-γ agonist pioglitazone protects against cisplatin-induced renal damage in mice. J Appl Toxicol. 2014;34:25–32. doi: 10.1002/jat.2818. [DOI] [PubMed] [Google Scholar]
- 50.Yadav DK, Kumar S, Choi EH, Chaudhary S, Kim MH. Molecular dynamic simulations of oxidized skin lipid bilayer and permeability of reactive oxygen species. Sci Rep. 2019;9:4496. doi: 10.1038/s41598-019-40913-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramesh G, Reeves WB. Salicylate reduces cisplatin nephrotoxicity by inhibition of tumor necrosis factor-alpha. Kidney Int. 2004;65:490–9. doi: 10.1111/j.1523-1755.2004.00413.x. [DOI] [PubMed] [Google Scholar]
- 52.Zhang B, Ramesh G, Norbury CC, Reeves WB. Cisplatin-induced nephrotoxicity is mediated by tumor necrosis factor-alpha produced by renal parenchymal cells. Kidney Int. 2007;72:37–44. doi: 10.1038/sj.ki.5002242. [DOI] [PubMed] [Google Scholar]
- 53.Ozkok A, Ravichandran K, Wang Q, Ljubanovic D, Edelstein CL. NF-kappa B transcriptional inhibition ameliorates cisplatin-induced acute kidney injury (AKI) Toxicol Lett. 2016;240:105–13. doi: 10.1016/j.toxlet.2015.10.028. [DOI] [PubMed] [Google Scholar]
- 54.Ramesh G, Reeves WB. p38 MAP kinase inhibition ameliorates cisplatin nephrotoxicity in mice. Am J Physiol Ren Physiol. 2005;289:F166–74. doi: 10.1152/ajprenal.00401.2004. [DOI] [PubMed] [Google Scholar]
- 55.dos Santos NA, Carvalho Rodrigues MA, Martins NM, dos Santos AC. Cisplatin-induced nephrotoxicity and targets of nephroprotection: an update. Arch Toxicol. 2012;86:1233–50. doi: 10.1007/s00204-012-0821-7. [DOI] [PubMed] [Google Scholar]
- 56.Huang S, You J, Wang K, Li Y, Zhang Y, Wei H, et al. N-acetylcysteine attenuates cisplatin-induced acute kidney injury by inhibiting the C5a receptor. Biomed Res Int. 2019;2019:4805853. doi: 10.1155/2019/4805853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cummings BS, McHowat J, Schnellmann RG. Role of an endoplasmic reticulum Ca2+-independent phospholipase A2 in cisplatin-induced renal cell apoptosis. J Pharmacol Exp Ther. 2004;308:921–8. doi: 10.1124/jpet.103.060541. [DOI] [PubMed] [Google Scholar]
- 58.Inoue K, Kuwana H, Shimamura Y, Ogata K, Taniguchi Y, Kagawa T, et al. Cisplatin-induced macroautophagy occurs prior to apoptosis in proximal tubules in vivo. Clin Exp Nephrol. 2010;14:112–22. doi: 10.1007/s10157-009-0254-7. [DOI] [PubMed] [Google Scholar]
- 59.Jiang M, Wei Q, Dong G, Komatsu M, Su Y, Dong Z. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int. 2012;82:1271–83. doi: 10.1038/ki.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaushal GP. Autophagy protects proximal tubular cells from injury and apoptosis. Kidney Int. 2012;82:1250–3. doi: 10.1038/ki.2012.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gong L, Pan Q, Yang N. Autophagy and inflammation regulation in acute kidney injury. Front Physiol. 2020;11:576463. doi: 10.3389/fphys.2020.576463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Su Y, Lu J, Gong P, Chen X, Liang C, Zhang J. Rapamycin induces autophagy to alleviate acute kidney injury following cerebral ischemia and reperfusion via the mTORC1/ATG13/ULK1 signaling pathway. Mol Med Rep. 2018;18:5445–54. doi: 10.3892/mmr.2018.9586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim TW, Kim YJ, Kim HT, Park SR, Lee MY, Park YD, et al. NQO1 deficiency leads enhanced autophagy in cisplatin-induced acute kidney injury through the AMPK/TSC2/mTOR signaling pathway. Antioxid Redox Signal. 2016;24:867–83. doi: 10.1089/ars.2015.6386. [DOI] [PubMed] [Google Scholar]
- 64.Katsuda H, Yamashita M, Katsura H, Yu J, Waki Y, Nagata N, et al. Protecting cisplatin-induced nephrotoxicity with cimetidine does not affect antitumor activity. Biol Pharm Bull. 2010;33:1867–71. doi: 10.1248/bpb.33.1867. [DOI] [PubMed] [Google Scholar]
- 65.Carvalho Rodrigues MA, Gobe G, Santos NA, Santos AC. Carvedilol protects against apoptotic cell death induced by cisplatin in renal tubular epithelial cells. J Toxicol Environ Health A. 2012;75:981–90. doi: 10.1080/15287394.2012.696512. [DOI] [PubMed] [Google Scholar]
- 66.Ozkaya O, Yavuz O, Can B, Dilek M, Savli E, Acikgoz Y, et al. Effect of rosiglitazone on cisplatin-induced nephrotoxicity. Ren Fail. 2010;32:368–71. doi: 10.3109/08860221003611729. [DOI] [PubMed] [Google Scholar]
- 67.Humanes B, Lazaro A, Camano S, Moreno-Gordaliza E, Lazaro JA, Blanco-Codesido M, et al. Cilastatin protects against cisplatin-induced nephrotoxicity without compromising its anticancer efficiency in rats. Kidney Int. 2012;82:652–63. doi: 10.1038/ki.2012.199. [DOI] [PubMed] [Google Scholar]
- 68.Duffy EA, Fitzgerald W, Boyle K, Rohatgi R. Nephrotoxicity: evidence in patients receiving cisplatin therapy. Clin J Oncol Nurs. 2018;22:175–83. doi: 10.1188/18.CJON.175-183. [DOI] [PubMed] [Google Scholar]
- 69.Shord SS, Thompson DM, Krempl GA, Hanigan MH. Effect of concurrent medications on cisplatin-induced nephrotoxicity in patients with head and neck cancer. Anticancer Drugs. 2006;17:207–15. doi: 10.1097/00001813-200602000-00013. [DOI] [PubMed] [Google Scholar]
- 70.Capizzi RL. Amifostine reduces the incidence of cumulative nephrotoxicity from cisplatin: laboratory and clinical aspects. Semin Oncol. 1999;26:72–81. [PubMed] [Google Scholar]
- 71.Cohen M, Hunter J. Complementary medicine products: interpreting the evidence base. Intern Med J. 2017;47:992–8. doi: 10.1111/imj.13534. [DOI] [PubMed] [Google Scholar]
- 72.Huang D, Wang C, Duan Y, Meng Q, Liu Z, Huo X, et al. Targeting Oct2 and P53: formononetin prevents cisplatin-induced acute kidney injury. Toxicol Appl Pharmacol. 2017;326:15–24. doi: 10.1016/j.taap.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 73.Ma X, Yan L, Zhu Q, Shao F. Puerarin attenuates cisplatin-induced rat nephrotoxicity: the involvement of TLR4/NF-kappaB signaling pathway. PLoS One. 2017;12:e0171612. doi: 10.1371/journal.pone.0171612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Athira KV, Madhana RM, Lahkar M. Flavonoids, the emerging dietary supplement against cisplatin-induced nephrotoxicity. Chem Biol Interact. 2016;248:18–20. doi: 10.1016/j.cbi.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 75.Ma P, Zhang S, Su X, Qiu G, Wu Z. Protective effects of icariin on cisplatin-induced acute renal injury in mice. Am J Transl Res. 2015;7:2105–14. [PMC free article] [PubMed] [Google Scholar]
- 76.Lou XY, Cheng JL, Zhang B. Therapeutic effect and mechanism of breviscapine on cisplatin-induced nephrotoxicity in mice. Asian Pac J Trop Med. 2015;8:873–7. doi: 10.1016/j.apjtm.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 77.Sahin K, Tuzcu M, Gencoglu H, Dogukan A, Timurkan M, Sahin N, et al. Epigallocatechin-3-gallate activates Nrf2/HO-1 signaling pathway in cisplatin-induced nephrotoxicity in rats. Life Sci. 2010;87:240–5. doi: 10.1016/j.lfs.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 78.Kanlaya R, Thongboonkerd V. Protective effects of epigallocatechin-3-gallate from green tea in various kidney diseases. Adv Nutr. 2019;10:112–21. doi: 10.1093/advances/nmy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kang L, Zhao H, Chen C, Zhang X, Xu M, Duan H. Sappanone A protects mice against cisplatin-induced kidney injury. Int Immunopharmacol. 2016;38:246–51. doi: 10.1016/j.intimp.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 80.Wei Z, He X, Kou J, Wang J, Chen L, Yao M, et al. Renoprotective mechanisms of morin in cisplatin-induced kidney injury. Int Immunopharmacol. 2015;28:500–6. doi: 10.1016/j.intimp.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 81.Tan RZ, Wang C, Deng C, Zhong X, Yan Y, Luo Y, et al. Quercetin protects against cisplatin-induced acute kidney injury by inhibiting Mincle/Syk/NF-kappaB signaling maintained macrophage inflammation. Phytother Res. 2020;34:139–52. [DOI] [PubMed]
- 82.Ninsontia C, Pongjit K, Chaotham C, Chanvorachote P. Silymarin selectively protects human renal cells from cisplatin-induced cell death. Pharm Biol. 2011;49:1082–90. doi: 10.3109/13880209.2011.568506. [DOI] [PubMed] [Google Scholar]
- 83.Tomar A, Kaushik S, Khan SI, Bisht K, Nag TC, Arya DS, et al. The dietary isoflavone daidzein mitigates oxidative stress, apoptosis, and inflammation in CDDP-induced kidney injury in rats: impact of the MAPK signaling pathway. J Biochem Mol Toxicol. 2020;34:e22431. [DOI] [PubMed]
- 84.Li F, Yao YY, Huang H, Hao H, Ying MZ. Xanthohumol attenuates cisplatin-induced nephrotoxicity through inhibiting NF-kappa B and activating Nrf2 signaling pathways. Int Immunopharmacol. 2018;61:277–82. doi: 10.1016/j.intimp.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 85.Badawy AM, El-Naga RN, Gad AM, Tadros MG, Fawzy HM. Wogonin pre-treatment attenuates cisplatin-induced nephrotoxicity in rats: Impact on PPAR-gamma, inflammation, apoptosis and Wnt/beta-catenin pathway. Chem Biol Interact. 2019;308:137–46. doi: 10.1016/j.cbi.2019.05.029. [DOI] [PubMed] [Google Scholar]
- 86.He X, Li C, Wei Z, Wang J, Kou J, Liu W, et al. Protective role of apigenin in cisplatin-induced renal injury. Eur J Pharmacol. 2016;789:215–21. doi: 10.1016/j.ejphar.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 87.Huang TH, Wu TH, Guo YH, Li TL, Chan YL, Wu CJ. The concurrent treatment of Scutellaria baicalensis Georgi enhances the therapeutic efficacy of cisplatin but also attenuates chemotherapy-induced cachexia and acute kidney injury. J Ethnopharmacol. 2019;243:112075. doi: 10.1016/j.jep.2019.112075. [DOI] [PubMed] [Google Scholar]
- 88.Wu CH, Chen AZ, Yen GC. Protective effects of glycyrrhizic acid and 18beta-Glycyrrhetinic acid against cisplatin-induced nephrotoxicity in BALB/c mice. J Agric Food Chem. 2015;63:1200–9. doi: 10.1021/jf505471a. [DOI] [PubMed] [Google Scholar]
- 89.Chao CS, Tsai CS, Chang YP, Chen JM, Chin HK, Yang SC. Hyperin inhibits nuclear factor kappa B and activates nuclear factor E2-related factor-2 signaling pathways in cisplatin-induced acute kidney injury in mice. Int Immunopharmacol. 2016;40:517–23. doi: 10.1016/j.intimp.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 90.Li CZ, Jin HH, Sun HX, Zhang ZZ, Zheng JX, Li SH, et al. Eriodictyol attenuates cisplatin-induced kidney injury by inhibiting oxidative stress and inflammation. Eur J Pharmacol. 2016;772:124–30. doi: 10.1016/j.ejphar.2015.12.042. [DOI] [PubMed] [Google Scholar]
- 91.Sadhukhan P, Saha S, Dutta S, Sil PC. Mangiferin ameliorates cisplatin induced acute kidney injury by upregulating Nrf-2 via the activation of PI3K and exhibits synergistic anticancer activity with cisplatin. Front Pharmacol. 2018;9:638. doi: 10.3389/fphar.2018.00638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Domitrovic R, Cvijanovic O, Pugel EP, Zagorac GB, Mahmutefendic H, Skoda M. Luteolin ameliorates cisplatin-induced nephrotoxicity in mice through inhibition of platinum accumulation, inflammation and apoptosis in the kidney. Toxicology. 2013;310:115–23. doi: 10.1016/j.tox.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 93.Sung MJ, Kim DH, Jung YJ, Kang KP, Lee AS, Lee S, et al. Genistein protects the kidney from cisplatin-induced injury. Kidney Int. 2008;74:1538–47. doi: 10.1038/ki.2008.409. [DOI] [PubMed] [Google Scholar]
- 94.Sun CY, Nie J, Zheng ZL, Zhao J, Wu LM, Zhu Y, et al. Renoprotective effect of scutellarin on cisplatin-induced renal injury in mice: Impact on inflammation, apoptosis, and autophagy. Biomed Pharmacother. 2019;112:108647. doi: 10.1016/j.biopha.2019.108647. [DOI] [PubMed] [Google Scholar]
- 95.Halliwell B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch Biochem Biophys. 2008;476:107–12. doi: 10.1016/j.abb.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 96.Rawat DS, Thakur BK, Semalty M, Semalty A, Badoni P, Rawat MS. Baicalein-phospholipid complex: a novel drug delivery technology for phytotherapeutics. Curr Drug Discov Technol. 2013;10:224–32. doi: 10.2174/1570163811310030005. [DOI] [PubMed] [Google Scholar]
- 97.Ma X, Dang C, Kang H, Dai Z, Lin S, Guan H, et al. Saikosaponin-D reduces cisplatin-induced nephrotoxicity by repressing ROS-mediated activation of MAPK and NF-kappaB signalling pathways. Int Immunopharmacol. 2015;28:399–408. doi: 10.1016/j.intimp.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 98.Li W, Yan MH, Liu Y, Liu Z, Wang Z, Chen C, et al. Ginsenoside Rg5 ameliorates cisplatin-induced nephrotoxicity in mice through inhibition of inflammation, oxidative stress, and apoptosis. Nutrients. 2016;8:566. [DOI] [PMC free article] [PubMed]
- 99.Zhang W, Hou J, Yan X, Leng J, Li R, Zhang J, et al. Platycodon grandiflorum saponins ameliorate cisplatin-induced acute nephrotoxicity through the NF-kappaB-mediated inflammation and PI3K/Akt/apoptosis signaling pathways. Nutrients. 2018;10:1328. [DOI] [PMC free article] [PubMed]
- 100.Sherif IO. Amelioration of cisplatin-induced nephrotoxicity in rats by triterpenoid saponin of Terminalia arjuna. Clin Exp Nephrol. 2015;19:591–7. doi: 10.1007/s10157-014-1056-0. [DOI] [PubMed] [Google Scholar]
- 101.Han MS, Han IH, Lee D, An JM, Kim SN, Shin MS, et al. Beneficial effects of fermented black ginseng and its ginsenoside 20(S)-Rg3 against cisplatin-induced nephrotoxicity in LLC-PK1 cells. J Ginseng Res. 2016;40:135–40. doi: 10.1016/j.jgr.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xing JJ, Hou JG, Ma ZN, Wang Z, Ren S, Wang YP, et al. Ginsenoside Rb3 provides protective effects against cisplatin-induced nephrotoxicity via regulation of AMPK-/mTOR-mediated autophagy and inhibition of apoptosis in vitro and in vivo. Cell Prolif. 2019;52:e12627. doi: 10.1111/cpr.12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang Y, Tao X, Yin L, Xu L, Xu Y, Qi Y, et al. Protective effects of dioscin against cisplatin-induced nephrotoxicity via the microRNA-34a/sirtuin 1 signalling pathway. Br J Pharmacol. 2017;174:2512–27. doi: 10.1111/bph.13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kang HR, Lee D, Eom HJ, Lee SR, Lee KR, Kang KS, et al. Identification and mechanism of action of renoprotective constituents from peat moss Sphagnum palustre in cisplatin-induced nephrotoxicity. J Funct Foods. 2016;20:358–68. doi: 10.1016/j.jff.2015.11.010. [DOI] [Google Scholar]
- 105.Jung K, Lee D, Yu JS, Namgung H, Kang KS, Kim KH. Protective effect and mechanism of action of saponins isolated from the seeds of gac (Momordica cochinchinensis Spreng.) against cisplatin-induced damage in LLC-PK1 kidney cells. Bioorg Med Chem Lett. 2016;26:1466–70. doi: 10.1016/j.bmcl.2016.01.056. [DOI] [PubMed] [Google Scholar]
- 106.Adewale OO, Oduyemi OI, Ayokunle O. Oral administration of leaf extracts of Momordica charantia affect reproductive hormones of adult female Wistar rats. Asian Pac J Trop Biomed. 2014;4:S521–4. doi: 10.12980/APJTB.4.2014C939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tam PP, Law LK, Yeung HW. Effects of alpha-momorcharin on preimplantation development in the mouse. J Reprod Fertil. 1984;71:33–8. doi: 10.1530/jrf.0.0710033. [DOI] [PubMed] [Google Scholar]
- 108.Patel JC, Dhirawani MK, Doshi JC. “Karella” in the treatment of diabetes mellitus. Indian J Med Sci. 1968;22:30–2. [PubMed] [Google Scholar]
- 109.Feng L, Ke N, Cheng F, Guo Y, Li S, Li Q, et al. The protective mechanism of ligustrazine against renal ischemia/reperfusion injury. J Surg Res. 2011;166:298–305. doi: 10.1016/j.jss.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 110.Michel HE, Menze ET. Tetramethylpyrazine guards against cisplatin-induced nephrotoxicity in rats through inhibiting HMGB1/TLR4/NF-kappaB and activating Nrf2 and PPAR-gamma signaling pathways. Eur J Pharmacol. 2019;857:172422. doi: 10.1016/j.ejphar.2019.172422. [DOI] [PubMed] [Google Scholar]
- 111.Yadav YC, Srivastava DN. Nephroprotective and curative effects of Ficus religiosa latex extract against cisplatin-induced acute renal failure. Pharm Biol. 2013;51:1480–5. doi: 10.3109/13880209.2013.793718. [DOI] [PubMed] [Google Scholar]
- 112.Domitrovic R, Cvijanovic O, Pernjak-Pugel E, Skoda M, Mikelic L, Crncevic-Orlic Z. Berberine exerts nephroprotective effect against cisplatin-induced kidney damage through inhibition of oxidative/nitrosative stress, inflammation, autophagy and apoptosis. Food Chem Toxicol. 2013;62:397–406. doi: 10.1016/j.fct.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 113.Hagar H, Medany AE, Salam R, Medany GE, Nayal OA. Betaine supplementation mitigates cisplatin-induced nephrotoxicity by abrogation of oxidative/nitrosative stress and suppression of inflammation and apoptosis in rats. Exp Toxicol Pathol. 2015;67:133–41. doi: 10.1016/j.etp.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 114.Chan TY. Aconite poisoning. Clin Toxicol (Philos) 2009;47:279–85. doi: 10.1080/15563650902904407. [DOI] [PubMed] [Google Scholar]
- 115.Wu JJ, Guo ZZ, Zhu YF, Huang ZJ, Gong X, Li YH, et al. A systematic review of pharmacokinetic studies on herbal drug Fuzi: Implications for Fuzi as personalized medicine. Phytomedicine. 2018;44:187–203. doi: 10.1016/j.phymed.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 116.Stegelmeier BL, Colegate SM, Brown AW. Dehydropyrrolizidine alkaloid toxicity, cytotoxicity, and carcinogenicity. Toxins (Basel). 2016;8:356. [DOI] [PMC free article] [PubMed]
- 117.Chen Q, Peng HX, Dong L, Chen LJ, Ma XB, Peng YP, et al. Activation of the NRF2-ARE signalling pathway by the Lentinula edodes polysaccharose LNT alleviates ROS-mediated cisplatin nephrotoxicity. Int Immunopharmacol. 2016;36:1–8. doi: 10.1016/j.intimp.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 118.Rjeibi I, Feriani A, Ben Saad A, Sdayria J, Saidi I, Ncib S, et al. Lycium europaeum extract: a new potential antioxidant source against cisplatin-induced liver and kidney injuries in mice. Oxid Med Cell Longev. 2018;2018:1630751. doi: 10.1155/2018/1630751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rjeibi I, Feriani A, Ben Saad A, Ncib S, Sdayria J, Hfaiedh N, et al. Lycium europaeum Linn as a source of polysaccharide with in vitro antioxidant activities and in vivo anti-inflammatory and hepato-nephroprotective potentials. J Ethnopharmacol. 2018;225:116–27. doi: 10.1016/j.jep.2018.06.036. [DOI] [PubMed] [Google Scholar]
- 120.Liu Q, Song J, Li H, Dong L, Dai S. Schizandrin B inhibits the cisDDP-induced apoptosis of HK2 cells by activating ERK/NFkappaB signaling to regulate the expression of survivin. Int J Mol Med. 2018;41:2108–16. doi: 10.3892/ijmm.2018.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mundhe NA, Kumar P, Ahmed S, Jamdade V, Mundhe S, Lahkar M. Nordihydroguaiaretic acid ameliorates cisplatin induced nephrotoxicity and potentiates its anti-tumor activity in DMBA induced breast cancer in female Sprague-Dawley rats. Int Immunopharmacol. 2015;28:634–42. doi: 10.1016/j.intimp.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 122.Jin J, Li M, Zhao Z, Sun X, Li J, Wang W, et al. Protective effect of Wuzhi tablet (Schisandra sphenanthera extract) against cisplatin-induced nephrotoxicity via Nrf2-mediated defense response. Phytomedicine. 2015;22:528–35. doi: 10.1016/j.phymed.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 123.Biessels GJ, Vermeij FH, Leijten FS. [Epileptic seizure after a cup of tea: intoxication with Japanese star anise] Ned Tijdschr Geneeskd. 2002;146:808–11. [PubMed] [Google Scholar]
- 124.Potocnjak I, Domitrovic R. Carvacrol attenuates acute kidney injury induced by cisplatin through suppression of ERK and PI3K/Akt activation. Food Chem Toxicol. 2016;98:251–61. doi: 10.1016/j.fct.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 125.Zang H, Yang Q, Li J. Eleutheroside B Protects against acute kidney injury by activating IGF pathway. Molecules. 2019;24:3876. [DOI] [PMC free article] [PubMed]
- 126.Domitrovic R, Cvijanovic O, Susnic V, Katalinic N. Renoprotective mechanisms of chlorogenic acid in cisplatin-induced kidney injury. Toxicology. 2014;324:98–107. doi: 10.1016/j.tox.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 127.Gao L, Wu WF, Dong L, Ren GL, Li HD, Yang Q, et al. Protocatechuic aldehyde attenuates cisplatin-induced acute kidney injury by suppressing Nox-mediated oxidative stress and renal inflammation. Front Pharmacol. 2016;7:479. doi: 10.3389/fphar.2016.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Eren H, Aydin HR, Tumkaya L, Kazaz IO, Kalkan Y, Kazaz SN, et al. Whortleberry protects kidney against the cisplatin-induced nephrotoxicity: an experimental study. Ren Fail. 2018;40:466–74. doi: 10.1080/0886022X.2018.1500292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nho JH, Jung HK, Lee MJ, Jang JH, Sim MO, Jeong DE, et al. Beneficial effects of cynaroside on cisplatin-induced kidney injury in vitro and in vivo. Toxicol Res. 2018;34:133–41. doi: 10.5487/TR.2018.34.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang R, Yin L, Zhang B, Shi H, Sun Y, Ji C, et al. Resveratrol improves human umbilical cord-derived mesenchymal stem cells repair for cisplatin-induced acute kidney injury. Cell Death Dis. 2018;9:965. doi: 10.1038/s41419-018-0959-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kim JS, Kim KS, Son JY, Kim HR, Park JH, Lee SH, et al. Protective effects of Dendropanax morbifera against cisplatin-induced nephrotoxicity without altering chemotherapeutic efficacy. Antioxidants (Basel). 2019;8:256. [DOI] [PMC free article] [PubMed]
- 132.Dehnamaki F, Karimi A, Pilevarian AA, Fatemi I, Hakimizadeh E, Kaeidi A, et al. Treatment with troxerutin protects against cisplatin-induced kidney injury in mice. Acta Chir Belg. 2019;119:31–7. doi: 10.1080/00015458.2018.1455418. [DOI] [PubMed] [Google Scholar]
- 133.Zhou P, Wu M, Ye C, Xu Q, Wang L. Meclofenamic acid promotes cisplatin-induced acute kidney injury by inhibiting fat mass and obesity-associated protein-mediated m(6)A abrogation in RNA. J Biol Chem. 2019;294:16908–17. doi: 10.1074/jbc.RA119.011009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sen Z, Jie M, Jingzhi Y, Dongjie W, Dongming Z, Xiaoguang C. Total coumarins from Hydrangea paniculata protect against cisplatin-induced acute kidney damage in mice by suppressing renal inflammation and apoptosis. Evid Based Complement Altern Med. 2017;2017:5350161. doi: 10.1155/2017/5350161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang C, Guo Y, Huang TS, Zhao J, Huang XJ, Tang HX, et al. Asiatic acid protects against cisplatin-induced acute kidney injury via anti-apoptosis and anti-inflammation. Biomed Pharmacother. 2018;107:1354–62. doi: 10.1016/j.biopha.2018.08.126. [DOI] [PubMed] [Google Scholar]
- 136.Yu X, Meng X, Xu M, Zhang X, Zhang Y, Ding G, et al. Celastrol ameliorates cisplatin nephrotoxicity by inhibiting NF-kappaB and improving mitochondrial function. EBioMedicine. 2018;36:266–80. doi: 10.1016/j.ebiom.2018.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ansari MA. Sinapic acid modulates Nrf2/HO-1 signaling pathway in cisplatin-induced nephrotoxicity in rats. Biomed Pharmacother. 2017;93:646–53. doi: 10.1016/j.biopha.2017.06.085. [DOI] [PubMed] [Google Scholar]
- 138.Zhang L, Gu Y, Li H, Cao H, Liu B, Zhang H, et al. Daphnetin protects against cisplatin-induced nephrotoxicity by inhibiting inflammatory and oxidative response. Int Immunopharmacol. 2018;65:402–7. doi: 10.1016/j.intimp.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 139.Kandemir FM, Yildirim S, Caglayan C, Kucukler S, Eser G. Protective effects of zingerone on cisplatin-induced nephrotoxicity in female rats. Environ Sci Pollut Res Int. 2019;26:22562–74. doi: 10.1007/s11356-019-05505-3. [DOI] [PubMed] [Google Scholar]
- 140.Tilyek A, Chai C, Hou X, Zhou B, Zhang C, Cao Z, et al. The protective effects of Ribes diacanthum Pall on cisplatin-induced nephrotoxicity in mice. J Ethnopharmacol. 2016;178:297–306. doi: 10.1016/j.jep.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 141.Yarijani ZM, Godini A, Madani SH, Najafi H. Reduction of cisplatin-induced renal and hepatic side effects in rat through antioxidative and anti-inflammatory properties of Malva sylvestris L. extract. Biomedicine Pharmacother. 2018;106:1767–74. doi: 10.1016/j.biopha.2018.07.115. [DOI] [PubMed] [Google Scholar]
- 142.Gulec M, Iraz M, Yilmaz HR, Ozyurt H, Temel I. The effects of Ginkgo biloba extract on tissue adenosine deaminase, xanthine oxidase, myeloperoxidase, malondialdehyde, and nitric oxide in cisplatin-induced nephrotoxicity. Toxicol Ind Health. 2006;22:125–30. doi: 10.1191/0748233705th255oa. [DOI] [PubMed] [Google Scholar]
- 143.Karwasra R, Kalra P, Gupta YK, Saini D, Kumar A, Singh S. Antioxidant and anti-inflammatory potential of pomegranate rind extract to ameliorate cisplatin-induced acute kidney injury. Food Funct. 2016;7:3091–101. doi: 10.1039/C6FO00188B. [DOI] [PubMed] [Google Scholar]
- 144.Jamshidzadeh A, Heidari R, Golzar T, Derakhshanfar A. Effect of Eisenia foetida extract against cisplatin-induced kidney injury in rats. J Diet Suppl. 2016;13:551–9. doi: 10.3109/19390211.2015.1124163. [DOI] [PubMed] [Google Scholar]
- 145.Guo Y, Wang M, Mou J, Zhao Z, Yang J, Zhu F, et al. Pretreatment of Huaiqihuang extractum protects against cisplatin-induced nephrotoxicity. Sci Rep. 2018;8:7333. doi: 10.1038/s41598-018-25610-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhou L, Wei XH, Pan CS, Yan L, Gu YY, Sun K, et al. QiShenYiQi Pills, a compound Chinese medicine, prevented cisplatin induced acute kidney injury via regulating mitochondrial function. Front Physiol. 2017;8:1090. doi: 10.3389/fphys.2017.01090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bratkov VM, Shkondrov AM, Zdraveva PK, Krasteva IN. Flavonoids from the Genus Astragalus: phytochemistry and biological activity. Pharmacogn Rev. 2016;10:11–32. doi: 10.4103/0973-7847.176550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sarker SD, Nahar L. An introduction to natural products isolation. Methods Mol Biol. 2012;864:1–25. doi: 10.1007/978-1-61779-624-1_1. [DOI] [PubMed] [Google Scholar]
- 149.D’Orazio N, Gemello E, Gammone MA, de Girolamo M, Ficoneri C, Riccioni G. Fucoxantin: a treasure from the sea. Mar Drugs. 2012;10:604–16. doi: 10.3390/md10030604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shi MJ, McMillan KL, Wu JX, Gillings N, Flores B, Moe OW, et al. Cisplatin nephrotoxicity as a model of chronic kidney disease. Lab Invest. 2018;98:1105–21. doi: 10.1038/s41374-018-0063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sharp CN, Doll MA, Megyesi J, Oropilla GB, Beverly LJ, Siskind LJ. Subclinical kidney injury induced by repeated cisplatin administration results in progressive chronic kidney disease. Am J Physiol Ren Physiol. 2018;315:F161–72. doi: 10.1152/ajprenal.00636.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Landau SI, Guo X, Velazquez H, Torres R, Olson E, Garcia-Milian R, et al. Regulated necrosis and failed repair in cisplatin-induced chronic kidney disease. Kidney Int. 2019;95:797–814. doi: 10.1016/j.kint.2018.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mercantepe F, Mercantepe T, Topcu A, Yilmaz A, Tumkaya L. Protective effects of amifostine, curcumin, and melatonin against cisplatin-induced acute kidney injury. Naunyn Schmiedebergs Arch Pharmacol. 2018;391:915–31. doi: 10.1007/s00210-018-1514-4. [DOI] [PubMed] [Google Scholar]
- 154.Ortega-Dominguez B, Aparicio-Trejo OE, Garcia-Arroyo FE, Leon-Contreras JC, Tapia E, Molina-Jijon E, et al. Curcumin prevents cisplatin-induced renal alterations in mitochondrial bioenergetics and dynamic. Food Chem Toxicol. 2017;107:373–85. doi: 10.1016/j.fct.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 155.Topcu-Tarladacalisir Y, Sapmaz-Metin M, Karaca T. Curcumin counteracts cisplatin-induced nephrotoxicity by preventing renal tubular cell apoptosis. Ren Fail. 2016;38:1741–8. doi: 10.1080/0886022X.2016.1229996. [DOI] [PubMed] [Google Scholar]
- 156.Ugur S, Ulu R, Dogukan A, Gurel A, Yigit IP, Gozel N, et al. The renoprotective effect of curcumin in cisplatin-induced nephrotoxicity. Ren Fail. 2015;37:332–6. doi: 10.3109/0886022X.2014.986005. [DOI] [PubMed] [Google Scholar]
- 157.Soetikno V, Sari SDP, Ul Maknun L, Sumbung NK, Rahmi DNI, Pandhita BAW, et al. Pre-treatment with Curcumin ameliorates cisplatin-induced kidney damage by suppressing kidney inflammation and apoptosis in rats. Drug Res (Stuttg) 2019;69:75–82. doi: 10.1055/a-0641-5148. [DOI] [PubMed] [Google Scholar]
- 158.Sayin VI, Ibrahim MX, Larsson E, Nilsson JA, Lindahl P, Bergo MO. Antioxidants accelerate lung cancer progression in mice. Sci Transl Med. 2014;6:221ra15. doi: 10.1126/scitranslmed.3007653. [DOI] [PubMed] [Google Scholar]
- 159.Sawyer D, Conner CS, Scalley R. Cimetidine: adverse reactions and acute toxicity. Am J Hosp Pharm. 1981;38:188–97. [PubMed] [Google Scholar]
- 160.Tejedor A, Torres AM, Castilla M, Lazaro JA, de Lucas C, Caramelo C. Cilastatin protection against cyclosporin A-induced nephrotoxicity: clinical evidence. Curr Med Res Opin. 2007;23:505–13. doi: 10.1185/030079906X167633. [DOI] [PubMed] [Google Scholar]
- 161.Crona DJ, Faso A, Nishijima TF, McGraw KA, Galsky MD, Milowsky MI. A systematic review of strategies to prevent cisplatin-induced nephrotoxicity. Oncologist. 2017;22:609–19. doi: 10.1634/theoncologist.2016-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chtourou Y, Aouey B, Aroui S, Kebieche M, Fetoui H. Anti-apoptotic and anti-inflammatory effects of naringin on cisplatin-induced renal injury in the rat. Chem Biol Interact. 2016;243:1–9. doi: 10.1016/j.cbi.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 163.Amini N, Sarkaki A, Dianat M, Mard SA, Ahangarpour A, Badavi M. Protective effects of naringin and trimetazidine on remote effect of acute renal injury on oxidative stress and myocardial injury through Nrf-2 regulation. Pharmacol Rep. 2019;71:1059–66. doi: 10.1016/j.pharep.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 164.Pan H, Chen J, Shen K, Wang X, Wang P, Fu G, et al. Mitochondrial modulation by Epigallocatechin 3-Gallate ameliorates cisplatin induced renal injury through decreasing oxidative/nitrative stress, inflammation and NF-κB in mice. PLoS One. 2015;10:e0124775. doi: 10.1371/journal.pone.0124775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fatima S, Al-Mohaimeed N, Al-Shaikh Y, Tyagi P, Banu N, Hasan S, et al. Combined treatment of epigallocatechin gallate and coenzyme Q10 attenuates cisplatin-induced nephrotoxicity via suppression of oxidative/nitrosative stress, inflammation and cellular damage. Food Chem Toxicol. 2016;94:213–20. doi: 10.1016/j.fct.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 166.Singh MP, Chauhan AK, Kang SC. Morin hydrate ameliorates cisplatin-induced ER stress, inflammation and autophagy in HEK-293 cells and mice kidney via PARP-1 regulation. Int Immunopharmacol. 2018;56:156–67. doi: 10.1016/j.intimp.2018.01.031. [DOI] [PubMed] [Google Scholar]
- 167.Lin CF, Kuo YT, Chen TY, Chien CT. Quercetin-rich guava (Psidium guajava) juice in combination with trehalose reduces autophagy, apoptosis and pyroptosis formation in the kidney and pancreas of type II diabetic rats. Molecules. 2016;21:334. doi: 10.3390/molecules21030334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang C, Pan Y, Zhang QY, Wang FM, Kong LD. Quercetin and allopurinol ameliorate kidney injury in STZ-treated rats with regulation of renal NLRP3 inflammasome activation and lipid accumulation. PLoS One. 2012;7:e38285. doi: 10.1371/journal.pone.0038285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Al Humayed S, Al-Ani B, El Karib AO, Shatoor AS, Eid RA, Aziz S, et al. Suppression of acetaminophen-induced hepatocyte ultrastructural alterations in rats using a combination of resveratrol and quercetin. Ultrastruct Pathol. 2019;43:162–9. doi: 10.1080/01913123.2019.1680585. [DOI] [PubMed] [Google Scholar]
- 170.Milton Prabu S, Shagirtha K, Renugadevi J. Quercetin in combination with vitamins (C and E) improves oxidative stress and renal injury in cadmium intoxicated rats. Eur Rev Med Pharm Sci. 2010;14:903–14. [PubMed] [Google Scholar]
- 171.Impellizzeri D, Bruschetta G, Ahmad A, Crupi R, Siracusa R, Di Paola R, et al. Effects of palmitoylethanolamide and silymarin combination treatment in an animal model of kidney ischemia and reperfusion. Eur J Pharmacol. 2015;762:136–49. doi: 10.1016/j.ejphar.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 172.Meng XM, Li HD, Wu WF, Ming-Kuen Tang P, Ren GL, Gao L, et al. Wogonin protects against cisplatin-induced acute kidney injury by targeting RIPK1-mediated necroptosis. Lab Invest. 2018;98:79–94. doi: 10.1038/labinvest.2017.115. [DOI] [PubMed] [Google Scholar]
- 173.Shimizu T, Shibuya N, Narukawa Y, Oshima N, Hada N, Kiuchi F. Synergistic effect of baicalein, wogonin and oroxylin A mixture: multistep inhibition of the NF-kappaB signalling pathway contributes to an anti-inflammatory effect of Scutellaria root flavonoids. J Nat Med. 2018;72:181–91. doi: 10.1007/s11418-017-1129-y. [DOI] [PubMed] [Google Scholar]
- 174.Sahu BD, Mahesh Kumar J, Sistla R. Baicalein, a bioflavonoid, prevents cisplatin-induced acute kidney injury by up-regulating antioxidant defenses and down-regulating the MAPKs and NF-kappaB pathways. PLoS One. 2015;10:e0134139. doi: 10.1371/journal.pone.0134139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tang D, Chen K, Huang L, Li J. Pharmacokinetic properties and drug interactions of apigenin, a natural flavone. Expert Opin Drug Metab Toxicol. 2017;13:323–30. doi: 10.1080/17425255.2017.1251903. [DOI] [PubMed] [Google Scholar]
- 176.Vasaikar N, Mahajan U, Patil KR, Suchal K, Patil CR, Ojha S, et al. D-pinitol attenuates cisplatin-induced nephrotoxicity in rats: Impact on pro-inflammatory cytokines. Chem Biol Interact. 2018;290:6–11. doi: 10.1016/j.cbi.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 177.Sahu AK, Verma VK, Mutneja E, Malik S, Nag TC, Dinda AK, et al. Mangiferin attenuates cisplatin-induced acute kidney injury in rats mediating modulation of MAPK pathway. Mol Cell Biochem. 2019;452:141–52. doi: 10.1007/s11010-018-3420-y. [DOI] [PubMed] [Google Scholar]
- 178.Abd-Elhakim YM, Ghoneim MH, Ebraheim LLM, Imam TS. Taurine and hesperidin rescues carbon tetrachloride-triggered testicular and kidney damage in rats via modulating oxidative stress and inflammation. Life Sci. 2020;254:117782. doi: 10.1016/j.lfs.2020.117782. [DOI] [PubMed] [Google Scholar]
- 179.Chen X, Wei W, Li Y, Huang J, Ci X. Hesperetin relieves cisplatin-induced acute kidney injury by mitigating oxidative stress, inflammation and apoptosis. Chem Biol Interact. 2019;308:269–78. doi: 10.1016/j.cbi.2019.05.040. [DOI] [PubMed] [Google Scholar]
- 180.Tomar A, Vasisth S, Khan SI, Malik S, Nag TC, Arya DS, et al. Galangin ameliorates cisplatin induced nephrotoxicity in vivo by modulation of oxidative stress, apoptosis and inflammation through interplay of MAPK signaling cascade. Phytomedicine. 2017;34:154–61. doi: 10.1016/j.phymed.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 181.Abdel-Daim MM, Abdellatief SA. Attenuating effects of caffeic acid phenethyl ester and betaine on abamectin-induced hepatotoxicity and nephrotoxicity. Environ Sci Pollut Res Int. 2018;25:15909–17. doi: 10.1007/s11356-018-1786-8. [DOI] [PubMed] [Google Scholar]
- 182.Suliman FA, Khodeer DM, Ibrahiem A, Mehanna ET, El-Kherbetawy MK, Mohammad HMF, et al. Renoprotective effect of the isoflavonoid biochanin A against cisplatin induced acute kidney injury in mice: effect on inflammatory burden and p53 apoptosis. Int Immunopharmacol. 2018;61:8–19. doi: 10.1016/j.intimp.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 183.Alauddin ChaturvediS, Malik MY, Azmi L, Shukla I, Naseem Z, et al. Formononetin and biochanin A protects against ritonavir induced hepatotoxicity via modulation of NF kappa B/pAkt signaling molecules. Life Sci. 2018;213:174–82. doi: 10.1016/j.lfs.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 184.Yuan Y, Zha H, Rangarajan P, Ling EA, Wu C. Anti-inflammatory effects of Edaravone and Scutellarin in activated microglia in experimentally induced ischemia injury in rats and in BV-2 microglia. BMC Neurosci. 2014;15:125. doi: 10.1186/s12868-014-0125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Qi ZL, Wang Z, Li W, Hou JG, Liu Y, Li XD, et al. Nephroprotective effects of anthocyanin from the fruits of Panax ginseng (GFA) on cisplatin-induced acute kidney injury in mice. Phytother Res. 2017;31:1400–9. doi: 10.1002/ptr.5867. [DOI] [PubMed] [Google Scholar]
- 186.Gao S, Li L, Li L, Ni J, Guo R, Mao J, et al. Effects of the combination of tanshinone IIA and puerarin on cardiac function and inflammatory response in myocardial ischemia mice. J Mol Cell Cardiol. 2019;137:59–70. doi: 10.1016/j.yjmcc.2019.09.012. [DOI] [PubMed] [Google Scholar]
- 187.Gao T, Zhao P, Yu XL, Cao SX, Zhang B, Dai M. Use of Saikosaponin D and JNK inhibitor SP600125, alone or in combination, inhibits malignant properties of human osteosarcoma U2 cells. Am J Transl Res. 2019;11:2070–80. [PMC free article] [PubMed] [Google Scholar]
- 188.Zhao Q, Yang M, Deng YP, Yu HT, Wang LL, Teng FK, et al. The safety evaluation of salvianolic acid B and ginsenoside Rg1 combination on mice. Int J Mol Sci. 2015;16:29345–56. doi: 10.3390/ijms161226176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yokozawa T, Liu ZW. The role of ginsenoside-Rd in cisplatin-induced acute renal failure. Ren Fail. 2000;22:115–27. doi: 10.1081/JDI-100100858. [DOI] [PubMed] [Google Scholar]
- 190.Kim YJ, Lee MY, Son HY, Park BK, Ryu SY, Jung JY. Red ginseng ameliorates acute cisplatin-induced nephropathy. Planta Med. 2014;80:645–54. doi: 10.1055/s-0034-1368571. [DOI] [PubMed] [Google Scholar]
- 191.Wang Z, Li YF, Han XY, Sun YS, Zhang LX, Liu W, et al. Kidney protection effect of ginsenoside Re and its underlying mechanisms on cisplatin-induced kidney injury. Cell Physiol Biochem. 2018;48:2219–29. doi: 10.1159/000492562. [DOI] [PubMed] [Google Scholar]
- 192.Qi Z, Li Z, Li W, Liu Y, Wang C, Lin H, et al. Pseudoginsengenin DQ exhibits therapeutic effects in cisplatin-induced acute kidney injury via Sirt1/NF-kappaB and caspase signaling pathway without compromising its antitumor activity in mice. Molecules. 2018;23:3038. [DOI] [PMC free article] [PubMed]
- 193.Qi Z, Li W, Tan J, Wang C, Lin H, Zhou B, et al. Effect of ginsenoside Rh2 on renal apoptosis in cisplatin-induced nephrotoxicity in vivo. Phytomedicine. 2019;61:152862. doi: 10.1016/j.phymed.2019.152862. [DOI] [PubMed] [Google Scholar]
- 194.Liang X, Yang Y, Huang Z, Zhou J, Li Y, Zhong X. Panax notoginseng saponins mitigate cisplatin induced nephrotoxicity by inducing mitophagy via HIF-1alpha. Oncotarget. 2017;8:102989–3003. doi: 10.18632/oncotarget.19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tian Z, Pang H, Du S, Lu Y, Zhang L, Wu H, et al. Effect of Panax notoginseng saponins on the pharmacokinetics of aspirin in rats. J Chromatogr B Anal Technol Biomed Life Sci. 2017;1040:136–43. doi: 10.1016/j.jchromb.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 196.Ma ZN, Liu Z, Wang Z, Ren S, Tang S, Wang YP, et al. Supplementation of American ginseng berry extract mitigated cisplatin-evoked nephrotoxicity by suppressing ROS-mediated activation of MAPK and NF-kappaB signaling pathways. Food Chem Toxicol. 2017;110:62–73. doi: 10.1016/j.fct.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 197.Wang L, Meng Q, Wang C, Liu Q, Peng J, Huo X, et al. Dioscin restores the activity of the anticancer agent adriamycin in multidrug-resistant human leukemia K562/adriamycin cells by down-regulating MDR1 via a mechanism involving NF-kappaB signaling inhibition. J Nat Prod. 2013;76:909–14. doi: 10.1021/np400071c. [DOI] [PubMed] [Google Scholar]
- 198.Chen L, Liu T, Wang Q, Liu J. Anti-inflammatory effect of combined tetramethylpyrazine, resveratrol and curcumin in vivo. BMC Complement Alter Med. 2017;17:233. doi: 10.1186/s12906-017-1739-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhang B, Lu C, Bai M, He X, Tan Y, Bian Y, et al. Tetramethylpyrazine identified by a network pharmacology approach ameliorates methotrexate-induced oxidative organ injury. J Ethnopharmacol. 2015;175:638–47. doi: 10.1016/j.jep.2015.09.034. [DOI] [PubMed] [Google Scholar]
- 200.Hussien NR, Al-Kuraishy HM, Al-Gareeb AI. Berberine and Pentoxifylline: a novel combination in amelioration of acute kidney injury. J Pak Med Assoc. 2019;69(Suppl 3):S98–S7. [PubMed] [Google Scholar]
- 201.Chen XY, Zhang Y, Zhu ZN, Liu HL, Guo HC, Xiong C, et al. Protective effect of berberine on doxorubicin-induced acute hepatorenal toxicity in rats. Mol Med Rep. 2016;13:3953–60. doi: 10.3892/mmr.2016.5017. [DOI] [PubMed] [Google Scholar]
- 202.Sun M, Zhao W, Xie Q, Zhan Y, Wu B. Lentinan reduces tumor progression by enhancing gemcitabine chemotherapy in urothelial bladder cancer. Surg Oncol. 2015;24:28–34. doi: 10.1016/j.suronc.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 203.Chen W, Cheng X, Chen J, Yi X, Nie D, Sun X, et al. Lycium barbarum polysaccharides prevent memory and neurogenesis impairments in scopolamine-treated rats. PLoS One. 2014;9:e88076. doi: 10.1371/journal.pone.0088076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bunel V, Antoine MH, Nortier J, Duez P, Stevigny C. Protective effects of schizandrin and schizandrin B towards cisplatin nephrotoxicity in vitro. J Appl Toxicol. 2014;34:1311–9. doi: 10.1002/jat.2951. [DOI] [PubMed] [Google Scholar]
- 205.Li YJ, Liu HT, Xue CJ, Xing XQ, Dong ST, Wang LS, et al. The synergistic anti-tumor effect of schisandrin B and apatinib. J Asian Nat Prod Res. 2020;22:839–49. doi: 10.1080/10286020.2019.1645131. [DOI] [PubMed] [Google Scholar]
- 206.Lee DW, Kwak IS, Lee SB, Song SH, Seong EY, Yang BY, et al. Post-treatment effects of erythropoietin and nordihydroguaiaretic acid on recovery from cisplatin-induced acute renal failure in the rat. J Korean Med Sci. 2009;24(Suppl):S170–5. doi: 10.3346/jkms.2009.24.S1.S170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Li JL, Chen SY, Qin XL, Fu Q, Bi HC, Zhang Y, et al. Wuzhi tablet (Schisandra sphenanthera extract) is a promising tacrolimus-sparing agent for renal transplant recipients who are CYP3A5 expressers: a two-phase prospective study. Drug Metab Dispos. 2017;45:1114–9. doi: 10.1124/dmd.117.076737. [DOI] [PubMed] [Google Scholar]
- 208.Qu X, Gao H, Tao L, Zhang Y, Zhai J, Sun J, et al. Astragaloside IV protects against cisplatin-induced liver and kidney injury via autophagy-mediated inhibition of NLRP3 in rats. J Toxicol Sci. 2019;44:167–75. doi: 10.2131/jts.44.167. [DOI] [PubMed] [Google Scholar]
- 209.Bunel V, Antoine MH, Nortier J, Duez P, Stevigny C. Nephroprotective effects of ferulic acid, Z-ligustilide and E-ligustilide isolated from Angelica sinensis against cisplatin toxicity in vitro. Toxicol Vitr. 2015;29:458–67. doi: 10.1016/j.tiv.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 210.Masoumi F, Youssefi MR, Tabari MA. Combination of carvacrol and thymol against the poultry red mite (Dermanyssus gallinae) Parasitol Res. 2016;115:4239–43. doi: 10.1007/s00436-016-5201-4. [DOI] [PubMed] [Google Scholar]
- 211.Diamond BJ, Shiflett SC, Feiwel N, Matheis RJ, Noskin O, Richards JA, et al. Ginkgo biloba extract: mechanisms and clinical indications. Arch Phys Med Rehabil. 2000;81:668–78. doi: 10.1016/s0003-9993(00)90052-2. [DOI] [PubMed] [Google Scholar]
- 212.Lee IC, Ko JW, Park SH, Shin NR, Shin IS, Kim YB, et al. Ameliorative effects of pine bark extract on cisplatin-induced acute kidney injury in rats. Ren Fail. 2017;39:363–71. doi: 10.1080/0886022X.2017.1282871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Potocnjak I, Broznic D, Kindl M, Kropek M, Vladimir-Knezevic S, Domitrovic R. Stevia and stevioside protect against cisplatin nephrotoxicity through inhibition of ERK1/2, STAT3, and NF-kappa B activation. Food Chem Toxicol. 2017;107:215–25. doi: 10.1016/j.fct.2017.06.043. [DOI] [PubMed] [Google Scholar]
- 214.Geyikoglu F, Emir M, Colak S, Koc K, Turkez H, Bakir M, et al. Effect of oleuropein against chemotherapy drug-induced histological changes, oxidative stress, and DNA damages in rat kidney injury. J Food Drug Anal. 2017;25:447–59. doi: 10.1016/j.jfda.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Przychodzen P, Wyszkowska R, Gorzynik-Debicka M, Kostrzewa T, Kuban-Jankowska A, Gorska-Ponikowska M. Anticancer potential of oleuropein, the polyphenol of olive oil, with 2-methoxyestradiol, separately or in combination, in human osteosarcoma cells. Anticancer Res. 2019;39:1243–51. doi: 10.21873/anticanres.13234. [DOI] [PubMed] [Google Scholar]
- 216.Elseweidy MM, Askar ME, Elswefy SE, Shawky M. Vanillin as a new modulator candidate for renal injury induced by cisplatin in experimental rats. Cytokine. 2017;99:260–5. doi: 10.1016/j.cyto.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 217.Nasr S, Varshosaz J, Hajhashemi V. Ortho-vanillin nanoparticle-doped glucan microspheres exacerbate the anti-arthritic effects of methotrexate in adjuvant-induced arthritis in rats. Pharmacol Rep. 2020;72:680–91. doi: 10.1007/s43440-020-00099-x. [DOI] [PubMed] [Google Scholar]
- 218.Ounjaijean S, Somsak V. Combination of zingerone and dihydroartemisinin presented synergistic antimalarial activity against Plasmodium berghei infection in BALB/c mice as in vivo model. Parasitol Int. 2020;76:102088. [DOI] [PubMed]
- 219.Yan SL, Wang ZH, Mong MC, Yang YC, Yin MC. Combination of carnosine and asiatic acid provided greater anti-inflammatory protection for HUVE cells and diabetic mice than individual treatments of carnosine or asiatic acid alone. Food Chem Toxicol. 2019;126:192–8. doi: 10.1016/j.fct.2019.02.027. [DOI] [PubMed] [Google Scholar]
- 220.Yan YY, Bi H, Zhang W, Wen Q, Liu H, Li JX, et al. Downregulation and subcellular distribution of HER2 involved in MDA-MB-453 breast cancer cell apoptosis induced by lapatinib/celastrol combination. J Buon. 2017;22:644–51. [PubMed] [Google Scholar]
- 221.Zhang JQ, Yao ZT, Liang GK, Chen X, Wu HH, Jin L, et al. [Combination of lapatinib with chlorogenic acid inhibits breast cancer metastasis by suppressing macrophage M2 polarization] Zhejiang Da Xue Xue Bao Yi Xue Ban. 2015;44:493–9. doi: 10.3785/j.issn.1008-9292.2015.09.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Potocnjak I, Simic L, Vukelic I, Domitrovic R. Oleanolic acid attenuates cisplatin-induced nephrotoxicity in mice and chemosensitizes human cervical cancer cells to cisplatin cytotoxicity. Food Chem Toxicol. 2019;132:110676. doi: 10.1016/j.fct.2019.110676. [DOI] [PubMed] [Google Scholar]
- 223.Minich DM, Bland JS, Katke J, Darland G, Hall A, Lerman RH, et al. Clinical safety and efficacy of NG440: a novel combination of rho iso-alpha acids from hops, rosemary, and oleanolic acid for inflammatory conditions. Can J Physiol Pharmacol. 2007;85:872–83. doi: 10.1139/Y07-055. [DOI] [PubMed] [Google Scholar]
- 224.Khan SA, Priyamvada S, Khan W, Khan S, Farooq N, Yusufi AN. Studies on the protective effect of green tea against cisplatin induced nephrotoxicity. Pharmacol Res. 2009;60:382–91. doi: 10.1016/j.phrs.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 225.Sahu BD, Rentam KK, Putcha UK, Kuncha M, Vegi GM, Sistla R. Carnosic acid attenuates renal injury in an experimental model of rat cisplatin-induced nephrotoxicity. Food Chem Toxicol. 2011;49:3090–7. doi: 10.1016/j.fct.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 226.Shi B, Wang LF, Meng WS, Chen L, Meng ZL. Carnosic acid and fisetin combination therapy enhances inhibition of lung cancer through apoptosis induction. Int J Oncol. 2017;50:2123–35. doi: 10.3892/ijo.2017.3970. [DOI] [PubMed] [Google Scholar]
- 227.Liu H, Gu LB, Tu Y, Hu H, Huang YR, Sun W. Emodin ameliorates cisplatin-induced apoptosis of rat renal tubular cells in vitro by activating autophagy. Acta Pharmacol Sin. 2016;37:235–45. doi: 10.1038/aps.2015.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Chen Y, Gan D, Huang Q, Luo X, Lin D, Hu J. Emodin and its combination with cytarabine induce apoptosis in resistant acute myeloid leukemia cells in vitro and in vivo. Cell Physiol Biochem. 2018;48:2061–73. doi: 10.1159/000492544. [DOI] [PubMed] [Google Scholar]
- 229.Hegazy MG, Emam MA. Ethanolic extract of Trigonella Foenum Graecum attenuates cisplatin-induced nephro- and hepatotoxicities in rats. Cell Mol Biol (Noisy-le-Gd) 2015;61:81–7. [PubMed] [Google Scholar]
- 230.Pradeep SR, Barman S, Srinivasan K. Attenuation of diabetic nephropathy by dietary fenugreek (Trigonella foenum-graecum) seeds and onion (Allium cepa) via suppression of glucose transporters and renin-angiotensin system. Nutrition. 2019;67-68:110543. doi: 10.1016/j.nut.2019.06.024. [DOI] [PubMed] [Google Scholar]
- 231.Jiang L, Liu Y, He P, Chen J, Liu S, Tan N. Geraniin ameliorates cisplatin-induced nephrotoxicity in mice. Free Radic Res. 2016;50:813–9. doi: 10.3109/10715762.2016.1173206. [DOI] [PubMed] [Google Scholar]
- 232.Boakye-Gyasi E, Kasanga EA, Ameyaw EO, Abotsi WKM, Biney RP, Agyare C, et al. An isobolographic analysis of the anti-nociceptive effect of geraniin in combination with morphine or diclofenac. J Basic Clin Physiol Pharmacol. 2018;29:201–9. doi: 10.1515/jbcpp-2017-0031. [DOI] [PubMed] [Google Scholar]
- 233.Divya MK, Lincy L, Raghavamenon AC, Babu TD. Ameliorative effect of Apodytes dimidiata on cisplatin-induced nephrotoxicity in Wistar rats. Pharm Biol. 2016;54:2149–57. doi: 10.3109/13880209.2016.1149494. [DOI] [PubMed] [Google Scholar]
- 234.Mahmoodnia L, Mohammadi K, Masumi R. Ameliorative effect of lycopene effect on cisplatin-induced nephropathy in patient. J Nephropathol. 2017;6:144–9. doi: 10.15171/jnp.2017.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Bayomy NA, Elbakary RH, Ibrahim MAA, Abdelaziz EZ. Effect of lycopene and rosmarinic acid on gentamicin induced renal cortical oxidative stress, apoptosis, and autophagy in adult male albino rat. Anat Rec (Hoboken) 2017;300:1137–49. doi: 10.1002/ar.23525. [DOI] [PubMed] [Google Scholar]
- 236.Mahgoub E, Kumaraswamy SM, Kader KH, Venkataraman B, Ojha S, Adeghate E, et al. Genipin attenuates cisplatin-induced nephrotoxicity by counteracting oxidative stress, inflammation, and apoptosis. Biomed Pharmacother. 2017;93:1083–97. doi: 10.1016/j.biopha.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 237.Kim BR, Jeong YA, Jo MJ, Park SH, Na YJ, Kim JL, et al. Genipin enhances the therapeutic effects of oxaliplatin by upregulating BIM in colorectal cancer. Mol Cancer Ther. 2019;18:751–61. doi: 10.1158/1535-7163.MCT-18-0196. [DOI] [PubMed] [Google Scholar]
- 238.Huang H, Shen Z, Geng Q, Wu Z, Shi P, Miao X. Protective effect of Schisandra chinensis bee pollen extract on liver and kidney injury induced by cisplatin in rats. Biomed Pharmacother. 2017;95:1765–76. doi: 10.1016/j.biopha.2017.09.083. [DOI] [PubMed] [Google Scholar]
- 239.Li YZ, Ren S, Yan XT, Li HP, Li W, Zheng B, et al. Improvement of cisplatin-induced renal dysfunction by Schisandra chinensis stems via anti-inflammation and anti-apoptosis effects. J Ethnopharmacol. 2018;217:228–37. doi: 10.1016/j.jep.2018.01.033. [DOI] [PubMed] [Google Scholar]
- 240.Katanic J, Matic S, Pferschy-Wenzig EM, Kretschmer N, Boroja T, Mihailovic V, et al. Filipendula ulmaria extracts attenuate cisplatin-induced liver and kidney oxidative stress in rats: In vivo investigation and LC-MS analysis. Food Chem Toxicol. 2017;99:86–102. doi: 10.1016/j.fct.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 241.Hosseinian S, Khajavi Rad A, Hadjzadeh MA, Mohamadian Roshan N, Havakhah S, Shafiee S. The protective effect of Nigella sativa against cisplatin-induced nephrotoxicity in rats. Avicenna J Phytomed. 2016;6:44–54. [PMC free article] [PubMed] [Google Scholar]
- 242.Mohebbati R, Shafei MN, Beheshti F, Soukhtanloo M, Roshan NM, Anaeigoudari A, et al. Mixed hydroalcoholic extracts of Nigella sativa and Curcuma longa improves adriamycin-induced renal injury in rat. Saudi J Kidney Dis Transpl. 2017;28:1270–81. doi: 10.4103/1319-2442.220880. [DOI] [PubMed] [Google Scholar]
- 243.Farooqui Z, Ahmed F, Rizwan S, Shahid F, Khan AA, Khan F. Protective effect of Nigella sativa oil on cisplatin induced nephrotoxicity and oxidative damage in rat kidney. Biomed Pharmacother. 2017;85:7–15. doi: 10.1016/j.biopha.2016.11.110. [DOI] [PubMed] [Google Scholar]
- 244.Al-Okbi SY, Mohamed DA, Hamed TE, Edris AE, Fouda K. Hepatic regeneration and reno-protection by fish oil, Nigella sativa Oil and combined fish oil/Nigella sativa volatiles in CCl4 treated rats. J Oleo Sci. 2018;67:345–53. doi: 10.5650/jos.ess17204. [DOI] [PubMed] [Google Scholar]
- 245.Cao SS, Yan M, Hou ZY, Chen Y, Jiang YS, Fan XR, et al. Danshen modulates Nrf2-mediated signaling pathway in cisplatin-induced renal injury. J Huazhong Univ Sci Technol Med Sci. 2017;37:761–5. doi: 10.1007/s11596-017-1801-1. [DOI] [PubMed] [Google Scholar]
- 246.Guan Y, Wu XX, Duan JL, Yin Y, Guo C, Wei G, et al. Effects and mechanism of combination of rhein and danshensu in the treatment of chronic kidney disease. Am J Chin Med. 2015;43:1381–400. doi: 10.1142/S0192415X15500780. [DOI] [PubMed] [Google Scholar]
- 247.Parhizgar S, Hosseinian S, Hadjzadeh MA, Soukhtanloo M, Ebrahimzadeh A, Mohebbati R, et al. Renoprotective effect of Plantago major against nephrotoxicity and oxidative stress induced by cisplatin. Iran J Kidney Dis. 2016;10:182–8. [PubMed] [Google Scholar]
- 248.Parhizgar S, Hosseinian S, Soukhtanloo M, Bideskan AE, Hadjzadeh MA, Shahraki S, et al. Plantago major protects against cisplatin-induced renal dysfunction and tissue damage in rats. Saudi J Kidney Dis Transpl. 2018;29:1057–64. doi: 10.4103/1319-2442.243960. [DOI] [PubMed] [Google Scholar]
- 249.Adeyemi OO, Ishola IO, Ajani ID. Citrullus colocynthis Linn. fruit extract ameliorates cisplatin-induced hepato-renal toxicity in rats. J Complement Integr Med. 2017;15:jcim-2017-0086. [DOI] [PubMed]
- 250.Adeoye BO, Asenuga ER, Oyagbemi AA, Omobowale TO, Adedapo AA. The protective effect of the ethanol leaf extract of Andrographis paniculata on cisplatin-induced acute kidney injury in rats through nrf2/KIM-1 signalling pathway. Drug Res (Stuttg) 2018;68:23–32. doi: 10.1055/s-0043-118179. [DOI] [PubMed] [Google Scholar]
- 251.Adeoye BO, Oyagbemi AA, Asenuga ER, Omobowale TO, Adedapo AA. The ethanol leaf extract of Andrographis paniculata blunts acute renal failure in cisplatin-induced injury in rats through inhibition of Kim-1 and upregulation of Nrf2 pathway. J Basic Clin Physiol Pharmacol. 2018;30:205–17. doi: 10.1515/jbcpp-2017-0120. [DOI] [PubMed] [Google Scholar]
- 252.Kim IH, Kwon MJ, Jung JH, Nam TJ. Protein extracted from Porphyra yezoensis prevents cisplatin-induced nephrotoxicity by downregulating the MAPK and NF-kappaB pathways. Int J Mol Med. 2018;41:511–20. doi: 10.3892/ijmm.2017.3214. [DOI] [PubMed] [Google Scholar]
- 253.Arab HH, Mohamed WR, Barakat BM, Arafa el SA. Tangeretin attenuates cisplatin-induced renal injury in rats: Impact on the inflammatory cascade and oxidative perturbations. Chem Biol Interact. 2016;258:205–13. doi: 10.1016/j.cbi.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 254.Funaro A, Wu X, Song M, Zheng J, Guo S, Rakariyatham K, et al. Enhanced anti-inflammatory activities by the combination of luteolin and tangeretin. J Food Sci. 2016;81:H1320–7. doi: 10.1111/1750-3841.13300. [DOI] [PubMed] [Google Scholar]
- 255.Do Amaral CL, Francescato HD, Coimbra TM, Costa RS, Darin JD, Antunes LM, et al. Resveratrol attenuates cisplatin-induced nephrotoxicity in rats. Arch Toxicol. 2008;82:363–70. doi: 10.1007/s00204-007-0262-x. [DOI] [PubMed] [Google Scholar]
- 256.Xian Y, Lin Y, Cao C, Li L, Wang J, Niu J, et al. Protective effect of umbilical cord mesenchymal stem cells combined with resveratrol against renal podocyte damage in NOD mice. Diabetes Res Clin Pract. 2019;156:107755. doi: 10.1016/j.diabres.2019.05.034. [DOI] [PubMed] [Google Scholar]
- 257.Dallak M, Dawood AF, Haidara MA, Abdel Kader DH, Eid RA, Kamar SS, et al. Suppression of glomerular damage and apoptosis and biomarkers of acute kidney injury induced by acetaminophen toxicity using a combination of resveratrol and quercetin. Drug Chem Toxicol. 2020;1–7. [DOI] [PubMed]
- 258.Xu GY, Tang XJ. Troxerutin (TXN) potentiated 5-Fluorouracil (5-Fu) treatment of human gastric cancer through suppressing STAT3/NF-kappa B and Bcl-2 signaling pathways. Biomed Pharmacother. 2017;92:95–107. doi: 10.1016/j.biopha.2017.04.059. [DOI] [PubMed] [Google Scholar]
- 259.Sekine Y, Nakayama H, Miyazawa Y, Kato H, Furuya Y, Arai S, et al. Simvastatin in combination with meclofenamic acid inhibits the proliferation and migration of human prostate cancer PC-3 cells via an AKR1C3 mechanism. Oncol Lett. 2018;15:3167–72. doi: 10.3892/ol.2017.7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Tan RZ, Liu J, Zhang YY, Wang HL, Li JC, Liu YH, et al. Curcumin relieved cisplatin-induced kidney inflammation through inhibiting Mincle-maintained M1 macrophage phenotype. Phytomedicine. 2019;52:284–94. doi: 10.1016/j.phymed.2018.09.210. [DOI] [PubMed] [Google Scholar]
- 261.Karahan MA, Yalcin S, Aydogan H, Buyukfirat E, Kucuk A, Kocarslan S, et al. Curcumin and dexmedetomidine prevents oxidative stress and renal injury in hind limb ischemia/reperfusion injury in a rat model. Ren Fail. 2016;38:693–8. doi: 10.3109/0886022X.2016.1157746. [DOI] [PubMed] [Google Scholar]
- 262.Cao L, Zhi D, Han J, Kumar Sah S, Xie Y. Combinational effect of curcumin and metformin against gentamicin-induced nephrotoxicity: Involvement of antioxidative, anti-inflammatory and antiapoptotic pathway. J Food Biochem. 2019;43:e12836. doi: 10.1111/jfbc.12836. [DOI] [PubMed] [Google Scholar]