Abstract
The content of intramuscular fat (IMF), that determines marbling levels is considered as one of the vital factors influencing beef sensory quality including tenderness, juiciness, flavour and colour. The IMF formation in cattle commences around six months after conception, and continuously grows throughout the life of the animal. The accumulation of marbling is remarkably affected by genetic, sexual, nutritional and management factors. In this review, the adipogenesis and lipogenesis process regulated by various factors and genes during fetal and growing stages is briefly presented. We also discuss the findings of recent studies on the effects of breed, gene, heritability and gender on the marbling accumulation. Various research reported that feeding during pregnancy, concentrate to roughage ratios and the supplementation or restriction of vitamin A, C, and D are crucial nutritional factors affecting the formation and development of IMF. Castration and early weaning combined with high energy feeding are effective management strategies for improving the accumulation of IMF. Furthermore, age and weight at slaughter are also reviewed because they have significant effects on marbling levels. The combination of several factors could positively affect the improvement of the IMF deposition. Therefore, advanced strategies that simultaneously apply genetic, sexual, nutritional and management factors to achieve desired IMF content without detrimental impacts on feed efficiency in high-marbling beef production are essential.
Keywords: Beef cattle, Genetics, Nutrition, Animal management, Intramuscular fat, Marbling
1. Introduction
Intramuscular fat (IMF) is the amount of visible fat that located between different skeletal muscle fibers in a same cut (Hocquette et al., 2010). Marbling is defined as the appearance of visible white flecks or streaks of IMF, detected visually between the bundles of muscle (Lee et al., 2018) and often assessed by ultrasound imaging, or visually evaluated by trained assessors on the interface of skeletal muscle (Harris et al., 2018, Nguyen et al., 2017). The IMF content in meat, including in skeletal muscle tissues (longissimus lumborum, biceps femoris, infraspinatus supraspinatus and semitendinosus), is one of the vital factors influencing beef palatability and quality (Chen et al., 2019, Harris et al., 2018, Malau‐Aduli et al., 2000). The marbling has a positive correlation with meat sensory traits including juiciness, colour, tenderness and taste (Stewart et al., 2021, ). Moreover, higher polyunsaturated fatty acid (PUFA) contents in IMF compared to visceral and subcutaneous fat depots are beneficial for human health (Nguyen et al., 2018). Therefore, a number of studies were recently conducted in order to enhance marbling levels in ruminants (Le et al., 2018, Ladeira et al., 2018, Nguyen et al., 2017). In some markets such as Japan, Australia, and South Korea, marbling score (MS) is considered as one of the pivotal criteria in classifying the grade of beef quality (Chen et al., 2019, Holló et al., 2018, Ladeira et al., 2018,).
The marbling is mainly accumulated through IMF cell hyperplasia and hypertrophy throughout the life of cattle. Harris et al. (2018) stated that the IMF cell hyperplasia during the fetal and neonatal stages is vital as it provides the places for the hypertrophy of IMF cells in later periods. The IMF deposition of beef cattle is influenced complexly by numerous genetic, nutritional and management factors (Chen et al., 2019, Hocquette et al., 2010, Wang et al., 2009). Therefore, the understanding of the metabolic mechanisms of adipogenesis and lipogenesis influencing the IMF deposition is crucial to improve beef marbling levels.
This review has an objective to briefly present the process of adipogenesis and lipogenesis, and discusses several main factors that influence marbling levels in beef cattle. These factors can be classified into three groups: genetics (breed, gene and heritability), gender, nutrition (fetal nutritional programming, the ratio of concentrate to roughage, and vitamins) and management (castration, age at weaning, age and weight at slaughter).
2. The formation and development of adipose tissues in beef cattle
In the animal body, fat is accumulated in four locations including visceral fat, subcutaneous fat, intermuscular fat, and IMF through adipogenesis and lipogenesis. The differentiation and biosynthesis of adipocytes occur during both fetal and neonatal stages (Ladeira et al., 2018, Pethick et al., 2004). The formation of adipocytes in beef cattle begins around three months after conception when embryonic cells are differentiated from the mesenchyme. Du et al. (2013) reported that visceral adipocytes are formed first followed by subcutaneous, intermuscular, and intramuscular adipocytes. The first intramuscular adipocytes are formed at 180 days of pregnancy (Taga et al., 2011). The hyperplasia phase of IMF cells lasts till 250 days old whereas the hyperplasia phases of other fat cells may complete at the early postweaning period (intermuscular and subcutaneous adipocytes), or at the neonatal stage (visceral adipocytes) (Keogh et al., 2021). The chronological variation in adipogenesis creates a chance to stimulate IMF cell formation without an increase in the overall adipose accumulation in cattle, which is called the “marbling window” (Du et al., 2017, Du et al., 2013). During the marbling window period, mainly intramuscular adipogenesis is active, leading to better IMF deposition and marbling levels (Du et al., 2015). From 250 days old to slaughter, the role of hypertrophy in the IMF accumulation is more important than that of hyperplasia (Du et al., 2013). Park et al. (2018a) stated that intramuscular adipocytes may continue to grow during the late fattening stage whereas the growth of other fat cells may be slow or largely cease. Thus, the purpose of fattening animals with high marbling potential is to increase the sizes of intramuscular adipocytes deposited among skeletal muscle fibres to obtain the desired quality beef.
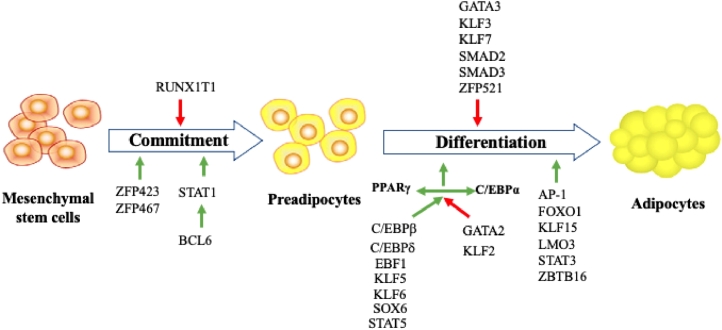
Adipogenesis is a complex and precisely orchestrated process including two separate stages: the commitment of mesenchymal stem cells (MSC) into preadipocytes and adipogenic differentiation (Du et al., 2015). This process is mediated by a number of adipogenic regulatory factors (Figure 1), in which cytidine-cytidine-adenosine-thymidine enhancer binding protein α (C/EBPα) and peroxisome proliferator-activated receptor γ (PPARγ), and zinc finger proteins are crucial determinants (Wei et al., 2019). In particular, zinc finger proteins (ZFP423 and ZFP467) principally promote the MSC commitment into preadipocytes, while RUNX1 partner transcriptional co-repressor 1 (RUNX1T1) inhibits the process. B-cell lymphoma 6 (BCL6) is reported to promote both the MSC commitment and the adipogenic differentiation by activating signal transducer and activator of transcription 1 (STAT1) downstream (Ambele et al. 2020). Additionally, PPARγ in cooperation with C/EBPα plays a crucial role in manipulating adipogenic differentiation because it is required for the activation of many adipogenic genes, such as C/EBPβ, C/EBPδ, early B-cell factor 1 (EBF1), Kruppel like factor 5 and 6 (KLF5 and KLF6), SRY-box transcription factor 6 (SOX6) and STAT5 (Urrutia et al., 2020; Ambele et al. 2020). In contrast, GATA-binding factor 2 (GATA2) and KLF2 inhibit PPARγ activation. Other transcription factors, including activating protein 1 (AP-1), forkhead box protein O1 (FOXO1), KLF15, LIM domain only 3 (LMO3), STAT3 and zinc finger and BTB domain containing 16 (ZBTB16) also promote the preadipocyte differentiation, while GATA3, KLF3, KLF7, SMAD family member 2 and 3 (SMAD2 and SMAD3) and ZFP521 restrict it (Ambele et al. 2020).
Figure 1.
The transition process from mesenchymal stem cells to adipocytes and transcriptional regulation of adipogenesis (adapted from Urrutia et al. (2020) and Ambele et al. (2020)). C/EBPα, C/EBPβ and C/EBPδ: cytidine-cytidine-adenosine-thymidine enhancer binding protein α, β, and δ; ZFP423, ZFP467 and ZFP521: zinc finger proteins 423, 467 and 521; RUNX1T1: RUNX1 partner transcriptional co-repressor 1; BCL6: B-cell lymphoma 6; STAT1 and STAT5: signal transducer and activator of transcription 1 and 5; PPARγ: peroxisome proliferator-activated receptor γ; EBF1: early B-cell factor 1; KLF2, KLF3, KLF5, KLF6, KLF7 and KLF15 Kruppel like factor 2, 3, 5, 6, 7 and 15; SOX6: SRY-box transcription factor 6; GATA2 and GATA3: GATA-binding factor 2 and 3; AP-1: activating protein 1; FOXO1: forkhead box protein O1; LMO3: LIM domain only 3; ZBTB16: zinc finger and BTB domain containing 16; SMAD2 and SMAD3: SMAD family member 2 and 3.
Lipogenesis is a physiological process of endogenous fatty acid (FA) synthesis and regulated by various factors (Ladeira et al., 2016). For the fat synthesis to take place, triglycerides must be incorporated into the animal's adipose tissue, after dietary FA uptake or de novo FA synthesis. Generally, the fat deposition rate is controlled by the animal's nutritional status. In ruminants, acetate and glucose are the main precursors for the FA biosynthesis. Subcutaneous adipocytes prefer acetate as lipogenic substrates whereas intramuscular adipocytes prefer glucose (May et al., 1995). However, Nayananjalie et al. (2015) and (Choi et al., 2015) found that acetate is the main precursor for lipid synthesis across fat depots.
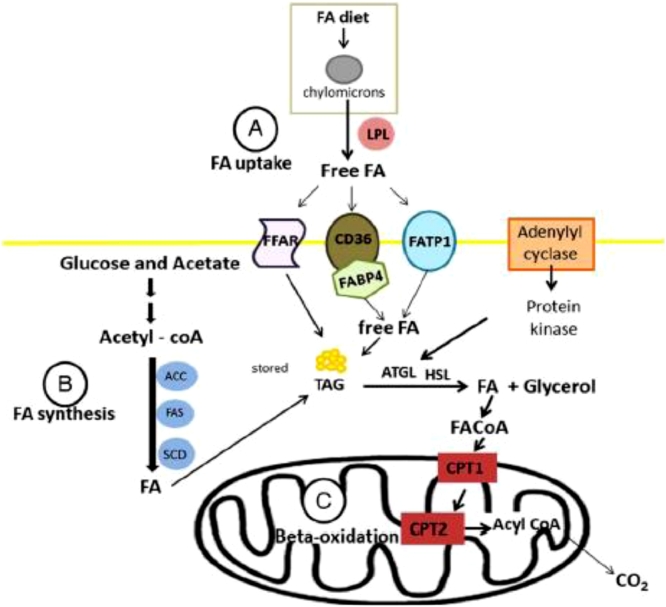
The FA uptake into the cell is facilitated by various associated proteins or fatty acid membrane transporters (Glatz et al., 2010). Fatty acids are transported into the cell by three groups of fatty acid transporters: fatty acid translocase (CD36), fatty acid transport protein 1 (FATP1) or fatty acid binding protein 4 (FABP4) in association with acyl-CoA synthase (Figure 2A). The de novo FA synthesis occurs by the action of the acetyl-CoA carboxylase, which is encoded by the acetyl-CoA carboxylase alpha (ACACA) gene and fatty acid synthase (FAS) (Ladeira et al., 2016). Following their synthesis or uptake by adipocytes, FA might be exposed to the action of the enzyme stearoyl-CoA desaturase (SCD) (Figure 2B) to insert double bounds in the chain. During lipolysis, FA need to be oxidized into the mitochondria via carnitine palmitoyl transferase enzyme (CPT1) (Bionaz et al., 2012). Inside the mitochondria, the long-chain acylcarnitine is converted back to long-chain acyl-CoA by CPT2, and then long-chain acyl-CoA enters β-oxidation pathway (Figure 2C). Therefore, changes in the balance between synthesis and degradation can influence the deposition of IMF. In other words, an increase in lipogenesis and FA uptake, and a decrease in lipolysis are associated with greater IMF deposition (Ladeira et al., 2018).
Figure 2.
Fatty acid (FA) uptake (A) synthesis (B) and oxidation (C) in ruminant adipose tissue (adapted from Ladeira et al. (2018)). LPL: lipoprotein lipase; ACC: acetyl-CoA carboxylase; FAS: fatty acid synthase, SCD: stearoyl-CoA desaturase; FFAR: free fatty acid receptors; CD36: fatty acid translocase; FATP1: fatty acid transport protein 1; FABP4: fatty acid binding protein 4; TAG: triacylglyceride; ATGL: adipose triglyceride lipase; HSL: hormone sensitive lipase; CPT: carnitine palmitoyltransferase.
As mentioned above, both hyperplasia and hypertrophy affect the deposition of IMF in beef cattle. Nutritional status during fetal, neonatal and early-postweaning stages influence the adipogenesis process and the number of adipocytes in adipose depots (Du et al., 2013). Therefore, maternal nutrition intervention during pregnancy is an effective approach to increase IMF biosynthesis in the fetus.
3. Genetic factors
3.1. Breed
In beef cattle, the IMF accumulation is considerably influenced by genetic backgrounds (Shahrai et al., 2021, Zhang et al., 2018, Wang et al., 2009, Pitchford et al., 2002, Malau‐Aduli et al., 2000). In Table 1, the longissimus muscle (LM) of Wagyu cattle contains the highest IMF content (31.8-37.8% from two publications of Irie et al. (2011) and Yamada et al. (2020). The second highest IMF content is observed in the LM of Hanwoo cattle (13.3-19.7%) (Beak et al., 2021, Choi et al., 2013). Bos taurus cattle generally have higher IMF contents than Bos indicus cattle (Teixeira et al., 2017, Flowers et al., 2018). For instance, the IMF content of Hereford (8.3%) (Greenwood et al., 2015) and Angus (6.5-7.5%) (Liu et al., 2021, Detweiler et al., 2019) is higher than that of Brahman and Nellore, which is mostly lower than 5% (Cesar et al. 2014, Miguel et al. 2011).
Table 1.
The intramuscular fat concentration of longissimus muscle in several cattle breeds
| Breed | Sex | No. of animals | Feeding system | Slaughter age (month) | Slaughter weight (kg) | IMF (%) | Reference |
|---|---|---|---|---|---|---|---|
| Wagyu | Steer | 51 | Feedlot | 30.7 | 717.7 | 31.8 | Yamada et al. (2020) |
| Wagyu | Heifer | 10 | Feedlot | 22 | 701 | 37.8 | Irie et al. (2011) |
| Wagyu crossbred | Steer | 167 | Feedlot | 25 | 731.4 | 27.8 | Connolly et al. (2020) |
| Wagyu×Angus | Mixed | 17 | Feedlot | NP | 576.5 | 10.0 | Liu et al. (2021) |
| Wagyu×Friesian | Heifer | 58 | Forage only | 26-30 | 483 | 7.5 | Burggraaf et al. (2020) |
| Hanwoo | Steer | 41 | Feedlot | 28.7 | 682 | 13.3 | Jeong et al. (2012) |
| Hanwoo | Steer | 20 | Feedlot | 31.7 | 752 | 15.3 | Choi et al. (2013) |
| Hanwoo | Steer | 43 | Feedlot | 34.2 | 425 | 19.7 | Beak et al. (2021) |
| Angus | Steer | 192 | Grazing-concentrate | 18 | 580 | 7.5 | Detweiler et al. (2019) |
| Angus | NP | 9 | Feedlot | NP | NP | 7.1 | Dinh et al. (2010) |
| Angus | Mixed | 12 | Feedlot | NP | 576.5 | 6.5 | Liu et al. (2021) |
| Angus | NP | 6 | Feedlot | 23 | NP | 3.5 | Martins et al. (2015) |
| Holstein-Friesian | Bull | 12 | Silage-concentrate | 24 | NP | 4.8 | Albrecht et al. (2006) |
| Hereford | Steer | 10 | Silage-concentrate | 27.5 | NP | 8.3 | Greenwood et al. (2015) |
| Hereford crossbred |
NP | 228 | Grazing-concentrate | 30 | 678 | 6.9 | Robinson et al. (2013) |
| Yunling | Bull | 20 | Grass-concentrate | 18 | 488 | 2.6 | Zhang et al. (2018) |
| Simmental | Bull | 20 | Grass-concentrate | 18 | 526 | 3.6 | Zhang et al. (2018) |
| Simmental | Bull | 46 | NP | 21 | 536 | 2.8 | Holló et al. (2018) |
| Nellore | Steer | 382 | Feedlot | 25 | NP | 2.8 | Cesar et al. (2014) |
| Nellore | NP | 6 | Feedlot | 23 | NP | 2.7 | Martins et al. (2015) |
| Brahman | Steer | 59 | Feedlot | 19 | 508 | 6.9 | Flowers et al. (2018) |
| Brahman | Steer | 50 | Grazing | 31 | 500 | 3.4 | Miguel et al. (2011) |
| Brahman | NP | 7 | Feedlot | NP | NP | 3.1 | Dinh et al. (2010) |
| Romosinuano | NP | 11 | Feedlot | NP | NP | 3.1 | Dinh et al. (2010) |
| Belgium Blue | Bull | 76 | Maize silage-concentrate | NP | 695 | 0.8 | De Smet et al. (2000) |
| Belgium Blue | Bull | 14 | Silage-concentrate | 24 | NP | 0.7 | Albrecht et al. (2006) |
IMF: intramuscular fat concentration; NP: not possible.
The IMF deposition differences between breeds may be caused by the differences in energy metabolism and FA synthesis in liver, and the expression of regulatory genes (Wang et al., 2017, Dinh et al., 2010). De Smet et al. (2004) suggested that the greater IMF content is associated with greater saturated fatty acid (SFA) and monounsaturated fatty acid (MUFA) contents whereas the PUFA content varied slightly. This possible genetic variation in FA metabolism is primarily due to the preferential incorporation of PUFA into phospholipids associated with cell membranes. In contrast, SFA and MUFA are included primarily in the triacylglycerol fraction, the main lipid fraction involved in tissue adiposity. When comparing IMF deposition in Wagyu and Angus skeletal muscle, Duarte et al. (2013) observed that the improvement of MS in Wagyu was associated with the upregulated mRNA expression of both early (ZFP423) and late adipogenic markers (PPARγ and C/EBPα). Similarly, Martins et al. (2015) observed that the greater levels PPARγ in the LM of Angus resulted in a higher IMF content compared to Nellore cattle. These findings indicate that enhanced adipogenesis was likely the driver of enhanced IMF content in skeletal muscle of high-marbled beef breed compared to low-marbled cattle.
3.2. Gene and heritability
For animals of the same breed, sex, similar age and fed the same diet, the content of IMF is regulated by different genes (Poleti et al., 2018). When fattening 20 Hanwoo cattle up to 30 months old and classifying them into high and low marbling groups, Shin & Chung (2016) reported that the triosephosphate isomerase 1 (TPI1) gene of animals from the group with high marbling was highly expressed, while animals from the low marbling group highly expressed troponin T type 1 (TNNT1), actin alpha 1 (ACTA1) and malate dehydrogenase 2 (MDH2) genes. Another study also demonstrated that the TPI1 gene was down-regulated in the high IMF group of Xiangxi×Angus crossbred cattle (Mao et al., 2016). Keady et al. (2013) found three genes, namely Enolase 3 (ENO3), pyruvate kinase M2 (PKM2), and glucose phosphate isomerase (GPI), in higher abundance in the LM of the Angus steer groups with high IMF content.
Wang et al. (2009) determined the correlation between genotype and fat in two groups of Wagyu×Hereford (WH) and Piedmontese×Hereford (PH) crossbred cattle and found that the LM intramuscular fat content of WH animals had stronger relationships with adiponectin, C1Q and collagen domain containing (ADIPOQ), FAS, SCD and thyroid hormone responsive SPOT14 (THRSP) genes compared with their PH counterparts. They also observed that the expression of C/EBPβ was relatively higher at 7 months of age, whereas PPARγ displayed a peak of expression at the period between 25 and 30 months old for WH animals. Poleti et al. (2018) found that two zinc finger proteins (zinc finger CCCH-type containing 6 (ZC3H6) and zinc finger GRF-type containing 1 (ZGRF1)) were identified in higher abundance in the Nellore male group with high IMF content. Chung et al. (2016) and Westbrook et al. (2021) reported that oleic acid stimulated the expression of G coupled protein receptor 43 (GPR43), which promotes the growth of IMF cells rather than other adipocytes.
The MS heritability of some beef cattle breeds has been estimated for a long period of time. By reviewing 72 studies (from 1962 to 2004), Utrera & Van Vleck (2004) found that the estimates of heritability of MS varied in a wide range (0.01-0.88). However, most of them were moderate, ranging from 0.30 to 0.57. Similarly, recent findings appeared to be consistent with the earlier studies, where MS heritability ranged from 0.34 to 0.68 (Table 2). Particularly, the MS heritability of Wagyu cattle was high ranging from 0.63 to 0.68 (Inoue et al., 2015, Takeda et al., 2017). The MS heritability of Hanwoo and Angus cattle ranged from 0.46 to 0.61 and from 0.35 to 0.61 respectively. Carvalho et al. (2019) and Moraes et al. (2019) showed that the estimated heritability for MS in Nellore cattle was from 0.34 and 0.47. The MS heritability of Brahman cattle ranged from 0.37 to 0.44 (Riley et al., 2002, Smith et al., 2007, Jeyaruban et al., 2017). When estimating the heritability for MS in Chikso cattle (a Korean brindle breed), Park et al. (2020a) reported that the MS heritability was 0.53.
Table 2.
The heritability of the marbling score in several cattle breeds
| Breed | No. of animals | Heritability | Reference |
|---|---|---|---|
| Wagyu | 3,128 | 0.68 | Takeda et al. (2017) |
| Wagyu | 5,788 | 0.63 | Inoue et al. (2015) |
| Hanwoo | 15,279 | 0.61 | Mehrban et al. (2021) |
| Hanwoo | 2,110 | 0.46 | Bedhane et al. (2019) |
| Hanwoo | 5,622 | 0.59 | Naserkheil et al. (2021) |
| Hanwoo | 9,952 | 0.56 | Park et al. (2020b) |
| Hanwoo | 6,092 | 0.56 | Alam et al. (2021) |
| Chikso | 2,377 | 0.53 | Park et al. (2020a) |
| Nellore | 896 | 0.34 | Carvalho et al. (2019) |
| Nellore | 940 | 0.47 | Moraes et al. (2019) |
| Red Angus | 15,260 | 0.35 | McAllister et al. (2011) |
| Red Angus | 142,146 | 0.40 | Boldt et al. (2018) |
| Australian Angus | 3,922 | 0.53 | Duff et al. (2021) |
| Australian Angus | 1,382 | 0.61 | Torres-Vázquez et al. (2018) |
| Australian Angus | 1,382 | 0.48 | Jeyaruban et al. (2017) |
| Brahman | 886 | 0.38 | Jeyaruban et al. (2017) |
| Brahman | 504 | 0.44 | Riley et al. (2002) |
| Brahman | 467 | 0.37 | Smith et al. (2007) |
An individual's genetic potential can also significantly affect its ability to accumulate IMF. Chen et al. (2019) stated that the marbling level was not uniform among individual animals in the same breed. Animals in the low IMF content potential group generally displayed lower marbling levels at the slaughter age than those in the high IMF content potential group (Black et al., 2015). The adipogenesis coincides with myogenesis during some stages of fetal development. Moreover, the animals with high marbling potential showed up-regulated the levels of mRNA associated with adipogenesis, and reduced mRNA levels linked to myogenesis. This explains why low MS was observed in cattle with enhanced muscle growth (Chen et al., 2019). Therefore, sire selection is very important for high-marbling beef production (Park et al., 2018a).
4. Gender
Gender is a major influence on the IMF contents of skeletal muscles in cattle. Generally, for a given slaughter weight, age and time on feed in the same breed, bulls have lower IMF contents than heifers (Harper & Pethick, 2004). Furthermore, Zhang et al. (2010) found that gender has effect on IMF content, FA profile and physiochemical properties of Qinchuan meat. These observations indicate that sex hormones may affect both hyperplasia and hypertrophy of intramuscular adipocytes in cattle. Picard et al. (2019) stated that lipid metabolism in the adipose tissue can be altered by controlling the sexual hormone status of cattle.
Testosterone and dihydrotestosterone regulate lineage determination in MSC by promoting their commitment to the myogenic lineage and inhibiting their differentiation into the adipogenic lineage through an androgen receptor-mediated pathway (Singh et al., 2003). Oh et al. (2005) suggested that testosterone inhibits differentiation of bovine intramuscular adipocytes by suppressing glycerol-3-phosphate dehydrogenase (GPDH) activity, a biomarker for adipogenesis. Testosterone also reduced adiponectin secretion, an adipose-specific secretory protein (Baharun et al., 2021).
On the other hand, 17β-estradiol and progesterone increase GPDH activity and reduce lipoprotein lipase (LPL) expression (Oh et al. 2005). Additionally, Lacasa et al. (2001) reported that progesterone upregulates the expression of sterol regulatory element-binding protein 1c (SREBP1c) gene which provides a potential mechanism for the lipogenic actions of progesterone on adipose tissue. The action of estrogens and their receptors, such as estrogen receptors α and β (ERα and ERβ), and G protein-coupled estrogen receptor 1 (GPER1), plays key roles in the regulation of energy metabolism pathways, including FA synthesis and β-oxidation (Xu & López, 2018). Estrogen is also through circulating adipokines as adiponectin and leptin which involve in fat deposition (Wyskida et al., 2017). Picard et al. (2019) reported that the differences in the abundance of triosephosphate isomerase 1 (TNNT1) between cows and steers could be the consequence of insulin sensitivity induced by estrogens. However, the regulatory mechanism of these hormones in beef cattle has not been fully studied and interpreted.
5. Nutritional factors
5.1. Fetal nutritional programming for marbling
Nutrient fluctuations during embryonic and fetal stages affect the development of the fetus, that permanently influences progeny's production performance and meat quality (Du et al., 2015). Other authors also indicated that both maternal under- and over-nutrition significantly affected intramuscular adipogenesis of offspring (Greenwood & Bell, 2019, Ladeira et al., 2018, Park et al., 2018a). The fetal stage is pivotal since it dictates how progenitor cells differentiate into muscle, fat or connective cells (Du et al., 2013).
All myocytes, fibroblasts and adipocytes stem from the same progenitor cell source during the early embryonic development (Du et al., 2015). According to Du et al. (2013), both endothelial and mesenchymal cells are initially differentiated from mesoderm-originated progenitors. Once formed, the MSC would differentiate into the precursors of fibroblasts and adipocytes. It may be considered that fibrogenesis and intramuscular adipogenesis is a competitive process. Thus, increasing adipogenic differentiation while decreasing fibrogenic differentiation from progenitor cells will increase both the tenderness and MS of beef (Du et al., 2017).
Numerous studies agreed that there is a positive correlation between the quantity of intramuscular adipocytes and the increased adipogenesis in fetal muscle (Yamada et al., 2020, Chen et al., 2019, Park et al., 2018a). Calves will form fewer intramuscular preadipocytes if they experience undernutrition conditions in late fetal and neonatal stages. This significantly restricts progeny's capability of marbling accumulation in later stages. Radunz et al. (2012) found that feeding pregnant dams with high-energy diets can promote fetal adipose tissue development resulting in long-term effects on progeny's IMF deposition. Some studies, therefore, concluded that enhancing intramuscular adipogenesis through good nutritional programming during pregnancy is a reasonable strategy for improving beef IMF contents (Du et al., 2015, Radunz et al., 2012).
In contrast, several studies concluded that the effects of fetal nutritional programming on progeny's marbling deposition are generally minor and blanketed by nutritional conditions during later stages (Ramírez et al., 2020, Greenwood & Bell, 2019, Robinson et al., 2013). Particularly, feeding dams with different nutrition levels at pasture from 80 days of pregnancy to parturition, Robinson et al. (2013) observed that there was no significant difference in MS among offspring. Ramírez et al. (2020) also concluded that restricting dietary energy levels of Angus dams (up to 50% of NRC requirement) during late gestation did not have significant impacts on the MS of castrated offspring.
Conflicting findings on the effects of fetal nutrition programming on the marbling level of progeny can be difficult to clearly interpret because they can be influenced by a variety of factors before and during pregnancy, and after parturition, including nutritional programming in fetal and postnatal stages and lactation performance (Greenwood & Bell, 2019). Moreover, high maternal nutrition during fetal and neonatal stages can lead to an overall improvement in progeny adiposity because it promotes the formation and development of adipocytes in all fat tissues (Du et al., 2017).
5.2. Concentrate to roughage ratios
Several studies concluded that beef cattle fed high-concentrate diets have higher IMF contents than those fed low concentrate diets (Khounsaknalath et al., 2021, Hwang & Joo, 2017, Pethick et al., 2004). Consuming excessive net energy is crucial for fat accumulation. Meanwhile, feeding cattle with concentrate (rice, barley, corn, cassava…) at high levels is one of the most popular strategies to increase net energy because these feed sources can be used in the digestive tract to create simple sugars and volatile fatty acids, that are a major source of net energy for ruminants. When finishing Angus crossbred steers, Duckett et al. (2013) observed that concentrate-finished steers had higher MS than their forage-finished counterparts. Moreover, Hwang & Joo (2017) suggested that high concentrate diets could increase the IMF content of Hanwoo steers. In Wagyu cattle, the IMF content of the LM in steers fed high concentrate diets from 4 to 10 months old and slaughtered at 31 months old, was higher than those fed only grass and slaughtered at the same period of time (Khounsaknalath et al., 2021).
Yamada & Nakanishi (2012) concluded that angiogenic growth factors expressed differently in fat depots and were affected by the concentrate to roughage ratio of fattening Wagyu steer diets. Particularly expression of adipogenic factors (C/EBPα, C/EBPβ and PPARγ) in both subcutaneous and intramuscular fat depots was lower in animals fed diets with low-concentrate levels than those fed diets of high-concentrate levels. They reported that because the differentiation of intramuscular preadipocytes in animals fed high-concentrate diets was stimulated, these animals might have higher MS than their counterparts fed low-concentrate diets. Additionally, the gene expression would vary depending on the adipose tissues and the duration of high-concentrate feeding (Li et al., 2018). These studies imply that the concentrate to roughage ratio has significant effects on the expression of adipogenic genes in fat depots.
On the contrary, there is a negative relationship between the dietary concentrate to forage ratio and the IMF content in Hanwoo cattle (Ku et al., 2021). Yang et al. (2020) also concluded that different whole-crop barley silage to concentrate ratio intakes during fattening periods did not cause any significant difference in MS of Hanwoo steers. Moreover, Teixeira et al. (2017) reported that bulls fed whole shelled corn without forage diets did not have higher IMF content in the LM compared to bulls fed diets containing ground corn and corn silage. They explained that extremely-high-concentrate diets reduced rumen pH and altered the microbiota and biohydrogenation pathway of the rumen, which increased the synthesis of trans10-cis12 conjugated linoleic acid (10,12 CLA) instead of cis9-trans11 conjugated linoleic acid. Various studies demonstrated that 10,12 CLA depresses the expression of SREBP1c and PPARγ genes (Teixeira et al., 2017, (Choi et al., 2015), Obsen et al., 2012). Consequently, the reducing expression of these genes has been responsible for reducing de novo FA synthesis by inhibiting the activity of SCD and FASN in bovine preadipocytes (Choi et al., 2015) while promoting the expression of genes associated with FA oxidation, such as CPT1 (Ladeira et al., 2018, Bionaz et al., 2012).
5.3. Vitamins
5.3.1. Vitamin A
Vitamin A (or known as retinol) is important for stimulating primary physiological functions and maintaining a healthy vision of cattle (Peng et al., 2021). Retinoic acid, a metabolite of vitamin A, plays major roles in both preadipocyte commitment and the differentiation of adipocytes (Wang et al., 2016). In an in vitro adipogenesis model using MSC, Dani et al. (1997) found that retinoic acid promoted adipogenic commitment of MSC. Consistently, retinoic acid treatment on MSC derived from embryoid bodies leads to prolonged activation of the extracellular signal-regulated kinase-1 (ERK) pathway, required for adipogenic commitment (Bost et al., 2002). Retinoic acid is also required for the expression of ZFP423 gene in adipocytes (Wang et al. 2016). However, numerous studies agreed that vitamin A is considered as a factor that restricts IMF deposition by reducing hyperplasia of adipocytes that still occur after 14 months of age or during the fattening period (Peng et al., 2019, Kruk et al., 2018, Pickworth et al., 2012b). Berry et al., (2012) observed that vitamin A influences the differentiation of adipocyte rather than affecting fat accumulation. This means that vitamin A specifically restricts IMF deposition, but it does not appear to affect subcutaneous fat deposition (Kruk et al., 2018, Berry et al., 2012, Oka et al. 1998). Ohyama et al. (1998) found that the inclusion of vitamin A reduced the number of preadipocytes and the activity of GPDH in Wagyu steers. Furthermore, retinoic acid hinders the differentiation of preadipocytes by inhibiting the expression of PPARγ (Mwangi et al., 2019) and C/EBPβ genes (Wang et al. 2016). Recent studies showed that vitamin A restriction during the fattening period increased the marbling levels of Angus steers (Knutson et al., 2020), and Wagyu cattle (Gotoh et al., 2018). However, other studies reported no significant effects of vitamin A restriction in Hanwoo steers (Peng et al., 2019) and Angus crossbred steers (Pickworth et al., 2012b).
The health and MS of the cattle are affected by the age at, or/and duration of restricting or supplementing vitamin A (Wang et al. 2016, Pickworth et al., 2012b). Wang et al. 2017 observed that maternal vitamin A enhances angiogenesis during fetal adipose tissue development in mice, which increases the density of adipogenic progenitor cells mediated by retinoic acid receptor signaling. Moreover, Harris et al., 2018 observed that calves treated 150,000 IU vitamin A had higher IMF content at harvest than untreated calves. This observation is because the injection of vitamin A to neonatal calves upregulated the expression of ZFP423, which resulted in an increased population of adipose progenitors. Thus, vitamin A administration during early development potentially provides beef cattle producers with an effective way to improve the efficiency of beef production and increase marbling levels (Harris et al., 2018).
On the contrary, Oka et al. (1998) reported that a vitamin A restriction in Wagyu diets from 15 months old to slaughter (29 months old) increased MS, but not in the duration from 23 months old to slaughter. They also suggested that dietary vitamin A is only restricted at the age of 14 to 23 months to improve MS. Kawachi (2006) demonstrated that there was an increase in the number of intramuscular adipocytes at the age of 13 to 19 months, so restricting dietary vitamin A during this period might promote MS. It is not recommended to restrict continuously vitamin A during the late fattening period because it may have adverse effects on animal health (arthritis, muscular edema, severe hepatic diseases and blindness) and reduce growth rates and carcass values (Gotoh et al., 2018, Oka et al., 1998). Currently, Japanese farmers usually fatten Wagyu cattle with concentrate-based diets from 10 to 30 months old, but they only restrict vitamin A during the middle fattening period (Gotoh et al., 2018). The level of vitamin A restriction is also an important factor to enhance MS and prevent health problems. Generally, animal nutritionists recommend that the concentration of dietary vitamin A varies from 3520 to15400 IU/kg for receiving stage, and 3300 to 7260 IU/kg for the fattening stage (Pyatt & Berger, 2005). Kawachi (2006) and Oka et al. (1998) suggested that the plasma retinol concentration in fattening cattle fed a vitamin A restricted diet should not be lower than 300 IU/l.
5.3.2. Vitamin C
Beef cattle normally do not receive the vitamin C (or L-ascorbic acid) supplementation in their diets because they can synthesize vitamin C in their liver (Mwangi et al., 2019, Park et al., 2018a). However, vitamin C plays an important role in collagen synthesis, oxidation-reduction reactions and infertility treatment (Ranjan et al., 2012). Vitamin C also helps to promote adipogenesis as a result of its positive effect on preadipocyte differentiation (Mwangi et al., 2019, Kawachi, 2006). In ruminant, dietary vitamin C supplements should be rumen-protected because the popular forms of vitamin C are degraded easily in the rumen (Ranjan et al., 2012). Jang et al. (2016) found that the MS of Hanwoo steers increased when supplementing saturated palm-oil coated vitamin C in their diets. Similarly, supplementing rumen-protected vitamin C has improved the MS of Wagyu steers (Kawachi, 2006) and Angus crossbred steers (Pogge & Hansen, 2013). In contrast, Pogge et al. (2015) concluded that the MS of Angus steers was not significantly affected by adding rumen-protected vitamin C. Therefore, more research is needed to determine appropriate levels and durations of dietary vitamin C supplementation in order to improve MS in beef cattle.
5.3.3. Vitamin D
Vitamin D, especially 1,25-dihydroxyvitamin D3 (an active form of vitamin D) is an important factor in maintaining calcium homeostasis and responds rapidly to calcium levels in diets (Kawachi, 2006). The impacts of vitamin D on the IMF deposition in ruminants have been reported by few studies. Mwangi et al. (2019) and Park et al. (2018a) stated that vitamin D restricts adipogenesis by inhibiting the differentiation of preadipocytes through the direct suppression of the adipogenic PPARγ gene. Feedlot cattle with low vitamin D3 levels had higher MS than those with higher vitamin D3 concentrations (Montgomery et al., 2004). In contrast, dietary vitamin D supplementation did not cause any significant difference in MS of short, black, British crossbred steers (Knobel-Graves et al., 2016), Angus crossbred steers (Pickworth et al., 2012a), Bos indicus crossbred cattle (Tipton et al., 2007), Angus, Brahman and their crossbred cattle (Reiling & Johnson, 2003).
6. Management factors
6.1. Castration
The castration of male calves generally may remarkably improve marbling levels in some cattle breeds. Castration profoundly increased IMF contents in Holstein calves (Marti et al., 2013), Hanwoo cattle (Bong et al., 2012), Chinese Qinchuan cattle (Zhang et al., 2017) and Nellore males (Anaruma et al., 2020). In fact, castration has been a popular practice in highly marbled Wagyu production in Japan (Gotoh et al., 2018). In South Korea, about 90.5% of Hanwoo bulls were castrated in 2015 (Chung et al., 2018). Bong et al. (2012) explained that castration can promote the IMF content because it can result in enhancing lipid uptake and lipogenesis, and reducing lipolysis. Park et al., (2018b) also reported that castration increased the IMF content, MS, and beef quality grade, and these changes were accompanied by the upregulation of the expression of PPARγ and FABP4 genes without changing that of fibrogenic genes.
Surgical castration, which removes both testicles, is a century-old technique for the control of male fertility, the improvement of carcass quality through increased fat deposition, reduced aggressive and sexual behaviours (Ahn et al., 2021, Mueller et al, 2019). However, this practice can be less effective on mass scale in stray animals because of vast area, limited resources, and animal welfare issues. Recently, alternatives to the traditional castration including hemi-castration (Ahn et al., 2021), immunocastration (vaccinated with gonadotropin-releasing hormone (GnRH)) (Mueller et al, 2019, Moreira et al., 2018) and chemical castration (treated with chemical agents such as NaCl, CaCl2, ethanol and lactic acid) (Oliveira et al., 2017) have been studied, and they have different effects on the IMF deposition of steers. The findings of Ahn et al. (2021) suggest that hemi-castration had positive effects on growth performance and meat yield traits than traditional castration due to the effect of testosterone secreted by one testis. However, hemi-castration lowers the MS as it inhibits the adipocyte differentiation and lipid synthesis. Mueller et al. (2019) and Moreira et al. (2018) suggested that immunocastration is an advantageous alternative to surgical castration because it is a welfare-friendly method, and improves fat deposition and meat quality. However, there is no found study investigating the effects of chemical castration on the IMF accumulation in cattle. This may be a knowledge gap, which needs to be filled in the future.
The castration timing may also have an effect on the marbling level. Several studies agreed that early castration increased MS, while late castration reduced MS (Chung et al., 2018, Marti et al., 2013). Nellore males castrated at weaning had higher MS than those castrated at 20 months old (Anaruma et al., 2020). Similarly, Marti et al. (2013) reported that there was a negative correlation between IMF content and age at castrating on Holstein cattle. However, Brown et al. (2015) observed no considerable difference in the MS of bull calves castrated near birth and at weaning. Hong et al. (2021) also concluded that the difference in castration age of Hanwoo bulls does not significantly affect MS. Additionally, early castration may have a negative effect on yield grade (Chung et al., 2018).
6.2. Age at weaning
In traditional cow-calf production systems, calves are weaned from their dams when reaching the age between 180 and 220 days. Early weaning is defined as removing offspring from their mothers prior to 180 days old, and it can be performed as early as 45 days after parturition (Rasby, 2007). Age at weaning is an important factor affecting the IMF deposition, and early weaning combined with a high-energy diet is considered as a promising method to improve beef marbling (Meyer et al., 2005, Reddy et al., 2017, Shoup et al., 2015). Particularly, Meyer et al. (2005) studied the effects of different weaning ages in Angus males and concluded that early-weaned steers (90 days of age) had better feed efficiency, higher average daily gain, hot carcass weight and fat percentage than traditionally weaned steers (174 days of age). In another Angus study, calves early weaned at 105 days old and fed a high-concentrate diet for 148 days increased hot carcass weight and MS compared with those weaned normally at 253 days old and grazed with their mothers on pasture (Scheffler et al., 2014). A study conducted on Angus and Angus×Simmental steers also observed that early weaning (141 days old) combined with a high-concentrate diet increased the IMF content compared to normal weaning (222 days old) combined with a roughage-based diet (Moisá et al., 2014). They explained that early weaning combined with a high-energy diet stimulated the differentiation of precocious preadipocytes and fat deposition by activating PPARγ and C/EBPα genes.
6.3. Age and weight at slaughter
The effects of different slaughter ages on the IMF content or MS have been evaluated in numerous cattle breeds, and most of them agreed that the IMF content increases with increasing age. The IMF content of the LM in Wagyu steers increased from 23.7% at 20 months old to 41.1% at 30 months old (Okumura et al., 2012). Greenwood et al. (2015) also observed increased IMF contents in Angus, Hereford, and Wagyu×Angus cattle when their slaughter age increased. Additionally, Holstein-Friesian bulls slaughtered 26 months of age had higher IMF content than those slaughtered at 20 months of age (Wang et al., 2019). A similar tendency was found in young Simmental bulls (Czyżak-Runowska et al., 2017) and Holstein calves (Marti et al., 2013). In research on the effect of different slaughter ages on the expression of adipogenic genes in Yanbian steers, Li et al. (2018) observed that acetyl-CoA carboxylase (ACC1), adipose tissue fatty acid-binding protein (FABP4), lipoprotein lipase (LPL), SREBP1, and SCD increasingly expressed, whereas FAS and PPARγ decreasingly expressed when the animals’ ages increased.
A number of studies agreed that the IMF content or MS has a positive relationship with slaughter weight in cattle breeds. In a review, Pethick et al. (2004) described that the IMF content increased when the hot carcass weight of Angus, Angus×Hereford and Wagyu×Holstein increased from 100 to 450 kg (Figure 3). A positive correlation between MS and slaughter weight in feedlot-finished Nellore heifers was observed (Rezende et al., 2019). Moreover, Nogalski et al. (2014) found that the IMF content of the LM in both Holstein×Limousin bulls and steers significantly increased when their slaughter weights increased from 450 to 600 kg. Similar results in Angus, Wagyu×Angus and Hereford cattle also were reported (Bruns et al., 2004, Greenwood et al., 2015).
Figure 3.
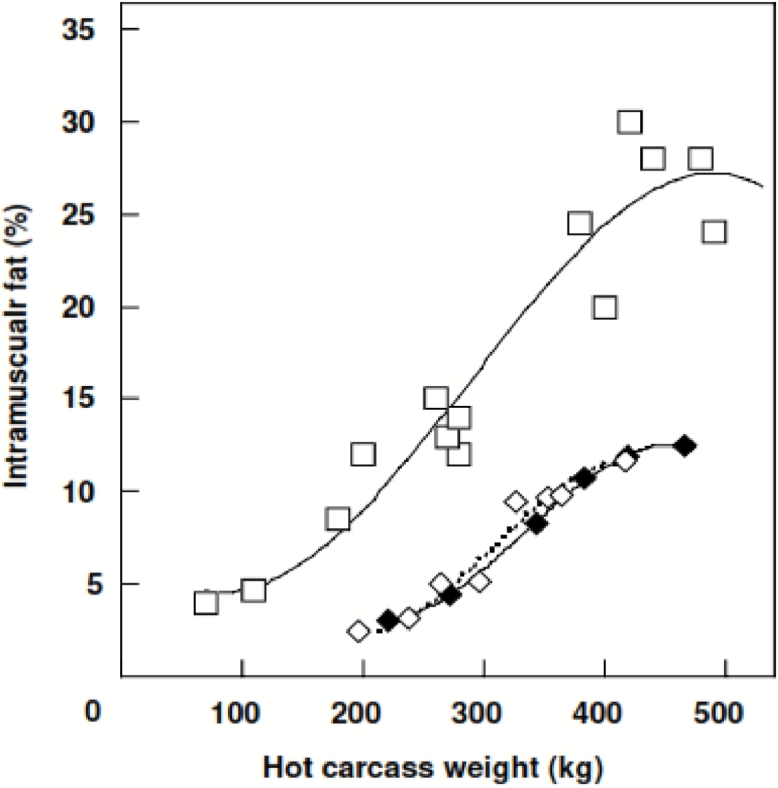
The correlation between hot carcass weight and intramuscular fat content of the Longissimus muscle of Angus (), Angus×Hereford () and Wagyu×Holstein callte () (adapted from Pethick et al. (2004))
The variation in the IMF content or MS between the different ages and weights at slaughter could be due to the impacts of numerous factors including genetics, nutrition and management. Pethick et al. (2004) stated that muscles need time to reach maturity, and extended fattening periods provide more time for the animal to achieve high levels of marbling. However, once animals reach certain slaughter ages and weights, the feed efficiency decreases because the muscle growth becomes slow (Agastin et al., 2013). Moreover, fattening animals for an extended period of time could lead to an increase in backfat thickness, which detrimentally influences yield grade (Park et al., 2018a). Gotoh et al. (2009) estimated that every 1% increase of the LM intramuscular fat content could relate to the increase of 3.0 kg of subcutaneous fat in Wagyu, 4.3 kg in Holstein-Friesian, 7.9 kg in Angus, and 10.7 kg in Belgian Blue. Thus, an extended fattening period is only recommended for animals with high genetic potential for marbling.
Apart from the above issues, a prolonged fattening period can lead to reduced carcass quality and economic efficiency because it increases the visceral and subcutaneous fat depositions, feed and labour costs, and the tenderness of the meat (Greenwood, 2021). High costs and inefficiencies exist in the current production systems for highly marbled Hanwoo (Chung et al., 2018) and Wagyu (Gotoh et al., 2018) beef. Greenwood (2021) reported that the Japanese Wagyu beef industry has to import 90% of the concentrate feed for fattening, and Wagyu cattle are fed a diet high in energy two or three times daily from 11 months old until slaughter at 28 to 30 months old.
Since the demand for higher MS increased, cattle are required to prolong longer feedlotting periods (100 to >350 days) (Greenwood, 2021). Chung et al. (2018) found that the average slaughtering age of Hanwoo steers in South Korea increased to 32.5 months in 2014 from 30.2 months in 2009. Motoyama et al. (2016) also reported that in Japan, Wagyu steers generally are slaughtered when they are 29 months old. Achievement of higher MS levels results in higher levels of inedible fat accumulation in the other depots and the associated costs and inefficiencies of feed use (Gotoh et al., 2018). Li et al. (2018) revealed that the IMF content rapidly enhanced between 12 and 28 months old, especially from 24 to 28 months old. Lee et al. (2013) observed that the profit of fattening Hanwoo steers was highest when the animals were slaughtered at 28 months of age, after that the profit decreased. As a result, slaughter age and weight together with carcass quality, feed and labour costs, and carcass prices are key criteria that need to be taken into account to obtain optimum production profits.
7. Conclusions
In this review, we briefly described the adipogenesis and lipogenesis processes in cattle and discussed the main factors (genetics, gender, nutrition and management) that affect the IMF deposition. The formation and development of adipocytes in cattle begin at the early embryonic developmental stage, and occur throughout the life of cattle. Bos taurus breeds generally have higher IMF content than Bos indicus cattle. The IMF accumulation is regulated by various associated genes, and the heritability for MS ranged from moderate to high. At the fetal and neonatal stages, nutrition and vitamin A supplementation mainly increase the number of intramuscular adipocytes by promoting preadipocyte commitment and the differentiation of adipocytes. During later growing stages, concentrate to roughage ratios greatly upregulate the hypertrophy of IMF cells. Dietary vitamin A and D restriction and vitamin C supplementation have positive effects on the IMF deposition. Castration and early weaning combined with high energy feeding are effective strategies for promoting marbling levels. Slaughter age and weight are positively correlated with the IMF content.
Some breeds with a high-marbling potential (Wagyu, Hanwoo) are often fattened with concentrate-based diets for a long period of time. A prolonged fattening period provides an opportunity to improve IMF deposition, but it results in a decrease in carcass quality and an increase in feed and management costs. Moreover, supplying mature animals with excessive energy diets during the late fattening stage can increase feed conversion ratio, the amount of inedible fat (visceral and subcutaneous) and feed residues which negatively affects the sustainability of beef production. The implementation of a single factor (e.g., genetic or nutritional) may be insufficient to improve the marbling level. Further research on developing new fusion technologies that simultaneously apply genetic, nutritional and management factors to improve feed efficiency and IMF content is essential.
Authors contributions
DVN conceptualised the layout, wrote the original draft, revised and finalised the manuscript. OCN and AEOM revised and finalised the manuscript. The final manuscript was approved by all authors.
Ethical considerations
I hereby, consciously certify that this submission fully follows the policies outlined in the Ethical Statement and the Guide for Authors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
No third-party support or funding was received during the writing and publication of the paper.
References
- Agastin A., Navès M., Farant A., Godard X., Bocage B., Alexandre G., et al. Effects of feeding system and slaughter age on the growth and carcass characteristics of tropical-breed steers. Journal of Animal Science. 2013;91:3997–4006. doi: 10.2527/jas.2012-5999. https://doi.org/10.2527/jas.2012-5999. [DOI] [PubMed] [Google Scholar]
- Ahn J.S., Kwon E.G., Lee H.J., Lee E.M., Hwang S.M., Cho S.R., et al. Effect of hemi-castration on the productivity, histological characteristics, and economic ffficacy of Korean beef cattle. Animals. 2021;11:2490. doi: 10.3390/ani11092490. https://doi.org/10.3390/ani11092490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M., Lee S.H., Lee D.H., Cho C., Park M.N. Genetic analysis of major carcass traits of Korean Hanwoo males raised for thirty months. Animals. 2021;11:1792. doi: 10.3390/ani11061792. https://doi.org/10.3390/ani11061792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht E., Teuscher F., Ender K., Wegner J. Growth-and breed-related changes of marbling characteristics in cattle. Journal of Animal Science. 2006;84:1067–1075. doi: 10.2527/2006.8451067x. https://doi.org/10.2527/2006.8451067x. [DOI] [PubMed] [Google Scholar]
- Ambele M.A., Dhanraj P., Giles R., Pepper M.S. Adipogenesis: a complex interplay of multiple molecular determinants and pathways. International Journal of Molecular Sciences. 2020;21:4283. doi: 10.3390/ijms21124283. https://doi.org/10.3390/ijms21124283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anaruma R., Reis L., De Felício P., Pflanzer S., Rossi S., Zanetti M., et al. Castration age and growth, meat production and meat quality of Nellore male cattle. Animal Production Science. 2020;60:725–731. https://doi.org/10.1071/AN18460. [Google Scholar]
- Baharun A., Said S., Arifiantini R.I., Karja N.W.K. Correlation between age, testosterone and adiponectin concentrations, and sperm abnormalities in Simmental bulls. Veterinary World. 2021;14(8):2124. doi: 10.14202/vetworld.2021.2124-2130. https://doi.org/10.14202/vetworld.2021.2124-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beak S.-H., Park S.J., Fassah D.M., Kim H.J., Kim M., Jo C., et al. Relationships among carcass traits, auction price, and image analysis traits of marbling characteristics in Korean cattle beef. Meat Science. 2021;171 doi: 10.1016/j.meatsci.2020.108268. https://doi.org/10.1016/j.meatsci.2020.108268. [DOI] [PubMed] [Google Scholar]
- Bedhane M., Van Der Werf J., Al Kalaldeh M., Lim D., Park B., Park M., et al. Assessment of genomic prediction accuracy for meat quality traits in Hanwoo cattle. Proceeding of Association for the Advancement of Animal Breeding and Genetics. 2019;23:278–281. [Google Scholar]
- Berry D.C., DeSantis D., Soltanian H., Croniger C.M., Noy N. Retinoic acid upregulates preadipocyte genes to block adipogenesis and suppress diet-induced obesity. Diabetes. 2012;61:1112–1121. doi: 10.2337/db11-1620. https://doi.org/10.2337/db11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bionaz M., Thering B.J., Loor J.J. Fine metabolic regulation in ruminants via nutrient–gene interactions: saturated long-chain fatty acids increase expression of genes involved in lipid metabolism and immune response partly through PPAR-α activation. British Journal of Nutrition. 2012;107:179–191. doi: 10.1017/S0007114511002777. https://doi.org/10.1017/S0007114511002777. [DOI] [PubMed] [Google Scholar]
- Black D., Neville B., Crosswhite M., Dahlen C. Evaluation of implant strategies in Angus-sired steers with high or low genetic potential for marbling and gain. Journal of Animal Science. 2015;93:5411–5418. doi: 10.2527/jas.2015-9296. https://doi.org/10.2527/jas.2015-9296. [DOI] [PubMed] [Google Scholar]
- Boldt R.J., Speidel S.E., Thomas M.G., Enns R.M. Genetic parameters for fertility and production traits in Red Angus cattle. Journal of Animal Science. 2018;96:4100–4111. doi: 10.1093/jas/sky294. https://doi.org/10.1093/jas/sky294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bong J.J., Jeong J.Y., Rajasekar P., Cho Y.M., Kwon E.G., Kim H.C., et al. Differential expression of genes associated with lipid metabolism in longissimus dorsi of Korean bulls and steers. Meat Science. 2012;91:284–293. doi: 10.1016/j.meatsci.2012.02.004. https://doi.org/10.1016/j.meatsci.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Bost F., Caron L., Marchetti I., Dani C., Marchand-Brustel Y.L., et al. Retinoic acid activation of the ERK pathway is required for embryonic stem cell commitment into the adipocyte lineage. Biochemical Journal. 2002;361:621–627. doi: 10.1042/0264-6021:3610621. https://doi.org/10.1042/bj3610621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A., Powell J., Kegley E., Gadberry M., Reynolds J., Hughes H., et al. Effect of castration timing and oral meloxicam administration on growth performance, inflammation, behavior, and carcass quality of beef calves. Journal of Animal Science. 2015;93:2460–2470. doi: 10.2527/jas.2014-8695. https://doi.org/10.2527/jas.2014-8695. [DOI] [PubMed] [Google Scholar]
- Bruns K., Pritchard R., Boggs D. The relationships among body weight, body composition, and intramuscular fat content in steers. Journal of Animal Science. 2004;82:1315–1322. doi: 10.2527/2004.8251315x. https://doi.org/10.2527/2004.8251315x. [DOI] [PubMed] [Google Scholar]
- Burggraaf V.T., Craigie C.R., Khan M.A., Muir P.D., Thomson B.C., Lowe K.A., et al. Effect of feeding forage or concentrate starter diets in early life on life-time growth, carcass traits and meat quality of Wagyu × Friesian cattle. Animal Production Science. 2020;60:1850–1860. https://doi.org/10.1071/AN19486. [Google Scholar]
- Carvalho M., Baldi F., Alexandre P., Santana M., Ventura R., Bueno R., et al. Genomic regions and genes associated with carcass quality in Nelore cattle. Genetics and Molecular Research. 2019;18:1–15. https://doi.org/10.4238/gmr18226. [Google Scholar]
- Cesar A.S., Regitano L.C., Mourão G.B., Tullio R.R., Lanna D.P., Nassu R.T., et al. Genome-wide association study for intramuscular fat deposition and composition in Nellore cattle. BMC genetics. 2014;15:1–15. doi: 10.1186/1471-2156-15-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Li W., Du M., Cao B. Adipogenesis, fibrogenesis and myogenesis related gene expression in longissimus muscle of high and low marbling beef cattle. Livestock Science. 2019;229:188–193. https://doi.org/10.1016/j.livsci.2019.09.032. [Google Scholar]
- Choi C., Jung K., Chung K., Yang B., Chin K., Suh S., et al. Administration of zilpaterol hydrochloride alters feedlot performance, carcass characteristics, muscle, and fat profiling in finishing Hanwoo steers. Livestock Science. 2013;157:435–441. http://dx.doi.org/10.1016/j.livsci.2013.06.035. [Google Scholar]
- Choi S.H., Park S.K., Johnson B.J., Chung K.Y., Choi C.W., Kim K.H., et al. AMPKα, C/EBPβ, CPT1β, GPR43, PPARγ, and SCD gene expression in single-and co-cultured bovine satellite cells and intramuscular preadipocytes treated with palmitic, stearic, oleic, and linoleic acid. Asian-Australasian Journal of Animal Sciences. 2015;28:411. doi: 10.5713/ajas.14.0598. https://doi.org/10.5713/ajas.14.0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K., Smith S., Choi S., Johnson B. Oleic acid enhances G protein coupled receptor 43 expression in bovine intramuscular adipocytes but not in subcutaneous adipocytes. Journal of Animal Science. 2016;94:1875–1883. doi: 10.2527/jas.2015-0010. https://doi.org/10.2527/jas.2015-0010. [DOI] [PubMed] [Google Scholar]
- Chung K.Y., Lee S.H., Cho S.H., Kwon E.G., Lee J.H. Current situation and future prospects for beef production in South Korea—A review. Asian-Australasian Journal of Animal Sciences. 2018;31:951. doi: 10.5713/ajas.18.0187. https://doi.org/10.5713/ajas.18.0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly S., Dona A., Hamblin D., Michael J., González L.A. Changes in the blood metabolome of Wagyu crossbred steers with time in the feedlot and relationships with marbling. Scientific Reports. 2020;10:1–11. doi: 10.1038/s41598-020-76101-6. https://doi.org/10.1038/s41598-020-76101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czyżak-Runowska G., Grześ B., Pospiech E., Komisarek J., Okulicz M., Stanisławski D., et al. Meat quality of Limousin young bulls slaughtered at 6, 9 and 12 months of age. Emirates Journal of Food and Agriculture. 2017:792–798. https://doi.org/10.9755/ejfa.2017.v29.i10.1297. [Google Scholar]
- Dani C., Smith A.G., Dessolin S., Leroy P., Staccini L., Villageois P., et al. Differentiation of embryonic stem cells into adipocytes in vitro. Journal of Cell Science. 1997;110:1279–1285. doi: 10.1242/jcs.110.11.1279. https://doi.org/10.1242/jcs.110.11.1279. [DOI] [PubMed] [Google Scholar]
- De Smet S., Raes K., Demeyer D. Meat fatty acid composition as affected by fatness and genetic factors: a review. Animal Research. 2004;53:81–98. https://doi.org/10.1051/animres:2004003. [Google Scholar]
- De Smet S., Webb E., Claeys E., Uytterhaegen L., Demeyer D. Effect of dietary energy and protein levels on fatty acid composition of intramuscular fat in double-muscled Belgian Blue bulls. Meat Science. 2000;56:73–79. doi: 10.1016/s0309-1740(00)00023-1. https://doi.org/10.1016/S0309-1740(00)00023-1. [DOI] [PubMed] [Google Scholar]
- Detweiler R.A., Pringle T.D., Rekaya R., Wells J.B., Segers J.R. The impact of selection using residual average daily gain and marbling EPDs on growth, performance, and carcass traits in Angus steers. Journal of Animal Science. 2019;97:2450–2459. doi: 10.1093/jas/skz124. https://doi.org/10.1093/jas/skz124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh T.T.N., Jr Blanton, J R., Riley D.G., Jr Chase, C C., Coleman S.W., Phillips W.A., et al. Intramuscular fat and fatty acid composition of longissimus muscle from divergent pure breeds of cattle. Journal of Animal Science. 2010;88:756–766. doi: 10.2527/jas.2009-1951. https://doi.org/10.2527/jas.2009-1951. [DOI] [PubMed] [Google Scholar]
- Du M., Ford S.P., Zhu M.-J. Optimizing livestock production efficiency through maternal nutritional management and fetal developmental programming. Animal Frontiers. 2017;7:5–11. https://doi.org/10.2527/af.2017-0122. [Google Scholar]
- Du M., Huang Y., Das A., Yang Q., Duarte M., Dodson M., et al. Manipulating mesenchymal progenitor cell differentiation to optimize performance and carcass value of beef cattle. Journal of Animal Science. 2013;91:1419–1427. doi: 10.2527/jas.2012-5670. https://doi.org/10.2527/jas.2012-5670. [DOI] [PubMed] [Google Scholar]
- Du M., Wang B., Fu X., Yang Q., Zhu M.-J. Fetal programming in meat production. Meat Science. 2015;109:40–47. doi: 10.1016/j.meatsci.2015.04.010. https://doi.org/10.1016/j.meatsci.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Duarte M.S., Paulino P.V.R., Das A.K., Wei S., Serao N.V.L., Fu X., et al. Enhancement of adipogenesis and fibrogenesis in skeletal muscle of Wagyu compared with Angus cattle. Journal of Animal Science. 2013;91:2938–2946. doi: 10.2527/jas.2012-5892. https://doi.org/10.2527/jas.2012-5892. [DOI] [PubMed] [Google Scholar]
- Duckett S., Neel J., Lewis R.M., Fontenot J., Clapham W. Effects of forage species or concentrate finishing on animal performance, carcass and meat quality. Journal of Animal Science. 2013;91:1454–1467. doi: 10.2527/jas.2012-5914. https://doi.org/10.2527/jas.2012-5914. [DOI] [PubMed] [Google Scholar]
- Duff C.J., Van Der Werf J.H.J., Parnell P.F., Clark S.A. Redefining residual feed intake to account for marbling fat in beef breeding programs. Animal Production Science. 2021 -. https://doi.org/10.1071/AN21107. [Google Scholar]
- Flowers S., Hamblen H., Leal-Gutiérrez J.D., Elzo M.A., Johnson D.D., Mateescu R.G. Fatty acid profile, mineral content, and palatability of beef from a multibreed Angus–Brahman population. Journal of Animal Science. 2018;96:4264–4275. doi: 10.1093/jas/sky300. https://doi.org/10.1093/jas/sky300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatz J.F., Luiken J.J., Bonen A. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiological Reviews. 2010;90:367–417. doi: 10.1152/physrev.00003.2009. https://doi.org/10.1152/physrev.00003.2009. [DOI] [PubMed] [Google Scholar]
- Gotoh T., Albrecht E., Teuscher F., Kawabata K., Sakashita K., Iwamoto H., et al. Differences in muscle and fat accretion in Japanese Black and European cattle. Meat Science. 2009;82:300–308. doi: 10.1016/j.meatsci.2009.01.026. https://doi.org/10.1016/j.meatsci.2009.01.026. [DOI] [PubMed] [Google Scholar]
- Gotoh T., Nishimura T., Kuchida K., Mannen H. The Japanese Wagyu beef industry: current situation and future prospects—a review. Asian-Australasian Journal of Animal Sciences. 2018;31:933. doi: 10.5713/ajas.18.0333. https://doi.org/10.5713/ajas.18.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood P.L. An overview of beef production from pasture and feedlot globally, as demand for beef and the need for sustainable practices increase. Animal. 2021 doi: 10.1016/j.animal.2021.100295. https://doi.org/10.1016/j.animal.2021.100295. [DOI] [PubMed] [Google Scholar]
- Greenwood P.L., Bell A.W. Developmental programming and growth of livestock tissues for meat production. Veterinary Clinics: Food Animal Practice. 2019;35:303–319. doi: 10.1016/j.cvfa.2019.02.008. https://doi.org/10.1016/j.cvfa.2019.02.008. [DOI] [PubMed] [Google Scholar]
- Greenwood P.L., Siddell J., Walmsley B., Geesink G., Pethick D., Mcphee M. Postweaning substitution of grazed forage with a high-energy concentrate has variable long-term effects on subcutaneous fat and marbling in Bos taurus genotypes. Journal of Animal Science. 2015;93:4132–4143. doi: 10.2527/jas.2015-8962. https://doi.org/10.2527/jas.2015-8962. [DOI] [PubMed] [Google Scholar]
- Harper G., Pethick D. How might marbling begin? Australian Journal of Experimental Agriculture. 2004;44:653–662. https://doi.org/10.1071/EA02114. [Google Scholar]
- Harris C.L., Wang B., Deavila J.M., Busboom J.R., Maquivar M., Parish S.M., et al. Vitamin A administration at birth promotes calf growth and intramuscular fat development in Angus beef cattle. Journal of Animal Science and Biotechnology. 2018;9:55. doi: 10.1186/s40104-018-0268-7. https://doi.org/10.1186/s40104-018-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocquette J.F., Gondret F., Baeza E., Medale F., Jurie C., Pethick D.W. Intramuscular fat content in meat-producing animals: development, genetic and nutritional control, and identification of putative markers. Animal. 2010;4:303–319. doi: 10.1017/S1751731109991091. https://doi.org/10.1017/S1751731109991091. [DOI] [PubMed] [Google Scholar]
- Holló G., Húth B., Holló I., Anton I. X-Ray computed tomography evaluation of intramuscular fat content in Hungarian Simmental cattle. Acta Alimentaria. 2018;47:220–228. https://doi.org/10.1556/066.2018.0002. [Google Scholar]
- Hong H., Baatar D., Hwang S.-G. The difference of castration timing of Korean Hanwoo bulls does not significantly affect the carcass characteristics. Journal of Animal Science and Technology. 2021;63:426. doi: 10.5187/jast.2021.e26. https://doi.org/10.5187/jast.2021.e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang Y.-H., Joo S.-T. Fatty acid profiles, meat quality, and sensory palatability of grain-fed and grass-fed beef from Hanwoo, American, and Australian crossbred cattle. Korean Journal for Food Science of Animal Resources. 2017;37:153. doi: 10.5851/kosfa.2017.37.2.153. https://doi.org/10.5851/kosfa.2017.37.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K., Honda T., Oyama K. Genetic relationships between internal diseases diagnosed at slaughter and carcass traits in Japanese Black cattle. Journal of Animal Science. 2015;93:2714–2721. doi: 10.2527/jas.2014-8497. https://doi.org/10.2527/jas.2014-8497. [DOI] [PubMed] [Google Scholar]
- Irie M., Kouda M., Matono H. Effect of ursodeoxycholic acid supplementation on growth, carcass characteristics, and meat quality of Wagyu heifers (Japanese Black cattle) Journal of Animal Science. 2011;89:4221–4226. doi: 10.2527/jas.2011-4211. https://doi.org/10.2527/jas.2011-4211. [DOI] [PubMed] [Google Scholar]
- Jang S., Chung K., Lee E., Yang S., Kwon E. Vitamin C supplement increased intramuscular adipose tissues but not affect myogenic development of Hanwoo steers. Journal of Animal Science. 2016;94:369. https://doi.org/10.2527/jam2016-0767. [Google Scholar]
- Jeong J., Kwon E., Im S., Seo K., Baik M. Expression of fat deposition and fat removal genes is associated with intramuscular fat content in longissimus dorsi muscle of Korean cattle steers. Journal of Animal Science. 2012;90:2044–2053. doi: 10.2527/jas.2011-4753. https://doi.org/10.2527/jas.2011-4753. [DOI] [PubMed] [Google Scholar]
- Jeyaruban M., Johnston D., Walmsley B. Genetic and phenotypic characterization of MSA index and its association with carcass and meat quality traits in Angus and Brahman cattle. Proceeding of Association for the Advancement of Animal Breeding and Genetics. 2017;22:313–316. [Google Scholar]
- Kawachi H. Micronutrients affecting adipogenesis in beef cattle. Animal Science Journal. 2006;77:463–471. https://doi.org/10.1111/j.1740-0929.2006.00373.x. [Google Scholar]
- Keady S.M., Kenny D.A., Ohlendieck K., Doyle S., Keane M., Waters S.M. Proteomic profiling of bovine M. longissimus lumborum from Crossbred Aberdeen Angus and Belgian Blue sired steers varying in genetic merit for carcass weight. Journal of Animal Science. 2013;91:654–665. doi: 10.2527/jas.2012-5850. https://doi.org/10.2527/jas.2012-5850. [DOI] [PubMed] [Google Scholar]
- Keogh K., Kelly A.K., Kenny D.A. Effect of plane of nutrition in early life on the transcriptome of visceral adipose tissue in Angus heifer calves. Scientific Reports. 2021;11:9716. doi: 10.1038/s41598-021-89252-x. https://doi.org/10.1038/s41598-021-89252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khounsaknalath S., Etoh K., Sakuma K., Saito K., Saito A., Abe T., et al. Effects of early high nutrition related to metabolic imprinting events on growth, carcass characteristics, and meat quality of grass-fed Wagyu (Japanese Black cattle) Journal of Animal Science. 2021;99 doi: 10.1093/jas/skab123. skab123https://doi.org/10.1093/jas/skab123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobel-Graves S., Brooks J., Johnson B., Starkey J., Beckett J., Hodgen J., et al. Effect of vitamin D3, zilpaterol hydrochloride supplementation, and postmortem aging on shear force measurements of three muscles in finishing beef steers. Journal of Animal Science. 2016;94:2637–2647. doi: 10.2527/jas.2015-0121. https://doi.org/10.2527/jas.2015-0121. [DOI] [PubMed] [Google Scholar]
- Knutson E.E., Menezes A.C.B., Sun X., Fontoura A.B.P., Liu J.H., Bauer M.L., et al. Effect of feeding a low-vitamin A diet on carcass and production characteristics of steers with a high or low propensity for marbling. animal. 2020;14:2308–2314. doi: 10.1017/S1751731120001135. https://doi.org/10.1017/S1751731120001135. [DOI] [PubMed] [Google Scholar]
- Kruk Z., Bottema M., Reyes-Veliz L., Forder R., Pitchford W., Bottema C. Vitamin A and marbling attributes: Intramuscular fat hyperplasia effects in cattle. Meat Science. 2018;137:139–146. doi: 10.1016/j.meatsci.2017.11.024. https://doi.org/10.1016/j.meatsci.2017.11.024. [DOI] [PubMed] [Google Scholar]
- Ku M.J., Mamuad L., Nam K.C., Cho Y.I., Kim S.H., Choi Y.S., et al. The effects of total mixed ration feeding with high roughage content on growth performance, carcass characteristics, and meat quality of Hanwoo steers. Food Science of Animal Resources. 2021;41:45–58. doi: 10.5851/kosfa.2020.e73. https://doi.org/10.5851/kosfa.2020.e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacasa D., Le Liepvre X., Ferre P., Dugail I. Progesterone stimulates adipocyte determination and differentiation 1/sterol regulatory element-binding protein 1c gene expression: potential mechanism for the lipogenic effect of progesterone in adipose tissue. Journal of Biological Chemistry. 2001;276:11512–11516. doi: 10.1074/jbc.M008556200. https://doi.org/10.1074/jbc.M008556200. [DOI] [PubMed] [Google Scholar]
- Ladeira M., Schoonmaker J., Gionbelli M., Dias J.C., Gionbelli T., Carvalho J.R., et al. Nutrigenomics and beef quality: A review about lipogenesis. International Journal of Molecular Sciences. 2016;17:918. doi: 10.3390/ijms17060918. https://doi.org/10.3390/ijms17060918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladeira M., Schoonmaker J., Swanson K., Duckett S., Gionbelli M., Rodrigues L., et al. Nutrigenomics of marbling and fatty acid profile in ruminant meat. Animal. 2018:1–13. doi: 10.1017/S1751731118001933. https://doi.org/10.1017/S1751731118001933. [DOI] [PubMed] [Google Scholar]
- Le H.V., Nguyen Q.V., Nguyen D.V., Otto J.R., Malau-Aduli B.S., Nichols P.D., et al. Enhanced omega-3 polyunsaturated fatty acid contents in muscle and edible organs of Australian prime lambs grazing lucerne and cocksfoot pastures. Nutrients. 2018;10:1985. doi: 10.3390/nu10121985. https://doi.org/10.3390/nu10121985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.-C., Choi H.-H., Shin J.-S., Kim K.-H., Oh Y.-K., Cheon D.-W. Carcass characteristics and profitability analysis based on slaughter age of Hanwoo steers. Journal of Animal Science and Technology. 2013;55:315–323. http://dx.doi.org/10.5187/JAST.2013.55.4.315. [Google Scholar]
- Lee Y., Lee B., Kim H., Yun Y., Kang S., Kim K., et al. Sensory quality characteristics with different beef quality grades and surface texture features assessed by dented area and firmness, and the relation to muscle fiber and bundle characteristics. Meat Science. 2018;145:195–201. doi: 10.1016/j.meatsci.2018.06.034. https://doi.org/10.1016/j.meatsci.2018.06.034. [DOI] [PubMed] [Google Scholar]
- Li X.Z., Yan C.G., Gao Q.S., Yan Y., Choi S.H., Smith S.B. Adipogenic/lipogenic gene expression and fatty acid composition in chuck, loin, and round muscles in response to grain feeding of Yanbian Yellow cattle. Journal of Animal Science. 2018;96:2698–2709. doi: 10.1093/jas/sky161. https://doi.org/10.1093/jas/sky161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.D., Moffitt-Hemmer N.R., Deavila J.M., Li A.N., Tian Q.T., Bravo-Iniguez A., et al. Wagyu–Angus cross improves meat tenderness compared to Angus cattle but unaffected by mild protein restriction during late gestation. Animal. 2021;15 doi: 10.1016/j.animal.2020.100144. https://doi.org/10.1016/j.animal.2020.100144. [DOI] [PubMed] [Google Scholar]
- Malau-Aduli A., Edriss M., Siebert B., Bottema C., Pitchford W. Breed differences and genetic parameters for melting point, marbling score and fatty acid composition of lot-fed cattle. Journal of Animal Physiology and Animal Nutrition. 2000;83:95–105. https://doi.org/10.1046/j.1439-0396.2000.00254.x. [Google Scholar]
- Mao Y., Hopkins D.L., Zhang Y., Li P., Zhu L., Dong P., et al. Beef quality with different intramuscular fat content and proteomic analysis using isobaric tag for relative and absolute quantitation of differentially expressed proteins. Meat Science. 2016;118:96–102. doi: 10.1016/j.meatsci.2016.03.028. https://doi.org/10.1016/j.meatsci.2016.03.028. [DOI] [PubMed] [Google Scholar]
- Marti S., Realini C., Bach A., Pérez-Juan M., Devant M. Effect of castration and slaughter age on performance, carcass, and meat quality traits of Holstein calves fed a high-concentrate diet. Journal of Animal Science. 2013;91:1129–1140. doi: 10.2527/jas.2012-5717. [DOI] [PubMed] [Google Scholar]
- Martins T.S., Sanglard L.M., Silva W., Chizzotti M.L., Rennó L.N., Serão N.V., et al. Molecular factors underlying the deposition of intramuscular fat and collagen in skeletal muscle of Nellore and Angus cattle. PLoS One. 2015;10 doi: 10.1371/journal.pone.0139943. https://doi.org/10.1371/journal.pone.0139943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May S.G., Burney N.S., Wilson J.J., Savell J.W., Herring A.D., Lunt D.K., et al. Lipogenic activity of intramuscular and subcutaneous adipose tissues from steers produced by different generations of Angus sires. Journal of Animal Science. 1995;73:1310–1317. doi: 10.2527/1995.7351310x. https://doi.org/10.2527/1995.7351310x. [DOI] [PubMed] [Google Scholar]
- Mcallister C., Speidel S., Crews Jr, D., Enns R. Genetic parameters for intramuscular fat percentage, marbling score, scrotal circumference, and heifer pregnancy in Red Angus cattle. Journal of Animal Science. 2011;89:2068–2072. doi: 10.2527/jas.2010-3538. https://doi.org/10.2527/jas.2010-3538. [DOI] [PubMed] [Google Scholar]
- Mehrban H., Naserkheil M., Lee D.H., Ibáñez-Escriche N. Genetic parameters and correlations of related feed efficiency, growth, and carcass traits in Hanwoo beef cattle. Animal Bioscience. 2021;34:824–832. doi: 10.5713/ajas.20.0135. https://doi.org/10.5713/ajas.20.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D., Kerley M., Walker E., Keisler D., Pierce V., Schmidt T., et al. Growth rate, body composition, and meat tenderness in early vs. traditionally weaned beef calves. Journal of Animal Science. 2005;83:2752–2761. doi: 10.2527/2005.83122752x. https://doi.org/10.2527/2005.83122752x. [DOI] [PubMed] [Google Scholar]
- Miguel J.A., Ciria J., Asenjo B., Pargas H., Colmenarez D. Chemical Composition of Meat in Castrated Male Brahman Cattle in Venezuela. Journal of Life Sciences. 2011;5 https://doi.org/10.17265/1934-7391/2011.07.013. [Google Scholar]
- Moisá S.J., Shike D.W., Faulkner D.B., Meteer W.T., Keisler D., Loor J.J. Central role of the PPARγ gene network in coordinating beef cattle intramuscular adipogenesis in response to weaning age and nutrition. Gene Regulation and Systems Biology. 2014;8:17–32. doi: 10.4137/GRSB.S11782. https://doi.org/10.4137/GRSB.S11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery J., Blanton Jr, J., Horst R., Galyean M., Morrow Jr, K., Wester D., et al. Effects of biological type of beef steers on vitamin D, calcium, and phosphorus status. Journal of Animal Science. 2004;82:2043–2049. doi: 10.2527/2004.8272043x. https://doi.org/10.2527/2004.8272050x. [DOI] [PubMed] [Google Scholar]
- Moraes G.F., Abreu L.R.A., Toral F.L.B., Ferreira I.C., Ventura H.T., Bergmann J.a.G., et al. Selection for feed efficiency does not change the selection for growth and carcass traits in Nellore cattle. Journal of Animal Breeding and Genetics. 2019;136:464–473. doi: 10.1111/jbg.12423. https://doi.org/10.1111/jbg.12423. [DOI] [PubMed] [Google Scholar]
- Moreira A.D., Siqueira G.R., Lage J.F., Benatti J.M.B., Moretti M.H., Miguel G.Z., et al. Castration methods in crossbred cattle raised on tropical pasture. Animal Production Science. 2018;58:1307–1315. https://doi.org/10.1071/AN16580. [Google Scholar]
- Motoyama M., Sasaki K., Watanabe A. Wagyu and the factors contributing to its beef quality: A Japanese industry overview. Meat Science. 2016;120:10–18. doi: 10.1016/j.meatsci.2016.04.026. https://doi.org/10.1016/j.meatsci.2016.04.026. [DOI] [PubMed] [Google Scholar]
- Mueller L.F., Balieiro J.C.C., Ferrinho A.M., Martins T.D.S., da Silva Corte R.R.P., de Amorim T.R., et al. Gender status effect on carcass and meat quality traits of feedlot Angus× Nellore cattle. Animal Science Journal. 2019;90:1078–1089. doi: 10.1111/asj.13250. https://doi.org/10.1111/asj.13250. [DOI] [PubMed] [Google Scholar]
- Mwangi F.W., Charmley E., Gardiner C.P., Malau-Aduli B.S., Kinobe R.T., Malau-Aduli A.E. Diet and genetics influence beef cattle performance and meat quality characteristics. Foods. 2019;8:648. doi: 10.3390/foods8120648. https://doi.org/10.3390/foods8120648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naserkheil M., Lee D.-H., Kong H.-S., Seong J., Mehrban H. Estimation of genetic parameters and correlation between yearling ultrasound measurements and carcass traits in Hanwoo cattle. Animals. 2021;11:1425. doi: 10.3390/ani11051425. https://doi.org/10.3390/ani11051425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayananjalie W.A.D., Wiles T.R., Gerrard D.E., McCann M.A., Hanigan M.D. Acetate and glucose incorporation into subcutaneous, intramuscular, and visceral fat of finishing steers. Journal of Animal Science. 2015;93:2451–2459. doi: 10.2527/jas.2014-8374. https://doi.org/10.2527/jas.2014-8374. [DOI] [PubMed] [Google Scholar]
- Nguyen D.V., Flakemore A.R., Otto J., Ives S.W., Smith R.W., Nichols P.D., et al. Nutritional value and sensory characteristics of meat eating quality of Australian prime lambs supplemented with pelleted canola and flaxseed oils: Fatty acid profiles of muscle and adipose tissues. Internal Medicine Review. 2017;3:1–21. http://dx.doi.org/10.18103/imr.v3i3.295. [Google Scholar]
- Nguyen D.V., Malau-Aduli B.S., Cavalieri J., Nichols P.D., Malau-Aduli A.E. Supplementation with plant-derived oils rich in omega-3 polyunsaturated fatty acids for lamb production. Veterinary and Animal Science. 2018;6:29–40. doi: 10.1016/j.vas.2018.08.001. https://doi.org/10.1016/j.vas.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogalski Z., Wielgosz-Groth Z., Purwin C., Sobczuk-Szul M., Mochol M., Pogorzelska-Przybytek P., et al. Effect of slaughter weight on the carcass value of young crossbred ('Polish Holstein Friesian'x'Limousin') steers and bulls. Chilean Journal of Agricultural Research. 2014;74:59–66. http://dx.doi.org/10.4067/S0718-58392014000100010. [Google Scholar]
- Obsen T., Faergeman N.J., Chung S., Martinez K., Gobern S., Loreau O., et al. Trans-10, cis-12 conjugated linoleic acid decreases de novo lipid synthesis in human adipocytes. The Journal of nutritional biochemistry. 2012;23(6):580–590. doi: 10.1016/j.jnutbio.2011.02.014. https://doi.org/10.1016/j.jnutbio.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh Y.S., Cho S.B., Baek K.H., Choi C.B. Effects of testosterone, 17β-estradiol, and progesterone on the differentiation of bovine intramuscular adipocytes. Asian-Australasian Journal of Animal Sciences. 2005;18:1589–1593. https://doi.org/10.5713/ajas.2005.1589. [Google Scholar]
- Ohyama M., Matsuda K., Torii S., Matsui T., Yano H., Kawada T., et al. The interaction between vitamin A and thiazolidinedione on bovine adipocyte differentiation in primary culture. Journal of Animal Science. 1998;76:61–65. doi: 10.2527/1998.76161x. https://doi.org/10.2527/1998.76161x. [DOI] [PubMed] [Google Scholar]
- Oka A., Maruo Y., Miki T., Yamasaki T., Saito T. Influence of vitamin A on the quality of beef from the Tajima strain of Japanese Black cattle. Meat Science. 1998;48:159–167. doi: 10.1016/s0309-1740(97)00086-7. [DOI] [PubMed] [Google Scholar]
- Okumura T., Saito K., Sowa T., Sakuma H., Ohhashi F., Tameoka N., et al. Changes in beef sensory traits as somatic-cell-cloned Japanese black steers increased in age from 20 to 30 months. Meat Science. 2012;90:159–163. doi: 10.1016/j.meatsci.2011.06.020. https://doi.org/10.1016/j.meatsci.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Oliveira F.C., Ferreira C.E., Haas C.S., Oliveira L.G., Mondadori R.G., Schneider A., et al. Chemical castration in cattle with intratesticular injection of sodium chloride: Effects on stress and inflammatory markers. Theriogenology. 2017;90:114–119. doi: 10.1016/j.theriogenology.2016.12.001. https://doi.org/10.1016/j.theriogenology.2016.12.001. [DOI] [PubMed] [Google Scholar]
- Park B., Choi T.J., Park M.N., Oh S.-H. Estimation of environmental effects and genetic parameters of carcass traits on Chikso (Korean brindle cattle) Asian-Australasian Journal of Animal Sciences. 2020;33:525. doi: 10.5713/ajas.19.0136. https://doi.org/10.5713/ajas.19.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M.N., Alam M., Kim S., Park B., Lee S.H., Lee S.S. Genomic selection through single-step genomic best linear unbiased prediction improves the accuracy of evaluation in Hanwoo cattle. Asian-Australasian Journal of Animal Sciences. 2020;33:1544. doi: 10.5713/ajas.18.0936. https://doi.org/10.5713/ajas.18.0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.J., Beak S.-H., Jung D.J.S., Kim I.H.J., Piao M.Y., Kang H.J., et al. Genetic, management, and nutritional factors affecting intramuscular fat deposition in beef cattle—a review. Asian-Australasian Journal of Animal Sciences. 2018;31:1043. doi: 10.5713/ajas.18.0310. https://doi.org/10.5713/ajas.18.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.J., Kang H.J., Na S., Lee S.H., Baik M. Differential expression of extracellular matrix and integrin genes in the longissimus thoracis between bulls and steers and their association with intramuscular fat contents. Meat Science. 2018;136:35–43. doi: 10.1016/j.meatsci.2017.10.008. [DOI] [PubMed] [Google Scholar]
- Peng D.-Q., Lee J.-S., Kim W.-S., Kim Y.-S., Bae M.-H., Jo Y.-H., et al. Effect of vitamin A restriction on carcass traits and blood metabolites in Korean native steers. Animal Production Science. 2019;59:2138–2146. https://doi.org/10.1071/AN17733. [Google Scholar]
- Peng D.Q., Smith S.B., Lee H.G. Vitamin A regulates intramuscular adipose tissue and muscle development: promoting high-quality beef production. Journal of Animal Science and Biotechnology. 2021;12:1–10. doi: 10.1186/s40104-021-00558-2. https://doi.org/10.1186/s40104-021-00558-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pethick D.W., Harper G.S., Oddy V.H. Growth, development and nutritional manipulation of marbling in cattle: a review. Australian Journal of Experimental Agriculture. 2004;44:705–715. https://doi.org/10.1071/EA02165. [Google Scholar]
- Picard B., Gagaoua M., Al Jammas M., Bonnet M. Beef tenderness and intramuscular fat proteomic biomarkers: Effect of gender and rearing practices. Journal of Proteomics. 2019;200:1–10. doi: 10.1016/j.jprot.2019.03.010. https://doi.org/10.1016/j.jprot.2019.03.010. [DOI] [PubMed] [Google Scholar]
- Pickworth C., Loerch S., Fluharty F. Restriction of vitamin A and D in beef cattle finishing diets on feedlot performance and adipose accretion. Journal of Animal Science. 2012;90:1866–1878. doi: 10.2527/jas.2010-3590. https://doi.org/10.2527/jas.2010-3590. [DOI] [PubMed] [Google Scholar]
- Pickworth C.L., Loerch S.C., Fluharty F.L. Effects of timing and duration of dietary vitamin A reduction on carcass quality of finishing beef cattle1. Journal of Animal Science. 2012;90:2677–2691. doi: 10.2527/jas.2011-4756. https://doi.org/10.2527/jas.2011-4756. [DOI] [PubMed] [Google Scholar]
- Pitchford W.S., Deland M.P.B., Siebert B.D., Malau-Aduliand A.E.O., Bottema C.D.K. Genetic variation in fatness and fatty acid composition of crossbred cattle1. Journal of Animal Science. 2002;80:2825–2832. doi: 10.2527/2002.80112825x. https://doi.org/10.2527/2002.80112825x. [DOI] [PubMed] [Google Scholar]
- Pogge D., Hansen S. Supplemental vitamin C improves marbling in feedlot cattle consuming high sulfur diets. Journal of Animal Science. 2013;91:4303–4314. doi: 10.2527/jas.2012-5638. https://doi.org/10.2527/jas.2012-5638. [DOI] [PubMed] [Google Scholar]
- Pogge D., Lonergan S., Hansen S. Impact of supplementing vitamin C for 56, 90, or 127 days on growth performance and carcass characteristics of steers fed a 0.31 or 0.59% sulfur diet. Journal of Animal Science. 2015;93:2297–2308. doi: 10.2527/jas.2014-7913. https://doi.org/10.2527/jas.2014-7913. [DOI] [PubMed] [Google Scholar]
- Poleti M.D., Regitano L.C., Souza G.H., Cesar A.S., Simas R.C., Silva-Vignato B., et al. Longissimus dorsi muscle label-free quantitative proteomic reveals biological mechanisms associated with intramuscular fat deposition. Journal of Proteomics. 2018;179:30–41. doi: 10.1016/j.jprot.2018.02.028. https://doi.org/10.1016/j.jprot.2018.02.028. [DOI] [PubMed] [Google Scholar]
- Pyatt N., Berger L. Potential effects of vitamins A and D on marbling deposition in beef cattle. The Professional Animal Scientist. 2005;21:174–181. https://doi.org/10.15232/S1080-7446(15)31199-2. [Google Scholar]
- Radunz A., Fluharty F., Relling A., Felix T., Shoup L., Zerby H., et al. Prepartum dietary energy source fed to beef cows: II. Effects on progeny postnatal growth, glucose tolerance, and carcass composition. Journal of Animal Science. 2012;90:4962–4974. doi: 10.2527/jas.2012-5098. https://doi.org/10.2527/jas.2012-5098. [DOI] [PubMed] [Google Scholar]
- Ramírez M., Testa L.M., Valiente S.L., Latorre M.E., Long N.M., Rodriguez A.M., et al. Maternal energy status during late gestation: Effects on growth performance, carcass characteristics and meat quality of steers progeny. Meat Science. 2020;164 doi: 10.1016/j.meatsci.2020.108095. https://doi.org/10.1016/j.meatsci.2020.108095. [DOI] [PubMed] [Google Scholar]
- Ranjan R., Ranjan A., Dhaliwal G., Patra R. L-Ascorbic acid (vitamin C) supplementation to optimize health and reproduction in cattle. Veterinary Quarterly. 2012;32:145–150. doi: 10.1080/01652176.2012.734640. https://doi.org/10.1080/01652176.2012.734640. [DOI] [PubMed] [Google Scholar]
- Rasby, R. (2007). Early weaning beef calves. Veterinary Clinics of North America: Food Animal Practice, 23,29-40. https://doi.org/10.1016/j.cvfa.2007.01.002. [DOI] [PubMed]
- Reddy K.E., Jeong J., Baek Y.-C., Oh Y.K., Kim M., So K.M., et al. Early weaning of calves after different dietary regimens affects later rumen development, growth, and carcass traits in Hanwoo cattle. Asian-Australasian Journal of Animal Sciences. 2017;30:1425–1434. doi: 10.5713/ajas.17.0315. https://doi.org/10.5713/ajas.17.0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiling B., Johnson D. Effects of implant regimens (trenbolone acetate-estradiol administered alone or in combination with zeranol) and vitamin D3 on fresh beef color and quality. Journal of Animal Science. 2003;81:135–142. doi: 10.2527/2003.811135x. https://doi.org/10.2527/2003.811135x. [DOI] [PubMed] [Google Scholar]
- Rezende P.L.D.P., Restle J., Bilego U.O., Fernandes J.J.D.R., Missio R.L., Guimarães T.P. Carcass characteristics of feedlot-finished Nellore heifers slaughtered at different weights. Acta Scientiarum. Animal Sciences. 2019;41 https://doi.org/10.4025/actascianimsci.v41i1.44826. [Google Scholar]
- Riley D.G., Chase C.C., Jr Hammond, A C., West R.L., Johnson D.D., Olson T.A., et al. Estimated genetic parameters for carcass traits of Brahman cattle. Journal of Animal Science. 2002;80:955–962. doi: 10.2527/2002.804955x. https://doi.org/10.2527/2002.804955x. [DOI] [PubMed] [Google Scholar]
- Robinson D., Cafe L., Greenwood P. Developmental programming in cattle: consequences for growth, efficiency, carcass, muscle and beef quality characteristics. Journal of Animal Science. 2013;91:1428–1442. doi: 10.2527/jas.2012-5799. https://doi.org/10.2527/jas.2012-5799. [DOI] [PubMed] [Google Scholar]
- Scheffler J., Mccann M., Greiner S., Jiang H., Hanigan M., Bridges G., et al. Early metabolic imprinting events increase marbling scores in fed cattle. Journal of Animal Science. 2014;92:320–324. doi: 10.2527/jas.2012-6209. https://doi.org/10.2527/jas.2012-6209. [DOI] [PubMed] [Google Scholar]
- Shahrai N.N., Babji A.S., Maskat M.Y., Razali A.F., Yusop S.M. Effects of marbling on physical and sensory characteristics of ribeye steaks from four different cattle breeds. Animal Bioscience. 2021;34:904–913. doi: 10.5713/ajas.20.0201. https://doi.org/10.5713/ajas.20.0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S., Chung E. Identification of differentially expressed genes between high and low marbling score grades of the longissimus lumborum muscle in Hanwoo (Korean cattle) Meat Science. 2016;121:114–118. doi: 10.1016/j.meatsci.2016.05.018. https://doi.org/10.1016/j.meatsci.2016.05.018. [DOI] [PubMed] [Google Scholar]
- Shoup L., Kloth A., Wilson T., González-Peña D., Ireland F., Rodriguez-Zas S., et al. Prepartum supplement level and age at weaning: I. Effects on pre-and postpartum beef cow performance and calf performance through weaning. Journal of Animal Science. 2015;93:4926–4935. doi: 10.2527/jas.2014-8564. https://doi.org/10.2527/jas.2014-8564. [DOI] [PubMed] [Google Scholar]
- Singh R., Artaza J.N., Taylor W.E., Gonzalez-Cadavid N.F., Bhasin S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology. 2003;144:5081–5088. doi: 10.1210/en.2003-0741. https://doi.org/10.1210/en.2003-0741. [DOI] [PubMed] [Google Scholar]
- Smith T., Domingue J., Paschal J., Franke D., Bidner T., Whipple G. Genetic parameters for growth and carcass traits of Brahman steers. Journal of Animal Science. 2007;85:1377–1384. doi: 10.2527/jas.2006-653. [DOI] [PubMed] [Google Scholar]
- Stewart S.M., Gardner G.E., Mcgilchrist P., Pethick D.W., Polkinghorne R., Thompson J.M., et al. Prediction of consumer palatability in beef using visual marbling scores and chemical intramuscular fat percentage. Meat Science. 2021;181 doi: 10.1016/j.meatsci.2020.108322. https://doi.org/10.1016/j.meatsci.2020.108322. [DOI] [PubMed] [Google Scholar]
- Taga H., Bonnet M., Picard B., Zingaretti M., Cassar-Malek I., Cinti S., et al. Adipocyte metabolism and cellularity are related to differences in adipose tissue maturity between Holstein and Charolais or Blond d'Aquitaine fetuses. Journal of Animal Science. 2011;89:711–721. doi: 10.2527/jas.2010-3234. https://doi.org/10.2527/jas.2010-3234. [DOI] [PubMed] [Google Scholar]
- Takeda K., Uchida H., Inoue K. Genetic relationships between temperament of calves at auction and carcass traits in Japanese Black cattle. Animal Science Journal. 2017;88:1475–1481. doi: 10.1111/asj.12787. https://doi.org/10.1111/asj.12787. [DOI] [PubMed] [Google Scholar]
- Teixeira P.D., Oliveira D.M., Chizzotti M.L., Chalfun-Junior A., Coelho T.C., Gionbelli M., et al. Subspecies and diet affect the expression of genes involved in lipid metabolism and chemical composition of muscle in beef cattle. Meat Science. 2017;133:110–118. doi: 10.1016/j.meatsci.2017.06.009. https://doi.org/10.1016/j.meatsci.2017.06.009. [DOI] [PubMed] [Google Scholar]
- Tipton N.C., King D.A., Paschal J.C., Hale D.S., Savell J.W. Effects of oral vitamin D3 supplementation and supplement withdrawal on the accumulation of magnesium, calcium, and vitamin D in the serum, liver, and muscle tissue and subsequent carcass and meat quality of Bos indicus influenced cattle. Meat Science. 2007;75:150–158. doi: 10.1016/j.meatsci.2006.06.024. https://doi.org/10.1016/j.meatsci.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Torres-Vázquez J.A., Van Der Werf J.H., Clark S.A. Genetic and phenotypic associations of feed efficiency with growth and carcass traits in Australian Angus cattle. Journal of Animal Science. 2018;96:4521–4531. doi: 10.1093/jas/sky325. https://doi.org/10.1093/jas/sky325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urrutia O., Mendizabal J.A., Alfonso L., Soret B., Insausti K., Arana A. Adipose tissue modification through feeding strategies and their implication on adipogenesis and adipose tissue metabolism in ruminants. International Journal of Molecular Sciences. 2020;21:3183. doi: 10.3390/ijms21093183. https://doi.org/10.3390/ijms21093183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utrera A.R., Van Vleck L.D. Heritability estimates for carcass traits of cattle: a review. Genetics and Molecular Research. 2004;3:380–394. [PubMed] [Google Scholar]
- Wang B., Yang Q., Harris C.L., Nelson M.L., Busboom J.R., Zhu M.J., et al. Nutrigenomic regulation of adipose tissue development—role of retinoic acid: a review. Meat science. 2016;120:100–106. doi: 10.1016/j.meatsci.2016.04.003. https://doi.org/10.1016/j.meatsci.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Li H., Wu F., Qiu X., Yu Z., Niu W., et al. Effects of dietary energy on growth performance, rumen fermentation and bacterial community, and meat quality of Holstein-Friesians bulls slaughtered at different ages. Animals. 2019;9:1123. doi: 10.3390/ani9121123. https://doi.org/10.3390/ani9121123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.H., Bower N., Reverter A., Tan S., De Jager N., Wang R., et al. Gene expression patterns during intramuscular fat development in cattle. Journal of Animal Science. 2009;87:119–130. doi: 10.2527/jas.2008-1082. https://doi.org/10.2527/jas.2008-1082. [DOI] [PubMed] [Google Scholar]
- Wei X., Han S., Wang S., Zheng Q., Li X., Du J., et al. ANGPTL8 regulates adipocytes differentiation and adipogenesis in bovine. Gene. 2019;707:93–99. doi: 10.1016/j.gene.2019.04.048. https://doi.org/10.1016/j.gene.2019.04.048. [DOI] [PubMed] [Google Scholar]
- Westbrook L., Johnson B.J., Gang G., Toyonaga K., Hwang J., Chung K., et al. Evidence for functional G-coupled protein receptors 43 and 120 in subcutaneous and intramuscular adipose tissue of Angus crossbred steers. Journal of Animal Science. 2021;99 doi: 10.1093/jas/skab125. skab125https://doi.org/10.1093/jas/skab125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyskida K., Franik G., Wikarek T., Owczarek A., Delroba A., Chudek J., et al. The levels of adipokines in relation to hormonal changes during the menstrual cycle in young, normal-weight women. Endocrine Connections. 2017;6:892–900. doi: 10.1530/EC-17-0186. https://doi.org/10.1530/EC-17-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., López M. Central regulation of energy metabolism by estrogens. Molecular Metabolism. 2018;15:104–115. doi: 10.1016/j.molmet.2018.05.012. https://doi.org/10.1016/j.molmet.2018.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Kamiya M., Higuchi M. Fat depot-specific effects of body fat distribution and adipocyte size on intramuscular fat accumulation in Wagyu cattle. Animal Science Journal. 2020;91:e13449. doi: 10.1111/asj.13449. https://doi.org/10.1111/asj.13449. [DOI] [PubMed] [Google Scholar]
- Yamada T., Nakanishi N. Effects of the roughage/concentrate ratio on the expression of angiogenic growth factors in adipose tissue of fattening Wagyu steers. Meat Science. 2012;90:807–813. doi: 10.1016/j.meatsci.2011.11.019. https://doi.org/10.1016/j.meatsci.2011.11.019. [DOI] [PubMed] [Google Scholar]
- Yang S.H., Kim W.H., Kang S.-N., Choi K.C., Kim D. A comparison of rice straw and whole-crop barley (Hordeum vulgare L.) silage supplements on performance and carcass characteristics of Hanwoo (Bos taurus coreanae) steers. Applied Sciences. 2020;10:7725. https://doi.org/10.3390/app10217725. [Google Scholar]
- Zhang H., Xia H., Jiang H., Mao Y., Qu K., Huang B., et al. Longissimus dorsi muscle transcriptomic analysis of Yunling and Chinese simmental cattle differing in intramuscular fat content and fatty acid composition. Genome. 2018;61:549–558. doi: 10.1139/gen-2017-0164. https://doi.org/10.1139/gen-2017-0164. [DOI] [PubMed] [Google Scholar]
- Zhang Y.-Y., Wang H.-B., Wang Y.-N., Wang H.-C., Zhang S., Hong J.-Y., et al. Transcriptome analysis of mRNA and microRNAs in intramuscular fat tissues of castrated and intact male Chinese Qinchuan cattle. PLoS One. 2017;12 doi: 10.1371/journal.pone.0185961. https://doi.org/10.1371/journal.pone.0185961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.-Y., Zan L.S., Wang H.B., Xin Y.P., Adoligbe C.M., Ujan J.A. Effect of sex on meat quality characteristics of Qinchuan cattle. African Journal of Biotechnology. 2010;9:4504–4509. [Google Scholar]
- Wang, B., Fu, X., Liang, X., Wang, Z., Yang, Q., Zou, T., et al. (2017). Maternal retinoids increase PDGFRα+ progenitor population and beige adipogenesis in progeny by stimulating vascular development. EBioMedicine, 18, 288-299. https://doi.org/10.1016/j.ebiom.2017.03.041. [DOI] [PMC free article] [PubMed]