Abstract
Background
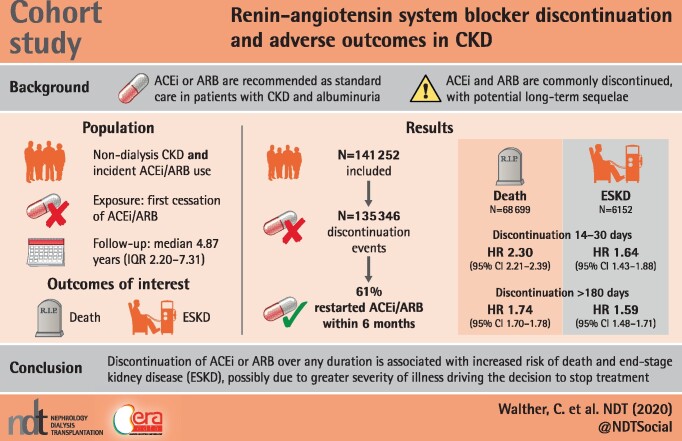
Treatment with renin–angiotensin system inhibitors (RASIs), angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) is the standard of care for those with chronic kidney disease (CKD) and albuminuria. However, ACEI/ARB treatment is often discontinued for various reasons. We investigated the association of ACEI/ARB discontinuation with outcomes among US veterans with non-dialysis-dependent CKD.
Methods
We performed a retrospective cohort study of patients in the Veterans Affairs healthcare system with non-dialysis-dependent CKD who subsequently were started on ACEI/ARB therapy (new user design). Discontinuation events were defined as a gap in ACEI/ARB therapy of ≥14 days and were classified further based on duration (14–30, 31–60, 61–90, 91–180 and >180 days). This was treated as a time-varying risk factor in adjusted Cox proportional hazards models for the outcomes of death and incident end-stage kidney disease (ESKD), which also adjusted for relevant confounders.
Results
We identified 141 252 people with CKD and incident ACEI/ARB use who met the inclusion criteria; these were followed for a mean 4.87 years. There were 135 356 discontinuation events, 68 699 deaths and 6152 incident ESKD events. Discontinuation of ACEI/ARB was associated with a higher risk of death [hazard ratio (HR) 2.3, 2.0, 1.99, 1.92 and 1.74 for those discontinued for 14–30, 31–60, 61–90, 91–180 and >180 days, respectively]. Similar associations were noted between ACEI and ARB discontinuation and ESKD (HR 1.64, 1.47, 1.54, 1.65 and 1.59 for those discontinued for 14–30, 31–60, 61–90, 91–180 and >180 days, respectively).
Conclusions
In a cohort of predominantly male veterans with CKD Stages 3 and 4, ACEI/ARB discontinuation was independently associated with an increased risk of subsequent death and ESKD. This may be due to the severity of illness factors that drive the decision to discontinue therapy. Further investigations to determine the causes of discontinuations and to provide an evidence base for discontinuation decisions are needed.
Keywords: ACEI, ARB, chronic kidney disease, discontinuation, ESKD, mortality
Graphical Abstract
KEY LEARNING POINTS
What is already known about this subject?
Angiotensin-converting enzyme inhibitors (ACEI)/angiotensin II receptor blockers (ARBs) are recommended as the standard of care for patients with albuminuria and chronic kidney disease (CKD).
ACEIs/ARBs are often discontinued due to adverse events or for other reasons and the impact of ACEI/ARB discontinuation on outcomes has been little studied.
What this study adds?
ACEI/ARB discontinuation is associated with an increased risk of mortality among those with CKD.
End-stage kidney disease risk also increases with ACEI/ARB discontinuation among those with CKD.
What impact this may have on practice or policy?
Physicians should assess eligible patients who are not on ACEI/ARB therapy and identify the reasons why these agents are not being used.
INTRODUCTION
Treatment with angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs), proven to slow the progression of diabetic and proteinuric kidney diseases, has become the standard of chronic kidney disease (CKD) care over the past 2 decades [1]. These agents likely provide benefit through several pathways, including a reduction in intraglomerular hydraulic pressure, improvement in endothelial function and modulation of inflammatory and fibrotic pathways [2]. Current clinical practice guidelines recommend the broad use of these agents in diabetic and proteinuric kidney diseases and as first-line agents for treatment of hypertension in people with and without kidney disease [1, 3, 4]. ACEI and ARB therapies have also been shown to be safe and effective even in advanced CKD [5].
Despite recommendations for widespread adoption, many patients with CKD do not receive ACEI/ARB therapy. Among nondialysis CKD patients with Medicare Part D in 2015, only 58% had at least one prescription filled for ACEIs/ARBs [6] and the proportion of patients with CKD who initiate or are maintained on ACEI/ARB therapy declines as CKD advances, a finding demonstrated repeatedly over decades [6, 7]. Over 5 years of follow-up in a Veterans Affairs (VA) cohort, even among those prescribed ACEI or ARB therapy, temporary or permanent discontinuations were frequent [7]. Reasons commonly cited for ACEI/ARB discontinuation include effects of their primary mechanism of action (serum creatinine elevations, hyperkalemia and hypotension) and side effects related to inhibition of kinin breakdown (cough and angioedema) [8]. They are also commonly discontinued with intercurrent illness, when volume depletion and/or hypotension are present or possible and among hospitalized patients, where reinstitution upon discharge is commonly not done [9]. Additionally, higher comorbidity burden itself, by placing the patient at risk for serum creatinine elevations classified as acute kidney injury (AKI), may lead to ACEI/ARB avoidance or discontinuation [10, 11].
To advance our understanding of the patterns of ACEI/ARB discontinuation and associated outcomes, we retrospectively studied outcomes of death and progression to end-stage kidney disease (ESKD) after temporary or permanent cessation of ACEI/ARB therapy among a nationwide cohort of veterans with CKD in the USA.
MATERIALS AND METHODS
Design overview
We designed a retrospective cohort study of patients with non-dialysis-dependent CKD receiving care through the VA health system, with ACEI/ARB discontinuation as the exposure and incident ESKD and death as outcomes of interest. To improve comparability, we limited the cohort to people who had initiated ACEI/ARB therapy after incident CKD (new user design) and who used statin therapy during follow-up. Adjustment for discontinuation of statin therapy—chosen as a commonly used, non-ACEI/ARB medication class—was done to reduce healthy user bias, whereby patients who continue to use a therapy may be generally behaviorally different and/or healthier.
Study population
We identified patients cared for in the VA health system who had a sustained estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 for >90 days (with measurements ≤410 days apart) from 1 January 2005 to 31 December 2015 estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) serum creatinine–based equation [12, 13]. Incident CKD date was taken as the date of sustained reduced eGFR [14]. We defined new ACEI/ARB use as the date of the first pharmacy fill, with no previous ACEI/ARB dispensations in the VA system (for up to 5 years dating back to 2000), to identify patients with CKD who were likely to be incident (‘new’) ACEI/ARB users. In an attempt to exclude persons who were not true incident ACEI/ARB users, but rather had switched pharmacies to the VA system, we excluded persons who had not filled other medications through the VA within 1 year prior to the incident ACEI/ARB dispensation. We also required the use of a commonly used medication class (statin) during the period of ACEI/ARB use, so that discontinuation of that medication could be adjusted for in analyses to try to reduce confounding related to general medication discontinuation and to isolate the associations with ACEI/ARB discontinuation itself. The study was approved by the Institutional Review Board of the Baylor College of Medicine (H-38697).
Data sources
Demographic characteristics were obtained from the VA Corporate Data Warehouse. We identified comorbid conditions based on International Classification of Diseases, Ninth and Tenth Revisions, Clinical Modification (ICD-9-CM and ICD-10-CM) diagnostic codes from the VA Inpatient and Outpatient Medical Statistical Analysis System Datasets, classified using the Deyo–Charlson comorbidity index [15, 16]. For instance, diabetes was identified using the diagnostic codes 250.0–250.9, E10, E11 and E13 and cardiovascular disease was identified using the diagnostic codes 410.0, 412, 413, 414, 429 and I21.09-21.10. Outpatient laboratory tests were accessed through the Corporate Data Warehouse LabChem data files. We identified pharmacy medication fills from the VA Pharmacy Benefits Management Database [17].
Outcomes
The outcomes of interest were, separately, death from any cause and ESKD. Dates of death were obtained from the VA Vital Status Files through 31 December 2016. ESKD was identified through linkage to the US Renal Data System database through 31 December 2016. For analysis of each outcome, censoring was performed at the time of the alternate outcome.
Exposure
The exposure of interest was first cessation of ACEI/ARB therapy. This was defined as nonoverlap of a pharmacy prescription fill of >14 days. We divided discontinuation duration into 14–<90, 90–180 and >180 days. We did not separately categorize permanent discontinuation, as this would introduce survival bias. We investigated the most recent outpatient serum creatinine and potassium levels (measured within the VA system) within the 6 months prior to ACEI/ARB discontinuation, along with hospitalizations within 6 months prior to ACEI/ARB discontinuation.
Kidney function
CKD was classified by GFR stages into G3a (eGFR 45–59 mL/min/1.73 m2), G3b (eGFR 30–44 mL/min/1.73 m2) and G4 (eGFR 15–29 mL/min/1.73 m2) using outpatient serum creatinine levels and the CKD-EPI creatinine equation [18].
Covariates
Demographics were age, sex and race/ethnicity categorized as white, black or other. Index year (year of incident ACEI/ARB use) was categorized as 2005–08, 2009–11 and 2012–15. Body mass index (BMI) was classified into underweight, normal weight, overweight and obese [19]. We included the following comorbidities in the model: diabetes, hypertension, congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), cerebrovascular disease, peripheral arterial disease, coronary artery disease (CAD), chronic liver disease and malignancy. Outpatient systolic blood pressure (SBP) was categorized as <120, 120–139 or ≥140 mmHg and diastolic blood pressure (DBP) as <80, 80–89 or ≥90 mmHg.
Statistical analysis
Baseline characteristics of the study population were tabulated. We used time-updated Cox proportional hazards models to evaluate the association of ACEI/ARB discontinuation with each of the primary outcomes separately. We used univariate and sequential multivariate models with covariates chosen based on clinical considerations. In Model 1, we adjusted for age, race and sex; Model 2 added eGFR and comorbidities [hypertension, CHF, COPD, cerebrovascular disease, peripheral vascular disease (PVD), CAD, chronic liver disease and malignancy]; the final model added SBP, DBP, BMI, year of incident ACEI/ARB use and statin discontinuation. ACEI/ARB discontinuation was treated as a time-updated exposure where participants contributed risk time to the no-discontinuation category prior to the first discontinuation event and to one of the discontinuation categories following the first discontinuation event. Only the first discontinuation events were considered in the analyses. We used multiple imputation for missing baseline covariates. Age, CKD stage (based on GFR), SBP and DBP, BMI and comorbidities were time-updated at the time of ACEI/ARB initiation. Analyses were conducted using SAS Enterprise Guide version 7.1 (SAS Institute, Cary, NC, USA; www.sas.com).
RESULTS
Patient characteristics
Among 1 371 075 veterans with CKD we identified 141 252 who met the inclusion criteria (Figure 1) from 2005 to 2015. Among them, 97% were males with a mean age of 73.7 years (SD 10.4) and a mean eGFR of 49.3 mL/min/1.73 m2. Diabetes mellitus was diagnosed prior to ACEI/ARB initiation in 42.5% and CHF in 17.4%. Other patient characteristics at the time of ACEI/ARB initiation are detailed in Table 1.
FIGURE 1.
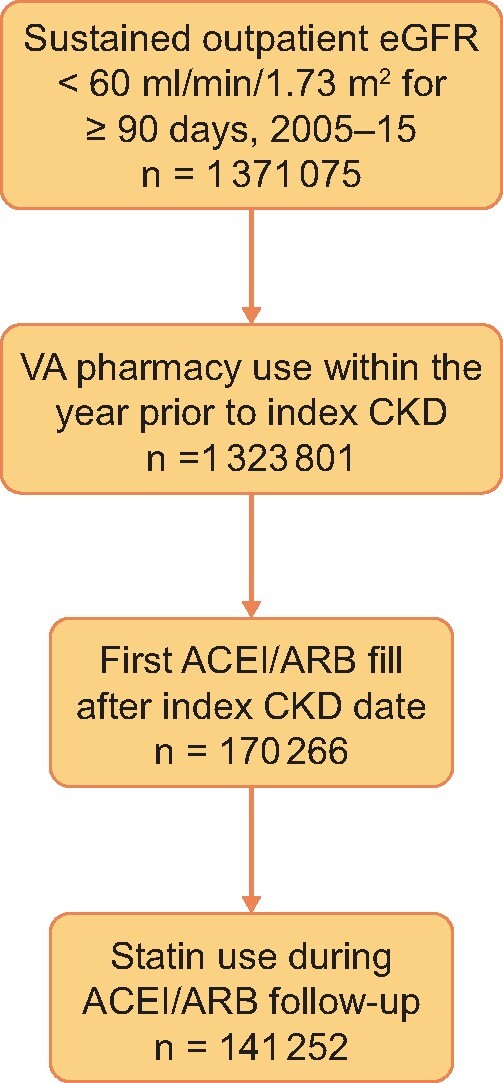
Flow chart showing how study participants were selected for this analysis.
Table 1.
Baseline characteristics (at the time of incident ACEI/ARB use)
| Characteristics | Valuesa |
|---|---|
| Age (years), % | 73.7 (10.4) |
| <40 | 0.23 |
| 40–49 | 1.27 |
| 50–59 | 8.06 |
| 60–69 | 27.44 |
| 70–79 | 31.06 |
| >80 | 31.93 |
| Male, n (%) | 164 629 (96.7) |
| Race, n (%) | |
| Black | 24 493 (14.39) |
| White | 4063 (2.39) |
| Other | 141 710 (83.23) |
| BMI (kg/m2), % | 29.2 (6.5) |
| <18.5 | 0.83 |
| 18.5–25 | 16.46 |
| 25.0–30 | 29.46 |
| >30 | 29.44 |
| SBP (mmHg), mean (SD) | 140.7 (22.8) |
| DBP (mmHg), mean (SD) | 75.5 (13.5) |
| eGFR (mL/min/1.73 m2), % | 49.3 (13.8) |
| 45–59 | 46.2 |
| 30–44 | 22.7 |
| 15–29 | 5.72 |
| <15 | 1.84 |
| Diabetes mellitus, n (%) | 72 382 (42.5) |
| CHF, n (%) | 29 659 (17.4) |
| CAD, n (%) | 19 803 (11.6) |
| PVD, n (%) | 38 229 (22.5) |
| COPD, n (%) | 56 112 (33.0) |
| Cerebrovascular disease, n (%) | 35 844 (21.1) |
| Chronic liver disease, n (%) | 8038 (4.7) |
| Malignancy, n (%) | 39 699 (23.3) |
| ACEI/ARB index year, % | |
| 2005–08 | 44.09 |
| 2009–11 | 25.40 |
| 2012–15 | 30.51 |
Missing data: BMI, 23.8%; SBP, 0.75%; DBP, 0.77%.
ACEI/ARB discontinuations
We identified 135 346 first discontinuation events, which occurred at a median of 0.52 years [interquartile range (IQR) 0.25–1.49] after ACEI/ARB initiation. About 14.5% of discontinuation events (19 289) were preceded by hospitalization within 6 months (Table 2). Serum potassium level was checked within the 6 months prior to discontinuation in 52 285 discontinuation events (38.6%). Serum potassium was ≥5.0 mEq/L in 5466 (10.5%) and ≥5.5 mEq/L in 1194 (2.3%) (Table 2). About 61% of patients who discontinued ACEIs/ARBs restarted within 6 months of discontinuation. Among them, 46.0% of patients had both creatinine and potassium tested in the first 90 days of ACEI/ARB resumption. An additional 0.8% had only creatinine measured in the same time period and an additional 2.3% had only potassium measured in that 90-day period.
Table 2.
Laboratory values and hospitalizations prior to ACEI/ARB discontinuation
| Hospitalizations within 6 months prior to discontinuation | n (%) |
| None | 116 057 (85.8) |
| Any hospitalization | 19 289 (14.5) |
| 0–30 days prior | 5774 (4.3) |
| 31–60 days prior | 3715 (2.7) |
| 61–90 days prior | 3504 (2.6) |
| 91–180 days prior | 6296 (4.7) |
| Outpatient laboratory values within 6 months prior to discontinuation | Measure |
| Serum creatinine (mg/dL) | |
| Median (IQR) | 1.4 (1.2–1.8) |
| Serum potassium (mEq/L)a | |
| Median (IQR) | 4.4 (4.0–4.7) |
| 5.0–5.4, n (%) | 4272 (8.2) |
| 5.5–6.0, n (%) | 859 (1.6) |
| >6.0, n (%) | 335 (0.64) |
Summarized for the 52 285 discontinuation events with serum potassium measurement within 6 months prior to discontinuation.
Outcomes
Death
Over 829 095 patient-years of follow-up there were 68 699 deaths and 6152 incident ESKD events. The median follow-up was 4.47 years (IQR 2.20–7.31). ACEI/ARB discontinuation was associated with a more than doubling of the risk of death in the univariate model for all durations of discontinuation studied (Table 3). Adjustment attenuated these associations slightly and in the fully adjusted model the hazard ratio (HR) for death ranged from a low of 1.74 [95% confidence interval (CI) 1.70–1.78] for discontinuations >180 days to a high of 2.30 (95% CI 2.21–2.39) for those who discontinued for 14–30 days. In all the models, the strength of the association with death was higher for shorter durations of discontinuation and the associations were statistically significant. Full model results are shown in Table 3.
Table 3.
HRs (95% CI) by duration of ACEI/ARB discontinuation (compared with no discontinuation)
| Discontinuation duration | Univariate | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|
| Death (days) | ||||
| 14–30 | 2.76 (2.66–2.87) | 2.74 (2.63–2.84) | 2.58 (2.48–2.68) | 2.30 (2.21–2.39) |
| 31–60 | 2.47 (2.36–2.58) | 2.41 (2.30–2.52) | 2.22 (2.12–2.32) | 2.00 (1.91–2.09) |
| 61–90 | 2.58 (2.45–2.71) | 2.48 (2.36–2.61) | 2.23 (2.12–2.34) | 1.99 (1.89–2.10) |
| 91–180 | 2.60 (2.51–2.70) | 2.47 (2.38–2.56) | 2.15 (2.07–2.23) | 1.92 (1.85–1.99) |
| >180 | 2.31 (2.26–2.37) | 2.12 (2.07–2.17) | 1.91 (1.87–1.96) | 1.74 (1.70–1.78) |
| Incident ESKD (days) | ||||
| 14–30 | 2.33 (2.04–2.67) | 2.20 (1.92–2.51) | 1.72 (1.50–1.96) | 1.64 (1.43–1.88) |
| 31–60 | 2.23 (1.92–2.60) | 2.11 (1.81–2.45) | 1.50 (1.29–1.74) | 1.47 (1.26–1.71) |
| 61–90 | 2.43 (2.05–2.87) | 2.37 (2.01–2.80) | 1.56 (1.32–1.84) | 1.54 (1.30–1.82) |
| 91–180 | 2.77 (2.47–3.10) | 2.82 (2.52–3.16) | 1.67 (1.49–1.87) | 1.65 (1.47–1.85) |
| >180 | 2.27 (2.12–2.44) | 2.58 (2.40–2.77) | 1.60 (1.49–1.72) | 1.59 (1.48–1.71) |
Model 1: Adjusted for age, race and sex.
Model 2: Model 1 + CKD G stage and comorbidities (hypertension, CHF, COPD, cerebrovascular disease, peripheral vascular disease, coronary artery disease, chronic liver disease and malignancy).
Model 3: Model 2 + SBP, DBP, BMI, year of incident ACEI/ARB use and statin discontinuation.
ESKD
The pattern of associations between discontinuation and risk of ESKD was similar to the associations of discontinuation with death. In the univariate model, the risk was more than doubled for all durations of discontinuation, with attenuation in the adjusted models, and risk ranged from 1.47 (95% CI 1.26–1.71) to 1.65 (95% CI 1.47–1.85) in the fully adjusted model for different durations of discontinuation (Table 3).
DISCUSSION
In this large, older, predominantly male veteran cohort with CKD and new ACEI/ARB use we found ACEI/ARB discontinuation to be associated with a substantially increased risk of death and ESKD. The risk of all-cause death approximately doubled following ACEI/ARB discontinuation and the risk of ESKD increased ∼50%. The risk of death was found to decrease with more prolonged discontinuation, whereas the risk of incident ESKD showed no clear pattern over the different durations. The strength of the association, especially with all-cause death, and the decrease in the association with more prolonged discontinuation suggest that the observed association is primarily due to factors driving discontinuation events rather than being caused by the discontinuation events themselves.
These results add new information to what is currently known about outcomes following ACEI/ARB discontinuation. Causal information about the effect of ACEI/ARB use patterns on the risk of all-cause death or ESKD in CKD can only be obtained from randomized trials. Randomized trials in people with CKD, with and without diabetes or albuminuria, comparing ACEI/ARB therapy to other antihypertensives or to placebo have shown varying degrees of benefit for all-cause mortality and ESKD [20]. Benefits for other outcomes (such as GFR change, doubling of serum creatinine and cardiovascular endpoints) and among certain subgroups (such as people with diabetes or albuminuria) have been more consistent and pronounced [20]. The benefit and safety of closely monitored ACEI/ARB use even in advanced CKD has been demonstrated [21–23]. However, trial results are not available to answer questions about the effects of temporary or permanent discontinuation of ACEIs/ARBs, as randomized studies comparing stoppage to continuation have not been performed, although one is ongoing in people with advanced CKD [24].
Observational evidence on the effects of ACEI/ARB discontinuation in CKD, either temporarily or permanently, is limited [25]. Using clinical data from 53 912 patients on ACEIs and ARBs, Qiao et al. [26] reported that ACEI/ARB discontinuation was common among those with Stage 4 CKD. Furthermore, hyperkalemia, acidosis, hypotension and hospitalization were associated with ACEI/ARB discontinuation. Furthermore, a recent analysis of 3909 patients with CKD cared for at a comprehensive health system found that ACEI/ARB discontinuation for >60 days within 6 months following an eGFR decline to <30 mL/min/1.73 m2 was associated with a 39% increased risk of death, 37% increased risk of major adverse cardiac events and a nonstatistically significant finding of 19% increased risk of ESKD [27]. The relative strength and direction of these findings accord with the findings of our study. In a propensity-matched cohort study of Chronic Renal Insufficiency Cohort (CRIC) study participants with eGFR <30 mL/min/1.73 m2, the pattern of renin–angiotensin system inhibitor use over 1 year (never, always, new and dynamic) was not associated with the risk of ESKD or death [28]. A recently published study analyzed ACEI/ARB use in CKD patients treated at the VA during a 3-year period before transition to dialysis [17]. The authors found that longer and continuous ACEI/ARB use prior to hemodialysis was associated with better postdialysis survival. This accords well with the findings of our study. Given the consistency of the findings, our data support and extend the notion that ACEIs/ARBs should generally be continued unless specific clinical situations (such as hypotension or AKI) preclude their use.
Possible factors driving discontinuation events and the associated mortality—only partially captured in our covariate data—include the severity of kidney disease, variability of kidney function, cause of kidney disease, frailty and limited projected life expectancy. It is notable that a significant portion of discontinuation events had no clearly identifiable cause based on lab results or severe intercurrent illness reflected in hospitalization. This suggests that most discontinuation events are related to other, more nuanced and difficult to discern factors, including those discussed above. Differentiating and understanding the burden of physiological and nonphysiological adverse events would aid clinicians in focusing on CKD patients who could be safely restarted on these agents. This is critical, as ACEI/ARB use in CKD appears to have plateaued in recent years and many people with CKD may be missing out on the important cardiovascular and kidney benefits of these agents [29]. It is also plausible that some of the low use of ACEIs or ARBs in CKD patients is related to clinical inertia (i.e. failure to initiate or intensify therapy when clinically indicated). Indeed, recent studies have shown that even among patients admitted with AKI, ACEI or ARB therapy can be initiated or reinitiated in most patients after discharge and is associated with improved mortality [30]. ACEI and ARB use remains foundational to CKD management, even as new effective therapies emerge for management of CKD.
The strengths of this analysis include the large sample size, the stringent CKD definition requiring sustained reduced eGFR and the availability of pharmacy data and key outcome measures. This analysis also expands the current knowledge regarding the association of ACE/ARB discontinuations with long-term outcomes. Limitations for this study include the inability to determine the exact circumstances of ACEI/ARB discontinuation, including what factors led to the discontinuations and what the intent of discontinuation events, and the inability to determine whether reinitiation of these agents is safe and offers kidney and cardiovascular benefits. Future investigations applying techniques such as natural language processing to large national registries may be able to shed additional light on these issues. Additionally, we were unable to investigate cause-specific deaths. Furthermore, we were unable to study the association of discontinuation with specific outcomes (such as major atherosclerotic cardiovascular events and CHF) due to a lack of comprehensive data for these outcomes. The observational nature of the study prevents any conclusions on causality. Due to the nature of the study cohort, whether these data are applicable to other settings is unclear. Proteinuria data were not available for the majority of people and were excluded from the model. We were unable to differentiate ACEI/ARB discontinuation with replacement with alternative antihypertensive medications from discontinuation without replacement.
In conclusion, we have demonstrated that ACEI/ARB discontinuation, even for a short period, is associated with a substantially increased risk of death and ESKD in a large cohort of patients with CKD. It remains to be determined what factors are driving the high frequency of discontinuations and, more importantly, what the appropriate stopping and restarting criteria are for ACEI/ARBs to optimize outcomes.
FUNDING
This work was supported by research funding from the US Department of Veterans Affairs Health Services Research & Development (1I01HX002917-01A1). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the Department of VA or the US government. C.P.W. is supported in part by the US National Institute of Diabetes and Digestive and KidneyDiseases (K23DK122131). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.”
CONFLICT OF INTEREST STATEMENT
S.D.N., P.A.R. and S.S.V. are employees of the US Department of Veterans Affairs. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the Department of Veterans Affais or the US government. S.D.N. reports personal fees from Bayer, Boehringer-Ingelheim, REATA and Tricida and grants from Keryx, outside the submitted work. W.C.W. reports personal fees from Akebia, AstraZeneca, Bayer, Merck, Janssen, Vifor Fresenius Medical Care and Renal Pharma (including Relypsa), outside the submitted work. All remaining authors have nothing to disclose. This work was presented as an oral abstract at Kidney Week 2019 held in Washington, DC. The results presented in this article have not been published previously in whole or part, except in abstract format.
(See related article by Rossignol and Agarwal. Should renin–angiotensin–aldosterone system inhibition enablement be a therapeutic target in CKD patients? Nephrol Dial Transplant 2021; 36: 1771–1772)
REFERENCES
- 1. Levin A, Stevens PE, Bilous RW. et al. Kidney Disease: Improving Global Outcomes CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150 [DOI] [PubMed] [Google Scholar]
- 2. Rüster C, Wolf G.. Renin-angiotensin-aldosterone system and progression of renal disease. J Am Soc Nephrol 2006; 17: 2985–2991 [DOI] [PubMed] [Google Scholar]
- 3.US Department of Veterans Affairs. Management of Chronic Kidney Disease (CKD) in Primary Care. Washington, DC: US Department of Veterans Affairs, 2014
- 4. Whelton PK, Carey RM, Aronow WS. et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018; 71: e13–e115 [DOI] [PubMed] [Google Scholar]
- 5. Shah AR, Sparks AM.. Renin–angiotensin system inhibition in advanced chronic kidney disease: how low can the kidney function go? Curr Opin Nephrol Hypertens 2019; 28: 171–177 [DOI] [PubMed] [Google Scholar]
- 6.US Renal Data System. Annual Data Report. Chapter 7: Prescription Drug Coverage in Patients with CKD. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2017
- 7. Navaneethan SD, Akeroyd JM, Ramsey D. et al. Facility-level variations in kidney disease care among veterans with diabetes and CKD. Clin J Am Soc Nephrol 2018; 13: 1842–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yildirim T, Arici M, Piskinpasa S. et al. Major barriers against renin-angiotensin-aldosterone system blocker use in chronic kidney disease stages 3–5 in clinical practice: a safety concern? Ren Fail 2012; 34: 1095–1099 [DOI] [PubMed] [Google Scholar]
- 9. Whiting P, Morden A, Tomlinson LA. et al. What are the risks and benefits of temporarily discontinuing medications to prevent acute kidney injury? A systematic review and meta-analysis. BMJ Open 2017; 7: e012674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mansfield KE, Nitsch D, Smeeth L. et al. Prescription of renin–angiotensin system blockers and risk of acute kidney injury: a population-based cohort study. BMJ Open 2016; 6: e012690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tomson C, Tomlinson LA.. Stopping RAS inhibitors to minimize AKI: more harm than good? Clin J Am Soc Nephrol 2019; 14: 617–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Levey AS, Stevens LA, Schmid CH.. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Walther CP, Richardson PA, Virani SS. et al. Association between intensity of statin therapy and mortality in persons with chronic kidney disease. Nephrol Dial Transplant 2020; 35: 312–319 [DOI] [PubMed] [Google Scholar]
- 14. Fung E, Chang TI, Chertow GM. et al. Receipt of nephrology care and clinical outcomes among veterans with advanced CKD. Am J Kidney Dis 2017; 70: 705–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deyo RA, Cherkin DC, Ciol MA.. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992; 45: 613–619 [DOI] [PubMed] [Google Scholar]
- 16. Sundararajan V, Henderson T, Perry C. et al. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol 2004; 57: 1288–1294 [DOI] [PubMed] [Google Scholar]
- 17. Gosmanova EO, Molnar MZ, Naseer A. et al. Longer predialysis ACEi/ARB utilization is associated with reduced postdialysis mortality. Am J Med 2020; 133: 1065–1073.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weiner DE, Tighiouart H, Vlagopoulos PT. et al. Effects of anemia and left ventricular hypertrophy on cardiovascular disease in patients with chronic kidney disease. J Am Soc Nephrol 2005; 16: 1803–1810 [DOI] [PubMed] [Google Scholar]
- 19. McCormick N, Bhole V, Lacaille D. et al. Validity of diagnostic codes for acute stroke in administrative databases: a systematic review. PLoS One 2015; 10: e0135834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Becker GJ, Wheeler DC, De Zeeuw D. et al. Kidney Disease: Improving Global Outcomes Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl 2012; 2: 337–414 [Google Scholar]
- 21. Hou FF, Zhang X, Zhang GH. et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med 2006; 354: 131–140 [DOI] [PubMed] [Google Scholar]
- 22. Remuzzi G, Ruggenenti P, Perna A. et al. Continuum of renoprotection with losartan at all stages of type 2 diabetic nephropathy: a post hoc analysis of the RENAAL trial results. J Am Soc Nephrol 2004; 15: 3117–3125 [DOI] [PubMed] [Google Scholar]
- 23. Ruggenenti P, Perna A, Remuzzi G.. ACE inhibitors to prevent end-stage renal disease: when to start and why possibly never to stop: a post hoc analysis of the REIN trial results. Ramipril Efficacy in Nephropathy. J Am Soc Nephrol 2001; 12: 2832–2837 [DOI] [PubMed] [Google Scholar]
- 24. Bhandari S, Ives N, Brettell EA. et al. Multicentre randomized controlled trial of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker withdrawal in advanced renal disease: the STOP-ACEi trial. Nephrol Dial Transplant 2016; 31: 255–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ahmed AK, Kamath NS, El Kossi M. et al. The impact of stopping inhibitors of the renin-angiotensin system in patients with advanced chronic kidney disease. Nephrol Dial Transplant 2010; 25: 3977–3982 [DOI] [PubMed] [Google Scholar]
- 26. Qiao Y, Shin JI, Sang Y. et al. Discontinuation of angiotensin converting enzyme inhibitors and angiotensin receptor blockers in chronic kidney disease. Mayo Clin Proc 2019; 94: 2220–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qiao Y, Shin JI, Chen TK. et al. Association between renin-angiotensin system blockade discontinuation and all-cause mortality among persons with low estimated glomerular filtration rate. JAMA Intern Med 2020; 180: 718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arora N, Katz R, Bansal N.. ACE inhibitor/angiotensin receptor blocker use patterns in advanced CKD and risk of kidney failure and death. Kidney Med 2020; 2: 248–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Murphy DP, Drawz PE, Foley RN.. Trends in angiotensin-converting enzyme inhibitor and angiotensin II receptor blocker use among those with impaired kidney function in the United States. J Am Soc Nephrol 2019; 30: 1314–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brar S, Ye F, James MT. et al. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with outcomes after acute kidney injury. JAMA Intern Med 2018; 178: 1681–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]