Abstract
Objectives
The aim was to compare the accuracy of colour Doppler ultrasonography (CDUS) and temporal artery biopsy (TAB) to establish the final diagnosis of GCA and to determine how the GCA probability score (GCAPS) performs as a risk stratification tool.
Methods
Descriptive statistics were performed on a retrospective cohort of patients referred to our vasculitis referral centre between 1 July 2017 and 1 October 2020 for suspected GCA. CDUS, TAB, centre-specific TAB (vasculitis centre vs referring hospitals) and GCAPS were compared against the final diagnosis of GCA as determined by a GCA expert; CDUS was also compared with TAB results.
Results
Data from 198 patients were included: 60 patients with GCA and 138 patients without GCA. Sixty-two patients had a TAB. Using the final diagnosis by a GCA expert as a reference, the sensitivity, specificity, positive predictive value and negative predictive value were 93.3%, 98.5%, 96.6% and 97.1% for CDUS and 69.2%, 100%, 100% and 81.8% for TAB, respectively. The false-negative rate was 6.7% for CDUS and 30.8% for TAB. False-negative TAB mostly occurred when performed in referring hospitals (57.1%) as opposed to our vasculitis centre (21.1%). With a cut-off at 9.5 points, sensitivity for GCAPS was 98.3% and specificity 74.3%.
Conclusion
CDUS of the temporal and axillary arteries showed a high sensitivity and specificity and helped to diagnose GCA in patients with negative TAB. We validated that GCAPS is a useful clinical tool, with a score of <9.5 making the diagnosis of GCA improbable.
Keywords: GCA, colour Doppler ultrasonography, temporal arteries, probability score
Key messages
Colour Doppler ultrasonography is a highly effective tool when performed by a skilled ultrasonographer.
Colour Doppler ultrasonography has a better sensitivity than temporal artery biopsy for the diagnosis of GCA.
GCA probability score is a useful risk stratification tool for the diagnosis of GCA.
Introduction
GCA is the most common primary systemic vasculitis, affecting more women than men. Incidence increases with age, occurring almost exclusively in patients >50 years old. Higher incidence rates have been reported in populations of northern European descent [1]. Patients can present with a range of non-specific symptoms, and the heterogeneous clinical phenotypes make its diagnosis challenging. Significant morbidity can be associated with GCA, particularly if permanent loss of vision occurs. This highlights the need for prompt diagnosis and the necessity of a reliable diagnostic tool. In the past, temporal artery biopsy (TAB) was the only diagnostic tool at our disposal; however, it entails an invasive procedure with possible false negatives. There is also important heterogeneity in how TAB is performed, including biopsy length, number of specimen sections, experience of the pathologist, surgical skill and centre expertise. All these variables have the potential to influence the TAB result.
In recent years, some non-invasive imaging modalities have shown superior results. Several studies have suggested that colour Doppler ultrasonography (CDUS) of the temporal and axillary arteries is a useful tool to diagnose GCA [2–4]. The 2018 EULAR recommendations for the use of imaging in large vessel vasculitis identify CDUS as the first-line imaging modality to diagnose GCA. These recommendations also state that a patient with a high clinical suspicion and a positive imaging test could forego TAB [5, 6].
Fast-track clinics for the diagnosis of GCA are being implemented in different countries to identify GCA rapidly and decrease associated complications. A study in Norway demonstrated that a fast-track outpatient approach with CDUS is associated with an 88% reduction in permanent visual impairment in patients with GCA when compared with a conventional referral route [7]. Reliability of the CDUS is crucial in these programmes and predicts their success. Thus, it is imperative to assess the performance and accuracy of ultrasonographers and to assess inter-reader variability.
The new GCA probability score (GCAPS) is intended to risk stratify patients with suspected GCA into low and high probability categories. When using a cut-off point of 9.5, the sensitivity and specificity are reported to be 95.7% and 86.7%, respectively. Items making up this probability score include demographics (age and sex), onset of symptoms, CRP level, symptoms (headache, polymyalgia, constitutional and ischaemic symptoms), visual signs (anterior ischaemic optic neuropathy, central and branch retinal artery occlusion, visual field loss and relative afferent pupillary defect), arterial abnormalities (temporal and extracranial arteries) and presence of an alternative diagnosis. Each item is attributed points between −3 and 3; final scores can range between 0 and 32, with a higher score associated with a higher clinical probability of GCA [8]. That study was completed in a single centre in the UK, and its external validity needs to be evaluated before GCAPS can be used reliably in other GCA fast-track centres.
The objective of this study was to compare CDUS and TAB with the final diagnosis of GCA in a Canadian vasculitis referral centre; to compare rates of false-negative TAB according to the centre where it was performed (our vasculitis centre with a dedicated experienced surgeon vs referring hospitals); and to validate the usefulness of the GCAPS in patients with suspected GCA.
Methods
Study design and population
We conducted a retrospective study of all adult patients referred to our department with suspected GCA and who had a CDUS performed as part of their clinical evaluation. Patients were identified from the CAPHECO-GCA (characteristics, phenotypes and complications of patients with GCA) database, which includes all patients referred with suspected GCA in our vasculitis referral centre (Hôpital du Sacré-Coeur de Montréal, Université de Montréal).
Inclusion criteria
Consecutive adult patients referred to our GCA US clinic for suspected GCA between 1 July 2017 and 1 October 2020.
Exclusion criteria
Patients who had a CDUS performed to evaluate disease recurrence and those without a comprehensive clinical assessment by one of our vasculitis experts were excluded.
GCA assessment and clinical data
Data collected included patient characteristics, clinical presentation, physical examination, CDUS of the temporal (superficial, frontal and parietal branches) and axillary arteries, TAB results, blood work and GCAPS. Normocytic anaemia was defined as a haemoglobin level <140 g/l for men and <120 g/l for women, with a mean corpuscular volume between 80 and 100 fl. Thrombocytosis was defined as platelets >400 × 109/l and leucocytosis as a white blood cell count >10 × 109/l. A CRP level >5 mg/l was considered elevated, as were ESR values >10 mm/h for men and >20 mm/h for women, using the Wintrobe method.
Colour Doppler ultrasonography was performed by the same ultrasonographer using the Zonare Z One Ultra Ultrasound System with a linear array 14L5 probe or the Canon Xario 200 Platinum series with an 18L7 probe. The ultrasonographer was trained in the use of CDUS for the diagnosis of GCA and had 4 years of experience. Longitudinal and transverse planes were obtained, and compression was applied on all temporal artery branches (common superficial, frontal and parietal branches). A positive CDUS result was defined by a hypoechoic circumferential intima–media thickening (halo sign) in at least two arterial segments and/or the presence of stenosis or thrombosis. These findings were confirmed with a positive compression sign (inability to compress the hypoechoic vessel wall oedema) [9–11].
Data on TAB performed both in our vasculitis centre and in referring hospitals were collected. In our centre, biopsies were performed by a single skilled vascular surgeon and analysed by the same experienced pathologist. Biopsies done in referring hospitals were performed by different surgery subspecialties (general surgery, ophthalmology, otorhinolaryngology or plastic surgery). The ultrasonographer, the surgeon and the pathologist were not blinded to the patient’s clinical characteristics or laboratory results.
If GCAPS was not documented specifically in the database, it was calculated retrospectively when all the required items were available. The final diagnosis as determined by the vasculitis specialist 6 months after the initial assessment was considered the gold standard for the diagnosis of GCA.
Statistical analysis
Analyses were performed using IBM SPSS, v.25.0. They included sensitivity, specificity, positive predictive value and negative predictive value for CDUS, TAB, centre-specific TAB (dedicated vasculitis centre vs referring hospitals) and GCAPS compared with the final diagnosis by a GCA expert. Although TAB is not the gold standard for the diagnosis of GCA, CDUS was also compared with TAB as a reference test. A receiver operating characteristic curve was plotted for GCAPS against the final diagnosis of GCA.
Categorical variables were presented as proportions (percentages) and continuous variables as mean values (S.d.) for normally distributed variables or medians [interquartile range (IQR)]. The χ2 test was used to compare categorical variables, and Fisher’s exact when appropriate. Student’s unpaired t-test was used for the comparison of continuous variables within two groups. A P-value of <0.05 was considered statistically significant.
Ethics and approval committee
This retrospective study was approved by the research ethics board of the Centre Intrégré Universitaire de santé et de services sociaux du Nord-de-l’Île de Montréal (study number 2019-1754). No modifications have been made to the research protocol, and approval was renewed annually.
Results
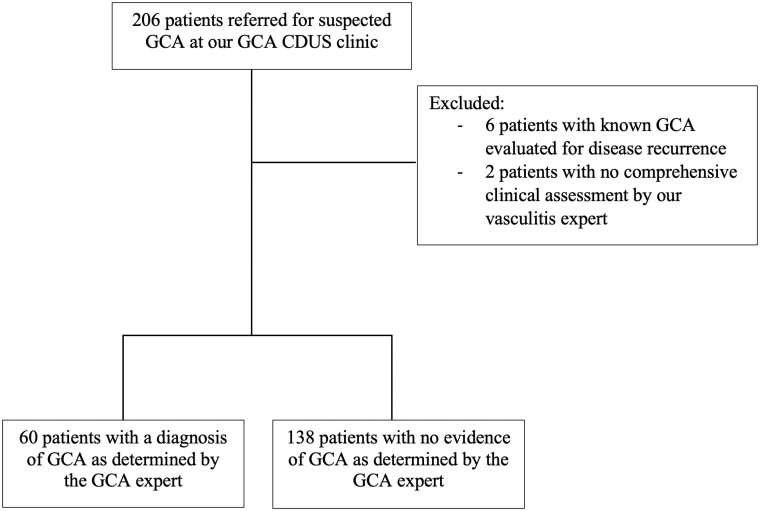
A total of 206 consecutive patients referred for suspected GCA were identified. Eight were excluded, leaving 198 patients included in the study (Fig. 1). Sixty patients had a final diagnosis of GCA as determined by the vasculitis expert, whereas 138 patients had an alternative diagnosis (Supplementary Table S1, available at Rheumatology Advances in Practice online). The main clinical characteristics of patients referred for suspected GCA are presented in Table 1. The mean age of patients diagnosed with GCA was 75.2 (7.3) years, and 70% were females. In patients with GCA, CRP was elevated in 95% of patients (mean value of 81.4 mg/l), and ESR, when performed, was elevated in 46.7% of patients (mean value of 45.3 mm/h). Common clinical manifestations reported by patients with GCA were headaches (81.7%) and constitutional symptoms (55.0%). New visual symptoms were reported by 36.7% of patients with GCA. On the initial assessment, none of the patients with GCA had suffered from stroke or transient ischaemic attack. Clinical features strongly associated with GCA included the presence of jaw claudication (likelihood ratio (LR) = 28.9; P < 0.001) and abnormal temporal artery on physical examination (LR = 50.88; P < 0.001). Normocytic anaemia, leucocytosis, thrombocytosis and elevated CRP were more often present in patients with GCA (Table 1).
Fig. 1.
Flowchart of patients included in the study.
CDUS: colour Doppler ultrasonography.
Table 1.
Baseline characteristics and initial presentation
| Parameter | GCA (n = 60) | No GCA (n = 138) | P-value |
|---|---|---|---|
| Age, mean (S.d.), years | 75.2 (7.3) | 70.7 (12.0) | 0.008 |
| Female, n (%) | 42 (70.0) | 99 (71.7) | 0.804 |
| Clinical features, n (%) | LR (P-value) | ||
| Headache | 49 (81.7) | 94 (68.1) | 4.03 (0.045) |
| Scalp tenderness | 21 (35.0) | 28 (20.3) | 5.63 (0.060) |
| Jaw claudication | 20 (33.3) | 6 (4.3) | 28.94 (<0.001) |
| Visual symptoms | 22 (36.7) | 30 (21.7) | 5.06 (0.073) |
| PMR | 18 (30.0) | 28 (20.3) | 2.60 (0.273) |
| Constitutional symptoms | 33 (55.0) | 28 (20.3) | 24.66 (<0.001) |
| Abnormal temporal artery on examination | 29 (48.3) | 7 (5.1) | 50.88 (<0.001) |
| Laboratory values, n (%) | |||
| Normocytic anaemiaa | 33 (55.0) | 39 (28.3) | 12.84 (0.002) |
| Elevated WBCb | 16 (26.7) | 16 (11.6) | 6.61 (0.037) |
| Thrombocytosisc | 19 (31.7) | 8 (5.8) | 21.73 (<0.001) |
| Elevated CRPd | 57 (95.0) | 82 (59.6) | 31.12 (<0.001) |
| Elevated ESRe | 28 (46.7) | 53 (38.4) | 6.09 (0.48) |
| Inflammatory markers | P-value | ||
| ESR, mean (S.d.), mm/h | 45.3 (23.5) | 36.1 (27.4) | 0.103 |
| CRP, mean (S.d.), mg/l | 81.4 (63.9) | 39.42 (60.6) | <0.001 |
| Temporal artery biopsy | |||
| Length, mean (S.d.), cm | 1.68 (0.33) | 1.53 (0.41) | 0.172 |
| Glucocorticoid use before CDUS, median (IQR), days | 4.00 (1.00–10.00) | 0.00 (0.00–5.00) | <0.001 |
| Cumulative dose of glucocorticoids before CDUS, mean (S.d.), mg | 772.73 (1135.0) | 232.05 (569.8) | <0.001 |
Haemoglobin <140 g/l for men and <120 g/l for women, with a mean corpuscular volume between 80 and 100 fl.
White blood cells >10 × 109/l.
Platelets >400 × 109/l.
CRP >5 mg/l.
ESR (Wintrobe method) >10 mm/h for men and >20 mm/h for women.
CDUS: colour Doppler ultrasonography; IQR: interquartile range; WBC: white blood cell.
All 198 patients included had a CDUS performed, and 62 patients had a TAB. Using the final diagnosis as determined by the vasculitis expert as a reference, the sensitivity, specificity, positive predictive value and negative predictive value were 93.3%, 98.5%, 96.6% and 97.1% for CDUS and 69.2%, 100%, 100% and 81.8% for TAB, respectively (Table 2). The false-negative rate of CDUS was 6.7%, as opposed to 30.8% for TAB. Two of the four patients with a diagnosis of GCA with a negative CDUS had a positive TAB. A negative TAB was observed in 8 of the 26 patients with GCA who had undergone TAB, all of whom had a positive CDUS except for one. Two false-positive results were observed with CDUS, whereas none was documented with TAB. When TAB was used as a reference for the diagnosis of GCA, CDUS had a sensitivity, specificity, positive predictive value and negative predictive value of 88.9%, 79.1%, 64% and 94.4%, respectively (Supplementary Table S2, available at Rheumatology Advances in Practice online). GCA was diagnosed in 42 patients when TAB was either negative or not performed (Supplementary Table S3, available at Rheumatology Advances in Practice online, for their baseline characteristics).
Table 2.
Comparison of results obtained by colour Doppler ultrasonography and temporal artery biopsy
| Parameter | GCA (n = 60), n/total (%) | No GCA (n = 138), n/total (%) |
|---|---|---|
| CDUS, n = 198 | ||
| Positive CDUSa | 56/60 (93.3) | 2/138 (1.4) |
| Negative CDUS | 4/60 (6.7) | 134/138 (97.1) |
| Inconclusive CDUSb | 0/60 (0.0) | 2/138 (1.4) |
| TAB, n = 62 | ||
| Positive TAB | 18/26 (69.2) | 0/36 (0.0) |
| Negative TAB | 8/26 (30.8) | 36/36 (100.0) |
| TAB performed in our vasculitis centre, n = 40 | ||
| Positive TAB | 15/19 (78.9) | 0/21 (0.0) |
| Negative TAB | 4/19 (21.1) | 21/21 (100.0) |
| TAB performed in referring hospitals, n = 22 | ||
| Positive TAB | 3/7 (42.9) | 0/15 (0.0) |
| Negative TAB | 4/7 (57.1) | 15/15 (100.0) |
Fifty-six patients with positive CDUS: 55 patients with positive CDUS of the temporal arteries, 12 patients with positive CDUS of axillary arteries, including 1 patient with positive CDUS of isolated axillary arteries without temporal artery involvement.
The US was either technically difficult to interpret or showed mild thickening of the intima–media complex at the upper limit of normal.
CDUS: colour Doppler ultrasonography; TAB: temporal artery biopsy.
The mean TAB specimen length was 1.68 and 1.53 cm (P = 0.172) in the GCA group and the non-GCA group, respectively. TAB specimens sampled in our vasculitis centre were longer than those obtained in referring hospitals, with an average length of 1.66 vs 1.30 cm (P = 0.014). The false-negative rate of TAB performed in our vasculitis centre was 21.1%, as opposed to 57.1% for those performed in the referring centre (Table 2).
Patients were on a glucocorticoid regimen for a median of 2 days (IQR 0.00–7.50) before they were referred to our US clinic and 7 days (IQR 4.75–13.50) before TAB was performed. Patients with a positive TAB were on glucocorticoids for a median of 9 days (IQR 4.00–21.50) before TAB was performed vs 7 days (IQR 4.00–13.00) when TAB was negative (P = 0.771). Patients with a TAB performed in our vasculitis centre tended to have a longer treatment course of glucocorticoid before TAB [median of 11.00 days (IQR 4.00–20.00)] compared with patients from referring hospitals [median of 5.00 days (IQR 5.00–7.00)], but this difference was not found to be statistically significant (P = 0.053).
With a cut-off of 9.5 points for the GCAPS, the sensitivity, specificity, positive predictive value and negative predictive value were 98.3%, 74.3%, 62.0% and 99.0%, respectively (Table 3). The area under the receiver operating characteristic curve for the 194 patients with a calculated GCAPS was 0.927 (95% CI: 0.892, 0.961). The optimal cut-off point for the curve was 9.5 points (Fig. 2).
Table 3.
Comparison of GCA probability score between patients with and without a diagnosis of GCA
| Parameter | GCA (n = 60) | No GCA (n = 138) | P-value |
|---|---|---|---|
| GCAPS points, mean (S.d.) | 14.3 (2.9) | 8.1 (3.1) | <0.001 |
| Patients with >9.5 points on GCAPS, n (%) | 57/58 (98.3) | 35/136 (25.7) | <0.001 |
| Patients with <9.5 points on GCAPS, n (%) | 1/58 (1.7) | 101/136 (74.3) |
GCAPS: GCA probability score.
Fig. 2.
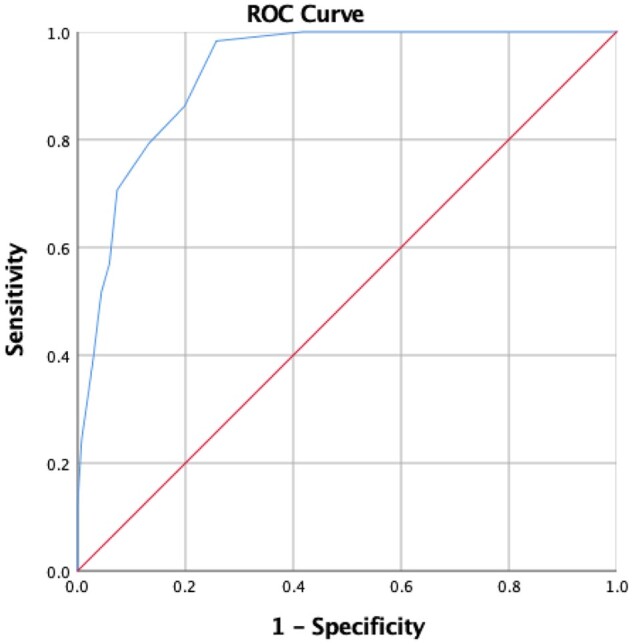
Receiver operating characteristic curve for GCA probability score
The area under the receiver operating characteristic curve for the 194 patients with a calculated GCAPS was 0.927 (95% CI: 0.892, 0.961). The optimal cut-off point for the curve was 9.5 points. GCAPS: GCA probability score.
Discussion
Increasing evidence in recent years indicates that CDUS is a helpful diagnostic tool for GCA. Our study demonstrated that CDUS has an excellent sensitivity and specificity when performed by a trained sonographer and that it performs better than TAB.
Wide ranges of sensitivities and specificities for CDUS have been reported, with superior results when performed by a trained sonographer using high-resolution equipment [3, 12, 13]. Many studies have shown that TAB is less sensitive than CDUS [14]. For instance, The Role of Ultrasound Compared with Biopsy of Temporal Arteries in the Diagnosis and Treatment of GCA (TABUL) study demonstrated that CDUS had a better sensitivity for the diagnosis of GCA in addition to being a cost-effective approach, whereas the sensitivity of TAB was reported to be as low as 39%. The specificity of CDUS was reported to be inferior to that of the TAB group, but these results were affected by the lack of experience of most sonographers [4]. A previous study suggested that the overall sensitivity and specificity of temporal artery US was >90% compared with the clinical diagnosis as established by the treating physician based on standard diagnostic criteria [3]. CDUS has also been proposed as a tool for the follow-up of patients with GCA; however, a previous study demonstrated no added value to this approach [15].
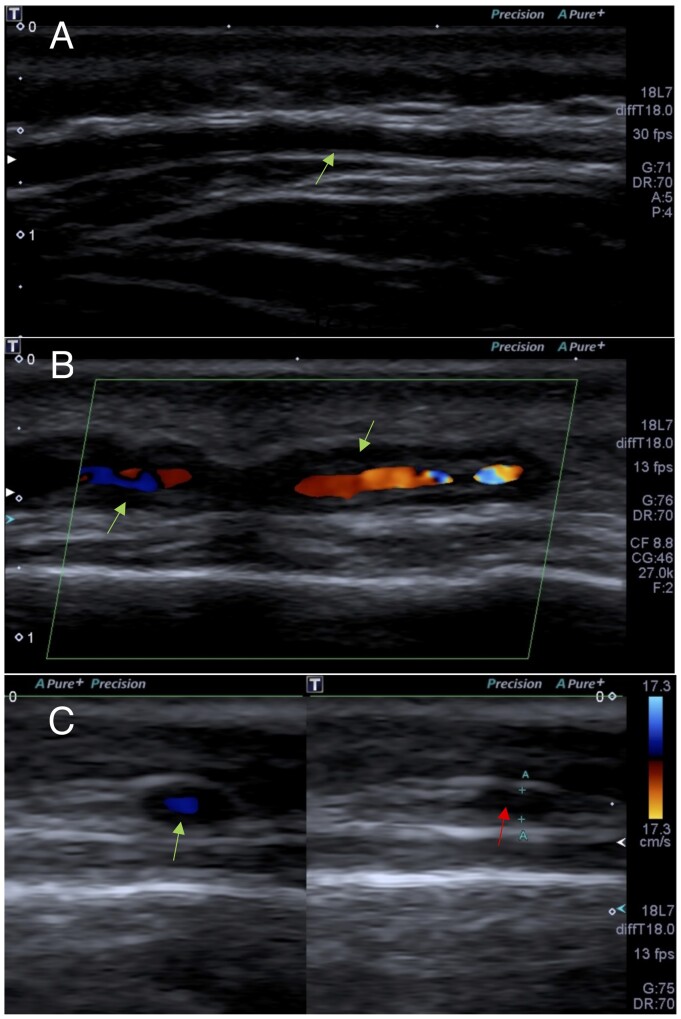
There are several advantages to CDUS, including the absence of radiation and procedural risks, easy access, rapid results, low cost and the possibility to identify skip lesions missed by TAB (Fig. 3). Skip lesions have been reported to occur in between 8.5 and 28% of TAB in patients with GCA [16–18]. The rate of false-negative TAB is reported to be as high as 15–60% [19]. We found similar data in our study, with a TAB false-negative rate of 30.8%. When TAB was performed in our vasculitis centre, the false-negative rate was 21.1%, compared with 57.1% in referring hospitals.
Fig. 3.
Colour Doppler ultrasonography of the temporal arteries
(A) Longitudinal view of a normal intima–media complex of the left frontal branch of the temporal artery (arrow). (B) Longitudinal view of a hypoechoic thickening of the intima–media (halo sign) of the left parietal branch of the temporal artery (arrow). (C) Transverse view of a halo sign using colour Doppler (green arrow) and positive compression sign in that location (red arrow).
Temporal artery biopsy performed in our centre has a false-negative rate in the lower range of that reported in the literature [14]. One explanation for this might be that in our centre, TAB is performed by a dedicated, experienced vascular surgeon and interpreted by a single pathologist. Most TABs in our centre were performed after CDUS, with a specific request regarding which temporal side and arterial segment to biopsy, whereas TABs outside our centre had been done without such guidance. Finally, TAB specimens were longer in our centre than those performed in referring hospitals, which is likely to enhance the diagnostic yield. TAB length was previously shown to be an important predictor of histopathological diagnosis; for every increase in biopsy length of 0.5 cm, there was an increase in the odds ratio for positive TAB up to 2.0 cm [20]. Overall, this highlights the importance of having a skilled surgeon to perform TAB and an experienced pathologist to analyse the tissue sample. Interestingly, a retrospective Canadian study collected data on TAB performed by 11 different subspecialties, each with a different level of experience depending on geographical location. The number of TABs done per specialty during a 10 year time frame ranged between 1 (emergency medicine) and 3791 (general surgery) [21].
GCAPS is an interesting tool for the risk stratification of patients with suspected GCA, particularly to exclude the diagnosis. The sensitivity of GCAPS in our study (with a cut-off of 9.5 points) was similar (98.3%) to the original publication (95.7%) but had a lower specificity of 74.3% vs 86.7%, respectively. In the previous study, 88.4% of patients were classified accurately using GCAPS, whereas 79.5% of our patient population was categorized correctly. This could be explained by the nature of our study, with certain GCAPS being calculated retrospectively. The receiver operating characteristic curve for GCAPS demonstrated an optimal cut-off point of 9.5 points, identical to what was previously reported [8].
Two patients in our study had a false-positive CDUS (1.47%), which is similar to what was observed in previous studies [22]. The first patient had a recent acute otitis media with mastoiditis; he had been referred to our clinic to rule out GCA because his inflammatory markers remained elevated despite the infectious episode presumably being resolved. Further investigations demonstrated an invasive ENT infection with endovasculitis. The second patient was referred with a high clinical probability of GCA (GCAPS = 17), with headaches, visual and constitutional symptoms, abnormal temporal artery examination and elevated inflammatory markers. CDUS was positive on temporal and axillary arteries. GCA was diagnosed, but the patient did not improve with glucocorticoids. Subsequent large vessel CT angiography revealed an aortic lesion compatible with angiosarcoma, which was confirmed on biopsy.
Four patients with GCA had a false-negative CDUS. Three of these four had a prolonged course of glucocorticoids before CDUS (ranging from 30 to 480 days before CDUS); GCA was confirmed with either TAB or extracranial large vessel imaging. The fourth patient underwent one of the first CDUS performed at our centre. He had been on CSs for 5 days before US examination and had a positive TAB. These cases highlight that the accuracy of CDUS decreases with glucocorticoid use [4, 23]. The TABUL study described optimal results when CDUS was performed on patients within 7 days of the start of glucocorticoids and, ideally, after they had received only a single dose of glucocorticoids. Additionally, vasculitides are heterogeneous diseases that can present with a range of symptoms, with many common diseases mimicking primary vasculitides [24, 25].
This study has several strengths. Patients were included consecutively, and most were assessed by a GCA specialist. The database was thorough, and we demonstrated that CDUS is highly performant in our referral centre, similar to previous studies published by CDUS pioneers. Although many factors influence TAB results, our study raises the question of whether having a consistent experienced surgical specialist and pathologist might improve the yield of TAB.
Our study has several limitations. The study was retrospective, and the ultrasonographer was not blinded to the patient’s characteristics, physical examination and laboratory values. However, the final diagnosis was confirmed 6 months after the original assessment. The external validity of our results might be affected by the fact that CDUS was performed by the same US expert and by the single-centre design of the study.
Conclusion
CDUS of the temporal and axillary arteries showed a high sensitivity and specificity and is a useful tool for the diagnosis of GCA when performed by an experienced sonographer. Good-quality TAB performed by a skilled surgeon and interpreted by an experienced pathologist can help to reduce the rate of false-negative TAB. The GCAPS is a useful clinical tool in our cohort of patients, with a score <9.5 points making the diagnosis of GCA unlikely.
Supplementary Material
Acknowledgements
We would like to thank the Canadian Network for Research on Vasculitides (CanVasc) for the support provided for this study. We gratefully thank our research coordinator, Ms Guylaine Marcotte, for her involvement throughout the study. We also thank Dr Alexandra Mereniuk for reviewing the manuscript. All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Study conception and design: Dr Makhzoum, Dr Ross. Acquisition of data: Dr Zarka, Dr Makhzoum. Analysis and interpretation of data: Dr Zarka, Dr Ross, Dr Makhzoum.
Funding: Funding was provided by CanVasc (Canadian network for research on vasculitides) to initiate the CAPHECO-GCA database.
Disclosure statement: J.-P.M. reports personal fees from Hoffmann-La Roche and GlaxoSmithKline outside the submitted work. The remaining authors have declared no conflicts of interest.
Data availability statement
The database used in this study is available from the corresponding author upon reasonable request. Dr Zarka and Dr Makhzoum had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Supplementary data
Supplementary data are available at Rheumatology Advances in Practice online.
References
- 1. Mohammad AJ, Englund M, Turesson C, Tomasson G, Merkel PA.. Rate of comorbidities in giant cell arteritis: a population-based study. J Rheumatol 2017;44:84–90. [DOI] [PubMed] [Google Scholar]
- 2. Duftner C, Dejaco C, Sepriano A. et al. Imaging in the diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open 2018;4:e000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schmidt WA, Kraft HE, Vorpahl K, Völker L, Gromnica-Ihle EJ.. Color duplex ultrasonography in the diagnosis of temporal arteritis. N Engl J Med 1997;337:1336–42. [DOI] [PubMed] [Google Scholar]
- 4. Luqmani R, Lee E, Singh S. et al. The role of ultrasound compared to biopsy of temporal arteries in the diagnosis and treatment of giant cell arteritis (TABUL): a diagnostic accuracy and cost-effectiveness study. Health Technol Assess 2016;20:1–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dejaco C, Ramiro S, Duftner C. et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis 2018;77:636–43. [DOI] [PubMed] [Google Scholar]
- 6. Hellmich B, Agueda A, Monti S. et al. Update of the EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis 2020;79:19–30. [DOI] [PubMed] [Google Scholar]
- 7. Diamantopoulos AP, Haugeberg G, Lindland A, Myklebust G.. The fast-track ultrasound clinic for early diagnosis of giant cell arteritis significantly reduces permanent visual impairment: towards a more effective strategy to improve clinical outcome in giant cell arteritis? Rheumatology (Oxford) 2016;55:66–70. [DOI] [PubMed] [Google Scholar]
- 8. Laskou F, Coath F, Mackie SL. et al. A probability score to aid the diagnosis of suspected giant cell arteritis. Clin Exp Rheumatol 2019;37(Suppl 117(2)): 104–8. [PubMed] [Google Scholar]
- 9. Chrysidis S, Duftner C, Dejaco C. et al. Definitions and reliability assessment of elementary ultrasound lesions in giant cell arteritis: a study from OMERACT Large Vessel Vasculitis Ultrasound Working Group. RMD Open 2018;4:e000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aschwanden M, Daikeler T, Kesten F. et al. Temporal artery compression sign – a novel ultrasound finding for the diagnosis of giant cell arteritis. Ultraschall Med 2013;34:47–50. [DOI] [PubMed] [Google Scholar]
- 11. Aschwanden M, Imfeld S, Staub D. et al. The ultrasound compression sign to diagnose temporal giant cell arteritis shows an excellent interobserver agreement. Clin Exp Rheumatol 2015;33:S-113–5. [PubMed] [Google Scholar]
- 12. Diamantopoulos AP, Haugeberg G, Hetland H. et al. Diagnostic value of color Doppler ultrasonography of the temporal arteries and large vessels in giant cell arteritis: a consecutive case series. Arthritis Care Res (Hoboken) 2014;66:113–9. [DOI] [PubMed] [Google Scholar]
- 13. Bley TA, Reinhard M, Hauenstein C. et al. Comparison of duplex sonography and high-resolution magnetic resonance imaging in the diagnosis of giant cell (temporal) arteritis. Arthritis Rheum 2008;58:2574–8. [DOI] [PubMed] [Google Scholar]
- 14. Rubenstein E, Maldini C, Gonzalez-Chiappe S, Chevret S, Mahr A.. Sensitivity of temporal artery biopsy in the diagnosis of giant cell arteritis: a systematic literature review and meta-analysis. Rheumatology (Oxford) 2020;59:1011–20. [DOI] [PubMed] [Google Scholar]
- 15. Monti S, Floris A, Ponte CB. et al. The proposed role of ultrasound in the management of giant cell arteritis in routine clinical practice. Rheumatology (Oxford) 2018;57:112–9. [DOI] [PubMed] [Google Scholar]
- 16. Poller DN, van Wyk Q, Jeffrey MJ.. The importance of skip lesions in temporal arteritis. J Clin Pathol 2000;53:137–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klein RG, Campbell RJ, Hunder GG, Carney JA.. Skip lesions in temporal arteritis. Mayo Clin Proc 1976;51:504–10. [PubMed] [Google Scholar]
- 18. Karassa FB, Matsagas MI, Schmidt WA, Ioannidis JP.. Meta-analysis: test performance of ultrasonography for giant-cell arteritis. Ann Intern Med 2005;142:359–69. [DOI] [PubMed] [Google Scholar]
- 19. Monti S, Floris A, Ponte C. et al. The use of ultrasound to assess giant cell arteritis: review of the current evidence and practical guide for the rheumatologist. Rheumatology (Oxford) 2018;57:227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chu R, Foster C, Ali M. et al. A ten-year retrospective review of temporal artery biopsy lengths in Alberta [abstract]. Arthritis Rheumatol 2019;71(Suppl 10). [Google Scholar]
- 21. Micieli JA, Micieli R, Margolin EA.. A review of specialties performing temporal artery biopsies in Ontario: a retrospective cohort study. CMAJ Open 2015;3:E281–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fernández-Fernández E, Monjo-Henry I, Bonilla G. et al. False positives in the ultrasound diagnosis of giant cell arteritis: some diseases can also show the halo sign. Rheumatology (Oxford) 2020;59:2443–7. [DOI] [PubMed] [Google Scholar]
- 23. Schmidt WA. Takayasu and temporal arteritis. Front Neurol Neurosci 2006;21:96–104. [DOI] [PubMed] [Google Scholar]
- 24. Zarka F, Veillette C, Makhzoum JP.. A review of the primary vasculitis mimickers based on the Chapel Hill consensus classification. Int J Rheumatol 2020;2020:8392542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Keser G, Aksu K.. Diagnosis and differential diagnosis of large-vessel vasculitides. Rheumatol Int 2019;39:169–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The database used in this study is available from the corresponding author upon reasonable request. Dr Zarka and Dr Makhzoum had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.