Abstract
In 2020, new vancomycin guidelines were released, recommending the transition from trough-based to AUC24 monitoring for adult and paediatric patients. Given the resources required to achieve this transition, there has been debate about the costs and benefits of AUC24-based monitoring. A recent narrative review of vancomycin therapeutic drug monitoring in paediatrics claims to have uncovered the methodological weaknesses of the data that informed the guidelines and advises against premature adoption of AUC24-guided monitoring. In this article, we present supporting arguments for AUC24-guided monitoring in children, which include that: (i) troughs alone are inadequate surrogates for AUC24; (ii) vancomycin-associated nephrotoxicity has significant consequences that warrant optimization of dosing; (iii) a substantial portion of children receiving vancomycin are at high risk for poor outcomes and deserve targeted monitoring; and (iv) limited efficacy data in support of AUC24 is not a justification to revert to a less supported monitoring approach.
Introduction
We read the recent narrative review of vancomycin therapeutic drug monitoring (TDM) in paediatrics by Jorgensen et al.1 with interest. In reviewing the same literature, we come to different conclusions and would like to present an alternative perspective supporting the need for AUC-based monitoring and dosing in children. Our primary contentions are that: (i) troughs alone are inadequate surrogates for exposure, specifically the 24 h AUC (AUC24), and do not safely guide dosing in children; (ii) vancomycin-associated nephrotoxicity has significant consequences in children and is best addressed with AUC24-directed precision dosing rather than simply lowering imprecise trough ranges; (iii) not all children prescribed vancomycin require TDM, but a substantial portion are at high risk for poor outcomes (i.e. nephrotoxicity) and deserve targeted monitoring and treatment; and (iv) limited efficacy data in support of AUC24 should not be used as a justification to revert to an even less supported monitoring approach, namely troughs.
Troughs are inadequate surrogates for exposure
The authors state that troughs have been shown to be a reasonable predictor of AUC24 in children from the reviewed literature, but the R2 between AUC24 and trough concentrations across cited paediatric studies ranges from 0.14 to 0.95 (median R2 = 0.66).1 There are important limitations to the design of many studies that have attempted to determine the correlation between troughs and AUC24 in children, including those deemed to show good or excellent correlation, and the variability across these studies precludes understanding what an individual patient’s true AUC24 is based on a measured trough. Furthermore, a trough will vary based on the dosing interval in that individual patient, a highly salient issue in paediatrics. AUCs, meanwhile, are agnostic to how frequently the drug is administered and will be the same whether daily doses are given as a continuous infusion or in divided doses. A trough-based approach also ignores the fact that clinically obtained troughs are often not true concentration minimums (more than 40% of the time in one study).2 Inappropriately timed troughs lead to difficult-to-interpret results, frequently requiring a repeat sample, or inaccurate interpretations by already busy clinicians. AUCs are not subject to such issues since Bayesian programs and two-point kinetic calculations both account for the timing of samples.3,4 To rely solely on a one-time trough measurement, the timing of which in itself is imprecise, limits clinicians’ ability to perform targeted dose adjustments given the limited pharmacokinetic information that can be developed from this single measurement without applying Bayesian methods.
Multiple studies using population models have shown that troughs are poor predictors of AUCs, resulting in high variabilities of AUC24. In a study of 306 neonates who underwent AUC monitoring at a single institution, a more than 3-fold variation in AUC24 was observed for any given trough value.5 Jorgensen et al.1 cite three references to support the statement that ‘in children troughs of 7–10 mg/L have correlated with AUCs of 400 mg·h/L’. But, these articles do not truly support this statement. Ploessl et al.6 report that 15% of children with troughs in the range of 7–10 mg/L had an AUC24 >600 mg·h/L. Frymoyer et al.7 found that AUC24 ranges from 300 to 800 mg·h/L for that same trough range. Finally, Le et al.8 showed that a mean trough of roughly 8–9 (95% CI 6–11) mg/L correlated with an AUC24 of approximately 400 mg·h/L via Monte Carlo simulation. The figure that the authors use to support this shows that troughs within that 95% CI of 6–11 mg/L could have an AUC24 from 250 to >525 mg·h/L (figure cut off at this value).5 Thus, while trough values of 7–10 mg/L may correspond to an AUC24 of approximately 400 mg·h/L on average, clinicians cannot apply population level correlations to individual patients given the imprecision in this relationship.
Vancomycin-associated nephrotoxicity has significant consequences
Central to the authors’ viewpoint is that incremental exposure to vancomycin is associated with increasing kidney injury and that there is not a hierarchy of pharmacokinetic indices that predict toxicity (i.e. they claim that AUC24 is not superior to trough concentrations). Since there is not a hierarchy, they suggest that TDM should be performed using troughs because they are simpler to implement clinically. Yet, as the authors have stated, causal inference is often derived from animal models, and animal models suggest that AUC24 is superior to trough for defining the causal relationship between vancomycin exposure and toxicity.9–12 While targeting lower trough values will reduce nephrotoxicity compared with use of trough targets of 15–20 mg/L, it is not predicted to reduce nephrotoxicity to the degree that targeting AUC24 will, owing to the imprecision of trough measurements to predict AUC24 in an individual and the superiority of the AUC–toxicity relationship.
Despite the perceived challenge of Bayesian software complexities and the time associated with two-point AUC24 calculations, AUC24 estimation is cost saving for hospitals (or at worst cost neutral) compared with trough-based dosing.13 In the analysis conducted by Lee et al.,13 both two-sample AUC24 calculations and single-sample Bayesian AUC24 estimation are associated with cost benefits in adults. The savings largely stem from nephrotoxicity reduction with AUC24-based dosing, as acute kidney injury (AKI) is associated with substantial increases in societal and hospital costs.14,15 Regardless of the financial impact, the health consequences of AKI in children cannot be understated. It has been previously reported that 70% of children who experience an AKI episode from high nephrotoxic medication exposure still have ongoing damage 6 months later.16 Additionally, AKI in sepsis or critically ill children is an independent risk factor for mortality.17 These effects of AKI on children cannot be discounted and efforts to reduce AKI, whether that be by avoiding unneeded vancomycin or providing therapy targeting the pharmacokinetic/pharmacodynamic parameter most closely associated with efficacy and toxicity, are justified.
Identifying children who deserve targeted monitoring and treatment
It is also argued that we should not extrapolate pharmacokinetic/pharmacodynamic targets derived from adults to children based on the claim that ‘children most commonly present with bone and joint infections’ and that ‘prolonged bacteraemia is the exception rather than the rule’, implying that children are less severely ill than adults.1 However, with the adoption of antibiotic stewardship programmes across the USA, vancomycin use has decreased overall, especially in previously healthy children, and many recent reports suggest that its primary use is for sepsis,18,19 not in children with bone and joint infections. Usage is now predominately in critical care units and in medically complex populations (congenital heart disease, oncology, transplant, short gut syndrome etc.). We do not espouse use of AUC monitoring in all children treated with vancomycin, but a larger proportion may benefit from this approach than Jorgensen et al.1 suggest.
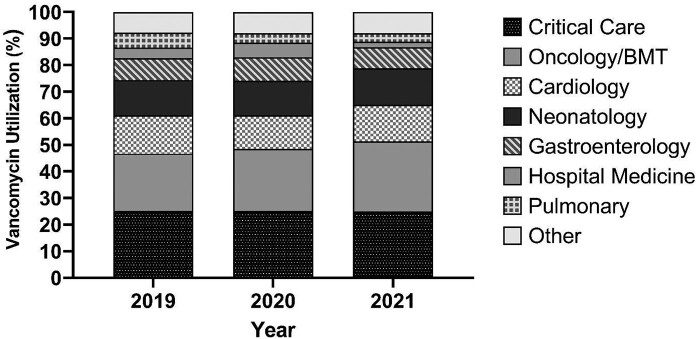
As is demonstrated by data from one of our tertiary care centres (Figure 1), since 2019 nearly 70% of vancomycin use for >48 h has been in four inpatient populations: Critical Care, Oncology/Bone Marrow Transplant, Gastroenterology and Cardiology. The children cared for on these units are vulnerable and at high risk for adverse drug events. Furthermore, the most common indications for vancomycin in these populations are for treatment of confirmed or suspected bacteraemia, febrile neutropenia, CNS infections and pneumonia, all of which carry significant morbidity and mortality risk if inadequately and not promptly treated. To ignore the adult and preclinical data for AUC24-targeted therapy would be a disservice to the medically complex, immunocompromised and critically ill patient populations who would most benefit from precision vancomycin dosing.
Figure 1.
Vancomycin utilization by specialty at Cincinnati Children’s Hospital Medical Center from January 2019 through March 2021. Only vancomycin use for >48 h was included. The Other category comprises 15 other subspecialties with varying vancomycin utilization. The figure was courteously provided by David Haslam, MD, Director of Antimicrobial Stewardship, Cincinnati Children's Hospital Medical Center.
We agree with the authors that AUC24 monitoring in all children prescribed vancomycin is unnecessary and would not be considered high-value care. Clinicians and hospitals implementing vancomycin TDM of any kind must prioritize identification of patients that would most benefit from this intervention. The 2020 vancomycin guidelines recommend AUC24-directed therapy for patients with suspected or confirmed serious MRSA infections, those at high risk of AKI, those with renal insufficiency and those receiving prolonged courses of therapy.20 We agree. As suggested by the vancomycin use data in Figure 1, this applies to a larger proportion of children than Jorgensen et al.1 recognize. Identifying children who are receiving longer durations of vancomycin, are at high risk for nephrotoxicity or have significant physiological changes that would affect AUC24 is paramount to maximize safety and reduce vancomycin-associated AKI through AUC24-targeted monitoring.
Efficacy of vancomycin
The authors also claim that there is a paucity of efficacy data supporting AUC24-directed vancomycin in paediatrics. While data are indeed limited, a recent paediatric study reported an association between persistent MRSA bacteraemia in children and AUC/MIC <300 but no association with troughs.21 The reasons for limited data stem from the fact that troughs have been utilized clinically over the past decade, not because AUC24 is not linked to efficacy in children. The inability to reliably estimate an individual patient’s AUC24 from their measured trough without Bayesian approaches has prevented large-scale efficacy studies using TDM data. And, despite extensive use of trough-based TDM for vancomycin, there remain zero studies that have demonstrated an association between specific trough concentrations and positive efficacy outcomes. To state that AUC24-directed therapy is not supported by efficacy data but claim that simply lowering trough goals is justified is fallacious.
As institutions transition from trough-based monitoring to AUC24-based monitoring, we will be able to harness the data prospectively to expand the already existing literature from adults on the safety benefits of AUC24-based monitoring.22 In fact, one paediatric institution reported that transitioning to AUC-based monitoring led to fewer dose adjustments, fewer concentration measurements and a 30% relative reduction in AKI per year.23 Equally important, we will also be able to generate the much-needed and long-awaited efficacy data. While it is possible that the efficacy benefit to controlling vancomycin exposures in children is limited, pursuing AUC24-based dosing is supportable on the basis of safety alone.22 The unknowns for efficacy should inspire institutions to collaborate and seek funding to support robust trials so that we can produce the data in a timely but rigorous manner. We should not use the lack of paediatric-specific data as an excuse to abandon the guideline-recommended AUC24-based monitoring and revert back to unsupported trough-based monitoring.
Ultimately, supporting a trough-based approach for vancomycin TDM fails to acknowledge the substantial impact that use of an imprecise measure has on our most vulnerable children. Simply dosing to achieve troughs of 5–15 mg/L may be sufficient for clinically stable and previously healthy children. But, again, these are not the majority of paediatric patients who are receiving longer-term vancomycin treatment courses. Pareto’s rule states that 80% of effects come from 20% of the causes. In the case of vancomycin, it would be reasonable to argue that 80% of children would have a similar outcome with use of AUCs, troughs or no monitoring at all. It is the remaining 20% of patients, however, who justify the cost and effort of targeted AUC24-based TDM, such as through model-informed precision dosing. Adoption of AUC24-directed therapy and robust clinical trials are simultaneously warranted. We will never know the correct target if we don’t start measuring the right thing.
Funding
This manuscript was written as part of our routine work. M.E.M. is supported by the National Institute of Child Health & Human Development Cincinnati Pediatric Clinical Pharmacology Postdoctoral Training Program (5T32HD069054-10). S.T.G. is supported by the Cincinnati Children’s Hospital Medical Center Child Health Research Career Development Award Program (5K12HD0-28827–29). J.L.G. is supported in part by a National Institute of General Medicine Sciences award (R01GM129783). M.H.S. is supported in part by a National Institute of Allergy and Infectious Diseases award (R21AI149026). K.J.D. is supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number K23HD091365.
Transparency declarations
M.H.S. has received research grants from and has consulting contracts with Nevakar and SuperTrans Medical and has patent US10688195B2 issued. K.J.D. has received research support from Merck & Co., Inc., unrelated to the current work. All other authors: none to declare.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1. Jorgensen SCJ, Dersch-Mills D, Timberlake K. et al. AUCs and 123s: a critical appraisal of vancomycin therapeutic drug monitoring in paediatrics. J Antimicrob Chemother 2021; 76: 2237–51. [DOI] [PubMed] [Google Scholar]
- 2. Morrison AP, Melanson SE, Carty MG. et al. What proportion of vancomycin trough levels are drawn too early?: frequency and impact on clinical actions. Am J Clin Pathol 2012; 137: 472–8. [DOI] [PubMed] [Google Scholar]
- 3. Suchartlikitwong P, Anugulruengkitt S, Wacharachaisurapol N. et al. Optimizing vancomycin use through 2-point AUC-based therapeutic drug monitoring in pediatric patients. J Clin Pharmacol 2019; 59: 1597–605. [DOI] [PubMed] [Google Scholar]
- 4. Neely M, Jelliffe R.. Practical, individualized dosing: 21st century therapeutics and the clinical pharmacometrician. J Clin Pharmacol 2010; 50: 842–7. [DOI] [PubMed] [Google Scholar]
- 5. Stockmann C, Sammons H, Starkey E. et al. Unanswered questions regarding optimal pediatric vancomycin use. Ther Drug Monit 2016; 38: 419–20. [DOI] [PubMed] [Google Scholar]
- 6. Ploessl C, White C, Manasco K.. Correlation of a vancomycin pharmacokinetic model and trough serum concentrations in pediatric patients. Pediatr Infect Dis J 2015; 34: e244–7. [DOI] [PubMed] [Google Scholar]
- 7. Frymoyer A, Guglielmo BJ, Hersh AL.. Desired vancomycin trough serum concentration for treating invasive methicillin-resistant staphylococcal infections. Pediatr Infect Dis J 2013; 32: 1077–9. [DOI] [PubMed] [Google Scholar]
- 8. Le J, Bradley JS, Murray W. et al. Improved vancomycin dosing in children using area under the curve exposure. Pediatr Infect Dis J 2013; 32: e155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Avedissian SN, Pais GM, O'Donnell JN. et al. Twenty-four hour pharmacokinetic relationships for intravenous vancomycin and novel urinary biomarkers of acute kidney injury in a rat model. J Antimicrob Chemother 2019; 74: 2326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Avedissian SN, Pais G, Liu J. et al. The pharmacodynamic-toxicodynamic relationship of AUC and Cmax in vancomycin-induced kidney injury in an animal model. Antimicrob Agents Chemother 2021; 65: e01945–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pais GM, Liu J, Zepcan S. et al. Vancomycin-induced kidney injury: animal models of toxicodynamics, mechanisms of injury, human translation, and potential strategies for prevention. Pharmacotherapy 2020; 40: 438–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scheetz MH, Pais G, Lodise TP. et al. Of rats and men, a translational model to understand vancomycin pharmacokinetic/toxicodynamic relationships. bioRxiv2021; doi:10.1101/2021.04.22.437975. [DOI] [PMC free article] [PubMed]
- 13. Lee BV, Fong G, Bolaris M. et al. Cost-benefit analysis comparing trough, two-level AUC and Bayesian AUC dosing for vancomycin. Clin Microbiol Infect 2020; doi:10.1016/j.cmi.2020.11.008. [DOI] [PubMed] [Google Scholar]
- 14. Ortiz-Soriano V, Donaldson K, Du G. et al. Incidence and cost of acute kidney injury in hospitalized patients with infective endocarditis. J Clin Med 2019; 8: 927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Silver SA, Long J, Zheng Y. et al. Cost of acute kidney injury in hospitalized patients. J Hosp Med 2017; 12: 70–6. [DOI] [PubMed] [Google Scholar]
- 16. Menon S, Kirkendall ES, Nguyen H. et al. Acute kidney injury associated with high nephrotoxic medication exposure leads to chronic kidney disease after 6 months. J Pediatr 2014; 165: 522–7.e2. [DOI] [PubMed] [Google Scholar]
- 17. Akcan-Arikan A, Zappitelli M, Loftis LL. et al. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int 2007; 71: 1028–35. [DOI] [PubMed] [Google Scholar]
- 18. Williams MC, Obermeier H, Hurst AL. et al. Hospital-wide description of clinical indications for pediatric anti-infective use. Clin Ther 2019; 41: 1605–11.e0. [DOI] [PubMed] [Google Scholar]
- 19. Scardina T, Stach L, Sun S. et al. Documentation of indications: agreement between order entry and clinical notes and effect on time to antibiotic administration. J Pharm Pract 2020; doi:10.1177/0897190020938225. [DOI] [PubMed] [Google Scholar]
- 20. Rybak MJ, Le J, Lodise TP. et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis 2020; 71: 1361–4. [DOI] [PubMed] [Google Scholar]
- 21. Yoo R, So H, Seo E. et al. Impact of initial vancomycin pharmacokinetic/pharmacodynamic parameters on the clinical and microbiological outcomes of methicillin-resistant Staphylococcus aureus bacteremia in children. PLoS One 2021; 16: e0247714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Neely MN, Kato L, Youn G. et al. Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob Agents Chemother 2018; 62: e02042-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Olson J, Hersh AL, Sorensen J. et al. Intravenous vancomycin therapeutic drug monitoring in children: evaluation of a pharmacy-driven protocol and collaborative practice agreement. J Pediatric Infect Dis Soc 2020; 9: 334–41. [DOI] [PubMed] [Google Scholar]