Abstract
We have produced a framework of general moral principles for animal research ethics in a book, Principles of Animal Research Ethics, which is forthcoming with Oxford University Press in fall 2019. This book includes a detailed statement and defense of our framework along with critical commentaries on our work from seven eminent scholars: Larry Carbone, Frans de Waal, Rebecca Dresser, Joseph Garner, Brian Hare, Margaret Landi, and Julian Savulescu. In the present paper, we explain the motivation for our project and present our framework of principles. The first section explains why a new framework is both needed and timely, on the basis of six important developments in recent decades. The second section challenges assertions of an unbridgeable gulf dividing the animal-research and animal-protection communities on the issue of animal research. It does so, first, by indicating common ground in the core values of social benefit and animal welfare and, then, by presenting and briefly defending our framework: three principles of social benefit and three principles of animal welfare. These six principles, we argue, constitute a more suitable framework than any other that is currently available, including the canonical 3 Rs advanced in 1959 by William M. S. Russell and Rex L. Burch.
Keywords: animal research, ethics, moral principles, 3 Rs, social benefit, animal welfare, IACUCs
Attention to the ethics of using animals in biomedical and behavioral research has surged over the past four decades. Mainstream journals in both the humanities and the sciences have increasingly published in this area after previously publishing little, if anything, on the subject. Meanwhile, codes of ethics formulated by professional societies1 as well as government regulations and guidelines in virtually every country have expanded or significantly improved during this period.2
Human research ethics has long benefited from widespread acceptance of general moral principles presented in documents such as the Belmont Report,3 but no comparable framework of principles displaying the core values of animal research ethics has been available to help in the guidance and proper oversight of animal research. (Later we will clarify why we do not regard the influential 3 Rs as constituting an adequate framework.) To fill this gap, we have produced a framework of general moral principles for animal research ethics in a book, Principles of Animal Research Ethics, to be published by Oxford University Press in the fall of 2019. This book includes a detailed statement and defense of our framework along with critical commentaries on our work from 7 eminent scholars representing an array of disciplines that grapple with animal research ethics: Larry Carbone, Frans de Waal, Rebecca Dresser, Joseph Garner, Brian Hare, Margaret Landi, and Julian Savulescu.
In this paper, we explain the motivation for our project and present our framework of principles, demonstrating its basis in shared values. The first section addresses 2 questions: Why is this new framework needed? and Why is it needed now? We answer by pointing to 6 developments that indicate the need for, and timeliness of, our framework: (1) growing public concerns about animal welfare; (2) advances in the scientific study of animals; (3) the development of animal ethics as a scholarly discipline; (4) significant gaps in the content of the 3 Rs conception of animal research ethics; (5) growing concerns among scientists about the reliability of nonhuman animals as models for humans; and (6) a persistent but unconstructive perception that fundamentally different moral perspectives on the ethics of animal research are irreconcilable.
Regarding development 6, in our second section we cast doubt on assertions of an unbridgeable gulf dividing the perspectives of persons deeply committed to biomedical research involving animals and individuals who identify strongly with animal protection. Our claim is that common ground exists between these 2 orientations that includes 2 core values: social benefit and animal welfare. On the basis of these 2 core values, we present, clarify, and briefly defend a new framework of moral principles containing 3 principles of social benefit and 3 principles of animal welfare. These 6 principles constitute a more suitable framework than any other currently available framework, including the influential one presented in Principles of Humane Experimental Technique, published in 1959 by zoologist and psychologist William M. S. Russell and microbiologist Rex L. Burch.4 Their principles are commonly referred to as the 3 Rs. We regard our set of principles as more comprehensive and more likely than theirs to foster extensive agreement among the many parties concerned to have a justified and practicable animal research ethics.
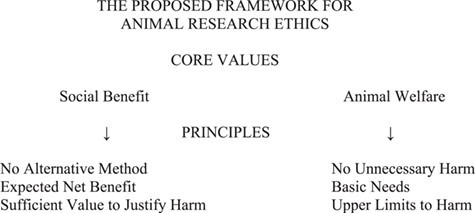
To summarize and anticipate our discussion below of the principles, here is a diagram of the structure of our framework:
The Need for a New Framework of Principles for Animal Research Ethics
Animal research currently lacks but needs a guiding ethical framework that can meet 3 demands. First, the framework must be ethically defensible, which requires being able to withstand well-informed scrutiny from specialists in ethics, investigators in science, and members of the informed general public. Second, the framework must be politically reasonable in offering a realistic chance of acceptance by persons interested in the advancement of animal research, persons interested in rigorous protection for animals involved in research, and the interested public. Third, the framework must be practically instructive by offering sound ethical guidance—even if only at a general level—to practitioners in the animal research enterprise. We believe our framework meets these demands for reasons we develop in the remainder of this paper.
Growing Public Concerns about Animal Welfare
Since roughly the 1970s, public concerns about animal welfare have increased substantially. This trend is reflected in numerous social, political, and institutional developments. For example, an increasing percentage of people have reduced or eliminated meat—and sometimes other animal products such as eggs and dairy products—from their diets in many countries.5 In addition, despite the fact that the use of animals in entertainment was rarely questioned for most of the twentieth century, we have recently seen the closing of circuses and various orca exhibits,6 the criminalization of cockfighting and dogfighting in all 50 US states and the District of Columbia,7 and protests against other forms of entertainment that involve rough treatment of animals, including rodeos.8 Meanwhile, we have seen the discontinuation of federally funded invasive research involving chimpanzees in the United States9 and involving great apes more generally in the European Union and beyond (though exceptions are sometimes permitted in the European Union).10 In all of these developments, concerns about animal welfare are prominent.
In light of these concerns about animal welfare, we maintain that the classic 3 Rs framework is no longer adequate by itself for animal research ethics. We also contend that our more comprehensive and defensible framework stands a better chance of sustaining public trust in animal research.
Advances in the Scientific Study of Animals
For much of the twentieth century, some leading schools of psychology and related disciplines encouraged a substantive view of nonhuman animals as (more or less) automata and/or a methodological view according to which the mental states of animals were not a fit topic of scientific investigation.11 Mental states such as intention were scarcely mentioned in these theories. These substantive and methodological approaches to animals’ mental lives have been heavily criticized and widely rejected in recent decades.12 Today the scientific study of animal consciousness and cognition continues to advance at a rapid pace, providing new insights into animal minds, bodies, behavior, and basic needs. Available evidence supports the attribution of consciousness or awareness to a wide range of species, probably including most vertebrate species and, among invertebrates, cephalopods.13 Higher order mental phenomena such as emotions,14 episodic memory,15 social self-awareness,16 self-recognition,17 meta-cognition,18 complex communication abilities,19 planning,20 and empathy21 are increasingly attributed to members of various nonhuman animal species on the basis of high-quality scientific studies and related analyses in the philosophy of cognitive science. Greater insight into animals’ mental lives has resulted in a higher estimation of the cognitive, psychological, and behavioral complexity of many animals, in turn fostering increased respect for them and thereby greater interest in ethical and scientific issues regarding their proper treatment, including their care and use in biomedical and behavioral research. We believe that at least one of our principles of animal welfare—the principle of basic needs (discussed in the next section)—is more responsive than the 3 Rs to the current state of scientific knowledge about animals and its ethical significance.
The Emergence and Growth of Animal Ethics as a Scholarly Field
Around the mid-1970s, a multidisciplinary scholarly literature began to emerge that focused on animals’ moral status (or inherent moral importance) and its implications for animal research ethics.22 This academic area has seen extraordinary growth, rising from obscurity to prominence and respectability in parallel with the growth of social concerns about animal welfare.23 Animal ethics today has its own academic centers, academic journals, at least one encyclopedia, and innumerable college and graduate school courses.
Some might interpret the emergence and growth of animal ethics as an idiosyncratic or temporary trend, but we agree with the consensus of scholars engaged in this area that these developments have helped to correct an unfortunate neglect of profoundly important questions about the moral status and welfare of animals. Given the maturation of animal ethics as a discipline, a framework for animal research ethics must be defensible from a standpoint that is well-informed both scientifically and ethically.
Gaps in the 3 Rs
Russell and Burch’s 3 Rs principles (or, perhaps more accurately, directives) call for replacing sentient animals with other models where possible, reducing the number of animal subjects to what is needed for statistical adequacy, and refining techniques to reduce animal pain and distress.24 Beyond government regulations and assorted codes of ethics, this text has been accorded something like canonical status for animal research ethics in many institutions and research contexts.
Historically the 3 Rs represent a landmark advance in the promotion of animal welfare and good science, and we do not underestimate this framework’s merit and historical influence. The objective of our book is not to replace it but to add complementary content for animal research ethics that the 3 Rs framework fails to provide. Russell and Burch’s principles neglect several important aspects of animal welfare as well as some important considerations pertaining to the human social benefits that justify animal research. Regarding animal welfare, the 3 Rs address this core value only in the context of “humane experimental technique”; that is, the welfare of animals is considered only insofar as they are used in scientific research procedures.25 Although the authors’ attention to this particular context is commendable, their narrow focus omits important areas of the welfare of animal subjects outside their use in scientific procedures—including matters of transport, housing, feeding, and companionship. In fairness to Russell and Burch, they note that they bracket such aspects of animals’ lives on the grounds that they are adequately addressed elsewhere.26 We maintain, by contrast, that principles of animal welfare should address all morally relevant aspects of animal research subjects’ lives. In addition to having a narrow conception of animal welfare, Russell and Burch’s framework omits important ethical considerations pertaining to human social benefit, including the likelihood of achieving benefit through animal studies and whether and how the prospect of benefit justifies anticipated costs and harms of research. Finally, the 3 Rs framework is presented without the support of ethical analysis.
The framework of core values and moral principles that we present in the next 2 sections of this paper (and more extensively in our book) fills in the gaps left by the 3 Rs pertaining to both animal welfare and social benefit. In addition, each principle is supported by explicit ethical analysis and argumentation.
Growing Scientific Concerns About the Reliability of Animal Models
Growing concerns have emerged in recent years from within the scientific community about the reliability of animals—especially rodents—as models for human biology and human disease. Some evidence indicates that attrition rates from successful animal studies to success in clinical application are at least 80%27 and possibly closer to 90%.28 One systematic review found that, among highly cited animal studies (a select group), only about one-third translated into successful human research leading to clinical use.29 A former Director of the National Institutes of Health lamented an overreliance on genetically modified mouse models,30 which have been unsuccessful in predicting effective treatments for Alzheimer’s disease after more than 200 successful animal studies (to mention only one of numerous examples).31 The Editor-in-Chief of The BMJ and co-editor of Peer Review in Health Sciences, Fiona Godlee, was sufficiently concerned about low translation rates that she stated that “funds might be better directed towards clinical rather than basic research, where there is a clearer return on investment in terms of effects on patient care.”32 Difficulties in translation have also been felt in some areas of pharmaceutical research.33 In response to growing evidence of attrition following successful animal studies, some scientists have called the animal research enterprise in general into question, at least implicitly,34 whereas other scientists have sought improvements in factors such as research methods, reporting, and journal practices.35 Also significant are claims advanced by some scientists that alternatives to the use of live animals—for example, in toxicity testing—are highly promising36 and might already be as reliable as animal models in some areas of research.37
The scientific questions involved in the evaluation of animal models are complex, and scientists should be leaders in the study of and deliberation about these issues, though professionals in other fields such as the history and philosophy of science can also contribute substantially. We believe that examination of these empirical, methodological, and philosophical matters are critically important for a proper understanding of the core value of social benefit. As we discuss in the next section, one necessary condition of the ethical justification of a prospective animal study is that the prospect of benefit—which takes into account not only the value of the prospective benefit, if it is achieved, but also the likelihood of achieving it—must be greater than the collective costs of the research. Otherwise, the study would not pass a reasonable cost-benefit test. The reliability of animal models, or particular types of animal models (e.g., primate models of treatments for infectious disease), is directly relevant to the likelihood of achieving sought-after benefits.38 The degree of reliability of the best alternatives to the use of live animals is also relevant to the core value of social benefit. As we discuss below, a second necessary condition of the ethical justification of a prospective animal study is that there be no alternative to the use of live animals that offers a reasonable way of answering the research question at hand.
Our concern in this subsection has been to point out that questions about the translation success of animal models and about the viability of alternative methods indicate the need for a more developed framework of principles for animal research ethics—one that, unlike the 3 Rs, includes principles of social benefit.
The Perception That Different Perspectives on Animal Research Are Irreconcilable
According to a widespread perception exhibited in literature on animal research ethics, a yawning chasm separates (1) an animal-research community committed to the scientific value and moral acceptability of laboratory animal research and (2) an animal-protection community that prioritizes the protection of animals’ interests.39 We understand the historical developments that have led to this perception, but we find it regrettable and dangerous to the extent that it suggests irreconcilable differences between 2 competing camps, thereby discouraging the acknowledgment or discovery of a common ground serviceable for animal research ethics. This perception can also, unhelpfully, encourage a view of animal research ethics as little more than a political battleground of competing ideologies.
To those who accept the view of entrenched, irreconcilable values in these 2 communities, it may seem naïvely optimistic for us to claim to have constructed a framework of principles for animal research ethics that can and should command the allegiance of both champions of the value of animal research and champions of expanded protections for animals in research. As we explain next, we reject this perception of irreconcilable differences and hold that one can champion both the value of well-designed scientific research with animals and the value of more rigorous protections of animal welfare.
To conclude this section, the 6 developments just described indicate the need for, and timeliness of, a new framework of principles for animal research ethics—to which we now turn.
The Basis and Content of a New Framework of Principles for Animal Research Ethics
In our view, reasonable representatives of both the animal research and animal protection communities should be able to agree on 3 pivotal moral norms that are intimately related to our framework of core values and basic principles: (1) sentient animals have moral status and are therefore not merely tools of research; (2) the only justification for (non-therapeutically) harming animal research subjects is the prospect of substantial and otherwise unattainable social benefits; and (3) permissible harming of animals in research is limited by identifiable considerations of animal welfare.
Consonant with these 3 claims is a thesis undergirding our framework of principles, namely that the 2 core values of animal research ethics are social benefit and animal welfare. Proceeding from this small set of moral norms and core values, we construct our framework of 6 moral principles—3 of social benefit and 3 of animal welfare. We believe they can be accepted by all parties who are enthusiastic about the history and promise of animal research and all parties who are enthusiastic about vigorous protection of animal research subjects’ welfare—without sacrifice of anyone’s basic commitments. Even those who believe that it is never, in principle, permissible to harm animal subjects in nontherapeutic research will, we hope, accept the present framework as a palatable compromise and one justified pragmatically as a major advance in animal protection.
Although other moral values or principles such as respect for animals and justice as fairness arguably deserve a place in animal research ethics, we have deliberately limited the values in our framework to those that virtually everyone can be expected to endorse. We believe that these values of social benefit and animal welfare are to a significant extent already embraced in the status quo of animal research regulation, practice, and philosophy—and that most, possibly all, of our framework’s 6 principles are already widely accepted even if they have not been explicitly captured in any previous framework of principles, ethical code, or body of regulations.
Principles of Social Benefit
We proceed now to a statement of each of the 6 principles followed by a clarification of its content and a brief defense of the claim that each presents a necessary condition of morally justified animal research. The domain to which these principles apply is research in which (1) sentient animal subjects live in captivity, (2) the animals are caused at least some harm in their living conditions or in scientific procedures associated with the research, and (3) the research is not therapeutic—that is, not intended to provide veterinary care to the animals for independently occurring health conditions (e.g., cancers that unexpectedly develop in cats living in human homes). Each principle in the framework is a necessary condition of morally justified animal research, meaning that failure to satisfy any 1 of the 6 principles entails a failure of moral justification. However, as explained below, we acknowledge the possibility that exceptions to the final (sixth) principle are justified in rare circumstances.40
The first set of principles—those of social benefit—begins with the Principle of No Alternative Method: use of animal subjects must be the sole ethically acceptable way to address a research problem whose solution offers the prospect of a social benefit. Because sentient animals have moral status, they should not be involved in research that is likely to harm them if viable alternative methods of answering the research question are at hand. (Sentient animals in the definition we propose are animals with the capacity to have pleasant or unpleasant experiences. As noted earlier, a reasonable working assumption is that at least most vertebrates and cephalopods are sentient.) An animal study must offer the prospect of some benefit to human society, and the research question it seeks to answer must be significant as opposed to trivial. The Principle of No Alternative Method is somewhat similar to the first of the 3 Rs, namely replacement. However, it is not sufficient merely to consider possible replacements, as some current codes require.41 Investigators have an obligation to search thoroughly for possible alternative methods and, where a scientifically viable alternative exists, forgo the use of live animals. The Principle of No Alternative Method can be viewed as a more robust version of the 3 Rs notion of replacement. When this principle is satisfied, an animal study offers the prospect of a unique benefit to society—that is, a benefit that is not reasonably attainable except through research involving animals.
If a prospective animal study satisfies this principle, the question arises whether the prospect of benefit that the study offers outweighs its predictable costs. In other words, a cost-benefit assessment is needed. In our framework, the overall cost-benefit appraisal proceeds in 2 steps—corresponding to our 2 further principles of social benefit. The first focuses on human interests (benefits from research involving animals as well as its costs) and the second relates these human interests to animals’ interests, as explained in the paragraphs that follow.
The first part of the cost-benefit assessment is the second principle of social benefit, the Principle of Expected Net Benefit: the prospect of human social benefit from a research study must outweigh the expected costs and risks to human beings. All animal studies involve certain costs (here using the term broadly to include anything that would count negatively in a cost-benefit assessment rather than including only financial costs), which must be outweighed by the prospect of benefits. With this principle matters are simplified by bracketing questions about the precise level of animals’ moral status. We are assuming that sentient animals have some level of moral status, but we set aside reasonable differences regarding how, precisely, to understand their moral status—or, equivalently, how heavily to weigh their interests, an issue to which we turn momentarily in connection with the third principle. Accordingly, the cost-benefit assessment required by this principle considers only costs to human beings and compares these costs to the prospect of benefit to human society. The costs to human beings include financial and opportunity costs associated with a prospective study and any risks that may be posed to human beings (e.g., in clinical trials) by reliance on animal models. Meanwhile, the prospect of benefit, which is to be compared with anticipated costs to human beings, is a function of (1) the magnitude of the benefit to society if the study eventually yields a benefit and (2) the likelihood of yielding this benefit.
It is often difficult to estimate this likelihood, but any rigorous cost-benefit comparison and appraisal will multiply the value of possible benefits by the estimated likelihood that the benefits will occur. Otherwise, the analysis will be distorted by inflation of the benefit side of the assessment. To use an analogy, a raffle ticket that purchases a 1-in-5 chance of winning a prize worth $20 is not itself worth $20; it is worth $4 because the chance of winning must be factored in. In this context, rates of successful translation from animal studies to eventual payoffs for human society are relevant and should be taken into account.
The second principle of social benefit states that a necessary condition of a morally justified animal study is that it offers an expectation of net benefit for human society (where “expectation” need not involve greater than a 50% chance of success but only that the likelihood of success times its value outweighs anticipated costs). If this principle is not satisfied, a proposed animal study is not justified even from a perspective that considers only human interests, without consideration of the interests of animal research subjects. If the first and second principles of social benefit are satisfied, then an animal study offers the prospect of a net benefit to human society (as required by the second principle) that is not feasibly available in any other way because there is “no alternative method” (as required by the first principle).
This conclusion calls attention to the need for a third principle of social benefit that considers animals’ moral status and interests. The question underlying this third principle is whether the study’s prospect of benefit is sufficient to outweigh the disvalue associated with harming animal subjects. A study is justified only if the answer is affirmative. This third principle of social benefit is the Principle of Sufficient Value to Justify Harm: the prospect of a net benefit for human society from a research study must be sufficiently valuable to justify expected harms to animal subjects.
What counts as sufficiently valuable? We believe the only sensible procedure to get an answer to this question is to leave it open for debate in review committee meetings and comparable deliberative settings. Reasonable differences exist regarding how to understand animals’ level of moral status—that is, how to assess how much moral weight animals’ interests should have. Our framework rests on the assumption that animals have a significant level of moral status or inherent moral importance, but that assumption leaves open exactly how much and which levels of protection are justified.
The application of the Principle of Sufficient Value to Justify Harm is sure to prove difficult in some cases, but the principle is necessary given the gap in reasoning between (1) an expectation of net benefit to human beings (as required by the second principle) that is not otherwise reasonably attainable (as required by the first principle) and (2) a conclusion that the expected benefit is sufficient to justify anticipated harms to animal subjects (as required by the third principle). In assessing a prospective study that satisfies the first 2 principles of social benefit, decision-makers tasked with assessing how to apply the third principle must consider whether the study’s anticipated net benefit is sufficiently valuable or large to justify anticipated harms. A consideration that will play an important role—in addition to the value of the expected net benefit to humanity and a judgment about animal subjects’ moral status—is how much harm the animal subjects are expected to undergo. This consideration takes us to the subject of animal welfare, the core value underlying the second set of 3 principles in our framework.
Principles of Animal Welfare
Sentient animals have a subjective quality of life, or experiential welfare, meaning that their lives can go well or badly for them in terms of the felt quality of their experiences. We assume that the animal subjects under consideration are sentient and therefore have an experiential welfare. The latter is a commonsensical basis for speaking about animal welfare. In our framework, a prospective animal study is morally justified only if it satisfies both the 3 principles of social benefit just discussed and the 3 principles pertaining to the welfare of animal subjects now to be discussed. What justifies harming animal subjects in general is a well-supported anticipation of social benefit, in particular, by showing that the demands of the 3 principles of social benefit have been met. However, harming, justified in general in this way, still requires due consideration of animal welfare and meeting the 3 principles of animal welfare in specific ways that will vary from study to study.
The first principle is the Principle of No Unnecessary Harm: animal subjects must not be harmed unless a particular harm is necessary for and morally justified by scientific purposes. Animal research that involves confining live animal subjects almost inevitably imposes some harm on its subjects—most notably pain, discomfort, or distress—whether in the course of scientific procedures or in handling, housing, or other circumstances of their lives. Harming others, whether human or animal, tends to be wrong. Accordingly, harming in particular instances is either unjustified, in which case it should not be done, or justified by appeal to some morally relevant and overriding consideration. Morally relevant considerations that may justify intentionally harming another individual include a need for self-defense (against an attacker), consent of the individual to be harmed (as in organized boxing), an expectation of significant benefit to the individual harmed (as in surgery and some therapeutic research), or an expectation of significant benefit to other individuals. The latter consideration plays a paramount role in the justification of (nontherapeutic) animal research.
The Principle of No Unnecessary Harm requires that harms to animal subjects in particular studies be limited to what is necessary given scientific purposes that have been shown to be legitimate by the satisfaction of the principles of social benefit. For example, a study that meets the principles of social benefit may require drawing blood samples from rodent subjects for genetic testing. Some pain is (we here stipulate) necessary given the need to draw blood. Under these circumstances, the Principle of No Unnecessary Harm requires making every reasonable effort not to cause more pain than is necessary—for example, by drawing blood more often than necessary or handling rodents more roughly than necessary. This principle overlaps with one of the 3 Rs—namely, refinement—but the present principle is broader in not being limited to scientific procedures. It requires, in addition, the minimization of harms associated with the feeding, housing, and transport of the rodent subjects in this imagined experiment.
Another implication of the Principle of No Unnecessary Harm is the prohibition of harm caused through negligence (as opposed to harms caused intentionally or knowingly). It may seem obvious that personnel should not harm animal subjects through negligence—for example, by failing to maintain a room temperature that is appropriate for the species—but in practice this application of the present principle is vitally important to the welfare of animal subjects.42
The second principle of animal welfare is the Principle of Basic Needs: animal subjects’ basic needs must be met in the conduct of studies unless failure to meet specific basic needs is both necessary for and morally justified by scientific purposes. This principle is similar to the Principle of No Unnecessary Harm but incorporates the critically important concept of animal subjects’ basic needs. We understand basic needs as general conditions of animals’ lives that are important for a good quality of life. They include nutritious food, clean water, safe shelter, species-appropriate housing and companionship, opportunities for stimulation and exercise, and freedom from experiential harm (e.g., pain, suffering), injury, and disease. (The relevant discussion in our book presents a more comprehensive list of basic needs.) When basic needs are not satisfied, an animal subject is harmed, resulting in a lower level of welfare or quality of life. Many studies call for some failure to meet basic needs—for example, by imposing pain on animal subjects—but such an imposition may nonetheless be justified by scientific purposes that satisfy the principles of social benefit.
In response to the foregoing, one might wonder both why animal subjects should be entitled to the satisfaction of their basic needs wherever compatible with scientific purposes and whether scientific personnel truly harm their subjects if they do not provide for all their basic needs. As a matter of common sense, we do not harm birds, squirrels, or deer living near our house if we fail to ensure that they have adequate nutrition and do not go hungry in the winter. But the relationship between personnel involved in animal research and their animal subjects is importantly different from the relationship between a homeowner and wild animals such as birds, squirrels, and deer living nearby. Investigators and others involved in the conduct of animal research have deliberately created a situation in which animals are compelled to be research subjects and are thereby rendered entirely dependent on their caretakers. This feature of the relationship creates relationship-based obligations to satisfy the basic needs of dependent animal subjects. Given this special relationship, failure to meet basic needs is tantamount to harm, just as one can harm one’s pet—or one’s young child—by failing to feed him or her. In this respect, the Principle of Basic Needs is closely related to the Principle of No Unnecessary Harm. They are linked by the fact that failure to meet an animal’s basic needs constitutes a type of harm if one places an animal in a situation of total dependency on one’s care. We note, finally, that the 3 Rs framework lacks any explicit statement of a general expectation to meet animal subjects’ basic needs.
The third and last principle of animal welfare is the Principle of Upper Limits to Harm: animal subjects must not be caused to endure severe suffering for a lengthy period of time. This principle, which sets a limit on the harm that may be imposed on animal subjects, might seem more controversial than the 2 principles that precede it, but this principle too is appropriately responsive to the recognition that sentient animals have moral status and must not be regarded as mere tools for research. The underlying premise is that animal research subjects should not be forced to endure prolonged agony, a conviction consistent with the acknowledgment that animal research subjects should be afforded decent lives when serving the interests of human society.
The 3 Rs set no limit on permissible harm to animal subjects. The same is true of US government principles and, as far as we know, every other code guiding publicly funded animal research in the United States. Current requirements pertaining to the use of anesthesia, analgesia, and sedatives—and to the performance of euthanasia on animal subjects whose suffering cannot otherwise be eliminated—do not set limits to permissible levels of harm because the pertinent requirements are suspended when critical scientific purposes call for withholding pain medications or euthanasia.
By contrast, the European Union and several individual nations have established upper limits on harm. The relevant EU directive states the following: “From an ethical standpoint, there should be an upper limit of pain, suffering and distress above which animals should not be subjected in scientific procedures. To that end, the performance of procedures that result in severe pain, suffering, or distress [that] is likely to be long-lasting and cannot be ameliorated, should be prohibited.”43 The eminent ethologist Sir Patrick Bateson, one of the first scholars to factor animal welfare explicitly into cost-benefit analyses of prospective animal experiments, supported a limit on animal suffering similar to that of the European Union.44 In our framework, the Principle of Upper Limits to Harm applies not only to experimental procedures, as in the EU directive, but to transportation, housing conditions, and other factors that affect animal subjects’ experience.
In view of the possibility of public health emergencies that might call for certain exceptions to the Principle of Upper Limits to Harm—such as a highly lethal epidemic for which no effective vaccine or treatment exists—we acknowledge that rare and extraordinary circumstances may sometimes justify overriding it. This acknowledgment should not be misconstrued as an opening to an extensive array of exceptions. The justification of any exception to this principle requires careful documentation of exceptionally important social interests and the infeasibility of pursuing those interests without involving live animal subjects in studies that are likely to impose severe suffering for an extended period of time.
Conclusion
In this article we have not discussed the role of ethics review committees in institutions that engage in animal research, but we do discuss this important subject in our forthcoming book, Principles of Animal Research Ethics. We regard the process of ethics review as comparable in importance in animal research ethics to the core values of social benefit and animal welfare, and we strongly endorse the role and functions of these committees. When they function properly, animal research ethics committees engage in sensitive, fair-minded interpretation and specification of applicable moral norms, laws, government regulations, scientific society guidelines, and the like. However, to say that this work of oversight and protocol review is massively important is not to endorse all features of the system of review as it now stands. Government guidelines in many countries need to be revised to implement a comprehensive ethical framework that can withstand critical scrutiny, and the practices of ethical review in many institutions should be improved to become more rigorous scientifically and ethically. Other issues such as conflict of interest in the approval of protocols also need serious consideration. The present article is not, however, the place to address these important subjects.
We hope that the framework of principles we have presented will provide valuable guidance to members of review committees and others involved in the evaluation of animal research. We also hope that our framework will serve as an instructive basis for improving government and international regulations and principles for the care and use of animal subjects in biomedical and behavioral research.
Acknowledgments and Declarations
The authors thank four anonymous reviewers of this article for helpful feedback and the editors of the special journal issue for their guidance and support.
Financial support. This research was supported, in part, by the Intramural Research Program of the NIH Clinical Center and by the National Science Foundation under Grant No. 1058186.
Disclaimer. The views presented here are the authors’ own and do not necessarily reflect the position or policy of NIH, NSF, or any other part of the federal government.
Footnotes
See, e.g., Council for the International Organizations of Medical Sciences (CIOMS) and The International Council for Laboratory Animal Science (ICLAS) (in partnership), International Guiding Principles for Biomedical Research Involving Animals (December 2012), available at: http://www.cioms.ch/images/stories/CIOMS/IGP2012.pdf, retrieved December 20, 2016.
See, e.g., Government of Australia (regulating studies funded by the National Health and Medical Research Council (NHMRC)), Australian Code for the Care and Use of Animals for Scientific Purposes (8th edition 2013, available at https://www.nhmrc.gov.au/guidelines-publications/ea28, retrieved March 25, 2017). In the U.S., government controls were more symbolic than substantive until the Animal Welfare Act was amended from 1985 through 2008. See further U. S. Interagency Research Committee, `U.S. Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training,’ in U.S. Department of Health and Human Services, National Institutes of Health, Office of Laboratory Animal Welfare (OLAW), as bundled with Public Health Service Policy on Humane Care and Use of Laboratory Animals, revision of 2015. In 2012 OLAW adopted the Guide for the Care and Use of Laboratory Animals, eighth edition released by the National Academy of Sciences, Institute for Laboratory Animal Research (ILAR), 2011. The British history of law and guidance is the longest and the most comprehensive. The British Animal Welfare Act 2006 is an Act of the Parliament of the UK that dates to the Protection of Animals Act of 1911, which it largely replaced.
National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research, The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research. Washington, DC: DHEW Publication OS 78–0012, 1978.
William M. S. Russell and Rex L. Burch, The Principles of Humane Experimental Technique (London: Methuen, 1959). Commissioned by Universities Federation for Animal Welfare (UFAW). Facsimile: Potters Bar, UK: Universities Federation for Animal Welfare, 1992. The history of the 3 Rs and the idea of “alternatives” precede Russell and Burch’s work but have strong connections to their work. Major Charles Hume founded UFAW in 1926 to promote scientific knowledge, explicitly including scientific knowledge of ways to promote the welfare of animals. On the direct connections between Hume, UFAW, and the early work on alternatives–including Russell and Burch’s book–see Michael Balls, `UFAW and Major Charles Hume: The Wisdom of Russell and Burch,' published February 27, 2014 in PILAS (Perspectives in Laboratory Animal Science), available at http://pilas.org.uk/ufaw-and-major-charles-hume/; and Balls,' The Principles of Humane Experimental Technique: Timeless Insights and Unheeded Warnings,’ Altex 27, Special Issue 2010: 19–23. Balls refers to the 3 Rs as ‘the Three Rs of UFAW.’
See, e.g., Vegetarian Research Group, ‘How Many Adults in the U.S. Are Vegetarian and Vegan?’ available at https://www.vrg.org/nutshell/Polls/2016_adults_veg.htm (accessed January 2, 2019); ‘Top Trends in Prepared Foods 2017: Exploring Trends in Meat, Fish and Seafood; Pasta, Noodles, and Rice; Prepared Meals; Savory Deli Food; Soup; and Meat Substitutes,’ PR Newswire, available at https://www.prnewswire.com/news-releases/top-trends-in-prepared-foods-2017-exploring-trends-in-meat-fish-and-seafood-pasta-noodles-and-rice-prepared-meals-savory-deli-food-soup-and-meat-substitutes-300478350.html (accessed January 2, 2019); Maria Chiorando, ‘Veganism Skyrockets to 7% of UK Population, Says New Survey,’ Plant Based News, available at https://www.plantbasednews.org/post/veganism-skyrockets-to-7-of-uk-population-says-new-survey (accessed January 2, 2019); and CTV News, ‘More than 3 Million Canadians Vegetarian or Vegan: Study,’ available at https://www.ctvnews.ca/canada/more-than-3-million-canadians-vegetarian-or-vegan-study-1.4027606 (accessed January 2, 2019).
See, e.g., Nick Marinoff, ‘Closing of Ringling Bros. Circus Reflects Changing Attitudes Toward Performance Animals,’ Planet Experts (January 23, 2017), available at http://www.planetexperts.com/circus-closure-reflects-changing-attitudes-on-performance-animals/ (accessed January 2, 2019); and Brian Clark Howard, ‘Controversial SeaWorld Orca Shows End in California, but Continue Elsewhere,’ National Geographic (January 4, 2017), available at https://news.nationalgeographic.com/2017/01/seaworld-final-orca-show-california-killer-whales/ (accessed January 2, 2019).
National Conference of State Legislatures, ‘Cockfighting Laws’ (by Jonathan Griffin), Briefing Papers on the Important Issues of the Day 22 (Jan. 2014), available at http://www.ncsl.org/LinkClick.aspx?fileticket=isGk5FRFdyE%3d&tabid=27698&portalid=1 (accessed July 11, 2018); Tom L. Beauchamp, F. Barbara Orlans, Rebecca Dresser, David B. Morton, and John P. Gluck, ‘The Sport of Rooster Fighting,’ chapter 5 in their The Human Use of Animals, second edition (New York and Oxford: Oxford University Press, 2008): 91–106; NBC News, ‘La. Finally Quits Cockfights, Last State to Ban It,’ as updated August 10, 2008, available at http://www.nbcnews.com/id/26123404/ns/us_news-life/t/la-finally-quits-cockfights-last-state-ban-it/#.W0Ya59JKhRY (accessed July 11, 2018). As of 2008, dogfighting became a felony in all 50 U.S, states and in the District of Columbia, Guam, Puerto Rico and the U.S. Virgin Islands. Being a spectator at a dogfight is likewise illegal in all states. For the history see ASPCA, ‘Dogfighting FAQ’ available at https://www.usatoday.com/story/money/2016/03/17/seaworld-orcas-killer-whales/81900498/ (accessed July 11, 2018); https://www.aspcapro.org/resource/disaster-cruelty-animal-cruelty-animal-fighting/dogfighting-faq (accessed July 11, 2018). On Orca shows and breeding, see Nathan Bomey, ‘Seaworld To Phase Out Killer Whale Shows, Captivity,’ USA Today, May 17, 2016, available at https://www.usatoday.com/story/money/2016/03/17/seaworld-orcas-killer-whales/81900498/; and Brian Clark Howard, ‘SeaWorld to End Controversial Orca Shows and Breeding,’ National Geographic, available at https://news.nationalgeographic.com/2016/03/160317-seaworld-orcas-killer-whales-captivity-breeding-shamu-tilikum/#/01seaworld.jpg (accessed July 11, 2018).
See, e.g., Ruwani Perera, ‘Rodeo Protests Dividing Town and Country,’ Newshub (December 31, 2017), available at https://www.newshub.co.nz/home/new-zealand/2017/12/rodeo-protests-dividing-town-and-country.html (accessed January 2, 2019); and Alexandra Nelson, ‘Death Sparks Renewed Fight Against Rodeos,’ Newshub (January 1, 2019), available at https://www.newshub.co.nz/home/new-zealand/2018/12/deaths-spark-renewed-fight-against-rodeos.html (accessed January 2, 2019).
See the Committee on the Use of Chimpanzees in Biomedical and Behavioral Research, Institute of Medicine (now Academy of Medicine), Chimpanzees in Biomedical and Behavioral Research: Assessing the Necessity (Washington, DC: National Academies Press, 2011), available at https://www.nap.edu/catalog/13257/chimpanzees-in-biomedical-and-behavioral-research-assessing-the-necessity, retrieved August 16, 2017; National Institutes of Health, Office of the Director, ‘Statement by NIH Director Dr. Francis Collins on the Institute of Medicine Report Addressing the Scientific Need for the Use of Chimpanzees in Research,’ Thursday, December 15, 2011, available at http://www.nih.gov/news/health/dec2011/od-15.htm, retrieved December 15, 2011; the follow-up report, Council of Councils, National Institutes of Health. Council of Councils Working Group on the Use of Chimpanzees in NIH-Supported Research: Report, 2013, available at https://dpcpsi.nih.gov/council/pdf/FNL_Report_WG_Chimpanzees.pdf, retrieved August 16, 2017; National Institutes of Health, Announcement of Agency Decision: Recommendations on the Use of Chimpanzees in NIH-Supported Research. Available at dpcpsi.nih.gov/council/pdf/NIHresponse_to_Council_of_Councils_recommendations_62513.pdf, retrieved July 28, 2013; and Tom L. Beauchamp, Hope Ferdowsian, and John Gluck, ‘Where Are We in the Justification of Research Involving Chimpanzees?’ Kennedy Institute of Ethics Journal 22 (September 2012): 211–42.
See Directive 2010/63/EU on the Protection of Animals Used for Scientific Purposes. Official Journal of the European Union L 276/33, especially Article 8, no. 3; adopted September 22, 2010; available at http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32010L0063, retrieved January 11, 2019.
A classic pre-20th-century work on the subject is T. H. Huxley, ‘On the Hypothesis that Animals are Automata, and its History,’ Nature 10 (1874): 362–366. In a famous essay soon thereafter, William James pushed further and asked about the human species: ‘Are We Automata?’ Mind 4 (1879): 1–22. For classic early 20th-century representatives of skepticism about animals’ minds or about their scientific investigation—which are commonly associated with a theory often labelled behaviorism—see John B. Watson, ‘Psychology as the Behaviorist Views It,’ Psychological Review 20 (1913): 158–177; and B. F. Skinner, The Behavior of Organisms (Oxford: Appleton-Century, 1938).
An influential critique of Skinner’s approach and behaviorism more generally (see previous note) appeared in Noam Chomsky, ‘A Review of B. F. Skinner’s Verbal Behavior,’ Language 35 (1959): 26–58. For influential work in the resurgence of the study of animal consciousness, see two books by Donald Griffin, The Question of Animal Awareness (New York: Rockefeller University Press, 1976) and Animal Minds: Beyond Cognition to Consciousness, revised ed. (Chicago: University of Chicago Press, 2001). Discussion of the contemporary, non-skeptical study of animal minds is found in Dale Jamieson and Marc Bekoff, ‘On the Aims and Methods of Cognitive Ethology,’ Philosophy of Science Association 2 (1992): 110–124. See also Marc Bekoff, Colin Allen, and Gordon M. Burghardt, The Cognitive Animal: Empirical and Theoretical Perspectives on Animal Cognition (Cambridge MA: MIT Press, 2002); Committee on Well-Being of Nonhuman Primates, U.S. Institute for Laboratory Animal Research (ILAR), Commission on Life Sciences, National Research Council, The Psychological Well-Being of Nonhuman Primates (Washington, D.C.: National Academies Press, 1998); and Frans de Waal, Are We Smart Enough to Know How Smart Animals Are? (New York: Norton, 2016).
For recent overviews of the evidence, see Colin Allen and Michael Trestman, ‘Animal Consciousness,’ Stanford Encyclopedia of Philosophy, substantive revision of October 24, 2016, especially sections 6–7, available at https://plato.stanford.edu/entries/consciousness-animal/, retrieved June 12, 2017; and David DeGrazia, ‘Sentience and Consciousness as Bases for Interests and Moral Status: Considering the Evidence,’ in Syd Johnson, Andrew Fenton, and Adam Shriver, eds., Neuroethics and Nonhuman Animals (New York: Springer, forthcoming 2020). See also, e.g., Marian Stamp Dawkins, Through Our Eyes Only: The Search for Animal Consciousness (Oxford: Freeman, 1993); Marian Stamp Dawkins, ‘Animal Minds and Animal Emotions,’ American Zoologist 40 (2000): 883–88; David Edelman, Bernard Baars, and Anil Seth, ‘Identifying Hallmarks of Consciousness in Non-Mammalian Species,’ Consciousness and Cognition 14 (2005): 169–187; Philip Low (drafter and editor), Jaak Panksepp, Diana Reiss, David Edelman, Bruno Van Swinderen, Christof Koch (editors), ‘The Cambridge Declaration on Consciousness,’ proclaimed in Cambridge, UK, 7 July 2012, at the Francis Crick Memorial Conference on Consciousness in Human and non-Human Animals, at Churchill College, University of Cambridge.
See, e.g., Jaak Panksepp, Affective Neuroscience: The Foundations of Human and Animal Emotions (New York: Oxford University Press, 2004); and Marc Bekoff, The Emotional Lives of Animals (Novato, CA: New World Press, 2007).
See, e.g., Selvino R. de Kort, Anthony Dickinson, and Nicola S. Clayton, ‘Retrospective Cognition by Food-Caching Western Scrub-Jays,’ Learning and Motivation 36 (2005): 159–176; Steffanie J. Babb and Jonathon D. Crystal, ‘Discrimination of What, When, and Where: Implications for Episodic-like Memory in Rats,’ Learning and Motivation 36 (2005): 177–189; and Bennett L. Schwartz, Megan L. Hoffman, and Sian Evans, ‘Episodic-like Memory in a Gorilla: A Review and New Findings,’ Learning and Motivation 36 (2005): 226–244.
See, e.g., Dorothy L. Cheney and Robert M. Seyfarth, How Monkeys See the World: Inside the Mind of Another Species (Chicago: University of Chicago Press, 1991); Janet Mann, Richard C. Connor, Peter L. Tyack, and Hal Whitehead (eds.), Cetacean Societies: Field Studies of Dolphins and Whales (Chicago: University of Chicago Press, 2000); Frans de Waal, Chimpanzee Politics, 25th anniversary edition (Baltimore: Johns Hopkins University Press, 2007); and G. Wittemyer and W. M. Getz, ‘Hierarchical Dominance Structure and Social Organization in African Elephants, Loxodonta Africana,’ Animal Behaviour 73 (2007): 671–681.
See, e.g., Gordon G. Gallup, ‘Self-Recognition in Primates: A Comparative Approach to the Bidirectional Properties of Consciousness,’ American Psychologist 32 (1977): 330–338; Diana Reiss and Lori Marino, ‘Mirror Self-Recognition in the Bottlenose Dolphin: A Case of Cognitive Convergence,’ PNAS 98 (2001): 5937–5942; and Joshua M. Plotnik, Frans B. M. de Waal, and Diana Reiss, ‘Self-Recognition in an Asian Elephant,’ PNAS 103 (2006): 17053–17057.
See, e.g., Robert R. Hampton, ‘Rhesus Monkeys Know When They Remember,’ PNAS 98 (2001): 5359–5362; J. David Smith and David A. Washburn, ‘Uncertainty Monitoring and Metacognition by Animals,’ Current Directions in Psychological Science 14 (2005): 19–24; Nate Kornell, Lisa S. Son, and Herbert S. Terrace, ‘Transfer of Metacognitive Skills and Hint Seeking in Monkeys,’ Psychological Science 18 (2007): 64–71; and Jonathon D. Crystal, and Allison L. Foote, ‘Metacognition in Animals,’ Comparative Cognition and Behavior Reviews 4 (2009): 1–16.
See, e.g., Sue Savage-Rumbaugh and Karen E. Brakke, ‘Animal Language: Methodological and Interpretive Issues,’ in Marc Bekoff and Dale Jamieson, eds., Interpretation and Explanation in the Study of Animal Behavior, vol. 1 (Boulder, CO: Westview, 1990): 313–343; Cheney and Seyfarth, How Monkeys See the World; and Peter L. Tyack, ‘Functional Aspects of Cetacean Communication,’ in Mann, Connor, Tyack, and Whitehead, Cetacean Societies: 270–307.
See, e.g., C. R. Raby, D. M. Alexis, A. Dickinson, and N. S. Clayton, ‘Planning for the Future by Western Scrub-Jays,’ Nature 445 (2007): 919–921. For an overview, see William A. Roberts, ‘Evidence for Future Cognition in Animals,’ Learning and Motivation 43 (2012): 169–180.
Frans de Waal, The Age of Empathy (New York: Three Rivers, 2009).
We analyze the concept of moral status as follows: A particular individual X has moral status if and only if (1) moral agents have obligations regarding their treatment of X, (2) X has interests, and (3) the obligations are based on X’s interests (from David DeGrazia, ‘Moral Status as a Matter of Degree?’ Southern Journal of Philosophy 46 [2008]: 181–198, at 183). See further on the subject of moral status Agnieszka Jaworska and Julie Tannenbaum, ‘The Grounds of Moral Status,’ Stanford Encyclopedia of Philosophy, ed. Edward Zalta (Stanford, CA: Stanford University Press, 2013), available at http://plato.stanford.edu/archives/sum2013/entries/grounds-moral-status/, retrieved July 7, 2017.
For two collections of original work that present current publications in animal ethics, see Tom L. Beauchamp and R. G. Frey, eds., The Oxford Handbook of Animal Ethics (New York: Oxford University Press, 2011); and Susan J. Armstrong and Richard G. Botsler, eds., The Animal Ethics Reader, third edition (New York: Routledge, 2017).
Russell and Burch, The Principles of Humane Experimental Technique.
Russell and Burch, The Principles of Humane Experimental Technique, p. 5.
Russell and Burch, The Principles of Humane Experimental Technique, p. 5.
S. Perrin, ‘Make Mouse Studies Work,’ Nature 507 (2014): 423–425.
See, e.g., Ismail Kola and John Landis, ‘Can the Pharmaceutical Industry Reduce Attrition Rates?’ Nature Reviews 3 (2004): 711–715; H. Bart van der Worp, David W. Howells, Emily S. Sena, et al., ‘Can Animal Models of Disease Reliably Inform Human Studies?’ PLOS Medicine 7 (3) (2010), available at https://doi.org/10/1371/journal.prmed.1000245, accessed January 5, 2019); and Joseph Garner, ‘The Significance of Meaning: Why Do Over 90% of Behavioral Neuroscience Results Fail to Translate to Humans, and What Can We Do to Fix It?’ ILAR Journal 55 (2014): 438–456.
Daniel G. Hackam and Donald A. Redelmeier, ‘Translation of Research Evidence From Animals to Humans,’ JAMA 296 (2006): 1731–1732.
See R. McManus, ‘Ex-Director Zerhouni Surveys Value of NIH Research,’ NIH Record 65 (13) (213): 1–2. For a general outline of problems in research involving animals, see Malcolm Macleod, ‘Why Animal Research Needs to Improve,’ Nature 477: 511 (2011), as published online, September 28, 2011, available at https://www.nature.com/collections/wjsrmrdnsm (accessed January 15, 2019). For an examination of widespread problems of data reproducibility in biomedical research, see C. Glenn Begley and John P. A. Ioannidis, ‘Reproducibility in Science: Improving the Standard for Basic and Preclinical Research,’ Circulation Research 116 (2015): 116–26, available at https://pdfs.semanticscholar.org/0f36/1241725540a1626a8e2cf21570711582682b.pdf (accessed January 15, 2019).
Kathleen R. Zahs and Karen H. Ashe, ‘“Too Much Good News”—Are Alzheimer Mouse Models Trying to Tell Us How to Prevent, Not Cure, Alzheimer’s Disease?’ Trends in Neuroscience 33 (2010): 381–389.
Fiona Godlee, ‘How Predictive and Productive is Animal Research?’ BMJ 348 (June 5, 2014), available at https://doi.org/10.1136/bmj.g3719 (accessed July 11, 2018). Godlee briefly discusses other articles of importance on this subject, including Pound, et al., 2004 and Pound and Bracken 2014, which we cite below.
See, e.g., Kola and Landis, ‘Can the Pharmaceutical Industry Reduce Attrition Rates?’ and Michael Rosenblatt, ‘An Incentive-based Approach for Improving Data Reproducibility,’ Science Translational Medicine 8 (27 April 2016): pp. 336ed5, available at http://stm.sciencemag.org/content/8/336/336ed5.full (accessed January 5, 2019).
See, e.g., Pandora Pound, Shah Ebrahim, Peter Sandercock, et al., ‘Where Is the Evidence that Animal Research Benefits Humans?’ BMJ 328 (2004): 514–517; and Pandora Pound and Michael Bracken, ‘Is Animal Research Sufficiently Evidence Based to be a Cornerstone of Biomedical Research?’ BMJ 348 (2014): g3387, available at https://www.bmj.com/content/348/bmj.g3387 (accessed January 5, 2019).
See, e.g., Ian Roberts, Irene Kwan, Phillip Evans, and Seven Haig, ‘Does Animal Experimentation Inform Human Healthcare? Observations from a Systematic Review of International Animal Experiments on Fluid Resuscitation,’ BMJ 324 (2002): 474–476; Daniel G. Hackam, ‘Translating Animal Research into Clinical Benefit,’ BMJ 334 (2007): 163–164; Pablo Perel, Ian Roberts, Emily Sena, et al., ‘Comparison of Treatment Effects Between Animal Experiments and Clinical Trials: Systematic Review,’ BMJ 334 (2007): 197–200; Valerie C. Henderson, Jonathan Kimmelman, Dean Fergusson, et al., ‘Threats to Validity in the Design and Conduct of Preclinical Efficacy Studies: A Systematic Review of Guidelines for in Vivo Animal Experiments,’ PLOS Medicine, July 29, 2013, available at https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1001489 (accessed January 5, 2019); Francis S. Collins and Lawrence A. Tabak, ‘NIH Plans to Enhance Reproducibility,’ Nature 505 (2014): 612–613; and Joseph P. Garner, Brianna N. Gaskill, Elin M. Weber, et al., ‘Introducing Therioepistemology: The Study of How Knowledge is Gained from Animal Research,’ Lab Animal 46 (2017): 103–113.
R. D. Combes, T. Berridge, J. Connelly, et al., ‘Early Microdose Drug Studies in Human Volunteers Can Minimise Animal Testing: Proceedings from a Workshop Organized by Volunteers in Research and Testing,’ European Journal of Pharmaceutical Sciences 19 (2003): 1–11.
See Andrew Rowan, ‘Ending the Use of Animals in Toxicity Testing and Risk Evaluation,’ Cambridge Quarterly of Healthcare Ethics 24 (2015): 448–458. A suggestive necessary condition of justified research on chimpanzees was formulated by the U.S. Institute of Medicine Committee on the Use of Chimpanzees in Biomedical and Behavioral Research: ‘There must be no other research model by which the knowledge could be obtained, and the research cannot be ethically performed on human subjects,’ Chimpanzees in Biomedical and Behavioral Research, p. 4. The many writings on the place and importance of alternatives to animal research include David Smyth, Alternatives to Animal Experiments (London: Scolar Press, 1978); Joanne Zurlo, Deborah Rudacille, and Alan M. Goldberg, Animals and Alternatives in Testing: History, Science, and Ethics (New York: Mary Ann Liebert, 1994); Ronald Hester and Roy Harrison (eds.), Alternatives to Animal Testing (Cambridge, U.K.: The Royal Society of Chemistry, 2006); and Sarah Adler, et al., ‘Alternative (Non-Animal) Methods for Cosmetics Testing: Current Status and Future Prospects—2010,’ Archives of Toxicology 85 (2011): 367–485. See also the discussions in Kathleen M. Conlee and Andrew Rowan, ‘The Case for Phasing Out Experiments on Primates,’ in Animal Research Ethics: Evolving Views and Practices, Hastings Center Report Special Report 42 (2012): S31-S34; and Hugh LaFollette and Niall Shanks, Brute Science: Dilemmas of Animal Experimentation (New York and Oxford: Routledge, 1996).
See Kimberly A. Phillips, Karen L. Bales, John P. Capitanio, et al., ‘Why Primate Models Matter,’ American Journal of Primatology 76 (2014): 801–27; Larry Carbone, ‘The Utility of Basic Animal Research,’ in Animal Research Ethics: Evolving Views and Practices, Hastings Center Report Special Report 42 (6) (2012): S12-S15; D. Eugene Redmond, Jr., ‘Using Monkeys to Understand and Cure Parkinson Disease,’ in Animal Research Ethics: Evolving Views and Practices, Hastings Center Report Special Report 42 (2012): S7-S1; and Simon Festing and Robin Wilkinson (Officers at the Research Defence Society in London), ‘The Ethics of Animal Research. Talking Point on the Use of Animals in Scientific Research,’ EMBO Reports 8 (2007): 526–530.
See, e.g., Bernard Rollin, ‘Ethics and Animal Research,’ in Jeremy R. Garrett (ed.), The Ethics of Animal Research: Exploring the Controversy (Cambridge, MA: MIT Press, 2012): 19–30, at 19–20.
See, in contrast to our framework, the U. S. Interagency Research Committee, ‘U.S. Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training,’ which implicitly invites exceptions to all of its principles, with no stated limits, in its formulation of its final principle: ‘where exceptions are required in relation to the provision of these Principles….’ (principle 9). We consider it a major advantage of our framework that it contains necessary conditions for justified research and no such global loophole.
See, e.g., U. S. Interagency Research Committee, ‘U.S. Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training,’ Principle III.
See the necessary condition of justified research on chimpanzees formulated by the U.S. Committee on the Use of Chimpanzees in Biomedical and Behavioral Research: ‘The animals used in the proposed research must be maintained either in ethologically appropriate physical and social environments or in natural habitats,’ in Chimpanzees in Biomedical and Behavioral Research, p. 4 (see also their related Conclusions and Recommendations, pp. 67–70).
Directive 2010/63/EU on the Protection of Animals Used for Scientific Purposes, Preamble 23; see also Preamble 22 and Articles 15, 38, 55, and Annex VIII. Compare the critical yet sympathetic appraisal of this Directive in Tom L. Beauchamp and David B. Morton, ‘The Upper Limits of Pain and Suffering in Animal Research: A Moral Assessment of The European Union’s Legislative Framework,’ Cambridge Quarterly of Healthcare Ethics 24 (October 2015), pp. 431–447.
Patrick Bateson, ‘Assessment of Pain in Animals,’ Animal Behaviour 42 (1991): 827–839.
Contributor Information
David DeGrazia, National Institutes of Health, Department of Bioethics; George Washington University, Department of Philosophy.
Tom L Beauchamp, Georgetown University, Department of Philosophy (emeritus).


