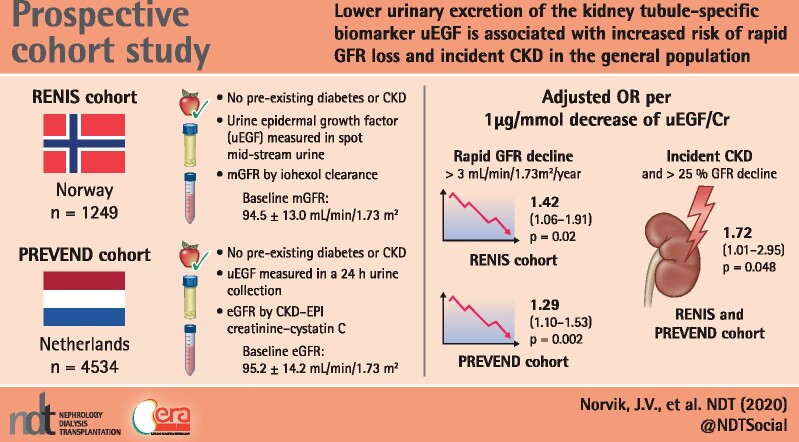
Abstract
Background
Lower urinary excretion of the kidney tubule–specific biomarker epidermal growth factor (uEGF) is associated with increased risk of renal function [glomerular filtration rate (GFR)] loss in diabetes and in patients with established chronic kidney disease (CKD). We investigated whether uEGF is associated with rapid GFR decline or incident CKD in the general population.
Methods
Subjects without CKD or diabetes were recruited from the general population in Tromso, Norway [Renal Iohexol Clearance Survey (RENIS); N = 1249] and Groningen, the Netherlands [Prevention of REnal and Vascular END-stage disease (PREVEND); N = 4534], with a median follow-up of 5.6 and 7.4 years, respectively. GFR was measured by iohexol clearance in the RENIS and estimated using the CKD Epidemiology Collaboration creatinine–cystatin C equation in the PREVEND study. Rapid GFR decline was defined as an annual GFR loss >3.0 mL/min/1.73 m2 and in sensitivity analyses as subjects with the 10% steepest GFR slope within each cohort.
Results
Lower baseline uEGF excretion was associated with rapid GFR loss in both cohorts {RENIS, odds ratio [OR] per 1 μg/mmol lower uEGF 1.42 [95% confidence interval (CI) 1.06–1.91], P = 0.02; PREVEND, OR 1.29 [95% CI 1.10–1.53], P < 0.01}, adjusted for baseline GFR, albumin:creatinine ratio and conventional CKD risk factors. Similar results were obtained using the outcome of the 10% steepest GFR slope in each cohort. Lower uEGF levels were associated with incident CKD in the combined analysis of both cohorts.
Conclusions
Lower uEGF levels are associated with increased risk of rapid GFR loss and incident CKD in the general population. This finding, together with previous findings in CKD and high-risk populations, supports that uEGF may serve as a broadly applicable biomarker representing the tubular component of the current glomerulus-centric clinical risk assessment system.
Keywords: chronic kidney disease, clinical epidemiology, epidermal growth factor, renal function decline, uEGF
Graphical Abstract
KEY LEARNING POINTS
What is already known about this subject?
Chronic kidney disease (CKD) is a major global public health problem with a high and increasing prevalence in recent decades. It is therefore vital to identify individuals at high CKD risk for early clinical intervention.
The current clinical in-use methods for CKD diagnosis and risk stratification are limited to measurement of the urinary albumin:creatinine ratio and the estimated glomerular filtration rate (eGFR). These variables cover glomerular damage and function of the kidney but are insensitive to early tubular dysfunction.
Lower urinary excretion of the kidney tubule–specific biomarker epidermal growth factor (uEGF) is associated with an increased risk of renal function (GFR) loss in diabetes and in patients with established CKD.
What this study adds?
In this longitudinal study, lower urinary excretion of uEGF was independently associated with rapid GFR loss and incident CKD in subjects without diabetes or established CKD in two European population cohorts.
What impact this may have on practice or policy?
uEGF may be a useful non-invasive biomarker that captures the tubular component of the function of the kidney in a manner beyond serum creatinine and proteinuria.
INTRODUCTION
Chronic kidney disease (CKD) is a major global public health problem with a high and increasing prevalence in recent decades [1]. It is an independent risk factor for cardiovascular disease, end-stage kidney disease (ESKD) and all-cause mortality [2–4], highlighting the importance of identifying individuals at high CKD risk for early clinical intervention [5, 6]. However, the current clinical in-use methods for CKD diagnosis and risk stratification are limited to the measurement of urinary albumin:creatinine ratio (ACR) and the estimated glomerular filtration rate (eGFR). These variables cover glomerular damage and function of the kidney but are insensitive to early tubular dysfunction [7].
Recently we used a kidney biopsy transcriptome-driven approach to develop nephron segment-specific, non-invasive prognostic biomarkers for CKD progression [8]. Messenger RNA levels of epidermal growth factor (EGF), a transcript selectively expressed by distal tubular epithelial cells and considered a marker of functional tubular mass and regeneration potential, correlated with GFR [8]. In patients with established CKD from various aetiologies, lower urinary excretion of EGF (uEGF) was associated with increased tubular atrophy and interstitial fibrosis. Urinary EGF levels improved the prediction of CKD progression independent of eGFR and ACR [8]. In patients with type 2 diabetes, lower uEGF was associated with an increased risk of new-onset GFR <60 mL/min/1.73 m2 and rapid kidney function decline, indicating that uEGF is applicable as a prognostic biomarker in subjects at high risk for CKD [9].
Tubular atrophy, interstitial fibrosis and loss of nephrons are hallmarks of impending renal dysfunction in apparently healthy subjects [10]. These early morphological changes correlate with the GFR decline in ageing individuals. Age-related GFR decline is a major contributor to the high prevalence of CKD in the elderly population, reaching ∼30–40% of people ≥65 years of age [11]. However, the rate of renal function decline differs significantly between people [12]. We hypothesized that uEGF is a biomarker for rapid kidney function loss where early kidney damage is silent due to a lack of sensitive and kidney-specific biomarkers. Therefore we investigated whether decreased uEGF excretion at baseline is associated with a rapid loss of GFR and incident CKD in two general population–based prospective cohorts with serial follow-up.
MATERIALS AND METHODS
Study populations
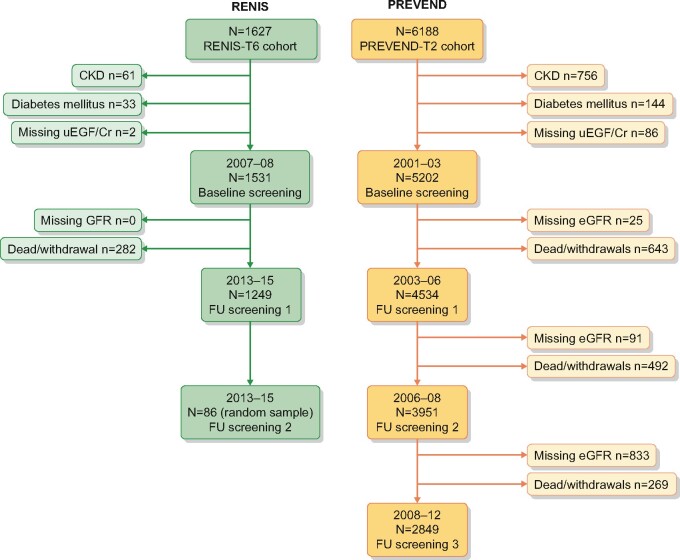
Figure 1 shows the flow charts of the Renal Iohexol Clearance Survey (RENIS) and the Prevention of REnal and Vascular END-stage disease (PREVEND) population-based cohort studies. Details have been described previously [13–15].
FIGURE 1.
Flowchart of the RENIS and PREVEND cohorts. FU, follow-up.
The RENIS (Tromsø, Norway) was designed to study determinants of change in measured GFR in middle-aged subjects from the general population and is an ancillary part of the sixth wave of the Tromsø Study, conducted in 2007–08. Subjects who did not report previous myocardial infarction, angina pectoris, stroke, diabetes mellitus (DM) or renal disease were invited to participate (N = 2825). A total of 2107 agreed and 1627 subjects were included according to a predetermined target of 1600 based on power calculations. Because uEGF has been shown to predict GFR decline in subjects with type 2 DM and pre-existing CKD [8, 9], for the current study we excluded subjects with DM (fasting glucose ≥7.0 mmol/L or haemoglobin A1c ≥6.5%; n = 33) or CKD [ACR ≥3.0 mg/mmol or measured GFR (mGFR) using iohexol clearance <60 mL/min/1.73 m2;n = 61] as well as subjects with missing data for uEGF (n = 2) or GFR at follow-up, mostly due to withdrawal (n = 282), leaving a cohort of 1249 subjects (Figure 1).
The PREVEND study (Groningen, the Netherlands) was designed to study the impact of albuminuria in subjects from the general population. A cohort of 8592 subjects was recruited from the inhabitants of the city of Groningen, ages 28–75 years, enriched for subjects with higher levels of albuminuria. For the current study, subjects that completed the second screening were used (2001–03, N = 6188) because urine samples from these subjects were available for uEGF measurement. We excluded subjects with DM (n = 144), CKD (ACR ≥3.0 mg/mmol or eGFR <60 mL/min/1.73 m2; n = 756) and missing data for uEGF (n = 86) or eGFR at baseline (n = 25) or eGFR at follow-up screening, mostly due to withdrawal (n = 643), leaving a cohort of 4534 subjects (Figure 1).
Both studies were approved by the local ethics committees and performed in accordance with the guidelines of the Declaration of Helsinki. All subjects provided written informed consent.
Data collection
The details of sample procurement in both the PREVEND study and RENIS have been previously described [13–15]. In both studies, the subjects completed a questionnaire regarding demographics, cardiovascular and renal disease history, smoking habits and medication use. Blood samples were drawn after an overnight fast for measurement of fasting serum glucose, triglycerides and cholesterol and stored at −80°C. For both studies, urinary albumin and creatinine concentration were measured in fresh urine samples.
Assessment of uEGF
In both studies, uEGF was assessed in duplicate. uEGF was normalized for urine creatinine concentration (uEGF/Cr) to adjust for differences in urine concentration. Hereafter, uEGF/Cr will be referred to as uEGF excretion. For the RENIS, uEGF was measured in the second void morning spot urine sample (stored at −80°C) using the Human EGF Immunoassay Quantikine enzyme-;inked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN, USA) on a 96-well format, with mean intra- and interassay coefficients of variation (COVs) of 3.5 and 4.6%, respectively. For the PREVEND study, uEGF was measured in a 24-h urine collection (stored at −20°C) using the Immunoassay Human EGF DuoSet ELISA (R&D Systems) on a 384-well format with mean intra- and interassay COVs of 4.7 and 7.9%, respectively. uEGF has been shown to be stable after prolonged frozen storage and after up to three freeze–thaw cycles [16]. Since uEGF was measured using different assay platforms in the two studies, 200 randomly selected urine samples were reanalysed using both platforms, showing a correlation of 0.93 and a high recovery within 70–130% and 80–120% boundaries of 94.0 and 86.1%, respectively.
Assessment of GFR
In the RENIS, GFR was measured at baseline and followed up with a single-sample plasma clearance of iohexol (mGFR). This method has been validated against gold standard methods and previously described in detail [13, 17]. Repeated follow-up measurements of GFR in a random sample of 87 subjects within 8 weeks showed that the mean COV for intra-individual GFR was 4.2% [interquartile range (IQR) 3.4–4.9] [18]. The subjects in the RENIS had one (baseline, n = 1531), two (follow-up, n = 1249) or three (repeated follow-up, n = 86) GFR measurements. In the PREVEND study, GFR was estimated with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation for creatinine and cystatin C (eGFR) [19], which has previously been described [20, 21]. Both measurements were calibrated against international standards. The subjects in the PREVEND study had one (baseline, n = 5202), two (follow-up, n = 4534), three (repeated follow-up, n = 3951) or four (repeated follow-up, n = 2849) eGFR assessments.
Statistical methods
Continuous data are presented as means with standard deviations (SDs) or as medians and IQRs in case of a skewed distribution. Categorical data are presented as percentages. We used Student’s t-test for normally distributed continuous variables, Wilcoxon's rank-sum test for skewed continuous variables and Pearson's chi-squared test for categorical variables to evaluate differences between the subjects below and above the combined uEGF median of both cohorts.
Rapid GFR decline was defined as an annual GFR loss >3.0 mL/min/1.73 m2, calculated by the difference in GFR from baseline to follow-up, divided by the observation time; a cut-off method used in previous studies [22–24]. For comparison, similar follow-up times for both cohorts were chosen (5.6 years in the RENIS and 5.0 years for the PREVEND study, limiting data from the baseline to the second follow-up screening for the latter cohort).
To utilize all available GFR measurements, we also defined rapid GFR decline as subjects with the 10% steepest GFR slope within each cohort [25, 26]. The GFR slope for each subject was calculated using all available GFRs in a linear mixed model with the baseline covariables age, sex, ACR, body mass index (BMI), systolic blood pressure (BP), fasting glucose, total cholesterol, triglycerides, current smoking and use of lipid- and BP-lowering drugs using random intercepts and slopes.
The association between uEGF excretion and rapid GFR loss was analysed using logistic regression models progressively adjusted for (i) sex, baseline age and GFR; (ii) ACR and (iii) the other aforementioned risk factors for CKD. Effect modification by age and sex was tested by including a two-way cross-product with uEGF excretion. Non-linear associations between uEGF excretion and rapid GFR decline were investigated by including a quadratic term of uEGF excretion in the logistic regression models. Logistic regression analyses with restricted cubic splines with three knots were used to visualize the association of uEGF excretion with the risk for rapid GFR decline.
In sensitivity analyses, we calculated the individual slope using a model adjusted for sex, baseline age and BMI only, because 8 and 252 subjects in the RENIS and PREVEND study, respectively, had missing values for the slope created using a model with all the baseline covariables listed above. Using this model, there were no missing values for the slope in either cohort.
To evaluate the discriminatory power of uEGF excretion for predicting a rapid decline in GFR during follow-up, we compared the area under the receiver operating characteristic curve (AUC) for nested logistic regression models using the likelihood ratio test and assessed the relative integrated discrimination index (rIDI) and the net reclassification index (NRI) [27]. The continuous NRI was used because it overcomes the problem of using categories that do not naturally exist and because we tested the same biomarker in two separate populations with different characteristics and age ranges [27–29].
The association between uEGF and the mean change in eGFR/GFR was analysed in linear mixed models with random intercept and random slope.
Finally, we combined the two cohorts to obtain power for interaction analyses with CKD risk factors and for the endpoint of incident CKD using multiple logistic regression. Incident CKD was defined as GFR <60 mL/min/1.73 m2 at the last follow-up or as GFR <60 mL/min/1.73 m2 accompanied by a GFR loss >10 mL/min/1.73 m2 or ≥25% relative to the baseline GFR, in accordance with recent proposals [30–33].
The analyses were performed using Stata version 14 (StataCorp, College Station, TX, USA) and R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org/). P-values <0.05 were considered statistically significant.
RESULTS
Baseline characteristics
The population characteristics of both studies are presented in Table 1. Baseline mGFR was 94.5 ± 13.0 mL/min/1.73 m2 in the RENIS and eGFR was 95.2 ± 14.2 mL/min/1.73 m2 in the PREVEND study. Because there was oversampling of subjects with higher albuminuria at baseline in the PREVEND cohort, the median ACR level was higher in the PREVEND study [0.7 mg/mmol (IQR 0.5–1.0)] compared with the RENIS [0.2 mg/mmol (IQR 0.1–0.5)].
Table 1.
Population characteristics
| Baseline variables | RENIS | PREVEND |
|---|---|---|
| (N = 1249) | (N = 4534) | |
| Sex (female), n % | 624 (50) | 2313 (51) |
| Age (years) | 57.9 ± 3.9 | 51.5 ± 10.9 |
| Race, n (%) | ||
| Caucasians | 1249 (100) | 4364 (96) |
| Asians | 0 | 86 (2) |
| Black | 0 | 35 (1) |
| Other | 0 | 49 (1) |
| BMI (kg/m2) | 27.1 ± 3.8 | 26.3 ± 4.1 |
| Systolic BP (mmHg) | 129.0 ± 17.4 | 123.4 ± 16.8 |
| Diastolic BP (mmHg) | 83.2 ± 9.7 | 72.6 ± 8.7 |
| Current smoker, n (%) | 232 (19) | 1203 (27) |
| Antihypertensive medication, n (%) | 209 (17) | 698 (15) |
| Lipid-lowering medication, n (%) | 74 (6) | 317 (7) |
| Fasting blood glucose (mmol/L) | 5.3 ± 0.5 | 4.8 ± 0.6 |
| Total cholesterol (mmol/L) | 5.6 ± 0.9 | 5.4 ± 1.0 |
| Triglycerides (mmol/L), median (IQR) | 1.0 (0.7–1.4) | 1.1 (0.8–1.6) |
| eGFRcyscrea (mL/min/1.73 m2) | 103.5 ± 10.7 | 95.2 ± 14.2 |
| mGFRiohexol (mL/min/1.73 m2) | 94.5 ± 13.0 | N/A |
| Urinary ACR (mg/mmol), median (IQR) | 0.21 (0.10–0.51) | 0.65 (0.48–1.01) |
| uEGF (µg/L), median (IQR) | 11.6 (5.9–22.0) | 10.5 (6.3–16.8) |
| uEGF/Cr (µg/mmol), median (IQR) | 1.8 (1.4–2.3) | 1.3 (0.9–1.9) |
Values are presented as mean ± SD unless stated otherwise. eGFR determined using the CKD-EPI creatinine–cystatin C equation. N/A: not available.
Associations between uEGF excretion and baseline characteristics
Table 2 presents the associations of uEGF excretion stratified by the median value (1.44 μg/mmol) with baseline demographics and established CKD risk factors. In both cohorts, subjects with a lower uEGF excretion were older and had higher BP and fasting blood glucose.
Table 2.
Associations of uEGF excretion stratified by the common uEGF/Cr median of both cohorts with baseline demographics and various established CKD risk factors
| Baseline variables | RENIS |
PREVEND |
||||
|---|---|---|---|---|---|---|
| uEGF/Cr < mediana | uEGF/Cr > mediana | P-value | uEGF/Cr < mediana | uEGF/Cr > mediana | P-value | |
| (n = 372) | (n = 877) | (n = 2520) | (n = 2014) | |||
| Sex (female), n % | 128 (34) | 496 (57) | <0.001 | 1054 (42) | 1259 (50) | <0.001 |
| Age (years) | 58.4 ± 3.8 | 57.7 ± 3.9 | 0.002 | 53.8 ± 10.9 | 48.6 ± 10.2 | <0.001 |
| BMI (kg/m2) | 27.4 ± 3.6 | 27.0 ± 3.9 | 0.08 | 27.0 ± 4.1 | 25.5 ± 4.0 | <0.001 |
| Systolic BP (mmHg) | 130.7 ± 17.1 | 128.2 ± 17.5 | 0.02 | 125.9 ± 17.1 | 120.3 ± 15.9 | <0.001 |
| Diastolic BP (mmHg) | 84.1 ± 9.4 | 82.8 ± 9.9 | 0.03 | 73.9 ± 8.9 | 70.8 ± 8.8 | <0.001 |
| Current smoker, n (%) | 62 (17) | 170 (19) | 0.26 | 630 (25) | 573 (28) | 0.001 |
| Antihypertensive medication, n (%) | 75 (20) | 134 (15) | 0.01 | 491 (19) | 207 (10) | <0.001 |
| Lipid-lowering medication, n (%) | 21 (6) | 53 (6) | 0.79 | 228 (9) | 89 (4) | <0.001 |
| Blood glucose (mmol/L) | 5.4 ± 0.5 | 5.3 ± 0.5 | 0.007 | 4.9 ± 0.6 | 4.7 ± 0.6 | <0.001 |
| Total cholesterol (mmol/L) | 5.6 ± 1.0 | 5.7 ± 0.9 | 0.13 | 5.5 ± 1.0 | 5.3 ± 1.0 | <0.001 |
| Triglycerides (mmol/L), median (IQR) | 1.1 (0.8–1.5) | 1.0 (0.7–1.4) | 0.1 | 1.2 (0.8–1.7) | 1.0 (0.7–1.3) | <0.001 |
| Urinary ACR (mg/mmol), median (IQR) | 0.20 (0.10–0.43) | 0.22 (0.10–0.54) | 0.14 | 0.65 (0.47–1.03) | 0.68 (0.49–0.99) | 0.07 |
| eGFRcyscrea (mL/min/1.73 m2) | 100.6 ± 11.4 | 104.7 ± 10.1 | <0.001 | 92.5 ± 14.4 | 98.6 ± 13.3 | <0.001 |
| mGFRiohexol (mL/min/1.73 m2) | 92.4 ± 13.0 | 95.4 ± 12.8 | <0.001 | N/A | N/A | N/A |
| uEGF (µg/L), median (IQR) | 6.3 (3.1–12.8) | 14.5 (7.6–26.0) | <0.001 | 7.3 (4.6–10.6) | 16.2 (11.4–22.7) | <0.001 |
| uEGF/Cr (µg/mmol), median (IQR) | 1.17 (0.99–1.31) | 2.06 (1.72–2.47) | <0.001 | 0.95 (0.69–1.19) | 2.01 (1.69–2.59) | <0.001 |
Values are presented as mean ± SD unless stated otherwise. eGFR was determined using the CKD-EPI creatinine–cystatin C equation. N/A: not available.
Urinary EGF/creatinine median was 1.44 µg/mmol.
A lower uEGF excretion level at baseline correlated with lower baseline GFR (r = 0.14, P < 0.001 and r = 0.22, P < 0.001, in the RENIS and PREVEND study, respectively). No significant correlation between uEGF excretion and ACR was observed in either cohort.
Association of uEGF excretion with rapid GFR decline
The median annual change in GFR was −0.92 (IQR −0.36 to −1.46) mL/min/1.73 m2/year in the RENIS (N = 1249, follow-up 5.6 years) and −0.85 (IQR −0.61 to −1.12) mL/min/1.73 m2/year in the PREVEND study (N = 4534, follow-up 7.4 years). Lower uEGF excretion was associated with an increased risk of rapid GFR decline in both cohorts (Table 3). All the models were well-calibrated, as judged by the Hosmer–Lemeshow statistic.
Table 3.
Logistic regression models with ORs for rapid GFR decline defined as a GFR decline >3.0 mL/min/1.73 m2/year a
| Model | RENIS |
PREVEND |
|||
|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | ||
| Crude | n = 1249 | n = 3951 | |||
| uEGF/Cr, per 1 µg/mmol decrease | 1.17 (0.89–1.53) | 0.30 | 1.32 (1.13–1.54) | 0.001 | |
| uEGF/Cr, fourth quartileb | Ref | Ref | |||
| uEGF/Cr, third quartileb | 1.15(0.72–1.82) | 0.57 | 1.47 (0.99–2.17) | 0.06 | |
| uEGF/Cr, second quartileb | 1.71 (1.06–2.75) | 0.03 | 1.51 (1.03–2.22) | 0.03 | |
| uEGF/Cr, first quartileb | 1.29 (0.62–2.68) | 0.50 | 1.87 (1.30–2.68) | 0.001 | |
| Model 1 | n = 1249 | n = 3951 | |||
| uEGF/Cr, per 1 µg/mmol decrease | 1.42 (1.06–1.90) | 0.02 | 1.31 (1.11–1.54) | 0.001 | |
| uEGF/Cr, fourth quartileb | Ref | Ref | |||
| uEGF/Cr, third quartileb | 1.46 (0.89–2.39) | 0.13 | 1.45 (0.98–2.16) | 0.07 | |
| uEGF/Cr, second quartileb | 2.38 (1.41–4.01) | 0.001 | 1.49 (1.00–2.21) | 0.048 | |
| uEGF/Cr, first quartileb | 1.95 (0.90–4.22) | 0.09 | 1.78 (1.22–2.62) | 0.003 | |
| Model 2 | n = 1245 | ||||
| uEGF/Cr, per 1 µg/mmol decrease | 1.42 (1.06–1.90) | 0.02 | 1.31 (1.12–1.55) | 0.001 | |
| uEGF/Cr, fourth quartileb | Ref | Ref | |||
| uEGF/Cr, third quartileb | 1.45 (0.89–2.37) | 0.14 | 1.47 (0.98–2.18) | 0.06 | |
| uEGF/Cr, second quartileb | 2.37 (1.40–4.01) | 0.001 | 1.52 (1.02–2.25) | 0.04 | |
| uEGF/Cr, first quartileb | 1.98 (0.91–4.28) | 0.08 | 1.81 (1.23–2.66) | 0.003 | |
| Model 3 | n = 1241 | n = 3758 | |||
| uEGF/Cr, per 1 µg/mmol decrease | 1.42 (1.06–1.91) | 0.02 | 1.29 (1.10–1.53) | 0.002 | |
| uEGF/Cr, fourth quartileb | Ref | Ref | |||
| uEGF/Cr, third quartileb | 1.43 (0.87–2.35) | 0.16 | 1.53 (1.02–2.31) | 0.04 | |
| uEGF/Cr, second quartileb | 2.39 (1.41–4.07) | 0.001 | 1.50 (0.99–2.26) | 0.05 | |
| uEGF/Cr, first quartileb | 1.93 (0.88–4.24) | 0.10 | 1.74 (1.16–2.61) | 0.01 | |
n = 127 for rapid decline in the RENIS; n = 293 for rapid decline in the PREVEND study.
Model 1: adjusted for sex, age and baseline GFR.
Model 2: adjusted for sex, age, baseline GFR and ACR.
Model 3: as in Model 2 and adjusted for BMI, systolic BP, fasting glucose, total cholesterol, triglycerides, current smoking, use of lipid-lowering drugs and use of antihypertensive medication.
Calculated by subtracting eGFR at baseline from eGFR at follow-up and dividing by the observation time (median 5.6 years in the RENIS and 5.0 years in the PREVEND study).
Common uEGF quartiles were used for both cohorts: 0.11–0.99, 0.99–1.44, 1.44–2.02 and 2.02–28.27 µg/mmol.
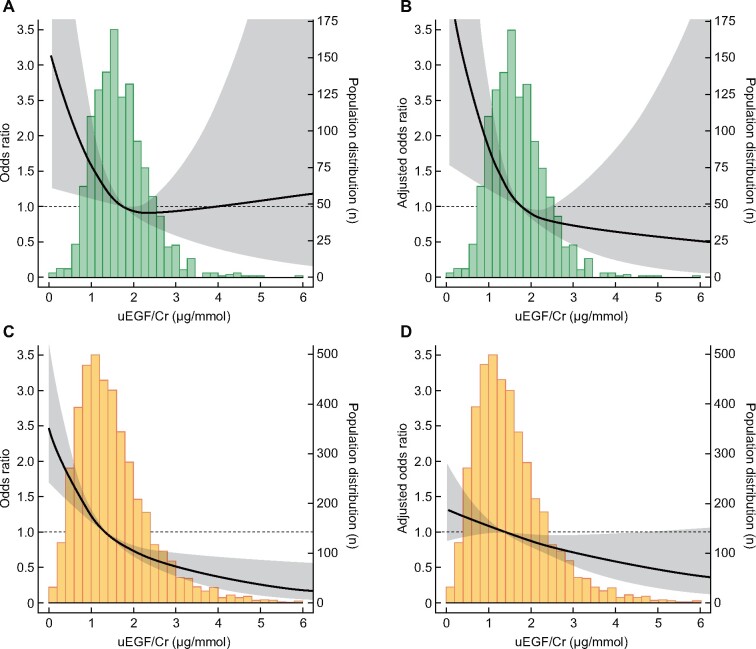
There was a borderline significant, non-linear quadratic association between uEGF excretion and rapid GFR decline in the RENIS (P = 0.07–0.1 in Models 1–4, Table 3) but not in the PREVEND study (P > 0.1). We repeated the analyses using an outcome of rapid GFR decline defined as subjects with the 10% steepest GFR slope within each cohort, calculated using linear mixed regression. The effect estimates were strengthened in the RENIS and attenuated in the PREVEND study, although they were statistically significant in both cohorts [odds ratio per 1 μg/mmol lower uEGF was 1.67 [95% confidence inetrval (CI) 1.23–2.27] and 1.20 (95% CI 1.04–1.38) in the RENIS and PREVEND study, respectively, in Model 3 (Figure 2 and Supplementary data, Table S1].
FIGURE 2.
Urinary EGF excretion and risk for rapid GFR decline defined as the 10% of subjects with the steepest GFR slope during follow-up per cohort (RENIS mean annual rate <−2.00 mL/min/1.73 m2 and PREVEND mean annual rate <−1.43 mL/min/1.73 m2). Upper panels show the association in subjects from the RENIS cohort (n = 1241): (A) crude and (B) adjusted for age, sex, mGFR and albuminuria. Lower panels show the association in subjects from the PREVEND cohort (n = 4282): (C) crude and (D) adjusted for age, sex, eGFR and albuminuria. Gray-shaded area denotes 95% CI.
We then investigated the association between baseline uEGF levels and the mean GFR change rates using linear mixed models. In the PREVEND study, there was a significant association between lower uEGF levels with a steeper GFR slope in the crude model only, and in the RENIS there were borderline significant associations with steeper slopes in the crude and adjusted models (Supplementary data, Table S2). The associations between tertiles of uEGF and GFR using linear mixed models with age as the time variable (age at baseline + observation time) are shown in the Supplementary data, Figure S1.
We tested the incremental value of adding uEGF to a statistical model with traditional CKD risk factors in predicting rapid GFR decline, defined as >3.0 mL/min/1.73 m2/year. The C-statistic increased only slightly from 0.71 to 0.72 in the RENIS and 0.65 to 0.66 in the PREVEND study (Supplementary data, Table S3). The improvement in the NRI was 0.30 (IQR 0.12–0.48) in the RENIS and 0.14 (IQR 0.02–0.26) in the PREVEND study (Supplementary data, Table S3). The event and non-event NRIs, as well as the rIDI, are presented in the Supplementary data, Table S3. The predicted versus observed risk of rapid GFR decline is shown in separate calibration plots for the RENIS and PREVEND study in the Supplementary data, Figure S2.
Subgroup analyses and incident CKD (GFR <60 mL/min/1.73 m2)
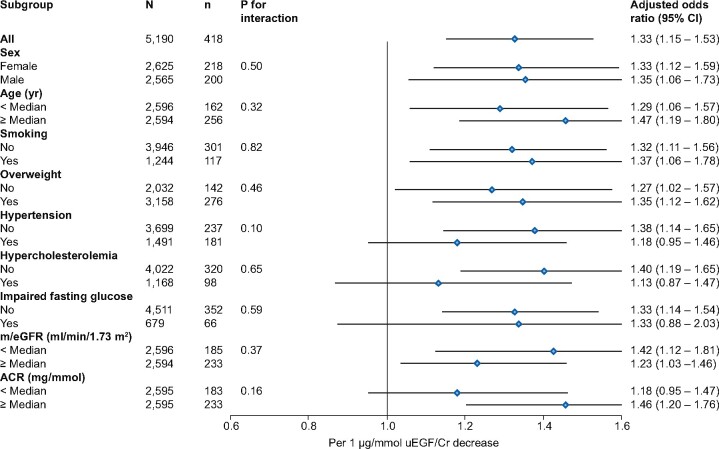
The cohorts were combined to improve power for analyses of the subgroups sex, current smoking, hypertension, hypercholesterolaemia, overweight, impaired fasting glucose, and age, e/mGFR and ACR above or below the median level and for analysis with incident CKD as an outcome.
No interaction was observed between uEGF excretion and CKD risk factors (Figure 3). The results seemed robust, as all subgroups showed increased risk when uEGF was lower, although due to impaired power significance was not obtained in all subgroups (Figure 3). Data on subgroup analysis for the RENIS and PREVEND study separately are provided in the Supplementary data, Figure S3.
FIGURE 3.
Logistic regression analyses with ORs for rapid GFR decline and interactions with various subgroups for both cohorts combined. Subgroups are defined by common medians or clinical class (hypertension defined as systolic BP ≥140 mmHg, diastolic BP ≥90 mmHg or use of BP-lowering drugs; hypercholesterolaemia defined as total cholesterol ≥6.5 mmol/L or use of lipid-lowering drugs; overweight defined as BMI ≥25 kg/m2; impaired fasting glucose defined as fasting glucose ≥5.6 mmol/L). The ORs are adjusted for baseline sex, age, m/eGFR and ACR. Common medians were age 53.3 years; m/eGFR 96.0 mL/min/1.73 m2; ACR 0.58 mg/mmol. N, number of subjects; n, number of events). Rapid GFR decline is defined as a GFR decline >3.0 mL/min/1.73 m2/year.
There were 189 cases of incident CKD defined as GFR <60 mL/min/1.73 m2 at the last follow-up and 96 and 41 cases of CKD defined as GFR <60 mL/min/1.73 m2 accompanied by a GFR loss >10 mL/min/1.73 m2 or ≥25% relative to baseline, respectively. The associations between uEGF and three separate definitions of incident CKD are presented in Table 4.
Table 4.
Logistic regression analyses with ORs for incident CKD ≤Stage 3a in the RENIS and PREVEND combined cohort a
| Incident GFR <60 mL/min/1.73 m2 |
Incident GFR <60 mL/min/1.73 m2 and baseline GFR >70 mL/min/1.73 m2 |
Incident GFR <60 mL/min/1.73 m2 and >25% GFR decline from baseline |
||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Model 1 | ||||||
| uEGF/Cr, per 1 µg/mmol decrease | 1.63 (1.26–2.10) | <0.001 | 1.70 (1.20–2.42) | 0.003 | 1.97 (1.14–3.40) | 0.02 |
| uEGF/Cr > medianb | Ref | Ref | Ref | |||
| uEGF/Cr < medianb | 2.02 (1.43–2.86) | <0.001 | 2.26 (1.41–3.64) | 0.001 | 2.22 (1.08–4.54) | 0.03 |
| Model 2 | ||||||
| uEGF/Cr, per 1 µg/mmol decrease | 1.23 (0.97–1.56) | 0.09 | 1.44 (1.02–2.05) | 0.04 | 1.83 (1.06–3.15) | 0.03 |
| uEGF/Cr > medianb | Ref | Ref | Ref | |||
| uEGF/Cr < medianb | 1.41 (0.98–2.04) | 0.07 | 1.91 (1.18–3.11) | 0.01 | 2.06 (1.00–4.24) | 0.049 |
| Model 3 | ||||||
| uEGF/Cr, per 1 µg/mmol decrease | 1.27 (0.99–1.62) | 0.06 | 1.39 (0.98–1.97) | 0.07 | 1.72 (1.01–2.95) | 0.048 |
| uEGF/Cr > medianb | Ref | Ref | Ref | |||
| uEGF/Cr < medianb | 1.44 (0.98–2.13) | 0.06 | 1.77 (1.07–2.93) | 0.03 | 1.95 (0.92–4.12) | 0.08 |
Model 1: adjusted for sex, age and cohort.
Model 2: adjusted for sex, age, cohort, baseline GFR and ACR.
Model 3: as in Model 2 and adjusted for BMI, systolic BP, fasting glucose, total cholesterol, triglycerides, current smoking, use of lipid-lowering drugs and use of antihypertensive medication.
There were 189, 96 and 41 incident cases of CKD according to the three different definitions, respectively.
The common urinary EGF/creatinine median was 1.44 µg/mmol.
Sensitivity analyses
To test the robustness of the results, we repeated the analyses using an outcome of rapid GFR decline defined as subjects with the top 20% steepest slopes in each cohort. The results were similar (Supplementary data, Table S4). Next, we recalculated the individual slopes using a less complex linear mixed model (adjusted for sex, baseline age and BMI only) and the results were similar (Supplementary data, Table S5).
When subjects with DM or CKD at baseline were not excluded from the analyses, similar associations were found (Supplementary data, Table S6). When subjects with ACR 3.0–30 mg/mmol were included, the association between uEGF and incident CKD became stronger and significant in all models (Supplementary data, Table S7).
Finally, in the RENIS cohort, we repeated the logistic regression analyses with eGFR calculated by the CKD-EPI creatinine–cystatin C equation in place of the mGFR. In these analyses, uEGF was similarly associated with rapid eGFR decline (Supplementary data, Table S8).
DISCUSSION
In two prospective general population–derived cohorts that excluded subjects with DM and CKD, lower urinary excretion of the kidney tubule–specific biomarker uEGF was associated with rapid kidney function decline and incident CKD.
GFR and albuminuria primarily cover the haemodynamic and glomerular state of the kidney but are insensitive to early tubular dysfunction. Tubular damage is increasingly recognized as an important factor in the development and progression of kidney disease [34]. Several urinary biomarkers of acute tubular injury have been identified, but only a few biomarkers reflect chronic tubular damage. EGF is expressed by tubular epithelial cells, represents functional tubular mass and is inversely associated with interstitial fibrosis and tubular atrophy [8, 35]. It also plays an important role in the regeneration of tubular cells through crosstalk with the Janus kinase–signal transducer and activator of transcription (JAK-STAT), extracellular signal-regulated kinase (ERK) and phosphoinositide 3-kinase (PI3K) pathways [36, 37]. Furthermore, intrarenal EGF is predicted to be the dominant upstream regulator of intrarenal transcripts significantly correlated with GFR decline in patients with CKD, providing a potential molecular mechanism that may underlie rapid loss of kidney function [8].
Our present clinical results build upon uEGF excretion as a predictor for kidney disease progression in patients with established CKD and DM [8, 38]. In CKD, reduced uEGF excretion is associated with progression to the composite endpoint of incident ESKD or a 40% reduction in kidney function [8, 38, 39]. In normoalbuminuric subjects with DM, lower uEGF showed an increased risk for future renal function decline [9].
EGF is only minimally detectable in plasma [40, 41] and urinary EGF excretion is a read-out of kidney tubular-specific expression of EGF [8, 42]. Hence, compared with serum creatinine, albuminuria and other biomarker candidates, the uEGF level is less likely to be confounded by extrarenal factors, which potentially contributes to its biomarker performance and sensitivity for early kidney injury. Moreover, uEGF is stable over extended periods of time and can be robustly detected using just a few microliters of urine sample, making it a clinically applicable biomarker in population-level settings.
Unlike the well-defined endpoints for CKD progression, that is, the incidence of ESKD, a doubling of serum creatinine or a decrease in GFR of 30–40% compared with baseline [43], there is no consensus on the definition of rapid kidney function decline in population-based studies. The aforementioned endpoints are unlikely to occur in healthy subjects from the general population within a limited duration of a typical cohort study. Endpoints defined by an absolute or percentage change from baseline to follow-up GFR have therefore been used in some studies [44]. However, as this usually relies on two GFR values, this method is more prone to misclassification and confounding compared with using a slope of GFR change calculated from all available GFR values throughout the follow-up duration. Therefore we also defined rapid GFR decline as the top 10% of subjects with the steepest GFR slopes as calculated by linear mixed models [43]. Similar results using this method further support the association between uEGF and rapid GFR decline.
Although uEGF levels were associated with rapid GFR decline in both cohorts, in adjusted models they were only borderline associated with the mean GFR decline in the RENIS. Notably, it is not necessarily a contradiction between the neutral effect of uEGF on the mean level of GFR and its association with rapid decline or incident CKD in a minority. uEGF may be an important contributing risk factor for CKD in combination with other genetic or environmental factors, thus affecting subgroups. For the youngest age group in the PREVEND study, the mean annual eGFR change rate decline was very small. Lower precision of eGFR in the normal range, confounding from non-GFR-related factors (such as muscle mass) and an abnormal increase in GFR in subgroups due to a phase of hyperfiltration may have influenced the results.
We defined incident CKD as incident GFR <60 mL/min/1.73 m2 accompanied by a GFR loss >10 mL/min/1.73 m2 or ≥25% loss of GFR from baseline [30–33]. By doing so, we ensure that incident CKD cases are likely to indeed have progressive CKD and preclude ‘contamination’ of incident CKD due to variability in measurements and biological day-to-day variability [45]. The association of uEGF with incident CKD is significant, independent of age, gender, cohort, baseline GFR and ACR. After adjusting for additional risk factors, the association either remains significant or on the borderline of significance (P = 0.08). An analysis including subjects with ACR 3.0–30 mg/mmol at baseline, which increased the sample size of the events, exhibited stronger and significant associations (Supplementary data, Table S7).
The addition of uEGF excretion to the conventional risk factors improved the prediction of rapid kidney function decline assessed by the NRI and IDI in both cohorts, but improvement of the AUC was limited. Continuous NRI has been proposed as a better metric to assess the discriminatory potential of a new biomarker, particularly when compared against models with existing baseline functionality [28, 29]. Although the use of continuous NRI has been criticized, in the current setting it is expected to be more sensitive than C-statistics and able to capture the enhanced detection of individuals at risk of a specific outcome where natural categories do not exist [28]. Our results build upon uEGF excretion as a predictor for kidney disease progression in patients with established CKD and DM [8, 38]. However, the exact place of measurement of uEGF to predict rapid kidney function decline has yet to be defined and is beyond the scope of the present study. However, it could be imagined that including uEGF into a risk assessment system may help to better predict which patients at high risk for CKD progression will actually progress. In addition, determination of this biomarker may also be of help to evaluate ‘intrarenal health’. This may be important for, for instance, kidney function assessment to select subjects to act as kidney transplant donors and before prescribing medications known to cause toxicity to kidney tubular cells, including certain antibiotics, mesalazine and human immunodeficiency virus medications [46, 47].
Our study has several limitations. Differences in the methods of urine sample collection and storage were present between the cohorts. However, it is unlikely that this affected the outcomes, as earlier studies showed that uEGF excretion was similar in spot and 24-h urine samples [40] and that uEGF was stable at different freezing conditions and during frozen storage [16]. A limitation regarding the uEGF measurements is the single measurement of uEGF. Intra-individual variation (day-to-day variation) in uEGF excretion may have allowed for misclassification bias when dividing the cohorts according to uEGF levels. Because both cohorts included predominantly white European subjects, validation of the utility of uEGF in general population cohorts of multi-ethnic origin will be required.
The strengths of the current study include comparable results in two independent, well-described cohorts from the general population [8, 9]. GFR was measured using an accurate method in the RENIS and estimated using the CKD-EPI creatinine–cystatin equation during long-term follow-up in the PREVEND study.
In conclusion, lower urinary excretion of the tubular biomarker uEGF was associated with rapid GFR loss in subjects without DM or CKD in two European population studies. Our study, together with previous reports in patients with CKD and in patients with diabetes and at high risk of CKD, indicates that uEGF may be a useful non-invasive biomarker that captures the tubular component of the function of the kidney in a manner beyond serum creatinine and proteinuria.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the subjects who participated in the RENIS and PREVEND study.
FUNDING
The RENIS is funded by the Northern Norway Regional Health Authority (SFP 1100-13), UiT The Arctic University of Norway and by an unrestricted grant from Boehringer-Ingelheim (1235.104 IIS). The PREVEND study is supported by several grants from the Dutch Kidney Foundation and the Dutch Heart Foundation (2001.005), the Dutch Government, the US National Institutes of Health and the University Medical Center Groningen, the Netherlands (E.033). This research project was supported in part by the Applied Systems Biology Core of the University of Michigan George M. O’Brien Kidney Research Core Center (P30-DK081943). None of the funding parties had any role in developing the protocol, performing the study or the publication process.
AUTHORS’ CONTRIBUTIONS
J.V.N., L.R.H., B.O.E., M.K., R.T.G., W.J. and T.M. were responsible for the conception and study design. J.V.N., L.R.H., V.N., K.S., R.T.G., B.O.E., W.J. and T.M. were responsible for the data analysis. J.V.N., L.R.H., V.N., K.S., M.D.S., B.O.E., M.K., R.T.G., W.J. and T.M. were responsible for the acquisition and interpretation of data. J.V.N., L.R.H., R.T.G., W.J. and T.M. drafted the manuscript. M.D.S., B.O.E., M.K. and R.T.G. critically revised the manuscript for important intellectual content. All authors read and approved the final manuscript. All authors agreed to be accountable for all aspects of the manuscript in ensuring that questions related to the accuracy or integrity of any part of it are appropriately investigated and resolved.
CONFLICT OF INTEREST STATEMENT
M.K., V.N. and W.J. have a patent pending on biomarkers for CKD progression (encompassing urinary excretion of epidermal growth factor as biomarkers of CKD progression). There are no other potential conflicts of interest relevant to this article.
(See related article by Nowak and Ärnlöv. Estimating tubular damage for predicting progression of chronic kidney disease—what are the implications for clinical practice and public health? Nephrol Dial Transplant 2021; 36: 1769–1770)
REFERENCES
- 1. Wang H, Naghavi M, Allen C. et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388: 1459–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gansevoort RT, Matsushita K, Van Der Velde M. et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 2011; 80: 93–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Go AS, Chertow GM, Fan D. et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296–1305 [DOI] [PubMed] [Google Scholar]
- 4. Levey AS, de Jong PE, Coresh J. et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int 2011; 80: 17–28 [DOI] [PubMed] [Google Scholar]
- 5. Gansevoort RT, Correa-Rotter R, Hemmelgarn BR. et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 2013; 382: 339–352 [DOI] [PubMed] [Google Scholar]
- 6. Weekley CC, Peralta CA.. Advances in the use of multimarker panels for renal risk stratification. Curr Opin Nephrol Hypertens 2012; 21: 301–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Earley A, Miskulin D, Lamb EJ. et al. Estimating equations for glomerular filtration rate in the era of creatinine standardization: a systematic review. Ann Intern Med 2012; 156: 785–795 [DOI] [PubMed] [Google Scholar]
- 8. Ju W, Nair V, Smith S. et al. Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci Transl Med 2015; 7: 316ra193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Betz BB, Jenks SJ, Cronshaw AD. et al. Urinary peptidomics in a rodent model of diabetic nephropathy highlights epidermal growth factor as a biomarker for renal deterioration in patients with type 2 diabetes. Kidney Int 2016; 89: 1125–1135 [DOI] [PubMed] [Google Scholar]
- 10. Hommos MS, Glassock RJ, Rule AD.. Structural and functional changes in human kidneys with healthy aging. J Am Soc Nephrol 2017; 28: 2838–2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen L, Yang T, Lu DW. et al. Central role of dysregulation of TGF-β/Smad in CKD progression and potential targets of its treatment. Biomed Pharmacother 2018; 101: 670–681 [DOI] [PubMed] [Google Scholar]
- 12. Choudhury D, Levi M.. Kidney aging—inevitable or preventable? Nat Rev Nephrol 2011; 7: 706–717 [DOI] [PubMed] [Google Scholar]
- 13. Eriksen BO, Mathisen UD, Melsom T. et al. Cystatin C is not a better estimator of GFR than plasma creatinine in the general population. Kidney Int 2010; 78: 1305–1311 [DOI] [PubMed] [Google Scholar]
- 14. Mathisen UD, Melsom T, Ingebretsen OC. et al. Estimated GFR associates with cardiovascular risk factors independently of measured GFR. J Am Soc Nephrol 2011; 22: 927–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lambers Heerspink HJ, Brantsma AH, De Zeeuw D et al. Albuminuria assessed from first-morning-void urine samples versus 24-hour urine collections as a predictor of cardiovascular morbidity and mortality. Am J Epidemiol 2008; 168: 897–905 [DOI] [PubMed] [Google Scholar]
- 16. Araki F, Nakamura H, Nojima N. et al. Stability of recombinant human epidermal growth factor in various solutions. Chem Pharm Bull 1989; 37: 404–406 [DOI] [PubMed] [Google Scholar]
- 17. Stevens LA, Levey AS.. Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol 2009; 20: 2305–2313 [DOI] [PubMed] [Google Scholar]
- 18. Melsom T, Stefansson V, Schei J. et al. Association of increasing GFR with change in albuminuria in the general population. Clin J Am Soc Nephrol 2016; 11: 2186–2194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Inker LA, Schmid CH, Tighiouart H. et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012; 367: 20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vart P, Bakker SJL, Schöttker B. et al. Relevance of correction for drift and day-to-day variation in cystatin C measurement: a post-hoc analysis of the PREVEND cohort, with independent replication in the ESTHER cohort. Clin Chem Lab Med 2015; 53: 1381–1390 [DOI] [PubMed] [Google Scholar]
- 21. Halbesma N, Brantsma AH, Bakker SJL. et al. Gender differences in predictors of the decline of renal function in the general population. Kidney Int 2008; 74: 505–512 [DOI] [PubMed] [Google Scholar]
- 22. Shlipak MG, Katz R, Kestenbaum B. et al. Rate of kidney function decline in older adults: a comparison using creatinine and cystatin C. Am J Nephrol 2009; 30: 171–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rifkin DE, Shlipak MG, Katz R. et al. Rapid kidney function decline and mortality risk in older adults. Arch Intern Med 2008; 168: 2212–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stefansson VTN, Schei J, Solbu MD. et al. Metabolic syndrome but not obesity measures are risk factors for accelerated age-related glomerular filtration rate decline in the general population. Kidney Int 2018; 93: 1183–1190 [DOI] [PubMed] [Google Scholar]
- 25. Grams ME, Sang Y, Ballew SH. et al. Evaluating glomerular filtration rate slope as a surrogate end point for ESKD in clinical trials: an individual participant meta-analysis of observational data. J Am Soc Nephrol 2019; 30: 1746–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boucquemont J, Heinze G, Jager KJ. et al. Regression methods for investigating risk factors of chronic kidney disease outcomes: the state of the art. BMC Nephrol 2014; 15: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Williamson JM, Lin HM, Kim HY.. Power and sample size calculations for current status survival analysis. Stat Med 2009; 28: 1999–2011 [DOI] [PubMed] [Google Scholar]
- 28. Pencina MJ, D'Agostino RB, Pencina KM. et al. Interpreting incremental value of markers added to risk prediction models. Am J Epidemiol 2012; 176: 473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leening MJG, Vedder MM, Witteman JCM. et al. Net reclassification improvement: computation, interpretation, and controversies: a literature review and clinician’s guide. Ann Intern Med 2014; 160: 122–131 [DOI] [PubMed] [Google Scholar]
- 30. Rebholz CM, Crews DC, Grams ME. et al. DASH (Dietary Approaches to Stop Hypertension) diet and risk of subsequent kidney disease. Am J Kidney Dis 2016; 68: 853–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grams ME, Rebholz CM, McMahon B. et al. Identification of incident CKD stage 3 in research studies. Am J Kidney Dis 2014; 64: 214–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bash LD, Coresh J, Köttgen A. et al. Defining incident chronic kidney disease in the research setting. Am J Epidemiol 2009; 170: 414–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shlipak MG, Day EC.. Biomarkers for incident CKD: a new framework for interpreting the literature. Nat Rev Nephrol 2013; 9: 478–483 [DOI] [PubMed] [Google Scholar]
- 34. Harris RC, Neilson EG.. Toward a unified theory of renal progression. Annu Rev Med 2006; 57: 365–380 [DOI] [PubMed] [Google Scholar]
- 35. Nowak G, Schnellmann RG.. Integrative effects of EGF on metabolism and proliferation in renal proximal tubular cells. Am J Physiol 1995; 269: C1317–25 [DOI] [PubMed] [Google Scholar]
- 36. Humes HD, Cieslinski DA, Coimbra TM. et al. Epidermal growth factor enhances renal tubule cell regeneration and repair and accelerates the recovery of renal function in postischemic acute renal failure. J Clin Invest 1989; 84: 1757–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lechner J, Malloth NA, Jennings P. et al. Opposing roles of EGF in IFN-α-induced epithelial barrier destabilization and tissue repair. Am J Physiol Cell Physiol 2007; 293: 1843–1850 [DOI] [PubMed] [Google Scholar]
- 38. Wu L, Li X-Q, Chang D-Y. et al. Associations of urinary epidermal growth factor and monocyte chemotactic protein-1 with kidney involvement in patients with diabetic kidney disease. Nephrol Dial Transplant 2018; [DOI] [PubMed] [Google Scholar]
- 39. Torres DD, Rossini M, Manno C. et al. The ratio of epidermal growth factor to monocyte chemotactic peptide-1 in the urine predicts renal prognosis in IgA nephropathy. Kidney Int 2008; 73: 327–333 [DOI] [PubMed] [Google Scholar]
- 40. Harskamp LR, Gansevoort RT, Boertien WE. et al. Urinary EGF receptor ligand excretion in patients with autosomal dominant polycystic kidney disease and response to tolvaptan. Clin J Am Soc Nephrol 2015; 10: 1749–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mattila AL, Viinikka L, Saario I. et al. Human epidermal growth factor: renal production and absence from plasma. Regul Pept 1988; 23: 89–93 [DOI] [PubMed] [Google Scholar]
- 42. Gesualdo L, Di Paolo S, Calabro A. et al. Expression of epidermal growth factor and its receptor in normal and diseased human kidney: an immunohistochemical and in situ hybridization study. Kidney Int 1996; 49: 656–665 [DOI] [PubMed] [Google Scholar]
- 43. Coresh J, Turin TC, Matsushita K. et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 2014; 311: 2518–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weldegiorgis M, de Zeeuw D, Li L. et al. Longitudinal estimated GFR trajectories in patients with and without type 2 diabetes and nephropathy. Am J Kidney Dis 2018; 71: 91–101 [DOI] [PubMed] [Google Scholar]
- 45. Gowans EMS, Fraser CG.. Biologial variation of serum and urine creatinine and creatinine clearance: ramifications for interpretation of results and patient care. Ann Clin Biochem 1988; 25: 259–263 [DOI] [PubMed] [Google Scholar]
- 46. Barnett LMA, Cummings BS.. Nephrotoxicity and renal pathophysiology: a contemporary perspective. Toxicol Sci 2018; 164: 379–390 [DOI] [PubMed] [Google Scholar]
- 47. Perazella MA. Tenofovir-induced kidney disease: an acquired renal tubular mitochondriopathy. Kidney Int 2010; 78: 1060–1063 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.