Abstract
Background
TP53 mutations occur in more than 50% of cancers. We sought to determine the effect of the intragenic P72R single nucleotide polymorphism (SNP; rs1042522) on the oncogenic properties of mutant p53.
Methods
P72R allelic selection in tumors was determined from genotype calls and a Gaussian distributed mixture model. The SNP effect on mutant p53 was determined in p53-negative cancer cell lines. RNA-sequencing, chromatin immunoprecipitation, and survival analysis were performed to describe the SNP effect. All statistical tests were 2-sided.
Results
Among 409 patients with germline heterozygous P72R SNP who harbored somatic mutations in TP53, we observed a selection bias against missense TP53 mutants encoding the P72 SNP (P = 1.64 x 10-13). Exogenously expressed hotspot p53 mutants with the P72 SNP were negatively selected in cancer cells. Gene expression analyses showed the enrichment of p53 pathway genes and inflammatory genes in cancer cells transduced with mutants encoding P72 SNP. Immune gene signature is enriched in patients harboring missense TP53 mutations with homozygous P72 SNP. These patients have improved overall survival as compared with those with the R72 SNP (P = .04).
Conclusion
This is the largest study demonstrating a selection against the P72 SNP. Missense p53 mutants with the P72 SNP retain partial wild-type tumor-suppressive functions, which may explain the selection bias against P72 SNP across cancer types. Ovarian cancer patients with the P72 SNP have a better prognosis than with the R72 SNP. Our study describes a previously unknown role through which the rs1042522 SNP modifies tumor suppressor activities of mutant p53 in patients.
TP53 mutations are a classical hallmark of human cancer (1,2). A majority of TP53 mutations are somatic and missense with single-base substitutions altering the encoded amino acid in the DNA binding domain of the protein p53 (3). The most frequent somatic mutations are referred to as “hotspot” mutations (4). In addition to somatic mutations, inherited germline polymorphisms in TP53 play a modifying role in p53 function (5). Most common TP53 single nucleotide polymorphisms (SNPs) are considered innocuous, but some may have biological and clinical significance in disease (6).
The most common TP53 SNP occurs at codon 72 (5), where the nucleotide sequence CCC or CGC encodes proline (P72) or arginine (R72) (rs1042522; P72R) and is found in the proline-rich domain (PRD). The ancestral P72 SNP is more common in equatorial populations, and the R72 SNP is more common in higher latitudes and colder climates (7,8). Cancers appear to select for TP53 mutations on the R72 SNP over the P72 SNP relative to their prevalence in the germline. Studies have demonstrated that some TP53 mutations with the R72 SNP have a growth advantage, which may partly explain this selection in cancers (5,9-14). However, to our knowledge, no studies have examined whether the P72 SNP is selected against because of antagonism toward a presumptive pathogenic missense mutation.
We uncovered that the oncogenic effect of TP53 hotspot mutations is inhibited by the P72 polymorphism. In 2 analyses performed on a large pan-cancer genomic dataset, we showed that TP53-mutated tumors that developed on the heterozygous P72R germline background preferentially harbor TP53 mutations in the R72 SNP allele and underwent loss of heterozygosity of the P72 SNP allele. Using RNA-Seq in cell lines, we demonstrated that p53 missense mutants with the P72 SNP show enrichment of p53 pathway genes. In contrast, the same p53 mutants with the R72 SNP induced gene sets associated with cell proliferation and pro-oncogenic pathways. We also show that p53 mutants with the P72 SNP transactivate canonical p53 targets, whereas mutants with the R72 SNP show no biologically significant binding or transactivation of these targets. We validated this effect using xenograft models by showing the growth of p53 missense mutants with the P72 SNP is much slower than tumors with the R72 SNP. Finally, high-grade serous ovarian carcinoma patients harboring p53 missense mutants with the P72 SNP have better overall survival than those with the R72 SNP. Altogether, we uncover a critical role for the P72R SNP in modulating the function of missense mutant p53, which has important implications for p53 biology and the prognostic effect of TP53 mutations in cancers.
Methods
Please see the Supplementary Methods (available online) for a detailed description of the methods used in this study.
The Cancer Genome Atlas (TCGA) Data Analysis
The initial query included 7980 patient samples with germline variant calls from TCGA dataset reported by Huang et al. (15). Subsequent analysis focused on 3956 patients with somatic mutations in TP53; 409 patients had germline P72R heterozygosity. P72R genotype calls were based on Huang et al. (TCGA genotype call) and by fitting a Gaussian-distributed finite mixture model.
Model-based clustering was used to generate the density distributions of patient samples. Clustering was conducted assuming a finite mixture of Gaussian distributions using the “normalmixEM” function in the R-package mixtools. The number of clusters was assumed to be k = 3 for the clustering analysis performed on the various data sets. Preference toward homozygygous R72 from homozygous P72 was performed by first computing the sample-wise difference in the estimated posterior probabilities of class membership between these 2 classes, followed by a nonparametric Wilcoxon rank-sum test on the differences to test the null hypothesis that the median difference is equal to 0.
RNA Sequencing Analysis From Tumors
Raw counts of RNA-sequencing (RNA-Seq) data of primary tumors from TCGA-ovarian cancer (OV) were obtained by the “TCGAbiolinks” R package “GDCdownload()” and “GDCprepare()” commands (16-18). Samples with RNA-Seq data available were filtered by those with P72 (n = 41) or R72 (n = 87) homozygous calls. Ensembl gene ids were converted to gene symbols by the “GeoTcgaData” R package “id_conversion()” command. Genes with less than 5 reads in 10% or less of samples were excluded. Filtered raw counts were normalized, and differential expression analyses were performed using the Bioconductor package DESeq2 (19). Differential expression analyses accounted for tumor purity as determined by Aran et al. (20). The complete DESeq2 results are available at https://osf.io/5ugv9/.
Gene set enrichment analysis (GSEA) was performed using GenePattern “GSEAPreranked” (v7.1.x) module using gene sets from the Molecular Signature Database (21,22). The ranking metric for GSEA-Preranked data was DESeq log2 fold-change values. The complete R code and results of GSEA analysis are available at https://osf.io/5ugv9/. Results of GSEA analysis show 5 representative enriched gene sets per genotype with a false discovery rate of no more than 25%, selected because of their relevance to the cancer pathways being evaluated in this study.
Immunoblotting
Immunoblotting was performed as previously described (23). Additional details are provided in the Supplementary Methods (available online).
Gene Expression Analysis
RNA isolation, library preparation, sequencing, analysis, and quantitative reverse transcription polymerase chain reaction were performed as previously described (24,25). Additional details are also provided in the Supplementary Methods (available online).
Growth Assays and Clonogenic Assays
On day 1, 2000 cells of each mutant were seeded, and plates were collected over 5 days. Cells were seeded, grown, and stained using Sulforhodamine B staining procedures as described previously (26,27).
Chromatin Immunoprecipitation (ChIP) and Polymerase Chain Reaction
ChIP was performed as previously described (28,29) with modifications (30).
Mouse Studies
Animal studies were performed according to the guidelines of the institutional animal care and local veterinary office and ethics committee of the University of California, Davis. Female NSG mice from Envigo (NOD.CB17-Prkdcscid/NCrHsd) were used. Xenografts were established by subcutaneous injection of SKOV3 cells freshly transduced with p53 mutants and selected with puromycin. Six mice per group for subcutaneous injection were included, for a total of 18 mice.
Statistical Analysis
The initial query included 7980 patients from TCGA database; 3956 patients had somatic TP53 mutations. Of the patients, 409 heterozygous for the P72R SNP were selected for analysis using genotype calls made by Huang et al. (15) and by fitting a Gaussian distributed finite mixture model to create 3 clusters (homozygous P72, heterozygous, and homozygous R72) based on R72 variant allele frequencies determined from tumor exome sequencing datasets. Follow-up statistical testing for preference toward the R/R allele was conducted. Additional details on the statistical tests that were performed can be found in the Supplementary Methods (available online). All statistical tests are 2-sided, and a P value of less than .05 was considered statistically significant.
Results
Effect of P72R SNP on Mutant TP53 Allelic Selection in Patients
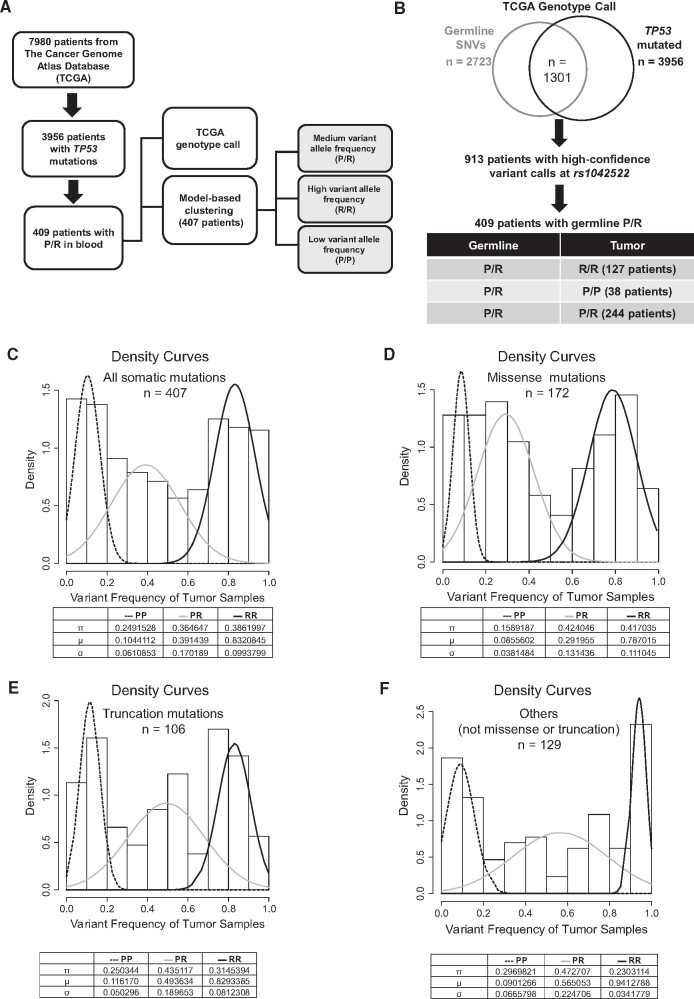
To assess the P72R SNP effect on the mutant TP53 allele selection in tumor samples, we identified patients with germline P72R heterozygosity from a published pan-cancer analysis (15). Among 3956 patients with somatic TP53 mutations, we identified 409 patients with the germline P72R heterozygous genotype and extracted the genotype calls from the corresponding tumors (TCGA legacy archive). We also developed an independent model-based clustering assuming a mixture Gaussian distribution of the P72R variant frequencies in tumors (Figure 1, A). Among 409 patients, 244 maintain heterozygosity, 127 show loss of P72 allele, and 38 show the loss of R72 allele in tumors. The enrichment of the R/R over the P/P genotype in tumors was found to be statistically significant (P = 1.64 x 10-13, multinomial proportions test) (Figure 1, B; Supplementary Figure 1, A, available online). Additionally, in high-grade serous ovarian cancer, which has the highest frequency of TP53 mutations, we observed a similar trend of bias against the P/P genotype in the tumors (P = 1.44 x 10-4, multinomial proportions test) (Supplementary Figure 1, F, available online).
Figure 1.
The effect of intragenic P72R SNP on mutant TP53 allele selection in patients. A) A schematic describing 2 methods used for the data analysis. B) The genotype calls were obtained from the Huang et al. (15) and The Cancer Genome Atlas (TCGA). Of these, 913 patients had high confidence genotype calls at the rs1042522. Tumor genotype calls for the rs1042522 and the proportion of patients for each genotype are shown. The proportion of patients with somatic mutations in TP53 with the homozygous P72 (P/P) genotype is statistically significantly lower than those with the homozygous R72 (R/R) genotype (P = 1.64 x 10-13). C) The rs1042522 variant frequencies from the tumor samples (n = 407) are fitted into a Gaussian distributed mixture model, and this analysis indicates the estimated fraction (π) of patients with the P/P genotype is statistically significantly lower than those with the R/R genotype (P = .003). D) In the subset of patients with TP53 missense mutations (n = 172), the estimated fraction of patients with the P/P genotype is statistically significantly lower than those with R/R genotype (P < .001). E) In the subset of tumors with TP53 truncation mutations (n = 106), the estimated fraction of patients with the P/P genotype is 0.250 compared with 0.314 for the R/R genotype (P = .27). F) In the remaining subset of tumors with other TP53 mutations, the estimated fraction of patients with P/P genotype is 0.296 compared with 0.230 for the R/R genotype (P = .30). All statistical analysis was performed using a multinomial proportions test. μ = mean variant allele frequency of the subpopulation; σ = standard deviation.
Next, P72R variant frequencies were determined from 407 tumor exome sequencing datasets, and posterior probabilities were calculated to assign the tumor genotype. The results of this analysis indicate a statistically significant loss of the P72 allele in tumors (P = .003, multinomial proportions test; Figure 1, C; Supplementary Figure 1, B, available online). Similarly, there is a statistically significant loss of the P72 allele in tumor samples with missense TP53 mutations (P < .001, multinomial proportions test; Figure 1, D; Supplementary Figure 1, C, available online). In contrast, no statistically significant bias for P72 allele loss is seen in tumors with nonmissense (truncation or nonsense) TP53 mutations (P = .27 and P = .30, respectively, multinomial proportions test; Figure 1, E and F; Supplementary Figure 1, D and E). Collectively, these results suggest that missense mutations in the P72 allele are negatively selected. Although the positive selection of missense mutations in the R72 allele is previously reported (31), our results provide an alternative, but not mutually exclusive, hypothesis that missense mutants in the P72 allele are negatively selected in tumors.
Effect of P72R SNP on Mutant p53-Induced Cell Growth
To gain mechanistic insights into the selection bias against the P72R SNP in tumors, we transduced p53-null SKOV3 and H1299 cells with 3 hotspot missense mutants (R248W, R248Q, R273H) with either P72 or R72 SNP. The short- and long-term growth assays revealed that hotspot mutants with the P72 SNP statistically significantly suppress cell growth vs the same mutants with the R72 SNP, compared with vector-transduced controls (Figure 2, A-F; Supplementary Figure 2, G and H, available online). This was reproduced in H1299 lung cancer cells (Supplementary Figure 2, A-F, available online). Using co-expressing green fluorescence protein (GFP) reporter and flow cytometry analysis, we observed gradual drift toward GFP-negative population in hotspot mutants with the P72 SNP (Chi (TX) > 4) (Figure 2, G; Supplementary Figure 2, I and J, available online), thus providing corroborating evidence that hotspot mutants with the P72 SNP exert negative selection pressure on cancer cells.
Figure 2.
The effect of missense TP53 mutants with the P72 SNP on cancer cell proliferation. A-C) SKOV3 cells were transduced with p53 mutants with either P72 or R72 SNP for 48 hours followed by a 10-day selection with 8 µg/ml of puromycin. Cells were subjected to cell growth assay with Sulforhodamine B (SRB) dye and colorimetric absorbance at 510 nm. Bottom panels show growth inhibition ratios of the P72/R72. D-F) TP53 mutants with the P72 SNP attenuate clonogenic growth relative to the R72 counterparts. Colonies were stained with SRB and counted after 14 days of culture. After the colony count, solubilized SRB was measured by absorbance at 510 nm. G) Cells expressing p53 mutant R248W with the P72 SNP undergo negative selection over time. Mutant cells were sorted (passage 0) and passaged 12 times over 40 days. Using green fluorescence protein (GFP) as a surrogate marker for p53 expression, the intensity of GFP expression was measured using flow cytometry at select passage intervals. Representative data at multiple passages is shown in panel G for p53 mutant R248W, and other mutants are shown in Supplementary Figure 2, I and J (available online). All data are shown as mean (SD). The statistical analysis was performed using a 2-tailed Student t test.
Effect of P72R SNP on Mutant p53-Induced Global and Canonical Target Gene Expression
RNA-Seq analysis of mutant pairs (Figure 3, A) identified 3528 and 2701 transcripts upregulated in cells transduced with hotspot mutants encoding P72 or R72 SNP, respectively (Figure 3, B; Supplementary Figure 3, A and B, available online). Metascape pathway analysis indicated upregulation of p53 pathway genes associated with apoptosis and endoplasmic reticulum (ER) stress response in mutants with the P72 SNP, whereas pro-oncogenic pathways genes associated with cell cycle progression, DNA repair, and DNA replication were upregulated in mutants with R72 SNP (Figure 3, C). Additionally, p53 canonical targets, like CDKN1A, GADD45α, GADD45β, and BBC3 were upregulated in p53 mutants with the P72 SNP (Figure 3, D and E; Supplementary Figure 3, C and D, available online). GSEA indicates that p53 pathway and apoptosis gene sets are enriched in mutants with the P72 SNP (Figure 3, F and G; Supplementary Figure 3, G and H, available online), whereas E2F, G2M, and MYC target genes are enriched in mutants with the R72 SNP (Figure 3, G; Supplementary Figure 3, E and F, available online). Altogether, our results suggest a tumor-suppressive role for p53 mutants with the P72 SNP and a tumor-promoting role for the same mutants with the R72 SNP.
Figure 3.
The effect of P72 SNP on the transcriptional regulation of canonical p53 target genes by TP53 missense mutants. A) Group design for the pairwise analysis of RNA transcripts from cells transduced with p53 mutants. SKOV3 cells were freshly transduced with p53 mutants R248Q, R248W, and R273H with the P72 and R72 SNP. Three mutant pairs are considered as experimental replicates, and 3 technical replicates for each mutant are included. After transduction and 5-8 days of puromycin selection, RNA was collected, and sequencing libraries were generated. DESeq2 and pair-wise analysis were used to analyze reads between the P72 and R72 groups (n = 18) and within each mutant pair. B) Differentially expressed transcripts in p53 mutants with the P72 SNP vs the R72 SNP. 3' Tag RNA-Seq was conducted on 9 p53 mutants with the P72 SNP and 9 p53 mutants with the R72 SNP; 20 982 transcripts show no statistically significant changes between the mutants with the P72 SNP and mutants with the R72 SNP, 3528 transcripts were upregulated in cells transduced with the mutants with the P72 SNP compared with those transduced with the mutants with the R72 SNP (false discovery rate [FDR] < 0.01 and fold change > 1.5), and 2701 transcripts upregulated in the mutants with the R72 SNP relative to the P72 SNP (FDR < 0.01 and fold change > 1.5). C) Metascape analysis of pathways associated with upregulated genes. D) Heatmap showing changes in the expression of select p53-target genes. E) MA plot of p53 mutant R248W indicates altered gene levels in select p53 targets. F) The number of gene sets predicted to be affected by p53 mutants with either the P72 or the R72 SNP. G) Select hallmark gene sets from the gene set enrichment analysis (GSEA) differentially enriched in mutants with either the P72 or R72 SNP. Representative enrichment plots of E2F targets and apoptosis gene sets differentially enriched in mutants with the R72 SNP or with the P72 SNP, respectively, are shown.
Gene Set Enrichment Analysis in Ovarian Carcinomas
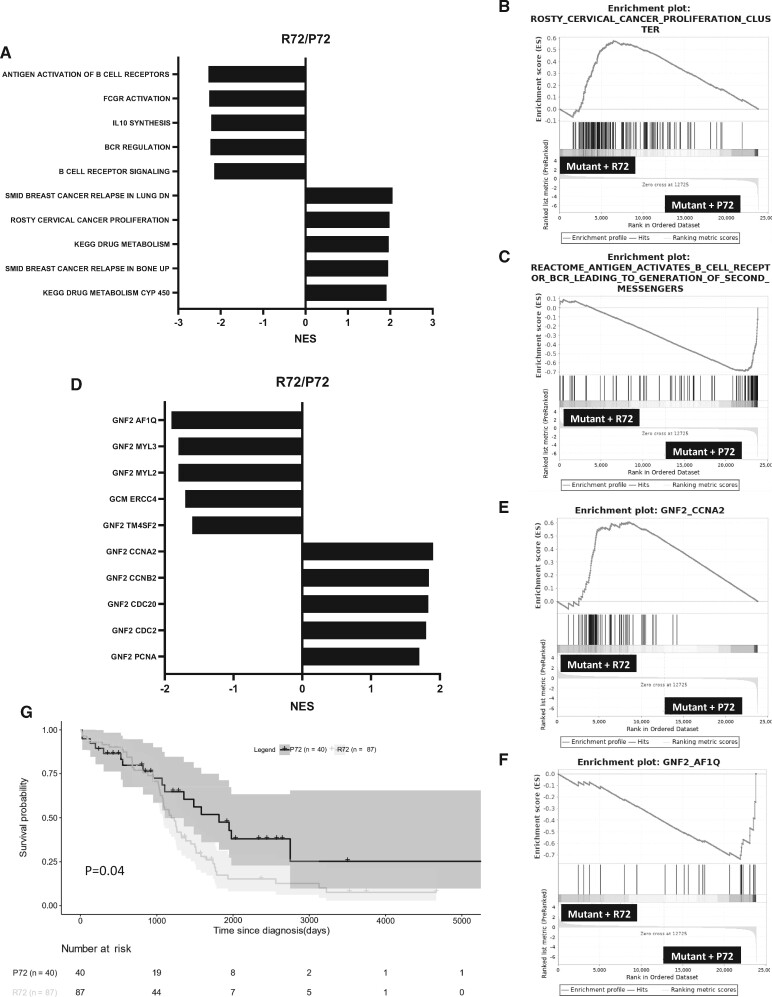
RNA-Seq analysis of ovarian carcinomas from TCGA showed statistically significant enrichment of cancer proliferation gene set in tumors with missense TP53 mutations homozygous for the R72 SNP (Figure 4, A and B), consistent with Figures 2 and 3. In contrast, tumors with missense TP53 mutations homozygous for the P72 SNP enriched for gene sets associated with antigen activation and cytokine signaling (Figure 4, A and C). Moreover, tumors with the R72 SNP showed statistically significant enrichment for cell proliferation gene signatures like PCNA, CDC2, CDC20, CCNA2, and CCNB2 (Figure 4, D and E). In contrast, tumors with the P72 SNP displayed statistically significant enrichment in gene sets associated with apoptotic signaling like AF1Q (Figure 4, D and F). Finally, ovarian cancer patients harboring TP53 missense mutations with the homozygous P72 SNP have better overall survival relative to patients harboring mutations with the homozygous R72 SNP (P = .04, log-rank test) (Figure 4, G). Collectively, these results corroborate the in vitro studies that demonstrate a tumor-suppressive role for p53 mutants with the P72 SNP vs a tumor-promoting role for mutants with the R72 SNP.
Figure 4.
Missense TP53 mutants with the P72 SNP and the enrichment for immune-related gene sets in high-grade serous carcinoma (HGSC) patients. A) Normalized enrichment scores (NES) of HGSC patient tumors with p53 mutations with the R72/P72 using gene set enrichment analysis (GSEA). The analysis was conducted using the curated gene set (C2) from the Molecular Signature Database. Five statistically significantly upregulated and downregulated gene sets are shown. B) A representative enrichment plot of cervical cancer proliferation clusters (Hallmark gene set) is shown. C) A representative enrichment plot of antigen-activated B-cell receptor gene sets (Hallmark gene set) is shown. D) NES of HGSC patient tumors with p53 mutations with the R72/P72 using GSEA. The analysis was conducted using the computational gene set (C4) from the Molecular Signature Database. Five statistically significantly upregulated and downregulated gene sets are shown. E) A representative enrichment plot of proliferation-associated CCNA2 (Hallmark gene set) is shown. F) A representative enrichment plot of apoptosis-associated AF1Q gene sets (Hallmark gene set) is shown. G) Kaplan-Meier plot for the overall survival of ovarian cancer patients with TP53 missense mutations grouped by the P72 or R72 variant.
Effect of P72R SNP on p53 Target Gene Expression
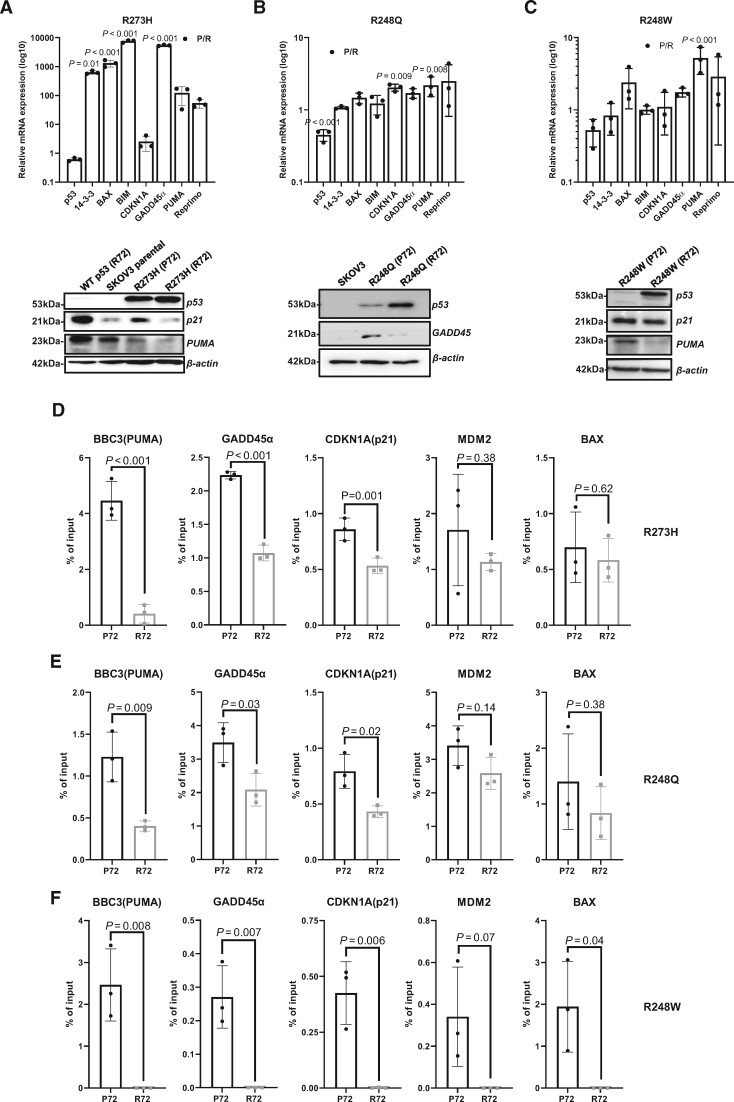
Using RNA expression analysis and immunoblotting, we found that p53 target genes involved in apoptosis, cell cycle regulation, and DNA damage response, such as BAX, PUMA, CDKN1A, GADD45α, and Reprimo, were upregulated in mutants with the P72 SNP compared with mutants with the R72 SNP (Figure 5, A-C; uncropped blots Supplementary Figure 4, A-C, available online). ChIP analysis indicates that mutants with the P72 SNP bind to promoters of p53 target genes like BIM, BAX, GADD45, CDKN1A (p21), and BBC3 (PUMA), whereas the same mutants with the R72 SNP show attenuated binding to the promoters (Figure 5, D-F). MCF-7 cells containing endogenous wild-type p53 activated by Nutlin 3 A treatment displayed higher but relatively comparable binding (compared with mutants with the P72 SNP) to the promoters of 3 statistically significantly altered genes: BBC3 (PUMA), GADD45α, and CDKN1A (p21) (Supplementary Figure 4, D, available online).
Figure 5.
The missense mutants with the P72 SNP maintain tumor suppressor function by binding to p53 canonical promoters. A-C) Quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis of select p53-target genes in SKOV3 cells transduced with mutants R273H, R248Q, or R248W with either P72 or R72 SNP. Cells were freshly transduced and selected for puromycin before total RNA was extracted for qRT-PCR. Data are shown as mean (SD) of P72 over R72 ratio in log10. Validation of altered transcripts was carried out by measuring protein levels using immunoblotting for p53 canonical targets. Whole-cell lysates were used for immunoblotting. D-F) Chromatin immunoprecipitation (ChIP) and quantitative PCR analysis of select p53-target genes. Immunoprecipitation was carried out using p53 antibody (DO-1), and enrichment was analyzed relative to percent of input. Experiments were conducted in 3 technical replicates and 3 independent biological replicates. All data are shown as mean (SD). The statistical analysis was performed using a 2-tailed Student t test. Wilcoxon sum ranked test for all graphs P ≥ .10.
Consistent with the ChIP and gene expression results, immunofluorescence using the antibody recognizing the wild-type p53 conformation (PAb1620) indicates a statistically significant increase in p53-positive nuclei in the R248W mutant with P72 SNP compared with the mutant with R72 (Supplementary Figure 5, available online). These results suggest that P72R polymorphism may alter p53 conformations, leading to differential p53 activities. Codon 72 is in the unstructured N-terminal PRD region (amino acids 40-90). To better understand how Pro vs Arg alters p53 conformation, we performed ab initio modeling of the PRD using QUARK protein structure prediction software (32). The proline vs arginine substitution produces distinct conformational changes because proline restricts backbone rotation, leading to a less tightly packed global structure that allows greater PRD flexibility (Supplementary Figure 6, A, left panel, available online), whereas arginine 72 (Supplementary Figure 6, A, right panel, available online) favors a tighter conformation of the PRD. Interestingly, modeling of full-length p53 using I-TASSER (33) suggests that the flexible PRD could potentially interact with R248, which is on the outer surface of p53 (Supplementary Figure 6, B, available online).
Effect of P72R SNP on Tumor Growth In Vivo
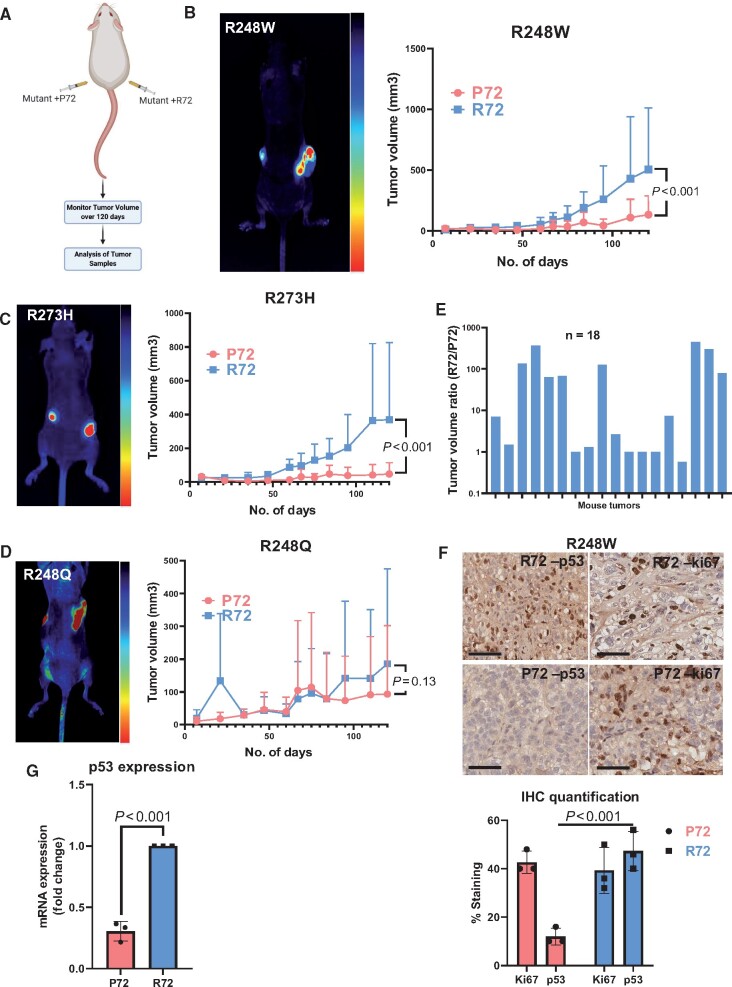
To assess the in vivo effect of P72R SNP on mutant p53 allele selection, we established a subcutaneous tumor model (Figure 6, A). After 120 days, mutants with the P72 SNP formed small or no tumors. This suppressive effect is statistically significantly stronger (P < .001, F test of variance) in R248W and R273H mutants (Figure 6, B and C; Supplementary Figure 7, A and B, available online) than in R248Q mutants (Figure 6, D; Supplementary Figure 7, C, available online), indicating the suppressive effect of the P72 SNP on mutant p53 behavior is mutation dependent. Although some mutants with the P72 SNP formed tumors, causing large experimental variations, these tumors displayed loss of p53 and GFP expression, suggesting that p53-silenced cancer cells or parental cells outcompeted cells transduced with the P72 SNP. In contrast, the same mutants with the R72 SNP maintained p53 and GFP expression (Figure 6, F and G; Supplementary Figure 7, A-D, available online). Tumor volume ratios (mutant + R72 SNP divided by mutant + P72 SNP) from each mouse at day 120 indicate an overall increase in tumor volume (12 of 18 mice; 66.7%) (Figure 6, E; Supplementary Figure 7, A-C, available online). Vector-transduced cells produced larger tumors than cells transduced with the R248W mutant encoding the P72 SNP (Supplementary Figure 8, available online). Ki-67 expression in tumors from mutants with P72 vs R72 was comparable (Figure 6, F). However, mutants with the P72 indicated a loss of p53 expression (Figure 6, G), recreating the negative selection observed in vitro and suggesting the outgrowth of p53-null cells.
Figure 6.
The P72 SNP regulates the negative selection of p53 mutants in vivo. A) Schematic describing experimental setup and subcutaneous injection pattern of mutant cell lines (n = 18). B-D) Comparison of in vivo tumor growth between P72 and R72 variants with select TP53 mutants. NSG nude mice were injected with 1 x 106 cells on each respective flank. Mutants with P72 were injected on the left flank, and mutants with R72 were injected on the right flank. Tumor volume was measured using calipers over a period of 120 days. The statistical analysis was performed using the F test of variance. Mean tumor volume (SD) is shown. E) Graph of tumor volume ratio in individual mice. Tumor ratios are displayed on a log10 scale. Tumor volume ratios were measured at end of the experiment (day 120). (F) Immunohistochemistry was performed on tumor tissues from the mutant with the P72 SNP or the mutant with R72 SNP groups. Ki-67 staining was used as a staining control for tumor cell growth. The p53 antibody (DO-1) was used for staining. Scale bars = 100 µm. Tissue samples were collected on day 120 after animal sacrifice and were fixed in formalin. P53 and Ki-67 staining in cells was quantified from 3 independent biological replicates. G) Immunohistochemistry (IHC) staining result was further validated with quantitative reverse transcription polymerase chain reaction analysis for p53 expression from 3 independent biological replicates for the mutants with the P72 SNP and mutants with the R72 SNP. The statistical analysis was performed using a 2-tailed Student t test. Mean (SD) is plotted.
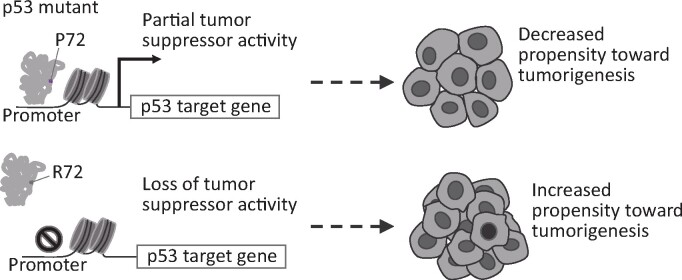
We propose a model to explain the modifying effect of the P72R SNP on mutant p53 behavior where mutants with the P72 SNP maintain partial tumor suppressor activity and its R72 counterpart would lose its transcriptional regulation of canonical p53 targets thus increasing its propensity toward tumor formation (Figure 7).
Figure 7.
Schematic describing the mechanism through which p53 mutants with the P72 SNP regulate canonical target gene expression.
Discussion
Previous studies have described the modifying role of the P72R SNP on mutant p53 behavior with emphasis on oncogenesis and metabolic activity (31,34-39). Using the largest pan-cancer cohort analyzed to date, we demonstrated that missense mutations in TP53 on the R72 allele are preferentially selected for tumor development, and mutations encoded by the P72 allele are negatively selected during tumorigenesis. These results may be in part because of the known gain-of-function effect of the hotspot mutations, such as their ability to interfere with p73 function (31) or to control tumor metabolism by regulating PGC-1α (39), which is permissive on the R72 allele. However, the novel result of our study is that some hotspot mutations on the P72 allele are not permissive because they maintain sufficient tumor suppressor activity. Therefore, the P72 SNP appears to be an intragenic suppressor of p53 gain-of-function effects. We observed this inhibitory effect in short- and long-term growth assays and in gene expression analyses in culture and high-grade serous carcinoma (HGSC) patients. p53 mutants with the P72 SNP bind to chromatin sites and induce a gene expression program consistent with the maintenance of wild-type p53 function. Furthermore, this tumor-suppressive effect of the P72 SNP is observed to a varying extent in xenograft tumors, producing smaller tumors compared with mutants with the R72 SNP.
The inhibitory activity of the P72 SNP has implications for understanding the molecular genetics of cancers with p53 missense mutations. Hotspot p53 mutants with the P72 SNP may be functionally wild type, or they may be functionally mutant because of other (epi)genetic or posttranslational modifications elsewhere on the p53 protein that prevent or negate the inhibitory effect of the P72 SNP that we observed in our experimental systems. Therefore, we analyzed the TCGA RNA-Seq data sets from ovarian cancer patients with TP53 missense mutations occurring together with homozygous P72 or R72 SNP and determined that missense mutants with the R72 SNP display proliferation-related gene expression patterns, consistent with mutant p53 gain-of-function activities. In contrast, missense mutants with the P72 SNP show enrichment of immune-related gene sets and better overall survival compared with the R72 SNP.
The activation of an inflammatory response gene expression program by p53 mutants with the P72 SNP, as suggested by GSEA in cell lines and patients, is potentially clinically significant. Increased tumor mutation burden correlates with tumor-infiltrating lymphocytes because of increased neoantigen formation and often correlates with response to immune checkpoint inhibitors (40,41). However, tumors with low tumor mutation burden can also have clinically significant immune infiltrates. For example, a subset of HGSC has clinically significant tumor-infiltrating lymphocytes, which correlate with the immunoreactive or C2 molecular subtype and improved prognosis (41-43). Therefore, our results showing an inflammatory gene signature in HGSC suggests a possible mechanistic link between p53 hotspot mutants on the P72 SNP, immune infiltrates, prognosis, and response to immune checkpoint inhibitors.
One limitation of our study is that we only tested a limited number of hotspot mutants with established gain-of-function activity. It is possible that the inhibitory effect of the P72 SNP on mutant p53 function is not generalizable to all missense mutants. With that said, we observed a bias against the P72 SNP with missense mutations in TP53 in pan-cancer data, which suggests the inhibitory effect of the P72 SNP may be generalizable to many missense p53 mutant proteins.
Funding
This work was supported by the Department of Defense (W81XWH-10-10386), Kansas Institute for Precision Medicine Centers of Biomedical Research Excellence (COBRE), supported by the National Institute of General Medical Science award (P20 GM130423), the Flow Cytometry Core Laboratory at the University of Kansas Medical Center, supported by the NIH/NIGMS COBRE grant (P30 GM103326) and the NIH/NCI Cancer Center grant (P30 CA168524), UNM Comprehensive Cancer Center Support Grant (P30 CA11810), and the UC Davis Comprehensive Cancer Center Support Grant (P30 CA093373).
Notes
Role of the funder: The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Disclosures: All authors declare no conflicts of interest.
Author contributions: CD, JM, and JC conceptualized and designed the experiments. JC supervised the experiments. CD, ANK, and JC wrote the manuscript. CD and JM performed the experiments. DJM, DK, JC, and DM performed bioinformatic and biostatistical analysis. CD collected and analyzed the data and generated figures. ANK, JM, DK, and JC edited the manuscript. ZZ and WX helped with animal experiments. AGR generated structural predictions. All other authors helped with lab experiments and data collection.
Data Availability
The cell line RNA-Seq analysis underlying this article is available in the Open Science Framework repository at https://osf.io/an4xr/. The ovarian tumor RNA-Seq analysis is available at https://osf.io/5ugv9/. Raw fastq files from RNA-Seq studies can be accessed via Sequence Read Archive (SRA) using the BioProject ID PRJNA689852.
Supplementary Material
References
- 1. Hollstein M, Sidransky D, Vogelstein B, Harris CC.. p53 mutations in human cancers. Science. 1991;253(5015):49–53. [DOI] [PubMed] [Google Scholar]
- 2. Levine AJ, Oren M.. The first 30 years of p53: rowing ever more complex. Nat Rev Cancer. 2009;9(10):749–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bouaoun L, Sonkin D, Ardin M, et al. TP53 variations in human cancers: new lessons from the IARC TP53 database and genomics data. Hum Mutat. 2016;37(9):865–876. [DOI] [PubMed] [Google Scholar]
- 4. Hainaut P, Hollstein M.. p53 and human cancer: the first ten thousand mutations. In: Vande Woude GF, Klein G, eds. Advances in Cancer Research. Vol. 77. London, UK: Academic Press; 2000:81–137. [DOI] [PubMed] [Google Scholar]
- 5. Whibley C, Pharoah PD, Hollstein M.. p53 polymorphisms: cancer implications. Nat Rev Cancer. 2009;9(2):95–107. [DOI] [PubMed] [Google Scholar]
- 6. Stracquadanio G, Wang X, Wallace MD, et al. The importance of p53 pathway genetics in inherited and somatic cancer genomes. Nat Rev Cancer. 2016;16(4):251–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shi H, Tan S-J, Zhong H, et al. Winter temperature and UV are tightly linked to genetic changes in the p53 tumor suppressor pathway in Eastern Asia. Am J Hum Genet. 2009;84(4):534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beckman G, Birgander R, Själander A, et al. Is p53 polymorphism maintained by natural selection? Hum Hered. 1994;44(5):266–270. [DOI] [PubMed] [Google Scholar]
- 9. Dumont P, Leu JI, Della Pietra AC 3rd, George DL, Murphy M.. The codon 72 polymorphic variants of p53 have markedly different apoptotic potential. Nat Genet. 2003;33(3):357–365. [DOI] [PubMed] [Google Scholar]
- 10. Azzam G, Wang X, Bell D, Murphy ME.. CSF1 is a novel p53 target gene whose protein product functions in a feed-forward manner to suppress apoptosis and enhance p53-mediated growth arrest. PLoS One. 2013;8(9):e74297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bergamaschi D, Samuels Y, Sullivan A, et al. iASPP preferentially binds p53 proline-rich region and modulates apoptotic function of codon 72-polymorphic p53. Nat Genet. 2006;38(10):1133–1141. [DOI] [PubMed] [Google Scholar]
- 12. Wegman P, Stal O, Askmalm MS, et al. p53 polymorphic variants at codon 72 and the outcome of therapy in randomized breast cancer patients. Pharmacogenet Genomics. 2006;16(5):347–351. [DOI] [PubMed] [Google Scholar]
- 13. Pim D, Banks L.. p53 polymorphic variants at codon 72 exert different effects on cell cycle progression. Int J Cancer. 2004;108(2):196–199. [DOI] [PubMed] [Google Scholar]
- 14. Thomas M, Kalita A, Labrecque S, et al. Two polymorphic variants of wild-type p53 differ biochemically and biologically. Mol Cell Biol. 1999;19(2):1092–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang K-L, Mashl RJ, Wu Y, et al. Pathogenic germline variants in 10,389 adult cancers. Cell. 2018;173(2):355–370.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Colaprico A, Silva TC, Olsen C, et al. TCGAbiolinks: an R/Bioconductor package for integrative analysis of TCGA data. Nucleic Acids Res. 2016;44(8):e71–e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Silva TC, Colaprico A, Olsen C, et al. TCGA workflow: analyze cancer genomics and epigenomics data using Bioconductor packages. F1000Res. 2016;5:1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mounir M, Lucchetta M, Silva TC, et al. New functionalities in the TCGAbiolinks package for the study and integration of cancer data from GDC and GTEx. PLoS Comput Biol. 2019;15(3):e1006701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Love MI, Huber W, Anders S.. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aran D, Sirota M, Butte AJ.. Systematic pan-cancer analysis of tumour purity. Nat Commun. 2015;6(1):8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102(43):15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reich M, Liefeld T, Gould J, et al. GenePattern 2.0. Nat Genet. 2006;38(5):500–501. [DOI] [PubMed] [Google Scholar]
- 23. Zhang X, Cheng L, Minn K, et al. Targeting of mutant p53-induced FoxM1 with thiostrepton induces cytotoxicity and enhances carboplatin sensitivity in cancer cells. Oncotarget. 2014;5(22):11365–11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen H, Gotimer K, De Souza C, et al. Short-term organoid culture for drug sensitivity testing of high-grade serous carcinoma. Gynecol Oncol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fang P, Souza CD, Minn K, Chien J.. Genome-scale CRISPR knockout screen identifies TIGAR as a modifier of PARP inhibitor sensitivity. Commun Biol. 2019;2(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vichai V, Kirtikara K.. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1(3):1112–1116. [DOI] [PubMed] [Google Scholar]
- 27. Bastola P, Neums L, Schoenen FJ, Chien J.. VCP inhibitors induce endoplasmic reticulum stress, cause cell cycle arrest, trigger caspase-mediated cell death and synergistically kill ovarian cancer cells in combination with Salubrinal. Mol Oncol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Johnson DS, Mortazavi A, Myers RM, Wold B.. Genome-wide mapping of in vivo protein-DNA interactions. Science. 2007;316(5830):1497–1502. [DOI] [PubMed] [Google Scholar]
- 29. Blecher-Gonen R, Barnett-Itzhaki Z, Jaitin D, et al. High-throughput chromatin immunoprecipitation for genome-wide mapping of in vivo protein-DNA interactions and epigenomic states. Nat Protoc. 2013;8(3):539–554. [DOI] [PubMed] [Google Scholar]
- 30. Fang P, Madden JA, Neums L, et al. Olaparib-induced adaptive response is disrupted by FOXM1 targeting that enhances sensitivity to PARP inhibition. Mol Cancer Res. 2018;16(6):961–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marin MC, Jost CA, Brooks LA, et al. A common polymorphism acts as an intragenic modifier of mutant p53 behaviour. Nat Genet. 2000;25(1):47–54. [DOI] [PubMed] [Google Scholar]
- 32. Xu D, Zhang Y.. Ab initio protein structure assembly using continuous structure fragments and optimized knowledge-based force field. Proteins. 2012;80(7):1715–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang J, Yan R, Roy A, et al. The I-TASSER Suite: protein structure and function prediction. Nat Methods. 2015;12(1):7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barnoud T, Parris JLD, Murphy ME.. Common genetic variants in the TP53 pathway and their impact on cancer. J Mol Cell Biol. 2019;11(7):578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cho J-H, Patel B, Bonala S, et al. The Codon 72 TP53 polymorphism contributes to TSC tumorigenesis through the Notch-Nodal axis. Mol Cancer Res. 2019;17(8):1639–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Singh KS, Leu JI-J, Barnoud T, et al. African-centric TP53 variant increases iron accumulation and bacterial pathogenesis but improves response to malaria toxin. Nat Commun. 2020;11(1):473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kung CP, Basu S, Murphy ME.. A link between TP53 polymorphisms and metabolism. Mol Cell Oncol. 2016;3(4):e1173769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kung CP, Liu Q, Murphy ME.. The codon 72 polymorphism of p53 influences cell fate following nutrient deprivation. Cancer Biol Ther. 2017;18(7):484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Basu S, Gnanapradeepan K, Barnoud T, et al. Mutant p53 controls tumor metabolism and metastasis by regulating PGC-1alpha. Genes Dev. 2018;32(3-4):230–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee S, Margolin K.. Tumor-infiltrating lymphocytes in melanoma. Curr Oncol Rep. 2012;14(5):468–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Garber K. Pursuit of tumor-infiltrating lymphocyte immunotherapy speeds up. Nat Biotechnol. 2019;37(9):969–971. [DOI] [PubMed] [Google Scholar]
- 42. Sinn M, Sinn BV, Treue D, et al. TP53 mutations predict sensitivity to adjuvant gemcitabine in patients with pancreatic ductal adenocarcinoma: next-generation sequencing results from the CONKO-001 Trial. Clin Cancer Res. 2020;26:3732–3739. [DOI] [PubMed] [Google Scholar]
- 43. Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The cell line RNA-Seq analysis underlying this article is available in the Open Science Framework repository at https://osf.io/an4xr/. The ovarian tumor RNA-Seq analysis is available at https://osf.io/5ugv9/. Raw fastq files from RNA-Seq studies can be accessed via Sequence Read Archive (SRA) using the BioProject ID PRJNA689852.