Abstract
Objective
Etiological classification of infantile spasms syndrome (ISS) is important, considering the influence on prognosis based on the presence or absence of a known etiology. This study was performed to describe the limitations and difficulties experienced within the South Asian region when classifying the etiology of ISS according to the current recommendation.
Method
Data on healthcare indices and facilities related to management of ISS for the nine countries in the South Asian region were gathered by the South Asian West Syndrome Research Group. A Google survey was performed among three hundred and thirty pediatric neurologists in the region. The capacity within each country for investigating etiology of ISS according to current described benchmarks was evaluated. The difficulties experienced in this regard and the potential solutions were investigated.
Results
One hundred and sixty pediatric neurologists (response rate 48%) from Bangladesh (19/25), India (94/255), Myanmar (11/11), Nepal (6/8), Pakistan (19/25), and from Sri Lanka (7/8) responded. Three countries had no pediatric neurology services. Fifty‐six percent attempted to classify ISS etiology according to classification outlined by International League Against Epilepsy in 2017. The facilities to perform metabolic, genetic, and immunological investigations were very limited. Lack of funding for investigations and poor laboratory support were the two most frequent barriers encountered. Sixty percent indicated that a separate classification is suitable for low‐income setting; 78% suggested inclusion of separate category as “incompletely investigated” as an alternative solution to mitigate the barrier of achieving a better understanding of the etiological subtypes seen more frequently in this region.
Significance
The resources in South Asian region are limited to meet the recommendations for investigating etiology of ISS. Including the etiological subcategory “incompletely investigated” is proposed as an alternative to understand the true proportions of children in this region, with a definite known etiology and those with an unknown etiology.
Keywords: etiological classification, infantile spasms syndrome, South Asian region
Key points.
The resources in the South Asian region are limited to meet the current recommendations for investigating etiology of ISS.
Lack of funding for investigations and poor laboratory support are the two most frequent barriers impeding a complete etiological evaluation.
Only about 50% of pediatric neurologists can attempt to classify etiology according to etiological subcategories in ILAE 2017 classification.
Including the etiological subcategory incompletely investigated was considered an option to reduce the limitation posed by “Investigation Gap” when investigating ISS in the region.
1. INTRODUCTION
Infantile spasms syndrome (ISS) is a developmental and epileptic encephalopathy (DEE) with a striking association with an underlying etiology in the majority. It may be related to abnormal brain structure and or abnormal function. The proportion with identifiable etiology, also referred to as “symptomatic,” ranges from 60% to 80% depending on the country of origin of study and year of publication. A large‐scale study from the National Consortium in Infantile Spasms in the United States reported that an underlying etiology is identifiable only in 64.4% of children even with detailed evaluation. 1 Those without a known cause are termed as unknown etiology or cryptogenic as in most previous publications. The benchmarks set in this study for the minimum investigations for each etiological category, suggested with reasonable precision, a two: one proportion between those with an identifiable etiology and those without. We will continue to refer to etiology known and etiology unknown to describe the categories symptomatic and cryptogenic etiologies, respectively.
Etiological classification of ISS is important, considering the prognosis variable on the presence or absence of a known etiology. 2 , 3 With advancement of technology, the understanding on etiologies underpinned with ISS has expanded. Thus, the etiological classification has grown from the basic symptomatic and cryptogenic types mentioned above to a much broader classification. The classification from the National Consortium in the United States had eight etiological subcategories. 1 Paciorkowski et al proposed a classification heavily weighted on genetic and biological subtypes. 4 These classifications expand our understanding of the different subtypes; however, the capacity for such detailed evaluation is limited to resourceful centers in few countries. Exhaustive classification does not influence the choice of initial treatment of ISS, except in those already known to have an etiology‐specific ISS such as pyridoxine‐dependent (ALDH7A1)‐DEE, pyridox(am)ine 5′‐phosphate deficiency (PNPO)‐DEE, and glucose transporter 1 deficiency syndrome (Glut1DS), treated with pyridoxine, pyridoxal phosphate, and ketogenic diet. In all other etiologies, the recommendation is for the use of steroids except for tuberous sclerosis (TSC) which is treated with vigabatrin. 5 TSC is essentially a diagnosis based on clinical and radiological features.
The World Bank ranks countries based on the Gross National Income per capita. Presently, there are seventy‐nine countries belonging to the lower two categories of low‐income and low‐middle income with more than 3.5 billion population. 6 These countries have severe limitations on government expenditure on healthcare services, many without free health care to all. Therefore, despite the relevance, applying these classifications to every child diagnosed with ISS is difficult in every setting. South Asia consists of nine countries, geographically located from the main continent to the Indian Ocean. It harbors 2 billion people, approximately 25% of the world's population. Except for Maldives (upper‐middle income), all other countries fall to or below lower middle income countries. 7 Apart from the economy, social and cultural background of this region also contributes toward several neurological disorders seen in children. High rates of consanguinity, perinatal complications, and congenital and acquired central nervous system (CNS) infections are some of these factors, closely related to the etiopathogenesis of ISS. Although a higher incidence of ISS across northern latitudes was proposed in 2018, 8 the lack of research on epidemiology of ISS from South Asia in this meta‐analysis was glaring. The higher incidence reported in the Scandinavian countries could be an expression of research capacity and comprehensive record‐keeping rather than a true increase of incidence in these countries. This cross‐sectional survey was undertaken to describe factors that influence investigating etiology of ISS in the South Asian region and to describe what the pediatric neurologists in this region experience when establishing etiology.
2. METHOD
This cross‐sectional survey was conducted by the South Asian West Syndrome Research Group (SAWSRG), a collaborative group of pediatric neurologists (Bangladesh, India, Myanmar, Nepal, Pakistan, Sri Lanka) and pediatricians with special interest in neurology (Bhutan and Maldives) and an adult neurologist with interest in pediatric epilepsies (Afghanistan) from the South Asian region. SAWSRG was formed in early 2019 and subsequently expanded to include the nine listed countries. The sole purpose of the group is to improve the care of children with ISS through collaborative research. 9 , 10 , 11
It was conducted from January to May 2021 and was granted ethical approval from the Ethics Review Committee of the Faculty of Medicine, University of Colombo (EC/21/45). Regional demographics and healthcare indexes related to childcare (a), availability of pediatric neurology services and facilities to classify etiology of ISS (b) were tabulated for each country by the study group members. The minimum requirements for classifying etiology were based on standards outlined for each etiological category by the National Consortium for Infantile spasms. 1 A literature survey was carried out to identify the etiologies and subtypes described within the South Asian region as of December 2020, which was resurveyed for new publications in June 2021 (c). Availability of consensus on management of ISS, government funding for investigating etiology, and public responsiveness toward investigations (d) were tabulated for each country by the study group members.
A Google survey was conducted among the pediatric neurologists, from the six countries in the region with established pediatric neurology services. The objective was to assess their practice regarding classifying etiology, the difficulties experienced during this process, and seek opinion regarding an achievable classification. A Delphi process, which elicited consensus among experts from different countries, was used to develop the questionnaire. The opinion of the experts which included judgment and agreement was obtained individually. JW acted as the moderator to accumulate these opinions. From an initial list of 24 questions, a final of 12 questions were selected based on consensus agreement by all 10 investigators. These questions had either one or multiple correct responses.
The pediatric neurologists were approached via email through professional groups by the respective members of the study group. Only those registered in respective professional bodies as pediatric neurologist or pediatrician with special training in neurology were contacted.
3. RESULTS
The socioeconomical demographics and health indices pertaining to childcare in the region are shown in Table 1. These indices varied across the region, that is, attendance of all births by skilled healthcare workers seen in Maldives and Sri Lanka to highest neonatal and infantile mortality rates reported from Afghanistan and Pakistan. The government expenditure on healthcare ranged from 1.8% to 9.4% of the individual Gross Domestic Product.
TABLE 1.
Summary of the demographic features and healthcare indices of the countries in the South Asian region
| Demographical data | Afghanistan | Bangladesh | Bhutan | India | Maldives | Myanmar | Nepal | Pakistan | Sri Lanka |
|---|---|---|---|---|---|---|---|---|---|
| Birth rate Per 1000 31 | 32 | 18 | 17 | 18 | 18 | 18 | 20 | 28 | 16 |
| Size of birth cohort (million) 32 | 1.08 | 3.04 | 0.01 | 25.6 | 0.06 | 0.9 | 0.6 | 5.4 | 0.3 |
| GDP (Billion USD) 33 | 19.29 | 30.25 | 2.53 | 2869 | 5.64 | 76 | 30.64 | 270 | 84 |
| *Per capita Income (USD) 34 | 507.1 | 1855.7 | 3316.2 | 2099.6 | 10 626.5 | 1407.8 | 1071.1 | 1284.7 | 3853.1 |
| Government expenditure on health (% of government expenditure) 35 | 1.8 | 2.98 | 7.61 | 3.39 | 9.41 b | 3.79 | 4.58 | 5.26 | 8.29 |
| Availability of free health care | Yes | Yes | Yes | Yes a | Yes | Yes | Partial | Yes | Yes |
| Healthcare indices | |||||||||
| Neonatal mortality (per 1000 live births) 36 | 35.9 | 19.1 | 16.6 | 21.7 | 4.9 | 22.4 | 19.8 | 41.2 | 4.3 |
| Infant mortality (per 1000 live births) 37 | 46.5 | 25.6 | 23.8 | 28.3 | 7.1 b | 35.76 | 25.8 | 55.7 | 6.1 |
| Exclusive breastfeeding rates for 6 months (%) 38 | 58 | 55 | 51.3 | 54.9 | 64 | 51.2 | 65 | 48 | 82 |
| DTP3 immunization coverage (%) 32 | 87 | 90 | 97 | 85 | 85 b | 91 | 91 | 72 | 99 |
| Unattended home delivery percentage (%) 39 | 49 | 47 | 4 | 19 | 0 | 40 | 42 | 31 | 0 |
| Literacy rate (adult) (2016‐18) 40 | 43 | 73.9 | 66.6 | 74.4 | 97.7 | 75.6 | 67.9 | 59.1 | 91.7 |
Limited to those below poverty line only.
Ministry of Health (MOH) [Maldives] and ICF. 2018. Maldives Demographic and Health Survey 2016‐17. Malé, Maldives, and Rockville, Maryland, USA: MOH and ICF.
Pediatric neurology services were available only in six countries. In Afghanistan and Bhutan, the services are provided by pediatricians or adult neurologists. Establishment of child neurology services in Maldives is still in its infancy. Availability of resources for performing electroencephalography (EEG) in children, neuroimaging, reference metabolic, and genetic laboratories in each country is listed in Table 2. Table 3 lists available literature in the region on ISS including described etiologies and Table S1 describes factors/measures that influence the evaluation of etiology.
TABLE 2.
Estimates of human resources and infrastructure for care of children with neurological diseases including infantile spasms syndrome
| Human resources and infrastructure | Afghanistan | Bangladesh | Bhutan | India | Maldives | Myanmar | Nepal | Pakistan | Sri Lanka |
|---|---|---|---|---|---|---|---|---|---|
| No of pediatricians | Unknown | 1300 | 16 | ~35 000 | 26+65 a | 1000 | 509 | 4000 | 520 |
| No of child neurologists | 0 | 25 | 0 | >250 a | 0 | 11 | 6 | 25 | 8 |
| Availability of EEG in children's hospital (available number) | None | Yes (16) | Yes (1) | Yes (many) | Yes (2) | Yes (1) | Yes (5) | Yes (many) | Yes (7) |
| Radiological services | |||||||||
| No of CT machines | 3 | 25 | 3 | >1000 | 6 | 100 | 60‐70 | 670 | 60 |
| No of MRI machines | 1 | 10 | 1 | >300 | 4 | 25 | 15 | 25 | 20 |
| No of Pediatric radiologists | 0 | Nil | 0 | ~400 a | 0 | 3 | Nil | 5 | 1 |
| Genetic services | |||||||||
| No of genetic laboratories | 0 | 2 | 0 | 10‐20 | 0 | 0 | 1 | 3 | 2 |
| No of clinical geneticists | 0 | 4 | 0 | ~100 a | 0 | 0 | 3 | 3 | 2 |
| Metabolic services | |||||||||
| No of reputed metabolic laboratories | 0 | 4 | 0 | ~10 | 0 | 0 | 5 | 2 | Nil |
| Metabolic specialists | 0 | 1 | 0 | >70 a | 0 | 0 | Nil | 2 | Nil |
| Average cost for single patient review, EEG and MRI brain (USD) | 175 | 100 | NA | 40‐50 b | 180 | 50 | 150 | 175‐200 | 150 |
NA—Not applicable as all services are free.
Based on number of members in these respective Indian associations/societies (Indian Academy of Pediatrics, Association of Child Neurology, Indian Society of Pediatric Radiology, Indian Academy of Medical Genetics, and Indian Society of inborn errors of metabolism).
Rough estimate.
TABLE 3.
Literature on Infantile spasms syndrome and its etiology from the countries in the South Asian region
| Afghanistan | Bangladesh | Bhutan | India | Maldives | Myanmar | Nepal | Pakistan | Sri Lanka | |
|---|---|---|---|---|---|---|---|---|---|
| No of articles on ISS indexed in PubMed or Scopus | None | 5 | 0 | >100 | None | 0 | 3 | 10 | 8 |
| Abstracts if no full texts | None | NA | None | NA | None | 2 | NA | NA | NA |
| Reported incidence/prevalence of WS per 1000 | None | None | None | Age specific prevalence: 0.0628/1000 | None | None | None | None | |
| Proportion with** | |||||||||
| Structural etiology | None | None | None | 82% (CI: 75%‐89%) | None | None | None | 75% (CI: 57%‐89%) | 68% (CI: 57%‐78%) |
| Acquired structural insult | None | None | None | 71% (CI: 61%‐80%) | None | None | None | 64% (CI: 55%‐73%) | 62% (51%‐73%) |
| Described etiologies of WS | None |
HIE/Perinatal asphyxia: 58% TORCH: 3.22% Brain malformation: 3.22%, Neonatal Hyperbilirubinemia: 3.22%, Neonatal sepsis: 3.2% Unknown: 6.5%* |
None |
Perinatal asphyxia/HIE (34.6%) Hypoglycemic brain injury (HBI) 16.7% Combined HIE and HBI (9%) Congenital brain malformations (3%) Other structural (10.7%) Infections (0.7%) Metabolic (0.7%) Proven genetic (7.2%) Not known/incompletely investigated (17.1%)^ |
None |
|
|
|
Known‐ 71% Unknown −18% Incompletely investigated −11% |
| Average age of onset (months) | NR | 5** | NR | NR | NR | 5.5# | 6### | 4‐6# | 5** |
| Average lead time to diagnosis/treatment (months) | NR | 7.5** | NR | 4.4** | NR | NR | NR | 6 | 1.4** |
| Ratio of male in comparison to female | NR | 2.1** | NR | 2.6** | NR | 1.44# | NR | NR | 1.36** |
Three hundred and thirty pediatric neurologists/pediatricians with special training in neurology were invited to participate. One hundred and sixty responded (response rate 48%). This included 19/25 (76%) from Bangladesh, 94/255 (36%) from India, 11/11 (100%) from Myanmar, 6/8 (75%) from Nepal, 19/25 (76%) from Pakistan, and 7/8 (87.5%) from Sri Lanka. The largest proportion (47%) were pediatricians who had completed a fellowship in pediatric neurology. There were 54 (34%) board‐certified pediatric neurologists and the others were pediatricians with a special training or interest in child neurology (17.7%). A majority (36.3%) had experience of over 10 years in pediatric neurology. The others had service of 5‐10 years (31%) and <5 years (32%). The average number of children newly diagnosed as ISS by each person for a year ranged from <10 in 16%, 10‐20 in 29%, 20‐50 in 32%, and >50 in 23%. A majority (47%) worked both in the government sector and in private healthcare facilities.
Almost all participants agreed on the importance of classifying the etiology of ISS; 71% indicated it as very important while 27% felt it was important. Fifty‐six percent indicated that they investigate to achieve the etiological subcategorization outlined in the International League Against Epilepsy (ILAE) consensus report of 2017 at present. Twenty‐five percent indicated that they could achieve only a simple classification as per the ILAE paper in 2010. 12 Only 18% indicated attempts to fulfill a more stringent etiological classification as proposed by Wirrell et al in 2015. 1 The availability of MRI imaging, metabolic, genetic, and immunological testing to them in all six countries is shown in Figure 1A; a comparison between India (more technically advanced) versus the other five countries is shown in 1B. The main barriers that prevent the neurologists from achieving a detailed etiological classification are shown in Figure 2. Lack of funding for investigations was the single most frequent obstacle. Projecting on how the investigation capacities would change over the next 5 years, a slightly higher percentage of 61% indicated confidence in reaching the ILAE etiological classification of 2017. Sixty percent of participants indicated that a separate classification is preferred for resource‐limited settings. As a compromise to overcome this difficult situation, 78% of participants proposed the inclusion of a separate subcategory as incompletely investigated to the current classification.
FIGURE 1.
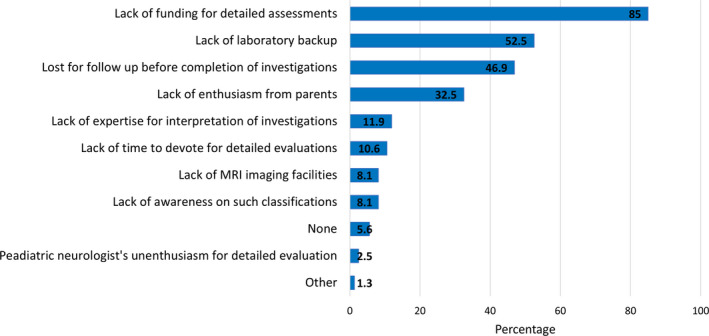
The availability of recommended investigations for establishing etiology according to ILAE etiological classification within the public and or private sector: A. in the hospitals of all participating pediatric neurologists and B. Distribution of these facilities across India and all other countries (n = 160)
FIGURE 2.
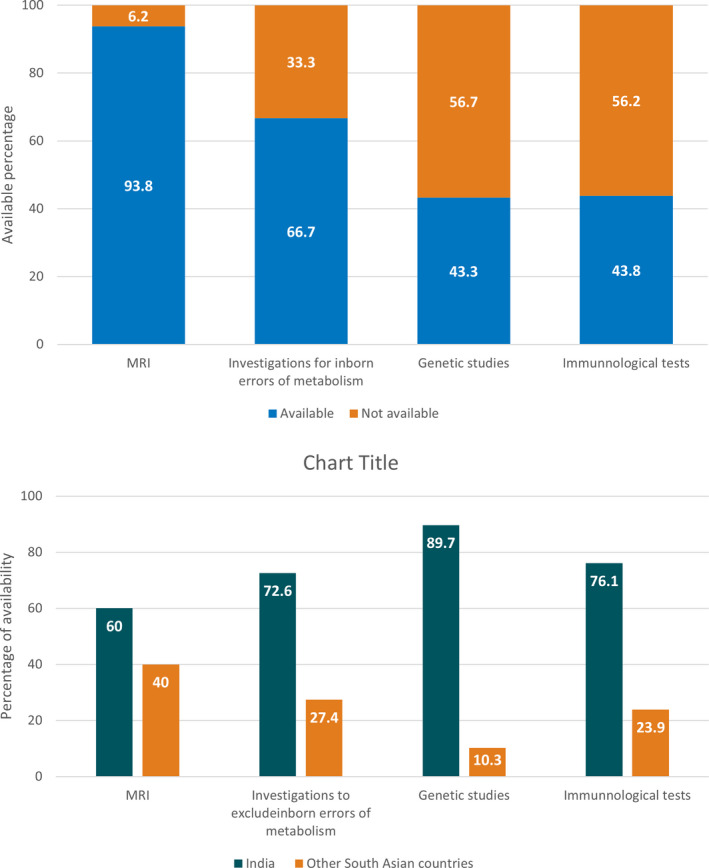
Difficulties and obstacles when investigating etiology of ISS by pediatric neurologists in South Asian countries (N = 160)
4. DISCUSSION
It is well established that etiology unknown or cryptogenic West syndrome is associated with better response to therapy, lesser number of relapses, better developmental outcome, and a possible mortality benefit. 13 , 14 Therefore, limitations for detailed investigation are an impediment to a clear understanding of the patient's prognosis. In fairness to the huge discrepancy of resources across the globe, it seems appropriate that a dialogue is generated on how etiology can be classified “adequately” by a pediatric neurologist anywhere in the world. In this study, we tried to illustrate the capacity of respective healthcare systems within South Asia and facilities available in each country to investigate etiology of ISS in par with the current recommendation. We found that the capacities were mostly below the requirement in general. Extreme variations were noted within the region. The study also reveals the difficulties experienced by child neurologists in the region in establishing etiology of ISS.
Infantile spasms syndrome literature predominates from the West, particularly North America and Europe. Data on incidence or prevalence of ISS from the East are limited. There is only a single study from India 15 to describe the prevalence of ISS from the South Asian region, despite one quarter of the world's population living within this geography. This results in a polarized understanding of etiologies particularly the proportions of etiology known and etiology unknown groups of ISS. The risks of pre‐, peri‐, and postnatal injury and infections being high due to greater consanguineous marriages, poor antenatal and natal care, and high rates of infections; the known etiologies could be expected to be higher in this region. The limited literature on ISS from this geographical setting is shown in Table 3, confirming this hypothesis. The average number of children with ISS newly diagnosed revealed in the survey also speaks of the disease burden in the region. The importance of understanding the etiologies more common to this region is to drive attention toward prevention of these risk factors. Strategies such as prepregnancy immunization for Rubella improved maternal nutrition and obstetric care, antenatal folic acid supplementation, vitamin K administration at birth, and improved vaccination coverage (BCG, HiB, and pneumococcal) for prevention of CNS infections are achievable objectives that would directly help to reduce the incidence of ISS. The higher proportion of etiology known group may also contribute to the lower response rates to therapy seen in this region, 16 requiring a greater vigil in treatment advocation. A similar situation of paucity of information on etiology due to investigative constraints as well as poor record‐keeping is likely in the South American 17 and African continents. 18 ILAE has recognized these difficulties in the upcoming proposed classification and definition of epilepsy syndromes in the neonate and infant. 19
The descriptions in yesteryears classified ISS etiology to three groups: symptomatic, cryptogenic, and idiopathic. Following the proposals from the ILAE in 2010, the classification was expanded to structural, genetic or metabolic, and unknown. 12 Though known etiology accounted for nearly 80% in earlier documentations, detailed evaluation by the National Infantile Spasms consortium, established that the proportion of children with symptomatic etiology was limited to 64.4%. 1 Interestingly, 85% of this group with known etiology had their etiology diagnosed with only a detailed clinical evaluation and neuroimaging. It is noticed that the 61% with identifiable etiology described in the UKISS trial 20 is rather close to the 55% reported by the US consortium. Unlike the US study, UKISS did not have an exhaustive strategy for investigation. The proximity of the percentages suggests that yield from extensive investigations will improve the proportion only by little. Since MRI was available in most settings evaluated in the current study, one could estimate that accurate diagnosis of etiology is achievable in 80%‐85% of ISS in this region using clinical assessments and imaging.
Recent literature highlights the increasing numbers of genetic variations, mostly de novo, being associated with ISS. Extensive classification scheme based on the type of variation has also been proposed. 4 This genetic subgroup is very unlikely to be diagnosed much in the geography of the current paper, as well as many other low‐resource settings. Epidemiologically, genetic and particularly metabolic 21 subcategories contribute to a small proportion of symptomatic ISS. In a recent study from India, genetic etiology was identified in 10% of children with ISS (39% of children with ISS and normal neuroimaging) even after detailed investigations, 22 while none had an identified metabolic etiology. Similarly, the proportions of ISS with immunogenic etiology is likely to be miniscule; there were none identified in the National Infantile Spasm Consortium. 1 On the other hand, even within the most exhaustive classifications, there are other etiologies of ISS such vitamin B12 deficiency, described within the region, 23 being unrecognized. There may be other ambiguous situations such as separating infective etiology over structural cause in babies who develop ISS due to intrauterine Zika and cytomegalovirus infections. 24
Contrary to figures from studies in the West, larger proportions with known etiology such as hypoxic ischemic encephalopathy (27%‐58%) 9 , 25 , 26 , 27 , and CNS infections (8%‐22%) 28 , 29 are reported in this geographical region (Table 3). Neonatal hypoglycemia is an important etiological factor in India. 22 , 30 Therefore, using detailed history and MRI imaging, a significant proportion of children in our setting can be identified to have an etiology. The proportion due to genetic and metabolic subgroups which are difficult to diagnose may be small. Introduction of an etiological subcategory as incompletely investigated, as done in the UKISS etiological subclassification, where these rare categories can be included will help the clinicians to accomplish a reasonable understanding of the prevailing etiologies in resource‐limited settings. This subdivision of the incompletely investigated category in the UKISS study accounted for only 6%. However, it facilitated a satisfactory understanding of the etiology in balance 94%. Majority of pediatric neurologists who participated in our study clearly favored inclusion of incompletely investigated as a separate subcategory in classifying etiology of ISS. It will improve the distinction of those definitely without a known etiology from those with no etiology due to incomplete evaluation. However, the incompletely investigated category will carry no weight for prognostication. We have proposed an algorithm that can be followed to achieve this subcategorization (Figure 3). This will facilitate distinction of the group with definite known etiology from the definite unknown group. This will enable a reliable comparison of etiologies across geographies.
FIGURE 3.
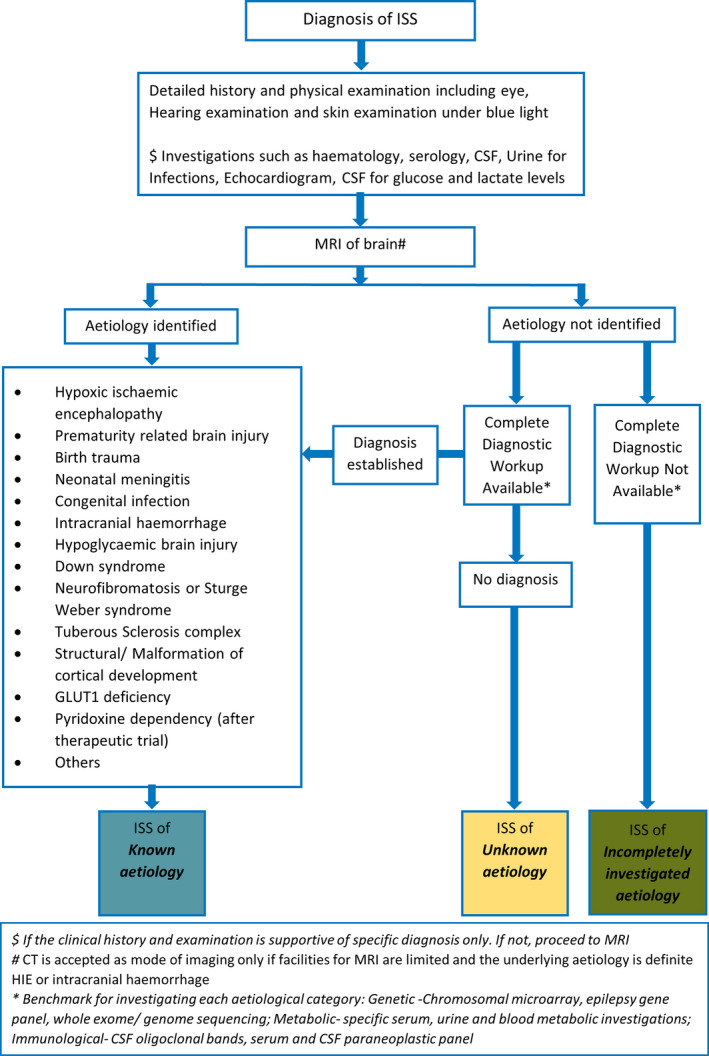
Algorithm for classifying etiology of infantile spams syndrome (ISS) in resource‐limited settings
One of the drawbacks in this study is that there is heterogeneity in the availability of services and expertise across countries investigated. This is directly influential in the availability and performance of investigative workup and the response to the survey. In this region, apart from resource limitations, other constraints contribute toward achieving a clear etiological classification. These include negative public attitude toward genetic etiologies, being lost for follow up and absence of systematic documentation of investigative findings. The survey did not explore different ways to mitigate the difficulty of investigation in the region, but only sorted opinion on usefulness of inclusion of subcategory as “incompletely investigated.”
5. CONCLUSION
This study describes the health‐related demographics and resources available within the nine South Asian countries linked to the management of ISS. It also investigated the practical difficulties faced by the child neurologists in this setting for investigating children with ISS. The majority of study participants proposed the inclusion of the subcategory “incompletely investigated” into the etiological classification of ISS. In the discussion, it focused on the impact of resource limitation on achieving a satisfactory classification of etiology of ISS and postulated for others in similar resource‐stricken countries. We envisage a dialogue on these observations of “investigation gap” in some parts of the world, so that a better understanding of the true proportions of etiologies of ISS across the globe will be facilitated.
CONFLICT OF INTEREST
Author JW receives support as principal investigator from NIH grant 1R21HD093563‐01. This grant is not related to infantile spasms syndrome. She has received speaker honoraria from Eisai. Authors PP, MM, SH, EH, PC, KL, KF, PM, and JS have no disclosures. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Table S1
App S1
ACKNOWLEDGMENTS
We wish to thank all pediatric neurologists who participated in this Google survey.
Wanigasinghe J, Sahu JK, Madaan P, et al. Classifying etiology of infantile spasms syndrome in resource‐limited settings: A study from the South Asian region. Epilepsia Open. 2021;6:e12548. 10.1002/epi4.12548
REFERENCES
- 1. Wirrell EC, Shellhaas RA, Joshi C, Keator C, Kumar S, Mitchell WG, et al. How should children with West syndrome be efficiently and accurately investigated? Results from the National Infantile Spasms Consortium. Epilepsia. 2015;56(4):617–25. [DOI] [PubMed] [Google Scholar]
- 2. Riikonen R. Long‐term outcome of patients with West syndrome. Brain Dev. 2001;23(7):683–7. [DOI] [PubMed] [Google Scholar]
- 3. Lagae L, Verhelst H, Ceulemans B, De Meirleir L, Nassogne MC, De Borchgrave V, et al. Treatment and long term outcome in West syndrome: the clinical reality. A multicentre follow up study. Seizure. 2010;19(3):159–64. [DOI] [PubMed] [Google Scholar]
- 4. Paciorkowski AR, Thio LL, Dobyns WB. Genetic and biologic classification of infantile spasms. Pediatr Neurol. 2011;45(6):355–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hancock EC, Osborne JP, Edwards SW. Treatment of infantile spasms. Cochrane Database Syst Rev. 2013;6:Cd001770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Population total in low and low‐Middle income settings [Internet]. [cited 2021 May 25]. Available from https://data.worldbank.org/country/XM
- 7. World Bank country and lending groups [Internet] . [cited 2021 May 25]. Available from https://datahelpdesk.worldbank.org/knowledgebase/articles/906519‐world‐bank‐country‐and‐lending‐groups
- 8. Jia JL, Chen S, Sivarajah V, Stephens D, Cortez MA. Latitudinal differences on the global epidemiology of infantile spasms: systematic review and meta‐analysis. Orphanet J Rare Dis. 2018;13(1):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Madaan P, Chand P, Linn K, Wanigasinghe J, Lhamu Mynak M, Poudel P, et al. Management practices for West syndrome in South Asia: a survey study and meta‐analysis. Epilepsia Open. 2020;5(3):461–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sahu JK, Madaan P, Chand P, Kumar A, Linn K, Mynak ML, et al. Management of West syndrome during COVID‐19 pandemic: a viewpoint from South Asian West Syndrome Research Group. Epilepsy Res. 2020;167:106453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Madaan P, Sahu JK, Wanigasinghe J, Fatema K, Linn K, Lhamu Mynak M, et al. Teleneurology based management of infantile spasms during COVID‐19 pandemic: a consensus report by the South Asia Allied West syndrome research group. Epilepsy Behav Rep. 2021;15:100423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde BW, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51(4):676–85. [DOI] [PubMed] [Google Scholar]
- 13. Harini C, Nagarajan E, Bergin AM, Pearl P, Loddenkemper T, Takeoka M, et al. Mortality in infantile spasms: a hospital‐based study. Epilepsia. 2020;61(4):702–13. [DOI] [PubMed] [Google Scholar]
- 14. Riikonen R. Infantile spasms: outcome in clinical studies. Pediatr Neurol. 2020;108:54–64. [DOI] [PubMed] [Google Scholar]
- 15. Koul R, Razdan S, Motta A. Prevalence and pattern of epilepsy (Lath/Mirgi/Laran) in rural Kashmir, India. Epilepsia. 1988;29(2):116–22. [DOI] [PubMed] [Google Scholar]
- 16. Wanigasinghe J, Arambepola C, Sri Ranganathan S, Sumanasena S, Attanapola G. Randomized, single‐blind, parallel clinical trial on efficacy of oral prednisolone versus intramuscular corticotropin on immediate and continued spasm control in west syndrome. Pediatr Neurol. 2015;53(3):193–9. [DOI] [PubMed] [Google Scholar]
- 17. Barbarrosa EP, de la Caridad Pérez Ferrer I, Tovani‐Palone MR. West syndrome: clinical characteristics, therapeutics, outcomes and prognosis. Electron J Gen Med. 2020;17(2):em190. [Google Scholar]
- 18. Keshave A, Yende‐Zuma N, Mubaiwa L, Adhikari M. The clinical profile and outcome of children with West syndrome in KwaZulu‐Natal Province, South Africa: a 10‐year retrospective review. S Afr J Child Health. 2017;11(3):135–40. [Google Scholar]
- 19. ILAE Classification & Definition of Epilepsy Syndromes in the Neonate and Infant. [cited 2021 May 25]. Available from https://www.ilae.org/guidelines/definition‐and‐classification/proposed‐classification‐and‐definition‐of‐epilepsy‐syndromes/proposed‐classification‐syndromes‐in‐neonates‐and‐infants
- 20. Osborne JP, Lux AL, Edwards SW, Hancock E, Johnson AL, Kennedy CR, et al. The underlying etiology of infantile spasms (West syndrome): information from the United Kingdom Infantile Spasms Study (UKISS) on contemporary causes and their classification. Epilepsia. 2010;51(10):2168–74. [DOI] [PubMed] [Google Scholar]
- 21. Salar S, Moshé SL, Galanopoulou AS. Metabolic etiologies in West syndrome. Epilepsia Open. 2018;3(2):134–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Surana P, Symonds JD, Srivastava P, Geetha TS, Jain R, Vedant R, et al. Infantile spasms: etiology, lead time and treatment response in a resource limited setting. Epilepsy Behav Rep. 2020;14:100397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meena MK, Sharma S, Bhasin H, Jain P, Kapoor S, Jain A, et al. Vitamin B(12) deficiency in children with infantile spasms: a case‐control study. J Child Neurol. 2018;33(12):767–71. [DOI] [PubMed] [Google Scholar]
- 24. Alves LV, Mello MJG, Bezerra PG, Alves JGB. Congenital Zika syndrome and infantile spasms: case series study. J Child Neurol. 2018;33(10):664–6. [DOI] [PubMed] [Google Scholar]
- 25. Sumanasena SP, Wanigasinghe J, Arambepola C, Sri Ranganathan S, Muhandiram E. Developmental profile at initial presentation in children with infantile spasms. Dev Med Child Neurol. 2019;61(11):1295–301. [DOI] [PubMed] [Google Scholar]
- 26. Kulsoom S, Ibrahim SH, Jafri SK, Moorani KN, Anjum M. Infantile Spasms: clinical profile and treatment outcomes. Pak J Med Sci. 2018;34(6):1424–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fatema K, Rahman MM, Akhter S, Shefa J. ACTH versus Vigabatrin as first line treatment for West syndrome – A prospective study. Eur Acad Res. 2017;8:3760–72. [Google Scholar]
- 28. Poudel P, Kafle SP, Pokharel R. Clinical profile and treatment outcome of epilepsy syndromes in children: a hospital‐based study in Eastern Nepal. Epilepsia Open. 2021;6(1):206–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aung HWW, Aye AH, Saan CS, Ko AM, See KMM, Mar MT, et al, eds. Response to oral prednisolone in infantile spasms: Myanmar Experience. 12th Asian and Oceanian Epilepsy Congress; 2018. [Google Scholar]
- 30. Sharma S, Kaushik JS, Srivastava K, Goswami JN, Sahu JK, Vinayan KP, et al. Association of Child Neurology (AOCN) — Indian Epilepsy Society (IES) Consensus Guidelines for the Diagnosis and Management of West Syndrome. Indian Pediatr. 2021;58(1):54–66. [PubMed] [Google Scholar]
- 31. Birth rate, crude (per 1,000 people) [Internet]. [cited 2021 May 25]. Available from https://data.worldbank.org/indicator/SP.DYN.CBRT.IN?view=chart
- 32. Programmes and Impact > South Asia [Internet] . [cited 2021 May 25]. Available from https://www.gavi.org/programmes‐impact/country‐hub/southeast‐asia
- 33. GDP (current US$) [Internet] . [cited 2021 May 25]. Available from https://data.worldbank.org/indicator/NY.GDP.MKTP.CD?end=2019&start=1960&view=chart
- 34. GDP per capita (current US$) [Internet] . [cited 2021 May 25]. Available from https://data.worldbank.org/indicator/NY.GDP.PCAP.CD
- 35. Domestic general government health expenditure (% of general government expenditure) [Internet]. [cited 2021 May 25]. Available from https://data.worldbank.org/indicator/SH.XPD.GHED.GE.ZS?view=chart
- 36. Mortality rate, neonatal (per 1,000 live births) [Internet]. [cited 2021 May 25]. Available from https://data.worldbank.org/indicator/SH.DYN.NMRT?view=chart
- 37. Mortality rate, infant (per 1,000 live births) [Internet]. [cited 2021 May 25]. Available from https://data.worldbank.org/indicator/SP.DYN.IMRT.IN?view=chart
- 38. Exclusive breastfeeding (% of children under 6 months) [Internet]. [cited 2021 May 25]. Available from https://data.worldbank.org/indicator/SH.STA.BFED.ZS
- 39. Births attended by skilled health personnel Data by country [Internet]. [cited 2021 May 25]. Available from https://apps.who.int/gho/data/node.main.SKILLEDBIRTHATTENDANTS?lang=en
- 40. Literacy rate, adult total (% of people ages 15 and above) [Internet]. [cited 2021 May 25]. Available from https://data.worldbank.org/indicator/SE.ADT.LITR.ZS?view=chart
- 41. Bhanudeep S, Madaan P, Sankhyan N, Saini L, Malhi P, Suthar R, et al. Long‐term epilepsy control, motor function, cognition, sleep and quality of life in children with West syndrome. Epilepsy Res. 2021;173:106629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
App S1


