Abstract
Multidrug-resistant Enterobacter aerogenes strains are increasingly isolated in Europe and especially in France. Treatment leads to imipenem resistance, because of a lack of porin. We studied the evolution of resistance in 29 strains isolated from four patients during their clinical course. These strains belonged to the prevalent epidemiological type observed in France in previous studies (C. Bosi, et al., J. Clin. Microbiol. 37:2165–2169, 1999; A. Davin-Regli et al., J. Clin. Microbiol. 34:1474–1480, 1996). They also harbored a TEM-24 extended-spectrum β-lactamase-coding gene. Thirteen strains were susceptible to gentamicin and resistant to imipenem and cefepime. All of the patients showed E. aerogenes strains with this resistance after an imipenem treatment. One patient showed resistance to imipenem after a treatment with cefpirome. Twelve of these 13 strains showed a lack of porin. Cessation of treatment with imipenem for three patients was followed by reversion of susceptibility to this antibiotic and the reappearance of porins, except in one case. For one patient, we observed three times in the same day the coexistence of resistant strains lacking porin and susceptible strains possessing porin. The emergence of multidrug-resistant E. aerogenes strains is very disquieting. In our study, infection by E. aerogenes increased the severity of the patients' illnesses, causing a 100% fatality rate.
Enterobacter aerogenes has emerged as an important hospital pathogen since 1992 (2, 6). This gram-negative bacterium is now the third leading cause of respiratory tract nosocomial infections by gram-negative bacteria, after Staphylococcus aureus and Pseudomonas aeruginosa (13). E. aerogenes strains isolated from hospitalized patients generally exhibit high resistance to broad-spectrum antibiotics. For instance, such high resistance against β-lactams is exhibited by three processes: enzymatic responses, such as plasmidic β-lactamase and chromosomic cephalosporinase; variability of the target of the antibiotic; and modification of the envelope permeability, including alteration of porin and expression of drug efflux (12, 20, 22).
Imipenem has been used successfully to treat multidrug-resistant organisms involved in nosocomial infections for more than a decade. Previously, resistance to imipenem was observed in Enterobacter cloacae and was shown to be mediated by a chromosomal cephalosporinase (21). In addition, in some cases altered outer membrane permeability has been reported for resistant isolates (15).
Previous studies have suggested that antibiotics (especially broad-spectrum cephalosporins) lead to a higher level of resistance in E. aerogenes (12). A broad-spectrum antimicrobial therapy promotes the selection of strains harboring resistance to cephalosporins, aminoglycosides, and fluoroquinolones. Imipenem resistance and cefepime resistance are often associated (9).
In the present study, we documented the step-by-step in vivo emergence of imipenem-resistant strains after prolonged antibiotic therapy. The antibiotic susceptibilities, molecular typing, and envelope permeabilities of all the clinical strains were studied along with antibiotic regimens. The emergence of imipenem resistance through a decrease in porin synthesis was found to correlate with imipenem therapy.
MATERIALS AND METHODS
Patient data.
The four patients were hospitalized in the south of France. Patients 2, 3, and 4 were in the same hospital.
Immunocompromised patient 1 was hospitalized for renal failure in a nephrologic intensive-care unit (ICU) between 1 April 1997 and 15 April 1997. He died on 15 April 1997 from multivisceral degradation. Patient 2 was hospitalized in an ICU between 26 January 1997 and 7 July 1997 for cardiorespiratory failure. In this case, the first strain of E. aerogenes was isolated on 14 April 1997. Patient 3, psychotic and suffering from an acute surrenalian failure, was hospitalized in an ICU for a Staphylococcus epidermidis respiratory infection between 3 August 1997 and 13 October 1997. He was then transferred to a pneumologic unit and died on 4 November 1997. From this patient, the first E. aerogenes strain was isolated on 1 September 1997. Patient 4, suffering from Parkinson's disease, was hospitalized for an acute asthma crisis in an ICU between 3 August 1997 and 17 September 1997. He was then transferred to a pneumologic unit, where he died on 14 October 1997. His first strain of E. aerogenes was isolated on 18 August 1997.
Patients 1 and 4 were treated with imipenem. Patients 2 and 3 were treated with imipenem and cefpirome (Fig. 1).
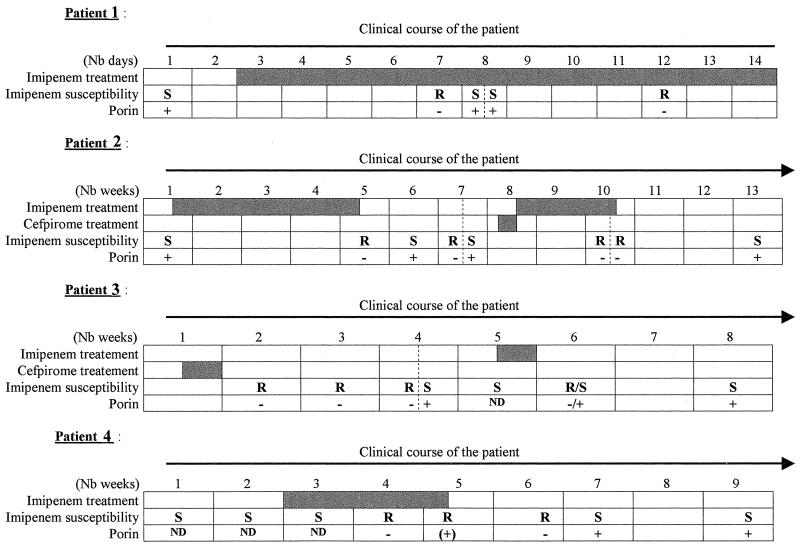
FIG. 1.
Clinical courses of patients 1, 2, 3, and 4. Shaded bars indicate patient treatment. Nb, number; ND, not done; +, present; −, absent; S, susceptible; R, resistant.
Bacterial strains and growth media.
Twenty-nine strains of E. aerogenes were isolated and studied during the infectious events. The strains were identified by the API 20E system (BioMérieux, Marcy l'Etoile, France). Initially, the strains were isolated in Mueller-Hinton agar (BioMérieux). In our laboratory, all bacterial strains were grown and subcultured in Luria-Bertani agar (Difco Laboratories, Detroit, Mich.). Subcultures in Luria-Bertani agar allowed us to investigate imipenem resistance stability. We subcultured one colony from the previous plate each time. We obtained five subcultures for each clinical strain.
Epidemiological typing.
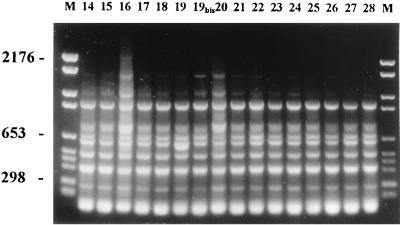
The strains were investigated by random amplification of polymorphic DNA (RAPD) with primer AP12H (5′-CGGCCCCTGT-3′) and enterobacterial repetitive intergenic consensus (ERIC)-PCR with primer ERIC2 (5′-AAGTAAGTGACTGGGGTGAGCG-3′). RAPD is based on the amplification of random DNA segments with a single primer of arbitrary nucleotide sequence. ERIC sequences represent an extragenic, highly conserved, and dispersed DNA sequence that has been observed in many eubacterial species. Consensus primers complementary to each end of a repeated sequence are oriented such that PCR amplification of DNA sequences proceeds between adjacent repeated ERIC elements. The PCR products have lengths reflecting distance polymorphisms between the 126-bp ERIC elements (7, 25, 26). With the RAPD methods, variation of intensity of PCR products was observed with faint bands but was not considered to be a difference (Fig. 2).
FIG. 2.
RAPD fingerprints of the 16 isolates of patient 3 (8) and patient 4 (8). Lane M, molecular weight marker (marker VI). The lane numbers are isolate designations.
(i) DNA preparation.
Strains were grown overnight at 37°C on Mueller-Hinton agar (BioMérieux). Total cellular DNA was extracted by the Chelex technique (10). Briefly a single colony was picked from a plate and resuspended in 2% (vol/vol) Chelex–0.05% (vol/vol) sodium dodecyl sulfate (SDS). The resuspension was heated at 98°C for 20 min. DNA concentrations were estimated on agarose gels.
(ii) Amplification conditions.
Amplification reactions were performed in a total volume of 50 μl containing 100 μM dATP, 100 μM dCTP, 100 μM dGTP, and 100 μM dTTP plus 0.2 μM primer, 25 ng of template DNA, and 1.25 U of Taq polymerase (Perkin-Elmer Cetus, Norwalk, Conn.) in 1× PCR buffer (10 mM Tris-HCl [pH 8.3], 50 mM KCl, 2.5 mM MgCl2, 0.001% [wt/vol] gelatin). A negative control without template DNA was included in each experiment. The reaction mixtures were subjected to amplification in a GenAmp PCR System 9600 (Perkin-Elmer Cetus) programmed for 45 cycles of 1 min at 94°C, 1 min at 37°C, and 1 min at 74°C. The amplification products (10-μl samples) were electrophoresed in 1.2% agarose gels in Tris acetate buffer (0.04 M Tris acetate, 0.001 M EDTA, pH 8.2), stained with ethidium bromide, and photographed on a UV light transilluminator. A molecular weight standard (marker VI; Boehringer, Mannheim, Germany) was included on each gel. We interpreted and compared the patterns without considering the origins of the strains. Heterogeneity with respect to the intensity and shape of bands was not considered a difference. Strains were considered different if their profiles differed by two or more bands according to previous studies (26).
(iii) Reproducibility.
For the two PCR-based techniques, RAPD and ERIC-PCR, reproducibility was determined by testing independent DNA preparations extracted from single-colony cultures at different times and amplified separately.
Antibiotic susceptibility tests.
Resistance to imipenem, cefepime, cefpirome, ciprofloxacin, chloramphenicol, and tetracycline was evaluated with the Walkaway 40 system (Sanofi Diagnostics Pasteur, Marnes-la-Coquette, France) and confirmed by the semimicromethod dilution method using Biomek 1000 (Beckman Instruments, Inc., Fullerton, Calif.). The Biomek 1000 is an automated laboratory workstation used as a liquid-handling robot. Imipenem (Merck Sharp Dohme and Chibret, Paris, France), cefepime (Bristol-Myers Squibb, Paris, France), cefpirome (Hoescht Marrion Roussel, Swindon, United Kingdom), ciprofloxacine (Sigma Chemical Co., St. Louis, Mo.), chloramphenicol (Sigma Chemical Co.), and tetracycline (Sigma Chemical Co.) were diluted at a concentration of 1,024 μg/ml. From this concentration, the Biomek 1000 made twofold serial dilutions (1/2, 1/4, 1/8, 1/16, 1/32, 1/64, 1/128, 1/256, 1/512, and 1/1,024) with Luria-Bertani broth (Difco Laboratories) on a microplate (Beckman Instruments, Inc.) to a total volume of 700 μl for each dilution. The robot also replaced antibiotics with distilled water in two places on the microplate, one as a growing test and one without inoculation as a negative test. The robot automatically inoculated the microplate with a solution containing 106 cells. The results were read after 24 h at 37°C (14). Bacterial strains were classified as susceptible, intermediate, and resistant, in keeping with the indications of the Antibiogram Committee of the French Society for Microbiology (1). Strains were classified as susceptible if they grew up to a concentration of 4 μg/ml for imipenem, 4 μg/ml for cefepime, 4 μg/ml for cefpirome, 1 μg/ml for ciprofloxacine, 8 μg/ml for chloramphenicol, and 4 μg/ml for tetracycline. Strains were classified as resistant if they grew under a concentration of 8 μg/ml for imipenem, 32 μg/ml for cefepime, 32 μg/ml for cefpirome, 2 μg/ml for ciprofloxacine, 16 μg/ml for chloramphenicol, and 8 μg/ml for tetracycline. Strains were classified as intermediate, for each antibiotic, if the MICs for them were between the limits of susceptibility and resistance.
TEM beta-lactamase identification.
PCR amplifications were performed as described previously (16). Amplification was achieved with an initial cycle of 5 min of denaturation at 95°C and then 30 cycles of 0.5 min at 94°C, 0.5 min at 55°C, and 0.5 min at 74°C. The primers were 5′-GACAGTTACCAATGCTTAATCA-3′ and 5′-TTGGGTGCACGAGTGGGTTA-3′ (16).
(i) DNA sequencing.
DNA sequencing was carried out by cycle sequencing with fluorescently labeled dideoxynucleotide terminators (Applied Biosystems Inc., Norwalk, Conn.). The sequencing reactions were carried out on a model 377 automated DNA sequencer (Applied Biosystems Inc.).
(ii) Amino acid sequence analysis.
Amino acid sequences were determined with Translate Tool on the ExPASy World Wide Web molecular biology server of the Swiss Institute of Bioinformatics (http://www.expasy.ch/tools/dna.html). Sequences were analyzed by using the table published by G. Jacoby and K. Bush on the World Wide Web server of the Lahey Clinic http://www.lahey.org/studies/webt.htm).
SDS-polyacrylamide gel electrophoresis and immunodetection of porins.
Porins were checked on the E. aerogenes strains showing a multidrug-resistant phenotype. Exponential-phase bacterial cells grown in Luria-Bertani broth were collected. Bacterial-cell pellets were solubilized in loading buffer at 96°C, and samples were loaded onto SDS-polyacrylamide gels (10% polyacrylamide, 0.1% SDS) as previously described (4). Electrotransfer to nitrocellulose membranes was performed in the presence of 0.05% SDS to achieve complete transfer of porins. An initial saturating step with Tris-buffered saline (50 mM Tris-HCl, 150 mM NaCl, pH 8) containing 10% (wt/vol) skim milk was carried out overnight at 4°C. The nitrocellulose membranes were then incubated in the same buffer containing 10% (wt/vol) skim milk and 0.2% Triton X-100 for 2 h at room temperature with polyclonal antibodies directed against denatured Escherichia coli porins (OmpF and OmpC). Polyclonal antibodies directed against the E. coli porins were able to recognize the E. aerogenes porins as reported previously (18). After successive washings in the same buffer, the antigen-antibody complexes were detected with alkaline phosphatase-conjugated affinitiPure goat anti-rabbit immunoglobulin G antibodies (Jackson ImmunoResearch, West Grove, Pa.). The polyclonal antibodies directed against the porin monomers OmpF and OmpC have been described previously (18).
RESULTS
Antibiotic susceptibility.
The antibiotic susceptibilities of strains ranged from a phenotype susceptible to cefepime, imipenem, and gentamicin to a phenotype susceptible to gentamicin only. Except for the first strain from patient 1, all of the strains were resistant to ciprofloxacin and chloramphenicol (Table 1). Half of the strains were considered resistant to tetracycline, but no correlation could be found with other antibiotic resistances.
TABLE 1.
Characteristics of 29 E. aerogenes isolates recovered from four patientsa
| Patient | Isolate no. | Date (day/month/year) | Source | RAPD type | Antimicrobial susceptibilityb
|
ESBLc | Porins | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FEP | CPO | IMP | CIP | CHL | TET | |||||||
| ATCC 13048 | 1 | 1 | 1 | 2 | 4 | 1 | ||||||
| 1 | 1 | 04/03/1997 | Tracheal aspiration | I | 1 | 1 | 1 | 2 | 8 | 1 | TEM-24 | Yes |
| 2 | 04/09/1997 | Tracheal aspiration | I | 64 | 64 | 16 | 32 | 256 | 4 | TEM-24 | No | |
| 3 | 04/10/1997 | Tracheal aspiration | I | 8 | 8 | 2 | 32 | 256 | 8 | TEM-24 | Yes | |
| 4 | 04/10/1997 | Urine | I | 16 | 16 | 2 | 32 | 256 | 8 | TEM-24 | Yes | |
| 5 | 04/14/1997 | Tracheal aspiration | I | 64 | 64 | 16 | 64 | 256 | 16 | TEM-24 | No | |
| 2 | 6 | 04/14/1997 | Urine | I | 2 | 2 | 1 | 128 | 512 | 4 | TEM-24 | Yes |
| 7 | 05/12/1997 | Urine and vaginal sample | I | 32 | 32 | 32 | 128 | 512 | 16 | TEM-24 | No | |
| 8 | 05/20/1997 | Sputum | I | 16 | 16 | 4 | 128 | 256 | 4 | TEM-24 | Yes | |
| 9 | 05/26/1997 | Urine | I | 128 | 128 | 8 | 128 | 512 | 16 | TEM-24 | No | |
| 10 | 05/26/1997 | Vaginal sample | I | 2 | 2 | 1 | 64 | 256 | 2 | TEM-24 | Yes | |
| 11 | 06/16/1997 | Vaginal sample | I | 64 | 64 | 8 | 128 | 512 | 8 | TEM-24 | No | |
| 12 | 06/17/1997 | Blood culture | I | 32 | 32 | 16 | 128 | 256 | 4 | TEM-24 | No | |
| 13 | 07/07/1997 | Tracheal aspiration | I | 4 | 4 | 2 | 64 | 256 | 8 | TEM-24 | Yes | |
| 3 | 14 | 09/01/1997 | Tracheal aspiration | I | 32 | 32 | 16 | 256 | 256 | 16 | TEM-24 | No |
| 15 | 09/08/1997 | Tracheal aspiration | I | 128 | 128 | 8 | 64 | 256 | 8 | TEM-24 | No | |
| 16 | 09/15/1997 | Urine | I | 64 | 64 | 16 | 256 | 256 | 8 | TEM-24 | No | |
| 17 | 09/15/1997 | Anal | I | 4 | 4 | 2 | 64 | 256 | 8 | TEM-24 | Yes | |
| 18 | 09/22/1997 | Tracheal aspiration | I | 2 | 2 | 1 | 64 | 256 | 4 | TEM-24 | ND | |
| 19 | 09/29/1997 | Tracheal aspiration | I | 128 | 128 | 8 | 64 | 256 | 8 | TEM-24 | No | |
| 19bis | 09/29/1997 | Tracheal aspiration | I | 2 | 2 | 4 | 32 | 512 | 8 | TEM-24 | Yes | |
| 20 | 10/13/1997 | Tracheal aspiration | I | 2 | 2 | 1 | 64 | 256 | 4 | TEM-24 | Yes | |
| 4 | 21 | 08/18/1997 | Urethral | I | 4 | 4 | 1 | 64 | 256 | 4 | TEM-24 | ND |
| 22 | 08/25/1997 | Urethral | I | 4 | 4 | 1 | 128 | 256 | 4 | TEM-24 | ND | |
| 23 | 09/01/1997 | Tracheal aspiration | I | 4 | 4 | 1 | 128 | 256 | 4 | TEM-24 | ND | |
| 24 | 09/08/1997 | Tracheal aspiration | I | 128 | 128 | 16 | 256 | 256 | 8 | TEM-24 | No | |
| 25 | 09/15/1997 | Tracheal aspiration | I | 32 | 32 | 16 | 256 | 128 | 4 | TEM-24 | Yes | |
| 26 | 09/25/1997 | Catheter | I | 32 | 32 | 16 | 256 | 128 | 4 | TEM-24 | No | |
| 27 | 10/01/1997 | Urine | I | 2 | 2 | 1 | 256 | 128 | 4 | TEM-24 | Yes | |
| 28 | 10/19/1997 | Urine | I | 2 | 2 | 1 | 256 | 128 | 4 | TEM-24 | Yes | |
ATCC 13048, 1, 3, 4, 6, 8, 10, 13, 17, 18, 19bis, 20, 21, 22, 23, 27, and 28 are susceptible isolates; 2, 5, (patient 1), 7, 9, 11, 12 (patient 2), 14, 15, 16, 19 (patient 3), 24, 25, and 26 (patient 4) show a resistant phenotype. Strains 19bis and 19 were isolated from the same agar plate, but 19bis showed a susceptible phenotype.
MIC (mg/liter). FEP, cefepime; CPO, cefpirome; IPM, imipenem; CIP, ciprofloxacin; CHL, chloramphenicol; TET, tetracycline; ND, not done.
ESBL, extended-spectrum β-lactamase.
β-Lactamase identification.
All of the strains harbored a TEM-24-type extended-spectrum β-lactamase-coding gene (Table 1).
Epidemiological typing.
The 29 different strains from the four patients belonged to the same epidemiological type (Fig. 2 and Table 1). There was no marked difference in pattern between these strains and the strain belonging to the type prevalent in Marseille since 1995, disseminated in 21 hospitals in France (3).
Immunodetection of porins.
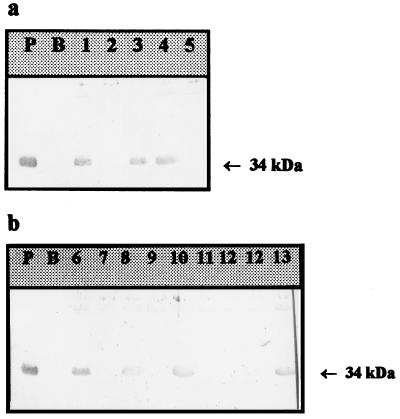
Polyclonal antibodies directed against denatured E. coli porins have been found to be able to recognize the E. aerogenes porin migrating at the 34-kDa position (17). Among the 29 strains, 12 showed a negative response with the immunological probes directed against the nonspecific enterobacterial porins, reflecting a porin-deficient phenotype (Table 1). These 12 strains were susceptible only to gentamicin. Twelve other strains showed a positive reaction (Fig. 3). These strains were susceptible only to cefepime, gentamicin, and imipenem. One strain showed a gentamicin-susceptible phenotype but also showed a porin. Four strains susceptible to cefepime, imipenem, and gentamicin were not studied for the presence of a porin.
FIG. 3.
Immunodetection of E. aerogenes outer membrane proteins. Immunostaining was done with polyclonal antibodies directed against the OmpF monomer or OmpC. PMY150 (P) and BZB1107 (B) were used as positive and negative samples, respectively. Isolates 2, 5, 7, 9, 11, and 12 from patients 1 (a) and 2 (b) presented porin-deficient phenotypes. The arrows indicate the postulated porin migration (34 kDa). The lane numbers are isolate designations.
Epidemiological evolution and patient data.
At the beginning of his clinical course, patient 1 harbored a strain susceptible to cefepime, imipenem, and gentamicin (Table 1). After 4 days of treatment including imipenem (day 7), an imipenem-resistant strain emerged. This first resistant strain was identified in a series of three strains; the other two strains conserved their susceptibility to imipenem. At that time, under the imipenem-induced pressure, two resistance phenotypes remained. Four days later, on day 12, only the imipenem-resistant phenotype could be isolated.
Like patient 1, patients 2 and 4 first presented an imipenem-, cefepime-, and gentamicin-susceptible strain. After imipenem treatment, these two patients presented isolates resistant to imipenem and cefepime but still susceptible to gentamicin. Stopping the imipenem treatment reversed the resistance to this antibiotic within 1.5 and 2 weeks for patients 2 and 4, respectively. Interestingly, on 26 May 1997, after the first imipenem treatment was discontinued, patient 2 showed two strains of E. aerogenes; one showed a cefepime, imipenem, and gentamicin susceptibility phenotype, and the other showed only gentamicin susceptibility.
As for patient 3, a strain resistant to imipenem was detected after a cefpirome treatment. This patient had not been treated with imipenem, and no E. aerogenes strain was identified before. We cannot conclude that this strain had not come from a horizontal acquisition, but no contact with other patients carrying this type of strain was noted. Cefpirome, like cefepime, has been shown to select for strains presenting an altered outer membrane (4). After the cefpirome treatment was stopped, a progressive recovery of imipenem susceptibility was evidenced in the isolated strains. During week 4, two isolates with different susceptibility patterns were isolated in patient 3. This could reflect the transition between the porin-deficient and porin-expressing cells. After the recovery of susceptibility, a 4-day imipenem treatment was carried out to eradicate bacterial colonization. The strain isolated 1 week after the beginning of this imipenem treatment was resistant to the antibiotic, but stopping the treatment led to a recovery of imipenem susceptibility, as for the other patients.
Moreover, in patient 3 during week 6, we isolated a strain that presented a peculiar phenotype and aspect relative to imipenem and cefepime susceptibilities and the presence of porin. Indeed, we could differentiate two types of colonies from the single isolate: the larger one corresponded to an imipenem- and cefepime-susceptible strain carrying porin; the smaller was the imipenem- and cefepime-resistant strains devoid of porin. Interestingly, the fifth subculture without antibiotics of the smallest resistant colonies allowed the isolation of a final large bacterial colony that was susceptible to imipenem.
Stability of imipenem resistance and porin-negative phenotype.
We subcultured the 12 strains showing imipenem resistance and a lack of porin several times on Luria-Bertani medium without antibiotics. After five reisolations, 11 strains showed imipenem susceptibility and porin expression. Only one was still imipenem resistant and porin negative.
DISCUSSION
We focused our analysis on resistance to cefepime and imipenem, which is associated with a lack of porin. For imipenem, the mechanism is not clearly understood, but it seems to be related to a lack of membrane permeability in E. aerogenes (4, 5, 8).
All of the patients were given imipenem therapy. Consequently, a few days later, an E. aerogenes strain appeared; it showed a cefepime resistance phenotype and imipenem resistance.
However, stopping treatment that includes imipenem leads systematically to a recovery of imipenem susceptibility. Furthermore, in three cases we observed heterogeneous populations in the same sample: patient 2 during week 7 and patient 3 during weeks 4 and 6.
After several in vitro subcultures, resistant clinical strains of E. aerogenes quickly recovered their porin content and susceptibility to imipenem (M. Mallea, unpublished results). Conversely, we observed that de novo use of imipenem induced a fast restart of imipenem resistance. We therefore cannot conclusively determine whether imipenem selected for new resistant clones in a population that was no longer exposed or if it directly activated physiological mechanisms preselected during previous treatment. The rapid and efficient modulation of porin expression that we observed during the therapy course suggests that this balance depends on a regulation cascade rather than a mutation on a porin gene or on a regulatory component.
The recovery of susceptibility and porin content could explain the apparent discrepancy concerning the isolation of an imipenem-resistant strain which expressed porin from patient 4 during week 5. In fact, this strain was submitted to several subcultures before we performed immunodetection of porins. To ascertain this point, we subcultured all of the porin-negative strains five times and reisolated them. After five reisolations in the absence of antibiotics, only 1 of the 12 original strains was still imipenem resistant and porin negative. The other strains recovered both imipenem susceptibility and porin expression.
This rapid regulation of porin synthesis is a significant advantage that confers on the pathogenic strain a prominent position during the therapy course compared to the other strains from patients or from environmental flora. Conversely, the rapid recovery of porins allows surviving bacteria to grow rapidly during the antibiotic cut. Although the strains recover antibiotic susceptibility after the cessation of treatment, the prognosis for infection due to such multidrug-resistant strains is very bad: deterioration of patients' conditions due to E. aerogenes had a part in the deaths of the four patients (De Gheldre et al. reported a crude fatality rate of 38% [10]).
The emergence of E. aerogenes strains with a decreased susceptibility to imipenem is of concern (4, 19, 23, 24). Arpin et al. reported that 4.6% of their E. aerogenes strains were resistant to imipenem (2). Relative to imipenem, this prevalent type of E. aerogenes can more rapidly adapt its regulation of permeability than other enterobacteria can. Moreover, Mallea et al. have described clinical E. aerogenes strains presenting a complex resistance strategy associating β-lactamase production, impermeability, and active efflux (17).
We found that 12 E. aerogenes strains showing such imipenem resistance exhibited an alteration of their porin content. This raises the question of whether permeability regulation is used as a resistance mechanism by E. aerogenes. It seems evident that E. aerogenes is able to perfectly adapt itself to antibiotic pressure and that the incidence of such mechanisms in this bacterium will probably increase with the use of imipenem in strains presenting the imipenem-gentamicin-sensitive phenotype (11).
ACKNOWLEDGMENTS
This work was supported by Assistance Publique de Marseille (Recherche Clinique) and the PACA region.
We thank B. Dussol for his help.
REFERENCES
- 1.Acar J, Carret G, Cavallo J D, Chardon H, Choutet P, Courvalin P, Dabernat H, Drugeon H, Dubreuil L, Goldstein F, Jarlier V, Leclercq R, Nicolas-Chanoine M H, Phillipon A, Rouveix B, Sirot J, Soussy C J, Thabaut A. Comité de l'Antibiogramme de la Société Française de Microbiologie. Communiqué 1998. Pathol Biol. 1998;46:I–XVI. [PubMed] [Google Scholar]
- 2.Arpin C, Coze C, Rogues A M, Gachie J P, Bebear C, Quentin C. Epidemiological study of an outbreak due to multidrug-resistant Enterobacter aerogenes in a medical intensive care unit. J Clin Microbiol. 1996;34:2163–2169. doi: 10.1128/jcm.34.9.2163-2169.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosi C, Davin-Regli A, Bornet C, Mallea M, Pages J M, Bollet C. Most Enterobacter aerogenes strains in France belong to a prevalent clone. J Clin Microbiol. 1999;37:2165–2169. doi: 10.1128/jcm.37.7.2165-2169.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charrel R N, Pages J M, De Micco P, Mallea M. Prevalence of outer membrane porin alteration in β-lactam-antibiotic-resistant Enterobacter aerogenes. Antimicrob Agents Chemother. 1996;40:2854–2858. doi: 10.1128/aac.40.12.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow J W, Shlaes D M. Imipenem resistance associated with the loss of a 40 kDa outer membrane protein of Enterobacter aerogenes. J Antimicrob Chemother. 1991;28:499–504. doi: 10.1093/jac/28.4.499. [DOI] [PubMed] [Google Scholar]
- 6.Davin-Regli A, Monnet D, Saux P, Bosi C, Charrel R N, Barthelemy A, Bollet C. Molecular epidemiology of Enterobacter aerogenes acquisition: 1-year prospective study in two intensive care units. J Clin Microbiol. 1996;34:1474–1480. doi: 10.1128/jcm.34.6.1474-1480.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davin-Regli A, Saux P, Bollet C, Gouin F, de Micco P. Investigation of a series of outbreaks of Enterobacter aerogenes in an intensive care unit by random amplification polymorphic DNA. J Med Microbiol. 1995;44:89–98. doi: 10.1099/00222615-44-2-89. [DOI] [PubMed] [Google Scholar]
- 8.De Champs C, Henquell C, Guelon D, Sirot D, Gazuy N, Sirot J. Clinical and bacteriological study of nosocomial infections due to Enterobacter aerogenes resistant to imipenem. J Clin Microbiol. 1993;31:123–127. doi: 10.1128/jcm.31.1.123-127.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Gheldre Y, Maes N, Rost F, De Ryck R, Clevenbergh P, Vincent J L, Struelens M J. Molecular epidemiology of an outbreak of multidrug-resistant Enterobacter aerogenes infections and in vivo emergence of imipenem resistance. J Clin Microbiol. 1997;35:152–160. doi: 10.1128/jcm.35.1.152-160.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Lamballerie X, Zandotti C, Vignoli C, Bollet C, de Micco P. A one-step microbial DNA extraction method using Chelex 100 suitable for gene amplification. Res Microbiol. 1992;143:785–790. doi: 10.1016/0923-2508(92)90107-y. [DOI] [PubMed] [Google Scholar]
- 11.Ehrhardt A F, Sanders C C, Thomson K S, Watanakunakorn C, Trulillano-Martin I. Emergence of resistance to Imipenem in Enterobacter strains masquerading as Klebsiella pneumoniae during therapy with Imipenem/Cilastatin. Clin Infect Dis. 1993;17:120–122. doi: 10.1093/clinids/17.1.120. [DOI] [PubMed] [Google Scholar]
- 12.Hopkins J M, Towner K J. Enhanced resistance to cefotaxime and imipenem associated with outer membrane protein alterations in Enterobacter aerogenes. J Antimicrob Chemother. 1990;25:49–55. doi: 10.1093/jac/25.1.49. [DOI] [PubMed] [Google Scholar]
- 13.Jarvis W R, Martone W J. Predominant pathogens in hospital infections. J Antimicrob Chemother. 1992;29:19–24. doi: 10.1093/jac/29.suppl_a.19. [DOI] [PubMed] [Google Scholar]
- 14.Kaayiere M G, Mahamoud A, Chevalier J, Soyfer J C, Cremieux A, Barbe J. Synthesis and antibacterial activity of the new 4-alkoxy, 4-aminoalkyl and 4-alkylthioquinoline derivatives. Eur J Med Chem. 1998;33:55–63. [Google Scholar]
- 15.Lee E H, Nicolas M H, Kitzis M D, Pialoux G, Collatz E, Gutmann L. Association of two resistance mechanisms in a clinical isolate of Enterobacter cloacae with high-level resistance to imipenem. Antimicrob Agents Chemother. 1991;35:1093–1098. doi: 10.1128/aac.35.6.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mabilat C, Goussard S. PCR detection and identification of genes for extended-spectrum β-lactamases. In: Persing D H, Smith T F, Tenover F C, White T J, editors. Diagnostic molecular microbiology: principles and applications. Washington, D.C.: American Society for Microbiology; 1994. pp. 553–559. [Google Scholar]
- 17.Mallea M, Chevallier J, Bornet C, Eyraud A, Davin-Regli A, Bollet C, Pages J M. Porin alteration and active efflux: two in vivo drug resistance strategies used by Enterobacter aerogenes. Microbiology. 1998;144:3003–3009. doi: 10.1099/00221287-144-11-3003. [DOI] [PubMed] [Google Scholar]
- 18.Mallea M, Simonet V, Eun-Hee L, Collatz E, Gervier R, Gutmann L, Pages J M. Biological and immunological comparisons of Enterobacter cloacae and Escherichia coli porins. FEMS Microbiol Lett. 1995;129:273–280. doi: 10.1111/j.1574-6968.1995.tb07592.x. [DOI] [PubMed] [Google Scholar]
- 19.Modakkas E M, Sanyal S C. Imipenem resistance in aerobic gram-negative bacteria. J Chemother. 1998;10:97–101. doi: 10.1179/joc.1998.10.2.97. [DOI] [PubMed] [Google Scholar]
- 20.Nikaido H. Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science. 1994;264:382–388. doi: 10.1126/science.8153625. [DOI] [PubMed] [Google Scholar]
- 21.Nordmann P, Mariotte S, Naas T, Labia R, Nicolas M H. Biochemical properties of a carbapenem-hydrolyzing beta-lactamase from Enterobacter cloacae and cloning of the gene into Escherichia coli. Antimicrob Agents Chemother. 1993;37:939–946. doi: 10.1128/aac.37.5.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pitout J D D, Thomson K S, Hanson N D, Ehrhardt A F, Coudron P, Sanders C C. Plasmid-mediated resistance to expanded-spectrum cephalosporins among Enterobacter aerogenes strains. Antimicrob Agents Chemother. 1998;42:596–600. doi: 10.1128/aac.42.3.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomson K S, Sanders C C, Chemel H. Imipenem resistance in Enterobacter. Eur J Clin Microbiol Infect Dis. 1993;12:610–613. doi: 10.1007/BF01973639. [DOI] [PubMed] [Google Scholar]
- 24.Tzouvelekis L S, Tzelepi E, Kaufmann M E, Mentis A F. Consecutive mutations leading to the emergence in vivo of imipenem resistance in a clinical strain of Enterobacter aerogenes. J Med Microbiol. 1994;40:403–407. doi: 10.1099/00222615-40-6-403. [DOI] [PubMed] [Google Scholar]
- 25.Versalovic J, Koeuth T, Lupski J R. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991;19:6823–6831. doi: 10.1093/nar/19.24.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woods C R, Jr, Versalovic J, Koeuth T, Lupski J R. Analysis of relationships among strains of Citrobacter diversus by using DNA fingerprints generated by repetitive sequence-based primers in the polymerase chain reaction. J Clin Microbiol. 1992;30:2921–2929. doi: 10.1128/jcm.30.11.2921-2929.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]