Tea, the second most popular nonalcoholic beverage consumed worldwide, is favoured by billions of consumers due to its special flavour and numerous health benefits (Higdon and Frei, 2003). Theanine, a unique secondary metabolite in the tea plant (Camellia sinensis L.), confers the umami taste of the tea infusion. Theanine also has many physiological beneficial effects, including promoting relaxation, improving sleep quality and immunity and protecting the cardiovascular system (Kanarek et al., 2011).
Theanine only accumulates at a high level in tea plants (Tadahiro and Shinsuke, 1984). This might be controlled by the specific presence of ethylamine in tea plants (Cheng et al., 2017), as theanine is primarily biosynthesized from ethylamine and glutamate by theanine synthetase in tea roots, with ethylamine being synthesized from alanine, by alanine decarboxylase (AlaDC) (Figure 1a,b), and CsAlaDC being specifically expressed in tea roots (Figure 1c). Although CsAlaDC exhibited AlaDC activity, in vitro (Bai et al., 2019), the in vivo role in tea plants has not been characterized.
Figure 1.
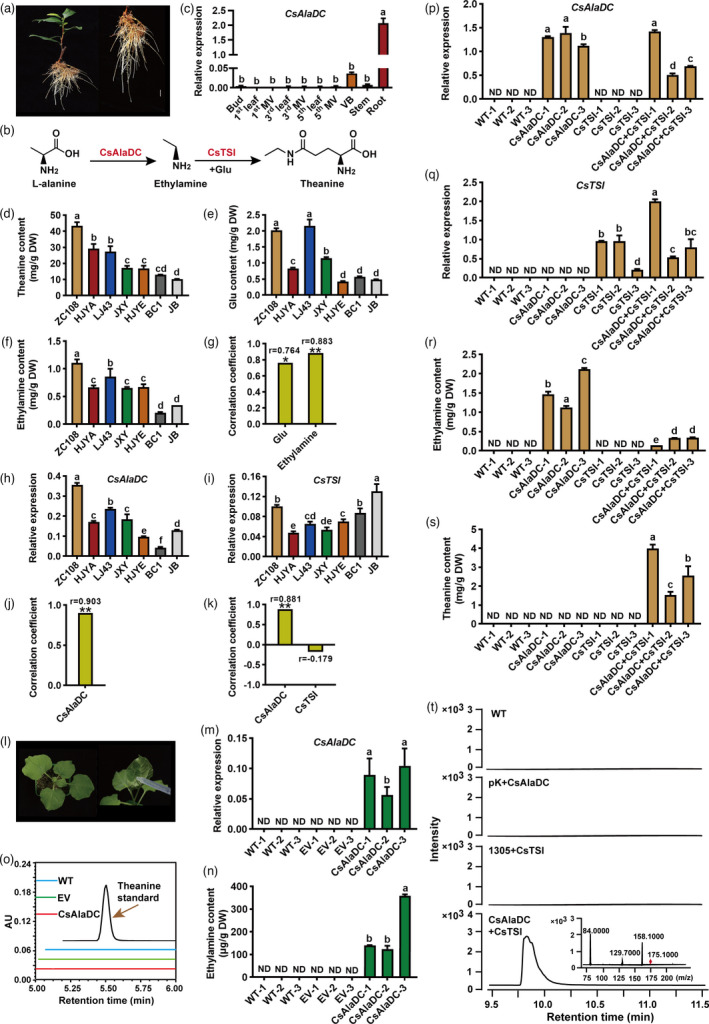
CsAlaDC expression was highly correlated with ethylamine and theanine contents in different tea plant cultivars and conferred a high level of theanine accumulation when co‐expressed with CsTSI in Nicotiana benthamiana. (a) Roots harvested for analysis. (b) Theanine biosynthesis pathway in the tea plant. CsAlaDC, alanine decarboxylase; CsTSI, theanine synthetase. (c) Tissue‐specific expression pattern of CsAlaDC. MV, major vein; VB, vascular bundle, peeled from the stem. (d–f) Theanine (d), glutamate (e), and ethylamine (f) contents in the roots of 7 tea plant cultivars. ZC108, Zhongcha 108; HJYA, Huangjinya; LJ43, Longjing 43; JXY, Jingxiangyu; HJYE, Huangjinye; BC1, Baicha 1; JB, Jibai. (g) The correlation coefficient of glutamate and ethylamine contents with theanine contents. *P < 0.05, **P < 0.01. (h, i) The relative expression of CsAlaDC (H) and CsTSI (I). CsGAPDH was used as an internal control. (j, k) The correlation coefficient of CsAlaDC expression levels with ethylamine contents (j), and the correlation coefficient of CsAlaDC and CsTSI expression levels with theanine contents (k). **P < 0.01. (l–o) Ectopic expression of CsAlaDC in N. benthamiana leaves. WT, wild‐type N. benthamiana without infiltration. EV, empty vector control; CsAlaDC, overexpression of CsAlaDC in N. benthamiana. NbGAPDH was used as an internal control. (p–t) Theanine contents in N. benthamiana leaves co‐expressing CsAlaDC and CsTSI. CsAlaDC, pK7WGF2+pCAMBIA1305‐CsAlaDC; CsTSI, pCAMBIA1305+pK7WGF2‐CsTSI; CsAlaDC+CsTSI, pCAMBIA1305‐ CsAlaDC+pK7WGF2‐CsTSI. Theanine in leaves of N. benthamiana was identified by mass chromatogram (UPLC‐QQQ‐MS). Characteristic theanine ions are located at m/z 158. Data represent means ± SD (n = 3), significant difference (P < 0.05) was labelled with different letters, according to Duncan's multiple range test.
Theanine content varies greatly among the different cultivars, and theanine levels appear to be genetically regulated in tea leaves (Fang et al., 2021). To investigate the in vivo role of ethylamine in theanine accumulation, we quantified the key metabolites in the theanine metabolic pathway, in the roots of seven tea plant cultivars (Figure 1d–f). As shown in Figure 1d, theanine contents in the roots of these cultivars varied greatly. Importantly, the ethylamine contents exhibited a similar pattern as the theanine contents (Figure 1F). The correlation coefficient between the contents of ethylamine and theanine was 0.883 (P < 0.01) (Figure 1g). Taken together, these findings provided in vivo evidence for the pivotal role of ethylamine in determining theanine accumulation in tea plants.
Next, we examined CsAlaDC expression in the roots of the seven cultivars (Figure 1h,i). Here, we found that its expression level was highly and positively correlated with the ethylamine contents, and the correlation coefficient reached 0.903 (P < 0.01) (Figure 1j). We also analysed the correlation between CsAlaDC expression and theanine content. Our findings indicated that CsAlaDC expression, in roots, was highly and positively correlated with the theanine contents, and the correlation coefficient was 0.881 (P < 0.01) (Figure 1k). Unexpectedly, the CsTSI expression level was not correlated with the theanine contents (Figure 1k). Taken together, these results suggested that CsAlaDC expression plays a critical role in determining theanine accumulation in roots of tea plants.
We used the Nicotiana benthamiana transient expression system to further characterize the function of CsAlaDC, in planta. To this end, A. tumefaciens strain GV3101 (pSoup‐p19), carrying pCAMBIA1305‐CsAlaDC‐GFP plasmid, was infiltrated into leaves of 5‐week‐old N. benthamiana plants (Figure 1l); the pCAMBIA1305 empty vector was used as the control (EV). We detected CsAlaDC expression at the mRNA level (Figure 1m) by qRT‐PCR, and a high level of ethylamine (Figure 1n; >100 μg/g dry weight) was also identified in the CsAlaDC‐expressing tobacco leaves. As anticipated, no ethylamine product was detected in WT tobacco leaves and those infiltrated with EV. Taken together, these findings offer support for the hypothesis that CsAlaDC has the capacity to synthesize ethylamine, in planta.
Theanine is highly demanded, by the market, due to its health effects and medicinal value, as a food constituent, in cosmetics and in other fields (Cheng et al., 2017). The theanine obtained both by direct extraction and chemical synthesis is of very poor quality (Gu et al., 2004). In addition, plant cell culture is disadvantaged by high cost, poor genetic stability and the low content of metabolites, hampering its use for industrial theanine production. An alternative, and intriguing possibility, would be to synthesize theanine in other crops to improve their health‐promoting effects of foods. Until now, however, the synthesis of theanine in non‐tea plants through synthetic biology has not been achieved. Tobacco (N. benthamiana) is generally used as the model plant for synthetic biology research (Forestier et al., 2021; Li et al., 2019). Thus, we felt it would be important to synthesize theanine in tobacco, as a model for theanine biosynthesis in non‐tea plants.
Given the biosynthesis of ethylamine in tobacco, we speculated whether theanine could be produced in the tobacco leaves in the presence of ethylamine. Unexpectedly, theanine production was not detected (Figure 1o), indicating that the presence of ethylamine did not lead to theanine synthesis, at least not in detectable quantities.
We then speculated that the combination of CsAlaDC and CsTSI, in tobacco leaves, could synthesize theanine. Based on this notion, we co‐infiltrated A. tumefaciens strains carrying pCAMBIA1305‐CsAlaDC and pK7WGF2‐CsTSI plasmids (CsAlaDC+CsTSI) into tobacco leaves. A. tumefaciens strains containing pCAMBIA1305 and pK7WGF2‐CsTSI (CsTSI) and pK7WGF2 and pCAMBIA1305‐CsAlaDC (CsAlaDC) were infiltrated as the controls. Both CsAlaDC and CsTSI were being expressed in the infiltrated tobacco leaves (Figure 1p,q). Ethylamine was produced in CsAlaDC‐ and CsAlaDC+CsTSI‐expressing leaves (Figure 1r). High levels of theanine were produced in leaves co‐expressing CsAlaDC and CsTSI, and the theanine content reached to ˜4 mg/g (Figure 1s,t), which is comparable with the theanine contents in tea plant leaves. Thus, theanine biosynthesis requires both the presence of ethylamine and the co‐action of CsAlaDC and CsTSI. To our knowledge, this is the first report of theanine biosynthesis, by synthetic biology, in a non‐tea plant.
In summary, we established that CsAlaDC works coordinately with CsTSI in determining the unique high theanine accumulation in the roots of tea plants. More importantly, we reported the first biosynthesis of theanine in a model plant, tobacco. Theanine production in this system does not require the provision of additional substrates, thereby greatly reducing costs and avoids causing environmental pollution. As theanine confers the umami taste, but also has various health effects, engineering its synthesis in non‐tea plants, including crops, may well contribute to improve the health‐promoting quality of the foods derived from such crops, as well as meeting the market demand for theanine.
Conflict of interest
The authors declare that they have no conflict of interest.
Author contributions
ZZ, TY, YW and SB conceived the study and designed the experiments; BZ, JG, CD, FL, SQ and SL carried out the experiments; BZ and ZZ wrote the manuscript, with editing by WJL; SB and WJL provided instructive comments. All authors reviewed and approved the final manuscript.
Acknowledgement
This work was supported by grants from the National Natural Science Foundation of China (32072624, 31770731), the Base of Introducing Talents for Tea Plant Biology and Quality Chemistry (D20026) and Outstanding Youth Project of the Natural Science Foundation of Anhui Province (2008085J18).
Zhu, B. , Guo, J. , Dong, C. , Li, F. , Qiao, S. , Lin, S. , Yang, T. , Wu, Y. , Bao, S. , Lucas, W. J. and Zhang, Z. (2021) CsAlaDC and CsTSI work coordinately to determine theanine biosynthesis in tea plants (Camellia sinensis L.) and confer high levels of L‐theanine accumulation in a non‐tea plant. Plant Biotechnol. J., 10.1111/pbi.13722
References
- Bai, P. , Wei, K. , Wang, L. , Zhang, F. , Ruan, L. , Li, H. , Wu, L. and et al. (2019) Identification of a novel gene encoding the specialized alanine decarboxylase in tea (Camellia sinensis) plants. Molecules, 24(3), 540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, S. , Fu, X. , Wang, X. , Liao, Y. and Yang, Z. (2017) Studies on the biochemical formation pathway of the amino acid L‐theanine in tea (Camellia sinensis) and other plants. J. Agr. Food Chem., 65(33), 7210–7216. [DOI] [PubMed] [Google Scholar]
- Fang, K. , Xia, Z. , Li, H. , Jiang, X. , Qin, D. , Wang, Q. , Wang, Q. et al. (2021) Genome‐wide association analysis identified molecular markers associated with important tea flavor‐related metabolites. Hortic. Res., 8(1), 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forestier, E. , Czechowski, T. , Cording, A.C. , Gilday, A.D. and Graham, I.A. (2021) Developing a Nicotiana benthamiana transgenic platform for high‐value diterpene production and candidate gene evaluation. Plant Biotechnol. J., 19(1), 98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, H. , Jiang, Y. and Wang, J. (2004) A practical synthesis of ethyl‐L‐glutamine (L‐Theanine). Org. Prep. Proced. Int., 36(2), 182–185. [Google Scholar]
- Higdon, J.V. and Frei, B. (2003) Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr., 43(1), 89–143. [DOI] [PubMed] [Google Scholar]
- Kanarek, R. , Mahoney, C. , Brunye, T. and Giles, G. (2011) Theanine mitigates caffeine‐accentuated stress response and reduces attentional processing biases following exposure to stress. Eur. J. Pharmacol., 668, e16. [Google Scholar]
- Li, J. , Mutanda, I. , Wang, K. , Yang, L. , Wang, J. and Wang, Y. (2019) Chloroplastic metabolic engineering coupled with isoprenoid pool enhancement for committed taxanes biosynthesis in Nicotiana benthamiana . Nat. Commun., 10(1), 4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadahiro, N. and Shinsuke, S. (1984) Differences in caffeine, flavanols and amino acids contents in leaves of cultivated species of Camellia . Japan J. Breed., 34(4), 459–467. [Google Scholar]


