Abstract
Cancer cells predominantly generate energy via glycolysis, even in the presence of oxygen, to support abnormal cell proliferation. Suppression of PDHA1 by PDK1 prevents the conversion of cytoplasmic pyruvate into Acetyl-CoA. Several PDK inhibitors have been identified, but their clinical applications have not been successful for unclear reasons. In this study, endogenous PDHA1 in A549 cells was silenced by the CRISPR/Cas9 system, and PDHA1WT and PDHA13SD were transduced. Since PDHA13SD cannot be phosphorylated by PDKs, it was used to evaluate the specific activity of PDK inhibitors. This study highlights that PDHA1WT and PDHA13SD A549 cells can be used as a cell-based PDK inhibitor-distinction system to examine the relationship between PDH activity and cell death by established PDK inhibitors. Leelamine, huzhangoside A and otobaphenol induced PDH activity-dependent apoptosis, whereas AZD7545, VER-246608 and DCA effectively enhanced PDHA1 activity but little toxic to cancer cells. Furthermore, the activity of phosphomimetic PDHA1 revealed the complexity of its regulation, which requires further in-depth investigation.
Keywords: Apoptosis, Glycolysis, PDHA1, PDK, Warburg effect
INTRODUCTION
Normal cells rely on mitochondrial oxidative phosphorylation (OXPHOS) to generate adenosine triphosphate (ATP) (1). Conversely, cancer cells mainly generate energy via glycolysis to support the abnormal cell proliferation even in the presence of oxygen (2, 3). Upregulation of glycolysis is one of the adaptation processes observed in tumors in response to environmental pressures such as hypoxia and acidosis (4). Therefore, inhibition of glycolysis has been regarded as a promising therapeutic stra-tegy against cancer cells (5).
In glucose metabolism, the pyruvate dehydrogenase (PDH) complex (PDC) mediates a major regulatory step, linking glycolysis to tricyclic acid cycle through catalyzing pyruvate to acetyl-CoA (6). Previous study suggested that inhibition of PDH activity provides advantages for cancer growth (7). PDH kinases (PDK1-4) regulate PDH activity by phosphorylating PDHA1 at three individual serine (Ser, S) residues (S293, S300 and S232) (8). PDK1 is frequently increased in cancer cells and plays an important role in tilting the energy balance in their favor by enhancing the Warburg effect (9). Suppression of PDK1 increases mitochondrial oxygen consumption and induces cancer cell death frequently by activating apoptosis (10). Therefore, PDK1 inhibition could serve as a novel therapeutic approach for treating cancers.
Through experiments or clinical trials, several PDK inhibitors have been developed, such as AZD7545, JX06, VER-246608, dichloroacetate (DCA) and Leelamine (11-13). We have also identified several novel PDK1 inhibitors including huzhangoside A, ilimaquinone and hemistepsin A, isolated from Anemone rivularis, Smenospongia cerebriformis and Hemistepta lyrate, respectively (14-16). These PDK inhibitors enhanced the metabolic shift from glycolysis to OXPHOS and consequently induced mitochondrial ROS-mediated apoptosis in several cancer cells (14-16). To date, there are no effective PDK inhibitors used for cancer treatment in clinical practice. Although extensive studies have been in progress to find novel PDK inhibitors, target accuracy and off-target toxicity of the inhibitors have made them controversial.
Aspartic acid (Asp, D) is electrically similar to phosphorylated Ser (17). To construct a phosphomimetic form of PDH E1 subunit alpha 1 (PDHA1), we replaced the above described three Ser residues into Asp (S293D, S300D and S232D; hereafter, PDHA13SD). A phosphomimetic PDHA1 is permanently similar to phosphorylated PDHA1 regardless of whether PDKs are active or not. Consequently, it could be resistant to PDK inhibitors. To ensure the function of PDHA13SD, endogenous PDHA1 was silenced by the CRISPR/Cas9 system. Subsequently, ectopic wildtype (PDHA1WT) and PDHA13SD were reconstructed. In this study, we aimed to discriminate the PDH activity-dependent cell death induced by established PDK inhibitors in human non-small cell lung cancer A549 cells.
RESULTS
Silencing of endogenous PDHA1 in A549 cells
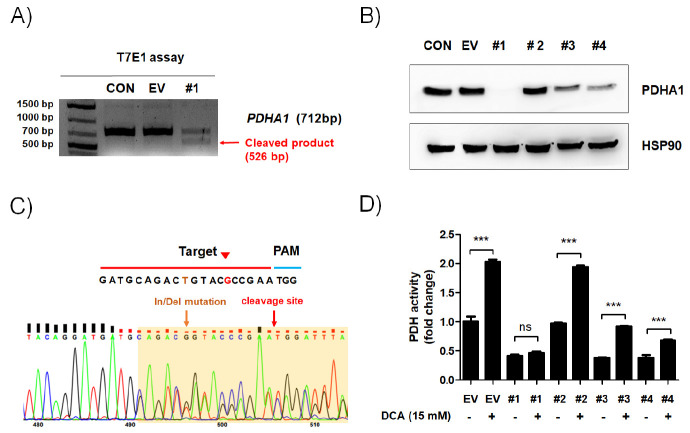
To establish a PDH activity-dependent cell death screening system, the CRISPR/Cas9 system was applied to knock out the PDHA1 gene in human non-small cell lung cancer A549 cells. Four lentiCRISPR vectors containing PDHA1-targeting single guide RNAs (sgRNAs) were transfected into A549 cells (Supplementary Fig. 1). Next, the T7E1 assay was conducted to evaluate the site-specific cleavage. The substantial cleavage was detected in A549 PDHA1KO #1 cells (Fig. 1A and Supplementary Fig. 2), but not in the #2, #3, or #4 group (data not shown). In parallel with the T7E1 assay, PDHA1 protein expression completely disappeared in A549 PDHA1KO #1 cells (Fig. 1B). Sequence analysis also revealed that In/Del mutation occurred at the sgRNA-targeted site in A549 PDHA1KO #1 cells (Fig. 1C). To further ensure PDH knockout in transfected A549 cells, PDH activity was measured with or without DCA treatment. PDH activity was not restored by DCA in A549 PDHA1KO #1 cells indicating that the deletion of PDHA1 successfully blocks Acetyl-CoA synthesis (Fig. 1D). Thus, A549 PDHA1KO #1 cells were chosen for further study.
Fig. 1.
CRISPR/Cas9-mediated PDHA1 knockout in A549 cells. PDHA1-targeting lentiCRISPR vectors were transiently transfected into A549 cells as indicated. Untransfected and empty vector-transfected A549 cells (CON and EV, respectively) were used as a negative control. (A) Genomic DNA was extracted, and the target region of PDHA1 was amplified by polymerase chain reaction (PCR). PCR products were extracted and incubated with T7 Endonuclease Ⅰ. The fragments are indicated by the red arrow. (B) Total PDHA1 expression was detected by western blot. HSP90 was used as an internal control. (C) Sequencing analysis was performed with genomic DNA derived from A549 PDHA1KO (#1) cells. The sgRNA-target sequence is marked with a red line; the PAM sequence is marked with a blue line; the yellow and red arrows indicate the in/del mutation and cleavage site in A549 PDHA1KO (#1) cells, respectively. (D) EV and PDHA1KO (#1, #2, #3 and #4) A549 cells were treated with or without DCA (15 mM) for 24 h. PDHA1 activity was determined using a commercially available PDH activity kit. The relative activities to DCA-free EV cells were calculated and are presented as means ± SEM. ***P < 0.001.
The comparison of PDHA1WT and PDHA13SD A549 cells
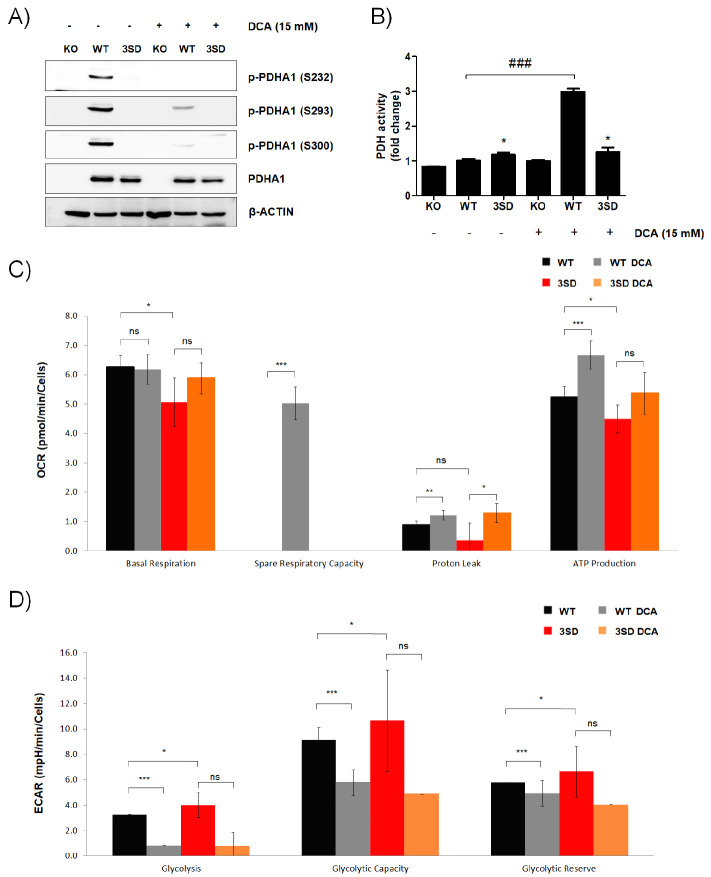
To establish the constitutively inactive PDHA1 vectors, three Ser residues - S232, S293 and S300 - were substituted with Asp to mimic their phosphorylations (18). WT and 3SD PDHA1 were re-introduced in A549 PDHA1KO #1 cells and named PDHA1WT and PDHA3SD cells, respectively. The phosphorylations of PDHA1 (S232, S293 and S300) were detected in PDHA1WT cells and phosphorylations of all Ser residues were suppressed by DCA (Fig. 2A). Following the phosphorylation, the PDH activity was increased by DCA in PDHA1WT cells, but not in PDHA13SD cells. However, the residual PDH activity of PDHA13SD cells was still observed regardless of DCA treatment (Fig. 2B). Next, OXPHOS levels, based on the oxygen consumption rate (OCR), were evaluated (Supplementary Fig. 3A). The basal respiration was higher in PDHA1WT cells than in PDHA13SD cells, and DCA significantly increased respiratory capacity in PDHA1WT cells compared to PDHA13SD cells. Moreover, PDHA1WT cells produced more ATP than PDHA3SD cells, and DCA significantly enhanced ATP production in PDHA1WT cells (Fig. 2C). Glycolysis was further examined by measuring the extracellular acidification rate (ECAR) (Supplementary Fig. 3B). The basal glycolytic capacity level was higher in PDHA13SD cells than in PDHA1WT cells, and DCA significantly reduced glycolytic capacity in PDHA1WT cells (Fig. 2D). These results indicate that ectopic PDHA1WT and PDHA13SD were successfully re-introduced into PDHA1KO cells and that PDHA13SD was less sensitive to DCA, a well-known PDK inhibitor.
Fig. 2.
Construction and analyses of phosphomimetic PDHA1-expressing A549 cells. PDHA1WT or phosphomimetic PDHA13SD were reintro-duced into PDHA1KO A549 cells. (A, B) PDHA1KO, PDHA1WT and PDHA13SD A549 cells were treated with DCA (15 mM) for 24 h as indicated. (A) The levels of phosphorylated serines in PDHA1 (S232, S293 and S300), and total PDHA1 were examined by western blot analysis. β-ACTIN was used as an internal loading control. (B) PDH activity was measured by a commercially available PDH activity assay kit. The relative activities on DCA-free PDHA1KO A549 cells were calculated and are shown as means ± SEM. *P < 0.05 compared with the KO control (1st line), ###P < 0.001 compared with the WT control (2nd line). (C, D) OCR (C) and ECAR (D) in PDHA1WT and PDHA13SD A549 cells were measured with or without DCA (15 mM) as indicated. Values are presented as means ± SD.
The activation of PDHA1 by different PDK inhibitors leads to different outcomes
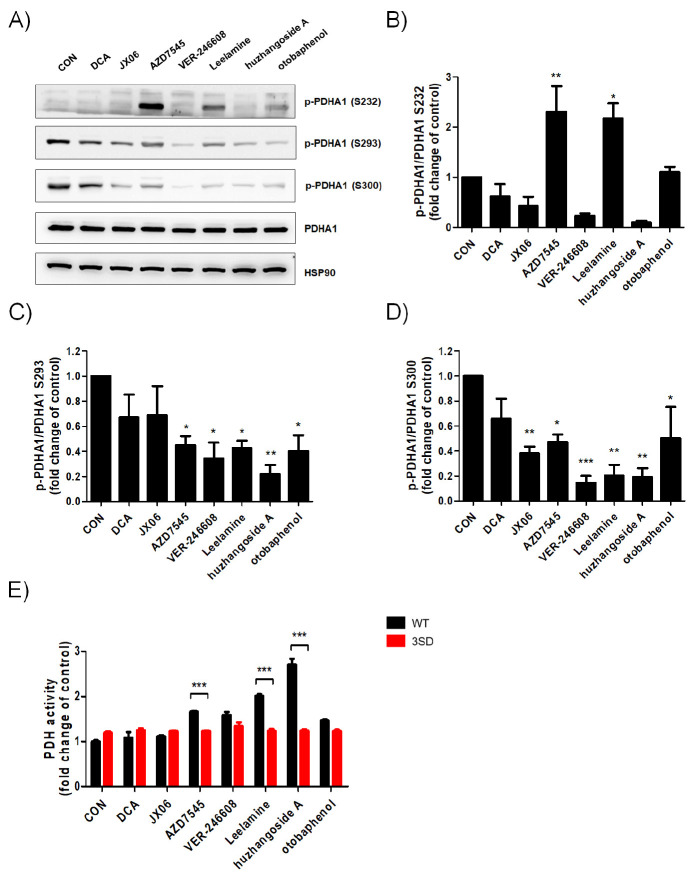
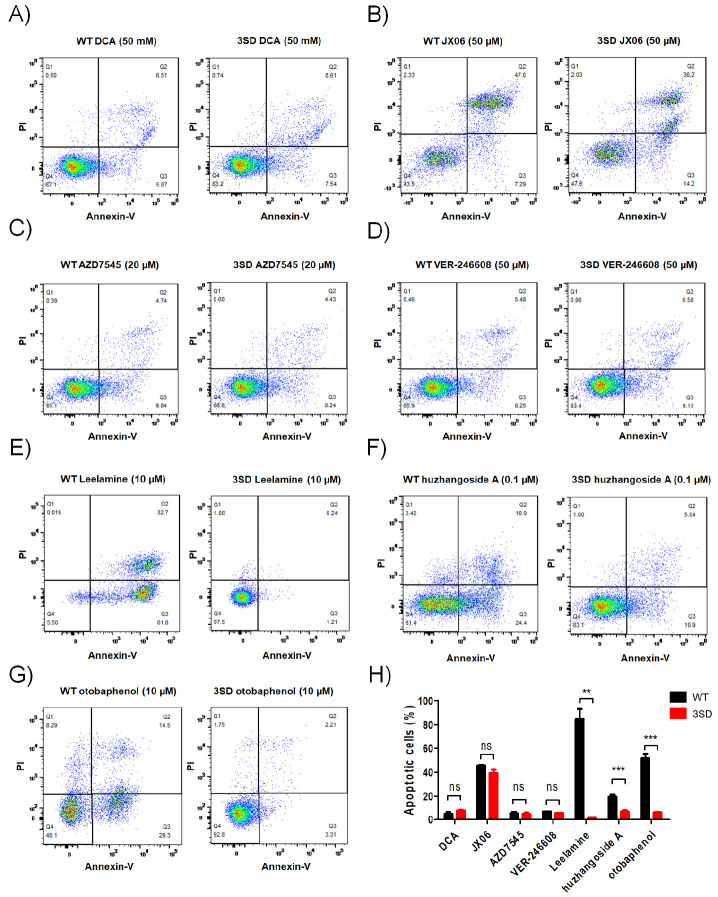
To analyze the correlation between PDHA1 phosphorylation status and activity alteration, the same concentration of PDK inhibitors of 10 μM was selected, except for huzhangoside A for which 1 μM was selected owing to its high cytotoxic sensitivity. AZD7545, VER-246608, Leelamine, huzhangoside A and otobaphenol markedly reduced the S293 and S300 phosphorylations of PDHA1 (Fig. 3A-D). Interestingly, S232 phosphorylation of PDHA1 was increased by AZD7545 and Leelamine. AZD7545, Leelamine, otobaphenol and huzhangoside A increased PDH activity in PDHA1WT cells that were inversely related to the S293 phosphorylation status of PDHA1 (Fig. 3E). However, dephosphorylations at S232 and S300 were not consistent with the enhancement of PDH activity (Fig. 3B, D, E). Additionally, PDH activity in PDHA13SD cells was not altered by PDK inhibitors (Fig. 3E). To further investigate whether cell death could be accompanied with PDH activation, cell viabil-ities were compared between PDK inhibitors treated PDHA1WT and PDHA3SD cells (Supplementary Fig. 4). Although the 50% cytotoxic concentration (CC50) was higher in PDHA13SD cells than in PDHA1WT cells, DCA, AZD7545 and VER-246608 were less toxic than other drugs, and JX06 showed similar CC50 in both PDHA1WT and PDHA13SD cells (Supplementary Table 3). Finally, apoptosis was measured by flow cytometry and the concentration of PDK inhibitors was determined as given in Sup-plementary Fig. 4. Consistent with the cell viability measurements, apoptosis was seldom induced by DCA, AZD7545 and VER-246608 (Fig. 4A-D). In contrast, Leelamine, huzhangoside A and otobaphenol-induced significantly higher apoptosis in PDHAWT cells than in PDHA13SD cells (Fig. 4E-G). Thus, Leela-mine, huzhangoside A and otobaphenol induced PDH activity dependent apoptosis. However, AZD7545, VER-246608 and DCA successfully enhanced PDH activity but failed to induce apoptotic cell death (Fig. 4H). Based on our results, PDH activation by different PDK inhibitors could lead to different outcomes. In summary, PDHA1WT and PDHA13SD cells can be used as a cell-based validation system for cancer treatment, however PDK inhibitors and the regulation of PDH activity requires more in-depth research.
Fig. 3.
Specificity assessments for targeting PDH using PDHA1-manipulated A549 cells. (A-D) PDHA1WT A549 cells were treated with DCA (10 μM), JX06 (10 μM), AZD7545 (10 μM), VER-246608 (10 μM), Leelamine (10 μM), huzhangoside A (1 μM) and otobaphenol (10 μM) for 4 h as indicated. (A) The levels of phosphorylated serines in PDHA1 (S232, S293 and S300) and total PDHA1 were examined by western blot analysis. HSP90 was used as an internal control. (B-D) The densitometric analyses (p-PDHA1/PDHA1) from three independent experiments were performed and are presented by means ± SEM. *P < 0.05, **P < 0.01 ***P < 0.001. (E) PDHA1WT and PDHA13SD A549 cells were treated with DCA (10 μM), JX06 (10 μM), AZD7545 (10 μM), VER-246608 (10 μM), Leelamine (10 μM), huzhangoside A (1 μM) and otobaphenol (10 μM) for 4 h as indicated. PDH activity was measured by a commercially available PDH activity assay kit. The relative activities to untreated PDHA1WT cells were calculated and are presented as means ± SEM. ***P < 0.001 compared with the WT control.
Fig. 4.
Validations for PDH-dependent apoptotic cell death using PDHA1-manipulated A549 cells. PDHA1WT (WT) and PDHA13SD (3SD) A549 cells were treated with DCA (50 mM; A), JX06 (50 μM; B), AZD7545 (20 μM; C), VER-246608 (50 μM; D), Leelamine (10 μM; E), huzhangoside A (0.1 μM; F) and otobaphenol (10 μM; G) for 24 h. The cells stained with Annexin Ⅴ and propidium iodide (PI) were analyzed by flow cytometry. (H) The percent frequency of Annexin Ⅴ-positive apoptotic cells is shown as means ± SEM. **P < 0.01; ***P < 0.001.
DISCUSSION
PDKs are potential targets for cancer and metabolic disease treatment (19). PDK1 functions as an oncogene that supports cancer cell proliferation and metastasis in non-small cell lung cancer (10). In our recent study, A549 cells were the most sensitive to ilimaquinone, one of our novel PDK inhibitors, among several cancer cell lines. Thus, we selected A549 cells to establish a cell-based screening system to validate PDH activity-dependent cell death. In addition, we aimed to discover new PDK inhibitors by evaluating our PDHA1WT and PDHA13SD cell-based screening system using well-known PDK inhibitors including DCA, JX06, AZD7545, VER-246608, Leelamine, huzhangoside A and otobaphenol. Therefore, at first these drugs were merely used to evaluate the PDHA1WT and PDHA13SD cells, rather than to validate their efficacy.
The PDC is a complex of three components, PDH (E1), dihydrolipoyl acetyltransferase (E2) and dihydrolipoyl dehydrogenase (E3) (20). The PDC activity is strictly regulated by two enzymes, PDK and PDP, mainly through phosphorylation (inhibition) and dephosphorylation (activation) of three well-known Ser residues (S232, S293 and S300) in PDHA1 (21). Phosphorylation(s) of any of the three sites can suppress PDH activity (22). In this study, we replaced all three Ser sites with Asp, and PDHA13SD was not affected by PDKs (Fig. 2B and 3C). Phosphorylation is responsible for rapid and timely inhibition, thus long-term phosphorylation, such as mutations, could result in unknown metabolic changes. In this study, there was residual PDH activity in PDHA13SD but not in PDHA1KO (Fig. 2B). A very recent study has shown that AMPK can activate PDC by phosphorylating S295 and S314 in PDHA1 (23). The level of p-AMPKα was higher in PDHA13SD cells than in PDHA1WT cells (data not shown). To this end, our data might reveal the complex regulation of PDH activity, more than the known regulating mechanism(s) by PDKs through the well-known three Ser residues. We suspect that the regulation of PDH activity requires more in-depth research. A vigorous study using our PDHA13SD cells could reveal the regulation of PDH activity by other pathway(s).
PDKs are engaged to the PDC by precise binding to the inner lipoamide domain of the PDH-E2/E3 binding protein (E3BP) core, and they effectively phosphorylate the PDHA1 (4). Elevated expressions of PDKs lead to a shift to glycolysis in cancer. PDK1 can phosphorylate all three phosphorylation sites, whereas PDK2, PDK3 and PDK4 can phosphorylate just two sites, S293 and S300 (24). The phosphorylation at S293 was faster than that at S300 and S232 (25), and the deac-tivating effect of phosphorylation at S300 or S232 was weaker than that at S293 (26). A previous study reported that reduction of phosphorylations at S232 and S300 did not affect PDH activity (27). Therefore, S293 is the major site for regulating PDH activity by phosphorylation (28). Consistent with many previous studies, our data indicate that the enhancement of PDH activity was most closely related to phosphorylation at S293 (Fig. 3). In this study, we found that phosphorylation of S232 was increased, rather than reduced, by AZD7545 and Leelamine through an unknown underlying mechanism(s) (Fig. 3B, C). Thus far, the mechanism of PDH activity regulation by phosphorylation at S232 and/or S300 in PDHA1 remains unclear.
There are four binding sites in PDKs those are critical for its activity regulation, including the pyruvate-binding domain (N-ter-minal regulatory domain), lipoamide-binding domain, nucleotide-binding domain (C-terminal catalytic domain) and allosteric CoA-binding site (29). DCA (30) binds to pyruvate-binding pocket; AZD7545 (31) targets the lipoamide-binding pocket; while JX06 (32), VER-246608 (33) and huzhangoside A (14) target the ATP-binding pocket. Although DCA is the most investigated PDK1 inhibitor, its enzymatic inhibition of PDKs requires the millimolar level, whereas the enzymatic inhibitions of JX06, AZD7545 and VER-246608 to PDKs are enough for nanomolar scales (Supplementary Table 4). However, the anti-cancer efficacy of VER-246608 and AZD7545 seemed obscure, consistent with previous studies (33, 34), and JX06 was little specific to PDHA1WT cells than to PDHA13SD cells. In another aspect, the in vitro inhibitory efficacy of huzhangoside A and otobaphenol on PDKs activity has not been explored. Meanwhile, AZD7545 and VER-2466608 sufficiently increased PDH activity without inducing severe cancer cell death. These results indicate that the correlation between PDH activation and acti-vity-related cancer cell death by PDK inhibitors could be poor, and more studies are necessary to confirm such correlation.
This study is not intended to validate the effectiveness of drugs, such as DCA, JX06, AZD7545, VER-246608, Leelamine and otobaphenol, and has several other limitations. First, only one NSCLC cell line was used. A further extensive study using various types of human cancer cells could solidify our cell-based validation system for cancer treatable PDK inhibitors. Second, the link between PDH activation and its activity-related cancer cell death induced by PDK inhibitors is unclear. Third, another way of PDH activity regulation(s) (except for S232, S293 and S300) by investigated PDK inhibitors is still possible. This also requires further experiments. Clinical use of all current PDK inhibitors face the challenge of pharmacokinetics, potency, selectivity and efficacy (19). Despite these limitations, our study can contribute to new insights and strategies for developing of efficient and low-toxic PDK inhibitors. In conclusion, phosphomimetic PDHA13SD reveals the complexity of its activity regulation, and PDHA1WT and PDHA13SD A549 cells can be used as a cell-based PDK inhibitor-evaluation system for cancer treatment and a valuable tool for PDHA1 biochemical research in cancer cell biology.
MATERIALS AND METHODS
Cell culture
A549 cells (Korean Cell Line Bank, Seoul, Korea; 10185) were cultured in RPMI 1640 medium (Welgene, Gyeongsan, Korea) with 10% heat-inactivated fetal bovine serum (FBS; Gibco, Thermo Fisher Scientific, Waltham, MA) and 1% penicillin/streptomycin (Gibco). Platinum-A (Plat-A) (Cell Biolabs, San Diego, CA; RV-102) cells were grown in Dulbecco’s Modified Eagle’s Medium (Welgene) supplemented with 10% FBS (Gibco) and 1% penicillin/streptomycin and selected by 5 μg/ml puromycin (Sigma-Aldrich) and 10 μg/mL blasticidin (Sigma-Aldrich) prior to produce retroviruses. All cells were incubated at 37°C in with 5% CO2.
Cellular energy metabolism by Seahorse XFe96 Analyzer
The OCR and ECAR were measured using an XFe96 Extracellular Flux Analyzer (Seahorse Bioscience, Billerica, MA) according to the manufacturer’s protocol. Briefly, cells were seeded in XFe96 culture plates at a density of 1.6 × 104 cells per well. The next day, the medium was replaced with XF base medium (pH 7.4, Seahorse Biosciences) supplemented with 25 mM D-glucose (Sigma-Aldrich; G7528), 1 mM sodium pyruvate (Sigma-Aldrich; S8636) and 1 X GlutaMAX (Gibco; 35050), or with XF Base Media (pH 7.4, Seahorse Biosciences) with 1 mM glutamine (Sigma-Aldrich; G8540) following the manufacturer’s protocol. To measure the OCR and ECAR, the compounds and metabolites used in this study were as follows: DCA (15 mM, Sigma-Aldrich; 347795), oligomycin A (1 μM, Sigma-Aldrich; 75351), trifluoromethoxy carbonylcyanide phenylhydrazone (FCCP; 2 μM, Sigma-Aldrich; C2920), rotenone (1 μM, Sigma-Aldrich; R8875) and antimycin A (1 μM, Sigma-Aldrich; A8674); D-glucose (10 mM, Sigma-Aldrich; G7528), oligomycin A (2 μM, Sigma-Aldrich; O75351) and 2-deoxy-D-glucose (2-DG; 50 mM, Sigma-Aldrich; D6134), for OCR and ECAR, respectively. For the normalization, DAPI stained cells were counted by the microscopy automatically (BioTek Lionheart FX, The Netherlands).
Detection of apoptotic cells by flow cytometry
PDHA1WT and PDHA13SD A549 cells were treated with various concentrations of drugs for 24 h. Apoptotic cells were examined using an apoptosis detection kit (BD Biosciences, San Jose, CA), detected by an Attune Acoustic Focusing Cytometer (Invitrogen), and analyzed using FlowJo software.
Statistical analysis
The CC50 of the drugs was calculated by excel curve fitting. The other analyses were carried out by GraphPad Prism software (GraphPad Software, San Diego, CA). The difference between the mean values of each group was analyzed using Student’s t-test, and multi comparisons among groups were analyzed using one-way analysis of variance with Dennett. The minimum level of statistical significance was set at a P < 0.05 for all the analyses. All the experiments were independently conducted thrice. Further details were shown in the supplementary material.
SUPPLEMENTARY MATERIALS
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF) grants funded by the Korean government (MIST; 2020R1C1C1003703 to Sung-Jin Bae, 2019R1A2C2003624 and 2021R1A4A1025662 to Ki-Tae Ha).
Footnotes
AUTHOR CONTRIBUTION
S.-J.B. and K.-T.H. conceived and supervised the project. L.J. performed the majority of experiments and collected the data. L.J. and M.C. performed the FACS analysis. B.-S.K. and J.H.H. cloned vectors used for genome editing and reconstructing PDHA1WT and PDHA13SD. S.P. and I.K.L examined OCR and ECAR. L.J. and S.-J.B. wrote the manuscript. D.R., J.H.K, I.K.L. and K.-T.H analyzed the data and revised the manuscript. All the authors reviewed the manuscript and agreed to the submission.
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Zheng J. Energy metabolism of cancer: glycolysis versus oxidative phosphorylation. Oncol Lett. 2012;4:1151–1157. doi: 10.3892/ol.2012.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kroemer G, Pouyssegur J. Tumor cell metabolism: Cancer's Achilles' heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 5.Pelicano H, Martin D, Xu R, and Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- 6.Jeoung NH. Pyruvate dehydrogenase kinases: therapeutic targets for diabetes and cancers. Diabetes Meta-b J. 2015;39:188. doi: 10.4093/dmj.2015.39.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golias T, Papandreou I, Sun R, et al. Hypoxic repression of pyruvate dehydrogenase activity is necessary for metabolic reprogramming and growth of model tumours. Sci Rep. 2016;6:1–11. doi: 10.1038/srep31146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolobova E, Tuganova A, Boulatnikov I, Popov KM. Regulation of pyruvate dehydrogenase activity through phosphorylation at multiple sites. Biochem J. 2001;358:69–77. doi: 10.1042/bj3580069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schell JC, Olson KA, Jiang L, et al. A role for the mitochondrial pyruvate carrier as a repressor of the Warburg effect and colon cancer cell growth. Mol Cell. 2014;56:400–413. doi: 10.1016/j.molcel.2014.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu T, Yin H. PDK1 promotes tumor cell proliferation and migration by enhancing the Warburg effect in non-small cell lung cancer. Oncol Rep. 2017;37:193–200. doi: 10.3892/or.2016.5253. [DOI] [PubMed] [Google Scholar]
- 11.Stacpoole PW. Therapeutic targeting of the pyruvate dehydrogenase complex/pyruvate dehydrogenase kinase (PDC/PDK) axis in cancer. JNCI: J Natl Cancer Inst. 2017;109:11. doi: 10.1093/jnci/djx071. [DOI] [PubMed] [Google Scholar]
- 12.Sun W, Xie Z, Liu Y, et al. JX06 selectively inhibits pyruvate dehydrogenase kinase PDK1 by a covalent cysteine modification. Cancer Res. 2015;75:4923–4936. doi: 10.1158/0008-5472.CAN-15-1023. [DOI] [PubMed] [Google Scholar]
- 13.Jeoung NH, Harris RA. Role of pyruvate dehydrogenase kinase 4 in regulation of blood glucose levels. Diabetes Metab J. 2010;34:274. doi: 10.4093/kdj.2010.34.5.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwak CH, Lee JH, Kim EY, et al. Huzhangoside A suppresses tumor growth through inhibition of pyruvate dehydrogenase kinase activity. Cancers. 2019;11:712. doi: 10.3390/cancers11050712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwak CH, Jin L, Han JH, et al. Ilimaquinone induces the apoptotic cell death of cancer cells by reducing pyruvate dehydrogenase kinase 1 activity. Int J Mol Sci. 2020;21:6021. doi: 10.3390/ijms21176021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin L, Kim EY, Chung T-W, et al. Hemistepsin A suppresses colorectal cancer growth through inhibiting pyruvate dehydrogenase kinase activity. Sci Rep. 2020;10:1–12. doi: 10.1038/s41598-020-79019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guerra-Castellano A, Díaz-Moreno I, Velázquez-Campoy A, Miguel A, Díaz-Quintana A. Structural and functional characterization of phosphomimetic mutants of cytochrome c at threonine 28 and serine 47. Biochim Biophys Acta-Bioenerg. 2016;1857:387–395. doi: 10.1016/j.bbabio.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Hitosugi T, Fan J, Chung TW, et al. Tyrosine phosphorylation of mitochondrial pyruvate dehydrogenase kinase 1 is important for cancer metabolism. Mol Cell. 2011;44:864–877. doi: 10.1016/j.molcel.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Shen X, Yan Y, Li H. Pyruvate dehydrogenase kinases (PDKs): an overview toward clinical applications. Biosci Rep. 2021;41:BSR20204402. doi: 10.1042/BSR20204402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McFate T, Mohyeldin A, Lu H, et al. Pyruvate dehydrogenase complex activity controls metabolic and malignant phenotype in cancer cells. J Biol Chem. 2008;283:22700–22708. doi: 10.1074/jbc.M801765200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmer AD, Walbrecq G, Kozar I, Behrmann I, Haan C. Phosphorylation of the pyruvate dehydrogenase complex precedes HIF-1-mediated effects and pyruvate dehydrogenase kinase 1 upregulation during the first hours of hypoxic treatment in hepatocellular carcinoma cells. Hypoxia. 2016;4:135. doi: 10.2147/HP.S99044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.SALE GJ, Randle PJ. Analysis of site occupancies in [32P] phosphorylated pyruvate dehydrogenase complexes by aspartyl‐prolyl cleavage of tryptic phosphopeptides. Eur J Biochem. 1981;120:535–540. doi: 10.1111/j.1432-1033.1981.tb05733.x. [DOI] [PubMed] [Google Scholar]
- 23.Cai Z, Li CF, Han F, et al. Phosphorylation of PDHA by AMPK drives TCA cycle to promote cancer metastasis. Mol Cell. 2020;80:263–278.:e267. doi: 10.1016/j.molcel.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.adhanjali S, Sr, Reddy MM. Inhibition of pyruvate dehydrogenase kinase as a therapeutic strategy against cancer. Curr Top Med Chem. 2018;18:444–453. doi: 10.2174/1568026618666180523105756. [DOI] [PubMed] [Google Scholar]
- 25.Korotchkina LG, Patel MS. Mutagenesis studies of the phosphorylation sites of recombinant human pyruvate dehydrogenase. Site-specific regulation. J Biol Chem. 1995;270:14297–14304. doi: 10.1074/jbc.270.24.14297. [DOI] [PubMed] [Google Scholar]
- 26.Korotchkina LG, Patel MS. Site specificity of four pyruvate dehydrogenase kinase isoenzymes toward the three phosphorylation sites of human pyruvate dehydrogenase. J Biol Chem. 2001;276:37223–37229. doi: 10.1074/jbc.M103069200. [DOI] [PubMed] [Google Scholar]
- 27.Jing E, O'Neill BT, Rardin MJ, et al. Sirt3 regulates metabolic flexibility of skeletal muscle through reversible enzymatic deacetylation. Diabetes. 2013;62:3404–3417. doi: 10.2337/db12-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kato M, Wynn RM, Chuang JL, et al. Structural basis for inactivation of the human pyruvate dehydrogenase complex by phosphorylation: role of disordered phosphorylation loops. Structure. 2008;16:1849–1859. doi: 10.1016/j.str.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeoung NH. Pyruvate dehydrogenase kinases: therapeutic targets for diabetes and cancers. Diabetes Metab J. 2015;39:188–197. doi: 10.4093/dmj.2015.39.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Kato M, Chuang DT. Pivotal role of the C-terminal DW-motif in mediating inhibition of pyruvate dehydrogenase kinase 2 by dichloroacetate. J Biol Chem. 2009;284:34458–34467. doi: 10.1074/jbc.M109.065557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kato M, Li J, Chuang JL, Chuang DT. Distinct structural mechanisms for inhibition of pyruvate dehydrogenase kinase isoforms by AZD7545, dichloroacetate, and radicicol. Structure. 2007;15:992–1004. doi: 10.1016/j.str.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun W, Xie Z, Liu Y, et al. JX06 selectively inhibits pyruvate dehydrogenase kinase PDK1 by a covalent cysteine modification. Cancer Res. 2015;75:4923–4936. doi: 10.1158/0008-5472.CAN-15-1023. [DOI] [PubMed] [Google Scholar]
- 33.Moore JD, Staniszewska A, Shaw T, et al. VER-246608, a novel pan-isoform ATP competitive inhibitor of pyruvate dehydrogenase kinase, disrupts Warburg metabolism and induces context-dependent cytostasis in cancer cells. Oncotarget. 2014;5:12862. doi: 10.18632/oncotarget.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayers R, Butlin R, Kilgour E, et al. AZD7545, a novel inhibitor of pyruvate dehydrogenase kinase 2 (PDHK2), activates pyruvate dehydrogenase in vivo and improves blood glucose control in obese (fa/fa) Zucker rats. Biochem Soc Trans. 2003;31:1165–1167. doi: 10.1042/bst0311165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.