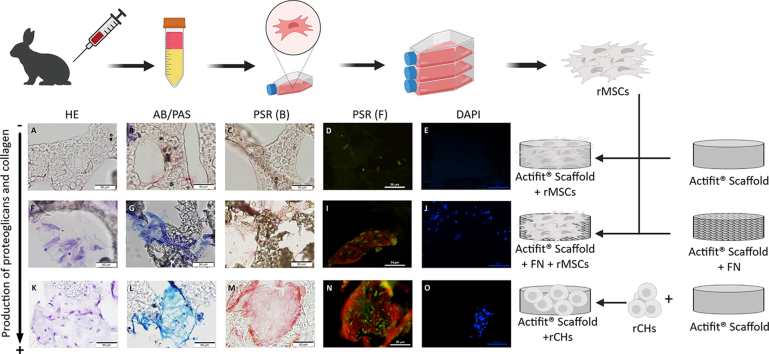
Abstract
Introduction
Partial meniscectomy is one of the most common surgical strategy for a meniscal injury, but sometimes, patients complain of knee pain due to an overload in the ablated compartment. In these cases, implantation of tissue engineering scaffold could be indicated. Currently, two commercial scaffolds, based on collagen or polycaprolactone-polyurethane (PCL-PU), are available for meniscus scaffolding. In short term follow-up assessments, both showed clinical improvement and tissue formation. However, long-term studies carried out in PCL-PU showed that the new tissue decreased in volume and assumed an irregular shape. Moreover, in some cases, the scaffold was totally reabsorbed, without new tissue formation.
Mesenchymal stem cells (MSCs) combined with scaffolds could represents a promising approach for treating meniscal defects because of their multipotency and self-renewal. In this work, we aimed to compare the behaviour of MSCs and chondrocytes on a PCL-PU scaffold in vitro. MSCs express integrins that binds to fibronectin (FN), so we also investigate the effect of a FN coating on the bioactivity of the scaffold.
Methods
We isolated rabbit bone marrow MSCs (rBM-MSCs) from two skeletally mature New Zealand white rabbits and stablished the optimum culture condition to expand them. Then, they were seeded over non-coated and FN-coated scaffolds and cultured in chondrogenic conditions. To evaluate cell functionality, we performed an MTS assay to compare cell proliferation between both conditions. Finally, a histologic study was performed to assess extracellular matrix (ECM) production in both samples, and to compare them with the ones obtained with rabbit chondrocytes (rCHs) seeded in a non-coated scaffold.
Results
A culture protocol based on low FBS concentration was set as the best for rBM-MSCs expansion. The MTS assay revealed that rBM-MSCs seeded on FN-coated scaffolds have more cells on proliferation (145%; 95% CI: 107%–182%) compared with rBM-MSCs seeded on non-coated scaffolds. Finally, the histologic study demonstrated that rCHs seeded on non-coated scaffolds displayed the highest production of ECM, followed by rBM-MSCs seeded on FN-coated scaffolds. Furthermore, both cell types produced a comparable ECM pattern.
Conclusion
These results suggest that MSCs have low capacity attachment to PCL-PU scaffolds, but the presence of integrin alpha5beta1 (FN-receptor) in MSCs allows them to interact with the FN-coated scaffolds. These results could be applied in the design of scaffolds, and might have important clinical implications in orthopaedic surgery of meniscal injuries.
Keywords: Meniscal injuries, Post-meniscectomy syndrome, Tissue engineering, Scaffolds, Fibronectin, Mesenchymal stem cell
Abbreviations: PCL-PU, polycaprolactone-polyurethane; MSCs, mesenchymal stem cells; FN, fibronectin; rBM, rabbit bone marrow; rCHs, rabbit chondrocytes; ECM, extracellular matrix; AMT, allograft meniscus transplantation; CMI, collagen meniscal implant; MNCs, mononuclear cells; RT, room temperature; ITS, Insulin Transferrin Selenium; PSR, picrosirius red
Graphical abstract
Highlights
-
•
Cultures with low FBS are more suitable to isolation and expansion of rBM-MSC.
-
•
PCL-PU scaffolds coated with FN show improve adhesion properties for rBM-MSCs.
-
•
rBM-MSCs seeded in PCL-PU + FN produce ECM similar to the one produced by chondrocytes.
1. Introduction
Meniscal injury is one of the most repeatedly treated damage in orthopaedic surgery. Meniscal tissue has limited ability to heal because of its low cellularity, dense ECM, and poor vascularization. In case of meniscal tear, meniscal repair is the preferred treatment to preserve a healthy joint. However, the torn tissue is often removed to ameliorate symptoms and some knees do not tolerate meniscectomy, resulting in the so-called post-meniscectomy syndrome (unicompartmental pain without significant articular cartilage wear) [1]. Therefore, several approaches have been proposed to substitute the missing tissue, including allograft meniscus transplantation (AMT) and tissue engineering.
While AMT is a reliable therapeutic option, its limited source and specific issues related to tissue banking policies make this treatment difficult in some countries. As for tissue engineering, several materials have been tested to construct meniscal scaffolds, which are mainly made of ceramic, biological, or synthetic polymers. All have specific advantages and disadvantages; therefore, new strategies consist in using composite scaffolds comprising different materials [2]. To enhance their biocompatibility, synthetic polymers are often covered with proteins (such as collagen, gelatine, fibronectin, and laminins), and/or peptides containing pro-adhesive sequences. These modifications enhance their physicochemical, mechanical, and degradability properties [3].
Currently, only two commercial scaffolds are available for meniscus repair: a collagen and glycosaminoglycan scaffold (Collagen Meniscal Implant (CMI®)) and a polycaprolactone-polyurethane (PCL-PU) scaffold (Actifit®). Both have bioresorbable and biocompatible characteristics, showing easy reabsorption and allowing adherence and proliferation of fibroblast and chondrocytes. CMI® is more biocompatible than Actifit®, but it also exhibits faster reabsorbing rate, leading to the disappearance of the scaffold before new tissue formation. In both cases, short term follow-up assessments showed a clinical improvement with meniscal-like tissue formation and presence of fibrochondrocytes [[4], [5], [6], [7]]. Cohort studies with 5 years or longer follow-up demonstrated that clinical improvement was maintained. However, the repaired meniscus area did not show a healthy morphology. Indeed, it showed a decrease in tissue volume, an irregular shape, and in some cases total reabsorption of the scaffold without new tissue formation [[8], [9], [10]]. Therefore, it might be assumed that scaffolds delay the clinical worsening, but the adverse effects at long term will be similar to those observed in partial meniscectomies.
Mesenchymal stem cells (MSCs) are multipotent stem cells that can differentiate into various cell lineages derived from the mesoderm. This characteristic, together with the fact that MSCs, in contrast to some differentiate cells, can be expanded in vitro, makes them a good resource for new tissue regenerative therapies. According to the U.S. National Institute of Health, MSCs are being used in more than 800 clinical trials for various conditions, including bone and cartilage defects [11]. However, no legislative authority has yet approved their use for the treatment of any disease. MSCs are a promising therapy for meniscal repair because they are able to differentiate into the corresponding cells, and to produce growth factors that induce tissue repair. Preclinical trials showed that the use of MSCs enhanced the repair of meniscal defects. These studies used fibrin clots, scaffold-free engineered meniscal tissue, and cell-seeded scaffolds, in combination with MSCs. The latter seem to be the most useful tool to ensure support long-term effect of the MSCs at the site of the defect [12].
A promising approach for meniscus repair could be to use a combination of MSCs and scaffolds with optimal degradation/reabsorption rate and bioactivity. MSCs lack several receptors already present in differentiated cells, limiting the binding to some surfaces. However, expression of fibronectin (FN) receptor have been reported in MSCs [13]. Therefore, the use of FN-coated scaffolds could increase their biocompatibility. The aim of the current work was to investigate in vitro the ability of rabbit MSCs to proliferate and differentiate into functional chondrocytes on a FN-coated PCL-PU scaffold. First, we assessed the multipotency of rabbit bone marrow mesenchymal stem cells (rBM-MSCs). Then, we evaluated the bioactivity of the modified scaffold by investigating the capability of MSCs to adhere to the surface and proliferate, differentiate, and produce an ECM that mimics the meniscal tissue. We hypothesized that FN improved the capacity of MSCs to adhere to the scaffold and did not impair their ability to differentiate into chondrocytes and produce ECM.
2. Materials and methods
2.1. Isolation and characterization of rBM-MSCs
To harvest MSCs, we used two skeletally mature New Zealand white rabbits. They were fully sedated by intra-muscular injection of Ketamine (35 mg/kg) and Xylazine (5 mg/kg), followed by sevoflurane inhalation (2%, rate 2 litres/min). Then, we performed a medial parapatellar approach on the right knee of the animals. After dislocating the kneecap, we made a puncture on the medial femoral condyle with an 18 G hypodermic needle. We aspirated the rabbit bone marrow (rBM) while making rotational needle movements. Finally, we collected the rBM in a syringe with citrate to avoid coagulation. The protocol including all animal care and experimental procedures was approved by the Ethical Committee of Animal Experimentation of our institution (CEEA-PRBB) and by the competing regional authorities.
We purified rBM mononuclear cells (MNCs) following the SepMate™ isolation tubes protocol. Briefly, after aspiration, rBM was diluted with an equal volume of PBS + 2% FBS and gently mixed. Such cell dilution was pulled on the SepMate™ tube previously filled with Ficoll. The tube was then centrifuged at 1200 g for 10 min at room temperature (RT). The enriched MNCs fraction was washed twice with 10% PBS + 2% FBS and centrifuged for 10 min at 400 g at RT. The obtained cells were then seeded at a density of 2 × 105/cm2 and their media replaced every 3–4 days, until the cells reached an 80%–90% confluence (8–10 days). At this point, highly enriched rBM-MSCs culture was obtained [[14], [15], [16]]. Therefore, rBM-MSCs were trypsinised and seeded in a 75 cm2 flask at a concentration of 5·104 cells/cm2. To expand them, rBM-MSCs were cultured with DMEM supplemented with 10% FBS for 14 days. Alternatively, they were cultured with StemPro® MSC SFM XenoFree (Gibco, life technologies) for 3 days and then with MesenPRO RS™ Medium (Gibco, life technologies) for 11 days. Cell media were replaced every 3–4 days. For our experiments, we only used cells from the first and the second passages.
The MSC multipotency test was performed in triplicate using commercial kits: “StemPro® Osteogenesis Differentiation Kit”, “StemPro™ Chondrogenesis Differentiation Kit”, and “StemPro™ Adipogenesis Differentiation Kit” (Gibco).
Cell count was performed with Neubauer Chamber using Trypan Blue exclusion and Flow Cytometry. For flow cytometry, we used an internal microsphere CountBright™ counting standard (Thermo Fischer Scientific), with settling properties similar to lymphocytes. We carried out each quantification in triplicate.
2.2. Establishment of rabbit chondrocyte (rCHs) culture
Cells were thawed from frozen stocks obtained from previous works [17]. rCHs were seeded in a 75 cm2 flask with a density of 5 × 104 cells/cm2 in DMEM medium supplemented with 10% FBS and 50 μg/ml Ascorbic Acid. They were grown for 14 days at 37 °C with 5% CO2 and 60% of relative humidity. The medium was replaced twice per week. For our experiments, we only used cells from the first and the second passages.
2.3. Scaffold preparation, cell seeding and culture
We cut a cylindrical piece with a diameter of 4 mm and a height of 2 mm from a commercial Actifit® structure. We sterilized the scaffolds by increasing ethanol concentration batch (50%, 70%, and absolute ethanol). Afterwards, they were washed three times in PBS, and finally immersed in DMEM medium for 24 h. For the scaffolds to be coated with FN, the DMEM medium included 1% FN. Scaffolds were then let dry for 24 h.
Cultured cells (rCHs or rBM-MSCs) reaching a confluence of 80–90%, were harvested and resuspended in DMEM at a concentration of 1 × 106 cells/μl. Afterwards, cells were seeded on sterilized scaffolds at a concentration of 5 × 107 cells/cm3 and cultured in wells of a non-adherent 48-well plate. rBM-MSCs were cultured with chondrocyte differentiation medium. Such medium consisted in DMEM supplemented with 10 μl/ml Insulin Transferrin Selenium (ITS), 50 μg/ml ascorbic acid, 10−7 M Dexamethasone, and 10 ng/ml TGF-β. For chondrocytes cultures on scaffolds, the culture medium used consisted in DMEM supplemented with 10% FBS and 50 μg/ml ascorbic acid. Cells were cultured for 3 weeks and medium was changed 3 times per week. A total of six scaffolds were cultured for each condition.
2.4. Evaluation of cell proliferation
To assess cell viability and proliferation on each scaffold, we performed an MTS assay (Abcam). Briefly, 10% MTS reagent was added to cell culture media and incubated for 3 h in standard culture conditions. Then, we briefly shook the plate and measured the absorbance at 490 nm. We evaluated each sample in triplicate.
2.5. Scaffold colonization and evaluation of ECM production
We evaluate the diffusion of the cells through the scaffold and their ECM production in three different conditions: FN-coated scaffold seeded with rBM-MSCs (FN-coated + rBM-MSCs) and non-coated scaffold seeded with rBM-MSCs (non-coated + rBM-MSCs) or rCHs (non-coated + rCHs). The evaluation was performed by histology study. Samples were fixed overnight with 10% formalin, and then put in a 15% sucrose (in PBS) bath, for 6 h. Finally, they were kept in a 30% sucrose bath for 18 h. Afterwards, scaffolds were embedded in OCT, cooled down in a bath of dry ice and isopropanol, and frozen at −20 °C. Three OCT blocks were prepared for each combination of cell + scaffold.
We obtained 4 μm transversal sections with Cryostat (Leica CM3050 S). These sections were then stuck to SuperFrostPlus® slides. Stains were performed in horizontal racks to avoid losing material because of the low adherence of the scaffold to the slide glass. We finally performed the following evaluations:
-
•
Scaffold colonization. We evaluated cell spreading along the scaffold through Haematoxylin – Eosin and DAPI staining. Sections were stained in Mayer's haematoxylin solution (30%) (Sigma Aldrich diagnostics®) for 15 s to 1 min. Afterwards, they were washed in running tap water for 10 min, soaked three times in 80% ethanol + 0.15% Hydrochloric Acid (HCl), and three times in 0.3% ammonia water. Then the samples were rinsed in distilled water and washed 5 min in 95% ethanol before counterstaining in 0.5% eosin Y alcoholic (Bio-Optica) solution for 15–30 s. Finally, slides were dehydrated and mounted with Dibutylphalate Polystyrene Xylene (DPX) new medium. For DAPI, the slides were washed with TPBS, mounted with an aqueous mounting agent containing DAPI (1:200).
-
•
Collagen stain. Sections were stained in Weighert's haematoxylin solution (Sigma–Aldrich) for 15 s to 1 min and washed in running tap water for 10 min. Then, they were stained for 1 h in picrosirius red (PSR) solution (Sirius red F3B Sigma–Aldrich “Direct Red 80”) in saturated aqueous picric acid (Sigma Aldrich), pH 2. Afterwards, the sections were washed twice in acidified water (0.1 N HCl), and three times in absolute ethanol (5 min each). Finally, they were cleared in xylene for 5 min and mounted with DPX new medium.
-
•
Proteoglycan stain. Sections were stained 5 min in 1% alcian blue (Merck Millipore) in 3% acetic acid. Then, they were soaked in 0.5% aqueous periodic acid solution for 5 min and, finally, they were bathed for 15 min in Schiff's reagent (Merck Millipore). At every step, the slides were washed with tap water for 3 min and rinsed in distilled water. Finally, they were stained with haematoxylin solution modified according to Gill III (Merck Millipore) for 20 s and washed for 3 min with tap water. After dehydration, samples were mounted with DPX new medium.
To observe the samples, we used an Automated Upright Microscope BX61 in bright light and took pictures with an Olympus digital camera using the software cell Sens Standard. We also observed the samples under an Epifluorescence Eclipse Ni-E Microscope. For PSR staining observation, we used 2 filters: FITC, and TxRed. Pictures were captured at 20× and 40× magnifications with a Nikon digital camera and processed with the photo program software Nikon NIS-E Advanced Research.
3. Results
3.1. Isolation of rBM-MSCs and multilineage differentiation
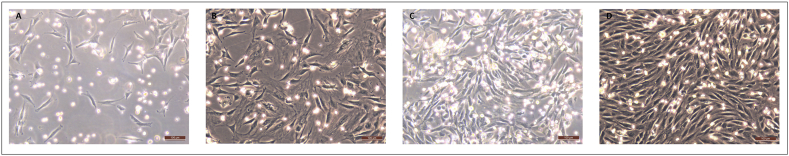
MNCs were obtained from the rBMs and cultured, showing the expected morphological features under light microscopy (Fig. 1). Surprisingly, while progressing to MSCs, cell morphology gradually became non-fibroblastic one (Fig. 2A–D). To discard the effect of possible differentiation inductors in the serum, we tested three different batches of FBS in the culture media. We did not observe any differences in cell morphology so differentiation inductors, if any, were shared by the three sera. To avoid the effect of FBS, we checked a culture protocol with low FBS concentration. rBM-MSCs were cultured in both cell culture conditions. Cells cultured in medium supplemented with FBS were fewer (approximately 25%) and had a larger size than cells cultured with low FBS concentration, although cultures were at similar confluence conditions (Fig. 2, Fig. 3). Furthermore, cells cultured with low FBS concentration showed the fusiform morphology and digital expansions typical for MSCs (Fig. 2E–H). Finally, we used the culture protocol with low FBS concentration for the subsequent isolation of rBM-MSCs.
Fig. 1.
MNCs' morphology observed at 4 (A), 7 (B), 9 (C), and 11 (D) days after BM aspiration. Culture media was DMEM+10% FBS. Magnification bar: 100 μm.
Fig. 2.
rBM-MSCs’ morphology observed at 4 (A, E), 7 (B, F), 9 (C, G), and 11 (D, H) days after mononuclear cell culture trypsinization. Culture media was DMEM+10%FBS in the upper row images (A–D), and low-FBS media (see Materials and Methods) in the lower row images (E–H). Magnification bar: 100 μm.
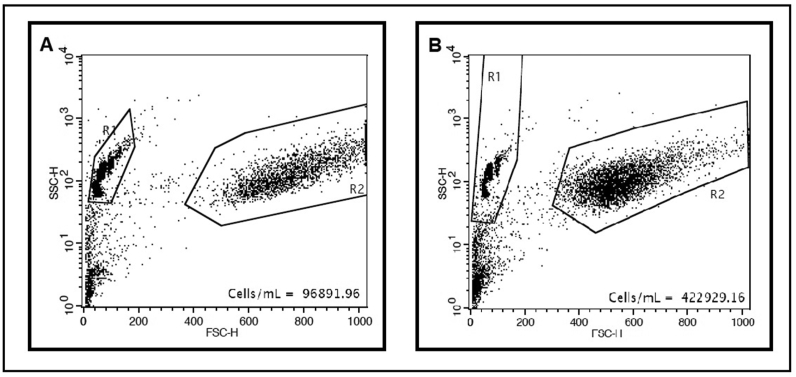
Fig. 3.
Cytometry results and cell count of rBM-MSCs cultured in DMEM+10%FBS (A) and low-FBS media (B) after 11 days of culture.
Before seeding the rBM-MSCs on the scaffolds, we assess their stemness by multipotency test and achieved differentiation to the three linages (chondrocytes, osteocytes, and adipocytes) (Suppl. Fig. 1).
3.2. Adhesion and proliferation of rBM-MSCs on non-coated and FN-coated scaffolds
Although rBM-MSCs were able to attach and proliferate both on non-coated and FN-coated scaffolds, these features were improved on the FN-coated scaffold. MTS assay showed that, 14 days after cell seeding, the number of proliferating cells present on FN-coated scaffolds were 145% (95% CI 107%–182%) higher than the proliferating cells grown on non-coated scaffolds.
3.3. Evaluation of rBM-MSCs differentiation potential and functionality cell spreading and ECM production
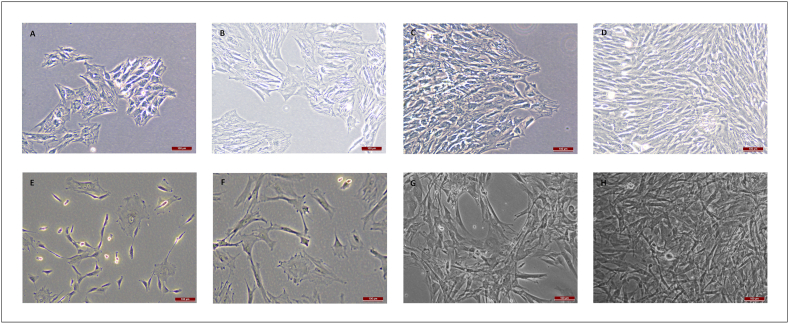
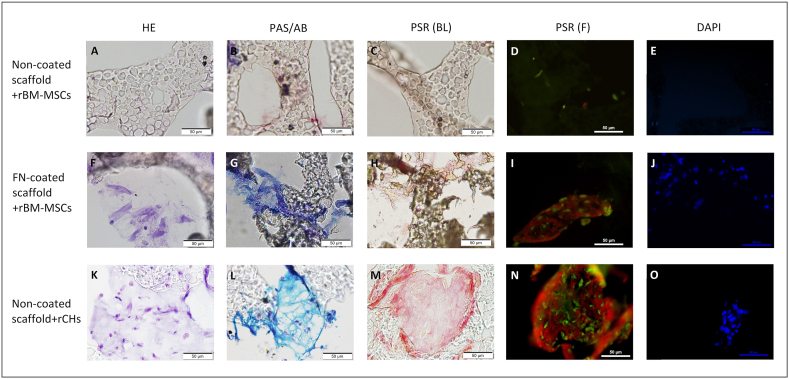
Three scaffolds were evaluated for each of the three conditions studied and similar results were observed in the slices obtained from them. The protocol followed to perform the histology studies caused loss of material from the slide. The surface properties of the scaffold prevented to perform a suitable bond to the surface, although the slide had a pre-treatment to improve its adhesion properties. This impeded to evaluate the cell migration along the complete sheet. Nevertheless, we could not observe any cell attached to the untreated scaffold seeded with rBM-MSCs (Fig. 4A–E); whereas the other two condition (FN-coated + rBM-MSCs, and non-coated + rCHs) present similar cell attachment and staining (Fig. 4F–J and K–O).
Fig. 4.
Representative histological images of uncoated scaffolds seeded with rCHs (A–E) and rBM-MSCs (F–J), and FN-coated scaffolds seeded with rBM-MSC (K–O), after 21 days of in vitro chondrogenesis. Haematoxylin-Eosin (HE) (A, F, K), PAS- Alcian blue (B, G, L), Picrosirius red (PRS) (C-D, H–I, M−N), and DAPI (E, J, O) stainings, observed in bright field or under fluorescence. Magnification bar: 50 μm.
The production of both proteoglycans and collagen could be evaluated as ECM presented good adhesion to the slide; therefore, it could be observed in the areas where it had been deposited (Fig. 4). As expected, rBM-MSCs seeded on untreated scaffolds exhibited no ECM formation (Fig. 4B–D), as no cells were observed. Big areas of tissue formation and high ECM production were obtained from rCHs seeded on untreated scaffolds (Fig. 4L–N); rBM-MSCs seeded on FN-coated scaffolds also produced ECM, but display a lower amount (Fig. 4G–I). Although rCHs produced more ECM than rBM-MSCs, the quality of the ECM produced was comparable. Indeed, both showed similar content of acidic and neutral mucins (Fig. 4G and L).
4. Discussion
In this study, we describe a protocol for rBM-MSCs culture (that is independent of the effect of FBS batches), proliferation, and differentiation into chondrocytes. We seeded rBM-MSCs on PCL-PU scaffold coated with FN and cultured them in a chondrocyte differentiation medium. In these conditions, they were able to attach, proliferate, and differentiate, producing an ECM that resembles the one produced by chondrocytes.
Repairment is the first option for injured meniscus. However, a partial or total meniscectomy is sometimes required to ameliorate pain and function, occasionally resulting in the post-meniscectomy syndrome. In order to avoid it, AMT and tissue engineering have been proposed to substitute the missing tissue. Regarding tissue engineering, several materials have been tested to produce meniscal scaffolds. It is well-known that synthetic polymers have higher lifespan than biological ones, but they present the disadvantage of displaying lower biocompatibility [2]. However, several works demonstrated that chondrocytes and fibrochondrocytes adhere and proliferate over synthetic polymers, producing an ECM that resembles the meniscal one, both in animal models [18,19], and in humans [7,20]. We observed similar results in vitro with chondrocytes. Nevertheless, we also showed that the bioactivity of the scaffolds for rBM-MSCs is much lower. In particular, rCHs proliferated over non-coated scaffolds forming colonies and synthetizing ECM, whereas rBM-MSCs proliferated much less and did not produce ECM. This difference between rBM-MSCs and rCHs could indicate that the cells colonizing the scaffold in vivo are differentiated cells migrating from adjacent areas of the meniscus, rather than MSCs from the synovial fluid. MSCs presence in synovial fluid increases after some knee pathologies or surgical procedures [[21], [22], [23]] and their presence is suggested to play a role in the healing of defects such as meniscal tears. However, it is still not clear whether this effect is a direct action of their proliferating and differentiating capacity or is rather mediated by secretion of trophic and immunomodulation factors [24]. Our results suggest that in surgeries for Actifit® implantation, the benefit of native MSCs presence in synovial fluid will not be related with their direct scaffold colonization and regeneration capacity, as their ability to adhere to Actifit® is low, but rather to a paracrine effect.
Despite the good results obtained with chondrocytes in the present study, there are several issues that discourage their use in scaffolds for tissue regeneration [25]. First of all, it is necessary to perform a cartilage lesion to isolate the chondrocytes. Secondly, healthy chondrocytes have low mitotic activity, limiting the number of cells that could be collected. Furthermore, chondrocytes should be expanded in a three-dimensional architecture to avoid dedifferentiation [26]. Finally, aging chondrocytes show declining synthetic activities, with production of smaller and less uniform aggrecan molecules and less functional link proteins [27]. On the other hand, MSC can be obtained from different sources, producing little harm to healthy tissues and maintain their expandability and multipotency after at least ten passages [28]. Integrins are adhesion receptors mediating cell–cell and cell–matrix interactions. They are not only implicated in cell binding but also in intracellular signalling. MSCs lack several integrins that are present in chondrocytes [13]. This could prevent MSCs to adhere and proliferate over non-coated scaffolds. However, fibronectin receptor (integrin alpha5beta1) is expressed by undifferentiated MSCs [13], allowing their interaction with FN-coated scaffolds, that will exhibit an improved bioactivity in terms of attachment and/or proliferation.
Although our work suggests that MSCs are not able to bind and proliferate over uncoated PCL-PU scaffold, other authors have succeeded to grow and differentiate them, using other techniques for cell seeding. Achatz et al. tested MSCs behaviour on Actifit® [29] and they observed excellent cell distribution through the polyurethane scaffold, with more than 75% of pores being cell-populated, extensive production of proteoglycans and collagen type II, and moderate production of collagen type I. They seeded the MSCs using a rotary valve vacuum pump, and therefore forcing the cells to occupy pores in the centre of the scaffold. So, MSCs located in pores were retained when culture media was changed, even they were not adhered to the material. Thus, MSCs could differentiate into chondrocytes (under suitable culture media) and produce ECM. Instead, we seeded the MSCs on the scaffold surface and let the cells diffuse naturally across the material. Hence, the number of MSCs captured inside the pores was low and most of them were removed with media change. We selected this seeding protocol to evaluate the capacity of the cells to migrate and invade coated and uncoated material. Unfortunately, this could not be evaluated because material was lost along the histology protocol.
The current work presents several limitations. First, we could not evaluate the colonization of the scaffold because it did not bind properly to the slide glass, so it was hardly observed in the histology preparations. Longer cultured time that allows rBM-MSC migration, cell differentiation, and ECM production could improve adherence of the material. Furthermore, other histology techniques, such as Methyl-methacrylate [30], used for hardness materials, could increase integrity of the sections obtained. Second, the experiments have been performed with rBM-MSCs harvested from a low number of donors (only two individuals). Nevertheless, the results are consistent between both cell lines. Finally, we used an animal model that is distant from humans on the phylogenetic scale. However, the expression of integrins in human MSCs has been extensively studied and fibronectin receptors are present in these cells.
5. Conclusion
We hypothesized that in orthopaedic surgery of meniscal injuries, scaffolds are colonized by differentiated cells (fibrochondrocytes and chondrocytes) migrating from adjacent areas of the meniscus. However, chondrocytes have low proliferative capacity and, furthermore, hypertrophic chondrocytes have altered protein expression, producing aberrant ECM that finally leads to the apoptosis of the cells [31]. This phenomenon might be behind the failure of the scaffold at long run [32]. The use of FN-coated scaffold allows MSCs to attach to Actifit® in vitro. This finally leads to MSC differentiation into new cells producing ECM similar to the ones produced by chondrocytes. Therefore, our results could have crucial implications in the design of scaffolds to improve their clinical use in tissue regeneration and functionality following orthopaedic surgery.
Declaration of competing interest
RT and SM have received funding from MBA for research projects in meniscal repair. The company is not financing the manuscript.
There are no other competing interests.
Acknowledgments
The authors would like to thank JF Sánchez for his assistance in rabbit surgeries and Laura Triginer for her assistance in histology techniques, and photography and image analysis. Authors also acknowledge Daniel Vivas and Joaquin Vives from Banc de Sang i Teixits de Catalunya for their support in the isolation and culture of rBM-MSCs. Finally, we want to thank The Paper Mill for the assistance in manuscript editing and English language support.
This work was supported by grant from AEI (Spanish State Research Agency) and FEDER (DPI2016-80283-C2-2-R). This work had been carried out in the Surgery and Morphological Sciences Doctorate framework of Universitat Autònoma de Barcelona.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.reth.2021.11.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Supplementary Figure 1.
rBM-MSC differentiation towards chondrogenic (E), adipogenic (F), and osteogenic (G, H) lineages. Staining of control cells (undifferentiated) is also shown (A-D). Magnification bar: 100 μm.
References
- 1.Bloch B., Getgood A., Parkinson B., Spalding T. In: Hulet C., Pereira H., Peretti G., Denti M., editors. Springer Berlin Heidelberg; Berlin, Heidelberg: 2016. Concepts in managing the patient with post-meniscectomy knee pain; pp. 437–446. (Surgery of the meniscus). 10.1007/978-3-662-49188-1_45. [Google Scholar]
- 2.O'Brien F.J. Biomaterials & scaffolds for tissue engineering. Mater Today. 2011;14:88–95. [Google Scholar]
- 3.Klimek K., Ginalska G. Proteins and peptides as important modifiers of the polymer scaffolds for tissue engineering applications—a review. Polymers. 2020;12 doi: 10.3390/polym12040844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baynat C., Andro C., Vincent J.P., Schiele P., Buisson P., Dubrana F., et al. Actifit synthetic meniscal substitute: experience with 18 patients in Brest, France. Orthop Traumatol Surg Res. 2014;100:S385–S389. doi: 10.1016/j.otsr.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Verdonk P., Beaufils P., Bellemans J., Djian P., Heinrichs E.L., Huysse W., et al. Successful treatment of painful irreparable partial meniscal defects with a polyurethane scaffold: two-year safety and clinical outcomes. Am J Sports Med. 2012;40:844–853. doi: 10.1177/0363546511433032. [DOI] [PubMed] [Google Scholar]
- 6.Verdonk R., Forsyth R. Histological analysis of tissue ingrowth and organization following implantation of a novel synthetic acellular meniscal scaffold for the treatment of irreparable partial meniscus tears and/or partial (SS-27A) Arthrosc J Arthrosc Relat Surg. 2011;27:e44–e45. [Google Scholar]
- 7.Verdonk R., Verdonk P., Huysse W., Forsyth R., Heinrichs E.L. Tissue ingrowth after implantation of a novel, biodegradable polyurethane scaffold for treatment of partial meniscal lesions. Am J Sports Med. 2011;39:774–782. doi: 10.1177/0363546511398040. [DOI] [PubMed] [Google Scholar]
- 8.Dhollander A., Verdonk P., Verdonk R. Treatment of painful, irreparable partial meniscal defects with a polyurethane scaffold: midterm clinical outcomes and survival analysis. Am J Sports Med. 2016;44:2615–2621. doi: 10.1177/0363546516652601. [DOI] [PubMed] [Google Scholar]
- 9.Monllau J.C., Gelber P.E., Abat F., Pelfort X., Abad R., Hinarejos P., et al. Outcome after partial medial meniscus substitution with the collagen meniscal implant at a minimum of 10 years' follow-up. Arthroscopy. 2011;27:933–943. doi: 10.1016/j.arthro.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Rodkey W.G., DeHaven K.E., Montgomery W.H., 3rd, Baker C.L., Jr., Beck C.L., Jr., Hormel S.E., et al. Comparison of the collagen meniscus implant with partial meniscectomy. A prospective randomized trial. J Bone Joint Surg Am. 2008;90:1413–1426. doi: 10.2106/JBJS.G.00656. [DOI] [PubMed] [Google Scholar]
- 11.Squillaro T., Peluso G., Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant. 2016;25:829–848. doi: 10.3727/096368915X689622. [DOI] [PubMed] [Google Scholar]
- 12.Angele P., Kujat R., Koch M., Zellner J. Role of mesenchymal stem cells in meniscal repair. J Exp Orthop. 2014;1:12. doi: 10.1186/s40634-014-0012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goessler U.R., Bugert P., Bieback K., Stern-Straeter J., Bran G., Hormann K., et al. Integrin expression in stem cells from bone marrow and adipose tissue during chondrogenic differentiation. Int J Mol Med. 2008;21:271–279. [PubMed] [Google Scholar]
- 14.Dolley-Sonneville P.J., Romeo L.E., Melkoumian Z.K. Synthetic surface for expansion of human mesenchymal stem cells in xeno-free, chemically defined culture conditions. PLoS One. 2013;8:e70263. doi: 10.1371/journal.pone.0070263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedenstein A.J., Chailakhyan R.K., Gerasimov U.V. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987;20:263–272. doi: 10.1111/j.1365-2184.1987.tb01309.x. [DOI] [PubMed] [Google Scholar]
- 16.Kuznetsov S.A., Friedenstein A.J., Robey P.G. Factors required for bone marrow stromal fibroblast colony formation in vitro. Br J Haematol. 1997;97:561–570. doi: 10.1046/j.1365-2141.1997.902904.x. [DOI] [PubMed] [Google Scholar]
- 17.Martinez-Diaz S., Garcia-Giralt N., Lebourg M., Gomez-Tejedor J.A., Vila G., Caceres E., et al. In vivo evaluation of 3-dimensional polycaprolactone scaffolds for cartilage repair in rabbits. Am J Sports Med. 2010;38:509–519. doi: 10.1177/0363546509352448. [DOI] [PubMed] [Google Scholar]
- 18.Tienen T.G., Heijkants R.G., de Groot J.H., Pennings A.J., Schouten A.J., Veth R.P., et al. Replacement of the knee meniscus by a porous polymer implant: a study in dogs. Am J Sports Med. 2006;34:64–71. doi: 10.1177/0363546505280905. [DOI] [PubMed] [Google Scholar]
- 19.Welsing R.T., van Tienen T.G., Ramrattan N., Heijkants R., Schouten A.J., Veth R.P., et al. Effect on tissue differentiation and articular cartilage degradation of a polymer meniscus implant: a 2-year follow-up study in dogs. Am J Sports Med. 2008;36:1978–1989. doi: 10.1177/0363546508319900. [DOI] [PubMed] [Google Scholar]
- 20.Bulgheroni E., Grassi A., Campagnolo M., Bulgheroni P., Mudhigere A., Gobbi A. Comparative study of collagen versus synthetic-based meniscal scaffolds in treating meniscal deficiency in young active population. Cartilage. 2016;7:29–38. doi: 10.1177/1947603515600219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watanabe N., Endo K., Komori K., Ozeki N., Mizuno M., Katano H., et al. Mesenchymal stem cells in synovial fluid increase in knees with degenerative meniscus injury after arthroscopic procedures through the endogenous effects of CGRP and HGF. Stem Cell Rev Rep. 2020;16:1305–1315. doi: 10.1007/s12015-020-10047-0. [DOI] [PubMed] [Google Scholar]
- 22.Morito T., Muneta T., Hara K., Ju Y.J., Mochizuki T., Makino H., et al. Synovial fluid-derived mesenchymal stem cells increase after intra-articular ligament injury in humans. Rheumatology. 2008;47:1137–1143. doi: 10.1093/rheumatology/ken114. [DOI] [PubMed] [Google Scholar]
- 23.Sekiya I., Ojima M., Suzuki S., Yamaga M., Horie M., Koga H., et al. Human mesenchymal stem cells in synovial fluid increase in the knee with degenerated cartilage and osteoarthritis. J Orthop Res. 2012;30:943–949. doi: 10.1002/jor.22029. [DOI] [PubMed] [Google Scholar]
- 24.Ayala-Cuellar A.P., Kang J.H., Jeung E.B., Choi K.C. Roles of mesenchymal stem cells in tissue regeneration and immunomodulation. Biomol Ther (Seoul) 2019;27:25–33. doi: 10.4062/biomolther.2017.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kessler M.W., Grande D.A. Tissue engineering and cartilage. Organogenesis. 2008;4:28–32. doi: 10.4161/org.6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giannoni P., Cancedda R. Articular chondrocyte culturing for cell-based cartilage repair: needs and perspectives. Cells Tissues Organs. 2006;184:1–15. doi: 10.1159/000096946. [DOI] [PubMed] [Google Scholar]
- 27.Adkisson H.D., Gillis M.P., Davis E.C., Maloney W., Hruska K.A. In vitro generation of scaffold independent neocartilage. Clin Orthop Relat Res. 2001:S280–S294. doi: 10.1097/00003086-200110001-00026. [DOI] [PubMed] [Google Scholar]
- 28.Sakaguchi Y., Sekiya I., Yagishita K., Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 29.Achatz F.P., Kujat R., Pfeifer C.G., Koch M., Nerlich M., Angele P., et al. In vitro testing of scaffolds for mesenchymal stem cell-based meniscus tissue engineering-introducing a new biocompatibility scoring system. Materials. 2016;9 doi: 10.3390/ma9040276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erben R.G. Embedding of bone samples in methylmethacrylate: an improved method suitable for bone histomorphometry, histochemistry, and immunohistochemistry. J Histochem Cytochem. 1997;45:307–313. doi: 10.1177/002215549704500215. [DOI] [PubMed] [Google Scholar]
- 31.van der Kraan P.M., van den Berg W.B. Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? Osteoarthritis Cartilage. 2012;20:223–232. doi: 10.1016/j.joca.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Monllau J.C., Poggioli F., Erquicia J., Ramirez E., Pelfort X., Gelber P., et al. Magnetic resonance imaging and functional outcomes after a polyurethane meniscal scaffold implantation: minimum 5-year follow-up. Arthroscopy. 2018;34:1621–1627. doi: 10.1016/j.arthro.2017.12.019. [DOI] [PubMed] [Google Scholar]