Abstract
As an emerging type of adult stem cell featuring non-invasive acquisition, urine-derived stem cells (USCs) have shown great potential for applications in tissue engineering and regenerative medicine. With a growing amount of research on the topic, the effectiveness of USCs in various disease models has been shown and the underlying mechanisms have also been explored, though many aspects still remain unclear. In this review, we aim to provide an up-to-date overview of the biological characteristics of USCs and their applications in skin, bone and articular cartilage repair. In addition to the identification procedure of USCs, we also summarize current knowledge of the underlying repair mechanisms and application modes of USCs. Potential concerns and perspectives have also been summarized.
Keywords: Urine-derived stem cells, Skin, Bone, Articular cartilage, Tissue engineering, Cell therapy
Highlights.
Provide an up-to-date overview of the biological characteristics of USCs.
Summarize current research and propose application modes of USCs in skin, bone and articular cartilage repair.
Summarize the understanding of the underlying repair mechanisms of USCs.
Propose further research directions to clarify the safety, efficacy, mechanisms and cost-effectiveness of applying USCs in the context of translational medicine.
Background
One of the main contributors to the global burden of disease is traumatic injury [1], while surgical wounds and burn injuries affect millions of people worldwide annually [2, 3]. In addition, chronic skin wounds are increasing, such as diabetic foot ulcers [3]. There is a huge demand for wound healing, defect repair and tissue regeneration. During the last decades, mesenchymal stem cells (MSCs) have shown great potential for tissue engineering and regenerative medicine. To date, such cells have been isolated from various tissues [4], e.g. bone marrow, adipose, tendon, placenta, etc. Though with different origins and behaviors, MSCs are believed to have similar characteristics such as cell surface antigen profiles. Adult MSCs are thought to be applicable for various diseases including skin, bone and articular cartilage injuries. Some MSCs have already been commercialized and approved by supervisory authorities [5, 6].
As for the roles that exogenous MSCs play in tissue repair, an early hypothesis was that MSCs could differentiate into functional cells to replace damaged cells. However, later studies showed that though in some cases MSCs did function as hypothesized, other mechanisms including MSC/cell fusion, paracrine effect, organelle transfer, extracellular vesicle-mediated active factors transfer and immune response were often more relevant [7, 8]. The overall outcome of repair is now believed to be a combined result involving multiple mechanisms.
Acquisition of MSCs from various tissues such as bone marrow and adipose tissue usually involves invasive procedures and injuries. As a result, researchers have set their sights on clinically discarded tissues such as the placenta and umbilical cord to isolate MSCs. Recently, urine-derived stem cells (USCs) have drawn much attention for their potential in regenerative medicine. Because of their similarity to MSCs, USCs have been applied in various disease models with promising results. Here, we discuss the biological characteristics of USCs, and then focus on their applications in skin, bone and articular cartilage repair. We also discuss the issues with regard to future studies and applications of USCs in regenerative medicine.
Review
Biological characteristics and applications of USCs
Urine has been considered as a new source of adult stem cells. Human kidneys produce ~180 L of primary filtrate daily, of which only ~1% is eventually excreted as urine [9]. This process ensures regular removal of metabolic wastes from the blood and maintenance of adequate blood pressure and pH value. Owing to the epithelial lining of the luminal surface in the urinary tract and a conservative estimation that 2000–7000 renal tubular cells are exfoliated daily [10], the sediment of urine is a major source of epithelial cells [11]. In 2008, Zhang et al. identified a stem cell population in urine, with an expansion potential for up to ten passages in vitro [12]. This stem cell population was later termed urine-derived stem cells or USCs. As the urine produced comes into contact with multiple tissues through the excretion process, the origin of USCs has remained controversial. USCs are positive for CD44, cytokeratin 13 and uroplakin Ia. These markers are also present in basal bladder cells [12]. Because basal cells can self-renew, proliferate and differentiate into intermediate and superficial cells, they are referred to as urothelial progenitor cells or stem cells [13, 14]. Accordingly, USCs are thought to be derived from basal cells [12]. However, subsequent research showed that the USCs derived from female donors who received male kidney transplantation showed X/Y chromosome characteristics, indicating that they are from the upper urinary system [15]. Immunofluorescence assay and real-time PCR assay suggested that USCs may originate from parietal cells or podocytes in the renal glomerulus [15]. In our previous work, according to morphology, we identified and characterized two subpopulations of USCs, and research showed that they have different origins: one of them may be from the renal mesenchyme near the loop of Henle and the distal convoluted tubule, while the other may originate from nephron tubules including Bowman’s capsule to the distal convoluted tubule, except the collecting duct [16]. Despite their multiple possible sites of the origin, USCs share similar marker profiles with MSCs, e.g. positive for CD73, CD90 and CD105, but negative for CD11b, CD14, CD19, CD34, CD45 and CD31 [12, 16–18]. Table 1 shows the typical cell surface markers of USCs.
Table 1.
The typical cell surface markers of USCs
| Ref. | CD105 | CD73 | CD90 | CD29 | CD146 | CD44 | CD166 | CD133 | CD24 | SSEA-4 | CD34 | CD45 | CD31 | HLA-DR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fu et al. [47] | \ | + | + | + | + | \ | \ | \ | \ | \ | − | \ | \ | − |
| Qin et al. [98] | \ | + | + | + | \ | \ | \ | \ | \ | \ | − | − | \ | \ |
| Pei et al. [64] | W | + | + | + | + | \ | \ | W | + | + | − | − | − | \ |
| Guan et al. [44] | W | + | + | + | \ | + | \ | \ | \ | \ | − | − | \ | \ |
| Guan et al. [99] | \ | + | + | + | \ | + | \ | − | \ | \ | − | − | \ | − |
| Chen et al. [61] | \ | + | + | + | \ | + | \ | \ | \ | \ | − | − | \ | \ |
| Zhang et al. [80] | W | + | + | + | \ | + | \ | − | \ | \ | − | − | \ | − |
| Chen et al. [46] | + | + | + | + | \ | \ | + | \ | \ | \ | − | − | \ | − |
| Xing et al. [101] | \ | \ | + | + | \ | + | \ | \ | \ | \ | \ | − | − | − |
| Cao et al. [81] | \ | + | + | + | + | \ | \ | − | \ | \ | − | − | \ | − |
| Zhang et al. [82] | \ | + | + | + | \ | + | \ | \ | \ | \ | − | − | \ | − |
| Sun et al. [100] | \ | + | + | + | \ | + | \ | \ | \ | \ | − | − | \ | \ |
+ positive expression, − negative expression, \ not tested, W weak-positive expression
However, it is much simpler to obtain USCs than MSCs. The major steps for the primary culture of USCs include centrifuging the collected urine samples, washing with PBS, resuspending the sediment in a culture medium, and then transferring to culture flasks. After a few days of culture, clones can be observed. Colonies usually appear ~3–9 days after plating, and the time of the colonies’ appearance may be not associated with the age or gender of the donor [19–21]. USCs are usually rice-shaped [17, 21, 22], although spindle-shaped USCs have also been observed [16, 19]. One report showed that urine samples collected continuously for 24 h from a healthy individual could generate up to 140 USC clones, and the average population doubling time from passage 0 forward to passage 8 of fresh USCs is 49.5 ± 7.2 h [21]. More than 1 × 108 USCs may be obtained over three passages, which will suffice for clinical applications [23].
Further analysis on the stemness features showed that the USCs displayed detectable levels of telomerase, and they expressed stemness-related genes, such as SOX2, OCT-3/4, C-MYC and KLF4 [17, 24], which are often of concern for subsequent applications. The USCs did not lead to teratomas or tumors [25], possibly because the mRNA levels of stemness-related genes in the USCs were significantly lower than those in embryonic stem cells [17]. In another study, the USCs did not express OCT4, and fully methylated CpG dinucleotides within the OCT4 promoter were observed [26]. Indeed, the USCs from various donors showed differential mRNA levels of OCT4 expression [17]. The differences are possibly due to the donor age. The expression of OCT4 at protein level in USCs is even weaker, down to a very low ratio/level [27] or undetectable [17] by immunofluorescence staining. Furthermore, USCs showed self-renewal in which inactive WNT/β-catenin signaling and active TGF-β/SMAD2/3 signaling may play an important role [26]. Previous studies proved that USCs are multipotent and could differentiate into bladder cell lineages such as urothelial, smooth muscle and endothelial cells. Other mesodermal cell lineages are also inducible, e.g. chondrocytes, adipocytes and osteocytes [17, 28]. In addition, they could also undergo neurogenic and skeletal myogenic differentiation [17].
Given the above features, USCs have been considered for application in regenerative medicine. They were first introduced in urinary tissue engineering [29, 30]. As adult stem cells, USCs are believed to have potential in regenerative medicine in addition to urological applications. Other potential application areas may include stress urinary incontinence [31, 32], erectile dysfunction [18, 33], acute kidney injury [24, 34], chronic kidney disease [35], vascular diseases [22], diabetes mellitus [36, 37], diabetic nephropathy [38, 39], chronic liver injury [40], inflammatory bowel diseases [41], neuron regeneration [42], osteonecrosis of the femoral head [43], bone [44, 45] and cartilage regeneration [46], and skin wounds healing [47]. Induced pluripotent stem cells generated from urine-derived cells or USCs can further expand the application areas [48], such as in cardiac repair [49], dental reconstruction [50], disease modeling and drug screening [51–57]. Moreover, USCs-derived extracellular vesicles also hold promise for the amelioration of various diseases [31, 58–63], and USCs-derived extracellular matrix promotes the differentiation of other stem cells into chondrogenic cells [64, 65].
The above application attempts are all based on the fact that various adult stem cell types have shown similarities in the context of tissue engineering and regenerative medicine. Therefore, it will be interesting to explore the feasibility of using USCs as substitutes for MSCs derived from other tissues in various situations. Since ‘MSC’ is a joint name, it is unreasonable to compare USCs with ‘MSCs’ unless a specific origin is defined. Of course, one may expect to find different features in USCs and ‘MSCs’, and current studies have indeed shown distinct properties of USCs in terms of proliferation, colony-formation and differentiation [66–69]. It is worth noting that most such differences are seen in in vitro results. A possible important mechanism in vivo is that USCs contain different secretomes [32, 65, 70] which can activate various downstream pathways. As mentioned above, the latest understanding is that differentiation ability is probably not the decisive factor, while the significant advantage of non-invasive acquirement has made USCs a very attractive cell type. It is possible that there will be tradeoffs between acquirement, efficacy and cost when multiple adult stem cell types are considered for particular situations.
In the following sections, the application of USCs in skin, bone and articular cartilage repair are discussed in detail, and the main studies in these fields are summarized in Table 2.
Table 2.
Applications of USCs in skin, bone and articular cartilage repair
| Applied tissue | Animal model | Defect | Dosage | Materials | Pretreatment | Outcome | Possible repair mechanisms | Ref. |
|---|---|---|---|---|---|---|---|---|
| Skin | New Zealand white rabbit | Full-thickness skin defects, 2 cm × 2 cm | 2 × 104 USCs/24-well plate-sized membranes | Polycaprolactone/gelatin nanofibrous (PCL/GT) membranes | N/A | Faster wound closure, increased re-epithelialization, collagen formation, and angiogenesis | USCs secrete VEGF and TGF-β1 promoting angiogenesis | [47] |
| Skin | BALB/C nude mouse | Full-thickness skin defect, 10 mm in diameter | 1 × 106 USCs/100 μL PBS (subcutaneous injection) | N/A | Treated by diluted bioglass ionic (BG) extracts | Better wound healing ability, improved angiogenesis, more collagen deposition, and the collagen structure is closer to that in the mouse | BG ionic extracts activate the paracrine effects (VEGF-KDR) between USCs and recipient cells (endothelial cells and fibroblasts) in wound healing | [80] |
| Skin | Streptozotocin-induced diabetic C57BL/6 mouse | Full-thickness skin defect, 6 mm in diameter | 200 μg USCs-Exos/100 μL PBS (intraperitoneal injection) | N/A | N/A | Accelerated wound healing, higher rates of re-epithelialization, more collagen deposition, improved cell (keratinocytes, fibroblasts and vascular endothelial) proliferation, less scar formation and improved angiogenesis | Exosomes (Exos) from USCs could effectively enhance the proliferation, migration and tube formation of vascular endothelial, promoting angiogenesis via transferring DMBT1 protein | [61] |
| Skin | Sprague–Dawley rats | Full-thickness skin defect, 2 cm in diameter | 5 × 103 USCs/96-well plate-sized membranes | Surface-structured bacterial cellulose nanofiber (S-BC) membranes | N/A | Accelerated wound healing, faster re-epithelialization, more collagen production and neovascularization | The substance secreted from USCs and the effect of S-BC on the adhesion and proliferation of vascular endothelial cells promote angiogenesis | [81] |
| Skin | BALB/C nude mouse | Full-thickness skin defect, 8 mm in diameter | 1 × 106 USCs/SIS membrane (10 mm in diameter) | Porcine small intestine submucosa (SIS) | The composites were pretreated with hypoxia (1% O2) for 24 h | Accelerated neovascularization, facilitated re-epithelialization, promoted skin appendage regeneration, improved the quality of collagen deposition and enhanced the wound healing | Hypoxic preconditioning enhanced composites secreting a large amount of growth factors (VEGF, EGF and bFGF) for enhancing wound angiogenesis at the early stage of wound healing | [82] |
| Bone | N/A | N/A | USCs | N/A | Fresh medium containing 4 μg/mL silver nanoparticles (AgNPs) treated for 24 h | Promoted osteogenic differentiation of USCs | The AgNPs themselves, rather than the released silver ions, lead USCs into osteogenic differentiation via activating RhoA, inducing actin polymerization and increasing cytoskeletal tension | [98] |
| Bone | Nude mouse | Ectopic bone formation (muscle pockets in hindlimbs) | 5 × 105 USCs/scaffold (5 × 5 × 3 mm) | Poly (lactic-co-glycolic acid)/calcium silicate composite (PLGA/CS) porous scaffold | One week culture in vitro | Induced osteogenic differentiation, ingrowth of blood vessels into scaffolds | CS induces the osteogenic differentiation of USCs through the Wnt/β-catenin signaling pathway | [44] |
| Bone | Sprague–Dawley rats | 6 mm critically sized femoral defect | 5 × 105 USCs/scaffold (5 × 5 × 6 mm) | β-TCP porous scaffold | Composites cultured in osteogenic differentiation media for 7 days | Increased new osseous formation, 5 out of 11 transplants completely bridged the critical-size bone defect | USCs can adhere, proliferate and differentiate into osteoblasts on a β-TCP scaffold | [45] |
| Bone | Nude mouse | Ectopic bone formation (muscle pockets in hindlimbs) | USCs (concentration not mentioned) | Porous ceramic scaffold made of β-tricalcium phosphate (β -TCP) | Lentiviral vectors-bone morphogenetic protein 2 (BMP2) gene transduction | Increased osteogenic activity of USCs, these transfected cells can undergo osteogenic differentiation without osteogenic medium in vitro, observed ectopic bone formation, USCs differentiate into osteoblasts | BMP2 gene transduction | [99] |
| Bone | New Zealand white rabbit | Critical-sized segmental bone defects model (the ulna bone together with the periosteum) | 6 × 105 USCs/scaffold (Φ 5 × 5 mm) | Surface mineralized biphasic calcium phosphate (BCPs) ceramics scaffold | The composites were cultured in osteogenic differentiation media for 7 days | Promoted the formation of new bone and accelerated the maturation of new bone in ulna defects | Scaffold provided a favorable microenvironment that enabled USCs to adhere and proliferate, early (ALP, BMP2, and RUNX2) and late (OCN) osteogenic gene marker were continuously and significantly upregulated | [101] |
| Bone | Sprague–Dawley rats | Skull defects | 1 × 105 USCs/hydrogel (5 mm in diameter) | Methacrylated solubilized decellularized cartilage (MeSDCC) hydrogel | USCs were infected with 10−6 mol/L BMP2 for 21 days | Increased bone formation, larger bone area | FAK plays a key role in regulating BMP2 enhanced osteogenic differentiation of USCs, the underlying mechanism might be the activation of AMPK and Wnt signaling pathways | [100] |
| Bone | Sprague–Dawley rat | Glucocorticoid-induced osteonecrosis of the femoral head | 500 μg USCs-EVs/200 μL PBS (tail intervenous injection) | N/A | N/A | Prevention of early stage osteonecrosis, rescued angiogenesis impairment, reduced apoptosis of cells, prevented trabecular bone destruction and improved bone microarchitecture | TIMP1 and DMBT1, respectively, partly mediate the anti-apoptotic and pro-angiogenic effects of extracellular vesicles from USCs (USCs-EVs) | [43] |
| Articular cartilage | N/A | N/A | BMSCs | N/A | Seeded on USCs-ECM for one passage | ECM deposited by USCs (USCs-ECM) could recharge senescent BMSCs toward chondrogenic differentiation | The Wnt11-mediated noncanonical signaling pathway might be responsible for USCs-ECM mediated BMSCs rejuvenation in terms of chondrogenic potential | [64] |
| Articular cartilage | New Zealand white rabbit | Knee-joint cartilage defect model, 5 mm in diameter | 1 × 107 USCs/1 mL HA (injection into cartilage-defect knee joints) | 1% Hyaluronic acid solution (HA) | N/A | More neocartilage formation that matures over time, showed the expression of collagen type II and synthesized proteoglycans | USCs are able to differentiate into chondrocytes with characteristic deposition of aggrecan and collagen II | [46] |
| Articular cartilage | N/A | N/A | 1 × 103 SDSCs/cm2 | UECM | N/A | Promoted proliferation and chondrogenic potential of SDSCs | Biophysical and biochemical cues (UECM is softer than others and contains different growth factors and collagen) | [65] |
N/A not applicable, BMSC bone marrow stromal cell, SDSC synovium-derived stem cells, β-TCP β-tricalcium phosphate, UECM ECM deposited by USCs, USCs urine-derived stem cells
Φ The diameter and height of 5 mm
Applications of USCs in skin repair
Skin injuries healing The skin consists of three main layers (epidermis, dermis and hypodermis) containing various appendages, e.g. hair, sweat glands and sebaceous glands. As the largest organ of the human body, it has multiple functions of great importance including protection against foreign pathogens, regulation of body temperature, prevention of dehydration and sensation, as well as production and activation of hormones, neuropeptides and cytokines [71, 72]. The common skin injuries include surgical incisions, burns and chronic ulcers, while wound healing is divided into four stages: hemostasis, inflammation, proliferation and remodeling [73, 74]. Angiogenesis of endothelial cells and collagen deposition of fibroblasts play important roles in the repair of skin defects. For injury healing, the endogenous stem cells of the skin can self-renew remarkably and produce daughter cells capable of differentiation into the relevant cell lineages that participate in the natural cutaneous wound healing process. However, when it comes to serious situations such as severe burn or diabetes mellitus, the repair process may be insufficient to achieve a satisfactory result. Usually, epidermal appendages are lost and scars are generated which are neither functional nor aesthetical. By contrast, exogenous stem cells under such conditions may result in better therapeutic outcomes. Many types of adult stem cells have already been tested for skin repair and regeneration in various acute and chronic skin injuries, including bone marrow-derived MSCs [75–78], adipose-derived stem cells [75], umbilical cord-derived MSCs [78] and placenta-derived stem cells [79].
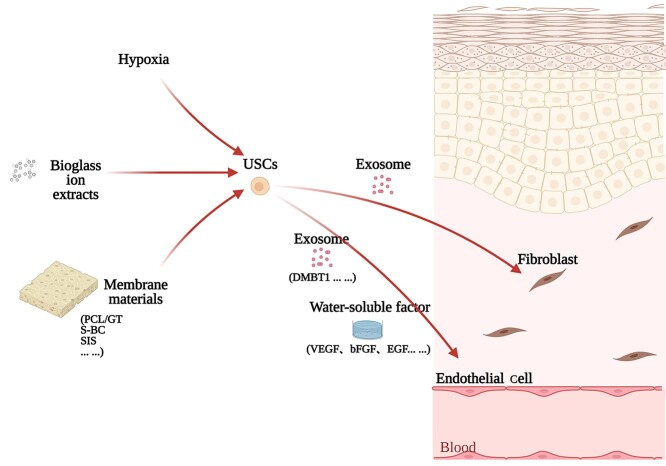
The application of USCs Several application strategies of USCs have been shown to be effective in full-thickness skin defect repair. Zhang et al. used the bioactivity of bioglass to enhance the skin defect repair ability of USCs by promoting the paracrine effect [80], which showed that pretreatment of USCs can improve their therapeutic efficacy. The use of USCs in conjunction with membrane materials has also shown therapeutic effectiveness. Fu et al. demonstrated that USCs seeded on polycaprolactone/gelatin nanofibrous membranes could enhance skin defect repair by promoting angiogenesis in the wound [47]. Cao et al. also reported that surface-structured bacterial cellulose loaded with the USCs could accelerate skin wound healing by promoting angiogenesis [81]. One of our recent studies showed that USCs-seeded porcine small intestine submucosa (USCs-SIS) biomaterial accelerated full-thickness skin defect repair in a streptozotocin-diabetic rat model. Moreover, such biomaterial promoted the regeneration of skin appendages at the center of the wound. Notably, further decellularization of the USCs-SIS did not significantly compromise the effectiveness of repair in the same in vivo model, which highlighted a greater application potential of such re-decellularized biomaterial as an off-the-shelf product for acute skin injuries (Wang Z. L. et al., in preparation). In another of our recent studies, a tissue-engineered skin patch that consisted of porcine SIS and hypoxic pretreated USCs could accelerate full-thickness skin defect repair and promote skin appendage regeneration in a nude mouse model [82]. Chen et al. also showed that the exosomes released by USCs could accelerate full-thickness skin defect healing in a streptozotocin-induced diabetic mouse model, and exosomal DMBT1 from USCs may play a crucial role through promoting angiogenic responses [61]. Taken together, it seems that USCs exert bioactivity in wound healing by paracrine effects and exosomes. Of note, membrane materials seem to be beneficial for the adhesion and proliferation of USCs, and pretreatment can enhance the effect of USCs. Moreover, combining the proper material forms (e.g. membrane-shaped materials) with certain pretreatments may create a synergistic effect. An effective application mode of USCs for skin defect repair is shown in Figure 1. However, other mechanisms may also be involved, and more experimental evidence is needed.
Figure 1.
An effective application mode of USCs in skin defect repair. Pretreatment can improve the performance of USCs. The secretions of USCs have therapeutic effects. USCs cultured with the membrane materials may show a synergistic effect. A variety of cellular strategies may impact on bioactivities through different pathways, and ultimately act on the effector cells to repair the skin defect. PCL/GT polycaprolactone/gelatin, S-BC surface-structured bacterial cellulose, SIS porcine small intestine submucosa
Applications of USCs in bone repair
Osteanagenesis Bone has an irregular and anisotropic hierarchical structure. Repeating osteon units of collagen fibers and calcium phosphate crystals make up the outer cortical bone, and an interconnecting framework of trabeculae surrounding a marrow space forms the inner cancellous bone [83]. Bone serves many key functions, such as load-bearing, movement, hematopoiesis, calcium homeostasis, acid/base buffering and cytokine storage [83, 84]. It also has high inherent regeneration capacity. However, nonunion and scar tissue formation can happen due to insufficient spontaneous healing under the situation of large bone defects that usually result from complex trauma, tumor resection and other diseases, i.e. critical-size defects (CSDs) which are defined as a deficiency of a length exceeding 2–2.5 times the diameter [85]. In such cases, surgical reconstruction with allogeneic or synthetic bone grafts is required. Although the gold standard for reconstructing large skeletal defects remains the transplantation of autogenous bone, the drawbacks are apparent, such as limited supply and secondary injuries. Instead, a promising alternative is tissue-engineered bone grafts.
For tissue-engineered bone, the essential scaffold material should be biocompatible, osteoconductive, osteoinductive, osteogenic, resorbable or degradable, and it should have proper mechanical properties [86]. Furthermore, different material properties can affect the behavior of the cells seeded onto the scaffold [87–89]. Meanwhile, much attention should be paid to the seeded cells themselves. Autologous osteoblasts are a choice, but their availability is hampered by prolonged timespan, limited source and sometimes bone-related disease [90]. In general, stem cells can be expanded remarkably, although adult stem cells have more advantages than embryonic stem cells with regard to ethics and safety concerns. As an adult stem cell type and derived from bone marrow, bone marrow-derived MSCs (BMSCs) are considered for bone tissue engineering. Bruder et al. [91, 92] provided the first proof for the possible application of BMSCs for the reconstruction of long segmental defects in larger animals. One of our previous studies showed that the BMSCs accelerated the repair of a tissue-engineered bone constructed in a rhesus monkey model [93]. And one our previous clinical case with 12-year follow-up of tissue-engineered ribs for chest wall reconstruction demonstrated the feasibility of BMSCs-seeded tissue-engineered bone construct for promoting functional bone regeneration in humans [94]. In addition to BMSCs, other adult stem cells such as adipose-derived stem cells [95], umbilical cord MSCs [96] and dental-derived MSCs have also been applied in bone tissue engineering [97]. The use of USCs has also been reported in bone tissue engineering.
The application of USCs Although Wu et al. have shown that USCs have inferior osteogenic differentiation capability [66], the abundant source and non-invasive acquisition of the USCs have endowed them with great potential for bone injury repair. Many studies have been conducted to enhance the osteogenic properties of USCs. Qin et al. showed that silver nanoparticles could enhance the osteogenic differentiation of USCs by activating RhoA, inducing actin polymerization and increasing cytoskeletal tension [98]. Guan et al. showed that BMP2 gene transduction could enhance the osteogenic potential of USCs [99]. Sun et al. showed that FAK could regulate BMP2-induced osteogenic differentiation of USCs in vitro and in vivo, and the activation of AMPK and Wnt signaling pathways might be responsible [100]. Furthermore, scaffolds with proper mechanical properties may be beneficial for osteogenesis. Guan et al. have successfully repaired a segmental femoral defect in a rat model by combining USCs with β-tricalcium phosphate scaffold [45]. They also used calcium silicate (CS) to induce the osteogenic differentiation of USCs, and the Wnt/β-catenin pathway is involved in the process. In in vivo implantation, the USCs-seeded poly(lactic-co-glycolic acid)/CS scaffold had high expression of osteocalcin [44]. Xing et al. used surface mineralized biphasic calcium phosphate ceramics seeded with USCs to repair segmental bone defects in a rabbit model, and showed that USCs/scaffold composites could promote the formation and maturation of new bone in ulna defects by providing a favorable microenvironment [101]. Moreover, the extracellular vesicles secreted by USCs have a certain effect of preventing osteonecrosis. Chen et al. showed that the extracellular vesicles secreted by USCs prevented early-stage glucocorticoid-induced osteonecrosis in a rat model, and the underlying mechanism could be the delivery of pro-angiogenic DMBT1 and anti-apoptotic TIMP1 [43]. Altogether, researchers have paid extensive attention to the effective osteogenic differentiation of USCs that already have great advantages as seed cells. Various strategies including treatment with nanoparticles, ion extracts of CS powder or BMP2 protein, as well as gene transduction (such as BMP2) can improve the osteogenic differentiation of USCs. Besides, scaffolds with proper mechanical properties are crucial for tissue-engineered bones. Furthermore, the extracellular vesicles of USCs may also be applied in bone injuries. The current effective application mode of USCs in bone defect repair is shown in Figure 2.
Figure 2.
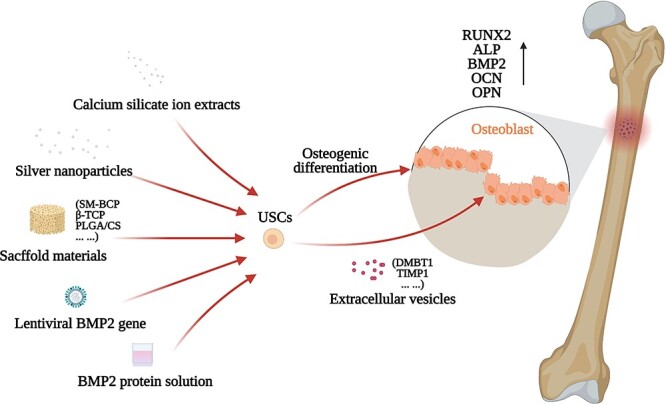
The current application mode of USCs in bone injuries healing. Pretreatment and transduction could improve the performance of USCs. The extracellular vesicles secreted by USCs have a therapeutic effect. USCs cultured with the scaffold materials may show a synergistic effect. A variety of cellular strategies may exert bioactivities through different pathways, and ultimately act on effector cells to repair the bone injury. SM-BCP surface mineralized biphasic calcium phosphate, β-TCP β-Tricalcium phosphate, PLGA/CS poly(lactic-co-glycolic acid)/calcium silicate composite
Applications of USCs in articular cartilage repair
Articular cartilage defect repair Cartilage consists of chondrocytes and the surrounding extracellular matrix. Chondrocytes embedded in articular cartilage receive nutrition diffused through the matrix. According to matrix composition, cartilages can be classified into three types (elastic cartilage, fibrocartilage and hyaline cartilage), and each of them makes up different cartilage tissues. Elastic cartilage is commonly seen in auricula and fibrocartilage in the intervertebral disk. Articular cartilage is hyaline cartilage which is aneural and avascular. The articular cartilage serves several critical functions for body movement, including providing a low-friction gliding surface, acting as a shock absorber and minimizing peak pressures on the subchondral bone. Articular cartilage has a very limited healing capacity, while damage from trauma or degeneration often results in gradual tissue deterioration, leading to debilitating joint pain, functional impairment and degenerative arthritis. The current treatment of articular cartilage defects includes total joint replacement for end-stage degenerative joint pathology, bone-marrow stimulating techniques and mosaicplasty for early lesions [102, 103]. To attain long-term clinical outcomes, cell-based strategies have been proposed, which hold promise for stimulating the regeneration of cartilage. Autologous chondrocytes are naturally considered for cell-based cartilage therapies. Indeed, there are commercially available products such as ChondroCelect and Carticel [104]. However, the challenge of obtaining high cell density and maintaining the differentiation state of the cells have prompted the quest for other cell sources. Likewise, MSCs are considered as alternatives for cell-based therapies in cartilage damage [105]. An important characteristic of MSCs is their chondrogenesis potential. The use of MSC-derived chondrocytes has been widely reported [106]. As discussed above, exogenous stem cells may participate in tissue repair in different ways. Usage of the MSCs per se has also been widely reported [107]. USCs have also been used for the repair of the cartilage defects.
The application of USCs Chen et al. made a compound by combining hyaluronic acid (HA) with USCs, and they injected the compound into the knee joint with a cartilage defect in a rabbit model. The result showed that the compound stimulated more neocartilage formation compared with single USCs, HA or normal saline [46]. Currently, USCs are mainly reported to be chondrogenic [17, 46, 47], but some are also reported to be non-chondrogenic [64]. This may be due to the difference in origin, status and culturing process of the cells. Our previous study also showed that different populations of USCs have different chondrogenic abilities [16]. Although some research showed that the USCs are non-chondrogenic, the extracellular matrix (ECM) deposited by the USCs could promote the chondrogenic capacity of the bone marrow stromal cells [64] and synovium-derived stem cells [65], indicating that the non-chondrogenic USCs can still participate in cartilage repair indirectly. Besides, Pei et al. showed that the supernatant of USCs contained an abundance of 31 human cytokines [64]. Li et al. showed that the USCs-derived ECM (USCs-ECM) is softer than the ECM deposited by adipose-derived stem cells, synovium-derived stem cells or dermal fibroblasts. Also, USCs-ECM contains different collagen (COL4A1, COL4A2) and growth factors (LOXL2, TGM2, PXDN) [65]. This may explain why USCs can promote chondrogenic differentiation of other stem cells. Taken together, USCs may be directly or indirectly involved in cartilage repair, and it may be beneficial to use a mixture of injectable materials with USCs. Furthermore, biophysical and biochemical cues may also contribute to the signals for the proliferation and chondrogenic differentiation of stem cells. As for the effectiveness of extracellular vesicles derived from USCs for cartilage regeneration, more research is still required. The current effective application mode of USCs is shown in Figure 3.
Figure 3.
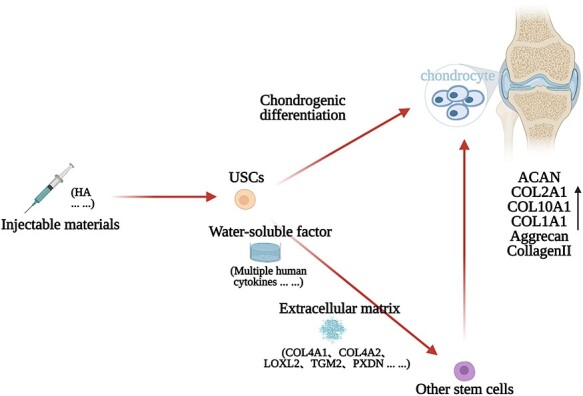
The current effective application mode of USCs in articular cartilage injuries healing. The secretions of USCs have therapeutic effects. USCs cultured with injectable materials may show a synergistic effect. A variety of cellular strategies may exert bioactivities through different pathways, and ultimately act on the effector cells to repair the articular cartilage injury. HA hyaluronic acid, USCs urine-derived stem cells
Perspectives
Basic biological characteristics of USCs Some fundamental biological issues, such as immunoregulatory activities and carcinogenesis risks, have not been fully explored for the understanding of the effectiveness and safety of USCs. Our recent study showed that USCs could not stimulate the proliferation of allogeneic peripheral blood mononuclear cells (PBMCs) but suppressed the phytohemagglutinin-induced activation of PBMCs, which demonstrated the low immunogenicity and moderate immunoregulatory activity of USCs (Gao et al., unpublished results). As the immunomodulatory effects are probably of great significance, such effects of USCs need to be addressed [41]. In addition, our previous study showed that subpopulations of the USCs have different characteristics [16]. Delineation of the unique features of USCs subpopulations is required to provide evidence for USC-based therapy. Meanwhile, basic research on the source of USCs is of significance, as such research may give some answers as to what kinds of people are suitable for supplying USCs, how to obtain USCs properly and whether there are some specific markers of USCs. We believe that single-cell sequencing combined with lineage tracing may offer some hints.
Flow-line production of USCs A flow-line production and application patterns of USCs are summarized in Figure 4. Production of clinical-grade stem cells should follow strict good manufacturing practice (GMP) of medical products guidelines. The safety and effectiveness of the USCs need to be monitored. In general, the production of USCs involves donor selection, cells harvesting method, medium formulation, cells amplification method, quality control criteria and so on. First, donors’ conditions such as age, disease and medication may influence the biological characteristics of the USCs obtained. Gao et al. found that the USCs from younger donors showed higher proliferation ability, less senescence and stronger osteogenic differentiation capacity, although USCs from all ages have shown potential for bone regeneration [108]. Schosserer et al. have noted a higher rate of success for isolating USCs from male donors compared with females (70 vs. 42%) [109]. Considering the difference between male and female genitals, attention should be paid to preventing the risk of contamination when collecting urine samples from females to avoid the period of menstruation and the first micturition of the day [48], and to cleaning the pudendum and the labia with pre-moistened wipes [48] and moist anti-bacterial toilettes [110]. Second, the method of cell harvesting can also influence the final therapeutic outcome [103], and it is necessary to optimize the culture protocol of the USCs [111]. Third, as medium formulation varies with laboratory, the differences mainly being related to the serum concentration and nutrient factor types, for instance, the presence or absence of adenine, epinephrine, or hydrocortisone [47, 61, 82], the necessary ingredients need to be determined.
Figure 4.
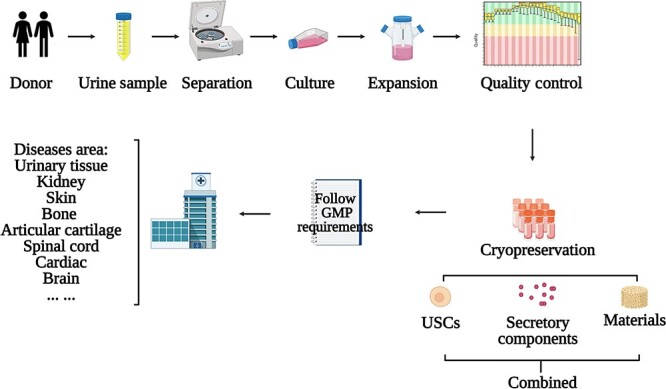
Workflow for application of USCs. USCs are obtained by centrifuging urine collected from donors, cultured in the appropriate medium, and then expanded into a large number by using the appropriate method. USCs or their secretory components can be used directly or coupled with the materials according to the target tissue types/diseases. GMP good manufacturing practice, USCs urine-derived stem cells
Clinical applications will usually require lots of cells, therefore, fast and vast proliferation of USCs for extensive usage have posed a great challenge. Microcarrier-based suspension culture may provide a solution [112]. However, whether USCs cultured by this method remain the same or whether the differences between USCs cultured by different methods lead to different therapeutic outcomes is still unknown. Another solution that faces the same dilemma is reconstituting the culture conditions of the USCs by adding certain nutritional supplements, seeding the USCs on other extracellular matrix components, and taking oxygen tension into account to improve the isolation and proliferation of the USCs [67, 113]. Considering batch-to-batch variation, the quality control of the USCs is of great significance. To meet the quality control criteria is a prerequisite for USCs-based cytotherapies and may involve an enormous amount of work. RNA detection of selected gene products, expression analysis of functionally relevant cell surface markers and protein detection of the secretome are suggested as an assay matrix [114].
Application modes of USCs When applying USCs in regenerative medicine, the scaffold materials should be considered simultaneously with the cell–scaffold complex depending on the clinical needs and the mechanisms by which stem cells may exert biological functions. For example, the materials used for skin defects should mimic the structure and biological function of the dermis [115]. Tan et al. showed that a hydrogel derived from acellular porcine adipose tissue could induce the regeneration of intradermal adipocytes and thereby accelerate wound healing in a nude mice model [116]. In addition to the materials, the strategies of cell manipulation may also vary with application situations. For example, the stem cells used in skin repair may be exempt from further induction, while osteoinduction of stem cells is usually included in bone repair. Moreover, in vitro pre-differentiation or in vivo differentiation of stem cells based on a controlled release system containing a cocktail of growth factors showed different effects [117]. Indeed, how USCs may participate in the repair process in various tissues remains to be clarified. Accordingly, whether cell induction or other manipulations are necessary remains uncertain. Furthermore, the application of USC secretions such as the extracellular vesicles, exosomes and extracellular matrix, may circumvent the potential risk of using the USCs themselves for treating the disease. It will also take a considerable amount of time to generate enough cells for autologous application. Therefore, it is more practical to apply USCs for treating chronic wound and elective surgical procedures but not acute burns, unless allogeneic applications of USCs prove to be safe, effective and cost-effective. For their non-invasive acquisition, low cost and tremendous application potential, USCs deserve more research and hold great promise for a broader range of applications.
Conclusions
The demands for wound healing, defect repair and tissue regeneration are ever-growing. Over the last decades, stem cells have shown great potential for regenerative medicine. As an emerging type of adult stem cell featuring non-invasive acquisition, USCs have been successfully applied for cytotherapies and tissue engineering in various disease models. With a few reports on skin, bone and articular cartilage repair, USCs have shown their effectiveness already. However, research on USCs is at its infancy stage, and more investigations are still required to answer the basic questions with regard to their origin, immunoregulatory activities and difference between their subpopulations, as well as optimization of their translational issues such as mode of application.
Supplementary Material
Contributor Information
Wenqian Zhang, Laboratory of Stem Cell and Tissue Engineering, Orthopedic Research Institute, Med-X Center for Materials, State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, Chengdu, Sichuan 610041, China.
Jungen Hu, Laboratory of Stem Cell and Tissue Engineering, Orthopedic Research Institute, Med-X Center for Materials, State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, Chengdu, Sichuan 610041, China.
Yizhou Huang, Laboratory of Stem Cell and Tissue Engineering, Orthopedic Research Institute, Med-X Center for Materials, State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, Chengdu, Sichuan 610041, China.
Chenyu Wu, Laboratory of Stem Cell and Tissue Engineering, Orthopedic Research Institute, Med-X Center for Materials, State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, Chengdu, Sichuan 610041, China.
Huiqi Xie, Laboratory of Stem Cell and Tissue Engineering, Orthopedic Research Institute, Med-X Center for Materials, State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, Chengdu, Sichuan 610041, China.
Funding
This work was financially supported by National Natural Science Foundation of China (Grant No. 31771065), the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (Grant No. ZYJC18002).
Authors’ contributions
All authors read and approved the final manuscript. WQZ and JGH contributed equally to the review, and should be viewed as co-first authors.
Conflicts of interest
None declared.
References
- 1. Gabbe BJ, Braaf S, Fitzgerald M, Judson R, Harrison JE, Lyons RA, et al. RESTORE: REcovery after serious trauma - outcomes, resource use and patient experiences study protocol. Inj Prev. 2015;21:348–54. [DOI] [PubMed] [Google Scholar]
- 2. Peck MD. Epidemiology of burns throughout the world. Part I: distribution and risk factors. Burns. 2011;37:1087–100. [DOI] [PubMed] [Google Scholar]
- 3. Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Snippert HJ, Clevers H. Tracking adult stem cells. EMBO Rep. 2011;12:113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Park YB, Ha CW, Lee CH, Yoon YC, Park YG. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: results from a clinical trial for safety and proof-of concept with 7 years of extended follow-up. Stem Cells Transl Med. 2016;5:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Childs PG, Reid S, Salmeron SM, Dalby MJ. Hurdles to uptake of mesenchymal stem cells and their progenitors in therapeutic products. Biochem J. 2020;477:3349–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vagnozzi RJ, Maillet M, Sargent MA, Khalil H, Johansen AK, Schwanekamp JA, et al. An acute immune response underlies the benefit of cardiac stem-cell therapy. Nature. 2019;577:405–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther. 2016;7:125–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Witzgall R. Are renal proximal tubular epithelial cells constantly prepared for an emergency? Focus on “the proliferation capacity of the renal proximal tubule involves the bulk of differentiated epithelial cells”. Am J Physiol Cell Physiol. 2008;294:C1–3. [DOI] [PubMed] [Google Scholar]
- 10. Ingelfinger JR. Nephrogenic adenomas as renal tubular outposts. N Engl J Med. 2002;347:684–6. [DOI] [PubMed] [Google Scholar]
- 11. Dörrenhaus A, Müller JIF, Golka K, Jedrusik P, Schulze H, Föllmann WJ. Cultures of exfoliated epithelial cells from different locations of the human urinary tract and the renal tubular system. Arch Toxicol. 2000;74:618–26. [DOI] [PubMed] [Google Scholar]
- 12. Zhang Y, McNeill E, Tian H, Soker S, Andersson KE, Yoo JJ, et al. Urine derived cells are a potential source for urological tissue reconstruction. J Urol. 2008;180:2226–33. [DOI] [PubMed] [Google Scholar]
- 13. Staack A, Hayward SW, Baskin LS, Cunha GR. Molecular, cellular and developmental biology of urothelium as a basis of bladder regeneration. Differentiation. 2005;73:121–33. [DOI] [PubMed] [Google Scholar]
- 14. Khandelwal P, Abraham SN, Apodaca G. Cell biology and physiology of the uroepithelium. Am J Physiol-Renal. 2009;297:1477–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu R, Liu G, Fan Y, Rohozinski J, Lu X, Rodriguez G, et al. 249 human urine-derived stem cells originate from parietal stem cells. J Urol. 2013;189:103. [Google Scholar]
- 16. Chen AJ, Pi JK, Hu JG, Huang YZ, Gao HW, Li SF, et al. Identification and characterization of two morphologically distinct stem cell subpopulations from human urine samples. Sci China Life Sci. 2020;63:712–23. [DOI] [PubMed] [Google Scholar]
- 17. Bharadwaj S, Liu G, Shi Y, Wu R, Yang B, He T, et al. Multipotential differentiation of human urine-derived stem cells: potential for therapeutic applications in urology. Stem Cells. 2013;31:1840–56. [DOI] [PubMed] [Google Scholar]
- 18. Ouyang B, Sun X, Han D, Chen S, Yao B, Gao Y, et al. Human urine-derived stem cells alone or genetically-modified with FGF2 improve type 2 diabetic erectile dysfunction in a rat model. PLoS One. 2014;9:e92825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burdeyron P, Giraud S, Hauet T, Steichen C. Urine-derived stem/progenitor cells: a focus on their characterization and potential. World J Stem Cells. 2020;12:1080–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu G, Pareta RA, Wu R, Shi Y, Zhou X, Liu H, et al. Skeletal myogenic differentiation of urine-derived stem cells and angiogenesis using microbeads loaded with growth factors. Biomaterials. 2013;34:1311–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lang R, Liu G, Shi Y, Bharadwaj S, Leng X, Zhou X, et al. Self-renewal and differentiation capacity of urine-derived stem cells after urine preservation for 24 hours. PLoS One. 2013;8:e53980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu G, Wu R, Yang B, Deng C, Lu X, Walker SJ, et al. Human urine-derived stem cell differentiation to endothelial cells with barrier function and nitric oxide production. Stem Cells Transl Med. 2018;7:686–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wan Q, Xiong G, Liu G, Shupe TD, Wei G, Zhang D, et al. Urothelium with barrier function differentiated from human urine-derived stem cells for potential use in urinary tract reconstruction. Stem Cell Res Ther. 2018;9:304–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li X, Liao J, Su X, Li W, Bi Z, Wang J, et al. Human urine-derived stem cells protect against renal ischemia/reperfusion injury in a rat model via exosomal miR-146a-5p which targets IRAK1. Theranostics. 2020;10:9561–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang D, Wei G, Li P, Zhou X, Zhang Y. Urine-derived stem cells: a novel and versatile progenitor source for cell-based therapy and regenerative medicine. Genes Dis. 2014;1:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rahman MS, Wruck W, Spitzhorn LS, Nguyen L, Bohndorf M, Martins S, et al. The FGF, TGFβ and WNT axis modulate self-renewal of human SIX2+ urine derived renal progenitor cells. Sci Rep-UK. 2020;10:739–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hwang YH, Cha SH, Hong Y, Jung AR, Jun HS. Direct differentiation of insulin-producing cells from human urine-derived stem cells. Int J Med Sci. 2019;16:1668–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu S, Liu Y, Bharadwaj S, Atala A, Zhang Y. Human urine-derived stem cells seeded in a modified 3D porous small intestinal submucosa scaffold for urethral tissue engineering. Biomaterials. 2011;32:1317–26. [DOI] [PubMed] [Google Scholar]
- 29. Bodin A, Bharadwaj S, Wu S, Gatenholm P, Atala A, Zhang Y. Tissue-engineered conduit using urine-derived stem cells seeded bacterial cellulose polymer in urinary reconstruction and diversion. Biomaterials. 2010;31:8889–901. [DOI] [PubMed] [Google Scholar]
- 30. Wu S, Wang Z, Bharadwaj S, Hodges SJ, Atala A, Zhang Y. Implantation of autologous urine derived stem cells expressing vascular endothelial growth factor for potential use in genitourinary reconstruction. J Urol. 2011;186:640–7. [DOI] [PubMed] [Google Scholar]
- 31. Wu R, Huang C, Wu Q, Jia X, Liu M, Xue Z, et al. Exosomes secreted by urine-derived stem cells improve stress urinary incontinence by promoting repair of pubococcygeus muscle injury in rats. Stem Cell Res Ther. 2019;10:80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu G, Wang X, Sun X, Deng C, Atala A, Zhang Y. The effect of urine-derived stem cells expressing VEGF loaded in collagen hydrogels on myogenesis and innervation following after subcutaneous implantation in nude mice. Biomaterials. 2013;34:8617–29. [DOI] [PubMed] [Google Scholar]
- 33. Zhang C, Luo D, Li T, Yang Q, Xie Y, Chen H, et al. Transplantation of human urine-derived stem cells ameliorates erectile function and cavernosal endothelial function by promoting autophagy of corpus cavernosal endothelial cells in diabetic erectile dysfunction rats. Stem Cells Int. 2019;2019:2168709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sun B, Luo X, Yang C, Liu P, Yang Y, Dong X, et al. Therapeutic effects of human urine-derived stem cells in a rat model of cisplatin-induced acute kidney injury in vivo and in vitro. Stem Cells Int. 2019;2019:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang C, George SK, Wu R, Thakker PU, Abolbashari M, Kim TH, et al. Reno-protection of urine-derived stem cells in a chronic kidney disease rat model induced by renal ischemia and nephrotoxicity. Int J Biol Sci. 2020;16:435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dong X, Zhang T, Liu Q, Zhu J, Zhao J, Li J, et al. Beneficial effects of urine-derived stem cells on fibrosis and apoptosis of myocardial, glomerular and bladder cells. Mol Cell Endocrinol. 2016;427:21–32. [DOI] [PubMed] [Google Scholar]
- 37. Zhao T, Luo D, Sun Y, Niu X, Wang Y, Wang C, et al. Human urine-derived stem cells play a novel role in the treatment of STZ-induced diabetic mice. J Mol Histol. 2018;49:419–28. [DOI] [PubMed] [Google Scholar]
- 38. Duan YR, Chen BP, Chen F, Yang SX, Zhu CY, Ma YL, et al. Exosomal microRNA-16-5p from human urine-derived stem cells ameliorates diabetic nephropathy through protection of podocyte. J Cell Mol Med. 2019;00:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xiong G, Tao L, Ma WJ, Gong MJ, Zhao L, Shen LJ, et al. Urine-derived stem cells for the therapy of diabetic nephropathy mouse model. Eur Rev Med Pharmacol Sci. 2020;24:1316–24. [DOI] [PubMed] [Google Scholar]
- 40. Hu C, He Y, Fang S, Tian N, Gong M, Xu X, et al. Urine-derived stem cells accelerate the recovery of injured mouse hepatic tissue. Am J Transl Res. 2020;12:5131–50. [PMC free article] [PubMed] [Google Scholar]
- 41. Zhou C, Wu XR, Liu HS, Liu XH, Liu GH, Zheng XB, et al. Immunomodulatory effect of urine-derived stem cells on inflammatory bowel diseases via downregulating Th1/Th17 immune responses in a PGE2-dependent manner. J Crohns Colitis. 2020;14:654–68. [DOI] [PubMed] [Google Scholar]
- 42. Guan JJ, Niu X, Gong FX, Hu B, Guo SC, Lou YL, et al. Biological characteristics of human-urine-derived stem cells: potential for cell-based therapy in neurology. Tissue Eng Part A. 2014;20:1794–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen CY, Du W, Rao SS, Tan YJ, Hu XK, Luo MJ, et al. Extracellular vesicles from human urine-derived stem cells inhibit glucocorticoid-induced osteonecrosis of the femoral head by transporting and releasing pro-angiogenic DMBT1 and anti-apoptotic TIMP1. Acta Biomater. 2020;111:208–20. [DOI] [PubMed] [Google Scholar]
- 44. Guan J, Zhang J, Guo S, Zhu H, Zhu Z, Li H, et al. Human urine-derived stem cells can be induced into osteogenic lineage by silicate bioceramics via activation of the Wnt/β-catenin signaling pathway. Biomaterials. 2015;55:1–11. [DOI] [PubMed] [Google Scholar]
- 45. Guan J, Zhang J, Li H, Zhu Z, Guo S, Niu X, et al. Human urine derived stem cells in combination with beta-TCP can be applied for bone regeneration. PLoS One. 2015;10:e0125253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen L, Li L, Xing F, Peng J, Peng K, Wang Y, et al. Human urine-derived stem cells: potential for cell-based therapy of cartilage defects. Stem Cells Int. 2018;2018:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fu Y, Guan J, Guo S, Guo F, Niu X, Liu Q, et al. Human urine-derived stem cells in combination with polycaprolactone/gelatin nanofibrous membranes enhance wound healing by promoting angiogenesis. J Transl Med. 2014;12:274–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhou T, Benda C, Duzinger S, Huang Y, Li X, Li Y, et al. Generation of induced pluripotent stem cells from urine. J Am Soc Nephrol. 2011;22:1221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jiang YF, Chen M, Zhang NN, Yang HJ, Rui Q, Zhou YF. In vitro and in vivo differentiation of induced pluripotent stem cells generated from urine-derived cells into cardiomyocytes. Biol Open. 2018;7:bio029157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cai J, Zhang Y, Liu P, Chen S, Wu X, Sun Y, et al. Generation of tooth-like structures from integration-free human urine induced pluripotent stem cells. Cell Regen. 2013;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jia B, Chen S, Zhao Z, Liu P, Cai J, Qin D, et al. Modeling of hemophilia a using patient-specific induced pluripotent stem cells derived from urine cells. Life Sci. 2014;108:22–9. [DOI] [PubMed] [Google Scholar]
- 52. Lee YM, Zampieri BL, Scott-McKean JJ, Johnson MW, Costa ACS. Generation of integration-free induced pluripotent stem cells from urine-derived cells isolated from individuals with down syndrome. Stem Cells Transl Med. 2017;6:1465–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ruiz-Babot G, Balyura M, Hadjidemetriou I, Ajodha SJ, Taylor DR, Ghataore L, et al. Modeling congenital adrenal hyperplasia and testing interventions for adrenal insufficiency using donor-specific reprogrammed cells. Cell Rep. 2018;22:1236–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li G, Xie B, He L, Zhou T, Gao G, Liu S, et al. Generation of retinal organoids with mature rods and cones from urine-derived human induced pluripotent stem cells. Stem Cells Int. 2018;2018:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sun G, Ding B, Wan M, Chen L, Jackson J, Atala A. Formation and optimization of three-dimensional organoids generated from urine-derived stem cells for renal function in vitro. Stem Cell Res Ther. 2020;11:309–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tang L, Wang H, Dai B, Wang X, Zhou D, Shen J, et al. Human induced pluripotent stem cell-derived cardiomyocytes reveal abnormal TGFβ signaling in type 2 diabetes mellitus. J Mol Cell Cardiol. 2020;142:53–64. [DOI] [PubMed] [Google Scholar]
- 57. Liu W, Zhang P, Tan J, Lin Y. Differentiation of urine-derived induced pluripotent stem cells to neurons, astrocytes, and microvascular endothelial cells from a diabetic patient. Cell Reprogram. 2020;22:147–55. [DOI] [PubMed] [Google Scholar]
- 58. Ouyang B, Xie Y, Zhang C, Deng C, Lv L, Yao J, et al. Extracellular vesicles from human urine-derived stem cells ameliorate erectile dysfunction in a diabetic rat model by delivering proangiogenic microRNA. Sex Med. 2019;7:241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhu Q, Li Q, Niu X, Zhang G, Ling X, Zhang J, et al. Extracellular vesicles secreted by human urine-derived stem cells promote ischemia repair in a mouse model of hind-limb ischemia. Cell Physiol Biochem. 2018;47:1181–92. [DOI] [PubMed] [Google Scholar]
- 60. Jiang ZZ, Liu YM, Niu X, Yin JY, Hu B, Guo SC, et al. Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res Ther. 2016;7:24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chen CY, Rao SS, Ren L, Hu XK, Tan YJ, Hu Y, et al. Exosomal DMBT1 from human urine-derived stem cells facilitates diabetic wound repair by promoting angiogenesis. Theranostics. 2018;8:1607–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Deng C, Xie Y, Zhang C, Ouyang B, Chen H, Lv L, et al. Urine-derived stem cells facilitate endogenous spermatogenesis restoration of Busulfan-induced nonobstructive azoospermic mice by paracrine exosomes. Stem Cells Dev. 2019;28:1322–33. [DOI] [PubMed] [Google Scholar]
- 63. Chen CY, Rao SS, Tan YJ, Luo MJ, Hu XK, Yin H, et al. Extracellular vesicles from human urine-derived stem cells prevent osteoporosis by transferring CTHRC1 and OPG. Bone Res. 2019;7:18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pei M, Li J, Zhang Y, Liu G, Wei L, Zhang Y. Expansion on a matrix deposited by nonchondrogenic urine stem cells strengthens the chondrogenic capacity of repeated-passage bone marrow stromal cells. Cell Tissue Res. 2014;356:391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Li J, Narayanan K, Zhang Y, Hill RC, He F, Hansen KC, et al. Role of lineage-specific matrix in stem cell chondrogenesis. Biomaterials. 2020;231:119681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wu C, Chen L, Huang YZ, Huang Y, Parolini O, Zhong Q, et al. Comparison of the proliferation and differentiation potential of human urine-, placenta decidua basalis-, and bone marrow-derived stem cells. Stem Cells Int. 2018;2018:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kim K, Gil M, Dayem AA, Choi S, Kang GH, Yang GM, et al. Improved isolation and culture of urine-derived stem cells (USCs) and enhanced production of immune cells from the USC-derived induced pluripotent stem cells. J Clin Med. 2020;9:827–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kang HS, Choi SH, Kim BS, Park GB, Kwon TG, Chun SY. Advanced properties of urine derived stem cells compared to adipose tissue derived stem cells in terms of cell proliferation, immune modulation and multi differentiation. J Korean Med Sci. 2015;30:1764–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sun JC, Xing F, Zou M, Gong M, Li L, Xiang Z. Comparison of chondrogenesis-related biological behaviors between human urine derived stem cells and human bone marrow mesenchymal stem cells from the same individual. Stem Cell Res Ther. 2021;12:366–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gabrielyan A, Neumann E, Gelinsky M, Wolff AR. Metabolically conditioned media derived from bone marrow stromal cells or human skin fibroblasts act as effective chemoattractants for mesenchymal stem cells. Stem Cell Res Ther. 2017;8:212–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mathes SH, Ruffner H, Graf-Hausner U. The use of skin models in drug development. Adv Drug Deliv Rev. 2014;69-70:81–102. [DOI] [PubMed] [Google Scholar]
- 72. Nejati R, Kovacic D, Slominski A. Neuro-immune-endocrine functions of the skin: an overview. Expert Rev Dermatol. 2013;8:581–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sun BK, Siprashvili Z, Khavari PA. Advances in skin grafting and treatment of cutaneous wounds. Science. 2014;346:941–5. [DOI] [PubMed] [Google Scholar]
- 74. Shpichka A, Butnaru D, Bezrukov EA, Sukhanov RB, Atala A, Burdukovskii V, et al. Skin tissue regeneration for burn injury. Stem Cell Res Ther. 2019;10:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Guo JM, Hu HD, Gorecka J, Bai HL, He H, Assi R, et al. Adipose-derived mesenchymal stem cells accelerate diabetic wound healing in a similar fashion as bone marrow-derived cells. Am J Physiol Cell Physiol. 2018;315:C885–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chen SX, Shi JB, Zhang M, Chen YH, Wang XE, Zhang L, et al. Mesenchymal stem cell-laden anti-inflammatory hydrogel enhances diabetic wound healing. Sci Rep. 2015;5:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lataillade JJ, Doucet C, Bey E, Carsin H, Huet C, Clairand I, et al. New approach to radiation burn treatment by dosimetry-guided surgery combined with autologous mesenchymal stem cell therapy. Regen Med. 2007;2:785–94. [DOI] [PubMed] [Google Scholar]
- 78. Xu HJ, Huang SH, Wang JJ, Lan Y, Feng LB, Zhu MS, et al. Enhanced cutaneous wound healing by functional injectable thermo-sensitive chitosan-based hydrogel encapsulated human umbilical cord-mesenchymal stem cells. Int J Biol Macromol. 2019;137:433–41. [DOI] [PubMed] [Google Scholar]
- 79. Wang HF, Chen LY, Liu Y, Luo BZ, Xie NZ, Tan T, et al. Implantation of placenta-derived mesenchymal stem cells accelerates murine dermal wound closure through immunomodulation. Am J Transl Res. 2016;8:4912–21. [PMC free article] [PubMed] [Google Scholar]
- 80. Zhang YL, Niu X, Dong X, Wang Y, Li HY. Bioglass enhanced wound healing ability of urine-derived stem cells through promoting paracrine effects between stem cells and recipient cells. J Tissue Eng Regen Med. 2018;12:E1609–22. [DOI] [PubMed] [Google Scholar]
- 81. Cao YM, Liu MY, Xue ZW, Qiu Y, Li J, Wang Y, et al. Surface-structured bacterial cellulose loaded with hUSCs accelerate skin wound healing by promoting angiogenesis in rats. Biochem Biophys Res Commun. 2019;516:1167–74. [DOI] [PubMed] [Google Scholar]
- 82. Zhang XR, Huang YZ, Gao HW, Jiang YL, Hu JG, Pi JK, et al. Hypoxic preconditioning of human urine-derived stem cell-laden small intestinal submucosa enhances wound healing potential. Stem Cell Res Ther. 2020;11:150–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Walmsley GG, Ransom RC, Zielins ER, Leavitt T, Flacco JS, Hu MS, et al. Stem cells in bone regeneration. Stem Cell Rev Rep. 2016;12:524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3:S131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Li Y, Chen SK, Li L, Qin L, Wang XL, Lai YX. Bone defect animal models for testing efficacy of bone substitute biomaterials. J Orthop Transl. 2015;3:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. El-Rashidy AA, Roether JA, Harhaus L, Kneser U, Boccaccini AR. Regenerating bone with bioactive glass scaffolds: a review of in vivo studies in bone defect models. Acta Biomater. 2017;62:1–28. [DOI] [PubMed] [Google Scholar]
- 87. Zhang K, Fan Y, Dunne N, Li X. Effect of microporosity on scaffolds for bone tissue engineering. Regen Biomater. 2018;5:115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Huang Y, Deng H, Fan Y, Zheng L, Che J, Li X, et al. Conductive nanostructured Si biomaterials enhance osteogeneration through electrical stimulation. Mater Sci Eng C. 2019;103:109748. [DOI] [PubMed] [Google Scholar]
- 89. Kasten P, Luginbuhl R, van Griensven M, Barkhausen T, Krettek C, Bohner M, et al. Comparison of human bone marrow stromal cells seeded on calcium-deficient hydroxyapatite, beta-tricalcium phosphate and demineralized bone matrix. Biomaterials. 2003;24:2593–603. [DOI] [PubMed] [Google Scholar]
- 90. Salgado AJ, Coutinho OP, Reis RL. Bone tissue engineering: state of the art and future trends. Macromol Biosci. 2004;4:743–65. [DOI] [PubMed] [Google Scholar]
- 91. Derubeis AR, Cancedda RJ. Bone marrow stromal cells (BMSCs) in bone engineering: limitations and recent advances. Ann Biomed Eng. 2004;32:160–5. [DOI] [PubMed] [Google Scholar]
- 92. Bruder SP, Kraus KH, Goldberg VM, Kadiyala S. The effect of implants loaded with autologous mesenchymal stem cells on the healing of canine segmental bone defects. J Bone Joint Surg Am. 1998;80:985–96. [DOI] [PubMed] [Google Scholar]
- 93. Xie HQ, Yang F, Deng L, Luo J, Qin T, Li X, et al. The performance of a bone-derived scaffold material in the repair of critical bone defects in a rhesus monkey model. Biomaterials. 2007;28:3314–24. [DOI] [PubMed] [Google Scholar]
- 94. Xie HQ, Huang FG, Zhao YF, Qin TW, Li XQ, Liu C, et al. Tissue-engineered ribs for chest wall reconstruction: a case with 12-year follow-up. Regen Med. 2014;9:431–6. [DOI] [PubMed] [Google Scholar]
- 95. Storti G, Scioli MG, Kim BS, Orlandi A, Cervelli V. Adipose-derived stem cells in bone tissue engineering: useful tools with new applications. Stem Cells Int. 2019;2019:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wang P, Liu X, Zhao L, Weir MD, Sun J, Chen W, et al. Bone tissue engineering via human induced pluripotent, umbilical cord and bone marrow mesenchymal stem cells in rat cranium. Acta Biomater. 2015;18:236–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Moshaverinia A, Chen C, Akiyama K, Xu X, Chee WW, Schricker SR, et al. Encapsulated dental-derived mesenchymal stem cells in an injectable and biodegradable scaffold for applications in bone tissue engineering. J Biomed Mater Res A. 2013;101:3285–94. [DOI] [PubMed] [Google Scholar]
- 98. Qin H, Zhu C, An Z, Jiang Y, Zhao Y, Wang J, et al. Silver nanoparticles promote osteogenic differentiation of human urine-derived stem cells at noncytotoxic concentrations. Int J Nanomedicine. 2014;9:2469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Guan J, Zhang J, Zhu Z, Niu X, Guo S, Wang Y, et al. Bone morphogenetic protein 2 gene transduction enhances the osteogenic potential of human urine-derived stem cells. Stem Cell Res Ther. 2015;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Sun X, Zheng W, Qian C, Wu Q, Hao Y, Lu G. Focal adhesion kinase promotes BMP2-induced osteogenic differentiation of human urinary stem cells via AMPK and Wnt signaling pathways. J Cell Physiol. 2020;235:4954–64. [DOI] [PubMed] [Google Scholar]
- 101. Xing F, Li L, Sun J, Liu G, Duan X, Chen J, et al. Surface mineralized biphasic calcium phosphate ceramics loaded with urine-derived stem cells are effective in bone regeneration. J Orthop Surg Res. 2019;14:419–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bhosale AM, Richardson JB. Articular cartilage: structure, injuries and review of management. Br Med Bull. 2008;87:77–95. [DOI] [PubMed] [Google Scholar]
- 103. Makris EA, Gomoll AH, Malizos KN, Hu JC, Athanasiou KA. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 2015;11:21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Madeira C, Santhagunam A, Salgueiro JB, Cabral JMS. Advanced cell therapies for articular cartilage regeneration. Trends Biotechnol. 2015;33:35–42. [DOI] [PubMed] [Google Scholar]
- 105. De Bari C, Roelofs AJ. Stem cell-based therapeutic strategies for cartilage defects and osteoarthritis. Curr Opin Pharmacol. 2018;40:74–80. [DOI] [PubMed] [Google Scholar]
- 106. Yin H, Wang Y, Sun Z, Sun X, Xu Y, Li P, et al. Induction of mesenchymal stem cell chondrogenic differentiation and functional cartilage microtissue formation for in vivo cartilage regeneration by cartilage extracellular matrix-derived particles. Acta Biomater. 2016;33:96–109. [DOI] [PubMed] [Google Scholar]
- 107. Qi Y, Du Y, Li W, Dai X, Zhao T, Yan W. Cartilage repair using mesenchymal stem cell (MSC) sheet and MSCs-loaded bilayer PLGA scaffold in a rabbit model. Knee Surg Sports Traumatol Arthrosc. 2014;22:1424–33. [DOI] [PubMed] [Google Scholar]
- 108. Gao P, Han P, Jiang D, Yang S, Cui Q, Li Z. Effects of the donor age on proliferation, senescence and osteogenic capacity of human urine-derived stem cells. Cytotechnology. 2017;69:751–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Schosserer M, Reynoso R, Wally V, Jug B, Kantner V, Weilner S, et al. Urine is a novel source of autologous mesenchymal stem cells for patients with epidermolysis bullosa. BMC Res Notes. 2015;8:767–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Afzal MZ, Strande JL. Generation of induced pluripotent stem cells from muscular dystrophy patients: efficient integration-free reprogramming of urine derived cells. J Vis Exp. 2015;28:52032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Liu Y, Ming L, Luo H, Liu W, Zhang Y, Liu H, et al. Integration of a calcined bovine bone and BMSC-sheet 3D scaffold and the promotion of bone regeneration in large defects. Biomaterials. 2013;34:9998–10006. [DOI] [PubMed] [Google Scholar]
- 112. Badenes SM, Fernandes-Platzgummer A, Rodrigues CAV, Diogo MM, da Silva CL, Cabral JMS. Chapter 4 - Microcarrier culture systems for stem cell manufacturing. In: Cabral JMS, Lobato de Silva C, Chase LG, Margarida Diogo M (eds). Stem Cell Manufacturing. Boston: Elsevier, 2016, 77–104. [Google Scholar]
- 113. Chun SY, Kim HT, Kwon SY, Kim J, Kim BS, Yoo ES, et al. The efficacy and safety of collagen-I and hypoxic conditions in urine-derived stem cell ex vivo culture. Tissue Eng Regen Med. 2016;13:403–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Galipeau J, Krampera M, Barrett J, Dazzi F, Deans RJ, DeBruijn J, et al. International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy. 2016;18:151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Murray RZ, West ZE, Cowin AJ, Farrugia BL. Development and use of biomaterials as wound healing therapies. Burns Trauma. 2019;7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Tan QW, Tang SL, Zhang Y, Yang JQ, Wang ZL, Xie HQ, et al. Hydrogel from acellular porcine adipose tissue accelerates wound healing by inducing intradermal adipocyte regeneration. J Invest Dermatol. 2019;139:455–63. [DOI] [PubMed] [Google Scholar]
- 117. Liu G, Wu R, Yang B, Shi Y, Deng C, Atala A, et al. A cocktail of growth factors released from a heparin hyaluronic-acid hydrogel promotes the myogenic potential of human urine-derived stem cells in vivo. Acta Biomater. 2020;107:50–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.