Abstract
Atopic dermatitis (AD) is one of the most prevalent chronic inflammatory skin diseases in the world. It is characterized by recurrent eczematous lesions and intense itch, and many cytokines are involved in the pathogenesis of AD. Among them, much attention has been paid to interleukin 31 (IL-31) as an AD-associated itch mediator. IL-31 is mainly produced by CD4+ helper T cells and transmits the signals via a heterodimeric receptor composed of IL-31 receptor A (IL-31RA) and oncostatin M receptor (OSMR), both of which are expressed in dorsal root ganglion (DRG) neurons. However, the molecular mechanisms of how IL-31 is produced in helper T cells upon stimulation and transmits the itch sensation to the brain were largely unknown. Recently, by using original mouse models of AD, we have identified endothelial PAS domain 1 (EPAS1) and neurokinin B (NKB) as key molecules critical for IL-31 production and IL-31-mediated itch transmission, respectively. These molecules could be novel drug targets for AD-associated itch. This review highlights our recent findings, which show the functional significance of these molecules in the IL-31-induced itch sensation, referring to their application to drug development.
Keywords: atopic dermatitis, chemical compounds, EPAS1, neurokinin B, SP1
Introduction
The atopic dermatitis (AD)-associated itch sensation induces scratching behavior and exacerbates skin inflammation, leading to an itch–scratch cycle (1). Therefore, chronic itch is a challenging clinical problem in the treatment of AD. Recently, interleukin 31 (IL-31) has been shown to play a major role in AD-associated itch; for example, serum IL-31 levels were correlated with disease severity in AD patients (2, 3). In addition, both mice treated with intra-dermal injections of IL-31 and transgenic mice over-expressing IL-31 exhibit scratching behavior and develop severe dermatitis (4, 5). After being secreted by type 2 helper T (Th2) cells, IL-31 transmits signals through a receptor complex consisting of IL-31 receptor A (IL-31RA) and oncostatin M receptor (OSMR) (5). Recent clinical studies have demonstrated that blockade of IL-31 signals by a humanized anti-human IL-31RA monoclonal antibody, nemolizumab, alleviates pruritus in patients with AD (6–8). Thus, the IL-31–IL-31RA axis is crucial for AD-associated itch; however, the mechanisms controlling the production of IL-31 and itch transmission via IL-31 signals are largely unknown.
Dedicator of cytokinesis (DOCK) family proteins are evolutionarily conserved guanine nucleotide exchange factors (GEFs). This family consists of 11 members (DOCK1–11), according to their sequence and substrate specificity (9). Among them, DOCK8 mediates Cdc42 activation through its DOCK homology region 2 (DHR-2) domain (10). Recently, it has been reported that bi-allelic loss-of-function mutations in DOCK8 cause a combined immunodeficiency characterized by AD in humans (11, 12). We found that DOCK8-deficient (Dock8–/–) mice spontaneously develop AD-like skin disease when crossed with transgenic mice expressing the AND T-cell receptor (TCR); these mice are designated ‘AND Tg mice’ (13). By analyzing these mice as AD model mice, we have identified key molecules essential for IL-31 production and itch transmission via IL-31RA–OSMR. In this review, we detail the functional significance of these molecules in the IL-31-induced itch sensation and refer to their applications to drug development.
The molecular mechanism of IL-31 production
DOCK8 negatively regulates IL-31 induction in helper T cells
To elucidate the molecular link between DOCK8 deficiency and atopic skin inflammation, we investigated the role of DOCK8 in antigen-specific T-cell responses. Initially, we developed Dock8–/– transgenic mice expressing the OTII TCR that recognizes ovalbumin peptide (OVA323-339) bound to I-Ab MHC molecules. Although OVA-specific T-cell proliferation and IL-2/IL-4 production were comparable between Dock8+/– and Dock8–/– OTII Tg mice, CD4+ T cells from Dock8–/– OTII Tg mice produced large amounts of IL-31 upon stimulation with OVA peptide (13). Dock8–/– OTII Tg mice did not develop skin inflammation; however, scratching behavior was induced when in vitro activated CD4+ T cells from Dock8–/– OTII Tg mice were adoptively transferred into CAG-OVA mice that ubiquitously express OVA protein under the control of the cytomegalovirus immediate early enhancer-chicken β-actin hybrid promoter (13).
To examine whether this effect could be extended to other CD4+ T cells with different antigen specificity, we developed Dock8–/– mice expressing the AND TCR. The AND TCR is a product of an artificial TCRαβ-chain combination, which recognizes the moth cytochrome C peptide (MCC88–103) in the context of I-Ek MHC molecules. Yet, it is known that CD4+CD8+ thymocytes expressing AND are also selected to mature in the presence of I-Ab MHC molecules (14). As seen in Dock8–/– OTII Tg mice, CD4+ T cells from Dock8–/– AND Tg mice produced IL-31 abundantly upon stimulation with MCC peptide (13). These results indicate that DOCK8 acts as a negative regulator for IL-31 induction in helper T cells.
The transcription factor EPAS1 functions as a master regulator for IL-31 induction
It has been reported that the AND TCR shows high self-reactivity to selecting I-Ab MHC molecules (15). Surprisingly, we discovered that Dock8–/– AND Tg mice developed severe skin inflammation spontaneously with scratching behavior and increased serum IL-31 levels (13). In addition, this skin inflammation was completely lost in Dock8–/– AND Tg mice when either OSMR or IL-31 was genetically deleted (13). These results demonstrate that Dock8–/– AND Tg mice spontaneously develop atopic skin inflammation through a mechanism dependent on IL-31 signaling.
By functionally analyzing 40 transcription factors that were up-regulated in Dock8–/– CD4+ T cells in microarray analysis, we identified endothelial PAS domain 1 (EPAS1) as a master regulator for IL-31 induction in CD4+ T cells (13). Indeed, induction of Il31 in Dock8–/– AND CD4+ T cells was markedly suppressed by knocking down Epas1 gene expression using small interfering RNA (siRNA) or genetically deleting EPAS1 expression in a T-cell-specific manner (designated CD4-Cre+Epas1lox/loxDock8–/– AND Tg mice). More importantly, scratching behavior, skin disease development and increased serum IL-31 levels were canceled in all CD4-Cre+Epas1lox/loxDock8–/– AND Tg mice tested (13). EPAS1 is known as hypoxia-inducible factor (HIF)-2α and controls hypoxic responses by forming a complex with aryl hydrocarbon receptor nuclear translocator (ARNT; also known as HIF-1β) (16). However, we demonstrated that EPAS1-mediated Il31 promoter activation was independent of ARNT and its activation occurred in collaboration with the transcription factor SP1 (specificity protein 1) (13).
Nuclear translocation of EPAS1 is regulated by DOCK8–MST1 interaction
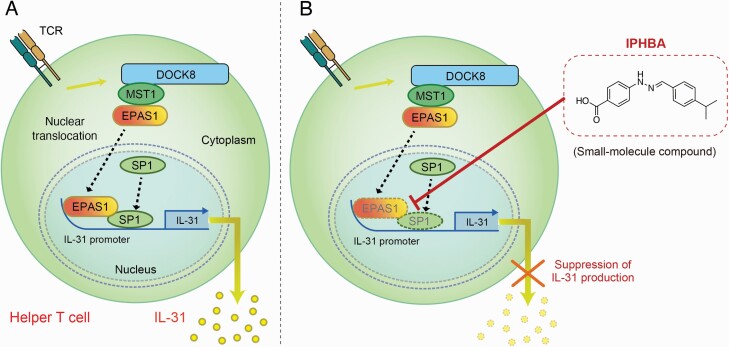
How EPAS1 is activated in helper T cells from AD patients is currently unknown. However, Elo et al. indicated that in human CD4+ T cells, EPAS1 is directly regulated by signal transducer and activator of transcription protein 6 (STAT6), a transcription factor required for IL-4-driven Th2 cell differentiation (17). Recently, another study demonstrated that EPAS1 expression in murine CD4+ T cells is regulated by mTOR complex 1 (mTORC1) and mTORC2 downstream from PI3K (18). The localization of EPAS1 in the cell is also important because EPAS1 mediates promoter activation of target genes in the nucleus after translocation from the cytoplasm (19). Thereby, we examined the effect of DOCK8 deficiency on subcellular localization of EPAS1 in mouse embryonic fibroblasts (MEFs). Nuclear localization of EPAS1 was notably augmented in Dock8–/– MEFs, and this effect of DOCK8 deficiency was canceled when wild-type (WT) DOCK8 was stably expressed in Dock8–/– MEFs, but not a DOCK8 mutant lacking the N-terminal 527 amino acid residues (13). Then, co-immunoprecipitation assay revealed that DOCK8 bound to mammalian ste-20 like kinase 1 (MST1) through the N-terminal region. We found that nuclear translocation of EPAS1 was significantly augmented by knocking down Mst1 gene expression in WT MEFs. Then, knockdown of Mst1 markedly induced Il31 gene expression in CD4+ T cells from Dock8+/– AND Tg mice (13). These findings show that the DOCK8–MST1 interaction negatively regulates IL-31 induction by inhibiting nuclear translocation of EPAS1 (Fig. 1A).
Fig. 1.
The effect on EPAS1-driven IL-31 production by the small-molecule compound IPHBA. (A) In helper T cells, DOCK8 binds to MST1 through the N-terminal region of DOCK8 and negatively regulates antigen-induced IL-31 production by inhibiting nuclear translocation of EPAS1. Upon stimulation, EPAS1 is translocated into the nucleus and induces Il31 gene expression in collaboration with the transcription factor SP1. (B) IPHBA inhibits the association between EPAS1 and SP1, resulting in defective recruitment of both transcription factors to the specific sites of the Il31 promoter. IPHBA could be a novel drug seed compound that suppresses IL-31 production in helper T cells.
Therapeutic approaches that combat AD-associated itch by suppressing IL-31 production
Identification of small-molecule inhibitors targeting EPAS1-driven IL-31 induction
Although blockade of IL-31 signals by an antibody specific for IL-31RA alleviates pruritus in AD patients (6–8), therapeutic approaches to inhibit IL-31 production by helper T cells remained unexploited. To identify small-molecule inhibitors of IL-31 production, we generated a luciferase reporter system using immortalized MEFs, in which EPAS1 was inducibly expressed in the presence of doxycycline to mediate Il31 promoter activation (20). By using this reporter system, we screened a chemical library consisting of 9600 compounds and identified 20 compounds as primary hits showing over 50% inhibition of Il31 promoter activity at a concentration of 10 μM. Then, after the subsequent phenotypic screening, we finally discovered that 4-(2-(4-isopropylbenzylidene)hydrazineyl)benzoic acid (IPHBA) effectively inhibited antigen-induced gene expression of Il31 in CD4+ T cells from Dock8–/– AND Tg mice. In addition, IPHBA treatment did not affect either the expression of Il2 and Il4 in these CD4+ T cells or non-specific T-cell proliferation induced by phorbol 12-myristate 13-acetate (PMA) plus ionomycin (20).
We also found that IPHBA could be detected in the blood for 12 hours in mice after being orally treated (20). Scratching behavior was significantly suppressed when IPHBA was orally administered after in vitro activated CD4+ T cells from Dock8–/– OTII Tg mice were adoptively transferred into CAG-OVA mice, indicating that oral administration of IPHBA could function in vivo. This treatment did not affect the body weight over 2 weeks, and the organs of treated mice exhibited no macroscopic abnormalities at autopsy (20).
IPHBA impairs recruitment of EPAS1 and SP1 to Il31 promotor
EPAS1 forms a complex with ARNT for cellular adaptations to low oxygen stress (16). Hypoxia-induced expression of VEGFA and GLUT1 was suppressed when a human cancer cell line was treated with FM19G11, which is an inhibitor that suppresses EPAS1 expression by acting on an undefined target (13, 20, 21). In contrast, no such inhibitory effect was seen for IPHBA, suggesting that ARNT-dependent EPAS1 functions are unaffected by IPHBA.
To understand the mechanism of how IPHBA regulates IL-31 induction in CD4+ T cells, we performed chromatin immunoprecipitation (ChIP) assays using CD4+ T cells from Dock8–/– AND Tg mice. We found that both EPAS1 and SP1 were recruited to Il31 promoter regions upon antigen stimulation, but their recruitment was significantly attenuated by IPHBA treatment (20). Luciferase reporter assays also showed that IPHBA treatment or the mutation of a consensus EPAS1-binding sequence in the reporter construct diminished EPAS1-mediated Il31 promoter activation (20, 22). It is indicated that EPAS1 forms an enhanceosome complex on the promoters of target genes through interactions with other transcription factors (23). Whereas the association between EPAS1 and SP1 was readily detected in human embryonic kidney 293T (HEK-293T) cells co-expressing FLAG-tagged EPAS1 and hemagglutinin (HA)-tagged SP1, such heterodimer formation was impaired in the presence of IPHBA (20). Thus, IPHBA inhibits the association between EPAS1 and SP1, resulting in defective recruitment of both transcription factors to the Il31 promoter (Fig. 1B).
IPHBA inhibits IL-31 production in human T cells from AD patients
We next examined whether IPHBA also acts in human T cells. Upon stimulation, CD4+ T cells from AD patients produced larger amounts of IL-31 than those from healthy controls and IPHBA treatment decreased the amount of IL-31 without affecting IL-2 production (20). Moreover, human IL31 promoter activation was induced in the presence of human EPAS1, but it was significantly suppressed by IPHBA. By deleting the IL31 promoter region, we identified the critical region for EPAS1-mediated transactivation (20), which included a consensus SP1 binding sequence (24). When this sequence was mutated, EPAS1-mediated IL31 promoter activation was diminished (20). These results suggest that EPAS1 also forms a heterodimer with SP1 to mediate IL-31 induction in human CD4+ T cells.
Therapeutic approaches that target the core mechanisms of the IL-31-induced itch
Neurokinin B is released from dorsal root ganglion neurons in response to IL-31
IL-31 produced by helper T cells transmits the signals via the IL-31RA–OSMR receptor complex. Previous reports showed that IL-31RA is most abundantly expressed in dorsal root ganglion (DRG) neurons in mice and humans (5, 25). Usoskin et al. performed single-cell RNA-based dissection of the mouse lumbar DRG for unbiased classification of sensory neuron types, which reveals that NP1, NP2 and NP3 neuronal types are likely to participate in pruritus (26). Among them, NP3 neurons expressed IL-31 receptor genes (Il31ra and Osmr) (26). Then, to identify candidate molecules that mediate the IL-31-induced itch, we performed microarray analysis of the DRG. The analysis revealed that Tac2 gene encoding neurokinin B (NKB) was expressed at much higher levels in Dock8–/– AND Tg mice than those in Dock8+/– AND Tg mice (27). Indeed, real-time PCR analysis for validation showed that the expression of Tac2 in the DRG neurons notably increased in Dock8–/– AND Tg mice and that Tac2 expression was canceled by genetically deleting OSMR or IL-31 in Dock8–/– AND Tg mice (27). Moreover, we confirmed that NKB peptide was expressed by the IL-31RA+ DRG neurons in Dock8–/– AND Tg mice and released in vitro from primary DRG neurons of WT mice in response to IL-31. Then, NKB release was completely abolished when primary DRG neurons lack OSMR expression (27). These findings indicate that NKB is released from DRG neurons in response to IL-31 stimulation.
NKB is selectively required for itch transmission by IL-31 signals
NKB is an endogenous peptide that belongs to the family of tachykinins including substance P (28). To examine the physiological significance of NKB in the IL-31-induced itch sensation, we developed NKB-deficient (Tac2–/–) mice (27). Intra-dermal injection of histamine, chloroquine or a protease-activated receptor 2 (PAR2) agonist (SLIGRL-NH2) comparably induced scratching in both Tac2–/– and Tac2+/– mice. However, whereas intra-dermal injection of IL-31 induced scratching in Tac2+/– mice, Tac2–/– mice were significantly less sensitive to IL-31. Interestingly, Tac2-deficiency markedly improved the skin inflammation and scratching behavior in Dock8–/– AND Tg mice without affecting serum IL-31 levels (27). Thus, NKB is selectively required for transmission of the IL-31-induced itch sensation.
NKB acts upstream of GRP to transmit IL-31-induced itch sensation
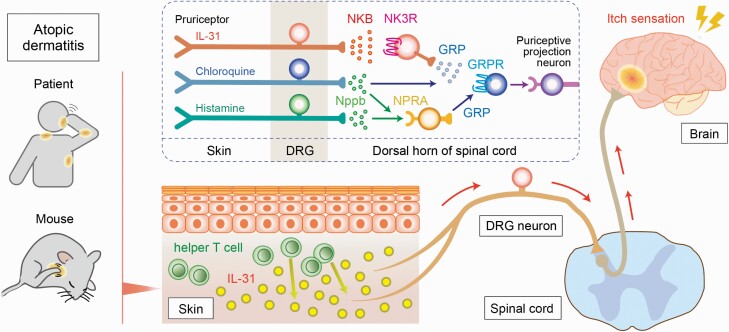
Natriuretic polypeptide b (Nppb) and gastrin-releasing peptide (GRP) in the spinal cord are known to be related with the transmission of the itch sensation (29); therefore, we examined whether itch transmission via IL-31 signals requires these peptides. Nppb has been implicated in IL-31-mediated skin inflammation in the periphery (29), but IL-31-induced scratching was unaffected when neurons expressing the Nppb receptor (natriuretic peptide receptor A; NPRA) were specifically ablated by intrathecal injection of toxin (saporin)-conjugated Nppb (27). In contrast, treatment with GRP-saporin reduced IL-31-induced scratching. Whereas GRP was released in vitro from WT DRG neurons in response to IL-31, GRP release was not detected when Tac2–/– primary DRG neurons were stimulated. In addition, the itch response induced by intrathecal injection of NKB was lost when neurons expressing the GRP receptor (GRPR) were ablated beforehand by toxin treatment (27). These results indicate that NKB acts upstream of GRP to transmit the IL-31-induced itch sensation (Fig. 2).
Fig. 2.
A scheme of the IL-31-mediated itch signaling pathway. In the atopic skin lesion, IL-31 is largely secreted by helper T cells and activates specific pruriceptors, resulting in the release of NKB from DRG neurons. NKB binds NK3R and induces the release of GRP to transmit the IL-31-induced itch sensation in the spinal cord. On the other hand, histamine-induced or chloroquine-induced pruritus is transmitted via Nppb and GRP, independent of the NKB–NK3R signaling pathway.
NK3R antagonists selectively inhibit the IL-31-induced itch response
NKB transmits the signals through neurokinin 3 receptor (NK3R), a G protein-coupled tachykinin receptor (28, 30). So far, several selective antagonists for NK3R such as osanetant and fezolinetant have been developed (31, 32). We found that intra-peritoneal injection of osanetant or oral administration of fezolinetant into WT mice attenuated IL-31-induced scratching behavior remarkably (26). On the other hand, treatment with NK3R antagonists failed to suppress the itch response induced by histamine, chloroquine or SLIGRL-NH2 (26). Thus, pharmacological inhibition of NK3R selectively attenuates the IL-31-induced itch sensation. Although NK3R antagonists suppress sex hormones by modulating gonadotropin secretion, the effect is transient and reversible, with no major side effects reported as yet (31, 32). Therefore, NK3R antagonists may be another option for treating AD-associated itch, particularly in adults.
Conclusion
We found that NKB is required for itch transmission via IL-31 signals and that NK3R antagonists may be useful for the control of IL-31-induced itch. Additionally, on the basis of our findings about how IL-31 is produced in helper T cells, we discovered the small-molecule compound IPHBA as a novel drug seed for the IL-31-induced itch sensation. Although the potency of IPHBA is not high enough, the design and synthesis of IPHBA analogs would lead to development of an oral drug for AD patients. These IL-31-targeted therapies might be applicable to not only AD but also other skin diseases that feature elevation of serum IL-31 levels, such as prurigo nodularis, psoriasis, cutaneous T-cell lymphoma and uremic pruritus (22, 33–38). To be noted, helper T cells may not be the only source of IL-31 in some contexts; for example, dermal conventional dendritic cells mainly produce IL-31 in response to the cytokine TGF-β1 during cutaneous wound healing (39). Besides, several studies revealed that IL-31 is also secreted by basophils, eosinophils, mast cells and macrophages (40–43). Whether IL-31 production is regulated by DOCK8–EPAS1/SP1 pathway in IL-31-expressing cells other than helper T cells or in various skin diseases is an important issue that should be investigated in future studies.
Funding
This work was supported by Leading Advanced Projects for Medical Innovation (grant no. JP19gm0010001) and Advanced Research and Development Programs for Medical Innovation (grant no. JP20gm1310005) from Japan Agency for Medical Research and Development (to Y.F.).
Conflicts of interest statement: the authors declared no conflicts of interest.
References
- 1. Weidinger, S., Beck, L. A., Bieber, T.et al. 2018. Atopic dermatitis. Nat. Rev. Dis. Primers 4:1. [DOI] [PubMed] [Google Scholar]
- 2. Raap, U., Wichmann, K., Bruder, M.et al. 2008. Correlation of IL-31 serum levels with severity of atopic dermatitis. J. Allergy Clin. Immunol. 122:421. [DOI] [PubMed] [Google Scholar]
- 3. Raap, U., Weißmantel, S., Gehring, M.et al. 2012. IL-31 significantly correlates with disease activity and Th2 cytokine levels in children with atopic dermatitis. Pediatr. Allergy Immunol. 23:285. [DOI] [PubMed] [Google Scholar]
- 4. Dillon, S. R., Sprecher, C., Hammond, A.et al. 2004. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat. Immunol. 5:752. [DOI] [PubMed] [Google Scholar]
- 5. Cevikbas, F., Wang, X., Akiyama, T.et al. 2014. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: involvement of TRPV1 and TRPA1. J. Allergy Clin. Immunol. 133:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kabashima, K., Matsumura, T., Komazaki, H.et al. ; Nemolizumab-JP01 Study Group. 2020. Trial of nemolizumab and topical agents for atopic dermatitis with pruritus. N. Engl. J. Med. 383:141. [DOI] [PubMed] [Google Scholar]
- 7. Silverberg, J. I., Pinter, A., Pulka, G.et al. 2020. Phase 2B randomized study of nemolizumab in adults with moderate-to-severe atopic dermatitis and severe pruritus. J. Allergy Clin. Immunol. 145:173. [DOI] [PubMed] [Google Scholar]
- 8. Mihara, R., Kabashima, K., Furue, M.et al. 2019. Nemolizumab in moderate to severe atopic dermatitis: an exploratory analysis of work productivity and activity impairment in a randomized phase II study. J. Dermatol. 46:662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kunimura, K., Uruno, T. and Fukui, Y. 2020. DOCK family proteins: key players in immune surveillance mechanisms. Int. Immunol. 32:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harada, Y., Tanaka, Y., Terasawa, M.et al. 2012. DOCK8 is a Cdc42 activator critical for interstitial dendritic cell migration during immune responses. Blood 119:4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang, Q., Davis, J. C., Lamborn, I. T.et al. 2009. Combined immunodeficiency associated with DOCK8 mutations. N. Engl. J. Med. 361:2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Engelhardt, K. R., Gertz, E. M., Keles, S.et al. 2015. The extended clinical phenotype of 64 patients with DOCK8 deficiency. J. Allergy Clin. Immunol. 136:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamamura, K., Uruno, T., Shiraishi, A.et al. 2017. The transcription factor EPAS1 links DOCK8 deficiency to atopic skin inflammation via IL-31 induction. Nat. Commun. 8:13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaye, J., Vasquez, N. J. and Hedrick, S. M. 1992. Involvement of the same region of the T cell antigen receptor in thymic selection and foreign peptide recognition. J. Immunol. 148:3342. [PubMed] [Google Scholar]
- 15. Mandl, J. N., Monteiro, J. P., Vrisekoop, N.et al. 2013. T cell-positive selection uses self-ligand binding strength to optimize repertoire recognition of foreign antigens. Immunity 38:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu, D., Potluri, N., Lu, J.et al. 2015. Structural integration in hypoxia-inducible factors. Nature 524:303. [DOI] [PubMed] [Google Scholar]
- 17. Elo, L. L., Järvenpää, H., Tuomela, S.et al. 2010. Genome-wide profiling of interleukin-4 and STAT6 transcription factor regulation of human Th2 cell programming. Immunity 32:852. [DOI] [PubMed] [Google Scholar]
- 18. Cho, S. H., Raybuck, A. L., Blagih, J.et al. 2019. Hypoxia-inducible factors in CD4 + T cells promote metabolism, switch cytokine secretion, and T cell help in humoral immunity. Proc. Natl Acad. Sci. USA 116:8975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Luo, J. C. and Shibuya, M. 2001. A variant of nuclear localization signal of bipartite-type is required for the nuclear translocation of hypoxia inducible factors (1alpha, 2alpha and 3alpha). Oncogene 20:1435. [DOI] [PubMed] [Google Scholar]
- 20. Kamikaseda, Y., Uruno, T., Kunimura, K.et al. 2021. Targeted inhibition of EPAS1-driven IL-31 production by a small-molecule compound. J. Allergy Clin. Immunol. 148:633. [DOI] [PubMed] [Google Scholar]
- 21. Moreno-Manzano, V., Rodríguez-Jiménez, F. J., Aceña-Bonilla, J. L.et al. 2010. FM19G11, a new hypoxia-inducible factor (HIF) modulator, affects stem cell differentiation status. J. Biol. Chem. 285:1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smythies, J. A., Sun, M., Masson, N.et al. 2019. Inherent DNA-binding specificities of the HIF-1α and HIF-2α transcription factors in chromatin. EMBO Rep. 20:e46401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pawlus, M. R. and Hu, C. J. 2013. Enhanceosomes as integrators of hypoxia inducible factor (HIF) and other transcription factors in the hypoxic transcriptional response. Cell. Signal. 25:1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mikulska, J. E. 2015. Analysis of response elements involved in the regulation of the human neonatal Fc Receptor Gene (FCGRT). PLoS One 10:e0135141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sonkoly, E., Muller, A., Lauerma, A. I.et al. 2006. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J. Allergy Clin. Immunol. 117:411. [DOI] [PubMed] [Google Scholar]
- 26. Usoskin, D., Furlan, A., Islam, S.et al. 2015. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci. 18:145. [DOI] [PubMed] [Google Scholar]
- 27. Sakata, D., Uruno, T., Matsubara, K.et al. 2019. Selective role of neurokinin B in IL-31-induced itch response in mice. J. Allergy Clin. Immunol. 144:1130. [DOI] [PubMed] [Google Scholar]
- 28. Zhang, W. W., Wang, Y. and Chu, Y. X. 2020. Tacr3/NK3R: beyond their roles in reproduction. ACS Chem. Neurosci. 11:2935. [DOI] [PubMed] [Google Scholar]
- 29. Bautista, D. M., Wilson, S. R. and Hoon, M. A. 2014. Why we scratch an itch: the molecules, cells and circuits of itch. Nat. Neurosci. 17:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Al Abed, A. S., Reynolds, N. J. and Dehorter, N. 2021. A second wave for the neurokinin Tac2 pathway in brain research. Biol. Psychiatry. 90:156. [DOI] [PubMed] [Google Scholar]
- 31. Spooren, W., Riemer, C. and Meltzer, H. 2005. NK3 receptor antagonists: the next generation of antipsychotics? Nat. Rev. Drug Discov. 4:967. [DOI] [PubMed] [Google Scholar]
- 32. Fraser, G. L., Ramael, S., Hoveyda, H. R.et al. 2016. The NK3 receptor antagonist ESN364 suppresses sex hormones in men and women. J. Clin. Endocrinol. Metab. 101:417. [DOI] [PubMed] [Google Scholar]
- 33. Ständer, S., Yosipovitch, G., Legat, F. J.et al. 2020. Trial of nemolizumab in moderate-to-severe prurigo nodularis. N. Engl. J. Med. 382:706. [DOI] [PubMed] [Google Scholar]
- 34. Narbutt, J., Olejniczak, I., Sobolewska-Sztychny, D.et al. 2013. Narrow band ultraviolet B irradiations cause alteration in interleukin-31 serum level in psoriatic patients. Arch. Dermatol. Res. 305:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Singer, E. M., Shin, D. B., Nattkemper, L. A.et al. 2013. IL-31 is produced by the malignant T-cell population in cutaneous T-cell lymphoma and correlates with CTCL pruritus. J. Invest. Dermatol. 133:2783. [DOI] [PubMed] [Google Scholar]
- 36. Oweis, A. O., Al-Qarqaz, F., Bodoor, K.et al. 2021. Elevated interleukin 31 serum levels in hemodialysis patients are associated with uremic pruritus. Cytokine 138:155369. [DOI] [PubMed] [Google Scholar]
- 37. Kabashima, K. and Irie, H. 2021. Interleukin-31 as a clinical target for pruritus treatment. Front. Med. (Lausanne) 8:638325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Furue, M. and Furue, M. 2021. Interleukin-31 and pruritic skin. J. Clin. Med. 10:1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xu, J., Zanvit, P., Hu, L.et al. 2020. The cytokine TGF-β induces interleukin-31 expression from dermal dendritic cells to activate sensory neurons and stimulate wound itching. Immunity 53:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Raap, U., Gehring, M., Kleiner, S.et al. 2017. Human basophils are a source of - and are differentially activated by - IL-31. Clin. Exp. Allergy. 47:499. [DOI] [PubMed] [Google Scholar]
- 41. Rüdrich, U., Gehring, M., Papakonstantinou, E.et al. 2018. Eosinophils are a major source of interleukin-31 in bullous pemphigoid. Acta Derm. Venereol. 98:766. [DOI] [PubMed] [Google Scholar]
- 42. Niyonsaba, F., Ushio, H., Hara, M.et al. 2010. Antimicrobial peptides human β-defensins and cathelicidin LL-37 induce the secretion of a pruritogenic cytokine IL-31 by human mast cells. J. Immunol. 184:3526. [DOI] [PubMed] [Google Scholar]
- 43. Hashimoto, T., Kursewicz, C. D., Fayne, R. A.et al. 2020. Mechanisms of itch in stasis dermatitis: significant role of IL-31 from macrophages. J. Invest. Dermatol. 140:850. [DOI] [PubMed] [Google Scholar]