Abstract
We describe the development of a capture enzyme-linked immunosorbent assay for the detection of the dengue virus nonstructural protein NS1. The assay employs rabbit polyclonal and monoclonal antibodies as the capture and detection antibodies, respectively. Immunoaffinity-purified NS1 derived from dengue 2 virus-infected cells was used as a standard to establish a detection sensitivity of approximately 4 ng/ml for an assay employing monoclonal antibodies recognizing a dengue 2 serotype-specific epitope. A number of serotype cross-reactive monoclonal antibodies were also shown to be suitable probes for the detection of NS1 expressed by the remaining three dengue virus serotypes. Examination of clinical samples demonstrated that the assay was able to detect NS1 with minimal interference from serum components at the test dilutions routinely used, suggesting that it could form the basis of a useful additional diagnostic test for dengue virus infection. Furthermore, quantitation of NS1 levels in patient sera may prove to be a valuable surrogate marker for viremia. Surprisingly high levels of NS1, as much as 15 μg/ml, were found in acute-phase sera taken from some of the patients experiencing serologically confirmed dengue 2 virus secondary infections but was not detected in the convalescent sera of these patients. In contrast, NS1 could not be detected in either acute-phase or convalescent serum samples taken from patients with serologically confirmed primary infection. The presence of high levels of secreted NS1 in the sera of patients experiencing secondary dengue virus infections, and in the context of an anamnestic antibody response, suggests that NS1 may contribute significantly to the formation of the circulating immune complexes that are suspected to play an important role in the pathogenesis of severe dengue disease.
Dengue viruses are a major public health problem in tropical and subtropical areas, being the cause of one of the most important mosquito-borne viral diseases. Up to 20 million people are infected globally each year (15). Infection with dengue virus can result in a relatively benign, acute febrile illness (dengue fever) or in severe disease with abnormalities in vascular permeability (dengue hemorrhagic fever [DHF]) which can sometimes lead to sudden and often fatal hypovolemic shock (dengue shock syndrome [DSS]) (10). All four dengue virus serotypes are capable of causing dengue fever, with the induction of an immune response that in most cases leads to lifelong protection against clinical disease arising from infection with the homologous serotype. Secondary infection with a heterologous serotype, however, may lead to the severe complications of DHF and DSS. Antibody-dependent enhancement of dengue virus growth in cells of the monocyte/macrophage lineage resulting from the presence of preexisting, nonneutralizing dengue virus-specific antibodies has been proposed as the pathogenetic mechanism that underlies DHF and DSS (12). However, the link between this enhanced replication and the vascular permeability that characterizes these diseases is still the subject of conjecture (24).
The dengue viruses are enveloped and contain a single, positive-sense RNA genome of about 11 kb that encodes a large polyprotein precursor. Co- and posttranslational processing gives rise to three structural and seven nonstructural proteins, encoded by genes in the order (from 5′ to 3′) C, prM, E, NS1, NS2a, NS2b, NS3, NS4a, NS4b, and NS5. NS1 is a 46- to 50-kilodalton glycoprotein which is expressed in both membrane associated (mNS1) and secreted (sNS1) forms (5, 30) and possesses both group-specific and type-specific determinants (6, 14). NS1 is unusual for a viral glycoprotein in that it does not form part of the virion structure but is expressed on the surface of infected cells. NS1 is initially translocated into the endoplasmic reticulum via a hydrophobic signal sequence encoded in the C-terminal region of E, where it rapidly dimerizes (30). While the function of NS1 is yet to be fully defined, preliminary evidence has shown it to be involved in viral RNA replication (18, 19).
NS1 was first described as a soluble complement-fixing (SCF) antigen in infected cell cultures (2, 3). The identity of SCF as the viral-encoded 46-kilodalton glycoprotein gp46 was established by Smith and Wright (28), and it was later renamed NS1 following the sequencing of the yellow fever virus genome (23). The flavivirus NS1 has been recognized as an important immunogen in infections (26) and has been shown to play a role in protection against disease (13, 27). However, a potential role for NS1 in immunopathogenesis has also been proposed based on the finding of anti-SCF antibodies in sera from patients undergoing secondary but not primary infections (8). The contribution of this antibody response to disease severity is not clearly understood, but it is now well established that circulating immune complexes and complement activation are integral features of DHF and DSS and are likely to play a significant role in pathogenesis (11, 24, 29). It is possible, therefore, that in addition to the virion itself (22), secreted NS1 may also contribute to immune complex formation.
In this study, we used our existing panel of monoclonal antibodies (MAbs) (6) to establish a sensitive capture enzyme-linked immunosorbent assay (ELISA) for NS1. Our aims were to assess the potential of using NS1 as a diagnostic marker of infection as well as to provide the assays necessary for investigating whether secreted NS1 might contribute to immune complex formation. The assay developed in this study was able to detect and quantify the levels of dengue 2 virus NS1 in both tissue culture harvests and a small panel of patient sera.
MATERIALS AND METHODS
Cells and viruses.
Vero cells were used to passage dengue viruses and were grown in 199 medium (Gibco BRL, Melbourne, Australia) supplemented with 5% fetal calf serum. Dengue type 2 virus (PR159) was originally obtained from Ernie Gould (Institute of Virology and Environmental Microbiology, NERC, Oxford, United Kingdom), and dengue types 1 (Hawaii), 2 (NGC), 3 (H87), and 4 (H241) viruses were from John Aaskov (Queensland University of Technology, Brisbane, Australia). Virus stocks were used to infect 80% confluent cell monolayers in 199 medium supplemented with 2% fetal calf serum and incubated at 37°C until cytopathic effect (CPE) was observed (between 3 and 6 days postinfection, depending on serotype), at which stage the supernatant and cell monolayers were harvested.
Immunoaffinity purification of NS1.
Purification of the secreted form of NS1 (sNS1) from media harvests of infected Vero cells was carried out as previously described by Falconar and Young (5). Briefly, harvests taken at peak CPE from Vero cells infected with dengue 2 virus (NGC) at a multiplicity of infection of 0.1 were first clarified by centrifugation at 2,000 × g in a bench-top centrifuge. After the addition of protease inhibitors, the clarified supernatant was then passed through a Sepharose 4B (Pharmacia, Uppsala, Sweden) column coupled with the MAb 5H4.4 (6). After washing the column with 5-bed volumes of TNE (10 mM Tris-HCl [pH 7.4], 150 mM NaCl, and 5 mM EDTA), sNS1 was eluted from the column in TNE containing 40 mM diethylamine (BDH, Kilsyth, Australia). Purity of the eluted fractions was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by silver staining. Peak fractions were pooled, protein concentration was determined by the bicinchoninic acid assay (Pierce), and fractions were stored as 50-μl aliquots at −70°C.
Monoclonal and polyclonal antibodies.
Previously characterized MAbs (6) raised against dengue 2 virus (PR159) NS1 were tested as probes for the capture ELISA. A selection of these MAbs was then chosen for further analysis based on preliminary binding data (Table 1). This selected panel includes both dengue group cross-reactive and dengue 2-specific MAbs. All were prepared as ascitic fluids by inoculation of hybridomas into pristane-primed mice and stored as 1:1 glycerol stocks at −20°C. Polyclonal, monospecific anti-NS1 antibody was raised in rabbits hyperimmunized with immunoaffinity-purified, dimeric dengue virus type 2 NS1. A human polyclonal serum with a high-titered anti-NS1 response was obtained from a primary infection case some 3 months after infection.
TABLE 1.
NS1-specific MAbs used as detection probes in the NS1 capture ELISA
| MAba | ELISA titerb | Linear epitopec | Cross-reactivity with other dengue serotypesd
|
|||
|---|---|---|---|---|---|---|
| DEN1 | DEN2 | DEN3 | DEN4 | |||
| 1H7.4 | 6.0 | + | − | +++ | − | − |
| 2C9.4 | 5.4 | + | − | ++ | − | − |
| 5H4.4 | 5.4 | + | − | +++ | − | − |
| 5H6.3 | 4.6 | − | − | +++ | − | + |
| 5B5.3 | 3.5 | − | − | ++ | +++ | + |
| 4H3.4 | 5.3 | + | +++ | +++ | +++ | +++ |
| 3D1.4 | 5.2 | + | +++ | +++ | +++ | +++ |
| 3A5.4 | 5.7 | + | +++ | +++ | +++ | +++ |
All MAbs used in this study are of the immunoglobulin G1 isotype and were described in detail by Falconar and Young (6).
Titers are expressed as log10 50% end points, as measured against purified NS1-coated ELISA plates.
Reactivity of the MAbs with linear epitopes was determined by dot blot analysis against reduced and carboxy-methylated, purified dengue 2 NS1. PEPSCAN analysis has identified the location of some of these epitopes and was reported by Falconar et al. (7). See text for details.
Cross-reactivities were determined by immunoblot analysis of SDS-PAGE-separated SDS lysates of Vero cells infected with the dengue virus serotype prototypes DEN1 (Hawaii), DEN2 (NGC), DEN3 (H87), and DEN4 (H241) and given arbitrary values based on intensity of staining.
ELISA capture assay.
The wells of a microtiter plate (Immulon 4; Dynatech) were coated with 50 μl of a 1:1,500 dilution of rabbit anti-NS1 serum in carbonate buffer (pH 9.6) and incubated overnight at 4°C. Between all subsequent incubation steps, the plates were washed three times with phosphate-buffered saline (PBS) containing 0.05% Tween 20 (PBST), and all dilutions were made in PBST containing 0.25% gelatin. The plates were blocked with 150 μl of PBS containing 1% gelatin and incubated at room temperature for 1 h. The antigen (immunoaffinity-purified NS1 or patient sera) was diluted in duplicate (in some cases, normal human sera [NHS] was incorporated in the diluent), and after removal of the blocking solution, 50 μl per well was added. The plates were incubated at 37°C for 1 h and washed, and 50 μl of the anti-NS1 MAb (diluted 1:1,000) was then added. Following a further incubation at 37°C for 1 h, the plates were washed and 50 μl of goat anti-mouse peroxidase-conjugated antibody (Jackson Immunoresearch) diluted 1:1,000 was added. Plates were incubated for 1 h at 37°C, washed, and developed with freshly prepared substrate solution (0.04% o-phenylenediamine.2HCl in citrate phosphate buffer, pH 5.0, containing 0.003% H2O2). The reaction was allowed to proceed in the dark for 10 min before being halted by the addition of 25 μl of 2 M H2SO4 and read on a microplate reader (MR5000; Dynatech Laboratories) at 490 nm.
Clinical samples.
Sera from dengue virus-infected patients collected during an epidemic in Thailand were kindly provided by C. Hoke and colleagues (AFRIMS, Bangkok, Thailand). Paired serum samples taken from individuals during the acute and convalescent phases of both primary and secondary dengue infection were tested for the presence of NS1 by using the type-specific MAb 1H7.4 as a probe. Sera were initially diluted 1/10 and then serially diluted twofold in the assay. Each microtiter plate included a titration of a standard preparation of purified NS1 (over the range, 10 to 100 ng/ml) diluted in PBST containing a 1/10 dilution of NHS.
RESULTS
Selection of capture and detection antibodies.
In order to establish a sensitive antigen capture ELISA for the dengue virus NS1 glycoprotein, we used a cross-reactive rabbit polyclonal antiserum raised against immunoaffinity-purified NS1 to capture NS1 from all four dengue virus serotypes and examined a panel of 35 MAbs (6) as selective detection probes. Checkerboard analyses of a dilution series of the rabbit antiserum against the full panel of MAbs and using a standard concentration of immunoaffinity-purified NS1 established an optimum dilution of 1:1,500 for the polyclonal capture antibody and 1:1,000 for those MAbs which yielded a signal in the assay. Using any of the MAbs, either singly or in combination as capture antibodies, resulted in a significantly reduced signal, and only a limited number of MAbs were effective as detection probes. Based on these results, a small panel of MAbs was chosen for further analysis. These are listed in Table 1 and include those recognizing both type-specific and cross-reactive epitopes.
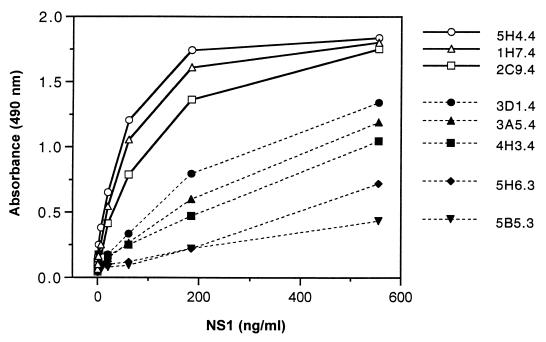
Figure 1 shows the results of using these MAbs as detection probes in the analysis of a dilution series of purified dengue 2 NS1. The results clearly indicate that the dengue 2-specific MAbs, 5H4.4, 1H7.4, and 2C9.4, clustered as a group showing the highest binding capacity. Not surprisingly, peptide-binding and competition studies have mapped these MAbs to the same epitope (25VHTWTEQYK33) (7, 31). The cross-reactive MAbs, 3D1.4, 3A5.4, and 4H3.4, also clustered as a group, reflecting their common epitope specificity (111LRYSWKTWGKA121) (7), and although less efficient as detection probes, they should prove useful for the analysis of NS1 encoded by each dengue virus serotype.
FIG. 1.
Reactivity of a panel of MAbs against serial dilutions of immunoaffinity-purified dengue 2 NS1 in the capture ELISA. NS1 was captured with ELISA plates coated with a 1:1,500 dilution of rabbit anti-NS1 polyclonal antisera and subsequently probed with the MAbs at a dilution of 1:1,000.
Given the identification of synergistic interactions between selected MAbs in competition analyses (31), we examined the effect of various combinations of MAbs on the detection sensitivity of the assay. No improvement in detection was observed with these cocktails when compared with 1H7.4 alone (results not shown), so this MAb was chosen for further assessment.
Sensitivity and reproducibility of the assay.
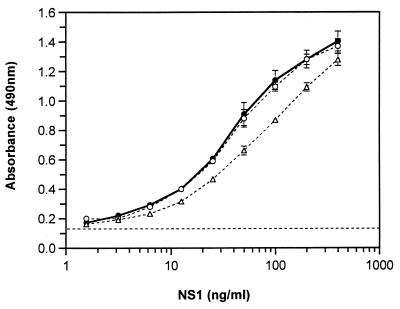
To determine the sensitivity of the NS1 capture assay, replicates of serially diluted immunoaffinity-purified NS1 of known concentration were probed with 1H7.4 (Fig. 2). Both PBS and pooled NHS were used as diluents in order to determine the effect that the presence of serum may have on detection sensitivity. The effect of NHS components on the capture and detection of NS1 was minimal at a 1-in-5 dilution and negligible at a 1-in-10 dilution (Fig. 2). As the subsequent analysis of patient sera was routinely carried out at dilutions of 1 in 10 or greater, it is assumed that serum components at these concentrations have little or no effect on detection sensitivity. The assay was considered positive if a sample yielded an optical density (OD) reading 3 standard deviations (0.036 OD492 [optical density at 492 nm] units) above the mean OD value for negative control samples (0.122 ± 0.012). Using these criteria, the limit of detection of NS1 with MAb 1H7.4 was approximately 4 ng/ml. The limit of detection of dengue 2 virus NS1 with the cross-reactive MAb probe 3D1.4 was approximately 15 ng/ml (data not shown).
FIG. 2.
Effect of human serum components on the detection of NS1 in the capture ELISA. Immunoaffinity-purified, secreted NS1 was serially diluted in the absence (●) or presence of NHS at dilutions of 1 in 5 (Δ) and 1 in 10 (○) and probed with the type-specific MAb 1H7.4. Data points represent the mean ± standard deviation for four replicates. The dashed line is the mean for the negative control samples (OD490, 0.122 ± 0.012).
The reproducibility of the assay was examined with multiple replicates assessed within the same test and by repeated testing over a 2-month period. Not surprisingly, the coefficient of variation results varied considerably depending on the level of NS1 present in the samples. In subsequent analyses of patient test sera (see below), samples were titrated and estimates of NS1 levels were determined by comparison of absorbance readings of appropriately diluted fractions with a standard curve derived from a titration series of purified NS1, within the range of 20 to 200 ng/ml. At a concentration of 100 ng/ml, the coefficient of variation for 12 replicates tested in the same assay was 4%, and the test-to-test coefficient of variation, using 16 replicates, was 12%. Samples stored at −70°C were stable over several months (data not shown).
Comparison of type-specific and group-reactive MAbs in the detection of secreted NS1 of all four dengue virus serotypes.
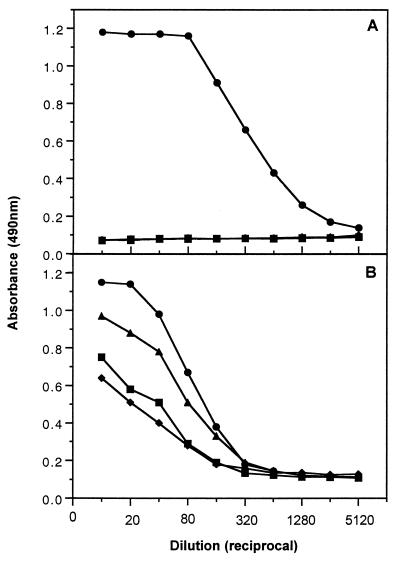
In order to examine the reactivity in the ELISA capture assay of NS1 derived from each of the four dengue virus serotypes, we tested media harvests taken from infected cell monolayers. Media harvests were taken at peak CPE (ranging from day 5 to 9 days postinfection for the different serotypes) from separate 25-cm2 flasks of Vero cell cultures infected at a multiplicity of infection of 0.1 with the four prototype dengue virus serotypes (DEN1 Hawaii, DEN2 NGC, DEN3 H87, and DEN4 H214). The 10-ml harvests were clarified by low-speed centrifugation for 10 min prior to analysis of the resulting supernatant fractions in the capture assay using either the type-specific (1H7.4) or group-reactive (3D1.4) MAbs as detection probes (Fig. 3). The specificity of the 1H7.4 probe for dengue 2 virus-derived NS1 was clearly demonstrated in Fig. 3A, as was the increased detection sensitivity of this MAb (approximately fourfold) compared with that of 3D1.4 (Fig. 3B). Comparison with a standard curve performed in parallel and prepared with serial dilutions of purified dengue 2 virus NS1 of known concentration indicated that between 5 and 7 μg/106 cells of NS1 was secreted from dengue 2 virus-infected Vero cells under these conditions. A time course analysis of NS1 secretion from infected cells paralleled virus titers as determined by a plaque assay (data not shown), suggesting that it may be possible to use secreted NS1 as a surrogate marker for viral infection.
FIG. 3.
Capture ELISA detection of NS1 secreted from Vero cells infected with all four dengue virus serotypes. Type-specific (1H7.4) (A) and group-reactive (3D1.4) (B) MAbs were used as the detection probes for media harvests from DEN1- (♦), DEN2- (●), DEN3- (■), and DEN4 (▴)-infected cells.
It is not known whether 3D1.4 recognizes the NS1 species derived from each serotype with equal avidity; however, it is interesting that the reactivity profile demonstrated in Fig. 3B reflects the antigenic pairings of the two known serotype subgroups, DEN2/4 and DEN1/3. It is also likely that the different levels of detection are in part due to the binding characteristics of the rabbit polyclonal capture antibody which was prepared by immunization with purified dengue 2 virus NS1. Differences in growth kinetics in Vero cells between the four dengue virus serotypes will also contribute to the varying levels of antigen detected in this assay. Currently, the assay employing 3D1.4 as the detecting probe can only provide evidence of the presence of NS1 in a sample. In order to provide quantitative estimates of NS1 derived from all four serotypes, purified preparations of each serotype NS1 will need to be generated to establish appropriate standard curves.
Detection of NS1 in patient sera.
In order to test the suitability of the assay for quantifying NS1 in clinical samples, a small panel of paired sera from dengue 2 virus-infected patients was examined. Sera taken during the acute phase of infection from 22 patients as well as 2 weeks later, during convalescence, were tested. Serum samples were serially diluted and processed in the ELISA capture assay (using 1H7.4 as the detecting antibody) in parallel with a titration of immunoaffinity-purified dengue 2 sNS1 as a standard. Absorbance readings of those dilutions that fell within the straight-line portion of the standard curve (10 to 100 ng/ml) were used to calculate the serum NS1 concentration (Table 2). Six of the patients were experiencing a primary infection with dengue 2 virus, and NS1 was not detected in any of the paired sera from this group. Of the 16 patients with a serologically confirmed secondary infection, seven were positive for NS1 with levels ranging from 70 ng/ml to as high as 15 μg/ml in the acute-phase sera. These values should be viewed in the context of the likely presence of anti-NS1 antibodies in the acute-phase sera of patients experiencing a secondary infection (4, 8, 17). They are presumably an underestimate of the true levels of secreted NS1, given that a significant proportion of NS1 may be trapped in immune complexes and not detected in our assay. Indeed, preincubation of purified NS1 with human sera containing antibodies to NS1 successfully competes with all of the MAb probes used in this assay (data not shown). We have tried a number of techniques reported to successfully dissociate immune complexes for subsequent antigen detection (16, 21), but to date without success, as each appears to lead to significant loss of NS1 antigenicity. None of the convalescent sera tested positive for NS1 (Table 2), presumably reflecting the transient nature of the viremia (9). Although there did not appear to be a significant difference in either the detection or in the levels of NS1 between those patients with dengue fever and those with dengue hemorrhagic fever, the small sample size precludes any definitive conclusions.
TABLE 2.
NS1 levels in the sera of dengue 2 virus-infected patients
| Type of infectiona | Gradeb | Serum phase | No. positive/no. tested (%) | [NS1] (μg/ml)c |
|---|---|---|---|---|
| Primary | DF | Acute | 0/6 (0) | —d |
| Convalescent | 0/6 (0) | — | ||
| Secondary | DF | Acute | 2/5 (40) | 0.6, 3.4 |
| Convalescent | 0/5 (0) | — | ||
| Secondary | DHF | Acute | 5/11 (45) | 0.07, 0.9, 0.8, 2.4, 15.0 |
| Convalescent | 0/11 (0) | — |
Defined by hemagglutination inhibition titers.
DF, dengue fever; DHF, dengue hemorrhagic fever.
NS1 levels were determined based on a comparison of the mean OD readings of diluted serum samples (three replicates for each dilution) in the ELISA capture assay with a standard curve performed in parallel.
NS1 level is below the detection sensitivity of the assay (4 ng/ml).
DISCUSSION
This paper describes the development of a capture ELISA for detection of the dengue virus protein NS1. Screening of an extensive panel of MAbs as detection probes and rabbit polyclonal anti-NS1 hyperimmune sera as capture antibody lead to the optimization of both type-specific and serotype cross-reactive assays. Despite the availability of a large number of MAbs specific for both linear and conformational epitopes (6), the majority were ineffective as probes in the capture ELISA. Of interest was the finding that only those MAbs specific for linear determinants, one type specific and the other cross-reactive among the dengue viruses, were effective as detection probes. The identity of these linear epitopes has been reported previously (7) and were defined by binding of the MAbs to a set of overlapping peptides. Although the amino acid sequence of the peptide identified by the dengue 2 virus-specific MAbs (25VHTWTEQYK33) is shared by the other three virus serotypes, the type specificity of their binding was clearly demonstrated in the present study (Fig. 3A). This finding supports our earlier demonstration that amino acids flanking this linear epitope appear to contribute conformational constraints that lead to the epitope specificity seen in the native protein (7). The sensitivity of the dengue 2 virus type-specific assay was estimated with immunoaffinity-purified NS1. Using this preparation as a reference standard, the assay was able to detect levels of NS1 down to 4 ng/ml. An analysis of infected Vero cells revealed that up to 7 μg of NS1 per ml was secreted into the media, with levels of secretion correlating with infectious virus titer.
We next tested the levels of NS1 in a panel of patient sera with the aim of assessing the potential of the capture ELISA as a diagnostic assay as well as the value of using NS1 as a surrogate marker of infection. However, in this study, NS1 was not detected in the limited panel of sera taken from patients experiencing a primary infection (Table 2). This result most likely reflects the sensitivity of the assay rather than the absence of NS1 in these samples, given that secreted NS1 is normally produced by infected eukaryotic cells and should therefore be present during the viremic phase of infection (30). The sensitivity of the assay will need to be improved beyond the current limit of 4 ng/ml to detect what must be lower levels of secretion of this protein in primary cases and in order for the assay to be of value in the routine diagnosis of dengue virus infections. These studies are currently underway. The low level of NS1 in these sera correlates well with earlier observations made by a number of groups of a poor antibody response to NS1 in most cases of primary infection (4, 8, 17). In contrast to these findings, relatively high levels of secreted NS1 were detected in the acute sera of patients experiencing a secondary infection (Table 2). This increase in the relative level of NS1 in turn suggests an increase in the infected cell population and, therefore, viral load in secondary versus primary infections. Taken together, these data provide the first direct evidence for enhanced virus replication in secondary infections in vivo, a hypothesis that has previously been supported by largely circumstantial data (24). We are presently examining a more extensive panel of patient sera to validate these preliminary findings.
Numerous studies have shown that in secondary dengue virus infections, both immune complexes and complement consumption vary linearly with disease severity pointing to a clear role for immune complexes in the pathogenesis of DHF and DSS (1, 20, 24, 25, 29). However, the identity of the antigen(s) involved in immune complex formation, while generally considered to be whole dengue virions (24), has yet to be determined. We have so far tested a relatively limited panel of patient sera, and the availability of only paired sera means that we have no data on the kinetics of appearance of NS1 and the antibody response to it. Nevertheless, the presence of both high levels of NS1 in the sera of secondarily infected patients, coupled with a vigorous anamnestic anti-NS1 response (4, 8, 17), identifies NS1 as a candidate antigen responsible for the formation of immune complexes in sufficient quantities to explain the massive complement activation seen in DHF and DSS (11, 24). Studies which address this possibility are presently in progress.
ACKNOWLEDGMENTS
We thank Charles Hoke and colleagues at AFRIMS, Bangkok, Thailand, for the generous provision of patient sera.
This work was funded in part by the World Health Organization and the National Health and Medical Research Council of Australia.
REFERENCES
- 1.Bokish V A, Top F H, Russell P K, Dixon F J, Muller-Eberhard H J. The potential pathogenic role of complement in dengue haemorragic fever syndrome. N Engl J Med. 1973;289:996–1000. doi: 10.1056/NEJM197311082891902. [DOI] [PubMed] [Google Scholar]
- 2.Brandt W E, Chiewslip D, Harris D L, Russell P K. Partial purification and characterization of a dengue virus soluble complement-fixing antigen. J Immunol. 1970;105:1565–1568. [PubMed] [Google Scholar]
- 3.Cardiff R D, Brandt W E, McCloud T G, Shapiro D, Russell P K. Immunological and biophysical separation of dengue-2 antigens. J Virol. 1971;7:15–23. doi: 10.1128/jvi.7.1.15-23.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Churdboonchart V, Bhamarapravati N, Peampramprecha S, Sirinavin S. Antibodies against dengue viral proteins in primary and secondary dengue hemorrhagic fever. Am J Trop Med Hyg. 1991;44:481–493. doi: 10.4269/ajtmh.1991.44.481. [DOI] [PubMed] [Google Scholar]
- 5.Falconar A K I, Young P R. Immunoaffinity purification of the native dimer forms of the flavivirus non-structural glycoprotein, NS1. J Virol Methods. 1990;30:323–332. doi: 10.1016/0166-0934(90)90075-q. [DOI] [PubMed] [Google Scholar]
- 6.Falconar A K I, Young P R. Production of dimer specific and dengue virus group cross reactive mouse monoclonal antibodies to the dengue 2 virus non-structural glycoprotein NS1. J Gen Virol. 1991;72:961–965. doi: 10.1099/0022-1317-72-4-961. [DOI] [PubMed] [Google Scholar]
- 7.Falconar A K I, Young P R, Miles M A. Precise location of sequential dengue virus subcomplex and complex B cell epitopes on the nonstructural-1 glycoprotein. Arch Virol. 1994;137:315–326. doi: 10.1007/BF01309478. [DOI] [PubMed] [Google Scholar]
- 8.Falkler W A, Diwan A R, Halstead S B. Human antibody to dengue soluble complement fixing (SCF) antigens. J Immunol. 1973;111:1804–1809. [PubMed] [Google Scholar]
- 9.Gubler D J, Suharyono W, Tan R, Abidin M, Sie A. Viraemia in patients with naturally acquired dengue infection. Bull W H O. 1981;59:623–630. [PMC free article] [PubMed] [Google Scholar]
- 10.Halstead S B. Immunological parameters of togavirus disease syndromes. In: Schlesinger R W, editor. The Togaviruses. New York, N. Y: Academic Press; 1980. pp. 107–173. [Google Scholar]
- 11.Halstead S B. Pathogenesis of dengue: challenges to molecular biology. Science. 1988;239:476–481. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- 12.Halstead S B. Antibody, macrophages, dengue virus infection, shock and hemorrhage: a pathogenic cascade. Rev Infect Dis. 1989;11(Suppl. 4):831–839. doi: 10.1093/clinids/11.supplement_4.s830. [DOI] [PubMed] [Google Scholar]
- 13.Henchal E A, Henchal L S, Schlesinger J J. Synergistic interactions of anti NS1 monoclonal antibodies protect passively immunized mice from lethal challenge with dengue 2 virus. J Gen Virol. 1988;69:2101–2107. doi: 10.1099/0022-1317-69-8-2101. [DOI] [PubMed] [Google Scholar]
- 14.Henchal E A, Henchal L S, Thaisomboonsuk B K. Topological mapping of unique epitopes on the dengue-2 virus NS1 protein using monoclonal antibodies. J Gen Virol. 1987;68:845–851. doi: 10.1099/0022-1317-68-3-845. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs M G, Young P R. Dengue: a continuing challenge for molecular biology. Curr Opin Infect Dis. 1998;11:319–324. [PubMed] [Google Scholar]
- 16.Kestens L, Hoofd G, Gigase P L, Deleys R, van der Groen G. HIV antigen detection in circulating immune complexes. J Virol Methods. 1991;31:67–76. doi: 10.1016/0166-0934(91)90145-p. [DOI] [PubMed] [Google Scholar]
- 17.Kuno G, Vorndam A V, Gubler D J, Gomez I. Study of anti-dengue NS1 antibody by western blot. J Med Virol. 1990;32:102–108. doi: 10.1002/jmv.1890320207. [DOI] [PubMed] [Google Scholar]
- 18.Lindenbach B D, Rice C M. Trans-complementation of yellow fever virus NS1 reveals a role in early RNA replication. J Virol. 1997;71:9608–9617. doi: 10.1128/jvi.71.12.9608-9617.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackenzie J, Jones M, Young P R. Immunolocalization of the dengue virus nonstructural glycoprotein NS1 suggests a role in viral RNA replication. J Virol Methods. 1996;220:232–240. doi: 10.1006/viro.1996.0307. [DOI] [PubMed] [Google Scholar]
- 20.Malasit P. Complement and dengue haemorrhagic fever/shock syndrome. Southeast Asian J Trop Med Public Health. 1987;18:316–320. [PubMed] [Google Scholar]
- 21.Miles S A, Balden E, Magpantay L, Wei L, Leiblein A, Hofheinz D, Toedter G, Stiehm E R, Bryson Y the Southern California Pediatric AIDS Consortium. Rapid serological testing with immune-complex-dissociated HIV p24 antigen for early detection of HIV infection in neonates. N Engl J Med. 1993;328:297–302. doi: 10.1056/NEJM199302043280501. [DOI] [PubMed] [Google Scholar]
- 22.Monath T P, Wands J R, Hill L J, Gentry M K, Gubler D J. Multisite monoclonal immunoassay for dengue viruses: Detection of viraemic sera and interference by heterologous antibody. J Gen Virol. 1986;67:639–650. doi: 10.1099/0022-1317-67-4-639. [DOI] [PubMed] [Google Scholar]
- 23.Rice C M, Lenches E M, Eddy S R, Shin S J, Sheets R L, Strauss J H. Nucleotide sequence of yellow fever virus: implications for flavivirus gene expression and evolution. Science. 1985;229:726–733. doi: 10.1126/science.4023707. [DOI] [PubMed] [Google Scholar]
- 24.Rothman A L, Ennis F A. Immunopathogenesis of dengue hemorrhagic fever. Virology. 1999;257:1–6. doi: 10.1006/viro.1999.9656. [DOI] [PubMed] [Google Scholar]
- 25.Ruangjirachuporn W, Boonpucknavig S, Nimmanitya S. Circulating immune complexes in serum from patients with dengue haemorrhagic fever. Clin Exp Immunol. 1979;36:46–53. [PMC free article] [PubMed] [Google Scholar]
- 26.Russell P K, Brandt W E, Dalrymple J M. Chemical and antigenic structure of flaviviruses. In: Schlesinger R W, editor. The Togaviruses. New York, N. Y: Academic Press; 1980. pp. 503–529. [Google Scholar]
- 27.Schlesinger J J, Brandriss M W, Walsh E E. Protection against 17D yellow fever encephalitis in mice by passive transfer of monoclonal antibodies to the nonstructural glycoprotein pg48 and by active immunization with gp48. J Immunol. 1985;135:2805–2809. [PubMed] [Google Scholar]
- 28.Smith G W, Wright P J. Synthesis of proteins and glycoproteins in dengue type 2 virus-infected Vero and Aedes albopictus cells. J Gen Virol. 1985;66:559–571. doi: 10.1099/0022-1317-66-3-559. [DOI] [PubMed] [Google Scholar]
- 29.Theofilopoulos A N, Wilson C B, Dixon F J. The Raji cell radioimmunoassay for detecting immune complexes in human serum. J Clin Investig. 1976;57:169–182. doi: 10.1172/JCI108257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winkler G, Maxwell S E, Ruemmler C, Stoller V. Newly synthesized dengue-2 virus non-structural protein NS1 is a soluble protein but becomes partially hydrophobic and membrane associated after dimerization. Virology. 1989;171:302–305. doi: 10.1016/0042-6822(89)90544-8. [DOI] [PubMed] [Google Scholar]
- 31.Young P, Bletchly C, Mackenzie J. Antigenic and structural analysis of the dengue virus glycoprotein, NS1. In: Uren M F, editor. Arbovirus research in Australia. 1993. pp. 138–142. Brisbane, Australia. [Google Scholar]