Abstract
The negative impacts of stress on gastrointestinal tract (GIT) barrier function can result in compromised animal growth and health. Aspirin is known to cause mucosal injury leading to increased gut permeability and tight junction damage and can be used as a model to study leaky gut in cattle. The objective of this study was to determine the long-term impact of aspirin-induced chronic leaky gut on cattle growth and carcass attributes. Two treatments were evaluated in two studies: control (no aspirin) or 0.25% of the diet dry matter (DM) aspirin fed daily. Diets consisted of 50% corn, 24% dried distillers grains, 20% corn silage, and 6% supplement on a DM basis. In experiment 1, sixteen Angus × Simmental heifers, allotted by body weight (BW) and breed composition, were fed diets for 154 d. On day 155, heifers were dosed with 1 liter of a 180-mM Cr–ethylenediaminetetraacetic acid solution using an esophageal tube and had urine collected every 1.5 to 3 h for 48 h for analysis of Cr as a measure of gut leakiness. In experiment 2, ninety-six Simmental × Angus steers (355.0 ± 14.8 kg) were allotted by BW and breed composition and fed treatment diets for 159 d. Weight was recorded monthly and serum was collected on day 159 and analyzed for lipopolysaccharide-binding protein (LBP), interleukin-6 (IL-6), serum amyloid A (SAA), haptoglobin, and aspartate aminotransferase (AST). Data were analyzed using the MIXED procedure of SAS. Heifers fed 0.25% aspirin in experiment 1 excreted more Cr into urine compared with heifers not fed aspirin (overall treatment effect, P = 0.01). In experiment 2, aspirin tended to increase serum LBP (P = 0.06) but had no effect on concentrations of IL-6, haptoglobin, SAA, or AST (P ≥ 0.25). Aspirin tended to decrease average daily gain (P = 0.10), decreased hot carcass weight and rib-eye area (P ≤ 0.05), and increased fat thickness, marbling score, and yield grade (P ≤ 0.02). Aspirin tended to increase kidney, pelvic, and heart fat percentage (P = 0.10) and had no effect on liver abscesses (P ≥ 0.80). This study indicates that leaky gut induced by long-term administration of aspirin has negative impacts on feedlot performance and carcass composition. The negative impact of aspirin-induced leaky gut on animal performance suggests that chronic leaky gut caused by other factors (subacute acidosis, stress) may be a significant problem for the feedlot industry.
Keywords: aspirin, beef feedlot, growth, inflammation, leaky gut
Introduction
The gastrointestinal tract (GIT) is in a constant state of controlled immune activation because of its close contact with the immunogenic contents of the lumen (Costa et al., 2011). Therefore, an adequate GIT barrier is considered essential for the host immune system and preservation of animal health and well-being. While cereal grains are the most cost effective and readily available energy source for beef feedlot producers around the world, grain-feeding cattle diminishes GIT microbial diversity, increases production of toxic and inflammatory compounds, weakens intestinal architecture, and compromises physical and chemical defense mechanisms (Petri et al., 2012; Saleem et al., 2012; Pederzolli et al., 2018). Nearly every grain-fed animal in the feedlot experiences acidic GIT pH that compromises a balanced inflammatory response. Indeed, others have noted a nonspecific immune response when cattle are fed high-grain finishing diets (Berry et al., 2004; Ametaj et al., 2009). Any interruption of normal, consistent consumption patterns of grain-fed cattle caused by stress, weather changes, or poor bunk management, for example, can further de-stabilize ruminal pH, shift microbial populations into dysbiosis, and cause GIT barrier dysfunction (Garcia et al., 2017). GIT dysfunction (leaky gut) causes inflammation and activates the immune system leading to decreased growth, increased morbidity and mortality (Mani et al., 2012), and negative impacts on producer profitability. Indeed, the GIT is one of the most metabolically active tissues in ruminants, accounting for approximately 20% of their oxygen consumption and 30% of metabolic processes and protein synthesis (McBride and Kelly, 1990; Cant et al., 1996). Kvidera et al. (2017a) observed that an activated immune system in the mature Holstein dairy cow used more than a kilogram of glucose in a 12-h period. The immune system’s energy demand adds to the maintenance requirement for cattle, thus pulling energy away from growth and production (NASEM, 2016). Because of the GIT’s central role in animal health, a deeper understanding of how GIT barrier function in cattle impacts growth and performance of feedlot cattle is needed. The exact repercussions of long-term leaky gut and maintenance of an activated immune system on beef cattle performance are currently unknown. However, it is difficult to study the impact of diet on intestinal barrier dysfunction without influencing energy intake and metabolic status.
Aspirin (acetylsalicylic acid) is a nonsteroidal anti-inflammatory drug (NSAID) known to covalently bind the active site of cyclooxygenase enzymes, resulting in diminished prostaglandin production and reduced intestinal mucosal and epithelial growth (Takeuchi and Amagase, 2018). Aspirin is well known to induce GIT mucosal injury in humans and laboratory animals (Lambert et al., 2012). By deregulating this physiological safeguard, NSAIDs render the gastric mucosa more susceptible to the damaging actions of the luminal contents of the GIT. Aspirin is used in humans and laboratory animals as a model to induce and study leaky gut (Oshima et al., 2008; Lambert et al., 2012) and is commonly used as an analgesic in cattle; the maximum recommended dosage is 100 mg/kg every 12 h (Gingerich et al., 1975) with a 24-h meat and milk withdrawal period (Smith et al., 2008). Briggs et al. (2020) reported that a one-time dose of 50 to 200 mg/kg body weight (BW) aspirin increased turnover of jejunal claudin-1, increased the appearance of Cr in the urine, and increased serum lipopolysaccharide-binding protein (LBP) indicating increased GIT leakiness. Thus, dietary administration of aspirin is a safe and effective method to intentionally induce leaky gut in cattle (Briggs et al., 2020). Our objective was to characterize the impact of long-term leaky gut and chronic immune activation on animal physiology, growth, and production. We hypothesized that long-term administration of 0.25% of the diet dry matter (DM) aspirin (equivalent to 50 mg/kg BW aspirin) will compromise GIT barrier function, leading to inflammation and immune system activation that will negatively impact animal performance and carcass characteristics.
Materials and Methods
All procedures performed in this study were approved by the Purdue University Animal Care and Use Committee and were in accordance with the Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching (FASS, 2010). The experiment was conducted at the Purdue University Animal Sciences Research and Education Center (ASREC) in West Lafayette, IN.
Experiment 1
Animals and diets
Sixteen Angus × Simmental heifers, sourced from Purdue ASREC, were allotted to 0% or 0.25% of diet DM aspirin to determine the effect of long-term administration of aspirin on Cr appearance in urine as a measure of GIT leakiness. Based on previous studies conducted with similar cattle at Purdue ASREC, we calculated that a prescribed dietary concentration of 0.25% of the diet DM would achieve an aspirin dose of 50 mg/kg BW, which has been demonstrated to initiate GIT barrier dysfunction in grain-fed feedlot cattle (Briggs et al., 2020). The actual overall aspirin intake on a BW basis for the current study was 49.2 mg/kg BW. Heifers were weighed at the start of the study and prior to urine collection with scales (480 Legend, Rice Lake Weighing Systems, Rice Lake, WI) that weighed to the nearest 0.5 kg. Scales were checked for accuracy at each weigh date. Heifers were allotted to treatments such that BW (336 ± 12.3 kg) and breed composition (% Simmental) was equal among treatments. Heifers were approximately 10 mo old at the start of dietary treatments and were fed diets for 154 d; average heifer weight at urine collection was 526 ± 24.2 kg. Heifers were vaccinated against Infectious Bovine Rhinotracheitis, Bovine Viral Diarrhea Types I and II, Parainfluenza-3, Bovine Respiratory Syncytial Virus, Mannheimia haemolytica, and Pasturella multocida (Vista Once, Merck Animal Health, Summit, NJ); vaccinated against Clostridia and Haemophilus somnus (Vision-7 Somnus; Merck Animal Health); treated with a pour-on (Cydectin, Bayer, Shawnee Mission, KS) for external parasites; and drenched with a de-wormer (Safeguard, Merck Animal Health, Madison, NJ) for internal parasites 3 mo prior to initiation of the study. Heifers were halter broken prior to initiation of the study. One heifer fed the control treatment was removed because of health reasons.
The control diet (Table 1) contained 50% corn, 24% dried distiller’s grains with solubles (DDGS), 20% corn silage, and 6% vitamin/mineral supplement (DM basis). Aspirin powder (VetOne MWI Animal Health, Boise, ID) was delivered to heifers in a corn/DDGS premix (38% corn, 57% DDGS, and 5% aspirin) that replaced a portion of DDGS and corn in the diet. Diets were formulated to meet or exceed NASEM (2016) requirements for crude protein, vitamins, and minerals. Heifers were fed in an open-sided barn with straw-bedded pens (3.4 × 9.1 m) over a concrete floor (two heifers per pen, eight heifers per treatment, and four pens per treatment). Feed was offered once daily at 0900 hours, and heifers were allowed ad libitum access to feed and water. Daily feed deliveries were adjusted using a 4-point bunk scoring system (Pritchard, 1993) to allow for ad libitum feed intake with little or no accumulation of unconsumed feed (score of ≤1). On day 155, the 16 heifers were restrained in a working chute and given 1 liter of a 180-mM Cr–ethylenediaminetetraacetic acid (EDTA) solution using an esophageal tube. Chromium–EDTA solution was prepared according to Binnerts et al. (1968). A latex Foley urinary catheter (C. R. Bard, Inc., Covington, GA) was inserted into the bladder, heifers were moved to individual stalls (1.1 × 2.1 m), tied to the front of the stall, and the urinary catheter line was attached to 4 liters drainage bags (Medline, Northfield, IL) which were fixed to the stall using string. Urine was completely drained from bags, weight was recorded, and a 45-mL subsample was collected every 1.5 h during the first 9 h and every 3 h thereafter for 48 h. Urine was frozen at −20 °C for subsequent analysis of Cr. The urine that was not subsampled was discarded to allow fresh urine to be collected for the next time period. During the sampling period, treatment diets and water were provided ad libitum for all animals.
Table 1.
Composition of diets (dry matter [DM] basis)
| Control1 | Aspirin1 | |
|---|---|---|
| Corn | 50 | 48 |
| Dried distillers grains with solubles | 24 | 21 |
| Corn silage | 20 | 20 |
| Vitamin/mineral supplement2 | 6 | 6 |
| Aspirin supplement3 | 0 | 5 |
| Diet composition, DM basis4 | ||
| Crude protein, % | 13.07 | 13.01 |
| Net energy for maintenance, Mcal/kg5 | 1.95 | 1.95 |
| Net energy for gain, Mcal/kg5 | 1.33 | 1.32 |
| Neutral detergent fiber, % | 22.85 | 22.79 |
| Calcium, % | 1.15 | 1.15 |
| Phosphorus, % | 0.49 | 0.49 |
| Sulfur, % | 0.25 | 0.25 |
1Control, no aspirin inclusion; aspirin, 0.25% of diet DM daily as aspirin.
2Vitamin/mineral supplement contained (DM basis): 18.25% Ca, 1.32% K, 0.44% Mg, 0.18% S, 563.91 ppm Zn, 522.90 ppm Fe, 440.41 ppm Mn, 183.33 ppm Cu, 9.66 ppm I, 4.48 ppm Se, 3.43 ppm Co, 42.19 IU/g vitamin A, 4.98 IU/g vitamin D, 0.155 IU/g vitamin E, and 413.6 ppm Rumensin (176.4 g/kg, Elanco Animal Health, Indianapolis, IN).
3Aspirin supplement contained 57% dried distillers grains with solubles, 38% corn, and 5% aspirin (DM basis).
4Analyzed at Cumberland Valley Analytical Services (Waynesboro, PA).
5Calculated based on NASEM (2016).
Chromium analysis
Approximately 2.5 mL of urine was centrifuged at 1,250 × g for 20 min, diluted to 25 mL with nanopure water, and analyzed for chromium by atomic absorption spectroscopy at 425.4 nm (SpectrAA 220 FS, Varian, Inc., Palo Alto, CA) according to the procedures of Vicente et al. (2004). Ground corn with a known Cr content served as a standard to check for accuracy and the coefficient of variation (CV) for Cr analysis was 5.7. Urinary Cr content at each 1.5 or 3 h time point was calculated as urine amount × Cr concentration.
Statistical analysis
Data were analyzed as a completely randomized design using the MIXED procedure of SAS (SAS Institute Inc., Cary, NC) with pen considered the experimental unit. The Shapiro–Wilk test was performed to test for normality, and urine Cr amount was analyzed as repeated measures over time. The model included the random effect of pen and the fixed effect of treatment, time, and the treatment × time interaction. Spatial power was the covariance structure used in the analysis. The LSMESTIMATE function of SAS was used to analyze only the within-time pairwise comparisons that were meaningful and to determine the simple effect of aspirin within time. Treatment comparisons were adjusted using the Bonferroni correction and the least square means statement was used to calculate adjusted means. Differences were considered significant when P ≤ 0.05, and 0.05 < P ≤ 0.10 was considered a tendency.
Experiment 2
Animals and diets
Ninety-six Angus × Simmental steers (355.0 ± 14.8 kg) were used to determine the effect of intentionally induced leaky gut on cattle performance and carcass characteristics. Leaky gut was induced by inclusion of 0.25% of the diet DM as aspirin (Briggs et al., 2020) over a 159-d period and was compared with a control diet of no aspirin inclusion. Based on previous studies conducted with similar cattle at Purdue ASREC, we calculated that a prescribed dietary concentration of 0.25% of the diet DM would achieve an aspirin dose of 50 mg/kg BW, which has been demonstrated to initiate GIT barrier dysfunction in grain-fed feedlot cattle (Briggs et al., 2020). The actual overall aspirin intake on a BW basis for the current study was 50.7 mg/kg BW. Steers were allotted to 16 pens (8 pens/treatment; 6 animals/pen; 48 animals/treatment) based on breed (% Simmental), BW, and source (Animal Science Research and Education Center or Feldun Purdue Agricultural Center). Pens (6.1 × 3.7 m) were located in a slatted floor, curtain-sided finishing barn and provided 0.55 m of bunk space. Diets (Table 1), feed delivery, and diet sampling were as described in experiment 1. Aspirin powder (VetOne MWI Animal Health, Boise, ID) was delivered to steers in a corn/DDGS premix (38% corn, 57% DDGS, 5% aspirin) that replaced a portion of DDGS and corn in the diet.
Upon entry into the feedlot, steers were vaccinated and treated for internal and external parasites as described in experiment 1. Steers were given booster vaccines 4 wk later. Steers were weighed twice on consecutive days at the initiation and termination of the study and weighed once monthly to monitor growth and health using the scales as described in experiment 1. Steers were implanted with Synovex-ONE Feedlot (200 mg of testosterone and 28 mg of estradiol benzoate; provided courtesy of Zoetis Animal Health, Kalamazoo, MI) at feedlot entry. Heavier pens of steers were weighed every other week as pen BWs approached 548 kg. Pens of steers that achieved an average BW of approximately 548 kg were fed 300 mg of Optaflexx (ractopamine hydrochloride; provided courtesy of Elanco, Greenfield, IN) daily during the last 42 d before slaughter. Performance data were analyzed for the first half (day 0 to 85), second half (day 86 to 159), and overall (day 0 to 159).
Five steers from every pen of six were transported 400 km to a USDA-inspected commercial abattoir (Tyson Foods Inc., Joslin, IL). One steer per pen was selected based on pen average BW for slaughter at Purdue University. Pens of steers were slaughtered at three different time points (147, 161, and 175 d) according to when 42 d of Optaflexx feeding was achieved (average pen BW of 617 ± 35.2 kg). All steers within a pen were slaughtered on the same day where 4, 2, and 2 pens of cattle fed the control treatment and 3, 2, and 3 pens of cattle fed the aspirin treatment were slaughtered on days 147, 161, and 175, respectively. Weighted average of days on feed (pen days) was 158 for steers fed the control treatment and 161 for steers fed the aspirin treatment. Aspirin powder was removed from diets 24 h prior to slaughter. Final BWs were not pencil shrunk. Hot carcass weight for all animals was recorded immediately after evisceration. All 96 carcasses were chilled for 24 h, and qualified University personnel measured subcutaneous fat thickness between the 12th and 13th rib; longissimus dorsi area via direct grid reading between the 12th and 13th rib; kidney, pelvic, and heart (KPH) fat as a percent of hot carcass weight (HCW); marbling score; and USDA quality and yield grades (YGs; USDA, 1997).
Blood analysis
Approximately, 10 mL of blood was collected from the jugular vein into tubes (Becton Dickinson, Franklin Lakes, NJ) 1 d prior to shipping to slaughter from two animals per pen (32 steers total, 16 per treatment). Steers were selected for blood collection such that average BW and breed composition (% Simmental) matched that of the pen average. Within 2 h of collection, blood samples were centrifuged at 1,250 × g for 20 min. Serum was harvested, separated into three aliquots, and frozen at −20 °C until analysis of LBP, interleukin-6 (IL-6), haptoglobin, serum amyloid A (SAA), and aspartate aminotransferase (AST). Serum concentrations of LBP (LSBio, LS-F7412, Seattle, WA), IL-6 (Abcam, ab205280, Cambridge, UK), SAA (Tridelta Development Ltd., TP 802, Maynooth, County Kildare, IE), and haptoglobin (ICL, E-10HPT, Inc., Portland, OR) were extracted using commercial enzyme-linked immunosorbent assay (ELISA) kits according to manufacturer instructions and measured at 450 nm on a Spark 10M plate reader (Tecan Life Sciences, Männedorf, Zürich, Switzerland). Enzyme-linked immunosorbent assay kits were bovine specific (IL-6, LBP, and haptoglobin) or designed to be used with multiple species (SAA) including bovine. Concentrations of bovine AST (Sigma-Aldrich, MAK055, St. Louis, MO) were determined using a colorimetric assay kit at 450 nm with the previously mentioned plate reader. Inter-assay CV for LBP, haptoglobin, SAA, and AST was 18.97, 12.31, 12.81, 8.97, and 3.42, respectively. Intra-assay CV for LBP, IL-6, haptoglobin, SAA, and AST was 5.70, 6.04, 3.47, 4.66, and 5.24, respectively.
Statistical analysis
Data were analyzed as a complete randomized design using the MIXED procedure of SAS (SAS Institute Inc., Cary, NC) with pen considered the experimental unit. The Shapiro–Wilk test was performed to test for normality. Performance data were analyzed as repeated measures over time. The repeated measures model included the random effect of pen and the fixed effect of treatment, time, and the interaction of treatment and time. Four covariance structures were compared for each variable and the structure that yielded the lowest Bayesian Information Criteria was used for all results. The LSMESTIMATE function of SAS was used to analyze only the within-time pairwise comparisons that were meaningful and to determine the simple effect of aspirin within time, which is what is presented in the Results section. Treatment comparisons were adjusted using the Bonferroni correction and the least square means statement was used to calculate adjusted means. Overall performance data (day 0 to 159), serum data, and carcass data were analyzed as a completely randomized design using the MIXED procedures of SAS without repeated measures. Serum data were not normally distributed and were log10 transformed for statistical analysis. Means and SE for transformed data were obtained by back-transformation using the delta method. The non-repeated measures model included the random effect of pen and the fixed effect of treatment. Treatment comparisons were made using Fisher’s protected least significant difference, and the least square means statement was used to calculate adjusted means. Differences were considered significant when P ≤ 0.05 and 0.05 < P ≤ 0.10 was considered a tendency.
Results and Discussion
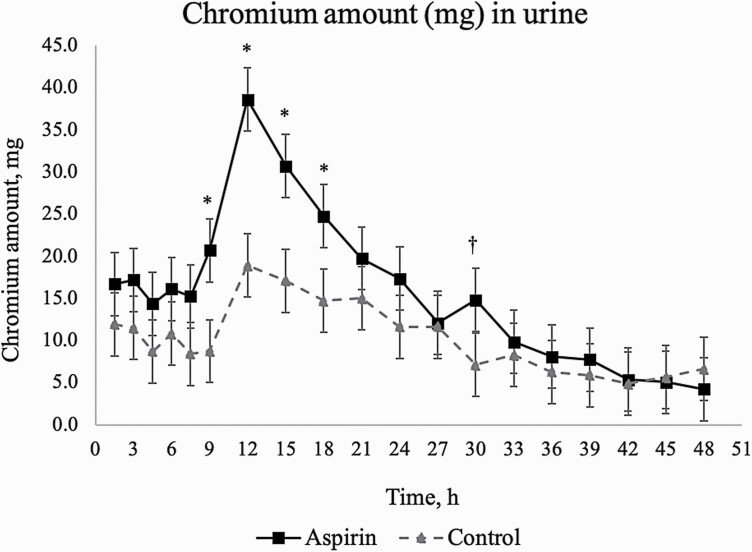
A number of conditions in the feedlot can cause leaky gut which likely stimulates the immune system, causes inflammation, and leads to losses in production. Well-known examples in ruminants include weaning, transportation stress, heat stress, feed deprivation, and acute or subacute ruminal acidosis (Baumgard and Rhoads, 2013; Zhang et al., 2013; Pederzolli et al., 2018). The objective of this study was to supplement aspirin in order to understand the effect of chronic intestinal barrier dysfunction on growth and carcass composition in otherwise healthy feedlot cattle without influencing energy intake and metabolic status. In experiment 1, there was an overall treatment effect and treatment × hour effect (P ≤ 0.03) of aspirin on urinary Cr excretion (Figure 1), where heifers fed 0.25% aspirin excreted more Cr into urine compared with heifers not fed aspirin. Heifers fed aspirin excreted more Cr into urine at 9, 12, 15, and 18 h after Cr-EDTA dosing (P ≤0.05) and tended to excrete more Cr into urine at 30 h after Cr-EDTA dosing (P = 0.07) compared with heifers not fed aspirin. Average urine volume was 1.0 and 1.7 liters for control and aspirin-treated heifers and did not differ (P = 0.14). Dosing with Cr-EDTA is an accepted technique for measuring GIT barrier function in vivo in ruminants (Schweigel et al., 2005; Zhang et al., 2013; Briggs et al., 2020). Chromium-EDTA is not digested or metabolized, has a high renal clearance rate (Bjarnason et al., 1986), permeates the GIT through the paracellular pathway, and appears in the urine (García-Lafuente et al., 2001; Schweigel et al., 2005; Ten Bruggencate et al., 2006). In healthy ruminants, approximately 2.5% of ruminally dosed Cr-EDTA is absorbed and excreted through urine (Shingfield et al., 2008), which is consistent with the current study where control and aspirin-fed heifers excreted 2.1% and 3.0% of their dose, respectively. The mass of an endotoxin is 2 to 70 kDa (Magalhães et al., 2007), whereas Cr–EDTA has a mass of 340 Da and a size of approximately 10 Å (García-Lafuente et al., 2001). Thus, Cr–EDTA is an indicator of gut barrier dysfunction, not necessarily of bacteria or endotoxin translocation. Briggs et al. (2020) reported that 200 mg/kg BW aspirin 24 to 36 h prior to slaughter increased turnover of jejunal claudin-1 and that aspirin doses from 50 to 200 mg/kg BW 24 to 36 h prior to slaughter linearly increased Cr-EDTA leakage into urine. Increased elimination of Cr-EDTA into urine caused by aspirin, as shown in the present study, indicates that long-term administration of aspirin causes mucosal damage that results in GIT leakiness. Aspirin did not affect BW (523 vs. 530 kg; P ≥ 0.84) or daily dry matter intake (DMI; 9.0 vs 8.5 kg/d, P = 0.29), for control compared with aspirin-fed heifers, respectively.
Figure 1.
Effect of long-term aspirin (50 mg/kg of body weight daily for 154 d) on chromium (mg) appearance in urine of beef heifers (experiment 1). Treatment effect (P = 0.01) and a treatment × hour effect (P = 0.03). Time points with * superscript indicate a timepoint effect (P ≤ 0.05); time points with † superscript indicate a tendency for a timepoint effect (0.05 < P ≤ 0.10). Control = no aspirin inclusion (eight heifers, four pens); aspirin = 0.25% of diet dry matter daily as aspirin (eight heifers, four pens). Average urine volume was 1.0 and 1.7 liters for control and aspirin-treated heifers, respectively (P = 0.14).
In experiment 2, aspirin tended (P = 0.06) to increase LBP in serum (Table 2), indicating that dietary aspirin compromised gut integrity, allowing lipopolysaccharide (LPS) to enter into circulation and cause an inflammatory response. However, serum concentrations of IL-6 (a pro- and anti-inflammatory cytokine), haptoglobin (acute phase protein), SAA (an acute-phase protein), and AST (a marker of liver stress) did not differ between treatments (P ≥ 0.25). The increase in serum concentrations of LBP in cattle fed aspirin is consistent with our previous report (Briggs et al., 2020). Because the LBP assay used in the current study detects both free LBP and LBP that is bound to LPS, our results are an indication of the host response induced by the presence of LPS in the bloodstream and suggest that greater amounts of LPS crossed the GIT barrier in cattle fed aspirin compared with cattle not fed aspirin. LPS is a component of Gram-negative bacterial cell walls that is recognized as an endotoxin because it has toxic effects on the host after it is shed from lysed bacteria. LPS accumulates in digestive contents during rapid microbial growth as well as during bacterial lysis. For example, grain feeding is associated with 18- to 20-fold increase in ruminal LPS concentrations compared with forage feeding and a 10-fold greater number of Fusobacterium necrophorum cells, the bacterium most closely linked with liver abscesses (Nagaraja and Lechtenberg, 2007). Increases in LPS also occur in the hindgut during grain-based subacute ruminal acidosis (SARA; Li et al., 2012). The combination of LPS and an acidic pH (5.0 to 5.5) acts synergistically to impair GIT barrier function and increases permeability as demonstrated in ex vivo studies (Gabel and Aschenbach, 2002; Emmanuel et al., 2007).
Table 2.
The effect of long-term aspirin (50 mg/kg of body weight daily for 159 d) on serum inflammatory markers of beef steers (experiment 2)
| Treatment1 | ||||
|---|---|---|---|---|
| Control | Aspirin | SEM | P-value | |
| n, individual (pen) | 16 (8) | 16 (8) | — | — |
| LPS2-binding protein, ug/mL | 26.9 | 38.0 | 2.05 | 0.06 |
| Interleukin-6, pg/mL | 40.6 | 33.9 | 2.83 | 0.42 |
| Haptoglobin, ug/mL | 2.71 | 2.34 | 0.165 | 0.47 |
| Serum amyloid A, ug/mL | 41.8 | 32.9 | 2.63 | 0.25 |
| Aspartate aminotransferase, mU/mL | 33.5 | 34.1 | 0.46 | 0.69 |
1Control, no aspirin inclusion; aspirin, 0.25% of diet DM daily as aspirin.
2LPS, lipopolysaccharide.
Bacterial LPS also contributes to systemic inflammation (Gozho et al., 2005; Li et al., 2012). The presence of LPS in the portal vein initiates a cascade of immune signaling, which results in production of inflammatory cytokines and acute-phase proteins, including LBP (Lu et al., 2008; Ceciliani et al., 2012). Indeed, Zhang et al. (2016) observed that in vitro administration of LPS increased mRNA expression of genes encoding for inflammatory cytokines IL-1β, IL-2, IL-6, and IL-8 and tumor necrosis factor (TNF)-α in tissue isolated from the rumen epithelium. LBP’s role is to signal for opsonization by phagocytes after binding with LPS (Lu et al., 2008; Ceciliani et al., 2012). Increases in circulating LBP have also been connected to a decrease in jejunum villus height-to-crypt depth ratio in lactating dairy cows, suggesting greater intestinal damage and decreased barrier function (Kvidera et al., 2017b). Interestingly, the magnitude of increased circulating LBP in aspirin-fed steers in the current study (41.2%) is similar to the 50% increase reported by Ametaj et al. (2009) in the first 3 wk after steers were transitioned to a high concentrate finishing diet and the 35.9% increases reported by Li et al. (2012) for dairy cows with SARA. Khafipour et al. (2009) observed a 2.9-fold increase in LBP for dairy cows with SARA compared with control cows. Briggs et al. (2020) similarly reported that administration of 200 mg/kg BW aspirin did not affect serum concentrations of IL-6 or AST. However, Briggs et al. (2020) reported that 200 mg/kg BW dosed 24 to 36 h prior to slaughter increased concentration of SAA in feedlot cattle. The fact that SAA and haptoglobin did not increase because of long-term aspirin administration in the present study is consistent with results of Kvidera et al. (2017b) who intentionally induced GIT barrier dysfunction with a gamma-secretase inhibitor and may be because acute-phase protein production in the liver is a secondary (non-local) response to toxic stimuli and can respond to a number of inflammatory conditions (Ceciliani et al., 2012). Ametaj et al. (2009) saw that SAA remained elevated but haptoglobin returned to baseline concentrations 6 wk after steers transitioned to high concentrate diets.
Performance results are presented in Table 3. The first half average daily gain (ADG) did not differ between treatments (P = 0.61). The second half and overall ADG tended to decrease for steers fed 0.25% of diet DM aspirin compared with steers fed no aspirin (P ≤ 0.10), but no difference in BW occurred (P ≥ 0.21). There were no differences between treatments for DMI, gain:feed, or days on feed (P ≥ 0.15). The tendency for a decrease in the second half and overall ADG indicates that long-term administration of aspirin had a negative effect on growth, which may be explained by leaky gut. Because of the close contact between the GIT barrier to immunogenic luminal contents, the GIT is in a constant state of controlled immune activation (Costa et al., 2011), which is energetically expensive. Indeed, Kvidera et al. (2017a) determined that an acutely activated immune system in Holstein cows will use more than a kilogram of glucose in a 12-h time period. For this reason, feeding aspirin to cattle and the resulting heightened immune activation could have a sufficient catabolic cost to cause nutritional resources to be directed away from anabolic processes. The fact that the negative impact on growth was delayed to the second half of the study suggests that the chronic immune activation took time to impact GIT barrier function or that other factors, such as warmer weather, increased fat deposition as animals mature, and/or β-agonist-induced lipolysis (discussed later) played a role.
Table 3.
Effect of long-term aspirin (50 mg/kg of body weight daily for 159 d) on performance of beef steers (experiment 2)
| Treatment1 | ||||
|---|---|---|---|---|
| Item | Control | Aspirin | SEM | P-value |
| n, individual (pen) | 48 (8) | 48 (8) | — | — |
| Body Weight2,4, kg | ||||
| Day 0 | 355.2 | 354.6 | 4.88 | 0.93 |
| Day 85 | 520.4 | 516.7 | 4.88 | 0.59 |
| Day 159 | 621.3 | 612.6 | 4.88 | 0.21 |
| Average daily gain3,4, kg/d | ||||
| First half (day 0 to 85) | 1.94 | 1.91 | 0.050 | 0.61 |
| Second half (day 86 to 159) | 1.39 | 1.27 | 0.050 | 0.09 |
| Overall (day 0 to 159) | 1.69 | 1.61 | 0.035 | 0.10 |
| Dry matter intake2,4, kg/d | ||||
| First half (day 0 to 85) | 9.4 | 9.5 | 0.21 | 0.71 |
| Second half (day 86 to 159) | 10.3 | 10.1 | 0.21 | 0.39 |
| Overall (day 0 to 159 | 9.8 | 9.8 | 0.18 | 0.84 |
| Gain:feed2,4 | ||||
| First half (day 0 to 85) | 0.206 | 0.200 | 0.0048 | 0.35 |
| Second half (day 86 to 159) | 0.135 | 0.126 | 0.0048 | 0.19 |
| Overall (day 0 to 159 | 0.173 | 0.164 | 0.0038 | 0.15 |
| Days on feed | 158 | 161 | 4.5 | 0.59 |
1Control, no aspirin inclusion; aspirin, 0.25% of diet dry matter daily as aspirin.
2No repeated measures treatment effect (P ≥ 0.19).
3Repeated treatment effect (P = 0.09).
4No repeated measures treatment × time effect (P ≥ 0.24).
Carcass data are presented in Table 4. There were no differences in dressing percentage, liver abscess score, or percent of liver abscesses (P ≥ 0.62). Cattle fed 0.25% aspirin produced carcasses with decreased hot carcass weight (P = 0.05) and rib-eye area (P = 0.01); increased fat thickness (P = 0.02), marbling score (P = 0.003), and YG (P = 0.01); and a tendency for increased KPH% (P = 0.10). Liver abscesses, caused by translocation of pathogenic bacteria across the digestive epithelium, are a common sequelae to SARA in feedlot cattle. Even though aspirin caused an inflammatory response and leaky gut similar to grain-induced SARA in the current study, it does not appear to induce liver abscesses. No difference in serum AST concentrations in the current study supports this and indicates that the liver was not inflamed. It may be that F. necrophorum was not present, was not able to translocate to the liver, or the feeding period was not long enough to allow for liver abscesses to present. The change in carcass composition as a result of aspirin administration is likely multifactorial. LPS that is bound to LBP in circulation is incorporated into lipoproteins and will migrate to the liver hepatocytes, potentially leading to fatty liver (Eckel and Ametaj, 2016). Lipopolysaccharides are removed from the liver and transported to white adipose tissue where they are stored and can later be neutralized by macrophages (Hersoug, 2016). Thus, the increase in fat deposition in the current study may be the result of greater need for body lipid stores to neutralize LPS. A switch toward fat production is consistent with data from Farney et al. (2013) who demonstrated that 1.95 g/L of sodium salicylate (also an NSAID) supplementation in a molasses carrier for 7 d in early postpartum dairy cows increased fat content in milk. The fact that LPS is stored in lipid and β-agonists are lipolytic may explain why aspirin-fed steers had a greater reduction in performance during the second half of the study compared with control steers. It is possible that greater amounts of LPS were released from fat stores in aspirin fed compared with control steers, which led to increased inflammation. While β2-agonists have been shown to attenuate the pro-inflammatory activities of a range of immune and inflammatory cells in vitro (Theron et al., 2013), Genther-Schroeder et al. (2016) observed that the β1-agonist ractopamine elicited an inflammatory response in feedlot steers. Inflammation-induced insulin resistance may have also contributed to increased fat deposition and in the present study. Circulating LPS has been demonstrated to generate a transient hyperglycemic response (Kvidera et al., 2017b), and whole body insulin resistance is a common outcome during infection. The whole body insulin resistance is designed to decrease peripheral tissue glucose use and the support glucose needs of the immune system (Horst et al., 2021). Decreased longissimus muscle area in the present study may have also been a result of adjustments in skeletal muscle structure intended to support immune cell glucose use, such as increased proteolysis to provide amino acids as substrates for gluconeogenesis and the biosynthesis of leukocytes and acute phase proteins (Horst et al., 2021).
Table 4.
Effect of long-term aspirin (50 mg/kg of body weight daily for 159 d) on carcass characteristics of beef steers (experiment 2)
| Treatment1 | ||||
|---|---|---|---|---|
| Item | Control | Aspirin | SEM | P-value |
| n, individual (pen) | 48 (8) | 48 (8) | — | — |
| Hot carcass weight, kg | 389.0 | 382.7 | 2.11 | 0.05 |
| Dressing percent, % | 62.6 | 62.5 | 0.19 | 0.62 |
| Fat thickness, cm | 1.35 | 1.53 | 0.049 | 0.02 |
| Rib-eye area, cm | 88.4 | 84.7 | 0.91 | 0.01 |
| Kidney, pelvis, and heart fat, % | 1.99 | 2.09 | 0.040 | 0.10 |
| Yield grade | 3.11 | 3.43 | 0.080 | 0.01 |
| Marbling score2 | 418.5 | 448.6 | 5.76 | <0.01 |
| Quality grade distribution | ||||
| Select, % | 4.3 | 2.1 | 2.48 | 0.55 |
| Choice−, % | 39.5 | 33.3 | 4.40 | 0.33 |
| Choice0, % | 35.4 | 33.4 | 6.07 | 0.82 |
| Choice+, % | 16.5 | 23.0 | 4.87 | 0.36 |
| Prime, % | 4.1 | 8.5 | 3.70 | 0.42 |
| Liver abscess score3 | 0.21 | 0.19 | 0.071 | 0.85 |
| Liver abscess, % | 18.8 | 16.8 | 5.62 | 0.80 |
1Control, no aspirin inclusion; aspirin, 0.25% of diet DM daily as aspirin.
2Practically devoid = 100 to 199; slight = 200 to 299; small = 300 to 399; modest = 400 to 499; and moderate = 500 to 599.
3Elanco Animal Health (Indianapolis, IN) liver abscess scores converted to numeric: 0, no abscesses present; −A or 1, one or two minor abscesses, 1; A or 2, two to four well-established abscesses; and +A or 3, large, active abscesses, may contain inflammation on the abscess periphery.
Long-term supplementation of aspirin to feedlot cattle increased leakage of Cr–EDTA into urine, increased serum LBP concentrations, and increased fat deposition while decreasing muscle deposition and daily gains. These results indicate that aspirin induced leaky gut and that leaky gut has a negative impact on feedlot cattle performance and carcass composition. Long-term use of aspirin can be used as a model to study the chronic effects of leaky gut in feedlot cattle on growth and carcass characteristics. Strategies that decrease leaky gut may be able to improve feedlot cattle performance.
Acknowledgment
Appreciation is extended to the employees of the Purdue University Beef Research and Educational Center for assistance in the conduction of this research.
Glossary
Abbreviations
- ADF
acid detergent fiber
- ADG
average daily gain
- AST
aspartate aminotransferase
- BW
body weight
- DDGS
dried distillers grains with solubles
- DM
dry matter
- DMI
dry matter intake
- EDTA
ethylenediaminetetraacetic acid
- GIT
gastrointestinal tract
- IL-6
interleukin-6
- KPH
kidney, pelvic, and heart fat
- LBP
lipopolysaccharide-binding protein
- LPS
lipopolysaccharide
- NDF
neutral detergent fiber
- NSAID
nonsteroidal anti-inflammatory drug
- SAA
serum amyloid A
- YG
yield grade
Conflict of interest statement
The authors report no conflicts of interest.
Literature Cited
- Ametaj, B. N., Koenig K. M., Dunn S. M., Yang W. Z., Zebeli Q., and Beauchemin K. A.. . 2009. Backgrounding and finishing diets are associated with inflammatory responses in feedlot steers. J. Anim. Sci. 87:1314–1320. doi: 10.2527/jas.2008-1196 [DOI] [PubMed] [Google Scholar]
- Baumgard, L. H., and R. P.Rhoads, Jr. 2013. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 1:311–337. doi: 10.1146/annurev-animal-031412-103644 [DOI] [PubMed] [Google Scholar]
- Berry, B. A., Confer A. W., Krehbiel C. R., Gill D. R., Smith R. A., and Montelongo M.. . 2004. Effects of dietary energy and starch concentrations for newly received feedlot calves: II. Acute-phase protein response. J. Anim. Sci. 82:845–850. doi: 10.2527/2004.823845x [DOI] [PubMed] [Google Scholar]
- Binnerts, W., Van′t Klooster A. T., and Frens A.. . 1968. Soluble chromium indicator measured by atomic absorption in digestion experiments. Vet. Rec. 82:470. [Google Scholar]
- Bjarnason, I., Williams P., Smethurst P., Peters T. J., and Levi A. J.. . 1986. Effect of non-steroidal anti-inflammatory drugs and prostaglandins on the permeability of the human small intestine. Gut 27:1292–1297. doi: 10.1136/gut.27.11.1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs, N. G., Brennan K. M., Funnell B. J., Nicholls G. T., and Schoonmaker J. P.. . 2020. Use of aspirin to intentionally induce gastrointestinal tract barrier dysfunction in feedlot cattle. J. Anim. Sci. 98(9):1–7. doi: 10.1093/jas/skaa264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cant, J. P., McBride B. W., and W. J.Croom, Jr. 1996. The regulation of intestinal metabolism and its impact on whole animal energetics. J. Anim. Sci. 74:2541–2553. doi: 10.2527/1996.74102541x [DOI] [PubMed] [Google Scholar]
- Ceciliani, F., Ceron J. J., Eckersall P. D., and Sauerwein H.. . 2012. Acute phase proteins in ruminants. J. Proteomics 75:4207–4231. doi: 10.1016/j.jprot.2012.04.004 [DOI] [PubMed] [Google Scholar]
- Costa, E., Uwiera R. R., Kastelic J. P., Selinger L. B., and Inglis G. D.. . 2011. Non-therapeutic administration of a model antimicrobial growth promoter modulates intestinal immune responses. Gut Pathog. 3:14. doi: 10.1186/1757-4749-3-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel, E. F., and Ametaj B. N.. . 2016. Invited Review: Role of bacterial endotoxins in the etiopathogenesis of periparturient diseases of transition dairy cows. J. Dairy Sci. 99:5967–5990. doi: 10.3168/jds.2015-10727 [DOI] [PubMed] [Google Scholar]
- Emmanuel, D. G., Madsen K. L., Churchill T. A., Dunn S. M., and Ametaj B. N.. . 2007. Acidosis and lipopolysaccharide from Escherichia coli B:055 cause hyperpermeability of rumen and colon tissues. J. Dairy Sci. 90:5552–5557. doi: 10.3168/jds.2007-0257 [DOI] [PubMed] [Google Scholar]
- Farney, J. K., Mamedova L. K., Coetzee J. F., Minton J. E., Hollis L. C., and Bradford B. J.. . 2013. Sodium salicylate treatment in early lactation increases whole-lactation milk and milk fat yield in mature dairy cows. J. Dairy Sci. 96:7709–7718. doi: 10.3168/jds.2013-7088 [DOI] [PubMed] [Google Scholar]
- FASS . 2010. Guide for the care and use of agricultural animals in agricultural research and teaching. 3rd ed. Champaign (IL):Consortium for Developing a Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching. [Google Scholar]
- Gabel, G., and Aschenbach J. R.. . 2002. Influence of food deprivation on the transport of 3-O-methyl-alpha-d-glucose across the isolated ruminal epithelium of sheep. J. Anim. Sci. 80:2740–2746. doi: 10.1093/ansci/80.10.2740 [DOI] [PubMed] [Google Scholar]
- Garcia, M., Bradford B., and Nagaraja T.. . 2017. Invited Review: Ruminal microbes, microbial products, and systemic inflammation. Prof. Anim. Sci. 33(6):635–650. doi: 10.15232/pas.2017-01663 [DOI] [Google Scholar]
- García-Lafuente, A., Antolín M., Guarner F., Crespo E., and Malagelada J. R.. . 2001. Modulation of colonic barrier function by the composition of the commensal flora in the rat. Gut 48:503–507. doi: 10.1136/gut.48.4.503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genther-Schroeder, O. N., Branine M. E., and Hansen S. L.. . 2016. The effects of increasing supplementation of zinc-amino acid complex on growth performance, carcass characteristics, and inflammatory response of beef cattle fed ractopamine hydrochloride. J. Anim. Sci. 94:3389–3398. doi: 10.2527/jas.2015-0209 [DOI] [PubMed] [Google Scholar]
- Gingerich, D. A., Baggot J. D., and Yeary R. A.. . 1975. Pharmacokinetics and dosage of aspirin in cattle. J. Am. Vet. Med. Assoc. 167:945–948. [PubMed] [Google Scholar]
- Gozho, G. N., Plaizier J. C., Krause D. O., Kennedy A. D., and Wittenberg K. M.. . 2005. Subacute ruminal acidosis induces ruminal lipopolysaccharide endotoxin release and triggers an inflammatory response. J. Dairy Sci. 88:1399–1403. doi: 10.3168/jds.S0022-0302(05)72807-1 [DOI] [PubMed] [Google Scholar]
- Hersoug, L. G., Møller P., and Loft S.. . 2016. Gut microbiota-derived lipopolysaccharide uptake and trafficking to adipose tissue: implications for inflammation and obesity. Obes. Rev. 17:297–312. doi: 10.1111/obr.12370 [DOI] [PubMed] [Google Scholar]
- Horst, E. A., Kvidera S. K., and Baumgard L. H.. . 2021. Invited Review: The influence of immune activation on transition cow health and performance–—a critical evaluation of traditional dogmas. J. Dairy Sci. 104:P8380–8410. doi: 10.3168/jds.2021-20330 [DOI] [PubMed] [Google Scholar]
- Khafipour, E., Krause D. O., and Plaizier J. C.. . 2009. A grain-based subacute ruminal acidosis challenge causes translocation of lipopolysaccharide and triggers inflammation. J. Dairy Sci. 92:1060–1070. doi: 10.3168/jds.2008-1389 [DOI] [PubMed] [Google Scholar]
- Kvidera, S. K., Dickson M. J., Abuajamieh M., Snider D. B., Fernandez M. V. S., Johnson J. S., Keating A. F., Gorden P. J., Green H. B., Schoenberg K. M., . et al. 2017a. Intentionally induced intestinal barrier dysfunction causes inflammation, affects metabolism, and reduces productivity in lactating Holstein cows. J. Dairy Sci. 100:4113–4127. doi: 10.3168/jds.2016-12349 [DOI] [PubMed] [Google Scholar]
- Kvidera, S. K., Horst E. A., Abuajamieh M., Mayorga E. J., Fernandez M. V., and Baumgard L. H.. . 2017b. Glucose requirements of an activated immune system in lactating Holstein cows. J. Dairy Sci. 100:2360–2374. doi: 10.3168/jds.2016-12001 [DOI] [PubMed] [Google Scholar]
- Lambert, G. P., Schmidt A., Schwarzkopf K., and Lanspa S.. . 2012. Effect of aspirin dose on gastrointestinal permeability. Int. J. Sports Med. 33:421–425. doi: 10.1055/s-0032-1301892 [DOI] [PubMed] [Google Scholar]
- Li, S., Khafipour E., Krause D. O., Kroeker A., Rodriguez-Lecompte J. C., Gozho G. N., and Plaizier J. C.. . 2012. Effects of subacute ruminal acidosis challenges on fermentation and endotoxins in the rumen and hindgut of dairy cows. J. Dairy Sci. 95:294–303. doi: 10.3168/jds.2011-4447 [DOI] [PubMed] [Google Scholar]
- Lu, Y. C., Yeh W. C., and Ohashi P. S.. . 2008. LPS/TLR4 signal transduction pathway. Cytokine 42:145–151. doi: 10.1016/j.cyto.2008.01.006 [DOI] [PubMed] [Google Scholar]
- Magalhães, P. O., Lopes A. M., Mazzola P. G., Rangel-Yagui C., Penna T. C., and A.Pessoa, Jr. 2007. Methods of endotoxin removal from biological preparations: a review. J. Pharm. Pharm. Sci. 10:388–404. [PubMed] [Google Scholar]
- Mani, V., Weber T. E., Baumgard L. H., and Gabler N. K.. . 2012. Growth and Development Symposium: Endotoxin, inflammation, and intestinal function in livestock. J. Anim. Sci. 90:1452–1465. doi: 10.2527/jas.2011-4627 [DOI] [PubMed] [Google Scholar]
- McBride, B. W., and Kelly J. M.. . 1990. Energy cost of absorption and metabolism in the ruminant gastrointestinal tract and liver: a review. J. Anim. Sci. 68:2997–3010. doi: 10.2527/1990.6892997x [DOI] [PubMed] [Google Scholar]
- Nagaraja, T. G., and Lechtenberg K. F.. . 2007. Liver abscesses in feedlot cattle. Vet. Clin. North Am. Food Anim. Pract. 23:351–369, ix. doi: 10.1016/j.cvfa.2007.05.002 [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering and Medicine (NASEM) . 2016. Nutrient requirements of beef cattle. 8th rev. ed. Washington (DC): The National Academies Press. [Google Scholar]
- Oshima, T., Miwa H., and Joh T.. . 2008. Aspirin induces gastric epithelial barrier dysfunction by activating p38 MAPK via claudin-7. Am. J. Physiol. Cell Physiol. 295:C800–C806. doi: 10.1152/ajpcell.00157.2008 [DOI] [PubMed] [Google Scholar]
- Pederzolli, R. A., Van Kessel A. G., Campbell J., Hendrick S., Wood K. M., and Penner G. B.. . 2018. Effect of ruminal acidosis and short-term low feed intake on indicators of gastrointestinal barrier function in Holstein steers. J. Anim. Sci. 96:108–125. doi: 10.1093/jas/skx049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri, R. M., Forster R. J., Yang W., McKinnon J. J., and McAllister T. A.. . 2012. Characterization of rumen bacterial diversity and fermentation parameters in concentrate fed cattle with and without forage. J. Appl. Microbiol. 112:1152–1162. doi: 10.1111/j.1365-2672.2012.05295.x [DOI] [PubMed] [Google Scholar]
- Pritchard, R. H. 1993. Bunk management. Proceedings of the Land O′Lakes Beef Seminar; Cedar Rapids (IA), Columbus (NE), Storm Lake (IA), and St. Paul (MN): Land O; ′Lakes Inc.; p. 4–15. [Google Scholar]
- Saleem, F., Ametaj B. N., Bouatra S., Mandal R., Zebeli Q., Dunn S. M., and Wishart D. S.. . 2012. A metabolomics approach to uncover the effects of grain diets on rumen health in dairy cows. J. Dairy Sci. 95:6606–6623. doi: 10.3168/jds.2012-5403 [DOI] [PubMed] [Google Scholar]
- Schweigel, M., Freyer M., Leclercq S., Etschmann B., Lodemann U., Böttcher A., and Martens H.. . 2005. Luminal hyperosmolarity decreases Na transport and impairs barrier function of sheep rumen epithelium. J. Comp. Physiol. B. 175:575–591. doi: 10.1007/s00360-005-0021-3 [DOI] [PubMed] [Google Scholar]
- Shingfield, K. J., Arölä A., Ahvenjärvi S., Vanhatalo A., Toivonen V., Griinari J. M., and Huhtanen P.. . 2008. Ruminal infusions of cobalt-EDTA reduce mammary Δ9-desaturase index and alter milk fatty acid composition in lactating cows. J. Nutr. 138:710–717. doi: 10.1093/jn/138.4.710 [DOI] [PubMed] [Google Scholar]
- Smith, G. W., Davis J. L., Tell L. A., Webb A. I., and Riviere J. E.. . 2008. Extralabel use of nonsteroidal anti-inflammatory drugs in cattle. J. Am. Vet. Med. Assoc. 232:697–701. doi: 10.2460/javma.232.5.697 [DOI] [PubMed] [Google Scholar]
- Takeuchi, K., and Amagase K.. . 2018. Roles of cyclooxygenase, prostaglandin E2 and EP receptors in mucosal protection and ulcer healing in the gastrointestinal tract. Curr. Pharm. Des. 24:2002–2011. doi: 10.2174/1381612824666180629111227 [DOI] [PubMed] [Google Scholar]
- Ten Bruggencate, S. J., Bovee-Oudenhoven I. M., Lettink-Wissink M. L., Katan M. B., and van der Meer R.. . 2006. Dietary fructooligosaccharides affect intestinal barrier function in healthy men. J. Nutr. 136:70–74. doi: 10.1093/jn/136.1.70 [DOI] [PubMed] [Google Scholar]
- Theron, A. J., Steel H. C., Tintinger G. R., Feldman C., and Anderson R.. . 2013. Can the anti-inflammatory activities of β2-agonists be harnessed in the clinical setting? Drug Des. Devel. Ther. 7:1387–1398. doi: 10.2147/DDDT.S50995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDA . 1997. Official United States standards for grades of carcass beef. Washington (DC): USDA. [Google Scholar]
- Vicente, F., Sarraseca A., de Vega A., and Guada J. A.. . 2004. Performance of several Cr and Yb analytical techniques applied to samples of different biological origin (digesta or faeces). J. Sci. Food Agric. 84:2035–2040. doi: 10.1002/jsfa.1908 [DOI] [Google Scholar]
- Zhang, S., Albornoz R. I., Aschenbach J. R., Barreda D. R., and Penner G. B.. . 2013. Short-term feed restriction impairs the absorptive function of the reticulo-rumen and total tract barrier function in beef cattle. J. Anim. Sci. 91:1685–1695. doi: 10.2527/jas.2012-5669 [DOI] [PubMed] [Google Scholar]
- Zhang, R., Zhu W., and Mao S.. . 2016. High-concentrate feeding upregulates the expression of inflammation-related genes in the ruminal epithelium of dairy cattle. J. Anim. Sci. Biotechnol. 7:42. doi: 10.1186/s40104-016-0100-1 [DOI] [PMC free article] [PubMed] [Google Scholar]