Abstract
Clinical and pathologic heterogeneity in type 1 diabetes is increasingly being recognized. Findings in the islets and pancreas of a 22-year-old male with 8 years of type 1 diabetes were discordant with expected results and clinical history (islet autoantibodies negative, hemoglobin A1c 11.9%) and led to comprehensive investigation to define the functional, molecular, genetic, and architectural features of the islets and pancreas to understand the cause of the donor’s diabetes. Examination of the donor’s pancreatic tissue found substantial but reduced β-cell mass with some islets devoid of β cells (29.3% of 311 islets) while other islets had many β cells. Surprisingly, isolated islets from the donor pancreas had substantial insulin secretion, which is uncommon for type 1 diabetes of this duration. Targeted and whole-genome sequencing and analysis did not uncover monogenic causes of diabetes but did identify high-risk human leukocyte antigen haplotypes and a genetic risk score suggestive of type 1 diabetes. Further review of pancreatic tissue found islet inflammation and some previously described α-cell molecular features seen in type 1 diabetes. By integrating analysis of isolated islets, histological evaluation of the pancreas, and genetic information, we concluded that the donor’s clinical insulin deficiency was most likely the result autoimmune-mediated β-cell loss but that the constellation of findings was not typical for type 1 diabetes. This report highlights the pathologic and functional heterogeneity that can be present in type 1 diabetes.
Keywords: pancreatic islet, type 1 diabetes, atypical, histology, endocrine, endotypes
Type 1 diabetes (T1D) is an autoimmune disease that results from selective destruction of islet β cells. Classically, T1D presents during childhood or adolescence; however, numerous patients do not fit these typical criteria [1,2]. For example, T1D genetic risk scores suggest T1D can develop within the first 6 months of life [3] and that as much as 40% of T1D develops after the age of 30 [4,5]. Beyond significant difference in age of onset, even among identical twins [6], phenotypic differences in timing to insulin dependence, residual C-peptide production, and seroconversion could result from variables such as environment, ethnicity, and genetics [7]. Furthermore, clinical insulin deficiency can present as T1D but instead result from single gene variants [8].
With the pathogenic heterogeneity of T1D being increasingly recognized [9], the concept of numerous endotypes, representing discrete, complex biological processes, responsible for the clinically observable T1D phenotype is emerging [1,10]. However, precise definitions for such endotypes do not exist. By studying pancreatic tissue and islets from the same donor, we report unexpected findings in the pancreas and islets of an individual with 8 years of clinical T1D supporting the concept of T1D heterogeneity.
Materials and Methods
Donor Information
Human tissue from 15 donors (1 with T1D and 14 without diabetes) were obtained through partnerships with organ donor organizations as previously described [11] (Table 1). The donor highlighted in this report was a Caucasian 22-year-old male (body mass index 25.7 kg/m2) with an 8-year history of T1D treated with Novolog and Lantus (hemoglobin A1c 11.9%) who died due to anoxic brain injury secondary to cardiac arrest from drug intoxication (Table 1, donor 1). Social history included cigarettes (1 pack/week), alcohol use (1×/week for 4 years), intravenous drug abuse, and daily THC use (5 years) prior to death. In the final admission, the donor presented in diabetic ketoacidosis with an anion-gap metabolic acidosis and admission glucose of 719 mg/dL. Toxicology screen was positive for tetrahydrocannabinol and opiates. He was treated with intravenous insulin for 4 days in the intensive care unit with respiratory support and corticosteroids, diuretics, antibiotics, and vasopressors prior to organ harvest. Renal (Cr 0.6 mg/dL) and hepatic (aspartate aminotransferase 14 u/L, alanine aminotransferase, 38 u/L, alkaline phosphatase 88 u/L) function were normal.
Table 1.
Human islet donor information
| Islet and/or tissue preparation | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unique identifier | DON57 | DON43 | DON41 | DON91 | DON5 | DON36 | DON138 | DON120 | DON126 | 08774469 | DON4 | DON54 | DON55 | DON42 | DON61 |
| Donor age, years | 22 | 7 | 8 | 8 | 9 | 11 | 16 | 19 | 19 | 19 | 10 | 20 | 24 | 35 | 55 |
| Donor sex | M | M | F | M | M | M | M | M | M | M | M | M | M | M | M |
| Donor BMI, kg/m2 | 25.7 | 26.6 | 16.1 | 17.2 | 15.5 | 18.3 | 23.2 | 20.1 | 21.2 | 34.1 | 19.3 | 19.4 | 35.5 | 26.8 | 35.6 |
| Donor HbA1c | 11.9 | N/A | N/A | N/A | N/A | 5.8 | 5.9 | 5.1 | 5.0 | 5.8 | N/A | 5.6 | 5.5 | 5.1 | N/A |
| Origin/source of islets | IIAM | NDRI | IIAM | NDRI | NDRI | IIAM | IIAM | NDRI | NDRI | IIDP | NDRI | IIAM | IIAM | IIAM | IIAM |
| Islet isolation center | AHN | AHN | AHN | AHN | AHN | AHN | AHN | AHN | AHN | Univ of Wis | AHN | AHN | AHN | AHN | AHN |
| Donor history of diabetes | Yes | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| Diabetes duration, years | 8 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Glucose-lowering therapy at time of death | Insulin (Novolog, Lantus) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Donor cause of death | Anoxia | Respiratory arrest | ICH | Anoxia | Head trauma | Anoxia | Head trauma | Head trauma | Anoxia | Head trauma | Head trauma | Head trauma | Head trauma | Head trauma | CVA |
| Warm ischaemia time, h | DBD | DBD | DBD | DBD | DBD | DBD | DBD | DBD | DBD | N/A | DBD | DBD | DBD | DBD | DBD |
| Cold ischaemia time, h | 12.9 | N/A | N/A | 12 | N/A | N/A | 10.77 | 16.38 | 11.9 | 7.3 | N/A | N/A | 12 | N/A | N/A |
| Estimated purity, % | 75 | 40-50 | 60 | 30 | N/A | 75 | 80 | 90 | 70 | 95 | N/A | N/A | 75 | N/A | N/A |
| Estimated viability, % | 90 | N/A | N/A | 90 | N/A | N/A | 75 | 83 | 70 | 98 | N/A | N/A | 90 | N/A | N/A |
| Culture time prior to shipment, h | N/A | N/A | N/A | 16 | N/A | N/A | 11 | 19 | 18 | 42 | N/A | N/A | 9 | N/A | 36 |
| Glucose-stimulated insulin secretion | Perifusion | Perifusion | Perifusion | Perifusion | Perifusion | Perifusion | Perifusion | Perifusion | Perifusion | Perifusion | Tissue only | Tissue only | Perifusion | Tissue only | Perifusion |
| Handpicked to purity? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Experiment used | In vitro, IHC | In vitro | In vitro | In vitro | In vitro | In vitro | In vitro | In vitro, IHC | In vitro, IHC | In vitro | IHC | IHC | IHC | IHC | IHC |
Samples included in this table have been previously published [1,2].
Abbreviations: AHN, Allegheny Health Network; BMI, body mass index; CVA, cerebrovascular accident (stroke); DBD, donation after brain death (warm ischemia time essentially 0 h); HbA1c, hemoglobin A1c; ICH, intracerebral hemorrhage; IHC, immunohistochemistry; IIAM, International Institute for the Advancement of Medicine; IIDP, Integrated Islet Distribution Program; N/A, not available; NDRI, National Disease Research Interchange; nPOD, Network of Pancreatic Organ Donors with Diabetes.
Reporting checklist from NJ Hart NJ, AC Powers, Progress, challenges, and suggestions for using human islets to understand islet biology and human diabetes, Diabetologia, 2019;62:212-222, and V Poitout, LS Satin, SE Kahn, A call for improved reporting of human islet characteristics in research articles, Diabetes, 68(2):239-240.
Human Pancreatic Islet and Tissue Procurement
Human pancreatic islet and tissue were procured in partnership with the International Institute for Advancement of Medicine, National Disease Research Interchange, Integrated Islet Distribution Program, and Network for Pancreatic Organ Donors with Diabetes using previously published methodology [8,11]. The Vanderbilt University Institutional Review Board declared studies on de-identified human pancreatic specimens do not qualify as human subject research.
Measurement of Endocrine Cell Populations
Islet dissociation, intracellular antibody staining, and population analysis on a BD Biosciences FACS Aria II Cell Sorter was performed using a previously described protocol [12].
DNA Sequencing
DNA was extracted from snap-frozen donor tissue using Wizard Genomic DNA purification kit (Promega, A1120). DNA sequencing was performed as previously described [8,11] using a custom-designed next-generation sequencing–targeted panel that includes 148 genes implicated in monogenic forms of diabetes (neonatal diabetes and maturity-onset diabetes of the young), insulin resistance, lipodystrophy, obesity, rare syndromic forms of diabetes, and diabetes candidate genes [13]. The T1D genetic risk score was calculated from 10 variants known to be associated with T1D [5].
Whole-exome Sequencing
Whole-exome sequencing was performed using the Agilent SureSelect Clinical Research Exome Kit (Agilent Technologies) on Illumina NextSeq technology with 150-bp paired end reads and mean depth of coverage over 150×. Variants with a global population frequency >1% in ExAC were excluded. Variants were filtered for relevance to human diabetes using diabetes-related keywords with Online Mendelian Inheritance in Man and Human Gene Mutation Database. All variants were interpreted according to the American College of Medical Genetics guidelines.
Pancreatic Islet T-cell Isolation
One hundred hand-picked whole islets were dissociated and immediately stained with viability dye and T-cell markers, which were used to detect T-cell populations by flow cytometry as previously described [14]. The gate was set for single cells, viable cells, and CD45+ (BD BioSciences, no. 560178, 1:100) [15] cells. All CD45+ cells were interrogated for either CD3+ (BD BioSciences, no. 555334, 1:100) [16] T cells or CD19+ (BD BioSciences, no. 555412, 1:25) [17] B cells. The CD3+ T cells were further evaluated for CD4+ (BD BioSciences, no. 555346, 1:25) [18] and CD8+ (BD BioSciences, no. 561953, 1:25) [19] cell subpopulations.
In Vitro Assessment of Pancreatic Islet Function
Islet function of the T1D donor (Table 1, donor 1) and normal controls (Table 1, donors 2-10) was evaluated with a dynamic cell perifusion system as previously described [8,11,20]. Insulin and glucagon concentrations in each perifusion fraction and islet extracts were measured by radioimmunoassay (Millipore).
Immunohistochemical Analysis
Immunohistochemical analysis of pancreas was performed on serial 5-μm cryosections from multiple blocks from head, body, and tail regions for islet cell mass, insulitis, and quantification of cellular protein expression as described [8,11]. Primary antibodies to insulin (Dako, no. A0564, 1:1000) [21], glucagon (abcam, ab-10988, 1:500; Cell Signaling, no. 2760, 1:500) [22,23], CD45 (BD Pharmigen, no. 555480, 1:100) [24], C-peptide (DSHB, GN-ID4, 1:500) [25], NKX6-1 (gift from Palle Serup, 1:2000), ARX (R&D Systems, AF7068, 1:1000) [26], MAFB (gift from Roland Stein, BL1228, 1:3000), somatostatin (Santa Cruz, sc-7819, 1:500) [27], and appropriate secondary antibodies (all to donkey and from Jackson ImmunoResearch: anti-guinea pig-Cy2, 706-225-148, 1:500; anti-rat-Cy2, 712-225-153 1:500; anti-mouse Cy3, 715-165-150, 1:500; anti-rabbit-Cy3, 711-165-152, 1:500; anti-mouse-Cy5, 715-175-151, 1:200; anti-goat-Cy5, 705-605-003, 1:200; anti-guinea pig-Cy5, 706-175148, 1:200) [28-34] were used. Based on recent reports, insulitis was defined by the presence of 15 or more CD45+ cells within the islet or the periphery. An islet was defined as greater than 80 μm. The presence of pseudoatrophic islets (ie, islets devoid of insulin positive cells) was confirmed by evaluation of the head, body, and tail regions. Percentage of islet cells was determined by number of cells expressing indicated marker over total cells in manually annotated islet region. The cumulative distribution of α cells, β cells, and CD45+ cells and correlation plots of hormone producing cells to CD45+ cells were assessed by simple linear regression using GraphPad Prism 8.0. Protein expression of nuclear factors in α cells was quantified using MetaMorph 7.1 imaging software (Molecular Devices) with manual counting and an average of 290 ± 57 α cells were counted per normal donor (Table 1, donors 4, 8-9, and 11-15) and an average of 763 ± 372 α cells were counted for this donor (Table 1, donor 1) for each transcription factor (MAFB, ARX, NKX6.1).
Statistical Analysis
Values are shown as mean ± SE of the mean for control samples. Data from a sample size of n = 1 for the donor precluded formal statistical analysis.
Results
Clinical Characteristics of Donor
We studied the pancreas and islets from a Caucasian 22-year-old male (body mass index = 25.7 kg/m2) with an 8-year history of T1D treated with insulin (Table 1, donor 1). At the time of pancreas procurement, the donor’s hemoglobin A1c was 107 mmol/mol (11.9%) and T1D-associated autoantibodies (mIAA, IA2A, GADA, and ZnT8) were negative. The serum C-peptide was 0.06 ng/mL, suggesting near absolute insulin deficiency but could also reflect impaired insulin secretion related to critical illness at the time of sample collection. No family history of diabetes was reported in the redacted medical chart.
Investigation of Pancreatic Tissue Revealed Typical and Atypical Features of T1D
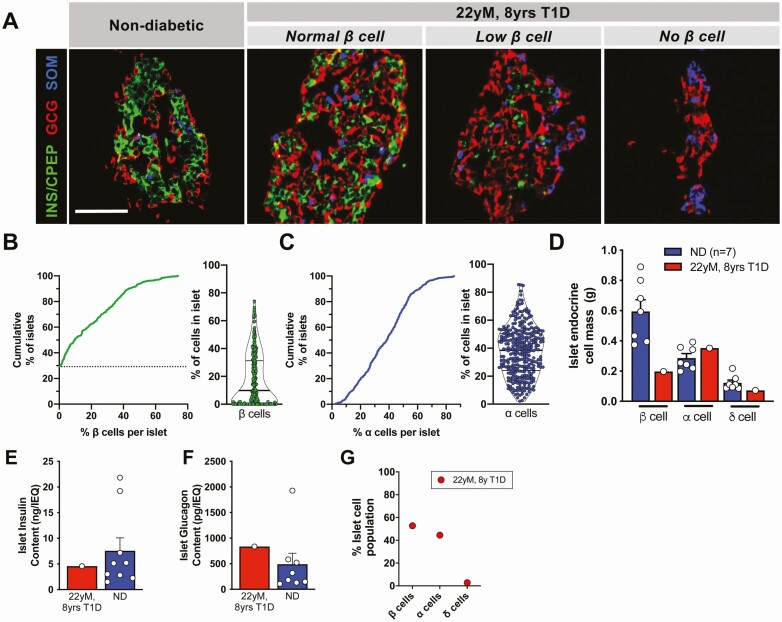
The donor pancreas was reduced in size and weighed 59.1g, similar to that seen for other T1D pancreata [35]. The pancreas was processed to obtain both tissue and isolated islets [32 000 islet equivalents (IEQs) at 65% purity, with greater than 90% viability] [11]. Surprisingly, systematic analysis of the head, body, and tail regions of the donor pancreas by immunofluorescence found that most islets (70.7%; 220 of 311 islets) contained at least 1 β cell, in contrast to tissue from donors with less than 10 years of T1D (17.8 ± 15.5%) [11] (Fig. 1A-1B). Despite remnant β cells in most islets, in aggregate, the distribution of the proportion of β cells per islet was strongly shifted downward, which was not seen with α cells (Fig. 1B-1C). Thus, the overall β-cell mass in the pancreas was lower than α-cell mass and less than that seen in donors without diabetes (Fig. 1D) but greater than in most individuals with T1D [11].
Figure 1.
Pancreatic islets from pancreas of 22-year-old donor with 8 years of type 1 diabetes had surprising β-cell numbers. (A) Expression of insulin (INS) or C-peptide (CPEP), glucagon (GCG), and somatostatin (SOM) in the donor’s (Table 1, donor 1) pancreatic tissue compared to a normal nondiabetic islet. Representative islets of varying β-cell numbers from donor are shown. Scale bar = 50 μm. (B-C) Cumulative distribution and violin plot of % β cells (B, green) per islet, % α cells (C, blue). Dashed line in B denotes nearly 30% of islets were devoid of all β cells. (D) β, α, and δ cell mass (grams) in donor pancreas compared to controls (Table 1, donors 4, 8-9, 11-15; control data published previously [8,11]). Each data point represents the average mass across the combined pancreatic head, body and tail regions of each donor. (E-F) Islet insulin (E) and glucagon (F) content compared to donors without diabetes [normal donors (ND)]. (G) Endocrine cell populations in dispersed isolated pancreatic islets from this donor contained 52.7% β cells, 44.4% α cells, and 2.7% δ cells. Normal control islets collected by this method had a range of 53.4 ± 2.6% β cells, 38.5 ± 2.7% α cells, and 7.5 ± 0.9% δ cells [11].
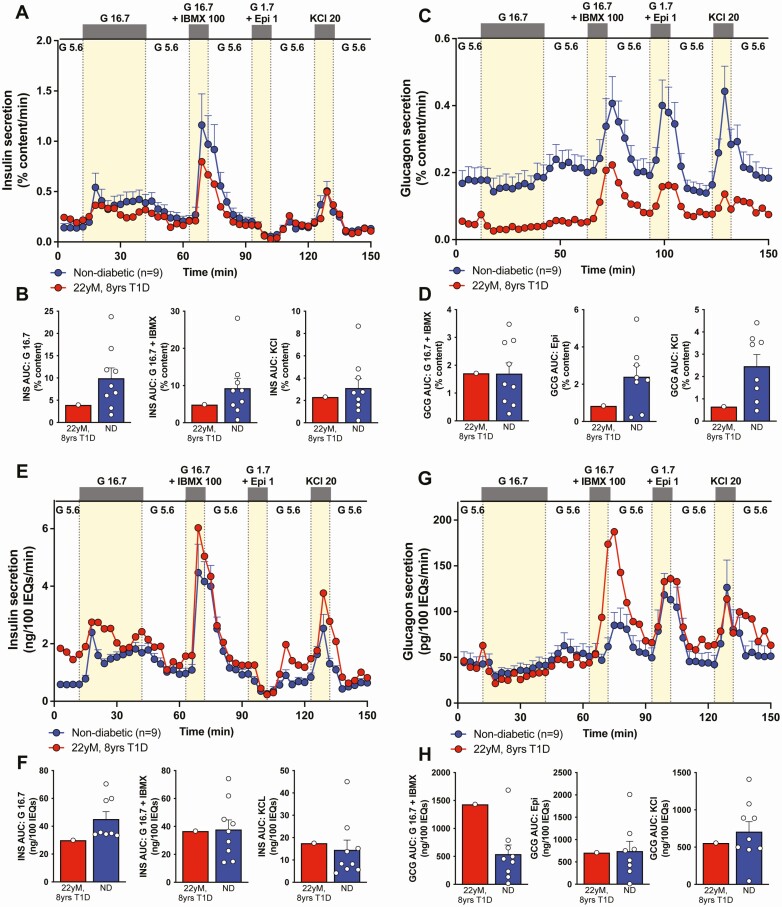
Isolated Islets Showed Substantial Insulin Secretion and Content
Surprisingly, both insulin and glucagon content per IEQ were similar to islets from nondiabetic donors (Fig. 1E-1F). Independent evaluation of isolated islet endocrine composition by flow cytometry (Fig. 1G) was also in the range of normal, unusual for T1D of this duration [11]. When normalized to % insulin content, these T1D donor islets had substantial glucose-stimulated insulin secretion in response to multiple secretagogues, suggesting that the function of individual β cells was relatively normal (Fig. 2A-2B). Consistent with the unexpectedly high β-cell numbers, the insulin secretion profile was more similar to nondiabetic controls and considerably more than reported for other T1D islet preparations when normalized by islet volume (IEQ) (Fig. 2E-2F) [11]. Interestingly, glucagon secretion was decreased when normalized by % glucagon content (Figs. 2C-2D and 2G-2H for normalization by islet volume), similar to findings in recent-onset T1D islets (<10-year T1D duration) and not described in the α cells from a donor with clinical insulin insufficiency due to HNF1A-associated monogenic diabetes [8,11].
Figure 2.
Pancreatic islets from pancreas of 22-year-old donor with 8 years of diabetes had considerable dynamic insulin secretion. (A, C) Insulin (A) or glucagon (C) secretion measured in islets isolated from donor (Table 1, donor 1, red) pancreas compared to controls without diabetes (Table 1, donors 2-10, blue; control data previously published [8,11]) normalized to % insulin content. G 5.6 − 5.6 mM glucose; G 16.7 − 16.7 mM glucose; G 16.7 + isobutylmethylxanthine (IBMX) 100 − 16.7 mM glucose + 100 μM IBMX; G 1.7 + Epi 1 − 1.7 mM glucose + 1 μM epinephrine; KCl 20 − 20 mM potassium chloride. (B, D) Integrated insulin (B) or glucagon (D) secretion was calculated as area under the curve (AUC) for the listed secretagogues. Analogous traces and analyses normalized by islet equivalents (IEQ), a measure of islet volume, are shown in Fig. 2E-2H. Results of the nondiabetic samples are expressed as mean ± SE of the mean.
Genetic Analysis Did Not Uncover Monogenic Causes of Diabetes
Because of the absence of T1D-associated antibodies and the greater than anticipated pancreatic β-cell mass, insulin secretion, and insulin content, all unexpected in T1D of this duration, we sequenced the donor DNA for variants in 148 diabetes-related genes and identified a heterozygous variant in the intronic region of glucokinase (GCK: c.209-8G > A) (Table 2). While this variant was previously reported [36], it is now known to be nonpathologic when evaluated by current in silico predictive tools (data not shown from Dr. R. Hegele, unpublished). The donor had the high-risk human leukocyte antigen (HLA) haplotypes DR4 and DQ8; further analysis of single nucleotide polymorphisms in HLA and non-HLA loci revealed a T1D genetic risk score of >75th percentile, which is reported to be indicative of T1D with 95% specificity and 50% sensitivity [5].
Table 2.
DNA sequencing of 22-year-old male donor with 8 years of type 1 diabetes for variants associated with monogenic diabetes
| Gene | Chr | Transcript ID (NCBI) | Nucleotide | Amino Acid Change | dbSNP ID | MAF | POLY Score |
|---|---|---|---|---|---|---|---|
| CDKN1C | 11 | NM_000076.2 | c.543_554del | p.Ala191_Pro194del | NA | 0 | 0 |
| CYP27B1 | 12 | NM_000785.3 | c.963 + 7T > G | — | NA | 0 | 0 |
| EIF2AK3 | 2 | NM_004836.5 | c.-201A > G | — | rs144057685 | 0.005 | 0 |
| FBN1 | 15 | NM_000138.4 | c.3294C > T | p.Asp1098Asp | rs140587 | 0.005 | 0 |
| GCK | 7 | NM_000162.3 | c.209-8G > A | — | rs144798843 | 0.001 | 0 |
DNA isolated from pancreatic sample of donor was subjected to DNA sequencing covering coding regions and splice junctions of 148 genes associated with monogenic diabetes [13].
Abbreviations: Chr, chromosome; MAF, minor allele frequency.
To further investigate the possibility of a diabetes related to a single gene variant, we performed whole-exome sequencing. All variants associated with diabetes were evaluated by keywords and assessed for pathogenicity and clinical phenotype, but no clear pathogenic variants in known genes related to β-cell identity or function were identified (Table 3). Interestingly, we did identify a previously unreported variant in SLC2A4 (c.811C > T), which encodes GLUT4, the insulin-regulated glucose transporter [37], that was predicted to be deleterious to protein function.
Table 3.
Variants associated with diabetes arising from whole exome sequencing of 22-year-old male donor with 8 years of type 1 diabetes
| Gene | Transcript ID (NCBI) | Nucleotide | Amino acid change | Zygosity | Allele Frequency (gnomAD), % | SIFT |
|---|---|---|---|---|---|---|
| ABCC9 | NM_005691.3 | c.3594G > A | Met1198Ile | Heterozygous | 0.0071 | Tolerated |
| COL6A5 | NM_153264.6 | c.2006T > G | Val669Gly | Heterozygous | 0.5300 | Deleterious |
| EPG5 | NM_020964.3 | c.3280G > A | Gly1094Ser | Heterozygous | 0.0250 | Tolerated |
| OAS1 | - | c.812A > T | Tyr271Phe | Heterozygous | 0.0016 | Tolerated |
| PPIP5K2 | NM_001345875.2 | c.3325A > G | Ile1109Val | Heterozygous | 0.0920 | Tolerated |
| SLC2A4 | — | c.811C > T | Arg271Trp | Heterozygous | 0.0000 | Deleterious |
| SOS1 | — | c.2593T > G | Leu865Val | Heterozygous | 0.0000 | Deleterious |
| UCP1 | — | c.169G > A | Gly57Ser | Heterozygous | 0.0290 | Tolerated |
| ZZEF1 | -— | c.8785C > G | Leu2929Val | Heterozygous | 0.0510 | Deleterious |
Donor DNA underwent whole-exome sequencing. Variants were filtered for relevance to human diabetes using key words with the top 9 variants reported here.
Abbreviation: SIFT, Sorting Intolerant From Tolerant.
Analysis of Islets and Pancreas Suggests Autoimmune Etiology for the Donor’s Diabetes
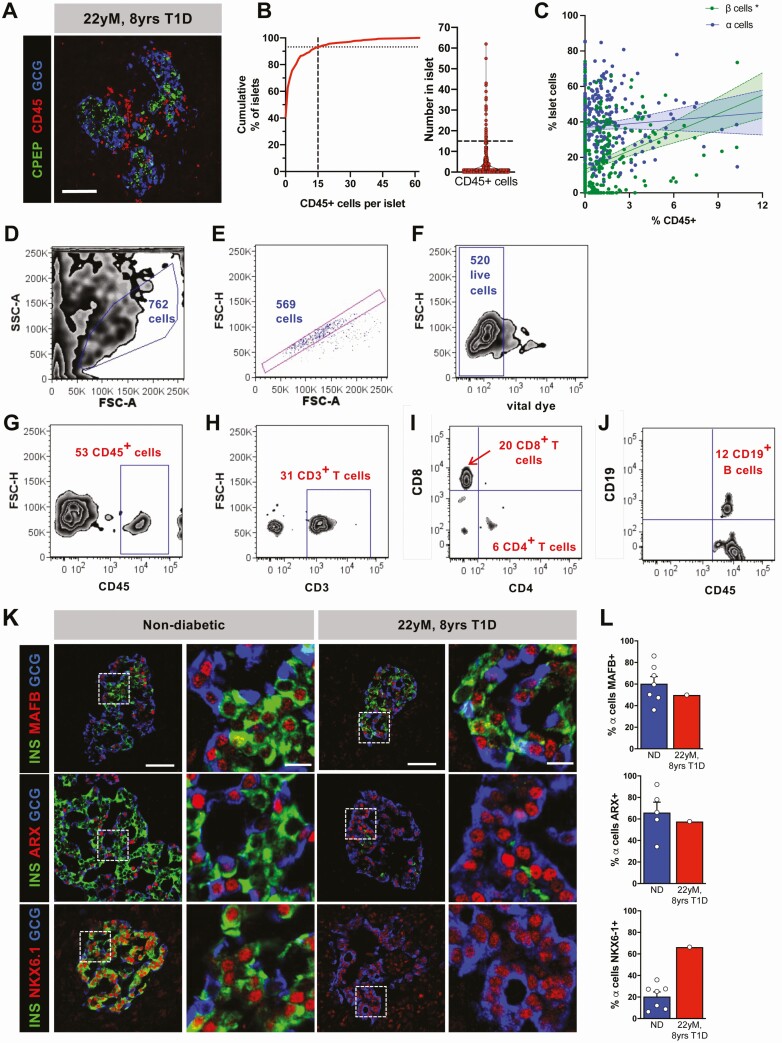
Despite the lack of humoral islet-directed autoimmunity, 21 of 311 islets (7%) evaluated from 8 blocks encompassing all regions of the donor pancreas had CD45+ infiltration with ≥15 CD45+ cells within the islet or at the islet periphery (Fig. 3A-3B) [9]. Notably, the degree of islet immune cell infiltration, measured by percentage of total islet cells expressing CD45, correlated with the proportion of islet β cells but not islet α cells (Fig. 3C) [9]. To identify these immune cells, lymphocytes were sorted from 100 hand-picked donor islets (Fig. 3D-3J) with 6 CD4+ T cells, 20 CD8+ T cells, and 12 CD19+ B cells identified, similar in distribution to samples with a >5-year duration of T1D (Dr. S. Kent, unpublished). We also looked for evidence of disordered islet gene expression recently described in T1D [11]. Misexpression of β-cell marker NKX6.1 transcription factor in α cells with maintained expression in β cells was seen in a pattern similar to that reported in T1D. However, we did not appreciate change in donor α-cell ARX or MAFB expression (Fig. 3K-3L).
Figure 3.
Pancreatic islets from pancreas of 22-year-old donor with 8 years of type 1 diabetes demonstrated immune and molecular features suggestive of type 1 diabetes. (A) Representative islet [C-peptide (CPEP), green; glucagon (GCG), blue) from donor showing significant immune cell (CD45, red) infiltration. Scale bar = 50 μm. (B) Cumulative distribution and violin plot of number of CD45+ cells per islet. Intersection of dashed lines in B indicates approximately 7% of islets (n = 311 islets evaluated) with 15 or greater CD45+ cells. (C) Correlation of % CD45+ cells vs % CPEP+ or % GCG+ cells in 311 islets reviewed from this donor analyzed by simple linear regression. * indicates significantly (P < 0.05) nonzero slope. (D-J) Lymphocytes from 100 hand-picked islets were dissociated and evaluated by flow cytometry. Initial gating for single, viable cells is shown in D-F. Cells were interrogated for CD45+ cells (G) and CD3+ T cells (H), which were evaluated for the subpopulations of CD8+ and CD4+ T cells (I) and for CD19+ B cells (J). (K-L) Immunohistochemistry (K) for expression of nuclear markers MAFB, ARX, and NKX6.1 in donor α cells and quantified (L) compared to the appropriate controls (Table 1, donors 4, 8-9, and 11-15; control data previously published [8, 11]). Scale bars in K represent 50 μm with corresponding inset scale bar 10 μm. Results of the nondiabetic samples are expressed as mean ± SE of the mean.
Discussion
In studies of the pancreas and islets from an individual with clinical T1D for 8 years, we found features that were surprising for T1D of this duration, including greater than anticipated β-cell mass in the pancreas and insulin secretion by isolated islets. These unexpected findings prompted a search for a single gene variant that contributed to the insulin deficiency; however, both targeted and whole-exome DNA sequencing found no clear monogenic cause for diabetes. Instead, further analysis of pancreatic tissue and islets revealed lymphocytic infiltration in islets with remnant β cells and molecular changes in α cells consistent with T1D. By integrating pancreatic islet histology, function, molecular analysis, and donor genetics, we conclude that this donor’s clinical insulin deficiency was most likely the result of immune-mediated loss of β cells, even though there were atypical features.
While the majority of T1D samples have nearly undetectable β cells by 10 years of disease, similar cases of substantial remnant β-cell mass (>10% of control) despite years of T1D have been reported [9,38]. However, substantial insulin secretion by isolated islets is quite unusual and differs from prior reports of T1D islets [11]. Interestingly, there was also discordance between β-cell numbers from isolated islets compared to in situ analysis of pancreatic tissue. Acknowledging this is a single case, possible explanations include (1) the function of the isolated islets did not reflect their in vivo function, perhaps as a result of glucotoxicity from the sustained hyperglycemia (A1c of 11.9), with recovery of islet function after isolation and during culture and (2) the isolated islets did not reflect those in the native pancreas; islets with more β cells and nearly normal insulin content/IEQ were overrepresented in the isolated islet preparation. Since most studies of T1D pancreas have examined only pancreatic tissue and not both islets and tissue, additional research is needed.
Clinically observed T1D in this donor with partially preserved β-cell mass and substantial insulin secretory function challenges perceived dogma regarding β-cell decline and threshold for clinical disease in T1D. While the variant in SLC2A4 is unlikely to cause the donor’s diabetes, it points out that other features could contribute to the clinical picture in the setting of inadequate β-cell mass in T1D. In addition, the presence of considerable functional β cells in ex vivo studies despite evidence of ongoing autoimmunity in this donor suggests that efforts to enhance and protect remaining β cells could be quite beneficial in such individuals. Moreover, while our evaluation of autoantibodies in this donor occurred at the time of death and may not be representative of this individual’s autoantibody status at disease diagnosis, this case highlights the importance and need for persistent biomarkers in T1D.
In sum, these findings demonstrate the value of integrating studies from both pancreatic tissue and isolated islets by highlighting unique aspects of pathologic and functional heterogeneity in T1D. This report adds to a growing consensus of heterogeneity in clinical presentation that may be indicative of multiple, distinct biological mechanisms that act to produce the clinical T1D phenotype [1,3,4]. Continued efforts directed at detailed clinical and immunologic phenotyping of individuals with T1D and in-depth analysis of the islets and tissue from donors with T1D are needed to understand this heterogeneity, precisely define the different forms of diabetes, and identify in vivo biomarkers that can help identify these cases early in disease progression [39].
Acknowledgments
We thank the organ donors and their families for their invaluable donation and the International Institute for Advancement of Medicine (IIAM), Organ Procurement Organizations, National Disease Research Exchange (NDRI), Network for Pancreatic Organ Donors with Diabetes (nPOD), and Integrated Islet Distribution Program (IIDP) for their partnership in studies of human pancreatic tissue and islets for research. C-peptide was analyzed at Northwest Lipid Metabolism & Diabetes Research Laboratories and autoantibodies were measured at the Barbara Davis Center for Childhood Diabetes. The authors apologize to investigators whose important work was not cited.
Financial Support: This research was performed using resources and/or funding provided by the NIDDK-supported Human Islet Research Network (HIRN, RRID:SCR_014393; https://hirnetwork.org; DK104211, DK108120, DK112232, DK123716, and DK120456), by DK106755, DK72473, DK89572, DK94199, DK116284, DK020595, DK104942, T32GM007347, F30DK112630, F30DK118830, and DK20593 (Vanderbilt Diabetes Research & Training Center), and by grants from the JDRF, the Leona M. and Harry B. Helmsley Charitable Trust, and the Department of Veterans Affairs (BX000666).
Author Contributions: R.H., J.T.W., M.S., S.C.K., D.M.H., R.B., L.H.P., M.B., and A.C.P. conceived and designed experiments. R.H., J.T.W., M.S., C.V.R., S.S., R.A., G.P., A.R., S.D.R., J.A.B.B., N.P., R.A.H., S.C.K., D.H.M., R.B., L.H.P., M.B., and A.C.P. performed experiments or analyzed the data and interpreted results. R.H., J.T.W., M.B., and A.C.P. wrote the manuscript. All co-authors reviewed, edited, and approved the final version.
Contributor Information
Marcela Brissova, Email: marcela.brissova@vanderbilt.edu.
Alvin C Powers, Email: al.powers@vumc.org.
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
All data generated or analyzed during this study are included in this published article or is available from the corresponding authors on reasonable request.
References
- 1. Redondo MJ, Hagopian WA, Oram R, et al. The clinical consequences of heterogeneity within and between different diabetes types. Diabetologia. 2020;63(10):2040-2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Powers AC. Type 1 diabetes mellitus: much progress, many opportunities. J Clin Invest. 2021;131(8):e142242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Consortium E-T, Johnson MB, Patel KA, et al. Type 1 diabetes can present before the age of 6 months and is characterised by autoimmunity and rapid loss of beta cells. Diabetologia. 2020;63(12):2605-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thomas NJ, Jones SE, Weedon MN, Shields BM, Oram RA, Hattersley AT. Frequency and phenotype of type 1 diabetes in the first six decades of life: a cross-sectional, genetically stratified survival analysis from UK Biobank. Lancet Diabetes Endocrinol. 2018;6(2):122-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oram RA, Patel K, Hill A, et al. A type 1 diabetes genetic risk score can aid discrimination between type 1 and type 2 diabetes in young adults. Diabetes Care. 2016;39(3):337-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Redondo MJ, Jeffrey J, Fain PR, Eisenbarth GS, Orban T. Concordance for islet autoimmunity among monozygotic twins. N Engl J Med. 2008;359(26):2849-2850. [DOI] [PubMed] [Google Scholar]
- 7. Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309(23):2473-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haliyur R, Tong X, Sanyoura M, et al. Human islets expressing HNF1A variant have defective β cell transcriptional regulatory networks. J Clin Invest. 2019;129(1):246-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Campbell-Thompson M, Fu A, Kaddis JS, et al. Insulitis and β-cell mass in the natural history of type 1 diabetes. Diabetes. 2016;65(3):719-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Battaglia M, Ahmed S, Anderson MS, et al. Introducing the endotype concept to address the challenge of disease heterogeneity in type 1 diabetes. Diabetes Care. 2020;43(1):5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brissova M, Haliyur R, Saunders D, et al. α cell function and gene expression are compromised in type 1 diabetes. Cell Rep. 2018;22(10):2667-2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blodgett DM, Nowosielska A, Afik S, et al. Novel observations from next-generation RNA sequencing of highly purified human adult and fetal islet cell subsets. Diabetes. 2015;64(9):3172-3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alkorta-Aranburu G, Sukhanova M, Carmody D, et al. Improved molecular diagnosis of patients with neonatal diabetes using a combined next-generation sequencing and MS-MLPA approach. J Pediatr Endocrinol Metab. 2016;29(5):523-531. [DOI] [PubMed] [Google Scholar]
- 14. Babon JA, DeNicola ME, Blodgett DM, et al. Analysis of self-antigen specificity of islet-infiltrating T cells from human donors with type 1 diabetes. Nat Med. 2016;22(12):1482-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.RRID:AB_1645479. https://scicrunch.org/resolver/AB_1645479
- 16.RRID:AB_395741. https://scicrunch.org/resolver/AB_395741
- 17.RRID:AB_395812. https://scicrunch.org/resolver/AB_395812
- 18.RRID:AB_395751. https://scicrunch.org/resolver/AB_395751
- 19.RRID:AAB_10896290. https://scicrunch.org/resolver/AB_10896290
- 20. Walker JT, Haliyur R, Nelson HA, et al. Integrated human pseudoislet system and microfluidic platform demonstrate differences in GPCR signaling in islet cells. JCI Insight. 2020;5(10):e137017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.RRID:AB_10013624. https://scicrunch.org/resolver/AB_10013624
- 22.RRID:AB_659831. https://scicrunch.org/resolver/AB_659831
- 23.RRID:AB_297642. https://scicrunch.org/resolver/AB_297642
- 24.RRID:AB_395872. https://scicrunch.org/resolver/AB_395872
- 25.RRID:AB_2255626. https://scicrunch.org/resolver/AB_2255626
- 26.RRID:AB_10973178. https://scicrunch.org/resolver/AB_10973178
- 27.RRID:AB_2302603. https://scicrunch.org/resolver/AB_2302603
- 28.RRID:AB_2340467. https://scicrunch.org/resolver/AB_2340467
- 29.RRID:AB_2340674. https://scicrunch.org/resolver/AB_2340674
- 30.RRID:AB_2340813. https://scicrunch.org/resolver/AB_2340813
- 31.RRID:AB_2307443. https://scicrunch.org/resolver/AB_2307443
- 32.RRID:AB_2340436. https://scicrunch.org/resolver/AB_2340436
- 33.RRID:AB_2340462. https://scicrunch.org/resolver/AB_2340462
- 34.RRID:AB_2340820. https://scicrunch.org/resolver/AB_2340820
- 35. Wright JJ, Saunders DC, Dai C, et al. Decreased pancreatic acinar cell number in type 1 diabetes. Diabetologia. 2020;63(7):1418-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cao H, Shorey S, Robinson J, et al. GCK and HNF1A mutations in Canadian families with maturity onset diabetes of the young (MODY). Hum Mutat. 2002;20(6):478-479. [DOI] [PubMed] [Google Scholar]
- 37. Klip A, McGraw TE, James DE. Thirty sweet years of GLUT4. J Biol Chem. 2019;294(30):11369-11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oram RA, Sims EK, Evans-Molina C. Beta cells in type 1 diabetes: mass and function; sleeping or dead? Diabetologia. 2019;62(4):567-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chung WK, Erion K, Florez JC, et al. Precision medicine in diabetes: a Consensus Report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2020;63(9):1671-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or is available from the corresponding authors on reasonable request.