Abstract
Background
Marantic endocarditis (non-bacterial thrombotic endocarditis) is a rare condition that involves non-infectious thrombotic lesions typically of the aortic and mitral valves. It is predominantly associated with malignancy and less commonly systemic lupus erythematosus. In this case, we report a patient with marantic endocarditis secondary to a renal cell carcinoma that was successfully treated with nephrectomy and anticoagulation.
Case summary
A 65-year-old male patient with embolic signs and symptoms was found to have non-infective thrombotic vegetations on three cardiac valves through transoesophageal echocardiography. Computed tomography revealed a 70 mm renal mass that confirmed to be a grade two clear-cell renal cell carcinoma. Nephrectomy and anticoagulation led to resolution of the embolic symptoms and of the valvular vegetations.
Discussion
The diagnosis of marantic endocarditis requires high clinical suspicion in a patient who presents with features of embolization. Incidence is highest in patients with an underlying malignancy, particularly adenocarcinoma. This case highlights the importance of echocardiography in diagnosis, removal of the source of thrombus, and prompt treatment with anticoagulation.
Keywords: Marantic, Endocarditis, Cancer, Embolism, Echocardiography, Stroke, Case report
Learning points
To demonstrate diagnosis and effective treatment in marantic endocarditis, particularly in the context of neoplasm.
To highlight the importance of echocardiography in diagnosing valvular disease.
Introduction
Marantic endocarditis (non-bacterial thrombotic endocarditis) is a rare condition that involves non-infectious thrombotic lesions typically of the aortic and mitral valves.1 It is predominantly associated with malignancy and less commonly systemic lupus erythematosus. It is often diagnosed post-mortem as an autopsy finding. Ante-mortem, it typically presents with embolic signs and symptoms. The highest incidence has been in patients with adenocarcinoma of the pancreas, lung, colon, ovary, and prostate.1–4
The prominent symptomatology of marantic endocarditis is embolization. This can manifest systemically as symptoms of stroke or transient ischaemic attack. The main stay of treatment in marantic endocarditis is removing the source of the immune-mediated deposition of thrombus, as despite therapeutic dose anticoagulation, new valvular vegetations can form.5,6 Early anticoagulation and removal of the source of thrombus can lead to reversal of symptoms and resolution of valvular lesions as shown in this clinical case. We emphasize the importance of clinical suspicion for marantic endocarditis as a differential diagnosis when a patient presents with signs of embolus and has valvular lesions on echocardiography, often on the background of malignancy.2–6
Timeline
| Days post admission | Events |
|---|---|
| −2 | Onset of neurology. |
| 0 | Admission day—negative non-contrast computed tomography (CT) of the head, blood cultures taken. |
| 2 | Magnetic resonance imaging (MRI) showing small occipital foci—embolus. |
| 4 | Decrease in Glasgow Coma Scale (GCS) to 3—intubated and ventilated. |
| 5 | Transthoracic echocardiography (TTE) showing valvular vegetations. Antibiotics commenced. Cerebral spinal fluid sample negative. |
| 7 | Transoesophageal echocardiography (TOE) confirming valvular vegetations. Autoimmune and neuronal panel of bloods sent. |
| 8 | CT chest/abdo/pelvis showing right renal mass—? Renal cell carcinoma. |
| 10 | Antibiotics stopped post-CT result, TOE result, and multiple negative blood cultures—marantic endocarditis diagnosis considered. |
| 11 | Right nephrectomy performed—returned to intensive care unit post-surgery. |
| 13 | Unfractionated heparin infusion commenced Day 2 post-operation. Alert, orientated, GCS now 15. |
| 14 | Switched over to low molecular weight heparin. |
| 17 | Discharged from hospital and bridged to warfarin. |
| Post discharge | |
| 2 months | Follow-up TOE showing healing valvular lesions. MRI brain showing no further emboli. |
| 4 months | Further TOE showing resolution of all valvular lesions. |
| 6 months | MRI brain showing no further emboli at 6 months. |
Case presentation
A 65-year-old Caucasian man presented with a 2-day history of sudden-onset headache, weakness, visual disturbance, and a background of unintentional weight loss. He experienced gait-disturbance, difficulty with word-finding, and left-sided visual impairment. He had associated nausea and vomiting. His wife describes confusion, muddled speech, and weakness. He had no history of head trauma preceding the neurological disturbance.
A systemic review revealed a 3-month history of an unintentional 15 kg weight loss.
Past medical history included: migraines in his 20s, with his last episode 40 years ago; type 2 diabetes mellitus diagnosed in his 20s (T2DM), treated with Vildagliptin and metformin since 2012, previously diet controlled; hypertension treated with Cilazapril; a left-sided cataract and progressive right wet macular degeneration.
On physical examination, significant findings were a heart rate of 69 beats per minute, blood pressure of 177/95 mmHg, respiratory rate 16 breaths per minute, oxygen saturations of 97%, while breathing room air, and a temperature of 36.7°C. He had no significant peripheral stigmata of infective endocarditis and was clinically euvolaemic. On cardiovascular examination, he had a new ejection systolic murmur heard loudest over the aortic region, radiating to the carotids, a clear chest on auscultation and no pitting oedema. On neurological examination, he had a left-sided hemianopia with left visual inattention. No focal deficit in tone, power, or sensation was identified. Left plantar reflex was up-going and he had impaired left-sided coordination characterized by past pointing and dysdiadochokinesia.
Initial investigations showed blood results with no remarkable features other than a creatinine of 120 µmol/L and an estimated glomerular filtration rate of 54 mL/min/1.73 m2, with a normal white cell count of 8.8 × 109/L and a C-reactive protein of <5 mg/L. His blood sugar was 9.7 mmol/L with serum ketones of 0.8 mmol/L, procalcitonin was negative.
Electrocardiogram showed sinus tachycardia at rate of 105 beats per minute, otherwise normal. His chest X-ray was normal.
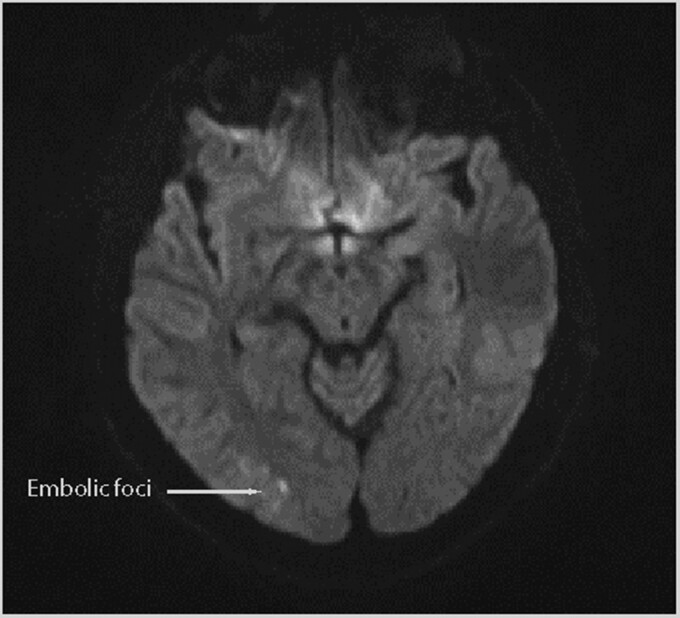
Non-contrast computed tomography (CT) of the head was performed which showed no acute intracranial findings. Magnetic resonance imaging (MRI) without IV contrast and magnetic resonance angiography of the head and neck with contrast showed a small foci of right occipital lobe restricted diffusion in keeping with embolic infarction (Figure 1). Time-of-flight sequencing demonstrated unremarkable cerebral vasculature.
Figure 1.
Diffusion weighted imaging sequence magnetic resonance imaging brain: small foci of diffusion restriction in the right occipital lobe.
On Day 4 of admission, the patient had a reduction in level of consciousness with a Glasgow Coma Scale (GCS) of 3 requiring intubation and mechanical ventilation. There was significant discordance between the relatively minor neuroimaging findings and the patient’s neurological status. Infectious/autoimmune encephalitis was considered in the differential diagnosis.
A cerebral spinal fluid (CSF) sample: including CSF protein study, Cryptococcus neoformans, N-methyl-D-aspartate (NMDA) receptor antibody, and a central nervous system polymerase chain reaction (PCR) screen were sent to query a cause for reduced consciousness. The CSF sample showed a raised protein level of 1.35 g/L (0.15–0.40) and glucose of 6.9 mmol/L (2.8–4.4). NMDA receptor antibodies were negative.
Transthoracic echocardiography (TTE) showed normal left ventricular systolic function with a subjective ejection fraction of >55%, and suspicious lesions on the aortic and mitral valves. Transoesophageal echocardiography (TOE) was performed which identified an 8 × 5 mm echogenic vegetation on the pulmonary valve and vegetations on the mitral and aortic valve (Figure 2). These findings were in keeping with the clinical finding of a new ejection systolic murmur heard loudest in the aortic region.
Figure 2.
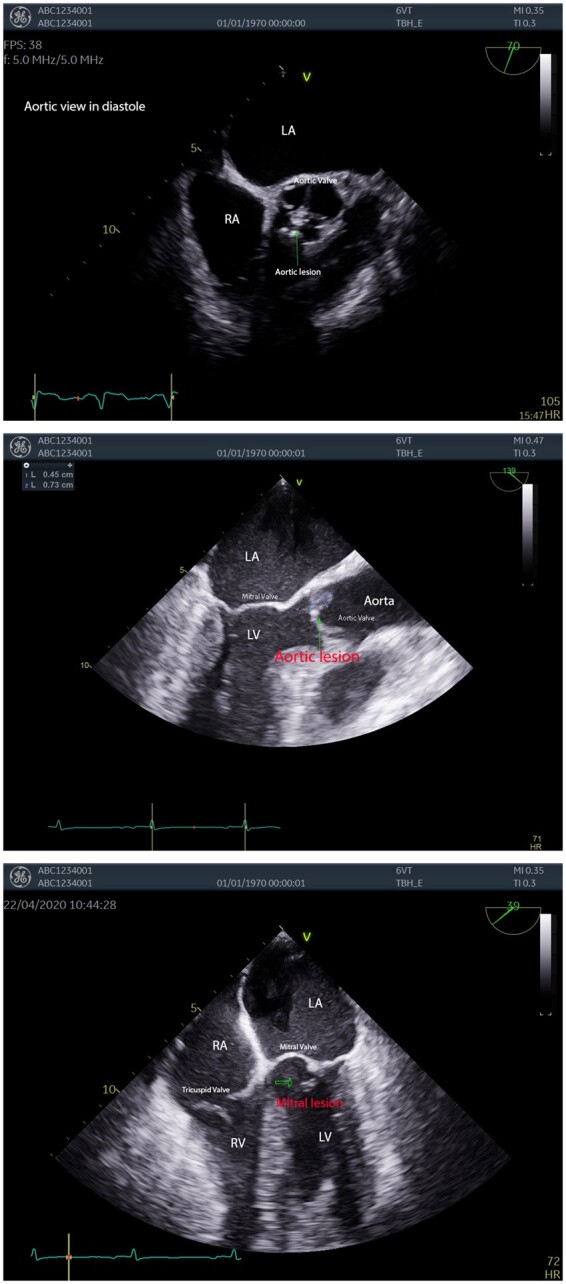
Initial transoesophageal echocardiography images showing valvular lesions. Labelled images of a mitral lesion, aortic lesion in diastole, and a long-axis view of an aortic lesion.
Intravenous amoxicillin 2 g Q4H and acyclovir were started after the TTE. After discussion with the infectious disease unit, ceftriaxone 2 g Q12h and was added while awaiting blood cultures. These returned negative.
A panel of bloods involving; antinuclear antibodies (ANA), antineutrophil cytoplasmic antibodies (ANCA), anti-rheumatoid factor, HIV screen, carcinoembryonic antigen (CEA), and CA19-9 were sent; all returned negative. Anti-neuronal antibodies were sent including anti-hu, Ri, Yo, Ma1, Ma2, anti-cv2/CRMP, amphiphysin, sox-1, zic-4, Tr, Tritin, and recoverin paraneoplastic antibody; all were negative.
Computed tomography chest/abdomen/pelvis was performed looking for neoplastic disease. A 61 × 63 × 70 mm right renal heterogeneous soft-tissue mass with a large necrotic centre was identified that was consistent with a right renal cell cancer without evidence of metastasis (Figure 3). Antibiotics were stopped on Day 10 of admission after CT result showing renal mass, multiple negative blood cultures, and TOE confirming valvular lesions, supporting a differential diagnosis of marantic endocarditis.
Figure 3.
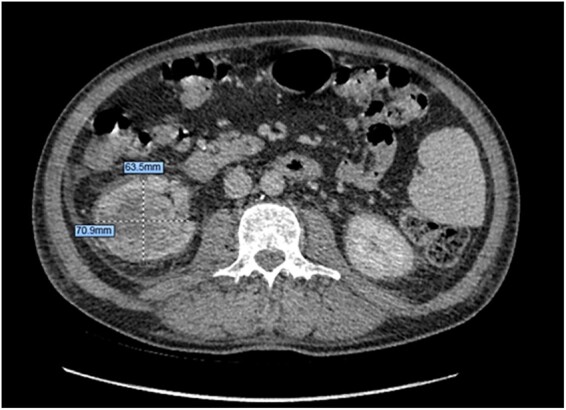
Computed tomography abdomen/pelvis—axial demonstrating right-sided renal mass with necrotic core.
On Day 11, the patient underwent nephrectomy, which was uncomplicated. An unfractionated heparin infusion was started on Day 2 post-operation, switching to therapeutic dose of low-molecular weight heparin after 24 h. The patient rapidly regained consciousness and returned to baseline neurological function post-nephrectomy. On Day 13 of admission, he was alert, orientated, and GCS 15 with no residual neurology.
On discharge, he was bridged onto warfarin for on-going anticoagulation. On a follow-up TOE, 2 and 4 months later the lesions steadily resolved (Figure 4). Anticoagulation was stopped at 4 months.
Figure 4.
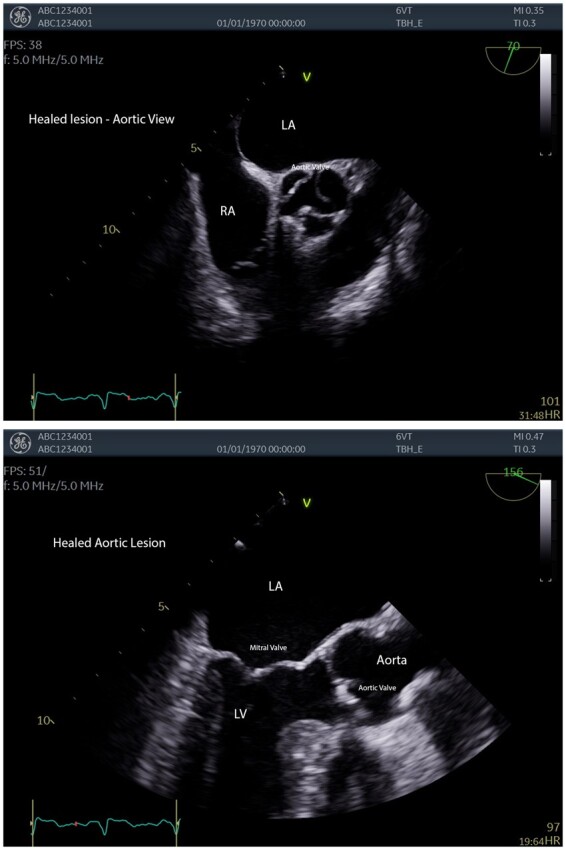
Images of aortic and mitral valves as lesions healed post anticoagulation.
Discussion—marantic endocarditis (non-bacterial thrombotic endocarditis)
Marantic endocarditis is characterized by sterile vegetations composed of fibrin-platelet thrombi which form on valvular leaflets.5 They primarily form on the aortic and mitral valves, and rarely form on the right side of heart.1 Marantic endocarditis typically occurs in patients with advanced cancer or SLE. Malignancies include adenocarcinoma of the pancreas, lung, colon, ovary, and prostate.1–4 We report a clinical first in renal cell carcinoma.
In an autopsy study, non-bacterial thrombotic endocarditis was found in 0.9–1.6% of their adult population.1 It is a difficult diagnosis to make ante-mortem due to patients remaining asymptomatic until an embolic event. Most cases of marantic endocarditis are diagnosed post-mortem.4,5
The prominent symptomology of marantic endocarditis is embolization. This can manifest systemically as symptoms of stroke or transient ischaemic attack.6 Ischaemic stroke is usually characterized by multiple small foci of infarction, most easily visualized on diffusion weighted MRI.6 Emboli to the nervous system, kidneys, spleen, extremities, and coronary arteries are most common.2,3 Systemic embolization is reported in 14–91% of cases with pulmonary embolism in 50% of all cases.6 Embolization is common due to the friable nature of the sterile thrombotic lesions; the lack of inflammation results in a poor adhesion to the valves they reside on.4–7
The pathogenesis centres around the release of pro-inflammatory cytokines which cause endothelial damage leading to formation and deposition of platelet and fibrin complexes, and immune complexes on the cardiac valves. This process is occurring on a background of a hyper-coagulative state caused by malignancies or systemic inflammation.1–3
Echocardiography is the gold standard for detecting valvular lesions ante-mortem. Transoesophageal echocardiogram is preferential to TTE as it is more sensitive.8 It can be difficult to differentiate sterile lesions from those of infective endocarditis. The features on echocardiography include small, sessile lesions with irregular borders, which are broad based, friable and are heterogeneous in density. Most commonly presenting on the mitral and aortic valves, and rarely the pulmonary; as seen in our reported case.9
Discussion of management and follow-up
The main stay of treatment in marantic endocarditis is removing the source of the immune-mediated deposition of thrombus, as despite therapeutic dose anticoagulation, new valvular vegetations can form.2,5 The initial choice of anticoagulation is unfractionated or low-molecular weight heparin, as it has been shown that patients on warfarin can have recurrent thromboembolism in cancer associated marantic endocarditis.7,10
Prior to results of the blood cultures, we commenced treatment with broad-spectrum intravenous antibiotics presuming an infectious cause of emboli.
Computed tomography abdominal scans revealed a soft-tissue mass with a large necrotic centre that was consistent with renal cell cancer (Figure 3). With multiple blood cultures remaining negative and evidence of multi-valvular thrombi on TOE/TTE, marantic endocarditis was put forward as a differential diagnosis.
Previous literature involving ovarian causes of marantic endocarditis have shown reduction in valvular thrombus after resection of the primary tumour, resolution of neurological symptoms, and no further thromboembolic events while on long-term anticoagulation.7
Follow-up MRI at 2- and 6 months demonstrated no new embolic infarcts, in keeping with clinical improvement.
Follow-up echocardiogram at 2 months showed remission of all cardiac vegetations. At 4 months, TOE demonstrated full resolution of vegetations of all affected valves, with no signs of destruction.
Conclusion
We present the first published case of marantic endocarditis secondary to renal cell carcinoma (see Table 1). This was treated through nephrectomy and therapeutic anticoagulation. We reflect on the importance of high clinical suspicion required in patients with signs of embolism, and valvular vegetations on echocardiography in the context of neoplasm. Due to the close correlation of marantic endocarditis and underlying neoplasms, it is vital to diagnose early so the underlying cause can be treated and therapeutic anticoagulation can be commenced.
Lead author biography
The lead author, Andrew Z. Harris, is a second year house officer from Christchurch, New Zealand. He completed a Bachelor of Science in Genetics before pursuing Medicine at the University of Auckland. He has a particular interest in cardiology and echocardiography.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Table 1.
Literature review summary
| Author/date | Title | Setting/context | Purpose | Findings |
|---|---|---|---|---|
| Deppisch, L.M. and Fayemi, A.O., 1976 | Non-bacterial thrombotic endocarditis (NBTE): clinicopathologic correlations | New York | Largest autopsy study over 10-year period investigating incidence and causes of NBTE |
|
| Gonzalez Q., Candela M.J., Vidal C., Roman J., and Aramburo P., 1991 | Non-bacterial thrombotic endocarditis in cancer patients | Mexico, Clinica Puerta de Hierro | Autopsy study of 1640 adult patients over 24-year period |
|
| el-Shami K., Griffiths E., and Streiff M., 2007 | Nonbacterial thrombotic endocarditis in cancer patients: pathogenesis, diagnosis, and treatment | The Sidney Kimmel Comprehensive Cancer Centre | A review of the causes, diagnosis, and treatment of Marantic Endocarditis |
|
| Umeojiako W.I., Kasouridis I., Sargent R., and Ghani S., 2019 | Atypical marantic endocarditis | UK, Darent Valley Hospital | Case report of a 44-year-old man with marantic endocarditis |
|
| Aryana A., Esterbrooks D.J., and Morris P.C., 2006 | Nonbacterial thrombotic endocarditis with recurrent embolic events as manifestation of ovarian neoplasm | USA, Massachusetts General Hospital | Case report of a 43-year-old woman with marantic endocarditis |
|
Supplementary Material
Acknowledgements
Permission obtained from all authors involved and the patient in the clinical case.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.
References
- 1. Deppisch LM, Fayemi AO.. Non-bacterial thrombotic endocarditis: clinicopathologic correlations. Am Heart J 1976;92:723–729. [DOI] [PubMed] [Google Scholar]
- 2. el-Shami K, Griffiths E, Streiff M.. Nonbacterial thrombotic endocarditis in cancer patients: pathogenesis, diagnosis, and treatment. Oncologist 2007;12:518–523. [DOI] [PubMed] [Google Scholar]
- 3. Borowski A, Ghodsizad A, Cohnen M, Gams E.. Recurrent embolism in the course of marantic endocarditis. Ann Thorac Surg 2005;79:2145–2147. [DOI] [PubMed] [Google Scholar]
- 4. Lopez JA, Ross RS, Fishbein MC, Siegel RJ.. Nonbacterial thrombotic endocarditis: a review. Am Heart J 1987;113:773–784. [DOI] [PubMed] [Google Scholar]
- 5. Umeojiako WI, Kasouridis I, Sargent R, Ghani S.. Atypical marantic endocarditis. BMJ Case Rep 2019;12:e232057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Singhal AB, Topcuoglu MA, Buonanno FS.. Acute ischemic stroke patterns in infective and nonbacterial thrombotic endocarditis: a diffusion-weighted magnetic resonance imaging study. Stroke 2002;33:1267–1273. [DOI] [PubMed] [Google Scholar]
- 7. Aryana A, Esterbrooks DJ, Morris PC.. Nonbacterial thrombotic endocarditis with recurrent embolic events as manifestation of ovarian neoplasm. J Gen Intern Med 2006;21:C12–C15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu J, Frishman WH.. Nonbacterial thrombotic endocarditis. Cardiol Rev 2016;24:244–247. [DOI] [PubMed] [Google Scholar]
- 9. Roldan CA, Qualls CR, Sopko KS, Sibbitt WL.. Transthoracic versus transesophageal echocardiography for detection of Libman-Sacks endocarditis: a randomized controlled study. The J Rheumatol 2008;35:224–229. [PubMed] [Google Scholar]
- 10. Salem DN, Stein PD, Al-Ahmad A, Bussey HI, Horstkotte D, Miller N. et al. Antithrombotic therapy in valvular heart disease—native and prosthetic: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004;126:457S–482S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.