Abstract
Background
Healthy lifestyle interventions offered at points of care, including support groups, may improve chronic disease management, especially in low-resource populations. We assessed the effectiveness of an educational intervention in type 2 diabetes (T2D) support groups to reduce cardiovascular disease (CVD) risk.
Methods
We recruited 518 participants to a parallel, two-arm, cluster-randomized, behavioural clinical trial across 22 clinics in Sonora, Mexico, between August 2016 and October 2018. We delivered a 13-week secondary prevention intervention, Meta Salud Diabetes (MSD), within the structure of a support group (GAM: Grupo de Ayuda Mutua) in government-run (community) Health Centres (Centros de Salud). The primary study outcomes were difference in Framingham CVD risk scores and hypertension between intervention (GAM+MSD) and control (GAM usual care) arms at 3 and 12 months.
Results
CVD risk was 3.17% age-points lower in the MSD arm versus control at 3 months [95% confidence interval (CI): −5.60, −0.75, P = 0.013); at 12 months the difference was 2.13% age-points (95% CI: −4.60, 0.34, P = 0.088). There was no evidence of a difference in hypertension rates between arms. Diabetes distress was also lower at 3 and 12 months in the MSD arm. Post-hoc analyses showed greater CVD risk reduction among men than women and among participants with HbA1c < 8.
Conclusions
MSD contributed to a positive trend in reducing CVD risk in a low-resource setting. This study introduced an evidence-based curriculum that provides T2D self-management strategies for those with controlled T2D (i.e. HbA1c < 8.0) and may improve quality of life.
Keywords: Cardiovascular disease, type 2 diabetes, diabetes support groups, cluster-randomized clinical trial
Key Messages
Meta Salud Diabetes contributed to a reduced cardiovascular disease risk among those in the intervention arm versus control, although the results were not sustained over the 1-year follow-up.
We found some evidence that greater risk reduction occurred among male participants than females.
Our findings add to existing evidence that support group strategies for type 2 diabetes management are effective and feasible in Latin American populations, even where resources are limited, while also highlighting the structural capacity of the Mexican public health system to implement tertiary prevention interventions in primary care settings.
Meta Salud Diabetes may confer a positive benefit for reducing cardiovascular disease risk and improving quality of life outcomes among Mexicans receiving ongoing support for type 2 diabetes management.
Introduction
As global health systems improve resources for the management of chronic diseases, the role of support group strategies is increasing. The World Health Organization (WHO) endorses peer support interventions for diabetes management,1 an approach which promotes management of modifiable risk factors for both type 2 diabetes (T2D) and comorbid conditions including cardiovascular disease (CVD). Sustained group support in T2D and CVD control is an impactful approach for health systems with limited medical resources and high prevalence of chronic conditions2 including obesity, high blood pressure, high cholesterol and tobacco use, all of which increase likelihood of CVD mortality among type 2 diabetics.3 Clinically-integrated lifestyle interventions that promote primary and secondary T2D control via self-help groups have been established as a priority for both the Mexican government and the WHO.1,4 Nonetheless, evidence for the feasibility, impact and sustainability of such programmes is not yet well established.
Primary care services for Mexican patients with T2D under the Ministry of Health includes self-help groups—Grupos de Ayuda Mutua (GAM)—as a general approach to address the growing burden of T2D. The GAM guidelines provide for weekly self-management care by providing a supportive group environment, glucose self-monitoring and health information via monthly meetings.4 Health centres formally register their GAMs with the state Ministry of Health, based on standard guidelines for participation and content.
However, there is no standardized approach across sites for patient education and patient support. We developed and evaluated the integration of a secondary prevention curriculum, Meta Salud Diabetes (MSD), within the structure of the GAM using a cluster-randomized trial. We hypothesized that GAM participants who participated in the MSD curriculum (GAM+MSD) would have lower CVD risk at each follow-up time point relative to individuals receiving standard GAM treatment at clinical sites where MSD was not offered.
The two aims for this research study were: (aim 1) testing the effectiveness of a CVD prevention intervention among patients with T2D attending a self-management support group (MSD+GAM) as compared with control (GAM usual care); and (aim 2) assessing the implementation of the MSD educational intervention to identify the strengths and limitations faced by each study-affiliated clinical site. This paper reports the results of the primary outcomes and selected secondary outcomes for aim 1.
Methods
Trial design
We designed a parallel, two-arm, cluster-randomized trial to evaluate the effectiveness of MSD+GAM for CVD prevention as compared with GAM usual care, where the unit of randomization was the health centre. We used a cluster trial because the intervention was administered at the health centre (cluster) level to reduce the risk of contamination in an individually randomized trial.
Participants
The inclusion criteria for health centres included: located in Sonora, Mexico; had a registered and active GAM; had the largest patient populations in three geographical regions (North, Central and South) of interest. Once the health centres were randomly assigned into the intervention or control sites, all GAM patients who met the inclusion criteria for our study were recruited to participate in the study. Study inclusion criteria for individual participants were: 18 years of age or older; a medical provider diagnosis of T2D; an established patient (i.e. receiving primary care) at the participating clinical site. Members of registered GAMs at each selected clinical site were individually screened for eligibility. The study protocol and consent process were approved by the University of Arizona Human Subjects Institutional Review Board under Protocol Number: 1508040144R003. The protocol was also approved by the Research Bioethics Committee at the University of Sonora.
Study arms
Intervention group
The MSD intervention consists of 2-h participatory workshop-style sessions delivered at the GAM level over 13 consecutive weeks and is described in detail in a previous publication.5 Participants received educational information and took part in empowerment-building discussions and interactive workshop activities to promote long-term behaviour change related to disease complications, diet and increased physical activity. Sessions maintained a basic structure throughout the 13 intervention weeks: blood pressure and glucose monitoring; readings, discussions and games related to each week’s topic; execution of a custom-designed physical activity routine; and follow-up exercise to meet a nutrition or physical activity goal. One or more health professionals (e.g. nurses, community health workers, doctors or clinic staff including interns) delivered each session during a regularly scheduled, face-to-face GAM meeting. Intervention fidelity was monitored at each clinic site throughout the trial by research staff.
Control group
The actions in the control group were the usual care activities, and these varied by GAM and by session. It was up to each control site to conduct their usual care activities, which included variations on the following: measuring patients' blood pressure and fasting blood glucose; providing general health information via invited speakers; assisting patients in making appointments with health centre staff to discuss their health issues
Randomization
The study biostatistician, masked to the identity of the clinics and with no contact with study participants, randomly allocated the clinics into intervention and control. We used the randomization module ‘ralloc’ of the statistical software Stata (StataCorp, College Station, TX), stratified by clinic location in the state of Sonora (North, Central, South), with permuted blocks to maintain balance between arms.
Measures
The primary outcomes were the Framingham CVD risk score (FRS) and hypertension, measured at baseline (T0), 3 months (T3) (post-intervention) and 12 months (T12). The FRS is the estimated probability of a cardiovascular event in 10 years, and was calculated separately for men and women using the coefficients from models reported in D’Agostino et al. for age, total cholesterol, high-density lipoprotein (HDL) cholesterol, non-treated systolic blood pressure (SBP), treated SBP, smoker (yes/no), T2D (yes/no) (Supplementary Table S1, available as Supplementary data at IJE online).6 Hypertension was defined as SBP over 130 or diastolic bloodpressure (DBP) greater than 80 mmHg. A small number of the lipid values were above or below detection limits (three total cholesterol values, four HDL values and seven triglyceride values). The assessment schedule of 3 and 12 months was based on literature that shows that behaviour takes at least 2 months to become habitual7; we assessed at 12 months to investigate sustained effects.
Socioeconomic status (SES) was measured by summing six items regarding possessions, resulting in an SES score with a possible range of 0–6, with higher scores indicating higher SES. Diabetes distress was measured using a modified version of the 20-item Problem Areas In Diabetes scale (PAID).8 We modified the response options from 0–4 to 0–3, as early participants had difficulties in discriminating between three (somewhat serious problem) and four (serious problem), as has sometimes been noted in low-literacy populations.9 Items were summed and multiplied by 4/3 (instead of 5/4) in order to get a possible range of 0–100, as in the original instrument, with higher values indicating greater problems.
Power and sample size
We calculated that a sample size of 10 clusters per arm, with an average of 16 participants per cluster, would yield 80% power to detect a standardized effect size of 0.4 for the FRS between the intervention and control arms at 3 months. We used an intra-cluster correlation (ICC) of 0.03, based on reviews of several studies with clustering where the median ICCs were found to be 0.01–0.02.10,11 To account for dropout we increased the number of clusters to 12 per arm and increased the number of participants per cluster by 25% to 20. Two clusters in the control arm were eventually dropped due to security concerns for the safety of research staff, leaving 12 and 10 in the intervention and control arms, respectively. This sample size also gives more than 80% power to detect a difference of 20% in hypertension rates between the groups, assuming rates of 60% and 40%.
Statistical methods
Summary statistics were used to describe the clusters and participants at baseline; t tests and chi square tests were used to assess baseline balance between the arms on participant-level variables, as randomization occurred at the cluster level only. Raw means and standard deviations or absolute frequency and percentages for each of the outcomes were computed.
Differences between the intervention and control arms at 3 (T3) and 12 months (T12) were estimated from linear mixed models. We used the repeated measures for mixed models, which uses an unstructured time and covariance matrix.12 Unadjusted models included fixed effects of strata (North, Central, South), the outcome at baseline (also known as analysis of covariance), time, treatment and time x treatment. A random effect for cluster was included and the Kenward-Roger adjustment to the denominator degrees of freedom was used. 13,14 These models: account for the correlation due to repeated measures on participants nested within clusters; allow for testing at specific time points as well as the difference in pattern of change over time between arms; and give valid results when data are missing completely at random and at random, if the models are specified correctly and include covariates associated with missingness.15 Binary outcomes were tested with generalized linear mixed models using the logit link and binomial distribution.16 To account for potential selection bias and/or imbalance of key covariates in individual participants within clinics, adjusted models were also fitted and included the baseline variables of age, gender, SES score, any CVD medication use (blood pressure, heart or stroke medication), smoking status and time in the GAM (just joined, <1 year, >1 year). These models were based on our background knowledge, literature and baseline tests of imbalance between arms. Secondary outcomes included haemoglobin A1c (HbA1c), glucose, cholesterol, triglycerides, SBP, body mass index (BMI) and diabetes distress (PAID scale), and were analysed similarly. Furthermore, to understand our CVD risk results, we performed two post-hoc subgroup analyses to investigate effect modification by sex and uncontrolled T2D (as measured by baseline HbA1c ≥ 8) using interaction terms. No corrections for multiple comparisons were made, so care must be used in interpretation.
Cronbach’s alpha was used to assess internal consistency at each time point for the PAID. Cronbach’s alpha for the PAID scale was 0.88, 0.86 and 0.90 at T0, T3 and T12, respectively, indicating high internal consistency.
Sensitivity analyses
We performed two sensitivity analyses. We first replaced the lipid levels that were beyond the limit of detection with missing values. The second sensitivity analysis used multiple imputation to fill in missing data in the primary analyses. Variables associated with missingness, the outcome or both were included in the imputation model, as well as all variables from the analysis model, including centre. Multiple imputation with chained equations using 100 imputations was used and the imputation was performed separately by arm.17 All analyses used Statistical Analysis Software (SAS version 9.4) (SAS Institute, Cary, NC).
Results
Table 1 gives baseline characteristics of the clinics (n = 22) and participants (n = 518). Clinics were located primarily in the Central region of Sonora (58.3% in MSD, 50.0% in control), and were mostly urban (83.3% in MSD, 80.0% in control). The median cluster size was 24 and 23 in the intervention and control arm, respectively. Participants were mostly female (85%); had a mean age of 57.8 years in the MSD arm and 62.4 years in the control arm; were mostly married or partnered (65.5%, 58.8%); with a median monthly income of $4000 pesos ($222.00 US dollars) and $3700 pesos ($205.00 US dollars) in the MSD and control arms, respectively. There were some differences between the arms at the participant level: MSD participants were slightly younger, had more years of education (7.3 versus 6.5), had spent less time in the GAM (34% just joined versus 9.4%) and had higher rates of smoking (11% versus 6%) and hypertension (55.1% versus 42.2%). Insurance, SES score, medication use and alcohol use were similar.
Table 1.
Baseline characteristics of health clinics (clusters) and participants
| Clinic characteristics |
Meta Salud Diabetes
K = 12 clinics |
Control
K = 10 clinics |
P -value |
|---|---|---|---|
| Location, n (%) | |||
| North | 3 (25.0) | 3 (30.0) | |
| Central | 7 (58.3) | 5 (50.0) | |
| South | 2 (16.7) | 2 (20.0) | |
| Urban, n (%) | 10 (83.3) | 8 (80.0) | |
| Cluster size, median (range) | 24 (19, 33) | 23 (12, 30) | |
| Participant characteristics | n = 293 participants | n = 225 participants | |
| Age (years), mean (SD) | 57.8 (11.2) | 62.4 (11.0) | <0.0001 |
| Female gender, n (%) | 249 (85.0) | 191 (84.9) | 0.98 |
| Married or partnered | 192 (65.5) | 131 (58.8) | 0.10 |
| Education (years), mean (SD) | 7.3 (3.7) | 6.5 (3.7) | 0.02 |
| Monthly household income, pesos, median (range) | 4000 (0-35 000) | 3700 (500-50 000) | 0.20 |
| Monthly income, pesos, n (%) | 0.56 | ||
| <1,000 | 18 (6.1) | 12 (5.4) | |
| 1000-2000 | 38 (12.9) | 43 (19.2) | |
| 2000-4000 | 102 (34.8) | 69 (30.8) | |
| 4000-6000 | 70 (23.9) | 50 (22.3) | |
| 6000-8000 | 22 (7.5) | 13 (5.8) | |
| >8000 | 31 (10.6) | 26 (11.6) | |
| SES score,a mean (SD) | 3.6 (1.2) | 3.6 (1.2) | 0.85 |
| Employed, n (%) | 128 (43.7) | 95 (42.4) | 0.77 |
| Insurance, n (%) | 0.85 | ||
| Public employee | 65 (22.3) | 56 (25.1) | |
| Seguro Popular | 222 (76.0) | 162 (72.7) | |
| Private | 2 (1.0) | 2 (0.9) | |
| No insurance | 3 (1.0) | 3 (1.4) | |
| Time in the GAM, n (%) | <0.0001 | ||
| Just joined | 100 (34.1) | 21 (9.4) | |
| <1 year | 59 (20.1) | 37 (16.5) | |
| ≥1 year | 134 (45.7) | 166 (74.1) | |
| Medications, n (%) | |||
| Diabetes | 271 (92.8) | 203 (91.4) | 0.33 |
| Cholesterol | 75 (25.7) | 74 (33.3) | 0.05 |
| Blood pressure | 200 (68.5) | 154 (69.4) | 0.83 |
| Heart | 34 (11.6) | 26 (11.3) | 0.96 |
| Stroke | 2 (0.8) | 2 (0.9) | 0.42 |
| Drinks any alcohol, n (%) | 89 (30.1) | 66 (29.4) | 0.51 |
| Current smoker, n (%) | 32 (11.0) | 13 (5.9) | 0.04 |
| Outcomes at baseline | |||
| CVD risk,b % | 20.8 (16.5) | 23.2 (17.2) | 0.11 |
| Hypertension, n (%) | 161 (55.1) | 95 (42.2) | 0.004 |
| Total cholesterol (mg/dL) | 191.6 (43.1) | 195.8 (51.2) | 0.33 |
| HDL cholesterol (mg/dL) | 45.8 (12.6) | 47.8 (14.0) | 0.08 |
| Triglycerides (mg/dL) | 203.1 (104.6) | 197.9 (93.3) | 0.56 |
| Systolic blood pressure (mm Hg) | 126.3 (20.3) | 125.1 (19.8) | 0.52 |
| Diabetes distressc | 22.6 (15.6) | 22.2 (16.3) | 0.78 |
SES, socioeconomic status; GAM, Grupo de Ayuda Mutua; CVD, cardiovascular disease; HDL, high-density lipoprotein; SD, standard deviation.
Possible range 0–6.
Framingham risk score, see Supplementary Material for calculation, available as Supplementary data at IJE online.
Possible range 0–100.
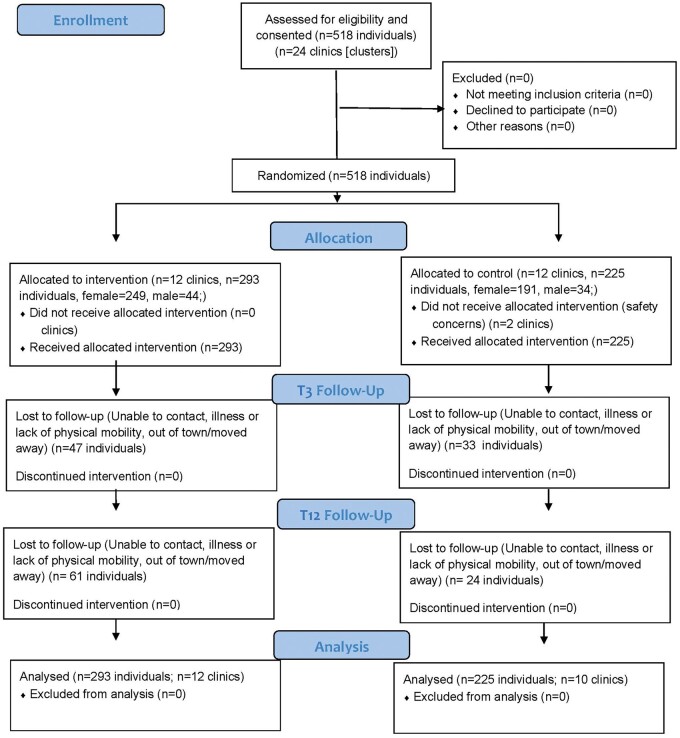
Figure 1 shows the participant flow. At T3, 47 (16%) of the 293 MSD participants had missing primary outcome data, as compared with 33 (15%) of the control participants (P = 0.67). At T12, the rates were higher in the MSD arm, 61 (21%) versus 24 (11%), P = 0.002.
Figure 1.
Participant Flow during the Meta Salud Diabetes Intervention Study, 215 x 279 mm (263 x 263 DPI)
Primary outcomes
Unadjusted and adjusted models gave similar results, as shown in Table 2; we report the unadjusted estimates. CVD risk was 3.17% age points lower in the MSD arm than the control arm at T3, (95% CI: −5.60, −0.75, P = 0.013); however, at 12 months the difference was −2.13% age points (95% CI: −4.60, 0.34, P = 0.088). There was no evidence of difference in hypertension at either time point. The unadjusted odds ratio (OR) was 1.16 (95% CI: 0.60, 2.07) at 3 months and 1.29 at 12 months (95% CI: 0.70, 2.40).
Table 2.
Primary outcomes. Difference between arms or odds ratio (OR) and 95% confidence intervals (CI)a
| Variable |
Time
(months) |
MSD
Mean (SD) |
Control
Mean (SD) |
Unadjusted difference (95% CI) | P | Adjusted differenceb (95% CI) | P |
|---|---|---|---|---|---|---|---|
| CVD riskc (%) | 3 | 18.9 (14.6) | 23.8 (18.4) | −3.17 (−5.60, −0.75) | 0.013 | −2.52 (−4.78, −0.24) | 0.031 |
| 12 | 18.9 (14.2) | 23.4 (18.3) | −2.13 (−4.60, 0.34) | 0.088 | −1.25 (−3.82, 1.32) | 0.276 | |
| n (%) | n (%) |
Unadjusted OR (95% CI) |
Adjusted ORb (95% CI) |
||||
| Hypertension | 3 | 131 (53.3) | 87 (45.1) | 1.16 (0.60, 2.07) | 0.727 | 1.03 (0.54, 1.98) | 0.920 |
| 12 | 120 (51.7) | 83 (41.1) | 1.29 (0.70, 2.40) | 0.419 | 1.39 (0.72, 2.67) | 0.322 |
SD, standard deviation; CVD, cardiovascular disease; SES, socioeconomic status; GAM, Grupo de Ayuda Mutua.
Estimated from analysis of covariance (ANCOVA) mixed models accounting for nested, longitudinal design.
Adjusted for baseline values of the outcome, age, gender, SES score, any CVD medication use (blood pressure, heart or stroke medication), smoking status, time in the GAM.
Framingham risk score, see Supplementary Material for calculation, available as Supplementary data at IJE online.
Post-hoc analyses showed that the effect of MSD was modified by gender, with larger effects for CVD risk among men than women (Supplementary Table S2, available as Supplementary data at IJE online). The difference between arms at T3 was −10.05 (95% CI: −15.01, −5.10, P < 0.0001) for men as compared with −1.79 (95% CI: −4.12, 0.53, P = 0.13) for women. At T12 the difference was −10.35 (95% CI: −15.60, −5.10, P < 0.0001) for men as compared with 0.07 (95% CI: −2.39, 2.53, P = 0.96) for women. The P-values for the sex by time and the treatment by sex interaction terms were 0.04 and <0.0001, respectively, but these results warrant cautious interpretation given the small sample size of men.
We also found evidence of effect modification by baseline HbA1c (P for interaction = 0.0006), with larger effects for CVD risk among participants whose T2D was controlled (baseline HbA1c <8). The difference between arms for this subgroup at T3 was −4.59 (95% CI: −7.22, −1.96, P = 0.001); at T12 the difference was −3.30 (95% CI: −6.02, −0.59, P = 0.019). There was no evidence of a difference in CVD risk between arms for participants with HbA1c ≥8.
Diabetes distress was lower at T3, with a difference of −4.36 (95% CI: −6.55, −2.18, P < 0.0001). There was no evidence of difference between arms for any of the other secondary outcomes (Table 3). The empirical ICC for CVD risk was 0.03. ICCs for each of the other outcomes are shown in Supplementary Table S4, available as Supplementary data at IJE online, and ranged from 0.001 to 0.06.
Table 3.
Secondary clinical outcomes
| Variable | Time (months) |
MSD
Mean (SD) |
Control
Mean (SD) |
Unadjusted difference (95% CI)a | P | Adjusted difference (95% CI)b | P |
|---|---|---|---|---|---|---|---|
| HbA1c (%) | 3 | 7.3 (1.8) | 7.1 (1.6) | −0.16 (−0.41, 0.09) | 0.209 | −0.14 (−0.39, 0.11) | 0.249 |
| 12 | 7.9 (2.1) | 7.8 (1.9) | −0.28 (−0.59, 0.04) | 0.086 | −0.27 (−0.58, 0.04) | 0.090 | |
| Glucose | 3 | 156.5 (63.5) | 162.3 (75.9) | −4.58 (−23.0, 13.8) | 0.61 | −3.92 (−19.9, 12.1) | 0.62 |
| 12 | 165.1 (74.3) | 173.3 (89.9) | −11.1 (−31.1, 8.9) | 0.27 | −11.5 (−29.4, 6.49) | 0.21 | |
| Total cholesterol (mg/dL) | 3 | 183.1 (40.7) | 188.6 (45.6) | −3.84 (−11.73, 4.05) | 0.326 | −4.72 (−12.5, 3.10) | 0.226 |
| 12 | 191.6 (44.6) | 193.6 (46.1) | −0.28 (−9.01, 8.44) | 0.948 | 0.33 (−8.38, 9.03) | 0.940 | |
| HDL cholesterol (mg/dL) | 3 | 47.8 (11.8) | 47.0 (13.4) | 1.45 (−0.71, 3.60) | 0.179 | 1.54 (−0.69, 3.76) | 0.167 |
| 12 | 46.8 (13.6) | 46.9 (13.7) | 1.28 (−1.06, 3.62) | 0.275 | 1.25 (−1.16, 3.66) | 0.299 | |
| Triglycerides (mg/dL) | 3 | 187.6 (108.6) | 183.6 (99.4) | 2.94 (−19.9, 25.8) | 0.796 | −5.95 (−29.0, 17.1) | 0.604 |
| 12 | 211.9 (115.8) | 212.5 (102.2) | −2.46 (−24.8, 19.9) | 0.823 | −10.3 (−32.6, 12.0) | 0.356 | |
| Systolic blood pressure (mmHg) | 3 | 126.4 (18.3) | 127.2 (20.9) | −2.21 (−5.68, 1.25) | 0.203 | −1.17 (−4.78, 2.45) | 0.516 |
| 12 | 124.9 (19.0) | 124.8 (20.1) | −0.33 (−3.79, 3.14) | 0.848 | 0.89 (−2.78, 4.56) | 0.626 | |
| Diabetes distressc | 3 | 15.1 (12.6) | 19.6 (13.8) | −4.36 (−6.55, −2.18) | <0.0001 | −5.13 (−7.30, −2.96) | <0.0001 |
| 12 | 15.3 (14.8) | 16.9 (15.9) | −2.21 (−4.69, 0.27) | 0.079 | −3.02 (−5.54, −0.50) | 0.019 |
HbA1C, haemoglobin A1C; HDL, high-density lipoprotein; SD, standard deviation; CI, confidence interval; CVD, cardiovascular disease; SES, socioeconomic status; GAM, Grupo de Ayuda Mutua.
Estimated from ANCOVA mixed models accounting for nested, longitudinal design.
Adjusted for baseline values of the outcome, age, gender, SES score, any CVD medication use (blood pressure, heart or stroke medication), smoking status, time in the GAM.
Possible range 0–100.
Sensitivity analyses
In the first sensitivity analysis, where limit-of-detection outcome values were replaced with missing values, results changed insignificantly from the primary analyses. The second sensitivity analysis results, where multiple imputation was used, gave CVD risk estimates that were similar to the primary analysis. CVD risk was 3.12% age points lower in the MSD arm than the control arm at T3, (95% CI: −5.67, −0.57, P = 0.013); at 12 months the difference was −2.40% age points (95% CI: −5.00, 0.17, P = 0.068); See Supplementary Material for more detail, available as Supplementary data at IJE online.
We found that in comparison with participants who had complete data, participants who dropped out were more likely to have just joined the GAM. All other variables were similar, except for health centre, where dropout rates ranged from 8% to 50%; see Supplementary Table S3, available as Supplementary data at IJE online.
Discussion
Relative to the primary outcome of changes in CVD risk measured by the FRS, members of the intervention arm demonstrated a positive trend toward improvement in CVD risk compared with the control arm. Though the absolute risk difference is small, it is important to recognize that there was a movement in a positive direction from high risk to moderate risk as a result of the MSD intervention, despite the challenges of conducting this study in Mexico. However, we did not see evidence of a difference in hypertension at either time point between the intervention arm and the usual care arm.
These findings demonstrate results similar to previous studies of community-based behavioural interventions.18 MSD, which is incorporated into existing clinic-based social support mechanisms within the Mexican health system, can moderately reduce CVD risk among individuals with T2D. In a study of mostly Hispanic participants, Ma et al. (2009) found that educational interventions led by health professionals targeting multifactor risk reduction can lead to modest improvements in CVD risk factors among high-risk patients in low-income, ethnic minority populations.19 Notably, the MSD intervention was provided by existing health professionals working within the health centres and thus, with adequate support, the programme could be expanded across the health system at relatively low cost. We also note that our study found larger improvements for CVD risk among participants whose baseline HbA1c was less than 8 as compared with participants with HbA1c ≥8 (i.e. uncontrolled T2D), although this was a post-hoc analysis. This is consistent with previous studies, which have found that individuals with HbA1c under 7 are consistently most likely to show benefit for CVD risk reduction.20 These findings further support increasing accessibility of health promotion interventions within primary care settings to encourage people with T2D to maintain control of their disease. Subgroup analyses revealed that MSD was very effective for men, but less so for women; this finding is particularly interesting given challenges recruiting men to participate in this study. Both of the subgroup analyses were unplanned, and care should be exercised in interpreting these results.
Diabetes distress was lower for participants of the intervention arm at the 3-month follow-up. Although not associated with increased metabolic control, these findings do underscore the importance of addressing mental health and quality of life among people with T2D.21 It is also possible that ongoing support, greater knowledge of the mechanisms of T2D on health status and increased confidence in one’s ability to practice self-management may result in greater control over time.22 Social support is a known protective factor against diabetes distress,23 especially in Mexican Hispanics.24 We also found that individuals who had recently joined a GAM were more likely to drop out of the intervention than those who had been members of a GAM for a longer period. Among previous educational interventions targeting individuals with T2D, predictors of dropout include lower self-efficacy,25 lack of interaction with a community health worker26 and low socioeconomic status,27 any of which may be associated with motivation to remain in the GAM and thus in our study.The population studied in this trial was predominantly older, low-income adults with limited mobility. Reasons given for dropout reflected these conditions when we were unable to contact enrollees, participants cited illness or lack of physical mobility or were out of town/moved away (Figure 1).
Strengths and limitations
Strengths of this study include that it was designed with the Secretaría de Salud (Health Ministry) and other health promotion experts, as well as adapted from previous evidence-based interventions such as Meta Salud and Pasos Adelante.28 Additionally, the academic-health system partnership approach we used promotes trust and respect among partners, which facilitates the research process,29 as well as the likelihood that positive results will lead to sustained integration of the intervention into the health care system. Ongoing support from the health system and health centres, including logistical support and intervention facilitation, lent itself to both the sustainability and the acceptability of the project for future implementation. An additional strength is that the MSD intervention was well received by the participants. We estimated a high attendance from participants that were not lost to follow-up at the conclusion of the intervention at T3 (n = 246), with attendance by participant on average 10 out of 13 sessions.
There were also weaknesses in the study design. The FRS has been demonstrated to overestimate CVD risk in U.S. Hispanic populations30–32; however, no alternative or more accurate measures of CVD risk among Hispanics, and more specifically among Mexicans, have been identified. Furthermore, overestimates of CVD risk are likely to be similar between the arms, yielding an unbiased estimate of the difference. Our participant sample may have suffered from selection bias in both arms (e.g. most participants were low-income women who traditionally participate more than men in the GAMs). Another limitation is that the FRS is both a surrogate and a composite outcome.33,34 Future research should consider actual CVD outcomes rather than risk scores. As pointed out by Freemantle et al., composite outcomes give increased statistical precision but can make interpretation more difficult.34 We tested the time-varying components of the FRS, but none was the apparent driver of the effect.
Another limitation in our study was the missing primary outcome data for the intervention arm at T12. Though this did constitute 21% of the intervention sample, our statistical analyses methods did adjust for missing data. We did not adjust for multiple comparisons, so it is possible that significant results are due to type I errors. Last, we did not conduct a health economic analysis to show the cost-effectiveness of our intervention. However, since our study was focused on establishing the effectiveness of the intervention, we felt that the cost-effectiveness analysis would be more appropriate for the scale-up phase.
Public health implications
This study introduced an evidence-based curriculum that provides strategies for T2D self-management among those with controlled T2D (i.e. those with an HbA1c < 8.0) and which may improve quality of life for individuals with chronic T2D, hypertensionand CVD risk. These findings demonstrate that existing health care staff can successfully deliver a health education curriculum within a clinical setting. Future studies focusing on the implementation of health promotion interventions across a variety of primary care settings are needed to improve dissemination and scale-up of evidence-based curricula. To support these efforts, development of risk prediction models that better estimate risk for Mexican populations are needed. In addition, new strategies are needed to improve involvement of participants in the support groups. Targeted recruitment of hard-to-reach subgroups including men may be warranted, given known difficulties recruiting and retaining men in T2D self-management interventions. Differing gender perspectives on both perceived intervention benefit and disease management self-efficacy35 should be considered among intersectional determinants of health including age, border-related stressors and comorbid health conditions. MSD is a theory-driven approach to improved diabetes control, and increased emphasis on personal and collective empowerment strategies that consider community and family support in diabetes stress management among Mexican men and women with T2D should be explored. These considerations, along with national improvements in tertiary public health services, should improve sustainability of MSD and similar programmes across GAMs.
Conclusions
Mexicans with T2D, particularly those facing day-to-day conditions of vulnerability, need quality care including effective health promotion strategies for chronic disease management. The T2D epidemic, declared a national emergency by the Mexican government in 2016,36 requires the health system to shift focus on several fronts: from infectious disease prevention to chronic disease care, and from disease prevention to health promotion. Addressing the epidemic entails using multiple approaches to create an integral strategy working on many structural levels. Health promotion programmes such as MSD may contribute to strengthening the ability of the health system to take these actions.
Supplementary data
Supplementary data are available at IJE online.
Funding
This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number R01HL125996. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary Material
Acknowledgements
We acknowledge the Ministry of Health of Sonora, Mexico, for their commitment to the study designed to improve the health of their patients with T2D.
Author contributions
C.R., C.D., M.L.B., E.C.V., M.I., M.dC.C.V. and J.G.dZ. contributed to conceptualization of the research design and methods. M.L.B. performed all statistical analyses. C.R., E.A. and T.N. led the writing and final review of the manuscript. C.D., M.L.B, E.C.V., M.I., M.dC.C.V., E.A. and J.G.dZ. contributed to writing and critical review of the paper. E.G.F., B.A. and T.N. contributed to critical review of the paper. C.R. and C.D. serve as co-principal investigators and approved final manuscript for publication.
Conflict of interest
None declared.
References
- 1.World Health Organization. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020. Geneva: WHO, 2013. [Google Scholar]
- 2. Doull M, O'Connor AM, Welch V, Tugwell P, Wells GA.. Peer support strategies for improving the health and well‐being of individuals with chronic diseases. Cochrane Database Syst Rev 2005, Issue 3, CD005352. [Google Scholar]
- 3. Lorber D. Importance of cardiovascular disease risk management in patients with type 2 diabetes mellitus. Diabetes Metab Syndr Obes 2014;7:169–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Estrategia Grupos de Ayuda Mutua . Enfermedades Crónicas. Lineamientos de Operación. Centro Nacional De Programas Preventivos Y Control De Enfermedades, CDMX, Mexico. [Mutual Aid Groups Strategy. Chronic Diseases. Operating Guidelines. National Center for Preventive Programs and Disease Control, Mexico City, Mexico].2016.
- 5. Sabo S, Champion CD, Bell ML. et al. Meta Salud Diabetes study protocol: a cluster-randomised trial to reduce cardiovascular risk among a diabetic population of Mexico. BMJ Open 2018;8:e020762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. D’Agostino RB, Vasan RS, Pencina MJ. et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117:743–53. [DOI] [PubMed] [Google Scholar]
- 7. Lally P, Van Jaarsveld C, Potts H, Wardle J.. How are habits formed: modelling habit formation in the real world. Eur J Soc Psychol 2010;40:998–1009. [Google Scholar]
- 8. Beléndez M, Hernández-Mijares A, Marco J, Domínguez JR, Pomares FJ.. Validation of the Spanish version of the Problem Areas in Diabetes (PAID-SP) Scale. Diabetes Res Clin Pract 2014;106:e93–e95. [DOI] [PubMed] [Google Scholar]
- 9. Chachamovich E, Fleck MP, Power M.. Literacy affected ability to adequately discriminate among categories in multipoint Likert Scales. J Clin Epidemiol 2009;62:37–46. [DOI] [PubMed] [Google Scholar]
- 10. Adams G, Gulliford MC, Ukoumunne OC, Eldridge S, Chinn S, Campbell MJ.. Patterns of intra-cluster correlation from primary care research to inform study design and analysis. J Clin Epidemiol 2004;57:785–94. [DOI] [PubMed] [Google Scholar]
- 11. Bell ML, McKenzie JE.. Designing psycho‐oncology randomised trials and cluster randomised trials: variance components and intra‐cluster correlation of commonly used psychosocial measures. Psychooncology 2013;22:1738–47. [DOI] [PubMed] [Google Scholar]
- 12. Mallinckrodt C, Lipkovich I.. Analyzing Longitudinal Clinical Trial Data: A Practical Guide. London: Chapman and Hall/CRC, 2016. [Google Scholar]
- 13. Leyrat C, Morgan KE, Leurent B, Kahan BC.. Cluster randomized trials with a small number of clusters: which analyses should be used? Int J Epidemiol 2018;47:321–31. [DOI] [PubMed] [Google Scholar]
- 14. Kenward MG, Roger JH.. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 1997;53:983–97. [PubMed] [Google Scholar]
- 15. Bell ML, Fairclough DL.. Practical and statistical issues in missing data for longitudinal patient-reported outcomes. Stat Methods Med Res 2014;23:440–59. [DOI] [PubMed] [Google Scholar]
- 16. Bell ML, Rabe BA.. The mixed model for repeated measures for cluster randomized trials: a simulation study investigating bias and type I error with missing continuous data. Trials 2020;21:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Azur MJ, Stuart EA, Frangakis C, Leaf PJ.. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res 2011;20:40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krantz MJ, Beaty B, Coronel-Mockler S, Leeman-Castillo B, Fletcher K, Estacio RO.. Reduction in cardiovascular risk among latino participants in a community-based intervention linked with clinical care. Am J Prev Med 2017;53:e71–e75. [DOI] [PubMed] [Google Scholar]
- 19. Ma J, Berra K, Haskell WL. et al. Case management to reduce risk of cardiovascular disease in a county health care system. Arch Intern Med 2009;169:1988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eeg‐Olofsson K, Cederholm J, Nilsson P. et al. New aspects of HbA1c as a risk factor for cardiovascular diseases in type 2 diabetes: an observational study from the Swedish National Diabetes Register (NDR). J Intern Med 2010;268:471–82. [DOI] [PubMed] [Google Scholar]
- 21. Paschalides C, Wearden A, Dunkerley R, Bundy C, Davies R, Dickens C.. The associations of anxiety, depression and personal illness representations with glycaemic control and health-related quality of life in patients with type 2 diabetes mellitus. J Psychosom Res 2004;57:557–64. [DOI] [PubMed] [Google Scholar]
- 22. Egede LE, Ellis C.. The effects of depression on diabetes knowledge, diabetes self-management, and perceived control in indigent patients with type 2 diabetes. Diabetes Technol Ther 2008;10:213–19. [DOI] [PubMed] [Google Scholar]
- 23. Baek RN, Tanenbaum ML, Gonzalez JS.. Diabetes burden and diabetes distress: the buffering effect of social support. Ann Behav Med 2014;48:145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McEwen MM, Pasvogel A, Gallegos G, Barrera L.. Type 2 diabetes self‐management social support intervention at the US‐Mexico border. Public Health Nurs 2010;27:310–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nam S, Dobrosielski DA, Stewart KJ.. Predictors of exercise intervention dropout in sedentary individuals with type 2 diabetes. J Cardiopulm Rehabil Prev 2012;32:370–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Corkery E, Palmer C, Foley ME, Schechter CB, Frisher L, Roman SH.. Effect of a bicultural community health worker on completion of diabetes education in a Hispanic population. Diabetes Care 1997;20:254–57. [DOI] [PubMed] [Google Scholar]
- 27. Roumen C, Feskens EJ, Corpeleijn E, Mensink M, Saris WH, Blaak EE.. Predictors of lifestyle intervention outcome and dropout: the SLIM study. Eur J Clin Nutr 2011;65:1141–47. [DOI] [PubMed] [Google Scholar]
- 28. Carvajal S, Miesfeld N, Chang J. et al. Evidence for long-term impact of Pasos Adelante: using a community-wide survey to evaluate chronic disease risk modification in prior program participants. Int J Environ Res Public Health 2013;10:4701–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Drahota A, Meza RD, Brikho B. et al. Community‐academic partnerships: a systematic review of the state of the literature and recommendations for future research. Milbank Q 2016;94:163–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. D'Agostino RB, Grundy S, Sullivan LM, Wilson P; CHD Risk Prediction Group. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA 2001;286:180–87. [DOI] [PubMed] [Google Scholar]
- 31. Matthews KA, Sowers MF, Derby CA. et al. Ethnic differences in cardiovascular risk factor burden among middle-aged women: Study of Women's Health Across the Nation (SWAN). Am Heart J 2005;149:1066–73. [DOI] [PubMed] [Google Scholar]
- 32. Abe Y, Rundek T, Sciacca RR. et al. Ultrasound assessment of subclinical cardiovascular disease in a community-based multiethnic population and comparison to the Framingham score. Am J Cardiol 2006;98:1374–78. [DOI] [PubMed] [Google Scholar]
- 33. Fleming TR, DeMets DL.. Surrogate end points in clinical trials: are we being misled? Ann Intern Med 1996;125:605–13. [DOI] [PubMed] [Google Scholar]
- 34. Freemantle N, Calvert M, Wood J, Eastaugh J, Griffin C.. Composite outcomes in randomized trials: greater precision but with greater uncertainty? JAMA 2003;289:2554–59. [DOI] [PubMed] [Google Scholar]
- 35. Van Dam HA, van der Horst FG, Knoops L, Ryckman RM, Crebolder HF, van den Borne BH.. Social support in diabetes: a systematic review of controlled intervention studies. Patient Educ Couns 2005;59:1–12. [DOI] [PubMed] [Google Scholar]
- 36.Centro Nacional de Programas Preventivos y Control de Enfermedades. Declaratoria de Emergencia Epidemiológica EE-4-2016 para todas las Entidades Federativas de México ante la magnitud y trascendencia de los casos de Diabetes Mellitus. [National Center for Preventive Programs and Disease Control. Epidemiological Emergency Declaration EE-4-2016 for all Federal Entities of Mexico in view of the magnitude and significance of Diabetes Mellitus cases].2016. http://www.cenaprece.salud.gob.mx/programas/interior/emergencias/descargas/pdf/EE_4.pdf (1 August, 2019, date last accessed).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.