Abstract
Objectives
To evaluate the time-dependent effects of tocilizumab on vascular inflammation as measured by 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) in GCA.
Methods
Patients with GCA treated with tocilizumab were selected from a prospective, observational cohort. Patients underwent FDG-PET at the baseline visit prior to initiation of tocilizumab and at subsequent follow-up visits performed at 6-month intervals. All imaging findings were interpreted blinded to clinical data. The PET vascular activity score (PETVAS) was used to quantify arterial FDG uptake. Wilcoxon signed rank test was used to compare change in PETVAS between visits. Linear regression was used to determine change in PETVAS over multiple timepoints.
Results
Twenty-five patients with GCA were included. All patients had physician-determined active vasculitis at the baseline visit by clinical assessment and FDG-PET interpretation. PETVAS was significantly reduced in association with tocilizumab treatment from the baseline to the most recent follow-up visit [24.0 (IQR 22.3–27.0) vs 18.5 (IQR 15.3–23.8); P <0.01]. A significant reduction in PETVAS was observed over a two-year treatment period (P <0.01 for linear trend), with a similar degree of improvement in both the first and second years of treatment. Repeat FDG-PET scans after tocilizumab discontinuation showed worsening PET activity in five out of six patients, with two patients subsequently experiencing clinical relapse.
Conclusion
Treatment of patients with GCA with tocilizumab was associated with both clinical improvement and reduction of vascular inflammation as measured by serial FDG-PET. Future clinical trials in GCA should study direct treatment effect on vascular inflammation as an outcome measure.
Keywords: large-vessel vasculitis, giant cell arteritis, tocilizumab, positron emission tomography
Rheumatology key messages
18F-fluorodeoxyglucose positron emission tomography (FDG-PET) activity is significantly reduced in response to treatment with tocilizumab.
Similar improvement in vascular inflammation occurred in the first and second year of tocilizumab treatment.
Repeat FDG-PET scans after tocilizumab discontinuation showed worsening vascular PET activity in most patients.
Introduction
Assessment of disease activity in GCA presents several challenges. Because symptoms of GCA are often non-specific (e.g. headache, malaise), clinical assessment of disease activity can be difficult and subjective [1]. Acute phase reactants may or may not track with disease activity, especially in the later phases of disease, and treatments may directly impact laboratory values independent of clinical effect [2]. While glucocorticoids have traditionally been the mainstay of treatment for GCA [3], two recent randomized controlled trials have demonstrated clinical efficacy of tocilizumab as a steroid-sparing agent [4, 5]. In both of these trials, response to treatment was defined by improvement in clinical and laboratory-based assessment of disease activity. Direct assessment of the large arteries by vascular imaging was not systematically studied as an outcome measure in either trial. Accordingly, the direct effect of tocilizumab on vascular inflammation and the utility of vascular imaging as an objective outcome measure in clinical trials of GCA has not been well characterized.
18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) is a diagnostic imaging modality in GCA that can be used to detect abnormal metabolic activity within the walls of large arteries as a surrogate for vascular inflammation [6]. Few studies have examined the utility of FDG-PET to monitor response to glucocorticoid-sparing treatments in GCA [7–9]. The objective of this study was to evaluate the time-dependent effects of tocilizumab on vascular inflammation as measured by FDG-PET in an observational cohort of patients with GCA. A more direct understanding of the effects of tocilizumab on vascular inflammation could inform treatment recommendations in GCA and impact future clinical trial designs in large-vessel vasculitis.
Methods
Study population and assessment
Complete details about the study population are included in Supplementary Data S1, available at Rheumatology online. Briefly, patients with GCA were recruited from across North America into an ongoing prospective, observational cohort study at the National Institutes of Health (NIH) in Bethesda, MD (ClinicalTrials.gov, NCT02257866). All patients fulfilled modified 1990 ACR Classification Criteria for GCA [10, 11]. To determine vascular response to tocilizumab, PET scan findings from the study visit immediately prior to the initiation of tocilizumab (baseline visit) were compared with imaging findings from all available subsequent follow-up visits, performed at ∼6-month intervals. For patients who discontinued tocilizumab at any point during the study, FDG-PET imaging was also compared before and after discontinuation of tocilizumab.
Clinical assessment and management
At each visit, patients underwent clinical, laboratory and imaging assessments within a 24-h time period at the NIH Clinical Center. Clinical and imaging assessments were performed blinded to each other. Definitions of disease activity are included in Supplementary Data S1, available at Rheumatology online.
FDG-PET imaging assessment
One nuclear medicine physician (M.A.A.) with >10 years clinical FDG-PET experience interpreted all of the PET studies, blinded to all clinical data. Each study was interpreted as active or inactive vasculitis based on overall subjective assessment by the reader with excellent reliability as previously reported [12–14]. Qualitative assessment of FDG uptake relative to liver uptake by visual assessment was assessed in nine arterial territories. A summary score, PET vascular activity score (PETVAS), was calculated (scale 0–27), with higher scores indicating a greater global burden of vascular inflammation [14] (see Supplementary Data S1, available at Rheumatology online, for additional details and imaging protocol).
Statistical analysis
The Wilcoxon signed rank test was used to compare change in PETVAS between two time points. Linear regression with P-value for linear trend was calculated to determine change in PETVAS over multiple timepoints. To determine whether the effect of tocilizumab on vascular inflammation was independent of glucocorticoid use, change in PETVAS was studied in a subset of patients treated with tocilizumab who were taking 10 mg daily prednisone or less at both imaging timepoints. The association between cumulative glucocorticoid dose and change in PETVAS was also assessed using Spearman’s rank-order correlation.
Ethics and informed consent
All patients provided written informed consent. This study was approved by the National Institute of Arthritis and Musculoskeletal and Skin Diseases institutional review board (NIAMS IRB Protocol: 14-AR-0200).
Results
Study population
Out of 74 patients with GCA enrolled in the cohort, 25 patients were treated with tocilizumab after study enrolment. Baseline demographics of the study population are shown in Supplementary Table S1, available at Rheumatology online. The majority of patients were female (76%) with a median age of 70.5 years. All patients had clinically active disease with associated active vasculitis on PET at the baseline visit. One baseline PET scan was excluded from further analyses due to poor quality. Fourteen (56%) patients previously received methotrexate, which was discontinued in all patients when tocilizumab was started.
Assessment of FDG-PET activity following tocilizumab treatment
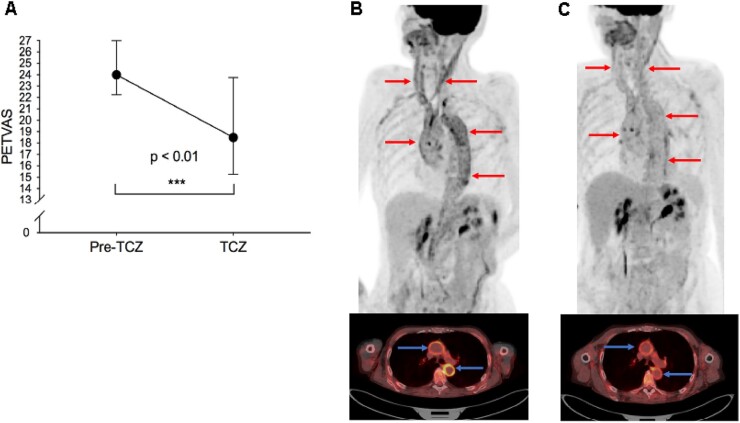
Three out of 25 (12%) patients remained clinically active at the most recent follow-up visit despite treatment with tocilizumab. Eleven out of 25 (44%) FDG-PET scans showed persistent active vasculitis by subjective assessment by the reader at the most recent follow-up visit, including the three patients who remained clinically active. The median imaging time interval while on tocilizumab was 1.1 years [interquartile range (IQR) 0.5–1.5 years]. There was significant reduction in PETVAS in response to tocilizumab treatment from the baseline visit (24.0, IQR 22.3–27.0) to the most recent follow-up visit (18.5, IQR 15.3–23.8); P < 0.01 (Fig. 1A).
Fig. 1.
18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) findings in response to tocilizumab in GCA
Patients underwent FDG-PET scan immediately prior to initiation of tocilizumab and a follow-up FDG-PET scan on tocilizumab treatment. PETVAS (median, IQR), a summary score of arterial FDG-uptake, improved in response to tocilizumab (A). Representative FDG-PET scans prior to (B) and during tocilizumab treatment (C) are shown in a patient with GCA. At the baseline study visit two years after diagnosis, she reported ongoing constitutional symptoms on prednisone 5 mg/day. FDG-PET showed moderate-severe FDG uptake throughout the aorta and arch vessels on whole-body imaging (red arrows) and axial views (blue arrows) (B). She was treated with tocilizumab yet remained on prednisone 5 mg/day. Six months later, she reported clinical improvement coinciding with significant improvement in arterial FDG uptake (C). IQR: interquartile range; PETVAS: PET vascular activity score; TCZ: tocilizumab.
Fourteen patients were treated with tocilizumab for relapsing disease without substantial concomitant glucocorticoid exposure (i.e. prednisone dose ≤10mg/day during baseline and follow-up scan). Significant reduction in PETVAS was still observed in this subset of patients [25.0, IQR 23.0–27.0–19.0, IQR 16.0–26.0; P = 0.04]. No significant association was found between cumulative glucocorticoid dose and change in PETVAS from baseline to most recent follow-up visit (r = 0.35, P = 0.11). Representative FDG-PET images from a patient treated with tocilizumab without substantial concomitant glucocorticoids are shown (Fig. 1B and C).
Longitudinal change in disease activity on FDG-PET
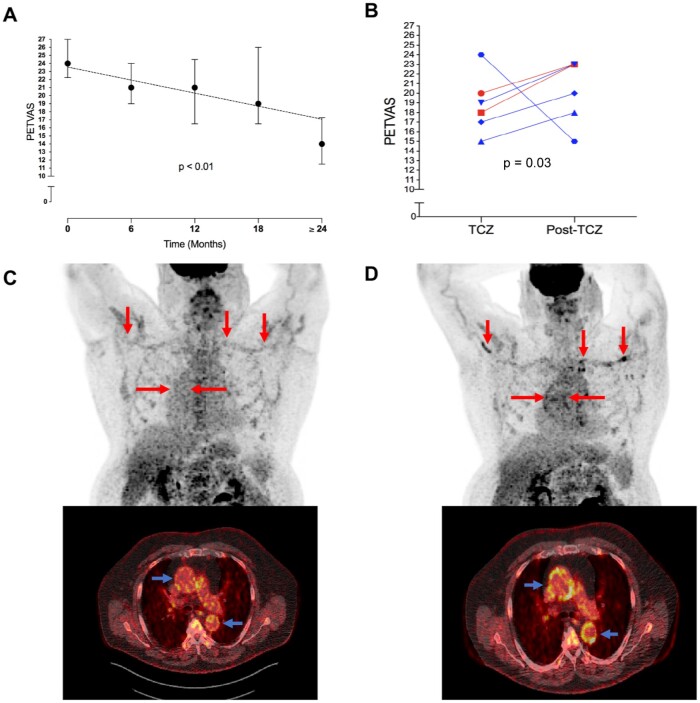
A significant reduction in PETVAS was observed over a two-year treatment period (P < 0.01 for linear trend) (Fig. 2A). All 25 patients had follow-up FDG-PET imaging studies after 6 months of tocilizumab treatment with significant improvement in median PETVAS [24.0, IQR 22.3–27.0–21.0, IQR 19.0–24.0; P < 0.01]. Nineteen patients (76%) had follow up FDG-PET imaging studies after 1 year of tocilizumab treatment and 12 patients (48%) had follow up FDG-PET imaging studies after ≥18 months of tocilizumab treatment. Importantly, there was a similar degree of improvement in vascular FDG uptake in both the first and the second years of treatment.
Fig. 2.
Longitudinal change in 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) activity during tocilizumab treatment
Patients who underwent multiple FDG-PET scans during tocilizumab treatment, continued to have reduction in PETVAS (median, IQR) over a two-year treatment period (A). Of the six patients who discontinued tocilizumab, five had worsening FDG-PET activity on subsequent imaging studies, and two patients experienced a clinical relapse in disease activity (red) (B). Representative FDG-PET scans during tocilizumab treatment (C) and post-tocilizumab treatment (D) are shown in a patient with biopsy-proven GCA treated with tocilizumab. While on treatment, there was minimal FDG uptake throughout the large arteries seen on whole-body and axial views (C). He discontinued tocilizumab after 18 months of therapy due to established clinical remission and subsequently developed fatigue, bilateral arm claudication, and elevations in acute phase reactants (ESR 28 and CRP 10.7 mg/L). A repeat FDG-PET scan off tocilizumab showed increased uptake in the large arteries on whole-body (red arrows) and axial views (blue arrows) (D). IQR: interquartile range; PETVAS: PET vascular activity score; TCZ: tocilizumab.
Six patients discontinued tocilizumab during the study. Five patients discontinued tocilizumab at the discretion of the treating physician after achieving clinical remission, and one patient discontinued due to persistent disease activity. The median time between tocilizumab discontinuation and the FDG-PET scan performed after tocilizumab treatment discontinuation was 0.5 years (IQR 0.5–0.9 years). At the time of tocilizumab discontinuation, the median PETVAS was 18.5 (IQR 16.5–21.0). A repeat FDG-PET scan at least 6 months after treatment discontinuation showed worsening PET activity in five out of six patients (median PETVAS 21.5, IQR 17.3–23.0, P = 0.03) (Fig. 2B). Two of the five patients with worsening vascular PET activity subsequently experienced a clinical relapse within 6 months (patient 1: PETVAS increased from 20 to 23; patient 2: PETVAS increased from 18 to 23). The two patients who experienced a clinical relapse were re-started on tocilizumab. The remaining three patients with worsening vascular PET activity without recurrence of clinical symptoms were not re-started on tocilizumab. Representative PET images from a patient who discontinued tocilizumab and subsequently experienced a clinical relapse are shown in Fig. 2C and D.
Discussion
This imaging study in patients with GCA treated with tocilizumab was nested within a prospective, observational cohort. Despite the limitations of observational cohort studies, these data support the concept that FDG-PET should be incorporated as a novel outcome measure into future randomized clinical trials in GCA. In patients with GCA treated with tocilizumab for active clinical disease, there was significant reduction in vascular inflammation as measured by serial FDG-PET imaging in parallel with clinical improvement in disease activity independent of glucocorticoid treatment. In a smaller subset of patients, there was a rebound of vascular inflammation upon discontinuation of tocilizumab with subsequent clinical relapse.
Findings from this study should inform future clinical trial design in GCA. FDG-PET can be used as an outcome measure to assess treatment response in patients with GCA with new or relapsing disease. Quantitative metrics to measure change in vascular PET activity should be studied. Incorporating more objective measures such as vascular PET activity into clinical trial designs in large-vessel vasculitis may provide a more nuanced understanding of treatment of inflammation at the vascular level and may enable the conduct of more efficient trials that require smaller sample sizes to demonstrate drug efficacy. Future clinical trials that use FDG-PET to study treatment effect of steroid-sparing therapies on vascular inflammation should be mindful of potential confounding effects of glucocorticoids.
The optimal duration of treatment with steroid-sparing agents is unknown in GCA. Randomized studies of treatment withdrawal in patients with GCA in the later stages of the disease could be employed to assess the optimal duration of glucocorticoid-sparing treatments and the potential risk of relapse once treatment is discontinued. These types of trials could incorporate vascular imaging assessment at the time of treatment discontinuation to determine whether vascular inflammation by FDG-PET may predict clinical relapse. Such trial designs could provide much-needed data to guide management decisions in the later phases of GCA.
In this study, vascular inflammation improved at the one-year time point in association with tocilizumab use, with a comparable rate of continued improvement also observed over the second year of treatment. Importantly, while the degree of arterial FDG uptake significantly improved with tocilizumab, 44% of patients treated with tocilizumab had evidence of persistent vasculitis on PET by subjective assessment by the reader over 1.1 years median follow-up. In a previous study of nine patients with GCA, vascular wall enhancement as assessed by magnetic resonance angiography (MRA) improved after one year of treatment with tocilizumab but did not resolve in one-third of patients [5, 15]. These findings suggest that tocilizumab improves, but does not normalize, vascular inflammation in GCA, and therefore may only be temporizing underlying disease activity. Another possibility is that treatment durations of longer than a year may be warranted for some patients with GCA.
In two prior studies, ∼50% of patients with GCA experienced a clinical relapse when tocilizumab was discontinued after one year of therapy [4, 5, 16]. Clinical, laboratory or MRA findings did not predict relapse. In this study, worsening PET activity was seen in five out of six patients following discontinuation of tocilizumab, and two of these patients experienced a clinical relapse. Studies about the utility of FDG-PET to predict clinical relapse have yielded conflicting results [14, 17]. Ultimately, randomized controlled trials could more definitively address this question.
There are several strengths to this study. Few previous studies have assessed the vascular response to glucocorticoid-sparing medications in GCA, thus providing a more nuanced understanding of the effect of steroid sparing therapies on the intended target tissue [7–9]. Unlike previous studies, a standardized imaging protocol was employed with assessment by a centralized reader to minimize information bias [8, 9]. Longitudinal clinical and imaging data were available and exceeded two years in some patients.
There were also some potential study limitations. This was a single-center study subject to referral bias. A comparator group of patients treated with glucocorticoids alone or with a different steroid-sparing therapy was not included. A delayed FDG-PET imaging protocol was used with images acquired two h after injection of the radiotracer, rather than the conventional one-h imaging acquisition. Because delayed imaging increases sensitivity to detect arterial FDG uptake, results from this study are not directly comparable to studies that use shorter imaging acquisition times [12]. However, all PET scans were obtained at the 2-h time point so timing of PET acquisition would not affect change in PETVAS over time within an individual patient. Finally, there were relatively small numbers of patients, particularly at later follow-up time points, but a significant reduction in PETVAS over time was still observed.
In conclusion, this study confirms that FDG-PET should be studied as a novel outcome measure in future clinical trials in GCA. FDG-PET could be employed to study treatment response in patients with active disease and potentially to assess relapse risk in patients in clinical remission. Further, the findings demonstrate in an observational cohort setting that treatment of patients with GCA during a time of clinically active disease with tocilizumab is associated with both clinical improvement and reduction of vascular inflammation. Future randomized trials in GCA that incorporate FDG-PET imaging as an outcome measure will likely provide much-needed data to guide management decisions in the early and late stages of disease.
Funding: This work was supported by the Intramural Research Program at the National Institute of Arthritis and Musculoskeletal and Skin Diseases at the National Institutes of Health.
Disclosure statements: The authors have declared no conflicts of interest.
Data availability statement
All data relevant to this study are included in the article or uploaded and in its online supplementary material.
Supplementary data
Supplementary data are available at Rheumatology online.
Supplementary Material
References
- 1. Michailidou D, Rosenblum JS, Rimland CA. et al. Clinical symptoms and associated vascular imaging findings in Takayasu's arteritis compared to giant cell arteritis. Ann Rheum Dis 2020;79:262–7. [DOI] [PubMed] [Google Scholar]
- 2. Kermani TA, Warrington KJ, Cuthbertson D. et al. Disease relapses among patients with giant cell arteritis: a prospective, longitudinal cohort study. J Rheumatol 2015;42:1213–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Proven A, Gabriel SE, Orces C, O'Fallon WM, Hunder GG.. Glucocorticoid therapy in giant cell arteritis: duration and adverse outcomes. Arthritis Rheum 2003;49:703–8. [DOI] [PubMed] [Google Scholar]
- 4. Stone JH, Tuckwell K, Dimonaco S. et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017;377:317–28. [DOI] [PubMed] [Google Scholar]
- 5. Villiger PM, Adler S, Kuchen S. et al. Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2016;387:1921–7. [DOI] [PubMed] [Google Scholar]
- 6. Slart R et al. FDG-PET/CT(A) imaging in large vessel vasculitis and polymyalgia rheumatica: joint procedural recommendation of the EANM, SNMMI, and the PET Interest Group (PIG), and endorsed by the ASNC. Eur J Nucl Med Mol Imaging 2018;45:1250–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Banerjee S, Quinn KA, Gribbons KB. et al. Effect of treatment on imaging, clinical, and serologic assessments of disease activity in large-vessel vasculitis. J Rheumatol 2020;47:99–107. [DOI] [PubMed] [Google Scholar]
- 8. Vitiello G, Orsi Battaglini C, Carli G. et al. Tocilizumab in giant cell arteritis: a real-life retrospective study. Angiology 2018;69:763–9. [DOI] [PubMed] [Google Scholar]
- 9. Regola F, Cerudelli E, Bosio G. et al. Long-term treatment with tocilizumab in giant cell arteritis: efficacy and safety in a monocentric cohort of patients. Rheumatol Adv Pract 2020;4:rkaa017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hunder GG, Bloch DA, Michel BA. et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum 2010;33:1122–8. [DOI] [PubMed] [Google Scholar]
- 11. Langford CA, Cuthbertson D, Ytterberg SR. et al. A randomized, double-blind trial of abatacept (CTLA-4Ig) for the treatment of giant cell arteritis. Arthritis Rheumatol 2017;69:837–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Quinn KA, Rosenblum JS, Rimland CA. et al. Imaging acquisition technique influences interpretation of positron emission tomography vascular activity in large-vessel vasculitis. Semin Arthritis Rheum 2020;50:71–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Quinn KA, Ahlman MA, Malayeri AA. et al. Comparison of magnetic resonance angiography and 18F-fluorodeoxyglucose positron emission tomography in large-vessel vasculitis. Ann Rheum Dis 2018;77:1165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grayson PC, Alehashemi S, Bagheri AA. et al. 18F-Fluorodeoxyglucose-positron emission tomography as an imaging biomarker in a prospective, longitudinal cohort of patients with large vessel vasculitis. Arthritis Rheumatol 2018;70:439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reichenbach S, Adler S, Bonel H. et al. Magnetic resonance angiography in giant cell arteritis: results of a randomized controlled trial of tocilizumab in giant cell arteritis. Rheumatology 2018;57:982–6. [DOI] [PubMed] [Google Scholar]
- 16. Adler S, Reichenbach S, Gloor A. et al. Risk of relapse after discontinuation of tocilizumab therapy in giant cell arteritis. Rheumatology 2019;58:1639–43. [DOI] [PubMed] [Google Scholar]
- 17. Blockmans D, de Ceuninck L, Vanderschueren S. et al. Repetitive 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: a prospective study of 35 patients. Arthritis Rheum 2006;55:131–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to this study are included in the article or uploaded and in its online supplementary material.