Abstract
Background
Fine-particulate-matter (i.e. with an aerodynamic diameter of ≤2.5 µm, PM2.5) air pollution is commonly treated as if it had ‘equivalent toxicity’, irrespective of the source and composition. We investigate the respective roles of fossil-fuel- and biomass-combustion particles in the PM2.5 relationship with cardiovascular morbidity and mortality using tracers of sources in Dhaka, Bangladesh. Results provide insight into the often observed levelling of the PM2.5 exposure–response curve at high-pollution levels.
Methods
A time-series regression model, adjusted for potentially confounding influences, was applied to 340 758 cardiovascular disease (CVD) emergency-department visits (EDVs) during January 2014 to December 2017, 253 407 hospital admissions during September 2013 to December 2017 and 16 858 CVD deaths during January 2014 to October 2017.
Results
Significant associations were confirmed between PM2.5-mass exposures and increased risk of cardiovascular EDV [0.27%, (0.07% to 0.47%)] at lag-0, hospitalizations [0.32% (0.08% to 0.55%)] at lag-0 and deaths [0.87%, (0.27% to 1.47%)] at lag-1 per 10-μg/m3 increase in PM2.5. However, the relationship of PM2.5 with morbidity and mortality effect slopes was less steep and non-significant at higher PM2.5 concentrations (during crop-burning-dominated exposures) and varied with PM2.5 source. Fossil-fuel-combustion PM2.5 had roughly a four times greater effect on CVD mortality and double the effect on CVD hospital admissions on a per-µg/m3 basis than did biomass-combustion PM2.5.
Conclusion
Biomass burning was responsible for most PM2.5 air pollution in Dhaka, but fossil-fuel-combustion PM2.5 dominated the CVD adverse health impacts. Such by-source variations in the health impacts of PM2.5 should be considered in conducting ambient particulate-matter risk assessments, as well as in prioritizing air-pollution-mitigation measures and clinical advice.
Keywords: PM2.5, source apportionment, fossil-fuel combustion, biomass combustion, crop burning, cardiovascular disease
Key Messages
This study found that, although biomass burning was associated with extreme fine-particulate-matter (i.e. with an aerodynamic diameter of ≤2.5 µm, PM2.5) exposures in Dhaka, cardiovascular hospital admissions and mortality outcomes were more strongly associated with fossil-fuel-combustion-related PM2.5, which is highly enriched in oxidative stress inducing transition metals and sulphur.
This study is the first to evaluate variations in pollution source and PM2.5 composition as a possible underlying cause of this sublinear health-effects relationship with particulate air pollution at high concentrations.
Plateauing of the exposure–response curve at higher PM2.5 exposures likely resulted from a lower cardiovascular toxicity from the biomass-burning PM2.5 that dominates the highest PM2.5-pollution days.
Study findings indicate that focusing on PM2.5 mass alone may not be the best particle-exposure metric to guide clinical or regulatory policies to achieve the greatest air-quality health benefits.
Source-specific air-pollution-control policies are needed to maximize the health benefits of clean-air action in South Asia and elsewhere.
Introduction
South Asian countries, including Bangladesh, experience extremely high air-pollution events, especially during the crop-burning seasons.1 Despite elevated exposures to fine particulate matter (i.e. with an aerodynamic diameter of ≤2.5 µm, PM2.5), few epidemiological studies have been performed to investigate the acute effects of region-specific PM2.5 air pollution on cardiovascular disease (CVD) health2,3 due in large measure to a lack of centralized health-outcome records to perform such analyses. As a result, doubt remains regarding the extent of health effects induced by air pollution in South Asia, even given the high exposures.4,5
Recent evidence suggests that there is variability in the adverse health effects associated with PM2.5 exposures around the world.6 However, because there are limited studies in the developing world, estimates of the health effects attributable to air pollution have largely relied on studies conducted in the developed world. Thus, there is uncertainty in health-effects estimations,7 since the dominant sources of air pollution differ by region, and available evidence suggests that particulate-matter (PM) toxicity can differ, depending on its source and chemical composition.8,9
Prior studies have found a plateauing, or ‘flattening-out’, of the exposure–health response curve at high PM2.5-concentration levels,10–14 but the reason for this is not yet well explained. This study is the first to evaluate varying pollution source and composition as a possible underlying cause of the non-linear health-effects behaviour of particulate air pollution in South Asia. In addition, this research investigates the appropriateness of the ‘equivalent-toxicity’ assumption commonly applied in PM2.5 health-impact analyses: all PM2.5 has the same health effect, irrespective of its local PM source or composition.15
We seek to determine the variability in the PM2.5-mass relationship with adverse CVD health outcomes as a function of particle source and composition. This will investigate whether quantitative estimations of fine-particle toxicity as a function of composition and source type might provide more informative evaluations of PM2.5 health effects than PM2.5 mass alone. This information will potentially enable more accurate predictions of the effects of PM2.5 mass on CVD around the world, as well as a more efficient CVD risk mitigation from PM2.5 exposure, once regional differences in the pollution composition and their respective health impacts are incorporated.
Materials and methods
Study area
The focused area for this research was the Greater Dhaka, which spreads over an area of 1500 km2 with a population of >18 million people in 2011.16 Greater Dhaka incorporates the districts of Dhaka, Gazipur and Narayanganj (Supplementary Figure 1, Supplementary Appendix A, available as Supplementary data at IJE online).
Environmental data
As described by us in more detail in the Supplemental Material, available as Supplementary data at IJE online, and elsewhere,17 daily weather and air-pollution records, including both fine-particulate-matter mass (PM2.5) and composition records, were procured from a variety of governmental and research resources. Daily 24-hr average fine particulate matter (PM2.5) was retrieved from the Department of the Environment (DoE, CASE project) in Dhaka, Bangladesh. Resource limitations restricted gravimetric PM2.5 sampling (24-hr) for trace analyses to generally twice per week on nuclepore filters using a Gent stacked-filter sampler at the Atomic Energy Center in Dhaka. The air pollution in this region is well known to be greatly affected by the crop-burning emissions during non-monsoon periods.17,18 As reported elsewhere, we developed an adjusted potassium index (Kadj) of biomass-burning PM2.5 after subtracting sea-salt and soil potassium PM2.5 from total potassium PM2.5.17 In addition, past studies have found sulphur (S) in PM2.5, which is primarily derived from fossil-fuel combustion.19–23 We therefore apportioned the PM2.5 mass, using the single key tracer regression method, across all sampling days into fossil-fuel-PM2.5 mass (predominantly from fossil-fuel combustion) and biomass-PM2.5 mass (predominantly from crop burning).17 The remaining unattributed PM2.5 mass (‘other PM2.5’; predominately from dust) was then calculated by subtracting the fossil-fuel-associated PM2.5 and biomass-associated PM2.5 from total PM2.5 mass. In Dhaka, fossil-fuel sources include brick kilns, traffic and diesel electric generators; biomass sources include household biomass cooking and agricultural crop burning; and other sources include soil dust, road dust, construction dust, industrial and incinerators.17,24
Health data
Unlike in the developing world, digitized records of daily deaths or visits/admissions to hospitals were not available for hospital records in Bangladesh. We therefore directly procured and then digitized daily written records from the National Institute of Cardiovascular Diseases (NICVD). The NICVD is the preeminent cardiovascular healthcare facility in Dhaka to which most CVD patients are sent.
The daily counts of CVD emergency-department visit (EDV) data for all ages and both sexes were from the NICVD for January 2014 to December 2017, and CVD hospitalizations data for all ages and both sexes for the period of September 2013 to December 2017. Although daily records were not available by cause, the annual counts of all patients from all ages and both sexes at NICVD in 2014 have been reported, including: acute myocardial infarction (30.3%); hypertensive heart disease (15.8%): congenital malfunctions of cardiac chambers and connections (9.4%); multiple-valve disease (8.7%); heart failure (8.6%); atherosclerosis (8.4%); acute and subacute infective endocarditis (6.8%); and other CVD diseases (12.0%).25 The total daily cardiovascular mortality counts data for all ages and both sexes for NICVD were provided in electronic form by the Directorate General of Health Services under the Ministry of Health and Family Welfare, Bangladesh for January 2014 to October 2017. The annual mortality counts for all ages and both sexes at NICVD in 2014 have been reported; these included acute myocardial infarction (33.3%), unstable angina (8.8%), cardiac arrest (8.6%), acute myocardial infarction, unspecified (7.4%), left-ventricular failure (4.7%), old myocardial infarction (4.4%), ischaemic cardiomyopathy (4.1%) and other cardiovascular causes (28.7%).25
Statistical analysis and modelling
Time-series modelling was applied to investigate associations between daily air-pollution variations and daily CVD hospital-care counts. Consistently with past analyses, a quasi-Poisson model, adjusted for overdispersion, was applied.26–28 We performed a single-day lag model (i.e. a linear parameterization for each of the individual lags) for total PM2.5 mass, PM2.5 constituents and sources. Further, an unconstrained distributed-lag model was also applied for the total PM2.5 mass only, in order to assess whether the cumulative effects were greater than the effects for the single highest-day lag. This distributed-lag approach requires everyday exposure data and so could not be applied to the constituent or source-specific analyses, as elemental analyses samples were only collected twice per week.
To address long-term trends and seasonality influences in the model, we included a smooth function of time with 12 degrees of freedom per year, yielding the lowest Akaike Information Criterion value. Terms were also included in the base model for weather, temperature and relative humidity, with 3 df.27,29–31 Day of week was included as a categorical variable and holidays as a dummy variable, including government-shutdown days. An autoregressive term addressed any remaining autocorrelation.32 An analysis was also conducted including a dummy variable for monsoon (June–September) vs non-monsoon (October–May) season, to test for the effect modification between seasons. We also addressed the potential influences of holidays. In each study year, the analyses included a dummy variable denoting that Ramadan period. In addition, there were several blockade days (locally called ‘Hartal’) in Dhaka during the study, so another dummy-variable ‘shutdown’ was added for these days, as identified from the national newspaper and records on the US Embassy website.
We conducted several sensitivity analyses to investigate the robustness of the association to model choice. One analysis was conducted by considering different degrees of freedom (6 to 13) per year (i.e. choice of smoothing function) and another was conducted by changing the degree of freedom for temperature and humidity. We also considered models that added a lag-1- to lag-3-day moving average of temperature and relative humidity with different degrees of freedom to control any delayed effects.
We determine the excess risks of CVD outcomes as percent excess per 10 µg/m3, for lag-0 (same day) through lag-3 for all exposure variables, as well as for a cumulative effect of lag-0 to lag-3 from a distributed-lag model for PM2.5 mass. Exposure–response (E–R) curves were also plotted with a natural spline, allowing a visual evaluation of potential non-linear relations. All analyses were done using R software, version 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria), using the stats, splines and dlnm packages.
Results
Descriptive analyses
We analysed 340 758 EDVs, 253 407 hospital admissions and 16 858 CVD deaths during the study period to evaluate possible associations of particulate air pollution with cardiovascular outcomes in Dhaka, Bangladesh (Table 1). There were higher mean values of EDV, hospital admissions and mortality during the non-monsoon period vs the monsoon season (Table 1). The day-of-week analysis showed that Friday (a religious day in Bangladesh) had a lower number of patients (Supplementary Figure 1, Supplementary Appendix B, available as Supplementary data at IJE online), as adjusted for in our statistical analyses.
Table 1.
Distribution of daily cardiovascular health outcomes, air pollution and weather variables
| Variables | All year |
Monsoon |
Non-monsoon |
|||
|---|---|---|---|---|---|---|
| Mean ± SD | IQR | Mean ± SD | IQR | Mean ± SD | IQR | |
| Health outcomes (counts/day) | ||||||
| ED visits (N = 340 758) | 233 ± 47 | 64 | 221 ± 51 | 74 | 242 ± 41 | 55 |
| Hospital admissions (N = 253 407) | 160 ± 43 | 62 | 148 ± 43 | 59 | 169 ± 40 | 58 |
| Mortality (N = 16 858) | 11 ± 12 | 5 | 9 ± 3 | 5 | 12 ± 16 | 6 |
| Weather (24-hr average) | ||||||
| Temperature (°C) | 26. 3 ± 4.1 | 6.2 | 28.8 ± 1.6 | 2.1 | 24.4 ± 4.3 | 6.7 |
| Relative humidity (%) | 72.4 ± 10.7 | 14 | 79.1 ± 7.1 | 10 | 67.5 ± 10.3 | 12 |
| Air pollution (24-hr average, µg/m3) | ||||||
| PM2.5 (µg/m3) (all 1582 days) | 87.9 ± 69.0 | 107.1 | 30.2 ± 15.1 | 22.4 | 115.9 ± 67.5 | 119.2 |
| PM2.5 (µg/m3) (sampling 388 days) | 90.2 ± 68.0 | 111.0 | 31.4 ± 15.4 | 18.5 | 117.5 ± 66.2 | 99.0 |
| BC (µg/m3) | 7.5 ± 4.6 | 5.6 | 5.2 ± 3.2 | 3.2 | 9.2 ± 4.7 | 5.7 |
| S (µg/m3) | 1.2 ± 1.05 | 0.8 | 0.8 ± 0.5 | 0.6 | 1.6 ± 1.3 | 0.9 |
| Kadj (µg/m3) | 0.2 ± 0.2 | 0.2 | 0.1 ± 0.1 | 0.1 | 0.3 ± 0.2 | 0.2 |
| Fossil-fuel-combustion PM2.5 (µg/m3) | 19.5 ± 11.8 | 12.4 | 13.9 ± 8.2 | 11.9 | 22.3 ± 12.6 | 13.3 |
| Biomass-burning PM2.5 (µg/m3) | 36.3 ± 29.9 | 36.2 | 11.8 ± 8.3 | 15.9 | 48.6 ± 32.3 | 40.2 |
BC, black carbon; ED, emergency department; IQR, interquartile range; Kadj, adjusted potassium (non-soil and non-sea-salt potassium); PM2.5, fine particulate matter with an aerodynamic diameter of ≤2.5 µm; S, sulphur; SD, standard deviation.
A strong seasonal pattern was identified in air-pollution levels, with higher concentrations during the non-monsoon period: the 24-hr average concentrations of PM2.5 during the monsoon and non-monsoon were 36.2 ± 21.5 and 126.1 ± 67.2 μg/m3, respectively (Table 1). Based on our source apportionment, fossil-fuel-related sulphur PM2.5 mass contributed an average of 21.6% (19.5 μg/m3) of the total PM2.5 mass on an annual basis, whereas biomass-burning-related adjusted potassium PM2.5 mass was larger, contributing 40.2% (36.3 μg/m3) (Table 1). Fossil-fuel-combustion-associated sulphur-related PM2.5 mass was 44.3% (13.9 μg/m3) of total fine particulate mass during the monsoon season, but fell to 18.9% (22.3 μg/m3) of total mass during the non-monsoon season. Alternatively, the biomass-burning-associated PM2.5 mass was 41.4% (48.6 μg/m3) of the total PM2.5 mass during the non-monsoon time (Table 1).
Health-effects analyses
Associations with PM2.5 mass
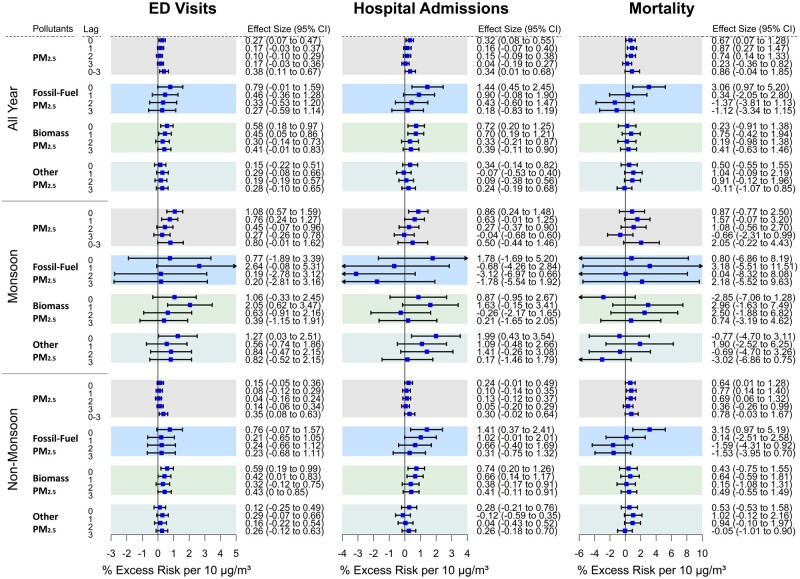
PM2.5 yielded significant associations for cardiovascular EDV, hospitalizations and mortality: a 10-μg/m3 increase in PM2.5 mass was associated with an estimated 0.27% (0.07% to 0.47%) at lag-0, 0.32% (0.08% to 0.55%) at lag-0 and 0.67% (0.07% to 1.28%) at lag-0 increase, respectively (Figure 1). PM2.5 mass was also significant for CVD mortality at lag-1 [0.87% (0.27% to 1.47%)] and lag-2 [0.74% (0.14% to 1.33%)]. The strongest PM2.5-mass association with CVD mortality was found at lag-1 and with hospital admission and ED visits at lag-0, which are consistent with prior studies elsewhere.19,31,33,34 The results from a distributed-lag model showed PM2.5 effect sizes (per 10 µg/m3) to be larger than for the single day for both EDV [0.38% (0.11% to 0.67%)] and hospitalizations [0.34% (0.01% to 0.68%)]. The larger effect sizes found from the distributed-lag model are consistent with cumulative effects from multi-day events. The associations with EDV, hospitalizations and mortality per interquartile increase in PM2.5 constituents S, Kadj and black carbon (BC) are presented in Supplementary Figures 2, 3 and 4, Supplementary Appendix B, available as Supplementary data at IJE online, respectively.
Figure 1.
The % Excess Risk for cardiovascular emergency departments visits, hospital admissions and mortality at single lag-0 to lag-3 (and lag 0–3 from a distributed lag model for PM2.5 mass) per 10 µg/m3 increase in PM2.5 mass, fossil-fuel PM2.5 mass, biomass PM2.5 mass and other-PM2.5 mass during study period, monsoon (June–September) and non-monsoon (October–May), adjusted for long-term trends and seasonality, day-of-week, holidays, Ramadan, blockade days and the temperature and relative humidity. For PM2.5 sources, single-day lag models were applied on 388 days during September 2013 to December 2017, 129 days during monsoon season and 259 days during non-monsoon season. ED, emergency department; PM2.5, fine particulate matter with an aerodynamic diameter of ≤2.5 µm.
PM2.5-effect modification by season
During the monsoon season, PM2.5 was significantly associated with EDV and hospital admissions, with an estimated 1.08% (0.57% to 1.59%) at lag-0 and 0.86% (0.24% to 1.48%) at lag-0 excess risk, respectively, and marginally significantly association with mortality, with an estimated 1.57% (–0.07% to 3.20%) at lag-1 per 10-μg/m3 increase in PM2.5 mass. During the non-monsoon period, these associations were reduced per unit mass, at 0.15% (–0.05% to 0.36%) at lag-1, 0.24% (–0.01% to 0.49%) at lag-0 and 0.77% (0.14% to 1.40%) at lag-1 excess risk for EDV, hospital admissions and mortality per 10 μg/m3 PM2.5, respectively. PM2.5 mass during non-monsoon season was also associated with mortality at lag-1 and lag-3. Although the PM2.5 association with increased EDV was not significant in the single-lag models, the result from the multi-day distributed-lag model showed the strongest association during the non-monsoon period [0.35% (0.08% to 0.63%)] excess risk per 10-μg/m3 increase in PM2.5 mass.
Effect modification as a function of the PM2.5-mass concentration level
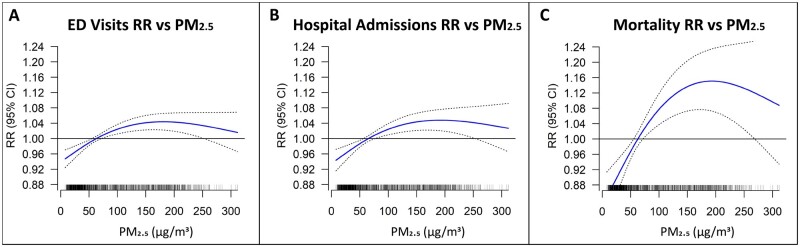
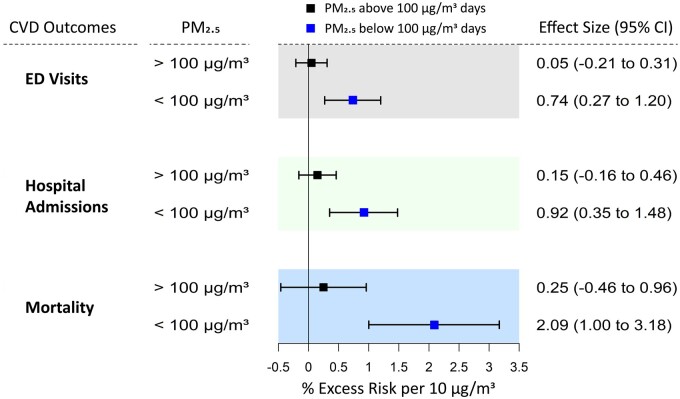
The steepest PM2.5-effect slopes were observed below the ∼80-μg/m3 PM2.5 level for EDV and hospital admissions, and below the ∼100-μg/m3 PM2.5 level for mortality (Figure 2A–C), consistently with a previous study.15 As we have published previously, concentration-weighted trajectories (CWTs), PM2.5 pollution rose and conditional bivariate probability function analyses have provided supportive evidence that high-pollution days (>100 μg/m3) were dominated by transboundary crop-burning pollution, whereas the <100-μg/m3 days are instead dominated by more local sources,17 so we also performed a hinge analysis evaluating PM2.5 health impacts of >100 μg/m3 vs <100 μg/m3. These results confirmed that there was significant decrease in the CVD-effect size per μg/m3 PM2.5 mass when the PM2.5 concentration was >100 μg/m3 (Figure 3). During <100-μg/m3 pollution days, the effect sizes per 10 μg/m3 in PM2.5 were 0.74% (0.27% to 1.20%) for ED visits, 0.92% (0.35% to 1.48%) for hospital admissions and 2.09% (1.00% to 3.18%) for mortality (Figure 3). In contrast, the effect sizes per 10-μg/m3 increase in PM2.5 during >100-μg/m3 pollution days were all non-significant: 0.05% (–0.21% to 0.31%) for ED visits, 0.15% (–0.16% to 0.46%) for hospital admissions and 0.25% (–0.46% to 0.96%) for mortality (Figure 3).
Figure 2.
(A–C) The exposure–response (E-R) curves with 3df for cardiovascular emergency visits (lag-0), hospital admissions (lag-0) and mortality (lag-1), respectively, against PM2.5 for full study period adjusted for temporal trends, holidays, Ramadan, blockade days, day-of-week, temperature and relative humidity. ED, emergency department; PM2.5, fine particulate matter with an aerodynamic diameter of ≤2.5 µm; RR, relative risk.
Figure 3.
Comparison of the effect sizes per 10 µg/m3 increase in PM2.5 mass concentration with all three cardiovascular health outcomes during all year, when PM2.5 was <100 µg/m3 (total 1019 days), and when PM2.5 was >100 µg/m3 (total 564 days). PM2.5 associations with emergency department visits and hospital admissions were at lag-0, and with mortality was at lag-1. The model was adjusted for long-term trends and seasonality, day-of-week, Ramadan, blockade days, holidays and the effect of temperature and relative humidity. CVD, cardiovascular disease; ED, emergency department; PM2.5, fine particulate matter with an aerodynamic diameter of ≤2.5 µm.
Supplementary Figure 5A, Supplementary Appendix B, available as Supplementary data at IJE online, documents that sulphur in the air, predominantly from fossil-fuel combustion, levelled off during the extremely high-pollution episodes, even though PM2.5 levels were rising. Thus, we found that there was not significantly more fossil-fuel-associated PM2.5 during the extreme PM2.5 pollution days, but there was, instead, more of the biomass-burning-related PM2.5, contributing strongly to the mass on the highest-PM2.5 days (Supplementary Figure 5B, Supplementary Appendix B, available as Supplementary data at IJE online) and coincident with the lowered PM toxicity per unit mass during the highest-pollution days.
PM2.5–health-effect modification by composition and source
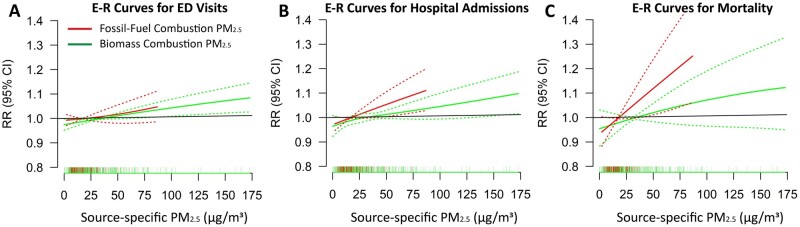
In order to further characterize the lower PM2.5 mass-effect estimates per µg/m3 of the three CVD health outcomes during crop-burning periods (i.e. during high-pollution episodes) as a function of PM2.5 source, we also conducted analyses comparing CVD health-effects estimates for the predominantly fossil-fuel sulphur-associated PM2.5 vs for the biomass-associated adjusted potassium PM2.5. In contrast to the PM2.5 mass E–R curves, the E–R curves for sulphur–PM with all three health outcomes showed a much more linear relationship (Supplementary Figure 6, Supplementary Appendix B, available as Supplementary data at IJE online), without ‘bending over’ during higher-concentration days. A 10-µg/m3 increase in fossil-fuel PM2.5 was associated with 0.79% (–0.01% to 1.59%) at lag-0, 1.44% (0.45% to 2.45%) at lag-0 and 3.06% (0.97% to 5.20%) at lag-0 excess risk for EDV, hospital admissions and mortality, respectively (Figure 1). In contrast, the biomass-burning-related PM2.5 mass-effect estimates for EDV, hospital admissions and mortality were 0.58% (0.18% to 0.97%) at lag-0, 0.72% (0.20% to 1.25%) at lag-0 and 0.75% (–0.42% to 1.94%) at lag-1, respectively, per 10-µg/m3 (Figure 1). We also evaluated associations by residual PM2.5 (i.e. ‘other PM2.5’) with CVD health outcomes. A 10-µg/m3 increase in residual PM2.5 was associated with 0.29% (–0.08% to 0.66%) at lag-1, 0.34% (–0.14% to 0.82%) lag-0 and 1.04% (–0.09% to 2.19%) at lag-1 increased risk of CVD EDV, hospital admissions and mortality, respectively. The season-specific models also provided a larger effect size for fossil-fuel PM2.5, but the wide confidence interval gets wider during monsoon season due to fewer PM2.5 samples.
The E–R relationship curves for EDV, hospital admissions and mortality with fossil-fuel-associated and biomass-associated PM2.5 are shown in Figure 4A–C, respectively. These curves indicate that the mortality and hospital admissions slope is steepest for the fossil-fuel-combustion-associated PM2.5 mass and much lower for the biomass-associated-PM2.5 mass, which is strongly impacted by crop burning at those times.
Figure 4.
The exposure–response (E-R) relationship curves with 3df for cardiovascular emergency department visits, hospital admissions and mortality with fossil-fuel combustion-related PM2.5 mass and biomass combustion-related PM2.5 mass. The strongest single-day fossil-fuel PM2.5 associations with cardiovascular emergency department visits and hospital admissions were at lag-0, and with mortality was at lag-1. The strongest single-day biomass PM2.5 associations with cardiovascular emergency department visits and hospital admissions were at lag-0, and with mortality was at lag-1. The model was adjusted for long-term trends and seasonality, day-of-week, Ramadan, blockade days, holidays and the effect of temperature and relative humidity. ED, emergency department; E-R, exposure–response; PM2.5, fine particulate matter with an aerodynamic diameter of ≤2.5 µm.
Sensitivity analyses indicated that the results were not sensitive to changes in df for the smooth functions of time, temperature and relative humidity, or to adding a lag-1- to lag-3-day moving average of temperature and relative humidity with different degrees of freedom (Supplementary Tables 1–3, Supplementary Appendix C, available as Supplementary data at IJE online).
Discussion
Our results are consistent with an effect of PM2.5 air pollution on CVD morbidity and mortality in Dhaka. In addition, our results further found that the PM2.5 impacts on the CVD health outcomes considered varied, depending on the composition and source of the PM2.5. CVD outcomes were most adversely impacted by the fossil-fuel-related-PM2.5 air pollution (primarily from diesel traffic and coal-burning brick kilns in Dhaka) per µg/m3 PM2.5.
The excess risk of acute cardiovascular events varies between 1% and 3% in developed-country studies.14,19,30,33,35–43 Our estimated overall PM2.5 mass–CVD-effect sizes are lower than generally found in those studies. This is likely because the PM2.5 air pollution in developed counties was more dominated by fossil-fuel-combustion sources (∼70–80%),44–46 whereas the contribution of fossil-fuel sources on total PM2.5 mass in Dhaka was only 21.6%. Dhaka PM2.5 mass was more dominated by biomass-burning PM2.5.17 It is important to note that we found comparable effect sizes with developed-country-study effect sizes by our fossil-fuel-burning-associated PM2.5 mass [1.44% (0.45% to 2.45%) for hospital admissions; and 3.07% (0.96% to 5.22%) for mortality] and by our <100-µg/m3 pollution days [0.92% (0.35% to 1.48%) for hospital admissions and 2.09% (1.00% to 3.18%) for mortality]. This indicates that the fossil-fuel-combustion-related PM2.5 in Dhaka has similar health risks to particles in the developed world, which are more similar in composition to this component of Dhaka PM2.5.
As found in several prior developing-world studies,10,11,13 the relative risk (RR) response to PM2.5-exposure curves for all three health outcomes exhibited non-linear relationships, flattening out at higher concentrations during pollution episodes, indicating a lower population health impact per μg/m3 by the PM2.5 pollution added on high-PM2.5 days. These episodes usually occurred during the rice-paddy-residue crop-burning period from November to February,17,47 as well as during the wheat-residue crop-burning in April–May.1 The ‘levelling-off’ of adverse CVD effects seen at high PM2.5 (Figure 2) could result from two possible factors: (i) composition changes as PM2.5 goes up; or (ii) there is a depletion of susceptible individuals by more moderate levels as they rise, so that there are fewer especially susceptible people left to be affected when the highest levels subsequently occur.48 But, the fact that neither the fossil-fuel-PM2.5 health-effects curves nor the biomass-PM2.5 health-effects curves bend over (Figure 4) like the PM2.5 curves do is inconsistent with a depletion in the population pool of susceptible individuals. If that form of ‘harvesting’ were happening, then the biomass- and the fossil-fuel-associated mortality and morbidity would also bend over at high levels, just like the PM2.5 curves do. Thus, it is indicated that a ‘depletion’ in susceptible individuals is not happening and that the bending-over is a result of lower-toxicity biomass particles dominating the PM2.5 exposures during the highest-PM2.5 days. In addition, comparisons between the overall fossil-fuel-PM2.5 vs biomass-PM2.5 mean effects on CVD health outcomes also indicate that the fossil-fuel-combustion-related PM2.5 mass is more responsible than the biomass-related PM2.5 for the overall adverse PM2.5–CVD health associations. When compared on an acute effect per µg/m3 mass basis, our results revealed that fossil-fuel-combustion-related PM2.5 mass had a roughly four times greater effect on CVD mortality and two times greater effect on CVD hospital admissions vs Biomass-combustion-related PM2.5 mass.
Although the acute health effects of biomass PM on acute respiratory health have been well documented,49–51 the CVD toxicity of biomass PM2.5 has also been indicated to be lower than PM from fossil-fuel combustion in the USA.21 This apparently lower CVD effect by biomass-combustion PM2.5 per unit mass is further supported by studies of indoor biomass burning, which experience very high PM2.5 exposures, but it has been found in biomass-cooking studies to have lower CVD-mortality effects per unit PM2.5 mass than found for respiratory effects.52
The biological mechanism for the apparently much higher CVD impact from fossil-fuel-combustion-related PM2.5 mass is not completely understood, but recent research indicates that it could be due to the enrichment of these particles in both transition metals and sulphur, and these constituents’ combined enhancement of PM2.5 oxidative-stress potential in the body. Oxidative stress and its associated inflammation are thought to be a major driving force in the adverse health effects of PM2.5 exposures.53–55 Whereas research has indicated that exposure to biomass-burning PM2.5 can add oxidative potential, comparisons have found the co-presence of sulphur with transition metals in fossil-fuel-combustion particles giving them much greater bioavailability and resulting oxidative-stress impacts, consistently with our results.56–58 The greater CVD impact of fossil-fuel particles, which are more enriched in oxidant transition-metal species and sulphur than biomass particles,58,59 is therefore consistent with a role by oxidative stress induced by transition metals in the effects of PM2.5. For example, burning coal in brick-kiln operations, which are common in the Dhaka area, results in the production of primary acidic sulphate, which can function as a ligand to mobilize the transition metals and consequent oxidative stress.56,57,60 Recent research also indicates that a diet rich in anti-oxidants is protective against the CVD-mortality effects of PM2.5, which is consistent with the importance of the oxidative-stress mechanism in PM2.5 cardiac health effects.61,62 Thus, there is a strong body of toxicological evidence consistent with the greater CVD toxicity that we find in fossil-fuel-combustion PM2.5 vs Biomass-combustion PM2.5, per mass basis.
Strengths and limitations
Our study also has multiple unique strengths, especially in the consideration of trace-element constituents and source health impacts in our PM2.5–health effects analyses, as well as providing research in an understudied area. However, we did not have detailed personal information for patients’ socio-economic status, status of home air-conditioning use (likely <1%) and status of using cooking stoves, but these characteristics should not change from day to day and are unlikely to confound our time-series analyses.63 Some other individual-level factors such as age and sex can be of importance, but were not able to be considered in this analysis. Also, we were unable to calculate the percentage of the population served by this hospital, as the electronic health data were not available from other hospitals in Dhaka. However, this percentage should not change from day to day and is unlikely to affect the results of the time-series analyses we conducted. Also, since we only had total daily CVD hospital counts, we also did not have count information for specific cardiovascular admission diagnoses on a daily basis or an ability to exclude scheduled hospital admissions, which may introduce error or ascertainment bias. Future research with more detailed health data should investigate these remaining issues. We were also not able to estimate the cumulative effect from distributed-lag models for PM2.5 constituents and sources, as those samples were not collected daily, and only sampled at most twice each week. Future work would benefit from daily PM2.5 chemical-characterization sampling at multiples sites within Dhaka.
Finally, a notable and novel strength of this research is that it provides PM2.5 health-impact estimates that are source-specific and therefore more generalizable than prior total PM2.5 mass health-effect estimates. In the past, PM2.5 health impacts have employed health-effect estimates that inherently assume that all PM2.5 mass has the same toxicity, irrespective of differences over time and locality in source mix and composition. In contrast, source-specific estimates, such as we provide here, can more appropriately be applied to other locations, once source-specific PM2.5 impacts are calculated, allowing the application of health-effect estimates of PM2.5 mass that more properly address PM2.5 source-mix changes over space and time.
Conclusion
This study provides confirming evidence of the detrimental CVD health effects of exposure to PM2.5 air pollution in a region where the PM is dominated by crop-burning air pollution. However, it also indicates that the CVD toxicity of PM2.5 mass varies with composition and source, and that greater adverse health impacts are from fossil-fuel-combustion-derived PM2.5 than from biomass-combustion-derived PM2.5, when compared on a per-unit-mass-exposure basis. These findings not only add to our overall scientific understanding of the role of PM and its composition in impacting cardiovascular health; they can also guide governments and healthcare professionals to adopt source- and composition-specific strategies to maximize the public-health benefits of PM2.5-mitigation actions.
Supplementary Data
Supplementary data are available at IJE online.
Supplementary Material
Acknowledgements
We would thank the ethics committee of the National Institute of Cardiovascular Diseases (NICVD) for approving our study protocol and allowing us to collect data. Special thanks to Dr Nur Alam, Associate Professor, NICVD, for helping and guiding us throughout the health-data collection at NICVD. We also would like to thank the registrar of the NICVD, Dhaka for guiding us during collecting the health data. We gratefully acknowledge the Department of Environment/CASE Project, Bangladesh for providing us with the air-pollution data. We gratefully acknowledge the Directorate General of Health Services (DGHS), Ministry of Family Health and Welfare, Bangladesh for providing us with the daily-mortality data. We would like to thank Dr Jeffrey Simonoff, Professor of Statistics, New York University for his valuable inputs on time-series modelling. The study was approved by the ethics committee of National Institute of Cardiovascular Disease (NICVD) (NICVD/Academic/Study/2016–17/3730). The data underlying this article will be shared on reasonable request to the corresponding author.
Author Contributions
M.M.R. and G.D.T. conceived of the study and contributed to the study design. M.M.R. and K.N. collected and cleaned the health data, air-pollution data and meteorological data. B.A.B. and P.K.H. collected PM2.5 samples for elemental analysis. M.M.R. did the statistical analysis and wrote the first draft. G.D.T., K.N., B.A.B., J.N. and P.K.H. helped to revise the manuscript, commented on the manuscript and contributed to discussion. G.D.T. contributed in interpreting the results and added intellectual content to the manuscript. All authors read and approved the manuscript.
Funding
This work was supported in part by the New York University National Institute of Environmental Health Sciences (NIEHS) Center Grant (ES00260).
Conflict of interest
None declared.
References
- 1. Bikkina S, Andersson A, Kirillova EN. et al. Air quality in megacity Delhi affected by countryside biomass burning. Nat Sustain 2019;2:200–05. [Google Scholar]
- 2. Gurung A, Son JY, Bell ML.. Particulate matter and risk of hospital admission in the Kathmandu Valley, Nepal: a case-crossover study. Am J Epidemiol 2017;186:573–80. [DOI] [PubMed] [Google Scholar]
- 3. Khan R, Konishi S, Fook C. et al. Environment Association between short-term exposure to fine particulate matter and daily emergency room visits at a cardiovascular hospital in. Sci Total Environ 2019;646:1030–36. [DOI] [PubMed] [Google Scholar]
- 4. Yamamoto SS, Phalkey R, Malik AA.. A systematic review of air pollution as a risk factor for cardiovascular disease in South Asia: limited evidence from India and Pakistan. Int J Hyg Environ Health 2014;217:133–44. [DOI] [PubMed] [Google Scholar]
- 5.HEI. Traffic-related air pollution: a critical review of the literature on emissions, exposure, and health effects. Heal Eff Inst 2010;Special Re(January):1–386. [Google Scholar]
- 6. Li X, Jin L, Kan H.. Air pollution: a global problem needs local fixes. Nature 2019;570:437–39. [DOI] [PubMed] [Google Scholar]
- 7. Forouzanfar MH, Afshin A, Alexander LT. et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1659–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rich DQ, Zhang W, Lin S. et al. Triggering of cardiovascular hospital admissions by source specific fine particle concentrations in urban centers of New York State. Environ Int 2019;126:387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Research Council. Research Priorities for Airborne Particulate Matter. IV. Continuing Research Progress. Washington, DC: National Academies Press, 2004. 10.17226/10957. [DOI] [Google Scholar]
- 10. Wong CM, Vichit-Vadakan N, Kan H. et al. Public Health and Air Pollution in Asia (PAPA): a multicity study of short-term effects of air pollution on mortality. Environ Health Perspect 2008;116:1195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen R, Yin P, Meng X. et al. Fine particulate air pollution and daily mortality: a nationwide analysis in 272 Chinese cities. Am J Respir Crit Care Med 2017;196:73–81. [DOI] [PubMed] [Google Scholar]
- 12. Yin P, Brauer M, Cohen A. et al. Long-term fine particulate matter exposure and nonaccidental and cause-specific mortality in a large national cohort of Chinese men. Environ Health Perspect 2017;125:117002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cifuentes LA, Vega J, Köpfer K, Lave LB.. Effect of the fine fraction of particulate matter versus the coarse mass and other pollutants on daily mortality in Santiago, Chile. J Air Waste Manag Assoc 2000;50:1287–98. [DOI] [PubMed] [Google Scholar]
- 14. Liu C, Chen R, Sera F. et al. Ambient particulate air pollution and daily mortality in 652 cities. N Engl J Med 2019;381:705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pope CA, Cohen AJ, Burnett RT.. Cardiovascular disease and fine particulate matter lessons and limitations of an integrated exposure-response approach. Circ Res 2018;122:1645–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bangladesh Bureau of Statistics. Age-Sex Composition of Bangladesh Population. Dhaka, Bangladesh, Ministry of Planning, Government of the people republic’s of Bangladesh; 2015. http://203.112.218.65:8008/WebTestApplication/userfiles/Image/PopMonographs/Volume-9_Age-Sex.pdf.
- 17. Rahman M, Begum BA, Hopke PK, Nahar K, George D.. Assessing the PM2.5 impact of biomass combustion in megacity Dhaka, Bangladesh. Environ Pollut 2020;264:114798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ommi A, Emami F, Zíková N, Hopke PK, Begum BA.. Trajectory-based models and remote sensing for biomass burning assessment in Bangladesh. Aerosol Air Qual Res 2017;17:465–75. [Google Scholar]
- 19. Ito K, Mathes R, Ross Z, Nádas A, Thurston G, Matte T.. Fine particulate matter constituents associated with cardiovascular hospitalizations and mortality in New York City. Environ Health Perspect 2011;119:467–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gwynn RC, Burnett RT, Thurston GD.. A time-series analysis of acidic particulate matter and daily mortality and morbidity in the Buffalo, New York, region. Environ Health Perspect 2000;108:125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thurston GD, Burnett RT, Turner MC. et al. Ischemic heart disease mortality and long-term exposure to source-related components of U.S. fine particle air pollution. Environ Health Perspect 2016;124:785–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dockery DW, Pope CA, Xu X. et al. An association between air pollution and mortality in six U.S. N Engl J Med 1993;329:1753–59. [DOI] [PubMed] [Google Scholar]
- 23. Peng RD, Bell ML, Geyh AS. et al. Emergency admissions for cardiovascular and respiratory diseases and the chemical composition of fine particle air pollution. Environ Health Perspect 2009;117:957–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Begum B, Nasiruddin M, Randal S, Sivertsen B, Hopke P.. Identification and apportionment of sources from air particulate matter at urban environments in Bangladesh. BJAST 2014;4:3930–55. [Google Scholar]
- 25.Bangladesh MOHFW. Ministry of Health and Family Welfare (MOHFW) HEALTH BULLETIN 2015 National Institute of Cardiovascular Disease (NICVD)—Sher-e-Bangla Nagar- Dhaka.2015. https://app.dghs.gov.bd/localhealthBulletin2015/publish/publish.php?org=10000007&year=2015 (1 March 2021, date last accessed).
- 26. Tian Y, Liu H, Liang T. et al. Fine particulate air pollution and adult hospital admissions in 200 Chinese cities: a time-series analysis. Int J Epidemiol 2019;48:1142–51. [DOI] [PubMed] [Google Scholar]
- 27. Tian Y, Liu H, Si Y. et al. Association between temperature variability and daily hospital admissions for cause- specific cardiovascular disease in urban China: a national time-series study. PLOS Med 2019;16:e1002738– 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ko FWS, Tam W, Wong TW. et al. Temporal relationship between air pollutants and hospital admissions for chronic obstructive pulmonary disease in Hong Kong. Thorax 2007;62:780–85. doi:10.1136/thx.2006.076166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Klemm RJ, Mason RM, Heilig CM. et al. Is daily mortality associated specifically with fine particles? Data reconstruction and replication of analyses. J Air Waste Manage Assoc 2000;50:1215–22. [DOI] [PubMed] [Google Scholar]
- 30. Jalaludin B, Morgan G, Lincoln D, Sheppeard V, Simpson R, Corbett S.. Associations between ambient air pollution and daily emergency department attendances for cardiovascular disease in the elderly (65 þ years ), Sydney, Australia. J Expo Sci Environ Epidemiol 2006;16:225–37. [DOI] [PubMed] [Google Scholar]
- 31. Zanobetti A, Schwartz J.. The effect of fine and coarse particulate air pollution on mortality: a national analysis. Environ Health Perspect 2009;117: 898–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barbette A, Louise M, Joel D, Lucas M, Transitional regression models, with application to environmental time series. Transitional Regres Model with Appl to Environ Time Ser, 2000.
- 33. Bell ML, Ebisu K, Peng RD. et al. Seasonal and regional short-term effects of fine particles on hospital admissions in 202 US counties, 1999-2005. Am J Epidemiol 2008;168:1301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cao J, Xu H, Xu Q, Chen B, Kan H.. Fine particulate matter constituents and cardiopulmonary mortality in a heavily polluted Chinese city. Environ Health Perspect 2012;120:373–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ostro B, Malig B, Hasheminassab S, Berger K, Chang E, Sioutas C.. Associations of source-specific fine particulate matter with emergency department visits in California. Am J Epidemiol 2016;184:450–59. [DOI] [PubMed] [Google Scholar]
- 36. Lanzinger S, Schneider A, Breitner S. et al. Ultra fine and fine particles and hospital admissions in central Europe: results from the UFIREG study. Am J Respir Crit Care Med 2016;194:1233–41. [DOI] [PubMed] [Google Scholar]
- 37. Le Tertre A, Medina S, Samoli E. et al. Short-term effects of particulate air pollution on cardiovascular diseases in eight European cities. J Epidemiol Community Health 2002;56:773–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ballester F, Rodríguez P, Iñíguez C. et al. Air pollution and cardiovascular admissions association in Spain: results within the EMECAS project. J Epidemiol Community Health 2006;60:328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Host S, Larrieu S, Pascal L. et al. Short-term associations between fine and coarse particles and hospital admissions for cardiorespiratory diseases in six French cities. Occup Environ Med 2007;65:544–51. [DOI] [PubMed] [Google Scholar]
- 40. Wang Y, Eliot MN, Wellenius GA.. Short-term changes in ambient particulate matter and risk of stroke: a systematic review and meta-analysis. Jaha 2014;3:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rajagopalan S, Al-Kindi SG, Brook RD.. Air pollution and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol 2018;72:2054–70. [DOI] [PubMed] [Google Scholar]
- 42. Mustafić H, Jabre P, Caussin C. et al. Main air pollutants and myocardial infarction: a systematic review and meta-analysis. JAMA 2012;307:713–21. [DOI] [PubMed] [Google Scholar]
- 43. Dai L, Zanobetti A, Koutrakis P, Schwartz JD.. Associations of fine particulate matter species with mortality in the united states: a multicity time-series analysis. Environ Health Perspect 2014;122:837–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Belis CA, Karagulian F, Larsen BR, Hopke PK.. Critical review and meta-analysis of ambient particulate matter source apportionment using receptor models in Europe. Atmos Environ 2013;69:94–108. [Google Scholar]
- 45. Viana M, Kuhlbusch TAJ, Querol X. et al. Source apportionment of particulate matter in Europe: a review of methods and results. Journal of Aerosol Science 2008;39:827–49. [Google Scholar]
- 46. Thurston GD, Ito K, Lall R.. A source apportionment of U.S. fine particulate matter air pollution. Atmos Environ 2011;45:3924–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Haider MZ, How to Stop the Pollution Caused by Burning Rice Residue? A Study from Bangladesh. Kathmandu, Nepal, The South Asian Network for Development and Environmental Economic (SANDEE); 2010, p. 71.
- 48. Zanobetti A. Generalized additive distributed lag models: quantifying mortality displacement. Biostatistics 2000;1:279–92. [DOI] [PubMed] [Google Scholar]
- 49. Martin KL, Hanigan IC, Morgan GG, Henderson SB, Johnston FH.. Air pollution from bushfires and their association with hospital admissions in Sydney, Newcastle and Wollongong, Australia 1994–2007. Aust N Z J Public Heal 2013;37:238–43. [DOI] [PubMed] [Google Scholar]
- 50. Henderson SB, Johnston FH.. Measures of forest fire smoke exposure and their associations with respiratory health outcomes. Curr Opin Allergy Clin Immunol 2012;12:221–27. [DOI] [PubMed] [Google Scholar]
- 51. Krall JR, Mulholland JA, Russell AG. et al. Emergency department visits for respiratory disease in four U.S. cities. Environemental Heal Perspect 2017;125:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Alam DS, Chowdhury MAH, Siddiquee AT. et al. Adult cardiopulmonary mortality and indoor air pollution: a 10-year retrospective cohort study in a low-income rural setting. Gh 2012;7:215–21. [DOI] [PubMed] [Google Scholar]
- 53. Brook RD, Rajagopalan S, Pope CA. et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 2010;121:2331–78. [DOI] [PubMed] [Google Scholar]
- 54. Miller MR, Shaw CA, Langrish JP.. From particles to patients: oxidative stress and the cardiovascular effects of air pollution. Future Cardiol 2012;8:577–602. [DOI] [PubMed] [Google Scholar]
- 55. Dreher K, Jaskot R, Kodavanti U, Lehmann J, Winsett D, Costa D.. Role of soluble metals in acute pulmonary toxicity of residual oil fly ash particles. Am J Respir Crit Care Med 1995;151:A265. [Google Scholar]
- 56. Fang T, Guo H, Zeng L, Verma V, Nenes A, Weber RJ.. Highly acidic ambient particles, soluble metals, and oxidative potential: a link between sulfate and aerosol toxicity. Environ Sci Technol 2017;51:2611–20. [DOI] [PubMed] [Google Scholar]
- 57. Brehmer C, Lai A, Clark S. et al. The oxidative potential of personal and household PM2.5 in a rural setting in southwestern China. Environ Sci Technol 2019;53:2788–98. [DOI] [PubMed] [Google Scholar]
- 58. Longhin E, Gualtieri M, Capasso L. et al. Physico-chemical properties and biological effects of diesel and biomass particles. Environ Pollut 2016;215:366–75. [DOI] [PubMed] [Google Scholar]
- 59. Finney KN, Szuhánszki J, Darvell LI. et al. Entrained metal aerosol emissions from air-fired biomass and coal combustion for carbon capture applications. Materials (Basel) 2018;11:1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ghio AJ, Stoneheurner J, McGee JK, Kinsey JS.. Sulfate content correlate with iron concentrations in ambient air pollution particles. Inhal Toxicol 1999;11:293–307. [DOI] [PubMed] [Google Scholar]
- 61. Lim CC, Hayes RB, Ahn J. et al. Mediterranean diet and the association between air pollution and cardiovascular disease mortality risk. Circulation 2019;139:1766–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Croft D, Block R, Cameron SJ. et al. Do elevated blood levels of omega-3 fatty acids modify effects of particulate air pollutants on fibrinogen? Air Qual Atmos Health 2018;11:791–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bell ML, Samet JM, Dominici F.. Time-series studies of particulate matter. Annu Rev Public Health 2004;25:247–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.