Abstract
Semaphorins were originally identified as axon-guidance molecules essential for neural development. In addition to their functions in the neural system, members of the semaphorin family have critical functions in many pathophysiological processes, including immune responses, bone homeostasis, cancer and metabolic disorders. In particular, several lines of evidence indicate that mammalian/mechanistic target of rapamycin (mTOR), a central regulator of cell metabolism, regulates the functions of semaphorins in various types of cells, revealing a novel link between semaphorins and cell metabolism. In this review, we discuss recent advances in the immunometabolic functions of semaphorins, with a particular focus on mTOR signaling.
Keywords: immunometabolism, mTOR, plexin, semaphorin
Introduction
The semaphorins are a large family of cell-surface and soluble proteins originally identified as axon-guidance molecules during the development of the nervous system. Outside the nervous system, semaphorin signaling also has multiple functions essential for tissue development and homeostasis, particularly in the immune system and metabolic system (1, 2). Work over the past quarter of a century has delineated the basic molecular mechanisms of semaphorins and their receptors. These proteins have widespread functional impacts, not only physiologically but also pathologically, as they participate in the development of autoimmune diseases and metabolic diseases. However, it remains to be elucidated whether semaphorin signaling directly regulates cell metabolism.
Metabolic adaptations are directly linked to regulation of cell functions, and cell metabolism changes dramatically during immune responses. In recent years, many researchers have focused on this on-demand metabolic shift as an essential part of immune responses. It is now clear that mammalian/mechanistic target of rapamycin (mTOR) signaling functions as an integrative hub that commands a broad network of cellular and metabolic activities that shape both innate and adaptive immune responses. During the past decade, progress has also been made in understanding the function of semaphorins in cell metabolism via mTOR signaling.
In this review, we first describe the general features of semaphorins, including their structure, receptors, signaling and downstream effects on pathophysiology in the immune system. We then summarize recent findings on the function of mTOR signaling in the innate and adaptive immune systems, highlighting the roles of mTOR signaling in immunometabolism. We also discuss recent developments that have enhanced our understanding of metabolic regulation via mTOR–semaphorin interactions. In this context, we focus on immunometabolic regulation by semaphorins.
Immune regulation by semaphorins
The semaphorin family is divided into eight subclasses based on the C-terminal structure: classes I and II (invertebrate semaphorins), classes III–VII (vertebrate semaphorins) and class VIII (viral semaphorins) (1, 2). Twenty semaphorins belonging to classes III–VII have been identified in mammals. All of these semaphorins have an extracellular N-terminal Sema domain (approximately 500 amino acid residues in length) with several cysteine-rich plexin–semaphorin–integrin (PSI) domains. Class III semaphorins are secreted proteins, whereas classes IV–VI semaphorins are transmembrane proteins and class VII semaphorins are glycophosphatidylinositol (GPI)-anchored membrane-bound proteins. Among the membrane-associated semaphorins, classes IV and VII semaphorins can be proteolytically cleaved from the membrane to generate soluble proteins.
The major mediators of semaphorin signaling are two groups of transmembrane proteins—plexins and neuropilins (Nrps). Nine plexins have been identified and subdivided into classes A, B, C and D. The structural feature of all plexins is the N-terminal Sema domain with PSI domains and IPT (immunoglobulin domain shared by plexins and transcription factors) domains in their extracellular regions. The cytoplasmic region of plexins consists of an R-Ras/M-Ras GTPase-activating protein (GAP) domain with a Rho GTPase-binding (RBD) domain. Class III secreted semaphorins require neuropilins as co-receptors to stabilize their interaction with plexins. By contrast, classes IV–VII membrane-associated semaphorins bind to plexins directly.
Accumulating evidence has shown that, in addition to axon guidance, semaphorin signaling plays critical roles in immune responses. Semaphorins with immunoregulatory functions are termed ‘immune semaphorins’ (1). The first immune semaphorin to be identified was Sema4D (also known as CD100), a class IV transmembrane-type semaphorin. Sema4D is indispensable for T cell priming. Sema4D on naive T cells interacts with CD72 on dendritic cells (DCs) and promotes their maturation, leading to T cell activation (3). Sema4d–/– mice have defects in the priming of antigen-specific T cells. In addition, Sema4D regulates immune responses in the central nervous system. T cell–microglia interactions mediated by Sema4D–Plexin-B1 promote the activation of microglia and amplify neuro-inflammation (4).
Sema4A, another class IV transmembrane-type semaphorin, is also essential for antigen-specific T cell priming and T helper cell (Th cell) differentiation (5). DCs and activated Th1 cells highly express Sema4A. TIM-2 is a major functional receptor for Sema4A in the immune system (6). Polarized Th2 cells preferentially express TIM-2. DC-derived Sema4A promotes T cell priming, whereas T cell-derived Sema4A is involved in the regulation of Th1 and Th2 responses. Sema4a–/– mice exhibit impaired Th1 responses to Propionibacterium acnes, a bacterium known to induce Th1 responses. Conversely, Sema4a–/– mice exhibit enhanced Th2 responses against Nippostrongylus brasiliensis, a Th2-inducing intestinal parasitic nematode. Consistent with this, Sema4a–/– mice are resistant to experimental autoimmune encephalomyelitis (EAE) because of their impaired Th1 responses.
Sema7A (also known as CD108), a class VII membrane-associated GPI-anchored semaphorin, has diverse functions in the immune system. Sema7A, which is expressed on activated T cells, promotes inflammatory cytokine production in myeloid cells through α1β1 integrin (7). Sema7a–/– mice are resistant to T cell-mediated inflammatory responses in EAE, contact hypersensitivity and pulmonary fibrosis, highlighting the importance of the interaction between Sema7A and α1β1 integrin in inflammatory responses. By contrast, intestinal epithelial cell (IEC)-derived Sema7A suppresses the development of dextran sodium sulfate (DSS)-induced colitis (8). The Sema7A–αvβ1 integrin signaling axis promotes interleukin 10 (IL-10) production by intestinal resident macrophages. Taken together, these observations indicate that Sema7A exerts a pro-inflammatory effect through T cell–monocyte interactions via α1β1 integrin and an anti-inflammatory effect through αvβ1 integrin expressed on intestinal macrophages.
Sema3A, a class III secreted semaphorin, affects immune responses in various ways. In the immune system, Sema3A is mainly expressed in activated DCs and T cells. In addition, tumor cells express Sema3A and inhibit T cell proliferation through suppression of the Ras/mitogen-activated protein kinase (MAPK) signaling pathway (9). Lymphatic endothelial cell-derived Sema3A mediates the navigation of migrating DCs via the Nrp-1–Plexin-A1 receptor complex (10). During DC migration, Sema3A binds to Plexin-A1 expressed on the rear side of cells and promotes phosphorylation of myosin light chain, increasing cell mobility by promoting actomyosin contraction. Indeed, Plexin-A1-deficient (Plxna1–/–) DCs or DCs with a mutant Nrp-1, in which the Sema3A-binding site is disrupted, exhibit defective transmigration across the lymphatics in vivo, highlighting the importance of Sema3A–Nrp-1–Plexin-A1 interaction in immune cell migration.
Sema3A exerts immune-suppressive effects through the Nrp-1–Plexin-A4 complex. Because of exaggerated T cell priming, Plxna4–/– mice exhibit exacerbated T cell-mediated immunoinflammatory responses, such as EAE (11). In contrast to the immune-suppressive function of Sema3A in the adaptive immune system, Sema3A augments innate immune responses (12). Plxna4–/– peritoneal macrophages exhibit defective inflammatory responses upon stimulation with Toll-like receptor (TLR) agonists. In mechanistic terms, Sema3A binding to Plexin-A4 promotes TLR-induced Rac1 activation accompanied by c-Jun N-terminal kinase (JNK) and NF-κB activation. Consistent with this, Plxna4–/– mice are markedly resistant to TLR-induced inflammation and sepsis induced by cecal ligation and puncture (CLP). Intra-peritoneal injection of Sema3A exacerbates the inflammatory responses caused by TLR stimulation and CLP, indicating that Sema3A is a positive modulator of innate immune responses.
Sema6D, a class VI transmembrane-type semaphorin, plays multifaceted roles in axon guidance, cardiogenesis, immune responses and bone homeostasis. Sema6D binds directly to the class-A plexins Plexin-A1 and Plexin-A4. In contrast to other semaphorins, Sema6D has a long cytoplasmic domain (390 amino acid residues) consisting of Src homology-3 (SH3) and zyxin-like domains. Sema6D signaling occurs in a bidirectional manner: in the canonical ‘forward’ direction as a ligand of Plexin-A1/Plexin-A4 and in the non-canonical ‘reverse’ direction as a receptor for these proteins. During chick cardiac morphogenesis, Sema6D forward signaling exerts region-specific activities via Plexin-A1, which forms receptor complexes with two receptor-type tyrosine kinases—Off-track (Otk) and vascular endothelial growth factor receptor type 2 (Vegfr2)—in the ventricle segment and conotruncal segment, respectively. Perturbed Sema6D expression results in abnormal cardiac tube formation (13). On the other hand, Plexin-A1-mediated Sema6D reverse signaling activates the tyrosine kinase c-Abl, leading to cytoskeletal rearrangement in chicken cardiac chamber formation (14).
Sema6D forward signaling is also critical for bone homeostasis (15). Sema6D promotes osteoclast differentiation through a receptor complex consisting of Plexin-A1, triggering receptor expressed on myeloid cells 2 (Trem-2) and the adaptor molecule DNAX-activation protein 12 (Dap12). Plxna1–/– mice exhibit defective osteoclast development, leading to impaired bone resorption and development of osteopetrosis.
In the immune system, Sema6D is expressed in various types of cells, including macrophages, DCs, T cells, B cells and natural killer (NK) cells. Sema6D promotes DC activation via the Plexin-A1–Trem-2–Dap12 receptor complex (15). Sema6D binds to Plexin-A1 expressed in DCs, inducing IL-12 production and up-regulation of MHC class II expression. Both Dap12–/– and Plxna1–/– mice are resistant to the development of EAE because of their reduced generation of antigen-specific T cells, demonstrating the importance of Plexin-A1–Trem-2–Dap12 signaling axis in DC activation (15, 16). In addition, Sema6D augments the production of type I interferons through the Plexin-A1–Trem-4–Dap12 receptor complex expressed in plasmacytoid DCs (17).
Control of immunometabolism by mTOR
In recent years, a growing body of research has revealed the importance of intracellular metabolic adaptations during immune responses, leading to the establishment of the flourishing field of immunometabolism (18).
The serine/threonine protein kinase mTOR, which is highly conserved from yeast to mammals, integrates extracellular and intracellular stimuli with cell metabolism and plays pivotal roles in a wide range of basic cellular and metabolic processes. mTOR is the central modulator of immunometabolism in both the innate and adaptive immune systems. mTOR is present in two structurally distinct signaling complexes—mTORC1 and mTORC2. mTORC1 is composed of mTOR, regulatory-associated protein of mTOR (RAPTOR), mammalian lethal with SEC13 protein 8 (mLST8; also known as GβL), proline-rich AKT1 substrate of 40 kDa (PRAS40) and DEP domain-containing mTOR-interacting protein (DEPTOR). On the other hand, mTORC2 consists of mTOR, rapamycin-insensitive companion of mTOR (RICTOR), mLST8, DEPTOR and mammalian stress-activated protein kinase-interacting protein 1 (mSIN1).
The key function of mTORC1 is promotion of cellular growth and proliferation via regulation of anabolic pathways. mTORC1 activation induces phosphorylation of ribosomal protein S6 kinase [S6K; upstream kinase for ribosomal protein S6 (S6)] and eukaryotic translation initiation factor 4E (eIF4E)-binding proteins (4E-BPs) to stimulate cap-dependent and cap-independent translation initiation (19). On the other hand, mTORC2 promotes metabolic reprogramming requisite for cell proliferation and survival through phosphorylation of AGC family kinases such as Akt and SGK. mTORC2-dependent SGK activation also regulates cytoskeletal dynamics important for cell movement.
In macrophages, inhibition of mTORC1 signaling by rapamycin enhances inflammatory responses via NF-κB and blunts the production of IL-10 via the transcription factor STAT3, suggesting an essential role for mTORC1 signaling in pro-inflammatory macrophage differentiation (20). In mechanistic terms, TLR4 signaling activates the phosphoinositide 3 kinase (PI3K)–Akt–mTORC1 pathway, which inhibits NF-κB activation to suppress pro-inflammatory cytokine production. Moreover, this pathway elicits the activation of CCAAT/enhancer-binding protein β (C/EBPβ) to drive a transcriptional program that promotes immune suppression during inflammation (21). Indeed, selective inhibition of PI3Kγ signaling in macrophages drives an immune stimulatory transcriptional program that augments antitumor CD8+ T cell responses.
On the other hand, mTORC2 signaling is indispensable for alternative activation of macrophages (22, 23). Glucose metabolism, which supports fatty acid synthesis and oxidation, plays a key role in anti-inflammatory macrophage polarization. mTORC2 signaling downstream of both IL-4 receptor (IL-4R) and CSF1R promotes glucose metabolism via induction of the transcription factor IRF4. Macrophage-specific deletion of mTORC2 activity suppresses tumor growth and exacerbates Heligmosomoides polygyrus bakeri (H. polygyrus) infection because it impairs alternative activation of macrophages.
mTOR signaling suppresses de novo differentiation and population expansion of regulatory CD4+ T cells (Treg cells). Rapamycin promotes Treg cell differentiation from naive T cells (24). Deletion of the phosphatase PTEN in Treg cells impairs Treg cell stability and effector function because it enhances Akt and mTORC2 activity (25). mTORC1 signaling and mTORC2 signaling promote Th1/Th17 and Th2 differentiation, respectively, whereas inhibition of both mTORC1 and mTORC2 leads to enhanced Treg cell differentiation (26). Paradoxically, mTORC1 signaling is also a pivotal positive regulator of Treg cell homeostasis. Treg cells have elevated steady-state mTORC1 activity, which in turn promotes cholesterol and lipid metabolism. In particular, these cells up-regulate the mevalonate pathway, which is essential for Treg cell functions. Indeed, disruption of mTORC1 signaling via Treg cell-specific deletion of Raptor leads to impaired immune-suppressive function of Treg cells and development of an inflammatory disorder (27).
mTORC1 and mTORC2 signaling is also deeply involved in the regulation of CD8+ T cell effector and memory differentiation. Activated effector T cells rely mainly on anabolic metabolism, such as aerobic glycolysis, whereas memory T cells up-regulate catabolic metabolism, like fatty acid oxidation (FAO) (28). T cell-specific depletion of tuberous sclerosis complex 2 (Tsc2), the negative regulator of mTORC1, promotes glycolysis, leading to exaggerated effector CD8+ T cell function and diminished memory CD8+ T cell development. By contrast, T cell-specific loss of RAS homolog enriched in brain (Rheb), a positive regulator of mTORC1, decreases glycolysis and increases FAO, leading to diminished effector CD8+ T cell function but enhanced development of memory CD8+ T cells (29).
On the other hand, loss of mTORC2 activity because of deletion of RICTOR results in the enhanced generation of memory CD8+ T cells via up-regulation of Cpt1α, which catalyzes the rate-limiting step of FAO. These data emphasize that mTORC1 and mTORC2 play distinct roles in the generation of effector and memory CD8+ T cells.
mTOR–semaphorin cross-talk
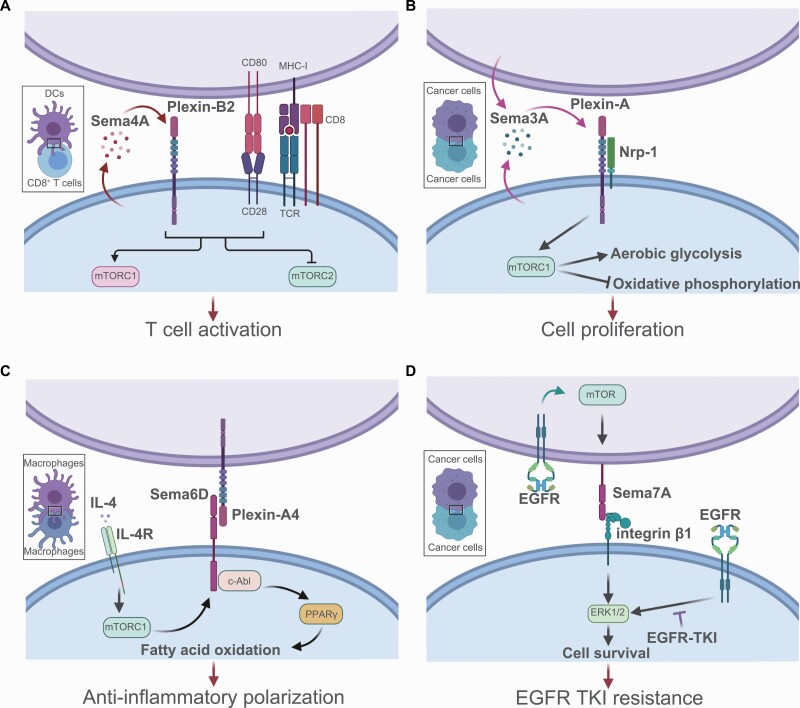
In 2011, the link between semaphorin input and metabolic signaling was first identified in Caenorhabditis elegans. Nukazuka et al. showed that semaphorin regulates epidermal cellular morphogenesis by modulating formation of the TOR complex formation (30). Semaphorin and plexin mutants exhibited reduced TORC1 activity and enhanced TORC2 activity, indicating that semaphorins regulate a shift in the TOR adaptor from RICTOR to RAPTOR. Moreover, in mammals, Ito et al. revealed that Sema4A–Plexin-B2 signaling augments mTORC1 signaling to promote activation and differentiation of CD8+ T cells (Fig. 1A) (31). Sema4a–/– CD8+ T cells have defects in effector functions because of reduced mTORC1 activity and elevated mTORC2 activity. Sema4A signaling also regulates Treg cell functions via modulation of Akt–mTOR signaling (32). Thymus-derived Treg cells express Nrp-1, which functions as a receptor of Sema4A. Binding of Sema4A to Nrp-1 activates the phosphatase PTEN to suppress Akt–mTOR signaling, leading to stabilization of the Treg cell program. Loss of Sema4A–Nrp-1 signaling in Treg cells promotes antitumor immune responses, suggesting that Sema4A and Nrp-1 are promising therapeutic targets for the treatment of cancer. The cross-talk between mTOR and semaphorin signaling is also indispensable for tumor biology. A recent study showed that autocrine Sema3A exerts an oncogenic effect via promotion of an mTORC1-mediated metabolic shift from oxidative phosphorylation to aerobic glycolysis in tumor cells (Fig. 1B) (33).
Fig. 1.
The interaction between semaphorins and mTOR signaling. The mTOR network collaborates with semaphorin signaling in immune cells and tumor cells. (A) CD8+ T cell-derived Sema4A augments mTORC1 signaling and promotes CD8+ T cell activation in an autocrine manner. Sema4A binds to Plexin-B2 on CD8+ T cells, leading to enhanced mTORC1 signaling downstream of TCR stimulation. (B) During cancer progression, tumor cells secrete Sema3A, which acts in an autocrine manner to promote mTORC1 signaling and a downstream metabolic shift to aerobic glycolysis. Consequently, Sema3A promotes tumor cell proliferation. (C) A metabolic shift to FAO is a hallmark of anti-inflammatory macrophage polarization. Sema6D functions as a positive modulator of FAO downstream of mTORC1 signaling. IL-4R–mTORC1 signaling induces Sema6D expression in macrophages. Sema6D, as a receptor for Plexin-A4, activates c-Abl and PPARγ, a master regulator of lipid metabolism. This Sema6D-mediated metabolic adaptation is a key determinant of alternative macrophage polarization. (D) In lung adenocarcinoma, the EGFR–mTOR signal induces expression of Sema7A, which binds to integrin β1 expressed on tumor cells and activates ERK signaling. This aberrant activation of ERK signaling results in a poor response to EGFR-TKI treatment because it inhibits tumor cell apoptosis. This image was created with BioRender (https://biorender.com/).
Recently, we reported that mTOR signaling regulates the expression of Sema6D and Sema7A in macrophages and lung tumor cells, respectively (34, 35). In macrophages, Sema6D reverse signaling is tightly coupled to metabolic regulation necessary for anti-inflammatory polarization (Fig. 1C) (34). We reported previously that amino acid sensing via the Lamtor1–v-ATPase–mTORC1 complex and downstream activation of liver X receptor (LXR) is essential for anti-inflammatory macrophage polarization (36). Indeed, Torin1, an inhibitor of mTORC1 and mTORC2, suppresses LXR signaling, leading to impaired anti-inflammatory macrophage polarization. Torin1-treated macrophages also exhibit impaired expression of Sema6D, indicating that mTORC1 activation induces Sema6D expression in macrophages (34). The lack of Sema6D on macrophages results in exaggerated inflammatory polarization and impaired anti-inflammatory polarization. In mechanistic terms, Sema6D reverse signaling mediated by Plexin-A4 and c-Abl kinase induces the expression of peroxisome proliferator-activated receptor γ (PPARγ), leading to lipid metabolic reprogramming required for anti-inflammatory polarization.
In the context of tumor treatment, our recent study showed that epidermal growth factor receptor (EGFR)–mTOR signaling up-regulates Sema7A expression in lung adenocarcinoma cells, allowing tumor cells to resist therapeutic EGFR tyrosine kinase inhibitor (EGFR-TKI) treatment by using the Sema7A–integrin β1 signaling axis (Fig. 1D) (35). Higher Sema7A expression in clinical lung adenocarcinoma specimens predicted a poorer response to EGFR-TKI treatment in patients with EGFR-mutant lung tumors. Another study showed that Sema7A also plays a critical role in the metabolic reprogramming of macrophages (37). Sema7A activates the mTOR and Akt2 signaling pathways, leading to metabolic reprogramming of anti-inflammatory macrophages; these changes are characterized by increases in FAO and oxidative phosphorylation, along with reductions in aerobic glycolysis and the pentose phosphate pathway. Taken together, these findings indicate that semaphorins and their receptors are critical for the regulation of cell metabolism, particularly mTOR-mediated immunometabolism.
Conclusions
As described above, semaphorin signaling has emerged as a pivotal factor controlling various pathophysiological responses beyond axon guidance. In this review, we have highlighted immunometabolic regulation mediated by semaphorins through mTOR signaling.
A clearer understanding of the mechanisms by which semaphorins and their receptors regulate the metabolic adaptations of immune cells should aid in the development of therapeutic targets for several human diseases, such as autoimmune diseases and metabolic diseases.
However, many questions remain to be answered. First, although the molecular mechanisms of mTOR signaling have been elucidated in recent years, it is still unclear how mTOR signaling regulates the expression and signaling of semaphorins. Second, we need to clarify whether semaphorin signaling is involved in the metabolism of other types of cells such as neurons, glial cells, adipocytes and endothelial cells, which express high levels of semaphorins. Recent work highlights the importance of Trem-2–mTOR signaling in the regulation of microglial autophagy, suggesting the involvement of semaphorin signaling in cell metabolism of the central nervous system (38). Furthermore, although targeting of semaphorin signaling represents a promising strategy for treating immunometabolic diseases, there are many limitations to this approach, including the redundancy of semaphorin–plexin system. Semaphorin signaling is context-dependent; thus, specific interventions would be necessary to target this pathway in therapeutic approaches. In summary, clarifying the mechanisms involved in semaphorin-mediated metabolic regulation of immune cells holds tremendous potential for both basic and clinical sciences.
Funding
This work was supported by research grants from the Japan Society for the Promotion of Science (JSPS) KAKENHI (JP20K22900 to Y.N., JP18H05282 to A.K.); the Japan Foundation for Applied Enzymology (TMFC) (to Y.N.); the Center of Innovation program (COISTREAM) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT) (to A.K.); the Japan Agency for Medical Research and Development (AMED)-CREST (15652237, to A.K.); the Japan Agency for Medical Research and Development (AMED) (J200705023, J200705710, J200705049, JP18cm016335, and JP18cm059042 to A.K.); the Kansai Economic Federation (KANKEIREN) (to A.K.); and Mitsubishi Zaidan (to A.K.).
Acknowledgements
We thank H. Matsushita and M. Takabatake for their excellent technical assistance.
Conflicts of interest statement: the authors declared no conflicts of interest.
References
- 1. Kumanogoh, A. and Kikutani, H. 2013. Immunological functions of the neuropilins and plexins as receptors for semaphorins. Nat. Rev. Immunol. 13:802. [DOI] [PubMed] [Google Scholar]
- 2. Worzfeld, T. and Offermanns, S. 2014. Semaphorins and plexins as therapeutic targets. Nat. Rev. Drug Discov. 13:603. [DOI] [PubMed] [Google Scholar]
- 3. Kumanogoh, A., Suzuki, K., Ch’ng, E.et al. 2002. Requirement for the lymphocyte semaphorin, CD100, in the induction of antigen-specific T cells and the maturation of dendritic cells. J. Immunol. 169:1175. [DOI] [PubMed] [Google Scholar]
- 4. Okuno, T., Nakatsuji, Y., Moriya, M.et al. 2010. Roles of Sema4D-plexin-B1 interactions in the central nervous system for pathogenesis of experimental autoimmune encephalomyelitis. J. Immunol. 184:1499. [DOI] [PubMed] [Google Scholar]
- 5. Kumanogoh, A., Shikina, T., Suzuki, K.et al. 2005. Nonredundant roles of Sema4A in the immune system: defective T cell priming and Th1/Th2 regulation in Sema4A-deficient mice. Immunity 22:305. [DOI] [PubMed] [Google Scholar]
- 6. Kumanogoh, A., Marukawa, S., Suzuki, K.et al. 2002. Class IV semaphorin Sema4A enhances T-cell activation and interacts with Tim-2. Nature 419:629. [DOI] [PubMed] [Google Scholar]
- 7. Suzuki, K., Okuno, T., Yamamoto, M.et al. 2007. Semaphorin 7A initiates T-cell-mediated inflammatory responses through alpha1beta1 integrin. Nature 446:680. [DOI] [PubMed] [Google Scholar]
- 8. Kang, S., Okuno, T., Takegahara, N.et al. 2012. Intestinal epithelial cell-derived semaphorin 7A negatively regulates development of colitis via αvβ1 integrin. J. Immunol. 188:1108. [DOI] [PubMed] [Google Scholar]
- 9. Catalano, A., Caprari, P., Moretti, S.et al. 2006. Semaphorin-3A is expressed by tumor cells and alters T-cell signal transduction and function. Blood 107:3321. [DOI] [PubMed] [Google Scholar]
- 10. Takamatsu, H., Takegahara, N., Nakagawa, Y.et al. 2010. Semaphorins guide the entry of dendritic cells into the lymphatics by activating myosin II. Nat. Immunol. 11:594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yamamoto, M., Suzuki, K., Okuno, T.et al. 2008. Plexin-A4 negatively regulates T lymphocyte responses. Int. Immunol. 20:413. [DOI] [PubMed] [Google Scholar]
- 12. Wen, H., Lei, Y., Eun, S. Y.et al. 2010. Plexin-A4-semaphorin 3A signaling is required for Toll-like receptor- and sepsis-induced cytokine storm. J. Exp. Med. 207:2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Toyofuku, T., Zhang, H., Kumanogoh, A.et al. 2004. Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, Plexin-A1, with off-track and vascular endothelial growth factor receptor type 2. Genes Dev. 18:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Toyofuku, T., Zhang, H., Kumanogoh, A.et al. 2004. Guidance of myocardial patterning in cardiac development by Sema6D reverse signalling. Nat. Cell Biol. 6:1204. [DOI] [PubMed] [Google Scholar]
- 15. Takegahara, N., Takamatsu, H., Toyofuku, T.et al. 2006. Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis. Nat. Cell Biol. 8:615. [DOI] [PubMed] [Google Scholar]
- 16. Bakker, A. B., Hoek, R. M., Cerwenka, A.et al. 2000. DAP12-deficient mice fail to develop autoimmunity due to impaired antigen priming. Immunity 13:345. [DOI] [PubMed] [Google Scholar]
- 17. Watarai, H., Sekine, E., Inoue, S.et al. 2008. PDC-TREM, a plasmacytoid dendritic cell-specific receptor, is responsible for augmented production of type I interferon. Proc. Natl Acad. Sci. USA 105:2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O’Neill, L. A., Kishton, R. J. and Rathmell, J. 2016. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 16:553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weichhart, T., Hengstschläger, M. and Linke, M. 2015. Regulation of innate immune cell function by mTOR. Nat. Rev. Immunol. 15:599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weichhart, T., Costantino, G., Poglitsch, M.et al. 2008. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity 29:565. [DOI] [PubMed] [Google Scholar]
- 21. Kaneda, M. M., Messer, K. S., Ralainirina, N.et al. 2016. PI3Kγ is a molecular switch that controls immune suppression. Nature 539:437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hallowell, R. W., Collins, S. L., Craig, J. M.et al. 2017. mTORC2 signalling regulates M2 macrophage differentiation in response to helminth infection and adaptive thermogenesis. Nat. Commun. 8:14208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang, S. C., Smith, A. M., Everts, B.et al. 2016. Metabolic reprogramming mediated by the mTORC2-IRF4 signaling axis is essential for macrophage alternative activation. Immunity 45:817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Valmori, D., Tosello, V., Souleimanian, N. E.et al. 2006. Rapamycin-mediated enrichment of T cells with regulatory activity in stimulated CD4+ T cell cultures is not due to the selective expansion of naturally occurring regulatory T cells but to the induction of regulatory functions in conventional CD4+ T cells. J. Immunol. 177:944. [DOI] [PubMed] [Google Scholar]
- 25. Shrestha, S., Yang, K., Guy, C.et al. 2015. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat. Immunol. 16:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Delgoffe, G. M., Pollizzi, K. N., Waickman, A. T.et al. 2011. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat. Immunol. 12:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zeng, H., Yang, K., Cloer, C.et al. 2013. mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature 499:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pearce, E. L. and Pearce, E. J. 2013. Metabolic pathways in immune cell activation and quiescence. Immunity 38:633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pollizzi, K. N., Patel, C. H., Sun, I. H.et al. 2015. mTORC1 and mTORC2 selectively regulate CD8⁺ T cell differentiation. J. Clin. Invest. 125:2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nukazuka, A., Tamaki, S., Matsumoto, K.et al. 2011. A shift of the TOR adaptor from Rictor towards Raptor by semaphorin in C. elegans. Nat. Commun. 2:484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ito, D., Nojima, S., Nishide, M.et al. 2015. mTOR complex signaling through the SEMA4A-Plexin B2 axis is required for optimal activation and differentiation of CD8+ T cells. J. Immunol. 195:934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Delgoffe, G. M., Woo, S. R., Turnis, M. E.et al. 2013. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature 501:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yamada, D., Kawahara, K. and Maeda, T. 2016. mTORC1 is a critical mediator of oncogenic Semaphorin3A signaling. Biochem. Biophys. Res. Commun. 476:475. [DOI] [PubMed] [Google Scholar]
- 34. Kang, S., Nakanishi, Y., Kioi, Y.et al. 2018. Semaphorin 6D reverse signaling controls macrophage lipid metabolism and anti-inflammatory polarization. Nat. Immunol. 19:561. [DOI] [PubMed] [Google Scholar]
- 35. Kinehara, Y., Nagatomo, I., Koyama, S., et al. 2018. Semaphorin 7A promotes EGFR-TKI resistance in EGFR mutant lung adenocarcinoma cells. JCI Insight 3:e123093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kimura, T., Nada, S., Takegahara, N.et al. 2016. Polarization of M2 macrophages requires Lamtor1 that integrates cytokine and amino-acid signals. Nat. Commun. 7:13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Korner, A., Bernard, A., Fitzgerald, J. C.et al. 2021. Sema7A is crucial for resolution of severe inflammation. Proc. Natl Acad. Sci. USA 118:e2017527118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ulland, T. K., Song, W. M., Huang, S. C.et al. 2017. TREM2 maintains microglial metabolic fitness in Alzheimer’s disease. Cell 170:649. [DOI] [PMC free article] [PubMed] [Google Scholar]