Highlights
-
•
Nuclear medicine theranostics have demonstrated success with a favourable safety and efficacy profile in several malignancies.
-
•
Kidneys being the primary excretory organ for most therapeutic radiopharmaceuticals are at risk of increased radiation exposure.
-
•
Recognition of the mechanisms of radiation induced nephropathy and associated risk factors can help in the development of appropriate interventions to prevent and limit renal toxicity.
-
•
Developments in reducing chronic radiation nephropathy following radionuclide therapies will help in avoiding the related morbidities, preserving the overall quality of life.
Keywords: Renal toxicity, Acute kidney injury, Chronic, CKD, PRRT, RLT, PSMA, 177Lu, 90Y, DOTATATE, DOTATOC, Radioimmunotherapy, Theranostics
Abstract
Radioligand therapies have opened new treatment avenues for cancer patients. They offer precise tumor targeting with a favorable efficacy-to-toxicity profile. Specifically, the kidneys, once regarded as the critical organ for radiation toxicity, also show excellent tolerance to radiation doses as high as 50–60 Gy in selected cases. However, the number of nephrons that form the structural and functional units of the kidney is determined before birth and is fixed. Thus, loss of nephrons secondary to any injury may lead to an irreversible decline in renal function over time. Our primary understanding of radiation-induced nephropathy is derived from the effects of external beam radiation on the renal tissue. With the growing adoption of radionuclide therapies, considerable evidence has been gained with regard to the occurrence of renal toxicity and its associated risk factors. In this review, we discuss the radionuclide therapies associated with the risk of nephrotoxicity, the present understanding of the factors and mechanisms that contribute to renal injury, and the current and potential methods for preventing, identifying, and managing nephrotoxicity, specifically acute onset nephropathies.
Graphical abstract
Introduction
Ever since the introduction of 131I (NaI) for treating hyperthyroidism due to Graves’ disease and differentiated thyroid cancer, the domain of radioligand therapies (RLT) has expanded rapidly. Today, the clinical indications of RLT cover a wide array of malignancies (Table 1). The concept of theranostics, emerging from using complementary molecules to achieve precision diagnostics and therapy, translating loosely to ‘seeing what you treat and treating what you see’ has shown the way forward in precision and personalized medicine.
Table 1.
Commonly practiced radionuclide therapies and their current clinical indications.
| Radionuclide therapy | Clinical Indication(s) |
|---|---|
| 131I (NaI) [1,2] | Hyperthyroidism due to Graves’ disease, Differentiated thyroid cancer |
| 131I mIBG [3] | Malignant Pheochromocytomas and Paragangliomas, Neuroendocrine neoplasms |
| Metastatic Osseous Pain Palliation [4] (eg. 177Lu-EDTMP, 153Sm-EDTMP) | For pain palliation from osteoblastic metastases (commonly prostate, breast primary cancers) |
| Selective Internal Radiation Therapy [5] (eg. 90Y/ 188Re - microspheres) | Primary hepatocellular carcinoma, hepatic metastases (commonly from colon primary cancers) |
| Peptide Receptor Radionuclide Therapy [6,7] (eg. 177Lu-DOTATATE, 90Y-DOTATOC) | Neuroendocrine neoplasms |
| Radioligand therapy [8], [9], [10] (eg. 177Lu-PSMA) | Prostate Cancer |
| Radioimmunotherapy [11] (eg. 90Y-Ibritumomab tiuxetan, 177Lu-Lilotomab satetraxetan) | Hematologic malignancies (lymphomas, leukemias), few solid malignancies |
| Radiation synovectomy [12] (eg. 90Y-Citrate) | Painful active synovial arthritis with effusion |
| Novel therapies [13], [14], [15], [16] (eg. 177Lu-FAPI, 177Lu-RGD, 177Lu-Pentixather) | Various malignancies |
Improving clinical experience led to a better understanding of the safety and efficacy profile of each of the aforementioned RLT. As most of the therapeutic radiopharmaceuticals have a renal-predominant mode of excretion, the kidneys become one of the major organs receiving high radiation absorbed doses. Additionally, several radiopharmaceuticals, by their inherent pharmacokinetic properties, are retained within the kidneys, further contributing to the increased radiation absorbed dose to the kidneys. The studies on somatostatin receptors targeting RLT performed with beta-emitting radionuclides such as 177Lu/ 90Y showed that nephrotoxicity (acute and chronic) was a dose-limiting adverse event in the absence of specific renal protection regimens [17]. This was a significant challenge to the successful outcomes of PRRT, as the dose-limiting nephrotoxicity narrows the therapeutic window and prevents sufficient administered radiation dose to achieve meaningful clinical outcomes. The introduction of amino-acid preparations in the PRRT protocol minimized the renal toxicity to a significant extent, although not eliminating it completely [18].
Our current understanding of renal toxicity mechanisms and patterns is based mainly on the early experiences with external beam radiation therapy (EBRT), where renal absorbed doses of 23 Gy cause nephropathy in ∼5% of patients within five years [19]. However, the radiation delivered with radionuclide therapies has some major differences with that of EBRT – the radiation being of relatively lower energy, delivered over a longer period of time and with inhomogeneous distribution among the renal tissues in the former [20]. Because of these key differences, it is pertinent to discuss the nephrotoxicity following radionuclide therapies as a separate entity.
In the present review, we discuss the renal toxicity patterns in patients undergoing radionuclide therapy, with regards to the determinants and mechanisms of nephrotoxicity, present and propose methods for its assessment, prevention, and management, emphasizing on acute-onset toxicities.
Radiopharmaceutical therapies–Factors determining risk of nephrotoxicity
Homogeneous exposure of the kidneys to ionizing radiation such as in the treatment of seminomas, retroperitoneal sarcomas and other abdominal solid tumors with traditional EBRT is known to cause classic radiation nephropathy. The radiation exposure with radiopharmaceuticals undergoing renal excretion is inhomogeneous, of lower energies, and with a lower dose rate. This is further complicated with radiopharmaceuticals that are bound and retained at specific sites in the nephron in addition to urinary excretion. Table 2 lists the common radiopharmaceuticals that carry a risk of radiation nephrotoxicity, and their mechanism of localization.
Table 2.
Mechanism of localization of radiopharmaceuticals with risk of nephrotoxicity.
| Radiopharmaceuticals | Mechanism of localization |
|---|---|
| Peptide Receptor Radionuclide Therapy [6] (eg. 177Lu-DOTATATE, 90Y-DOTATOC) | Somatostatin Receptor (SSTR) targeting on cell membrane [21,22]; renal tubular binding (also in glomerulus, vasa recta) |
| Radioligand therapy [[8], [9], [10],23] (eg. 177Lu-PSMA, 225Ac-PSMA) | Prostate Specific membrane antigen (PSMA) targeting on cell membrane; proximal renal tubular binding |
| Radioimmunotherapy [11] (eg. 90Y-Ibritumomab tiuxetan, 177Lu-Lilotomab satetraxetan) | Targeting of specific cellular antigens (eg. CD20, CD30, CD37); retention of radiolabel in proximal tubular cells |
Both the choice of radionuclide and the peptide/ carrier molecule determine the occurrence, and severity of radiation nephropathy. It is pertinent to discuss the residualizing versus non-residualizing property of the radionuclides. Once internalized, the radiopharmaceutical is catabolized, releasing the constituent radionuclide which depending on its physical properties either freely washes out of the cell (non-residualizing) or is retained intracellularly (residualizing). Thus, residualizing radiolabels will irradiate the target cell for a longer time in comparison to the non-residualizing radiolabels. Radiometals such as 177Lu, 90Y, 188Re are residualizing radiolabels, whereas iodides are non-residualizing. Residualizing radiolabels are advantageous from the viewpoint of tumor cytotoxicity, but at the same time they increase the toxicity profile due to off-target localization in the normal tissues [24]. Next, the physical decay properties of the radionuclide determine the extent and severity of their effects. Alpha-emitters (e.g. 225Ac, 211At, 213Bi, 223Ra) deposit very high energy over a limited area, reflected by their high linear energy transfer ∼100 keV/µm, in comparison to beta-emitters (e.g. 90Y, 188Re/ 186Re, 131I, 177Lu,) which have a longer range and a low LET of ∼0.2 keV/µm [25]. Thus, the tissues adjacent to the target, with no direct binding of the radiopharmaceutical receive negligible radiation dose from alpha-emitters in contrast to higher irradiation with beta-emitters. The beta-emitters themselves differ significantly in terms of their energy, and path length. PRRT commonly employs either 90Y or 177Lu labelled with a SSTR targeting peptide. 90Y emits higher energy beta-particles with a longer tissue range (Eβmax 2.3 MeV; Rmax 1.1 cm, Rmean 0.2 cm) in comparison to 177Lu (Eβmax 0.5 MeV; Rmax 0.2 cm, Rmean 0.05 cm). The higher path length of 90Y beta-particles is sufficient to cause irradiation of the glomerulus over a larger extent from the primary tubular binding site, in comparison to 177Lu [26]. As discussed in the later sections, this contributes to a higher risk of radiation nephropathy with the same absorbed doses of 90Y compared to 177Lu.
The choice of molecule labelled with the radionuclide is an important determinant for occurrence of radiation induced nephropathy. The size, in terms of radius and molecular weight, charge and protein binding of a molecule determine its extent of glomerular filtration. Particles weighing > 70 kDa or > 4.2 nm in radius, and those bound to plasma proteins (such as albumin) undergo negligible glomerular filtration [24]. Most radiolabeled peptides and small antibody fragments are sufficiently small in size with no significant protein binding and are thus filtered at the glomerulus. Subsequently, most radiopeptides are re-absorbed at the proximal renal tubule where they undergo further catabolism.
Radiolabeled somatostatin receptor targeting peptides (e.g. 177Lu-DOTATATE, 90Y-DOTATOC) are actively reabsorbed from the apical membrane of the proximal renal tubule via the megalin and cubilin receptor mediated endocytosis [24]. The somatostatin targeting peptides also bind directly to the somatostatin receptors that are physiologically expressed in the kidney (predominantly in the tubular epithelial cells), which contributes (although much lesser than tubular reabsorption) to the renal retention [27,28]. Further, since different peptides have varying binding affinity to the somatostatin receptor subtypes, using the same activity of the same radionuclide with different peptides gives us different dose distributions in the kidney [20]. Prostate specific membrane antigen (PSMA) is a type II glycoprotein with increased expression on prostate tumor cells as well as in the neovasculature of non-prostate malignancies and in several normal tissues like salivary glands and kidney [29], [30], [31], [32], [33]. The currently used therapeutic agents, such as 177Lu-PSMA-617 and 177Lu-PSMA-I&T bind to PSMA expressed on the proximal renal tubules and are subsequently internalized [29]. Radioimmunotherapy involves labeling a radionuclide with an intact antibody, or a small fragment. The small antibody fragments undergo renal clearance and therefore have the advantage of rapid background clearance and superior tumor to background ratios in diagnostic imaging. However, the same renal clearance, and retention in the renal tubules becomes a source of significant radiation exposure in the therapeutic setting [34].
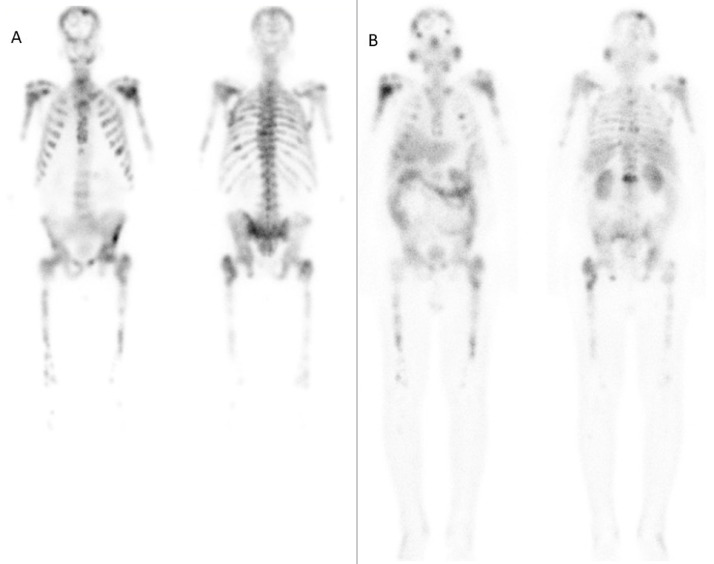
An important factor that alters the biodistribution of radiopharmaceuticals in radionuclide therapy is the tumor sink effect. It refers to the sequestration of majority of the radiotracer in the tumor sites, leading to significantly reduced uptake in the physiologic tissues and organs. The mechanism is analogous to the ‘super-scan’ picture seen on 99mTc-methylene diphosphonate bone scintigraphy in metabolic or metastatic bone diseases, where the radiotracer is preferentially taken up by the extensive tumor sites, leading to poor soft tissue and renal visualization [35]. Extensive tumor burden on somatostatin receptor or PSMA imaging leads to a reduced radiotracer uptake in the normal organs, including the kidneys (Fig. 1). Thus, personalized dosimetry in such patients can help in increasing the maximum deliverable tumor dose while maintaining the limits for renal toxicity [36].
Fig. 1.
A - Post-therapy images obtained in the anterior (left) and posterior (right) projections at 24 h after the administration of 4.6 GBq 177Lu-PSMA (1st cycle) showing extremely high tumor burden in the skeletal system, resulting in negligible tracer activity in the kidneys and the intestine. B - Post-therapy images obtained in the anterior (left) and posterior (right) projections at 24 h after the administration of 4.4 GBq 177Lu-PSMA (7th cycle) in the same patient, showing favourable treatment response as seen by the reduction in skeletal tumor burden, and visualization of tracer activity in the kidneys and the intestine. The patient showed a significant reduction in Serum PSA (from 2681 mcg/L to 40.1 mcg/L), alng with improvement in the bone marrow and renal function.
Mechanisms of radiation induced nephrotoxicity
The broad goal of radiopharmaceutical therapy and for any radiation therapy in oncology is depositing sufficient energy in the tumor cells to render them sterile. Several intracellular targets contribute to this process, but the most important, and effective target is deoxyribonucleic acid (DNA). Ionizing radiation can cause both direct and indirect DNA damage, with the latter being the predominant process. Indirect DNA damage results from the production of reactive oxygen species that can lead to base damage, cross-linking of bases, and strand breakage among other mechanisms. Direct or indirect damage that leads to double-stranded DNA breaks is most effective for cellular killing [37]. While increasing the extent of such DNA damage is favourable for the therapeutic goal, the simultaneous radiation induced toxicity in the normal tissues presents as a dose-limiting factor.
The tissue-based factors determining radiosensitivity of normal tissues are the level of differentiation and the proliferation rate. This was explained by Bergonié and Tribondeau, and subsequently named as the ‘Law of Bergonié and Tribondeau.’ The essence of their law is that cells having a higher proliferative rate and lower degree of differentiation (or specialization) are more radiosensitive than their specialized, non-proliferating counterparts. Thus, spermatogonia, which have high mitotic activity and are undifferentiated exhibit high radiosensitivity in comparison to relatively radioresistant Sertoli cells, which are terminally differentiated and the mature cells showing no proliferative activity [38]. However, it is crucial to understand that the law lays more emphasis on the rapidity rather than the severity of the radiation induced toxic effects in its description of radiosensitivity. Simply put, the rapidly dividing cells show the effects of radiation induced damage much earlier than the more nascent tissues which demonstrate the detrimental changes over a longer period. Recognizing this caveat helps us in understanding the radiosensitivity of the kidneys.
Although initially regarded as relatively radioresistant, in keeping with their specialized and mitotically stable status, it was gradually realized that the kidneys are one of the most radiosensitive organs in the abdomen [39,40]. Nephron is the structural and functional unit of a kidney. The number of nephrons in a normal kidney range from 1–1.5 million (in each kidney) and this number is fixed for a given individual, i.e. nephron as a unit does not have the capacity to repopulate. Any insult to the kidney causing loss of nephrons, leads to a permanent decline in the renal function. However, the scenario is different for the individual constituents of a nephron. Renal tubular cells have shown to regenerate and repopulate following several types of cellular injuries [41]. This is observed in acute tubular necrosis where a multitude of factors ultimately result in the sloughing off of the tubular epithelium. However, if the inciting factor is timely managed, the tubular cells regenerate leading to recovery of renal function. The distinction between temporary and permanent loss of renal function is made by the loss of individual tubular cells versus the loss of whole nephrons, respectively. As previously outlined, much of our understanding of radiation induced nephrotoxicity is based on patients undergoing external beam radiation therapy. Histologic studies of radiation (EBRT) induced nephropathy identified the glomerular-vascular region as the primarily affected compartment over the tubular region [42]. This is reflected in the glomerular and vascular predominant changes following acute radiation injury in the form of loss of endothelial cells, sub-endothelial widening of the glomerular basement membrane, and mesangiolysis. Glomerulosclerosis, vascular occlusion, and interstitial fibrosis are the late onset changes [43]. Early descriptions of radiation nephritis included separate syndromes – acute and chronic radiation nephritis, benign and malignant hypertension and asymptomatic proteinuria [44]. As is consistent with the delayed effects of radiation based renal injury, the first clinical signs and symptoms appear at least six months after exposure to the therapeutic ranges of radiation doses. Acute radiation nephropathy, manifesting at 6–18 months after radiation therapy can present as new-onset hypertension, azotemia, asymptomatic proteinuria, fatigue or anemia [43]. Chronic radiation nephropathy, on the other hand manifests at least 18 months after radiation therapy and presents with signs of chronic kidney disease with renal volume loss, hypertension, proteinuria and anemia.
Tumor lysis syndrome (TLS) is an emergency condition involving rapid killing of tumor cells that release a surge of metabolites, such as uric acid, phosphate, and potassium into the blood stream. This metabolic imbalance can lead to renal insufficiency, cardiac arrhythmias, seizures and can culminate with death due to multi-organ failure [45]. Factors increasing the risk of TLS include the presence of hematologic malignancies, bulky tumors and effectiveness of therapy. Crystals of calcium phosphate, xanthine and sodium urate precipitate in the renal tubules causing obstruction and inflammation, leading to AKI. Around 2% of patients undergoing radionuclide therapy with either 177Lu-DOTATATE/ DOTATOC or 177Lu-PSMA were reported to develop clinical TLS in a retrospective review of 205 patients [46]. Another study of 1109 patients treated with 90Y-DOTATOC had two patients developing TLS with acute reversible kidney injury [47]. Even in VISION trial testing 177Lu-PSMA-617 in mCRPC patients AKI was noted in 0.4% of the patients and TLS in 0.2% of patients [48]. The underlying principle of AKI following TLS post radionuclide therapy is similar to that described in the setting of traditional anti-cancer therapies. Acute kidney disease (AKD) occurring within 3 months of 177Lu-PSMA therapy has also been reported in 2/195 (1%) of patients [49].
Patients undergoing radionuclide therapies often have pre-existing medical/ surgical co-morbidities and exposure to several cycles of prior cytotoxic chemotherapy, targeted therapy, radiation therapy or a combination of these. Several additional risk factors thus predispose these patients to a risk of AKI following radionuclide therapy (Table 3).
Table 3.
| Additional risk factors for developing nephrotoxicity following radionuclide therapy |
| Elderly age-group (> 60 years) |
| Longstanding diabetes mellitus, hypertension |
| Medical/ Surgical co-morbidities (nephrotic syndrome, congestive cardiac failure, renal insufficiency, sepsis, hypovolemia, prior nephrectomy, obstructive uropathy) |
| Nephrotoxic medications – non-steroidal anti-inflammatory drugs, aminoglycoside antibiotics etc. |
| Nephrotoxic anti-cancer medications – cisplatin, mitomycin-C, methotrexate etc. |
| Thrombotic microangiopathy |
Nephrotoxicity after radiopharmaceutical therapies
Nephrotoxicity following radionuclide therapies can present as acute/ subacute events with reversibility or permanent deficits in renal function. The common terminology criteria for adverse events (CTCAE) by the National Cancer Institute provides for standardized reporting and grading of the adverse events following anti-cancer therapy, to ensure consistency and comparability across studies. The CTCAE grade a given adverse event from 1 to 5, with grades 3 and above denoting a severe event [52].
In this section, we will discuss the evidence of nephrotoxicity with the commonly performed radionuclide therapies. It is important to note that the CTCAE descriptions are updated with time and hence there might be discrepancies of the version used among studies, especially over a large time-frame. For example, CTCAE v4.03 reported grades 1–3 of acute kidney injury in terms of rise in creatinine, whereas this was eliminated in CTCAE v5.0. To ensure clarity, the CTCAE version followed by a study is provided in parentheses alongside the toxicity grade.
Somatostatin receptor targeting RLT
Somatostatin receptor targeting RLT initially began with 111In-pentetreotide, utilizing the gamma emissions of 111In for imaging, and auger electron emissions for therapy. No major renal toxicity was observed with 111In-pentetreotide at cumulative injected activities of up to 160 GBq. Of the 40 evaluable patients, five developed grade 1 renal toxicity. However, four of these five patients already had grade I creatinine values at baseline. Mean creatinine values showed no significant change from baseline onwards [53]. The most likely explanation for this observation would be the limited tissue penetration of the auger electrons (upto 10 µm), insufficient to irradiate a significant area of normal renal tissue. The downside of using the auger electrons for therapy was the limited treatment efficacy.
The beta-emitters with higher energy emerged as attractive options for PRRT. 90Y is a pure beta-emitter with half-life of 2.7 days, maximum beta-particle energy of 2.3 MeV and maximum range of 1.1 cm in soft tissue. The earliest clinical results with 90Y-DOTATOC based PRRT in patients with advanced somatostatin receptor expressing malignancies reported occurrence of nephrotoxicity in 4/29 (13.8%) patients. All the four patients had received cumulative doses of > 7400 MBq/m2 with no renal protection in the form of Hartmann-Hepa 8% solution. Two of the four patients required haemodialysis post PRRT [54]. Renal biopsies performed after treatment with 90Y-DOTATOC therapy have shown the presence of thrombotic microangiopathy, which is classical for radiation induced nephropathy, and additional findings such as mesangiolysis, glomerular sclerosis, tubular atrophy and interstitial fibrosis [55,56]. Amino-acid infusion based renal protection became a norm with PRRT since its introduction in 1999, which has been reported to reduce the renal absorbed radiation dose by upto 65% [57]. Studies on PRRT performed with 90Y-based radiopharmaceuticals have reported severe nephrotoxicity rates upto 14% [58]. A median 25% (range: 11–45%) reduction in GFR was noted in eight out of 25 patients (32%) in whom GFR was measured at 6–12 months after 90Y-DOTATOC treatment [59]. A study of 1109 patients treated with 90Y-DOTATOC had 102 (9.2%) patients developing severe grade 4 or 5 (CTCAE v3.0) permanent renal toxicity [47]. They reported older age, baseline renal insufficiency, and high renal uptake of the radiopharmaceutical as risk factors for development of severe nephrotoxicity.
177Lu is a beta-emitter with half-life of 6.7 days, maximum beta-particle energy of 0.5 MeV and maximum range of 0.2 cm in soft tissue. As previously discussed, the lower range of beta-particles emitted by 177Lu in comparison to those of 90Y leads to lower irradiation of the glomerulus and an overall lower risk of nephrotoxicity post PRRT with the former, with all the other factors remaining constant. Retrospective evaluation of 323 patients treated with 177Lu-DOTATATE based PRRT showed no occurrence of grade 3 or 4 (CTCAE v4.03) (sub)acute renal toxicities. 14 (4%) patients had a grade I (sub)acute renal toxicity. Three patients (1%) experienced a grade 2 (sub)acute renal toxicity which was found not related to the therapy. The mean radiation dose delivered to the kidneys was 20.1 ± 4.9 Gy. Overall, none of the patients had an annual reduction in renal function, in terms of creatinine clearance by > 20% [60]. Another study of 504 patients reported two patients with severe delayed nephrotoxicity, with both being likely unrelated to the therapy – one patient had pre-existent renal insufficiency and the other had progressive tricuspid regurgitation [61]. The highest reported occurrence of severe nephrotoxicity (CTCAE v3.0 grade 4 or 5) following 177Lu-based PRRT is 9.2% in a study of 141 patients treated with 259 cycles of 177Lu-DOTATOC [62]. However, the study included patients with baseline reduced renal function who would already be at a higher risk for developing further renal function impairment. Additionally, the long follow-up period (median – 9 months, range: 1–80.1 months), could have permitted for natural decline of GFR as well as intervention by another nephrotoxic treatment, data on both of which were not captured by the study. A study of 807 patients reported that 177Lu- based PRRT had a lower risk of nephrotoxicity in comparison to that with 90Y- based radiopharmaceuticals. The rate of occurrence of any grade or persistent nephrotoxicity in patients treated with 177Lu-based PRRT was 13.4% in comparison to 33.6% with 90Y-based PRRT [63]. The long term overall survival analyses as well as an update on the toxicity profile after 177Lu-DOTATATE did not raise any further concerns. The percentage of patients developing Grade 3 or higher renal toxicity was observed in 5.4% of patients [64].
PSMA based RLT
The initial radioligand therapies in prostate cancer were performed using J591, which is a monoclonal antibody targeting PSMA and associated with significantly higher renal toxicity [65]. The use of small molecule inhibitors, commonly PSMA-617 and PSMA-I&T lowered the occurrence of nephrotoxicity. Dosimetry comparisons between 177Lu-PSMA-617 and 177Lu-PSMA-I&T have shown a higher renal uptake and a resultant higher renal absorbed dose with the latter [66]. A systematic review and meta-analysis reported nephrotoxicity of any grade in 9.5% of 744 patients post 177Lu-PSMA (PSMA-617 or PSMA-I&T) RLT [67]. Similarly, a study of 32 patients treated with 177Lu-PSMA-617 (mean cumulative administered activity: 21.3 ± 5.2 GBq) showed a change from grade 1 or 2 to grade 3 nephrotoxicity (CTCAE v3.0) in 10%. Severe nephrotoxicity was not reported in any of the patients [68]. A study of 100 patients treated with a cumulative 319 cycles of 177Lu-PSMA-I&T reported no grade 3 or 4 non-hematologic toxicities. Specifically, no nephrotoxicity was reported in any of the patients with a median follow-up of 9.5 months (range: 7–16.3 months) [69]. A retrospective review of 195 patients showed a grade 1 or 2 nephrotoxicity (CTCAE v5.0) in 4.5% after treatment with 177Lu-PSMA (PSMA-617 or PSMA-I&T) based RLT, much lower than that reported in the previous studies. They reported two patients who developed acute renal insufficiency with significant reduction in GFR (31% and 24%) within 3 months after RLT, with recovery to near baseline levels by one month. Three patients developed CKD G3a (GFR 45–59 mL/min/1.73 m2), one of whom had a baseline G2 CKD (GFR 60–89 mL/min/1.73 m2). The authors reported pre-existing CKD as the most significant risk factor for developing post-RLT nephrotoxicity over other factors such as hypertension, diabetes or history of AKI [70]. Retrospective review of the data from 119 patients at Bad Berka, Germany, who underwent 300 cycles of 177Lu-PSMA RLT (median activity 6 GBq/cycle; range: 2–9.7 GBq) with a follow-up of 34 months showed no nephrotoxicity associated with the treatment [66]. The phase two randomised controlled trial comparing 177Lu-PSMA-617 RLT versus cabazitaxel in 200 patients with metastatic castration resistant prostate cancer showed no acute renal toxicity post RLT. Delayed nephrotoxicity was prudently not commented upon due to the limited follow-up period (median 18.4 months) [71]. The recently concluded VISION trial comparing 177Lu-PSMA-617 RLT with standard of care versus standard care alone reported renal adverse effects (CTCAE v5.0) of any grade in 8.7% in the intervention arm (versus 5.9% in control arm) and grades 3–5 renal effects in 3.4% patients in the intervention arm (versus 2.9% in control arm) [72].
It has to be recognised that several studies have a relatively small follow-up period, which although adequate for detecting acute and sub-acute changes in renal function, might be insufficient for capturing the chronic renal insufficiency. Additionally, factors such as the natural decline in GFR with increasing age, and institution of other nephrotoxic treatments after completion of RLT also need to be accounted for. A challenge in obtaining longer term follow-up in metastatic castration resistant prostate cancer is the short overall survival of these patients (median 13.7 months; range: 8–14 months) [67].
Methods for renal protection in radionuclide therapies
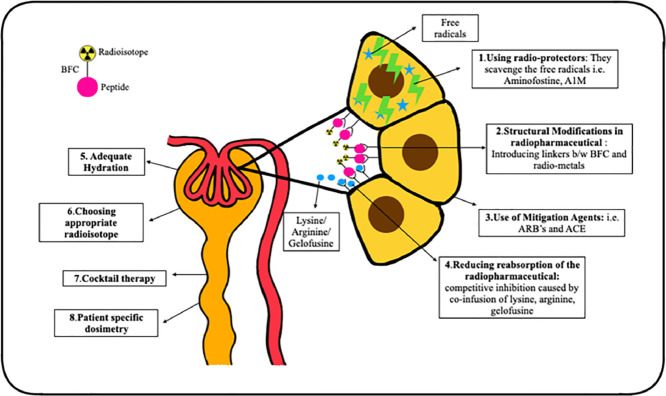
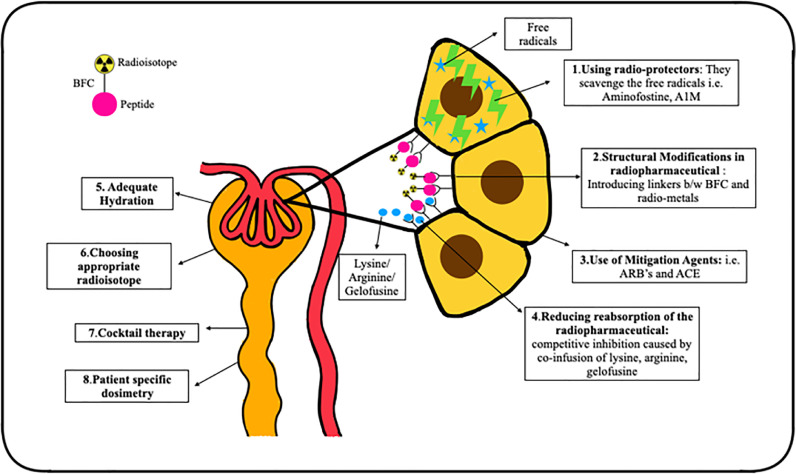
Various approaches have been employed to decrease the risk of nephrotoxicity with radionuclide therapies, with the majority focusing on reducing the radiation dose to the kidneys (Fig. 2).
Fig. 2.
Various methods for reducing the nephrotoxicity associated with radionuclide therapies.
Competitive inhibition by saturation of somatostatin receptors
As the therapeutic radiopharmaceuticals in PRRT are localized in the proximal tubular cells, competitive inhibition of the amino acid transporters has been the most successful technique in reducing PRRT associated nephrotoxicity [73]. The simultaneous infusion of positively charged amino acids, such as L-arginine and/or L-lysine, competitively inhibits the reabsorption of radiotracer from the negatively charged proximal tubular membrane, leading to 9–53% reduction in the renal radiation dose [73]. In the present clinical practice, the duration of amino acid infusion is 4 h. However, studies have shown that increasing the infusion time by 10 and 48 h leads to a further reduction in the renal absorbed doses by 39% and 65%, respectively [74].
Gelofusine is a plasma expander that competitively inhibits the reabsorption of proteins and peptides by increasing the excretion of megalin ligands, and hence, can be used for renal protection. Studies have shown that use of lysine and gelofusine alone caused ∼40% reduction in the renal uptake of 177Lu-DOTA- [Tyr3] octreotate by whereas, combination of gelofusine and lysine cause 62% reduction in tracer uptake by kidneys [75]. Gelofusine is given as bolus of 1 mL/kg body weight for 10 min prior to the therapy, followed by infusion at 0.02 mL/kg/min for 3 h after the radiolabeled peptide infusion [76].
Use of radio-protectors
Radio-protectors reduce the radiation damage by scavenging the free radicals formed as the result of interaction of radiations. Amifostine is a commonly used radio-protector in EBRT and its role in PRRT is being evaluated. It is a free radical scavenger, protecting the healthy tissue from radiation damage. In a preclinical study, Amifostine or its active metabolite WR-1065 proved to be an effective renal protector, preventing any derangements in S.Cr, proteinuria and histopathological renal damage after treatment with 177Lu-DOTA- [Tyr3] octreotate [77]. Kristiansson et al. evaluated the role of recombinant human protein α1-microglobulin (A1M), an antioxidant and free radical scavenger, as a potential radio-protector of the kidneys during PRRT. They observed that the co-administration of A1M reduced 177Lu-DOTATATE induced DNA damage, and structural renal damage, prevented reduction in GFR, and proteinuria up to six months after injection [78].
Structural modification of radiopharmaceutical
The addition of specific linker moieties between the radiometal-bifunctional chelator and peptide can help in the renal protection. Their molecular structure is either cleaved via hydrolyses in the brush border membrane in the PCT, hence causing it's urinary excretion instead of endocytosis or by lysosomal enzymes which release the complex from the cell after endocytosis. Uehara et al. introduced a glycyl-lysine bond containing linker moiety in 188Re-tricarbonyl-(cyclopentadienyl)-glycyl-lysine-Fab which caused reduction in the renal uptake without effecting the tumor tracer avidity [79].
Use of mitigation agents
Angiotensin II receptor blockers (ARBs) and angiotensin-converting enzyme (ACE) inhibitors are radiation mitigators that inhibit the counterproductive tissue reactions to radiation-induced damage, such as the renin-angiotensin-aldosterone system mediated renal injury. It has been observed that inhibition of the renin-angiotensin-aldosterone system during renal irradiation reduced the incidence of nephrotoxicity post EBRT. In a preliminary study by Rolleman et al. a combination of ARB with lysine reduced renal toxicity in rats after high activities of 177Lu-DOTA- [Tyr3] octreotate [80].
Choice of radioisotope
177Lu- or 90Y-labeled somatostatin receptor analogues are used in PRRT, and as previously highlighted, the incidence of nephrotoxicity is lower with 177Lu-labeled peptides compared to those labeled with 90Y [81]. The choice of radionuclide thus influences the renal outcomes, and can accordingly be tailored to patient-specific needs.
Combination or cocktail therapies
Combination or cocktail therapies have shown a survival benefit and a reduced incidence of renal toxicity by the sequential application of 90Y and 177Lu- labeled peptides. Baum et al. reported that the patients on combination therapy showed a lower long-term median yearly decline in TER values (6.2%) in comparison to those on 177Lu- or 90Y-based therapies alone (7% and 6.7%, respectively) [82].
Adequate hydration
Adequate hydration and frequent micturition ensures faster biological elimination of the radiotracer via the kidneys which further helps in reducing the renal absorbed dose. As per the recommendations for 177Lu-PSMA RLT, the patients should be well hydrated pre- and post-therapy with 1–1.5 liters saline or water for renal protection [83].
Personalized dosimetry for renal dose estimation
As previously discussed, the maximum dose limits for PRRT cannot be directly derived from the EBRT models. Bodei et al. recommended biological effective dose of less than 40 Gy as safe in patients without any risk factors and a dose of less than 28 Gy in patients with known risk factors. The dose received by the kidneys can vary between patients with a standard deviation as large as 50% [84]. In view of the inter-patient variability, individualized renal dosimetry should be adopted in routine clinal practice in PRRT which can help in delivering larger therapeutic doses with minimum nephrotoxicity.
Dosimetry based on planar/ SPECT imaging using MIRD remains one of the oldest and most preferred method [85]. Many semi-quantitative softwares based on the MIRD formulations such as MIRDose and OLINDA are in use which help in the pre-therapeutic dosimetry for PRRT [79]. Dose planning for therapy with 111In/90Y-peptides based on SPECT imaging using Monte Carlo simulations has also been evaluated [86]. Violet et al. performed whole-body dosimetry based on MIRD formulations for 177Lu-PSMA-617 RLT by acquiring SPECT/CT images at 4, 24, and 96 hrs post therapy [87]. Hou et al. developed an automated, observer-independent method for small volume dosimetry in kidneys. The dose estimates obtained using this method were compared with those from a manual selection of small volume locations and with doses obtained using whole kidney segmentations. An evident linear relationship was observed between small volume and whole-kidney dosimetry with an average ratio of 1.8 for majority patient datasets [88].
Patient-specific dosimetry can help replacing fixed dose treatment regime for improved therapeutic outcome.
Dose fractionation in patients with risk factors for nephrotoxicity
Poor renal function, hypertension, and diabetes at baseline were among the risk factors identified for developing renal toxicity after PRRT [89,90]. However, in patients with dialysis-dependent end-stage renal disease, a modified protocol (University Ulm) should be used for PRRT.
-
•
Dose Considerations for PRRT: As 177Lu-DOTATATE shows predominant uptake and excretion by the kidneys, patients undergoing chronic hemodialysis may potentially receive higher radiation exposure compared to the patients with normal renal function. Hence, in hemodialysis patients, the dose should be reduced to 1.5–2 GBq due to increased circulation time in the blood-pool and severely reduced renal excretion; hematotoxicity remains the primary concern.
-
•
Timing of therapy: PRRT should be planned immediately after dialysis, preferably on the same day of dialysis, or within 24 h after the last dialysis. The patient may go for the next planned dialysis two days after therapy administration.
-
•
Amino-acids and intravenous fluid infusion: The infusion of amino acids is not recommended in these patients, due to the obviated requirements of renal protection. The fluid volume should be adjusted according to the patient's cardiovascular status / resting renal function.
-
•
Radiation protection for dialysis after therapy: All the dialysis bags and tubing must be collected as they are radioactive due to the presence of 177Lu. The dialysis unit should be informed about the relevant radiation protection requirements.
-
•
Therapy cycle interval: There is no requirement to alter the therapy intervals for patients with end-stage renal disease.
Kalogianni et al. studied PRRT in dialysis-dependent patients by delivering 177Lu-DOTATATE in three fractions over a 15-month period. The administered activity was reduced to 50% for the first two fractions to ensure that the whole-body radiation doses were within safe limits of the treatments. They concluded that 177Lu-DOTATATE PRRT using dose reduction protocol appears to be safe in patients with end-stage renal failure on haemodialysis [91].
Patients with a solitary functioning kidney pose a clinical challenge for administration of PRRT. Ranade et al. evaluated renal toxicity profile in patients who underwent three cycles of 177Lu-DOTATATE therapy with single functioning kidney. They concluded that acute or chronic renal toxicity was not seen with co-administration of amino acids and dose fractionation [92]. The results were similar with 177Lu-PSMA RLT. In an analysis of 16 patients with a single functioning kidney, RLT with 177Lu-PSMA did not lead to any signs of acute or subacute nephrotoxicity during a mean follow-up of nearly two years [93].
In-silico analysis for treatment optimization
The tumor uptake of a radiopharmaceutical depends primarily on the delivery of the radio-tracer to the target site, expression of the relevant receptors/ transporters and the target-specific affinity of the radiopharmaceutical [94]. Decreased perfusion can be one of the major limiting factors for reduced tracer uptake and hence, reduced tumor absorbed doses. Jiménez et al. calculated the minimal receptor density and tumor perfusion in 177Lu-DOTATATE PRRT using nine physiologically based pharmacokinetic (PBPK) modeling with tumor control probability (TCP) of 99% and a maximal tolerated biologically effective dose (BEDmax) for organs at risk (OARs) for treating NET's and meningioma. It was observed that PBPK modeling can allow development and tumor specific selection of the radiotracer to better predict the therapeutic outcome based on receptor density and tumor perfusion [95].
Conclusion
Nuclear Medicine theranostics have demonstrated significant success with a favorable safety and efficacy profile in several malignancies. The kidneys present as a major critical organ, receiving high radiation absorbed dose from radiopharmaceuticals undergoing renal excretion. Nephrotoxicity becomes one of the dose-limiting toxicities in therapies with renal retention of the radiopharmaceutical, such as in somatostatin receptor targeting peptide receptor radionuclide therapy and PSMA targeting radioligand therapy. Recognition of the mechanisms of radiation induced nephropathy and associated risk factors can help us in the development of appropriate interventions to prevent and limit renal toxicity. Reduction of the renal absorbed radiation doses can help in increasing the therapeutic window, facilitating delivery of higher radiopharmaceutical activities and resultant higher delivered radiation dose to the tumors. With further gains in evidence, it is expected that in the future, the radiopharmaceutical therapies would be incorporated earlier in the management algorithm of patients. This would also translate to longer survival periods following therapy and in turn a longer time for delayed nephrotoxicity to manifest. Developments in reducing chronic radiation nephropathy following radionuclide therapies will help in avoiding the related morbidities, preserving the overall quality of life.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Ashwin Singh Parihar: Conceptualization, Methodology, Investigation, Writing – original draft, Writing – review & editing. Sejal Chopra: Methodology, Investigation, Writing – original draft, Writing – review & editing. Vikas Prasad: Conceptualization, Methodology, Supervision, Writing – review & editing, Project administration.
CRediT authorship contribution statement
Ashwin Singh Parihar: Conceptualization, Methodology, Investigation, Writing – original draft, Writing – review & editing. Sejal Chopra: Methodology, Investigation, Writing – original draft, Writing – review & editing. Vikas Prasad: Conceptualization, Methodology, Supervision, Writing – review & editing, Project administration.
Declaration of Competing Interest
None.
Acknowledgements
None
References
- 1.Mumtaz M., Lin L.S., Hui K.C., et al. Radioiodine I-131 for the therapy of graves’ disease. Malays. J. Med. Sci. 2009;16:25–33. [PMC free article] [PubMed] [Google Scholar]
- 2.Sherman S.I. Thyroid carcinoma. Lancet. 2003;361:501–511. doi: 10.1016/s0140-6736(03)12488-9. [DOI] [PubMed] [Google Scholar]
- 3.Carrasquillo J.A., Chen C.C., et al. Neuroendocrine tumors: therapy with 131I-MIBG. Strauss H, Mariani G, Volterrani D, et al., editors. Neuroendocrine tumors: therapy with 131I-MIBGNucl. Oncol. 2017:1269–1306. Eds. page. [Google Scholar]
- 4.Finlay I.G., Mason M.D., Shelley M. Radioisotopes for the palliation of metastatic bone cancer: a systematic review. Lancet Oncol. 2005;6:392–400. doi: 10.1016/S1470-2045(05)70206-0. [DOI] [PubMed] [Google Scholar]
- 5.Titano J., Voutsinas N., Kim E. The role of radioembolization in bridging and downstaging hepatocellular carcinoma to curative therapy. Semin. Nucl. Med. 2019;49:189–196. doi: 10.1053/j.semnuclmed.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Strosberg J., El-Haddad G., Wolin E., et al. Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumors. N. Engl. J. Med. 2017;376:125–135. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashwathanarayana A.G., Biswal C.K., Sood A., et al. Imaging-guided use of combined 177Lu-DOTATATE and capecitabine therapy in metastatic mediastinal paraganglioma. J. Nucl. Med. Technol. 2017;45:314–316. doi: 10.2967/jnmt.117.197400. [DOI] [PubMed] [Google Scholar]
- 8.Baum R.P., Kulkarni H.R., Schuchardt C., et al. 177Lu-labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-resistant prostate cancer: safety and efficacy. J. Nucl. Med. 2016;57:1006–1013. doi: 10.2967/jnumed.115.168443. [DOI] [PubMed] [Google Scholar]
- 9.Parihar A.S., Chandekar K., Singh H., et al. Orbital and brain metastases on 68Ga-PSMA PET/CT in a patient with prostate carcinoma refractory to 177Lu-PSMA and 225Ac-PSMA therapy. Asia Ocean J. Nucl. Med. Biol. 2021;9:67–70. doi: 10.22038/AOJNMB.2020.50820.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vadi S.K., Kumar R., Mittal B.R., et al. Unusual Case of Diffuse penile metastasis of prostate cancer on 68Ga PSMA PET/CT imaging and 177Lu PSMA posttherapy scintigraphy. Clin. Nucl. Med. 2018;43:276–278. doi: 10.1097/RLU.0000000000002001. [DOI] [PubMed] [Google Scholar]
- 11.Larson S.M., Carrasquillo J.A., Cheung N.K .V.., et al. Radioimmunotherapy of human tumours. Nat. Rev. Cancer. 2015;15:347–360. doi: 10.1038/nrc3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeiadin N., Rampakakis E., Turcotte E., et al. Safety and therapeutic value of radiosynoviorthesis with yttrium-90: a Canadian single-centre experience. Rheumatology. 2020 doi: 10.1093/rheumatology/keaa637. [DOI] [PubMed] [Google Scholar]
- 13.Syed M., Flechsig P., Liermann J., et al. Fibroblast activation protein inhibitor (FAPI) PET for diagnostics and advanced targeted radiotherapy in head and neck cancers. Eur. J. Nucl. Med. Mol. Imaging. 2020;47:2836–2845. doi: 10.1007/s00259-020-04859-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrmann K., Schottelius M., Lapa C., et al. First-in-human experience of CXCR4-directed endoradiotherapy with 177Lu-and 90Y-labeled pentixather in advanced-stage multiple myeloma with extensive intra-and extramedullary disease. J. Nucl. Med. 2016;57:248–251. doi: 10.2967/jnumed.115.167361. [DOI] [PubMed] [Google Scholar]
- 15.Parihar A.S., Mittal B.R., Kumar R., et al. 68Ga-DOTA-RGD2 positron emission tomography/computed tomography in radioiodine refractory thyroid cancer: prospective comparison of diagnostic accuracy with 18F-FDG positron emission tomography/computed tomography and evaluation toward potential theranostics. Thyroid. 2020;30:557–567. doi: 10.1089/thy.2019.0450. [DOI] [PubMed] [Google Scholar]
- 16.Parihar A.S., Sood A., Kumar R., et al. Novel use of 177Lu-DOTA-RGD2 in treatment of 68Ga-DOTA-RGD2-avid lesions in papillary thyroid cancer with TENIS. Eur. J. Nucl. Med. Mol. Imaging. 2018;45:1836–1837. doi: 10.1007/s00259-018-4036-x. [DOI] [PubMed] [Google Scholar]
- 17.Cybulla M., Weiner S., Otte A. End-stage renal disease after treatment with 90 Y-DOTATOC. Eur. J. Nucl. Med. Mol. Imaging. 2001;28:1552–1554. doi: 10.1007/s002590100599. [DOI] [PubMed] [Google Scholar]
- 18.Rolleman E., Valkema R., de Jong M., et al. Safe and effective inhibition of renal uptake of radiolabelled octreotide by a combination of lysine and arginine. Eur. J. Nucl. Med. Mol. Imaging. 2003;30:9–15. doi: 10.1007/s00259-002-0982-3. [DOI] [PubMed] [Google Scholar]
- 19.Emami B., Lyman J., Brown A., et al. Tolerance of normal tissue to therapeutic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 20.Lambert B., Cybulla M., Weiner S.M., et al. Renal toxicity after radionuclide therapy. Radiat. Res. 2004;161:607–611. doi: 10.1667/rr3105. [DOI] [PubMed] [Google Scholar]
- 21.Cives M., Strosberg J. Radionuclide therapy for neuroendocrine tumors. Curr. Oncol. Rep. 2017:19. doi: 10.1007/s11912-017-0567-8. [DOI] [PubMed] [Google Scholar]
- 22.Parihar A.S., Sood A., Sood A., et al. Demonstration of focal physiologic in-vivo somatostatin receptor expression in the caput epididymis of the testes on 68Ga-DOTANOC PET/CT and 177Lu-DOTATATE post-therapy whole body scintigraphy. Asia Ocean J. Nucl. Med. Biol. 2020;8:132–135. doi: 10.22038/AOJNMB.2020.44324.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Satapathy S., Das C.K., Parihar A.S., et al. Response to Concomitant enzalutamide and 177Lu-PSMA-617 radioligand therapy in atm-mutated metastatic castration resistant prostate cancer. Clin. Nucl. Med. 2021;46:582–583. doi: 10.1097/RLU.0000000000003541. [DOI] [PubMed] [Google Scholar]
- 24.Vegt E., De Jong M, Wetzels J.F.M., et al. Renal toxicity of radiolabeled peptides and antibody fragments: mechanisms, impact on radionuclide therapy, and strategies for prevention. J. Nucl. Med. 2010;51:1049–1058. doi: 10.2967/jnumed.110.075101. [DOI] [PubMed] [Google Scholar]
- 25.Mulford D.A., Scheinberg D.A., Jurcic J.G. The promise of targeted {alpha}-particle therapy. J. Nucl. Med. 2005;(46 Suppl 1):199S–204S. [PubMed] [Google Scholar]
- 26.Bodei L., Cremonesi M., Ferrari M., et al. Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90Y-DOTATOC and 177Lu-DOTATATE: the role of associated risk factors. Eur. J. Nucl. Med. Mol. Imaging. 2008;35:1847–1856. doi: 10.1007/s00259-008-0778-1. [DOI] [PubMed] [Google Scholar]
- 27.Reubi J.C., Horisberger U., Studer U.E., et al. Human kidney as target for somatostatin: high affinity receptors in tubules and vasa recta. J. Clin. Endocrinol. Metab. 1993;77:1323–1328. doi: 10.1210/jcem.77.5.7915721. [DOI] [PubMed] [Google Scholar]
- 28.Bhandari S., Watson N., Long E., et al. Expression of somatostatin and somatostatin receptor subtypes 1-5 in human normal and diseased kidney. J. Histochem. Cytochem. 2008;56:733–743. doi: 10.1369/jhc.2008.950998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baccala A., Sercia L., Li J., et al. Expression of prostate-specific membrane antigen in tumor-associated neovasculature of renal neoplasms. Urology. 2007;70:385–390. doi: 10.1016/j.urology.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 30.Parihar A.S., Vadi S.K., Mittal B.R., et al. 68Ga-PSMA-HBED-CC-avid synchronous urinary bladder paraganglioma in a patient with metastatic prostate carcinoma. Clin. Nucl. Med. 2018;43:e329–e330. doi: 10.1097/RLU.0000000000002172. [DOI] [PubMed] [Google Scholar]
- 31.Parihar A.S., Sood A., Mittal B.R., et al. 68Ga-PSMA-HBED-CC PET/CT and 18F-FDG PET/CT in Ewing sarcoma. Clin. Nucl. Med. 2020;45:e57–e58. doi: 10.1097/RLU.0000000000002764. [DOI] [PubMed] [Google Scholar]
- 32.Parihar A.S., Mittal B.R., Sood A., et al. 68Ga-prostate-specific membrane antigen PET/CT and 18F-FDG PET/CT of primary signet ring cell breast adenocarcinoma. Clin. Nucl. Med. 2018;43:e414–e416. doi: 10.1097/RLU.0000000000002265. [DOI] [PubMed] [Google Scholar]
- 33.Osman M.M., Iravani A., Hicks R.J., et al. Detection of Synchronous primary malignancies with68Ga-labeled prostate-specific membrane antigen PET/CT in patients with prostate cancer: frequency in 764 patients. J. Nucl. Med. 2017;58:1938–1942. doi: 10.2967/jnumed.117.190215. [DOI] [PubMed] [Google Scholar]
- 34.Behr T.M., Sharkey R.M., Sgouros G., et al. Overcoming the nephrotoxicity of radiometal-labeled immunoconjugates: improved canner therapy administered to a nude mouse model in relation to the internal radiation dosimetry. Cancer. 1997:2591–2610. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2591::aid-cncr35>3.3.co;2-a. page. [DOI] [PubMed] [Google Scholar]
- 35.Parihar A.S., Sood A., Lukose T.T., et al. Metabolic Bone superscan in carcinoma breast with occult graves’ disease: looking beyond skeletal metastases. Indian J. Nucl. Med. 2018;33:145–147. doi: 10.4103/ijnm.IJNM_144_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beauregard J.M., Hofman M.S., Kong G., et al. The tumour sink effect on the biodistribution of 68Ga-DOTA- octreotate: implications for peptide receptor radionuclide therapy. Eur. J. Nucl. Med. Mol. Imaging. 2012;39:50–56. doi: 10.1007/s00259-011-1937-3. [DOI] [PubMed] [Google Scholar]
- 37.Santivasi W.L., Xia F. Ionizing radiation-induced DNA damage, response, and repair. Antioxid. Redox Signal. 2014;21:251–259. doi: 10.1089/ars.2013.5668. [DOI] [PubMed] [Google Scholar]
- 38.Bergonie J., Tribondeau L. Interpretation of some results of radiotherapy and an attempt at determining a logical technique of treatment. Radiat. Res. 1959;10:587–588. [PubMed] [Google Scholar]
- 39.Hartman F.W., Bolliger A., Doub H.P. Functional studies throughout the course of roentgen-ray nephritis in dogs. J. Am. Med. Assoc. 1927;88:139–145. [Google Scholar]
- 40.Doub H.P., Bolliger A., Hartman F.W. The relative sensitivity of the kidney to irradiation. Radiology. 1927;8:142–148. [Google Scholar]
- 41.Rinkevich Y., Montoro D.T., Contreras-Trujillo H., et al. In vivo clonal analysis reveals lineage-restricted progenitor characteristics in mammalian kidney development, maintenance, and regeneration. Cell Rep. 2014;7:1270–1283. doi: 10.1016/j.celrep.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valkema R., Pauwels S.A., Kvols L.K., et al. Long-term follow-up of renal function after peptide receptor radiation therapy with (90)Y-DOTA(0),Tyr(3)-octreotide and (177)Lu-DOTA(0), Tyr(3)-octreotate. J. Nucl. Med. 2005;(46 Suppl 1):83S–91S. [PubMed] [Google Scholar]
- 43.Klaus R., Niyazi M., Lange-Sperandio B. Radiation-induced kidney toxicity: molecular and cellular pathogenesis. Radiat. Oncol. 2021;16:43. doi: 10.1186/s13014-021-01764-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luxton R.W., Kunkler P.B. Radiation nephritis. Acta Radiol. Ther. Phys. Biol. 1964;2:169–178. doi: 10.3109/02841866409134143. [DOI] [PubMed] [Google Scholar]
- 45.Howard S.C., Jones D.P., Pui C.-.H. The tumor lysis syndrome. N. Engl. J. Med. 2011;364:1844–1854. doi: 10.1056/NEJMra0904569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang K., Brenner W., Prasad V. Tumor lysis syndrome: a rare but serious complication of radioligand therapies. J. Nucl. Med. 2019;60:752–755. doi: 10.2967/jnumed.118.217380. [DOI] [PubMed] [Google Scholar]
- 47.Imhof A., Brunner P., Marincek N., et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue[90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J. Clin. Oncol. 2011;29:2416–2423. doi: 10.1200/JCO.2010.33.7873. [DOI] [PubMed] [Google Scholar]
- 48.Sartor O., de Bono J., KN Chi, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2021;385:1091–1103. doi: 10.1056/NEJMoa2107322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gallyamov M., Meyrick D., Barley J., et al. Renal outcomes of radioligand therapy: experience of 177lutetium—Prostate-specific membrane antigen ligand therapy in metastatic castrate-resistant prostate cancer. Clin. Kidney J. 2020;13(6):1049–1055. doi: 10.1093/ckj/sfz101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Małyszko J., Kozłowska K., Kozłowski L., et al. Nephrotoxicity of anticancer treatment. Nephrol. Dial. Transplant. 2017;32:924–936. doi: 10.1093/ndt/gfw338. [DOI] [PubMed] [Google Scholar]
- 51.Leblanc M., Kellum J.A., Gibney R.T.N., et al. Risk factors for acute renal failure: inherent and modifiable risks. Curr. Opin. Crit. Care. 2005;11:533–536. doi: 10.1097/01.ccx.0000183666.54717.3d. [DOI] [PubMed] [Google Scholar]
- 52.National Cancer Institute. Common Terminology Criteria For Adverse Events (CTCAE) Common Terminology Criteria for Adverse Events (CTCAE) v5.0 [Internet]. 2017.
- 53.Valkema R., De Jong M., Bakker W.H., et al. Phase I study of peptide receptor radionuclide therapy with [111In-DTPA0]octreotide: the Rotterdam experience. Semin. Nucl. Med. 2002;32:110–122. doi: 10.1053/snuc/2002.31025. [DOI] [PubMed] [Google Scholar]
- 54.Otte A., Herrmann R., Heppeler A., et al. Yttrium-90 DOTATOC: first clinical results. Eur. J. Nucl. Med. 1999;26:1439–1447. [PubMed] [Google Scholar]
- 55.Stoffel M.P., Pollok M., Fries J., et al. Radiation nephropathy after radiotherapy in metastatic medullary thyroid carcinoma. Nephrol. Dial. Transplant. 2001;16:1082–1083. doi: 10.1093/ndt/16.5.1082. [DOI] [PubMed] [Google Scholar]
- 56.Moll S., Nickeleit V., Mueller-Brand J., et al. A new cause of renal thrombotic microangiopathy: yttrium 90-DOTATOC internal radiotherapy. Am. J. Kidney Dis. 2001;37:847–851. doi: 10.1016/s0272-6386(01)80135-9. [DOI] [PubMed] [Google Scholar]
- 57.Bodei L., Cremonesi M., Grana C., et al. Receptor radionuclide therapy with 90Y- [DOTA] 0-Tyr3-octreotide (90Y-DOTATOC) in neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging. 2004;31:1038–1046. doi: 10.1007/s00259-004-1571-4. [DOI] [PubMed] [Google Scholar]
- 58.Marincek N., Jörg A.C., Brunner P., et al. Somatostatin-based radiotherapy with [90Y-DOTA]-TOC in neuroendocrine tumors: long-term outcome of a phase I dose escalation study. J. Transl. Med. 2013;11:11–17. doi: 10.1186/1479-5876-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pfeifer A.K., Gregersen T., Grønbæk H., et al. Peptide receptor radionuclide therapy with 90Y-DOTATOC and 177Lu-DOTATOC in advanced neuroendocrine tumors: results from a Danish cohort treated in Switzerland. Neuroendocrinology. 2011;93:189–196. doi: 10.1159/000324096. [DOI] [PubMed] [Google Scholar]
- 60.Bergsma H., Konijnenberg M.W., van der Zwan W.A., et al. Nephrotoxicity after PRRT with 177Lu-DOTA-octreotate. Eur. J. Nucl. Med. Mol. Imaging. 2016;43:1802–1811. doi: 10.1007/s00259-016-3382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kwekkeboom D.J., De Herder W.W., Kam B.L., et al. Treatment with the radiolabeled somatostatin analog [177Lu- DOTA0,Tyr3]octreotate: toxicity, efficacy, and survival. J. Clin. Oncol. 2008;26:2124–2130. doi: 10.1200/JCO.2007.15.2553. [DOI] [PubMed] [Google Scholar]
- 62.Romer A., Seiler D., Marincek N., et al. Somatostatin-based radiopeptide therapy with [177Lu-DOTA]-TOC versus [90Y-DOTA]-TOC in neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging. 2014;41:214–222. doi: 10.1007/s00259-013-2559-8. [DOI] [PubMed] [Google Scholar]
- 63.Bodei L., Kidd M., Paganelli G., et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur. J. Nucl. Med. Mol. Imaging. 2015;42:5–19. doi: 10.1007/s00259-014-2893-5. [DOI] [PubMed] [Google Scholar]
- 64.Strosberg J., et al. Oral presentation at the 2021 ASCO annual meeting. 2021. June 4Abstract 4112. [Google Scholar]
- 65.Eyben FE von, Roviello G., Kiljunen T., et al. Third-line treatment and 177 Lu-PSMA radioligand therapy of metastatic castration-resistant prostate cancer: a systematic review. Eur. J. Nucl. Med. Mol. Imaging. 2017;45:496–508. doi: 10.1007/s00259-017-3895-x. 2017 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kulkarni H.R., Singh A., Schuchardt C., et al. PSMA-based radioligand therapy for metastatic castration-resistant prostate cancer: the bad berka experience since 2013. J. Nucl. Med. 2016;57:97S–104S. doi: 10.2967/jnumed.115.170167. [DOI] [PubMed] [Google Scholar]
- 67.Yadav M.P., Ballal S., Sahoo R.K., et al. Radioligand therapy with 177Lu-PSMA for metastatic castration-resistant prostate cancer: a systematic review and meta-analysis. Am. J. Roentgenol. 2019;213:275–285. doi: 10.2214/AJR.18.20845. [DOI] [PubMed] [Google Scholar]
- 68.Maffey-Steffan J., Scarpa L., Svirydenka A., et al. The 68Ga/177Lu-theragnostic concept in PSMA-targeting of metastatic castration–resistant prostate cancer: impact of post-therapeutic whole-body scintigraphy in the follow-up. Eur. J. Nucl. Med. Mol. Imaging. 2020;47:695–712. doi: 10.1007/s00259-019-04583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heck M.M., Tauber R., Schwaiger S., et al. Treatment outcome, toxicity, and predictive factors for radioligand therapy with 177Lu-PSMA-I&T in metastatic castration-resistant prostate cancer. Eur. Urol. 2019;75:920–926. doi: 10.1016/j.eururo.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 70.Gallyamov M., Meyrick D., Barley J., et al. Renal outcomes of radioligand therapy: experience of 177lutetium-prostate-specific membrane antigen ligand therapy in metastatic castrate-resistant prostate cancer. Clin. Kidney J. 2021;13:1049–1055. doi: 10.1093/ckj/sfz101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hofman M.S., Emmett L., Sandhu S., et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397:797–804. doi: 10.1016/S0140-6736(21)00237-3. [DOI] [PubMed] [Google Scholar]
- 72.Sartor O., de Bono J., Chi K.N., et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2021:1–13. doi: 10.1056/NEJMoa2107322. Ahead of P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bernard B.F., Krenning E.P., Breeman W.A., Rolleman E.J., Bakker W.H., Visser T.J., et al. D-lysine reduction of indium-111 octreotide and yttrium-90 octreotide renal uptake. J. Nucl. Med. 1997;38(12):1929–1933. 11. [PubMed] [Google Scholar]
- 74.Guerriero F., Ferrari M.E., Botta F., et al. Kidney dosimetry in (177)Lu and (90)Y peptide receptor radionuclide therapy: influence of image timing, time-activity integration method, and risk factors. Biomed. Res. Int. 2013;2013 doi: 10.1155/2013/935351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rolleman E.J., Bernard B.F., Breeman W.A., et al. Molecular imaging of reduced renal uptake of radiolabelled [DOTA0,Tyr3]octreotate by the combination of lysine and Gelofusine in rats. Nuklearmedizin. 2008;47:110–115. doi: 10.3413/nukmed-0069. [DOI] [PubMed] [Google Scholar]
- 76.Watkins J. Reactions to gelatin plasma expanders. Lancet. 1994;344:328–329. [author reply 9-30] [PubMed] [Google Scholar]
- 77.Behr T.M., Sharkey R.M., Juweid M.E., et al. Reduction of the renal uptake of radiolabeled monoclonal antibody fragments by cationic amino acids and their derivatives. Cancer Res. 1995;55:3825–3834. [PubMed] [Google Scholar]
- 78.Melis M., Valkema R., Krenning E.P., et al. Reduction of renal uptake of radiolabeled octreotate by amifostine coadministration. J. Nucl. Med. 2012;53:749–753. doi: 10.2967/jnumed.111.098665. [DOI] [PubMed] [Google Scholar]
- 79.Uehara T., Koike M., Nakata H., et al. Design, synthesis, and evaluation of [188Re]organorhenium-labeled antibody fragments with renal enzyme-cleavable linkage for low renal radioactivity levels. Bioconjug. Chem. 2007;18:190–198. doi: 10.1021/bc0602329. [DOI] [PubMed] [Google Scholar]
- 80.Rolleman E.J., Valkema R., Bernard B., et al. Additive effect of an angiontensin II blocker to kidney protection by lysine in a rat model of radiation nephropathy. Eur. J. Nucl. Med. Mol. Imaging. 2007;34(Suppl.2):S240. [Google Scholar]
- 81.Sierra M.L., Agazzi A., Bodei L., et al. Lymphatic toxicity in patients after peptide-receptor radionuclide therapy (PRRT) with 177Lu-DOTATATE and 90Y-DOTATOC. Cancer Biother. Radiopharm. 2009;24:659. doi: 10.1089/cbr.2009.0641. [DOI] [PubMed] [Google Scholar]
- 82.Baum R.P., Kulkarni H.R., Carreras C. Peptides and receptors in image-guided therapy: theranostics for neuroendocrine neoplasms. Semin. Nucl. Med. 2012;42:190–207. doi: 10.1053/j.semnuclmed.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 83.Emmett L., Willowson K., Violet J., Shin J., Blanksby A., Lee J. Lutetium 177 PSMA radionuclide therapy for men with prostate cancer: a review of the current literature and discussion of practical aspects of therapy. J. Med. Radiat. Sci. 2017;64(1):52–60. doi: 10.1002/jmrs.227. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bodei L., Cremonesi M., Ferrari M., et al. Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90Y-DOTATOC and 177Lu-DOTATATE: the role of associated risk factors. Eur. J. Nucl. Med. Mol. Imaging. 2008;35:1847–1856. doi: 10.1007/s00259-008-0778-1. [DOI] [PubMed] [Google Scholar]
- 85.Kwekkeboom D.J., Bakker W.H., Kooij P.P., Konijnenberg M.W., Srinivasan A., Erion J.L., et al. [177Lu-DOTAOTyr3]octreotate: comparison with [111In-DTPA0]octreotide in patients. Eur. J. Nucl. Med. 2001;28:1319–1325. doi: 10.1007/s002590100574. [DOI] [PubMed] [Google Scholar]
- 86.Ljungberg M., Frey E., Sjogreen K., Liu X., Dewaraja Y., Strand S.E. 3D absorbed dose calculations based on SPECT: evaluation for 111In/90Y therapy using Monte Carlo simulations. Cancer Biother. Radiopharm. 2003;18:99–107. doi: 10.1089/108497803321269377. [DOI] [PubMed] [Google Scholar]
- 87.Violet J., Jackson P., Ferdinandus J., Sandhu S., Akhurst T., Iravani A., Kong G., Kumar A.R., Thang S.P., Eu P., Scalzo M. Dosimetry of 177Lu-PSMA-617 in metastatic castration-resistant prostate cancer: correlations between pretherapeutic imaging and whole-body tumor dosimetry with treatment outcomes. J. Nucl. Med. 2019;60(4):517–523. doi: 10.2967/jnumed.118.219352. Apr 1. [DOI] [PubMed] [Google Scholar]
- 88.Hou X., Zhao W., Beauregard J.M., Celler A. Personalized kidney dosimetry in 177Lu-octreotate treatment of neuroendocrine tumours: a comparison of kidney dosimetry estimates based on a whole organ and small volume segmentations. Phys. Med. Biol. 2019;64(17) doi: 10.1088/1361-6560/ab32a1. Aug 28. [DOI] [PubMed] [Google Scholar]
- 89.Waldherr C., Pless M., Maecke H.R., Schumacher T., Crazzolara A., Nitzsche E.U., et al. Tumor response and clinical benefit in neuroendocrine tumors after 7.4 GBq (90)Y-DOTATOC. J. Nucl. Med. 2002;43(5):610–616. [PubMed] [Google Scholar]
- 90.Ezziddin S., Sabet A., Heinemann F., Yong-Hing C.J., Ahmadzadehfar H., Guhlke S., et al. Response and long-term control of bone metastases after peptide receptor radionuclide therapy with (177)Lu-octreotate. J. Nucl. Med. 2011;52(8):1197–1203. doi: 10.2967/jnumed.111.090373. [DOI] [PubMed] [Google Scholar]
- 91.Kalogianni E., Ruiz D.L., Corcoran B.J., Devlin L.A., Vivian G.C., Mulholland N.J. 177Lu-Dotatate therapy for the treatment of metastatic neuroendocrine tumours in a patient on haemodialysis—Dosimetric considerations. BJR case reports. 2015 Aug 18:20150177. [DOI] [PMC free article] [PubMed]
- 92.Ranade R., Basu S. 177Lu-DOTATATE PRRT in patients with metastatic neuroendocrine tumor and a single functioning kidney: tolerability and effect on renal function. J. Nucl. Med. Technol. 2016;44(2):65–69. doi: 10.2967/jnmt.115.168146. Jun 1. [DOI] [PubMed] [Google Scholar]
- 93.Zhang J., Kulkarni H.R., Singh A., Schuchardt C., Niepsch K., Langbein T., Baum R.P. 177Lu-PSMA-617 radioligand therapy in metastatic castration-resistant prostate cancer patients with a single functioning kidney. J. Nucl. Med. 2019;60(11):1579–1586. doi: 10.2967/jnumed.118.223149. . Epub 2019 Mar 8. PMID: 30. [DOI] [PubMed] [Google Scholar]
- 94.Park S., Parihar A.S., Bodei L., Hope T.A., Mallak N., Millo C., Prasad K., Wilson D., Zukotynski K., Mittra E. Somatostatin receptor imaging and theranostics: current practice and future prospects. J. Nucl. Med. 2021;62(10):1323–1329. doi: 10.2967/jnumed.120.251512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jiménez-Franco L.D., Glatting G., Prasad V., Weber W.A., Beer A.J., Kletting P. Effect of tumor perfusion and receptor density on tumor control probability in 177Lu-DOTATATE therapy: an in silico analysis for standard and optimized treatment. J. Nucl. Med. 2021;62(1):92–98. doi: 10.2967/jnumed.120.245068. Jan 1. [DOI] [PubMed] [Google Scholar]