Abstract
Indigenous chickens (IC) contribute socioeconomically to household food security in the region of East Africa. However, their potential and improvement are not well documented. This review is aimed at exploring the production and potential of indigenous chickens in East Africa. The various tools for literature search such as google search and Google scholars, agricultural journals, animal sciences and health journals, poultry related journals, and country online databases were used to gather information. IC were primarily reared by women and were kept predominantly under scavenging systems where the conditions of management (feeding, housing, and health care) are poor. They presented a high variation in their reproduction and production characteristics. The products (meat and eggs) were of good quality and preferred by the local consumers. Despite the variation and potential of IC, improvements in the village system were constrained by diseases and loss due to Newcastle, Gumboro, and Ecto-endo parasites and predators. Farmers primarily used traditional methods to control the diseases, and some used conventional medications and vaccines. Due to the potential of IC, the exploration of various strategies for improvement supported by the details of their genetic variability and adaptation as well as different management conditions was a goal of this review.
Key words: East African region, indigenous chicken, management, production, potential
INTRODUCTION
Chickens have been a widespread poultry species worldwide due to the cultural, social, and economic role they play in the daily livelihoods of the population. Africa counts about 1.3 billion of chickens and approximately 80% were indigenous varieties kept in rural areas (Gueye, 1998). Similarly, Goodger et al. (2002), found the indigenous chickens (IC) in Africa made up 80% of the chickens. Among all chickens raised in sub-Saharan Africa, Dessie (2003), stated 78% were IC. Nearly 100.8 million IC, the focus of the review have been estimated to reside in East Africa (F.A.O. STAT, 2007). In East Africa, the distribution of IC varied. For example, Ethiopia counted nearly 65 million chickens (FAO, 2000; Dessie, 2003) from which more than 95% were composed of village chickens. The poultry population in Rwanda was about 5.3 million birds among which nearly 70% were indigenous chickens (NISR, 2015). In Kenya, a report from the Ministry of Agriculture, Livestock, and Fisheries show the presence of 32 million chickens among which over 70% were IC (MALF, 2015). In 2001, Zambia counted 26 million chickens with 11 million being indigenous varieties (Bwalya, 2014). Indigenous chickens have played a substantial role by contributing to the food security of rural households across the developing world. Their products, meat, and eggs often constituted the main source of protein and income, and they served as a source of investment and security for rural households (Dessie, 2003; Muchadeyi et al., 2007; Alders and Pym, 2009). Women and children in tropical countries on small farms kept IC as a main source of investment. Alongside the development of rural livelihoods, IC played a significant role in peri- and urban households where they have been integrated into daily livelihood strategies (Dana et al., 2010). In the review by Gueye (2000), it was indicated that in central Tanzania, women added an additional 10% to their annual income with an average flock size of 5 chickens (3 adult females and 2 adult males). Dessie (1996, 2003) reported in Ethiopia, IC production made up 90% of the national poultry meat and egg production. Findings by Mekonnen et al. (2010), mentioned that in the same country, the annual consumption per capita of eggs and meat averaged 75 eggs and 2.5 chickens, respectively. According to Okello et al. (2010) and Magothe et al. (2012), IC as a source of both protein and income, has been shown to contribute to the economic and nutritional welfare of poor rural households in Kenya. In Zambia, low-income households consume per month 2.34 times more chicken compared to 1.9 times per month for high-income households (Bwalya, 2014). Alders and Pym (2009) and Simainga et al. (2010, 2011) reported that, in Mozambique and Zambia, the households headed by children and widows considered the IC easy to rear and provided high-quality protein and other micronutrients. It has been shown that in Zimbabwe, IC constituted a tool to combat HIV and AIDS especially in children and women-headed households where they were considered an alternative food source when income decreased (Mutenje et al., 2008). In Uganda, IC were kept primarily for egg and meat production, however, they were also used in traditional ceremonies such as childbirth as well as naming babies, marriage, etc. (Kyarishma et al., 2004). Most IC are produced under extensive or village scavenging systems with few inputs. Many studies in the East Africa region have shown that indigenous birds are accommodated in the same house with the family and feeding is scavenge-based (Kingori et al., 2003; Dessie, 2003; Bwalya, 2014; Mahoro et al., 2017). Despite the advantages, the production of indigenous chicken faces many challenges. The poor productivity of birds, a shortage and poor quality of feeds consumed by village chickens, frequent disease outbreaks, and inappropriate housing were the main constraints which have been reported across East Africa (Mapiye et al., 2008; Dana et al., 2010; Mekonnen et al., 2010; Magothe et al., 2012; Okeno et al., 2012; Bwalya, 2014; Mahoro et al., 2017). Mahoro et al. 2017 reported predation as a serious constraint in Rwanda. Furthermore, numerous socioeconomic challenges have been reported including lack of markets, and poor institutional, as well as infrastructural support (Mapiye et al., 2008). Numerous strategies have been suggested to help farmers cope with these challenges. These have included farmer training in improved production, breeding, feeding practices, housing, health management, and entrepreneurship skills, as well as the development of the feed industry (Mahoro et al., 2017). The use of locally available feeds and ethnoveterinary medicines and the intensification of farmers’ education have been suggested by Mapiye et al. (2008) as viable strategies that can be used for improved production of IC. Although IC seems to have the similar advantages and constraints in the region, they are kept under different environmental or ecological regions. This leads to varied management systems and outputs. The main objective of this paper was to explore the potential of IC and their production in the East African region. This review was based on the information gathered from different sources, published and unpublished works that were considered relevant for explaining the potential of IC in the region and the ways for further improvement and conservation.
SOURCE OF INFORMATION
As a review paper, data sources including Google search and Google scholar, agricultural journals, animal sciences and health journals, poultry related journals and country online databases were used to find the relevant research articles and reports. The specific areas used to categorize the information and studies were country names, chicken production systems, poultry production and reproduction performances, chicken/poultry management, chicken health, and diseases. The indigenous chicken was added where necessary as a key word. These areas are keys in succeeding in poultry industry and helped in general to understand the production and potential of IC in the East African Region.
PRODUCTION DYNAMICS OF IC IN EAST AFRICA
Management Systems of IC in Different Countries of the East African Region
IC present a range of variability in genotypes and phenotypes. The environment in which individuals are living affects the expression of most metric traits (Falconer and Mackay, 1996; Wiener and Rouvier, 2009). The heterogeneity of the environment has been derived from a range of components including temperature, feed resources, micro, and macro-ecology. These components were associated with the production objective, specifically whether the birds raised were in a commercial or subsistence system as reported by Khobondo et al. (2015). Farmers’ practices and their involvement in decision-making (Mapiye et al., 2008; Khobondo et al., 2015) affected the reproductive and productive performance of IC and often determined how the latter are managed. For management optimization, the research showed that the role of household and gender, the flock size and structure, the objective of production, production system, housing, and feeding must be considered.
Household and Gender Involvement in Indigenous Chicken Production
In order to foster farm development, the introduction of new technologies is required. The level of understanding of farmers and the implementation of these technologies requires the analysis of the role of household members and gender in village chicken production. Mapiye et al. (2008) reported that gender analysis could help understand who needs the knowledge on-farm technologies to avoid their misdirection. Moreover, this analysis might explain the role and responsibilities of men and women in farm activities, resource access, and their use, and decision making on the farm (Kusina et al., 2001). Indigenous chicken production often involves all household members (husband, wife, and children) according to specific and sociocultural interests (Dessie and Ogle, 2001; Mapiye et al., 2008). The size and composition of the household are one of the important factors of chicken management in terms of labor and activities assignment. In East African countries, this factor varies from one country to another and region to another. In Rwanda, household size varied from 5 to 6 people (NISR, 2012; Mbuza et al., 2016; Hirwa et al., 2019). The same family size of 6 people was reported in North Wollo, Amhara Region, Ethiopia by Hailu et al. (2013), and in West Amhara (Worku et al., 2012) and Bure district (Fisseha et al., 2010). However, a family size of 4 people was observed in the Metekel zone (Solomon et al., 2013). It was revealed that the household members involved in IC are of different ages. In Zimbabwe, Mlambo et al. (2011) reported the farmers who were involved in village indigenous chicken production average 46 yr old. The studies in Ethiopia reported the average age of indigenous chicken farmers ranging between 37 and 46 yr (Fisseha et al. 2010; Worku et al, 2012; Hailu et al., 2013; Solomon et al., 2013; Tadesse et al., 2013; Zemelak et al. 2016). In Rwanda the average age reported is 35 yr varying between 17 and 52 yr indicating that the youth were involved in the IC production (Mbuza et al., 2016; Hirwa et al., 2019). The NISR (2016) reported that more than 39% of the population is youth, ranging from 14 to 35 yr. The educational level of the farmers also likely has an effect on IC production. Studies in Rwanda reported 75.2 to 84.5% of farmers attended formal education but 15.5 to 25.8% were illiterate and had some informal education (Mbuza et al., 2016; Mahoro et al., 2017; Hirwa et al., 2019). Ethiopia has shown a high proportion of illiterate farmers with average ranging from 35 to 42% (Hailu et al., 2013; Solomon et al., 2013). However, the study did not report the cause of this high illiteracy, but the proportions vary from one region to another within the same country. Therefore, the level of education could be a factor in understanding and adopting new technologies to improve farm practices. Regarding the participation and responsibilities of household members in farm activities, the studies of Kusina and Kusina (1999) and Maphosa et al. (2004) reported in Zimbabwe more than 90% of the population kept chickens, with 95% household birds belonging to women. Women were responsible for many farm activities including feed distribution, housecleaning, bird watering, sales of eggs, and a live chicken. Muchadeyi et al. (2004) reported that because the eggs and chickens constitute the key source of their income, women manage chickens and farm outputs, and make decisions on the production system (Kusina et al., 2001). The chicken processing and construction of birds’ shelter are the main activities of men, however, the joint decision making by men and women could be observed in some communities (Muchadeyi et al., 2004). The higher involvement of women might be due to their low literacy, allowing them to remain at the house looking after livestock, while the more literate men are involved in other professional activities or business (Kitalyi, 1999). Mbuza et al. (2016), suggested that improved development of IC could be in parallel with increasing the education level of farmers. Mapiye et al. 2008 stated focusing on women could more effectively improve IC production. Feeding chickens (81%), selling birds and eggs (47 and 57%, respectively), and house cleaning with the proportion of 39% were reported in Ethopia as being the responsibilities of women (Fisseha et al., 2010). The same author reported children participated in farm activities by helping their mothers in cleaning the chicken house, feeding, and watering birds. Another study by Zewdu et al. (2013), reported different findings in the Metekel zone, Northwest Ethiopia, where the marketing decisions were made by men (61%), joint decision making of men and women (16%), and the proportion of women who independently make a decision is low (13%). Nevertheless, they mentioned that all household members participle actively in farm activities and men and women have equal ownership over the chickens. However, even with equal ownership of birds, men still made the final decisions but women were responsible for marketing and often had control of the benefits from the farm (Fetsum et al., 2009). Mbuza et al. (2016) reported that in Rwanda the IC depend mainly on family labor (65%) but some farmers can use both families and hired labor. They mentioned production that depends only on hired labor was not economically viable; noting only 15% of farmers in Rwanda used hired labor. Mahoro et al. (2017) similarly reported high responsibility of women and children for IC farm activities with 78 and 19%, respectively of all farmers in their study among whom 66% cite farming as the main source of income. The study of Ochieng et al. (2011) in Kenya, using the log-linear regression model with different variables in farm activities, showed the positive and highly significant coefficient of the female gender. The study showed that women were the dominant labor force but farm productivity was higher with men. The women were more often at the house looking after birds with 76% of females involved in IC production (Ochieng, 2010). However, to improve IC production, women should be considered the target point for education and training (Ochieng et al., 2011). Similar findings in Kenya on the success of women in farming productivity were reported (Okitoi et al., 2007). In Zimbabwe, women followed by children owned most of the chickens (Kitalyi, 1999; Mlambo et al., 2011) with different findings in Sudan where most of the chickens were owned by men (Khalafalla et al., 2002). In Rwanda, Hirwa et al. (2019) reported the children owned IC (31%) followed by joint-family ownership (30%). In this study the number of IC owned by women is low (17%). The chicken management and ownership were determined by the purpose of their keeping and production system.
The Purpose of Keeping Indigenous Chickens by Rural Households
In developing countries, IC are generally reared for egg and meat production with the aim of home consumption, reproduction, or income generation. Sonaiya and Swan (2004) reported that the primary purpose of the rearing of local chickens in developing countries is to sell live chickens and eggs for income generation. The production of both egg and meat for home consumption and income generation were the main purposes of keeping IC in a study by Mapiye and Sibanda 2005. However, surveys conducted in some African and Asian countries, in 2002, showed that more than 76% of eggs produced are naturally incubated to produce chicks for future breeding birds (Ekue et al., 2002; Khalafalla et al., 2002; Njue et al., 2002; Nqindi, 2002). In Ethiopia, cash income represented 27 to 51%, home consumption 19 to 44%, sacrifice 25% and the replacement of birds represents 20% (Tadelle and Ogle, 1996; Fisseha et al., 2010; Hailu et al., 2013). Zewdu et al. (2013), reported in the Metekel zone, Northwest Ethiopia, cash income was ranked as the priority of IC keeping by 93% of interviewees. This involved the sale of live birds representing 44 and 78% in Fogera and Dale districts of Ethiopia (Fisseha et al., 2010). The main functions of egg production in Ethiopia were hatching for chicken replacement (52–72%) and home consumption ranging from 20 to 69% (Tadelle and Ogle, 1996; Fisseha et al. 2010; Zewdu et al., 2013). Egg consumption was ranked as a priority in Farta, Mandura, Horro, Konso, and Cheka followed by meat consumption (Dana et al., 2010). Other chicken functions in Ethiopia comprise additional farm activities, job opportunities, and gifts (Zewdu et al., 2013). In Rwanda, the sale of chickens and eggs for household income represents 72% of their use (Mbuza et al., 2016). This function was supported by Hirwa et al. (2019), but according to Mahoro et al. (2017), the main functions of keeping IC include egg production at 47% and home consumption representing 39%. The variability of the use of IC was influenced by location, ownership and economic status of the family. In Zimbabwe, 65% of farmers kept IC to generate cash income (Mlambo et al., 2011). The live IC play the role of a bank for some financial needs such as paying for school fees, medical costs for household members, and taxes (Mapiye et al., 2008). However, this function differed depending on the socioeconomic status of households (Muchadeyi et al., 2004). The chicken also played an important sociocultural role including starter capital for youth and young women after marriage, for gratitude of services rendered (Kusina and Kusina, 1999), special food for guests, use in cultural ceremonies (Mapiye et al., 2008), and as gifts for strengthening the relationship between in-law relatives or families (Muchadeyi et al., 2004). Maphosa et al. (2004) and Muchadeyi et al. (2004) mentioned that production of manure, in Zimbabwe, is considered an important function of indigenous chicken keeping in a rural area because it constitutes a high-value fertilizer for vegetable gardens compared to manure from goat and cattle. Gondwe and Wollny (2007) reported 2 sources of chicken off-take in Malawi, with household and social functions representing 56% while the remaining 44% constitute the off-take due to losses. Therefore, these authors reported that use of IC by households and communities were home consumption, sociocultural functions, cash income from the selling of live chickens, and breeding stock exchange between farmers. For home consumption and gifts, male chickens were more often used than females which were kept for egg production and further reproduction functions (natural incubation and brooding). The purpose of selling birds was for cash (68% of all sold chickens), for barter, mats, and clothes (23%) as well as for wages for labor hired at the farm (Gondwe and Wollny, 2007).
Food security is a requirement of rural households in developing countries (Sonaiya, 2007). In Kenya, income generation is the main aim of chicken rearing followed by the use of meat and eggs as the main feeds stuff for rich, cheap, and accessible protein sources (Magothe et al., 2012). Home consumption constituted 18% of the eggs laid and for meat 30% of the flock at the household level (Okitoi, 2000; Kaudia and Kitalyi, 2002). A study of Okeno et al. (2012) conducted in 6 counties of Kenya such as Siaya, Kakumaga, Bomet, Narok, West Pokot, and Turkana reported 21.5% of household consumption of all chicken off-take followed by 17% of sales for income and 9.5% for sociocultural activities (donations and exchange). The remaining 52% of the chickens’ exit from the household was due to diseases and infections, predation, and theft. The same authors also reported that 84% of the total eggs laid were set for hatching to produce chicks. Njenga (2005) reported other functions of IC in Kenya including sociocultural and spiritual events (entertainment, funerals, and spiritual cleaning) and gifts. Similar findings were observed in Uganda, where chickens are used for household food, cash, gifts, ceremonies, and others representing 36, 33, 13, 16, and 2%, respectively (Ssewannyana et al., 2008a). These authors also reported that production of chicks (45%), food (33%), and cash income (20%) were the main uses of eggs laid by IC.
In Tanzania, production of IC represented 38% of the total income earned by the household income-generating activities (Oswin and Kalista, 2017). This was the highest source of household income, followed by other livestock (33%), crop production (18%) exotic chicken (2%), and petty business (9%). The income was from the sale of eggs and chickens to local (51%) and open (16%) markets. Home consumption constituted about 33% of use. In East Africa the demand for IC meat and eggs has been higher than that of exotic varieties of chickens, such as commercial broilers and hybrid layers raised intensively because the local products were preferred by the consumers due to their pigmentation and taste (Ssewannyana et al., 2008a; Oswin and Kalista, 2017). A variety of studies showed that the availability of eggs and meat at the household level, constituted a crucial source of protein to improve the nutritional status of children under 5 yr old (Kingori et al., 2010; Oswin and Kalista, 2017). The study of Oswin and Kalista (2017) conducted in Bangalala, Kirinjiko, Vumari, and Masandare, reported that, in Tanzania, IC contribute to household income (37–40%), the source of animal protein (30–32%), traditional values (19–22%), social function (11–14%), and manure for garden fertilization (70–80%). In summary, IC have played a crucial role in providing income and improved nutrition in rural households.
The Size and Structure of the Indigenous Chicken Flock
Flock size of IC has played an important role in raising household incomes. Mbuza et al. 2016, in Rwanda, found that small flock sizes limited income in rural households.
In the past 2 decades, several studies have estimated the different typical ratios of adults: growers: chicks ranging from 2:1:1 (Khalafalla et al., 2002; Njue et al., 2002), to 2:1:2 (Babiker et al., 2009). Table 1 shows the flock size and structure of IC in certain countries of East Africa. The average flock size across the 8 countries ranges from 6 to 57.5 chickens per household. The lowest flock size can be observed in Malawi at 12.9, and the highest flock size was in Uganda ranging from 20.5 to 57.5 birds. Except for Uganda, in all other countries, the flock sizes were similar in rural households. The flock structure in function of ratios, cock: hens: pullets: cockerels: chicks in these countries range from 1:3:3:1:5 (in Ethiopia) to 1:6:2:1:5 (in Zimbabwe), 1:4:4:3:6 (in Kenya), and 1:4:5:4:8 (in Uganda). These figures show that the pullets and chicks were the main flock components available at the household, and were supposed to serve as future breeding birds. A higher proportion of cockerels were kept in Kenya and Uganda. This may have been due to the aim of farmers to slaughter or sell them at an advanced age for meat production and/or income generation. Pullets remained at the household for reproduction and egg production for the market or consumption. However, the low number of chicks per sire and the low flock sizes in the region may have been due to the low reproduction of indigenous chicken and the high exploitation (consumption or selling) of live birds for petty cash (Muchadeyi et al., 2007).
Table 1.
The flock size and structure of indigenous chickens in some countries of East African region.
| Country | Flock size (number) | Flock structure (number) |
Reference | |||||
|---|---|---|---|---|---|---|---|---|
| Hens | Cocks | Pullets | Cockerels | Chicks | Chicks+growers | |||
| Rwanda | 9.7–23.3 | 4.8–11.5 | 1.3–2.8 | 2.2–6.2 | 2.2–8.0 | 6.3–15 | - | Hirwa et al. 2019 |
| Ethiopia | 6.23–16 | 2.5–3.0 | 1–1.6 | 2.3-2.72 | 0.9–1.64 | 5.2–5.6 | - | Fisseha et al. 2010; Nebiyu et al. 2013; Zewdu et al. 2013 |
| Malawi | 12.9 | 5.17 | 0.81 | - | - | - | 7.11 | Gondwe and Wollny 2007 |
| Zimbabwe | 8–20.7 | 6–6.8 | 1–1.7 | 0.6–4.0 | 0.7–1.0 | 1.6–8.5 | - | Muchadeyi et al. 2004; Muchadeyi et al. 2007; Mlambo et al. 2011; Nkululeko and Ndiweni 2013 |
| Kenya | 15–23 | 4.32–25.5 | 1.5–6.4 | 4.0–30.1 | 2.32–17.5 | 8.2–40.4 | - | Olwande et al. 2010; Magothe et al. 2012; Okeno et al. 2012; Ochieng et al. 2013 |
| Tanzania | 13.2 | - | - | - | - | - | - | Swai et al. 2007 |
| Uganda | 20.2–57.5 | 6–16 | 2–3 | 8.0–20.5 | 4.0–20.5 | 7.5–37.5 | - | Ssewannyana et al. 2008a |
| Sudan | 18.8–34.0 | - | - | - | - | - | - | Khalafalla et al. 2002; Sayda 2012 |
Indigenous Chicken Production Systems
In characterizing the chicken production systems, some farm components must be determined: the type and number of chickens kept, availability and types of facilities (housing, equipment such as feeders, troughs, nests), relative expenses (feeds, vaccines, and medicines, detergents or antiseptics), practices related to feeds and water provision and type of production (meat or eggs) and the application of biosecurity measures. In addition, the income and production costs were important factors in determining the production system. In the study of Menge et al. (2005), 3 chicken production systems were categorized in Kenya: scavenging (free-range or extensive), semi-intensive, and intensive systems. However, assuming that the conditions of production in Kenya are not different from those in other countries in the region, the factors used to classify the indigenous production systems could be valid in all Eastern African countries. Subsistence or commercial production as reported by Khobondo et al. (2015), affected the kind and level of inputs used for production, farm practices, and types and level of outputs. The scavenging systems, prevalent in rural areas with low human density in households with low income, were characterized by a low number of local chickens (scarcely more than thirty adult chickens) per family (Nzioka, 2000), where the night shelters included human habitats, kitchens, rudimentary coops, or stores (Khobondo et al., 2015). No disease preventive measures or care were provided with them especially when IC were outside of the shelter, which resulted in a greater loss due to predation and disease. In this system the chickens walked around looking for feeds on their own including various seeds, insects, earthworms, and grasses (Birech, 2002) with little to no supplementation provided (Nzioka, 2000), except for supplementation during cropping season when the birds were confined. Supplementation in this system includes kitchen leftovers, maize, and other sources of feeds available at the household level (Khobondo et al., 2015). The purpose of these production systems relied on meat and egg production for subsistence, sociocultural functions and rarely source of income (Njenga, 2005), and the production costs per unit of outputs (eggs or/and meat), were quite insignificant (Birech, 2002). At 50 birds per household, some forms of housing such as simple or proper shelters were provided (Khobondo et al., 2015). These authors reported that this system was dominant in regions of higher human density in rural and periurban areas. The feed resources were scavenging around the homestead or inside a fenced area comprising forage, kitchen waste, and insects (Kingori et al., 2010). The supplementation of water and feeds depended on the commercial value given to chickens, and this system was characterized by low to medium inputs (Khobondo et al., 2015). When improvements were seen in housing and feeding by some supplementation, under this system the production per household increased by decreasing losses due to predation, but also by the increased flock size. Khobondo et al. (2015) characterized the intensive production system as a system with a larger flock size, up to 500 adult birds. A fully confined shelter of deep litter or slatted floor types constructed by the farmer was provided to accommodate these birds (Magothe et al., 2012). Due to the higher level of inputs required and a high level of bird management, this system was predominant in urban and peri-urban zones, practiced by farmers with a shortage of land resources for cropping (Menge et al., 2005). Urban and periurban areas had high demand for chicken products, and the investment in chicken production was a preferred activity for income generation for people able to afford the high costs of required inputs and management. The main feeds used were commercial feeds or home-made feeds accompanied by kales, spinach, cabbage, and other young grasses (Kingori et al., 2010). In an intensive system, high biosecurity and disease control were provided which resulted in lower mortality and higher production in terms of egg production and growth rates (Khobondo et al., 2015).
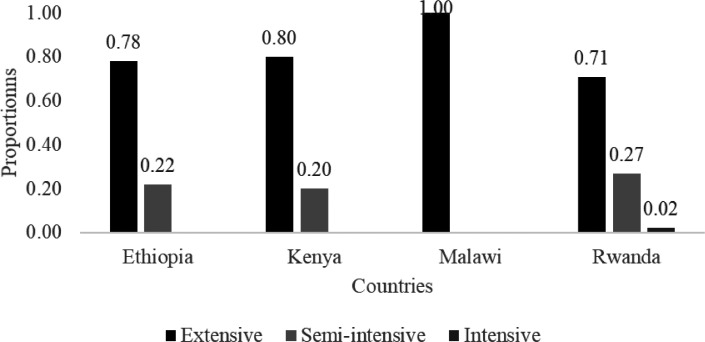
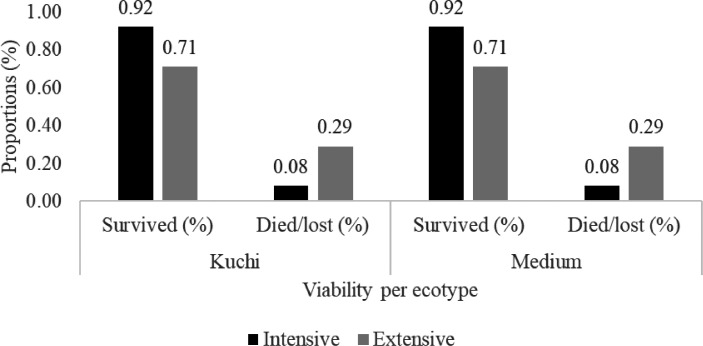
Figure 1 shows the frequency of different indigenous chicken production systems in some countries in the region. A free-range or extensive system of managing chickens is predominant in all countries ranging from 71% in Rwanda to 100% in Malawi. It was followed by a semi-intensive system of raising chickens ranging from 20% of all flocks in Kenya to 27% in Rwanda. These studies help explain the generally typically poor management of IC. Several studies on IC were done in Ethiopia and Kenya which resulted in the improvement of bird management shown by the decrease of extensive systems favoring semi-intensive systems (Gondwe and Wollny, 2007; Zemelak et al., 2016) compared to other countries. These results had a positive impact on the improvement of management especially in terms of feed supplementation and housing. In Ethiopia, Dana et al. (2010) carried out a study in 5 districts; Farta, Mandura, Horro, Konso, and Sheka. They reported that on average 83% of farmers provided a feed supplement to scavenging by the birds. The same practice was observed in the Metekel zone, Northern Ethiopia where 92 and 7% of farmers provided feed supplements in dry and rainy seasons, respectively (Zewdu et al., 2013). In all of the East African countries, where the intensive system was practiced, IC were often reared together with crosses or exotic varieties. In Rwanda intensive systems of management represented only 2% of total number of poultry operations (Mahoro et al., 2017; Hirwa et al., 2019). In some countries, such as Malawi (Gondwe and Wollny, 2007) and Zimbabwe (Mlambo et al., 2011) reported that all farmers practiced the extensive system where it represented 100% of all flocks. However, according to the same authors, in Zimbabwe, 95% of farmers provided shelter for their birds. Taking into account the loss of birds due to diseases, theft, and/or predation there was a significant difference from one management system to another (Figure 2). The study of Lwelamira et al. (2008) in Tanzania showed that the loss in extensive systems varied from 8 to 29% of birds. This study reported no different impacts of production systems on chicken ecotypes. The loss was very similar for the Kuchi and Medium ecotypes with 29 and 8% in the extensive system and intensive system, respectively.
Figure 1.
Indigenous chicken production systems in certain countries in East African region (Adapted from Gondwe and Wollny, 2007; Ochieng et al., 2013; Zemelak et al., 2016 and Hirwa et al., 2019).
Figure 2.
Viability of indigenous chickens managed in intensive and extensive systems in Tanzania (Source: Lwelamira et al., 2008).
This indicated if the management system improved, losses would decrease with the adoption of the intensive system, where improvements were made in feeding, biosecurity, housing, and disease control. This may also increase the survivability of IC and result in the improvement of productivity, as well as income. The bird viability can increase from 71 to 92%. Studies showed in Table 2, the improvement of management conditions were key to increase the production performance of IC. The study of Magothe et al. (2012) in Kenya reported that reproductive and production performances are affected positively by the production systems. Kingori et al. (2010) and Khobondo et al. (2015), in their studies, reported that the values of the age at the first egg in days, number of clutches per year, number of eggs per clutch, annual egg production, adult male body weight (g), and adult female body weight (g) were higher in intensive and semi-intensive systems than their values in the extensive system, showing the importance of improved feeding practice and application of biosecurity measures. However, the extensive system showed high egg hatchability where it reached 84% compared to 74 to 77% for intensive and semi-intensive, respectively.
Table 2.
Reproduction and production performance of indigenous chickens in intensive, semi-intensive and free-range systems in Kenya and Tanzania.
| Country | Performance traits | Intensive system | Semi-intensive system | Free-range system | References |
|---|---|---|---|---|---|
| Kenya | Age at first egg (days) | 166 | 203 | 224 | Magothe et al. 2012 |
| Number of clutches per year | 4 | 3 | 2.5 | ||
| Number of eggs per clutch | 30 | 21.2 | 11.1 | ||
| Annual egg production | 120 | 75 | 40 | ||
| Hatchability (%) | 74.2 | 77 | 84 | ||
| Adult male body weight (g) | 2210 | - | 1,770 | ||
| Adult female body weight (g) | 1,660 | - | 1,320 | ||
| Tanzania | Growth and feed conversion | Sanka and Mbaga 2014 | |||
| Slaughter weight (g) | 1,556 | 1,414 | - | ||
| Average daily gain (g/day) | 11.95 | 9.84 | - | ||
| Feed conversion efficiency (g feed/gain) | 8.25 | 5.49 | - | ||
| Carcass traits | |||||
| Dressing (%) | 65.18 | 65.71 | - | ||
| Breast (%) | 23.56 | 24.93 | - | ||
| Thigh (%) | 17.28 | 17.36 | - | ||
| Drumstick (%) | 15.36 | 15.32 | - |
Magothe et al. 2012 noted that natural incubation with brooding hens was adopted in all production systems. The reason for high hatchability in an extensive system might be due to the approach used for calculations of eggs set or eggs laid. Another reason could be the reliability of the temperature for hatching under a hen, the number of eggs set per brooding hen, and the storage conditions of incubating eggs (Kirunda and Muwereza, 2011). In the extensive system, the exchange of cocks was part of the breeding system. The three systems did not show much difference in the growth performance of IC. In Tanzania, all of the birds managed in different systems showed nearly the same performance in average daily gain and slaughter weight as well as the same carcass yield (Sanka and Mbaga, 2014). The feed intake and growth performance of IC between the free-range, semi-range and intensive systems do not show a great difference (Table 3). The difference observed was due to the feed composition, especially energy content, which decreases feed intake when it increases in the diet (Menge et al., 2005). This means that with the same opportunity to have a balanced diet, the IC should be able to perform equally in all production systems. The challenge appeared to be increasing the flock size and improved management strategies at the household level to improve income. In intensive and semi-intensive systems, it was possible to increase the flock size, but the input costs were considered very high compared to free-range systems where the flock size is small and the efforts of farmers to manage the IC are negligible. Due to these constraints, the intensive and semi-intensive systems were considered economically inefficient as reported by Menge et al. (2005) and Okeno et al. (2012). In their studies on the development modeling of IC in Kenya, they revealed that the production costs in the 2 first systems were too high for most households compared to those in free-range systems. There was an economic loss in the intensive system by small farmers whereas a profit was realized by small farmers in the free-range system. This statement in contrast to the statements above that says the performance was the same in all systems. Here we are talking about the economic performance or economic loss. When the IC are kept in the same conditions across all production systems, there is no big difference in their production performance, but when you try a benefit-cost analysis there is a big difference because you invest more in semi-intensive and intensive systems which render them more inefficient than extensive (scavenging) system. This poor performance was caused by the poor genetic potential of the IC rather than the poor performance of systems. However, further research is needed with a large sample size to determine whether there is a real difference in the performance of IC between different systems and look at the impact of including the improved chickens.
Table 3.
Estimated feed intake, chicken growth performance and economic parameters from simulation model applied to indigenous chicken in intensive, semi-intensive, and free-range systems.
| Performance parameters | Intensive system | Semi-intensive system | Free-range system |
|---|---|---|---|
| Feed intake | |||
| TFI per chick (kg DM) for 6 wk | 1.01–2.14 | 0.83–2.14 | 1.13–2.26 |
| TFI per pullet (kg DM) for 15 wk | 7.63–8.27 | 7.61–11.11 | 8.42–8.64 |
| TFI per cockerel (kg DM) for 15 wk | 7.06–8.24 | 8.20–9.65 | 7.70–8.80 |
| TFI per hen (kg DM)/yr | 44.21–47.71 | 32.72–43.29 | 29.0–44.12 |
| TFI per cock (kg DM)/yr | 46.42 | 45.06 | 45.72 |
| Chicken weights | |||
| Male ADG - 21 wk (g) | 9.96–10.43 | 9.24–9.90 | 9.07–9.23 |
| Female ADG - 21 wk (g) | 9.00–9.14 | 8.61–8.70 | 7.68–8.35 |
| Cockerel LWT - 21 wk (kg) | 1.50–1.56 | 1.39–1.48 | 1.36–1.39 |
| Pullet LWT - 21 wk (kg) | 1.38 - 1.35 | 1.30–1.31 | 1.16–1.26 |
| Cock mature LWT (kg) | 1.63–1.74 | 1.57–1.66 | 1.51–1.57 |
| Hen mature LWT (kg) | 1.43–1.47 | 1.40–1.47 | 1.22–1.36 |
| Economic parameters | |||
| Total revenues (KSh) | 2,577.37 to 7,912.01 | 1,613.85 to 2,766.61 | 1,156.35 to 1,237.35 |
| Total costs (KSh) | 5645.64 to 12,879.08 | 1760.88 to 2,639.47 | 508.83 to 624.46 |
| Profit (KSh) | −3,068.27 to −4,969.50 | −87.04 to 127.14 | 612.86 to 647.52 |
Source: Menge et al., 2005 and Okeno et al., 2012.
Abbreviations: ADG, average daily gain; DM, dry matter; LWT, live weight; Ksh, Kenyan shillings; TFI, total feed intake.
Housing Systems
The chicken house and other required equipment such as feed and water troughs as well as brooders constitute the main assets in chicken production (Ochieng et al., 2011). In many countries of the region, the housing of IC was very basic as has been confirmed by Mlambo et al. (2011) in Zimbabwe. The main reasons farmers stated they provided chicken housing was to ensure the security of the flocks, protect them from the severe weather, thieves, predators, and diseases as well as allowing improved practices of feeding (Mlambo et al., 2011; Ochieng et al. 2011, Magothe et al., 2012). Chicken productivity was higher and income increased in the households able to provide adequate facilities to their birds. Various housing systems of IC can be distinguished depending on the practices in different regions (Table 4). IC across the region were often accommodated in the household with humans ranging from 37% of the flocks in Rwanda (Mahoro et al., 2017) to 84% of flocks in Malawi (Gondwe and Wollny, 2007). This system was predominant and represented 56% of all flocks. In the household, chickens have been noted to have a separate room, or they are covered on the floor by the wooden baskets, perched inside the house or housed on ceilings of the houses (Fisseha et al., 2010; Hailu et al., 2013; Nebiyu et al., 2013; Zewdu et al., 2013). This was supported by an example from the study of Fisseha et al. (2010) in North West Ethiopia, where 46% of flocks have perches inside the family house, 27% covered on the floor by bamboo made-baskets, 4% on ceilings and 1% under sitting facilities. Hailu et al. (2013) also in Ethiopia, in North Wollo, Amhara Region, and Nebiyu et al. (2013) in the Halaba district of Southern Ethiopia reported that 57 and 12%, respectively of the chicken flocks are sheltered overnight in a room inside the family house.
Table 4.
Housing systems of indigenous chickens in some countries of Eastern African Region.
| Country | Household house (%) | In the kitchen (%) | Separate shelter (%) | Other (%) | Reference |
|---|---|---|---|---|---|
| Ethiopia | 76.5 | - | 22.1 | 1.4 | Fisseha et al. 2010 |
| 54 | - | 45 | 1 | Dana et al. 2010 | |
| 55.4 | 12.65 | 15.5 | 16.45 | Hailu et al. 2013 | |
| Kenya | 59 | - | 22.1 | 19.9 | Okeno et al. 2012 |
| Malawi | 84.5 | 8.1 | 7.4 | - | Gondwe and Wollny 2007 |
| Rwanda | 45.8 | - | 47.7 | 6.5 | Mbuza et al. 2016 |
| 37.4 | 40.3 | 13.6 | 8.7 | Mahoro et al., 2017 | |
| Tanzania | 48 | 0.8 | 51.2 | - | Swai et al. 2007 |
| 45.4 | 0.7 | 49.6 | 4.6 | Marwa and Lukuyu 2015 | |
| Zimbabwe | - | 18 | 82 | - | Muchadeyi et al. 2004 |
Other flocks varying from 1% in Tanzania (Marwa and Lukuyu, 2015) to 40% in Rwanda (Mahoro et al., 2017) with an average of 13% were sheltered in the kitchen. Hirwa et al. (2019) observed 3% of households in Eastern province of Rwanda that keep their birds in trees. In Zimbabwe, some chickens housed in the kitchen or granaries were covered with woven baskets (Muchadeyi et al., 2004). The importance of providing a separate house to chickens has been noted in the research. Disease control through the application of biosecurity measures (Mbuza et al., 2016), protection against predators, and realizing a higher profit from IC (Swai et al., 2007) have been the main reasons farmers adopt separate housing for chickens. About 36% of farmers, ranging from 7% in Malawi (Gondwe and Wollny, 2007) to 82% in Zimbabwe (Muchadeyi et al., 2004) house the birds in a separate house or in other facilities to ensure a minimum control of bird health. Tanzania had an average of 50% of farmers report keeping the chickens housed separately from humans in separate housing (Swai et al., 2007; Marwa and Lukuyu, 2015). The research on poultry housing is expected to increase as the studies on IC improved management strategies increase in rural areas.
Poultry housing in East Africa consists of local materials including; bamboo, wooden poles, bricks, branches of trees, mud blocks, thatched roofs and poultry houses with steel sheet roofing (Mapiye and Sibanda, 2005; Zewdu et al., 2013). The materials used were a function of flock size, land available around the main house, capital, ease of cleaning and durability, as well as the level of knowledge and attitude of farmers (Kusina and Kusina, 1999). Chicken houses vary in East Africa depending on the materials available and the types of threats to the health of birds faced in the region (Muchadeyi et al., 2004). They can be either built upon the ground or raised as reported in studies by Kusina and Kusina (1999) and Mapiye and Sibanda (2005). In Zimbabwe, straw or grass, compacted soil, wood, and wood shavings are used for the floors but feed and water troughs are rare (Maphosa et al., 2004). Some farmers provide specific shelters to chicks, that is, cages overnight for a period of 2 wk after hatching (Maphosa et al., 2004) and handwoven basket for broody hens and their chicks (Nebiyu et al., 2013). In most cases, the chickens scavenge during the day and housing facilities are provided during the night. However, this showed the evolution in providing shelter to IC targeting the well-being and the higher production of the birds, as well as efforts to minimize zoonotic disease exposure to household members by living separately from their animals (Mbuza et al., 2016). Thus, further studies on the effects of these housing structures on local chicken productivity are required. For more information on the housing facilities in the region many studies are available: Dana et al. (2010), Fisseha et al. (2010), Hailu et al. (2013), Nebiyu et al. (2013), Zewdu et al. (2013), and Zemelak et al. (2016) in Ethiopia, Ochieng et al. (2011), Okeno et al. (2012) in Kenya, Gondwe and Wollny (2007) in Malawi, Mbuza et al., (2016) and Mahoro et al. (2017) in Rwanda, Swai et al. (2007), Marwa and Lukuyu (2015) in Tanzania, Muchadeyi et al. (2004) and Mlambo et al. (2011) in Zimbabwe. In general, the housing system seems to be a key factor for improving the productivity of chickens, especially aiming in decreasing the losses.
Nutrition and Feeding Systems of Indigenous Chickens
Beyond housing and production systems, there is a strong correlation with improved housing and the need for improved feeding systems to increase productivity. The housing also often determined the feeding system favored. While IC have the capacity to adapt to a harsh environment scavenging for feed, the unanswered question is whether the free-range scavenging system meets the birds nutritional requirements. The chickens have nutritional requirements genetically predetermined (Khobondo et al., 2015). Like other animals, chickens require energy, proteins, minerals, and vitamins for their maintenance and production. These nutrients must be contained in the diet in correct proportions and accessible in sufficient amounts (Khobondo et al., 2015). Before feeding them, especially in intensive and semi-intensive systems understanding their daily requirements and the nutritional value of the feed resources is mandatory. The challenge for many small flock owners in feeding IC is to provide nutrients according to the birds' physiological stages (Khobondo et al., 2015). Table 5 illustrates the energy and protein requirements for different categories of IC kept in different production systems. From the information provided in the table, there appeared to be a great gap in the nutritional requirement of different age categories within each system. However, the nutrient requirements of IC were described by Khobondo et al. (2015), but further studies are required.
Table 5.
Proteins and energy intake of IC of different age categories in scavenging, semi-intensive and intensive systems.
| Categories/Age in weeks | Scavenging |
Semi-intensive |
Intensive |
References | |||
|---|---|---|---|---|---|---|---|
| Proteins (%) | Energy (Kj) | Proteins (%) | Energy (Kj) | Proteins (%) | Energy (Kj) | ||
| From 5 to 8 | - | - | - | - | 17.79 | 365.02 | Chemjor 1998; Kingori et al. 2003 |
| From 8 to 14 | - | - | - | - | 14.36 | 540.09 | |
| From 14 to 21 | - | - | - | - | 13.85 | 832.21 | |
| From 14 to 21 | - | - | - | - | 10.9 | 971 | |
| From 14 to 21 | 8.5 | 910 | - | - | - | - | Kingori et al. 2007 |
| From 13 to 25 | - | - | 11.7 | 949.04 | - | - | |
| Growers and mature birds | 8.5 | 910 | - | - | - | - | Birech 2002 |
| Laying hens (46−54) | - | - | - | - | 9.7 | 1,100 | Kingori et al. 2010 |
| Laying hens (42–50) | - | - | - | - | 14.77 | 949.04 | Kingori et al. 2014 |
Adapted from Khobondo et al. (2015).
The importance of balancing the diet of IC was explained by Magala et al. (2012) in Uganda. They showed a decrease of 42 g in weight gain was realized with an increase of 200 Kcal ME/kg (from 2,800 to 3,000 Kcal) at 18% crude protein (CP). An increase of 2% CP (18–20% CP) with the diet providing 2,800 Kcal/kg decreased the weight of 74 g while it realized a slight increase when the energy increases simultaneously with proteins of 200 Kcal and 2% respectively. This could, however, indicate the importance of an energy-protein balanced diet, a result of a good ration formulation (Khobondo et al., 2015). In the study by Kingori (2004), on the effect of 5 levels of CP (10, 12, 14, 16, and 18%) on 2 feeding parameters (feed intake and feed conversion ratio) together with the bodyweight of IC from 14 to 21 wk of age, reported the increase of both feed intake and live weight gain and a decrease of feed conversion efficiency as the protein level in the diet per kg is increased. However, the inclusion of 16 and 18% didn't show a significant difference in all of the parameters under study, and thereby the conclusion of 16% CP as adequate was drawn. These results were not different from those reported by Ndegwa et al. (2001) suggesting that 17% CP sufficed for good growth rates in IC chickens. It is of great importance to note that the nutrient requirement might vary in function of body size, age, sex, and production systems depending on the production purpose of the farmer (Khobondo et al., 2015). There is no exact feed intake in dry matter (DMI) and energy intake as metabolizable energy (MEI) known for IC especially in scavenging systems. These parameters are estimated using the following formula suggested by Birech (2002):
In the East Africa, as in most of Africa, most IC are reared in an extensive or free-range system in which they wander around looking for feed on their own during the day and accommodated in the shelter provided the night. Green grass, vegetable leaves, leguminous grains, food waste, grains, snails, and insects were the primary scavenged feeds (Kingori 2004; Muchadeyi et al., 2004; Hailu et al., 2013). They have a wide variation in raw materials and nutrient content levels (Birech, 2002), based on place and time (Sonaiya, 2002). Except for crude fiber, they do not regularly meet the nutritional requirements of the chickens (Khobondo et al., 2015). Their dry matter (DM) content was shown to be low in the rainy season resulting in a decrease of chicken productivity due to the restriction of DM intake (Kingori et al., 2007). It is also crucial to mention that during the rainy season the scavenging for feeds was also limited by the time the chickens spend under shelter. The energy supply is critical throughout the year, but limited during the rainy season in contrast to protein supply, which is particularly critical during the dry season (Khobondo et al., 2015). Barua and Yoshimura (1997) categorized the IC as good foragers. This could result in the various sources of pigments (i.e., carotenoids) which play a big role in egg yolk color. Scavenging by IC can usually provide maintenance requirements and a certain low growth rate and/or egg production (Kingori et al., 2014). However, it has been noted that this source of feed was not adequate to satisfy the true nutrient requirements of chickens (Mwalusanya, 1999). Deficits in protein and energy intake were reported by Birech (2002) in Kenya. Some studies were conducted in Africa to increase the quality and biomass of feeds used in free-range systems. Specifically, the production of termites and maggots was a feeding technique that showed potential improvement of the nutritional quality and quantity of feeds used in the scavenging system in Togo and Burkina Faso (Khobondo et al., 2015). These authors mentioned that it was more important to supplement when scavenged feed sources were limited. The scavenging supplementation was adopted by farmers to improve the quality of the diet and to increase the productivity of IC. Mustafa et al. (2012) suggested that during diet formulation, to optimize the profile of amino acids, protein inclusion should go up to 20% respecting the ratio animal to plant protein of 1:1. As the protein sources are the most expensive of all raw materials of poultry diets, the utilization of insects presents great potential as feeds in an extensive production system (Maciorowski et al., 2007). This ingredient constituted a cheaper protein source to meet the chicken protein requirements, whereby it contained a high level of proteins, fat as a source of energy, vitamins, and minerals (Khobondo et al., 2015). The feedstuffs available locally and utilized by IC farmers for protein, energy, vitamins, and mineral supplementation were identified by (Kingori et al., 2014). The ingredients available at the household level for protein supplementation comprised 2 origins: 1) plant origin such oilseed meals (sunflower meal, cottonseed meal, groundnut meal, soya bean meal), corn gluten meal, maize germ, peas, beans; 2) animal origin including meat, blood meal, fish meal, earthworms, and insects. The inclusion of 75% of energy ingredients in diet formulation was recommended. The energy ingredients available at African households for chicken diet supplementation included roots and tubers, millet, maize, sorghum, pollard, maize bran, rice bran, and wheat bran. The supply of vitamins were thought to be provided from weeds, green-young grass, cabbage, kale, spinach, fresh cow dung, and synthetic vitamins, and minerals were supplied by eggshells, bone meal, and commercial minerals. The improvement of feeding practices in the Eastern Africa region is promising (Table 6). The interest in scavenging supplementation is growing as it is seen as an important possible supplement in IC farming in many countries of the East African region where for example 83 to 97.5% (Dana et al., 2010; Fisseha et al., 2010) and 90.5 to 100% (Olwande et al., 2010; Okeno et al., 2012) of farmers in Ethiopia and Kenya respectively provide supplements to their birds.
Table 6.
Feeding practices in some countries of East African region (percentage of farmers).
| Countries | Scavenging only (%) | Scavenging + Supplements (%) | Watering (%) | References |
|---|---|---|---|---|
| Ethiopia | 17 | 83 | - | Dana et al. 2010 |
| 2.5 | 97.5 | 78.9 | Fisseha et al. 2010 | |
| 10.13 | 89.87 | 100 | Hailu et al. 2013 | |
| 7.5 | 92.5 | 92.5 | Zewdu et al. 2013 | |
| Kenya | 0 | 100 | - | Olwande et al. 2010 |
| 9.5 | 90.5 | 95.8 | Okeno et al. 2012 | |
| Malawi | 22.4 | 77.6 | - | Gondwe and Wollny 2007 |
| Rwanda | 67.6 | 32.4 | - | Mbuza et al. 2016 |
| 46.6 | 53.4 | 55.3 | Mahoro et al. 2017 | |
| Zimbabwe | 4 | 96 | - | Muchadeyi et al. 2004 |
| 10 | 90 | 100 | Mlambo et al. 2011 |
The conditions in which the supplements are provided differ. The cropping, rainy, and dry seasons were considered critical periods for feed supplementation (Muchadeyi et al., 2004; Mapiye et al. 2008; Fisseha et al., 2010; Hailu et al., 2013; Zewdu et al., 2013; Mahoro et al, 2017). This could be because during the cropping season the birds were confined at households to protect the crops while in the dry season the scavenging resources were scarce. Particular attention was paid to broody hens with limited time for scavenging and chicks not yet able to satisfy their needs with scavenging feeds (Nebiyu et al., 2013). Another factor governing the supplementation was the type and amount of the crop available in the agroecological zones (Muchadeyi et al., 2007; Okeno et al., 2012; Zemelak et al., 2016). Although there has been some progress, feeding practices in indigenous chicken production encounter many challenges included; limited knowledge of farmers, their awareness and technical know-how (Marwa and Lukuyu, 2015; Mbuza et al., 2016), low availability of feeds and their high cost, competition between human and livestock for feed resources (Mahoro et al., 2017), a sex-limited activity where it was considered the job of women and children (Olwande et al., 2010; Mlambo et al., 2011), spoilage and poor quality of the raw materials due to lack of storage facilities (Marwa and Lukuyu, 2015), supplementation without taking into account of the flock size (Maphosa et al., 2004; Mlambo et al., 2011), and the quality and quantity of water provided to birds (Mlambo et al., 2011). Consequently, under these conditions, IC have been underfed and undernourished so that the production and reproduction performances was limited (Butcher and Miles, 2002; Smith et al., 2005; Mbuza et al., 2016). Strategies for improving the feeding practices was also related to government policy involving all actors and stakeholders concerned in value chain development. Furthermore, the improvement of feeding systems could be associated with the genetic improvement of the chickens for optimizing the efforts and costs of indigenous chicken production for a satisfying benefit by rural farmers.
Breeding and Production of Indigenous Chickens
As explained above, the birds were mostly kept in an extensive system where they have been typically free throughout the day scavenging for feeds. This system affects the breeding and production of local chickens. Due to this system's characteristics, the level of awareness and knowledge of farmers, their advantages and disadvantages, the control mechanism of breeding practices turn out to be complicated. As noted earlier, the IC system has been subsistence-based with low inputs and poor infrastructure and has also been characterized by small flock size (Hailu et al., 2013). The small flock size favors increased inbreeding, possibly causing a loss of fitness (Falconer and Mackay, 1996). In many countries, the inbreeding depression is exacerbated by other factors such as feed shortage, diseases, and predators (Hailu et al., 2013; Khobondo et al., 2015; Mahoro et al., 2017) which together constitute many limiting factors of flock development in rural area. The absence of involvement or active participation of the government and other stakeholders in genetic improvement at the rural household level is another factor which limits the development of the indigenous chicken value chain, rendering it sometimes an ineffective activity and qualified to be practiced by the vulnerable people (women, children, orphans). However, at the rural household level, farmers have their breeding and reproduction practices to maintain their flocks.
Reproduction and Production of ICs
The mating in IC was between the hens or pullets kept by the household farm with either the household cock or the cock from outside the household. Ideally, culling underproductive chickens, those with unwanted characteristics, and retaining the best cock and hen, and preventing the mating with unwanted cock were the mating control criteria used by farmers (Hailu et al., 2013). These authors reported that when the farmer wanted to control the mating using the cock selected for desired traits, this restriction mating was applied for a given number of eggs (3 or more) selected for incubation. After mating, the hens or pullets were able to mate with other cocks met during scavenging. The eggs produced along this period were used in production rather than in reproduction. It is often difficult to differentiate reproduction and egg production in poultry because the eggs can serve both functions. The objective of reproduction has been to have the offspring or a new generation of both sexes to become the future parents (Falconer and Mackay, 1996). Table 7 illustrates the reproduction performance of IC. The sexual maturity age of cockerels and pullets varies between 22 and 40 wk and between 20 and 40 wk, respectively. The average age observed in Kenya for both sexes was 24 to 40 wk and 25 to 40 wk for females and males, respectively (Olwande et al., 2010). These authors reported the poor management and the extra production time wasted before mating (Sonaiya and Swan, 2004) as the cause of the delay of sexual maturity. The birds are mated at an earlier age in Ethiopia where the pullets are mated at 21 to 24 wk and cockerels at 22 to 24 wk age-old (Hailu et al., 2013; Zewdu et al., 2013). The age at first egg laid ranged from 24 to 40 wk. There was no real difference in average age at first laying between the indigenous chicken populations in the countries. Egg fertility in IC varied between 53 and 62% (Shanawany and Banerjee, 1991; Mebratu, 1997; Kingori, 2004; Njenga, 2005) however, some eggs set had a hatchability of 80% (Table 7). The lowest hatchability was observed in Ethiopia in 1997s at the rate of 9%, but a study conducted in 2014 reported a hatchability of 84% (Nebiyu et al., 2014). This data showed significant progress in IC reproduction over two past decades. Kenya had a hatchability of 70 to 84% (Kingori, 2004; Njenga, 2005; Olwande et al., 2010) and Rwanda was 81% (Mahoro et al., 2017), Uganda was 40 to 100% (Kyarishma et al., 2004; Ssewannyana et al., 2008a,b); while Tanzania had a hatchability of 84% (Mwalusanya et al., 2002) and 20 to 70% in Zimbabwe (Pedersen, 2002; Muchadeyi et al., 2004). A low rate of chicks survival (10%) was observed in Kenya in 2010 (Olwande et al., 2010) whereas the highest rate (75–99%) was observed in Uganda in 2008 (Ssewannyana et al., 2008b). Compared to exotic chicken varieties, IC showed a high reproduction potential as confirmed by Katule (1990) and Yami (1995). Lemlem and Tesfay (2010) reported that the hatchability of Rhode Island Red (RIR) and White Leghorn was 39 and 76%, respectively. The genetic potential, high ratio hens/cock, and richness of the scavenge feed were reported as the causal factors of high fertility and egg hatchability in IC in comparison with exotic varieties with a recommended hens/cock ratio of 10:1 (Mwalusanya et al., 2002). Therefore, this reproduction potential of indigenous chicken provides an expectation of a good product when the breeding objective is well defined.
Table 7.
Reproduction performance of indigenous chickens in some countries of East African region.
| Country | Age at first mating male (wk) | Age at first mating female (wk) | Age at first laying (wk) | Number of eggs set | Fertility (%) | Hatchability (%) | Survival (%) | Reproductive life span male (yr) | Reproductive life span female (yr) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Ethiopia | - | - | 23.7–32.8 | - | 56 | 39 | - | - | - | Shanawany and Banerjee 1991 |
| - | - | 23.7–32.8 | - | 53-60 | 9.0–44.4 | - | - | - | Mebratu 1997 | |
| 24.25 | 23.84 | 26.11 | 11.7 | - | 86 | 45 | - | - | Hailu et al. 2013 | |
| 21.76 | 20.88 | - | 11.8–14.74 | - | 78.62–84.74 | - | 3.79 | 3.56 | Zewdu et al. 2013 | |
| - | - | 26 | 12 | - | 83.7 | 52.30 | - | - | Nebiyu et al., 2013 | |
| Kenya | 24–40 | 25–40 | - | - | - | 70.16–80.61 | 9.73–13.43 | - | - | Olwande et al. 2010 |
| 23.71–32.0 | - | - | - | - | - | - | - | Ndegwa and Kimani 1996; Siamba et al. 2000 | ||
| - | - | - | - | 61.8 | 74.2–84.0 | - | - | - | Kingori, 2004; Njenga 2005 | |
| Rwanda | 24 | - | 28 | - | - | - | - | - | - | Mbuza et al. 2016 |
| - | 25.2 | 27.4 | 10.3 | - | 81.5 | 58.30 | - | - | Mahoro et al. 2017 | |
| 23–36 | 23.5–36.3 | 24.6–30.4 | 6.6–14.7 | 52.53–85.3 | Hirwa et al. 2019 | |||||
| Uganda | - | 20–28 | - | - | - | 40–100 | - | - | - | Kyarishma et al. 2004 |
| - | - | 29.2 | - | - | 87.1 | 6.3 (chicks) | - | - | Ssewannyana et al. 2008a | |
| - | - | - | - | - | 66.7–90.2 | 75–99 | - | - | Ssewannyana et al. 2008b | |
| Tanzania | - | - | 24–40 | - | - | 83.6 | 59.7 | - | - | Mwalusanya et al. 2002 |
| Zimbabwe | - | - | 26–30 | 8.0–14.0 | - | - | - | - | - | Kusina and Kusina 1999; Pedersen, 2002 |
| - | - | - | - | - | 20–70 | 20–70 | - | - | Pedersen 2002; Muchadeyi et al. 2004 |
The production performance of IC is presented in Table 8. The number of clutches per hen per year ranges from 2 to 4.3 with an average of 5 to 28 eggs per clutch. The lowest number of eggs per clutch (5 eggs per clutch) was observed in Rwanda (Mbuza et al., 2016), the highest (28 eggs per clutch) observed in Tanzania (Mwalusanya et al., 2002), while the low number of clutches per hen per year (2 clutches) was observed in Tanzania (Mwalusanya et al., 2002), in Uganda (Ssewannyana et al., 2008a) and Kenya (Olwande et al., 2010). The highest (4.3 clutches) was observed in Ethiopia (Zewdu et al., 2013). The number of clutches can differ according to weather conditions. The example is in Tanzania where the number is reduced to 2 clutches per year in wet and cool zones (Mwalusanya et al., 2002). The number of eggs produced per indigenous hen per year varied from 20 to 82 eggs. The IC in Ethiopia showed egg production ranging from 52 eggs to 82 eggs per hen per year (Shanawany and Banerjee, 1991; Mebratu, 1997; Hailu et al., 2013; Zewdu et al., 2013; Nebiyu et al., 2013). The egg production in Uganda and Rwanda seemed to be similar to an average of 35 eggs per hen per year (Kyarishma et al., 2004; Mahoro et al., 2017). These figures indicate the low egg production of IC when compared to that of exotic varieties. White Leghorn and Rhode Island Reds can lay up to 173 and 185 eggs per hen per year when kept in improved local conditions (Lemlem and Tesfay, 2010). The genetic potential, management level, natural incubation, and brooding are the main causes of the low production in IC. The improvement of genetics and management practices could offer some of the fastest improvements to the use of IC. Egg weights varied between 32 and 60 g. The heaviest egg weights (60 g) were observed in Zimbabwe (Muchenje and Sibanda, 1997) while the lighter egg weights (32 g) were observed in Tanzania (Mwalusanya et al., 2002). The adult body weight of cock and hens were between 1.3 and 2.7 kg and between 1.0 and 3.15 kg, respectively. In all countries, cocks were on average heavier than hens. The body weight at the hatch of chicks was approximately 32 to 33 g (Magothe and Kahi, 2010). IC grow slowly, due to their genetic potential and the systems in which they were kept, generally under poor management conditions. The data on growth rate provided in Table 8 are all less than 10.2 g per day observed on immature Leghorn of 10 wk of age (Mwalusanya et al. 2002), however, Kingori (2004) reported that the growth rate of local chickens is similar to that of egg-type crosses. The variability of IC populations resulted in the difference in productivity reported between different ecotypes (Table 9).
Table 8.
Production performance of indigenous chickens in some countries of East African region.
| Country | Clutch size (eggs per clutch) | Number of clutches per hen per year | Number of eggs per hen per year | Egg weight (g) | Body weight male (kg) | Body weight female (kg) | Live weight gain (g/day); at 6–10 wk | Body weight at hatch (g/bird) male | Body weight at hatch (g/bird) female | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Ethiopia | - | - | 54–82 | 44–49 | 1.3–1.7 | 1.0–1.2 | 5.0–6.4 | - | - | Shanawany and Banerjee 1991; Mebratu 1997 |
| 12.64 | 3.62 | 50 | - | - | - | - | - | - | Hailu et al. 2013 | |
| 13.56 | 4.29 | 60 | - | - | - | - | - | - | Zewdu et al. 2013 | |
| 13 | 3.8 | 52 | 39.4 | - | - | - | - | - | Nebiyu et al. 2013 | |
| Kenya | 6.0–16.0 | 2.0–3.0 | - | 37–53 | 1.5–2.5 | 1.0–2.25 | 3.8–4.7 | - | - | Olwande et al. 2010 |
| - | - | - | - | - | - | 4.9–5.2 | 32–33 | 32–33 | Magothe and Kahi, 2010 | |
| 14.8–18.4 | 3.1–3.52 | - | - | 1.97–2.58 | 1.46–2.0 | - | - | - | Okeno et al. 2012 | |
| - | - | - | 49.3–53.9 | - | 1.73–1.87 | - | - | - | Kingori et al. 2014 | |
| Rwanda | 5.0–18.0 | - | - | - | - | - | - | - | - | Mbuza et al. 2016 |
| 16.4–19.6 | 2.5–2.7 | 30–40 | 33.2–47.9 | - | - | - | - | - | Mahoro et al. 2017 | |
| Uganda | 6.0–20 | 2.5–3.0 | 20–50 | 40–50 | 1.5–2.5 | 1.0–1.5 | - | - | - | Kyarishma et al. 2004 |
| 13–15 | 2.0–2.4 | - | - | 1.7–2.4 | 1.2–1.6 | - | - | - | Ssewannyana et al. 2008a | |
| 12–22.8 | - | - | - | - | - | - | - | - | Ssewannyana et al. 2008b | |
| Tanzania | 06–28.0 | 2.0–4.0 | - | 32–57 | - | 1.15–3.15 | 1.2–9.1 | - | - | Mwalusanya et al. 2002 |
| Sudan | - | - | - | 37.9–39.9 | - | 1.2–1.5 | - | - | - | Mekki et al 2005 |
| Zimbabwe | - | - | - | 35–60 | - | - | - | - | - | Muchenje and Sibanda, 1997; Mapiye and Sibanda 2005), |
| - | - | - | - | 2.4–2.7 | 1.5–1.8 | 3.7–4.4 | - | - | Pedersen 2002; Maphosa et al. 2004 |
Table 9.
Reproductive and production performance of some indigenous ecotypes in East African region.
| Trait | Ethiopia |
Sudan |
Kenya |
Reference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tikur | Melata | Kei | Gebsima | Netch | Large Baladi | Bare-neck | Betwi | Normal feathered | Crested -head | Frizzle | Naked neck | ||
| Reproduction | |||||||||||||
| Age at first egg (wk) | 24.7 | 29.1 | 23.7 | 32.8 | 31 | - | - | - | - | - | - | - | Abebe, 1992; Dessie et al., 2000, Wilson, 2010 |
| Fertility (%) | 56 | 60 | 57 | 53 | 56 | - | - | - | - | - | - | - | Mebratu, 1997; Dessie et al., 2000, Wilson, 2010 |
| Hatchability (%) | 42 | 41.8 | 44.3 | 39.3 | 9 | - | - | - | - | - | - | - | Mebratu 1997; Dessie et al. 2000, Wilson 2010 |
| Production | |||||||||||||
| Weight of day-old chick (g) | 27–32 | 35 | 27–32 | 26–31 | 32 | - | - | - | - | - | - | - | Mebratu 1997; Dessie et al., 2000 |
| 32.6 | 33.0 | 33.0 | 33.0 | Mekki et al., 2005 | |||||||||
| Weight at 24 wk (kg) | 0.77–1.35 | 1.48 | 1.0–1.36 | 0.97–1.3 | 1.42 | - | - | - | - | - | - | - | Forssido, 1986; Abebe, 1992;Dessie et al., 2000 |
| - | - | - | - | - | - | - | - | 1.57 | 1.46 | 1.50 | 1.45 | Mekki et al., 2005 | |
| Weight gain at 24 wk (g/day) | 5.2 | 5.4 | 5.0 | 5.1 | 6.4 | - | - | - | - | - | - | - | Shanawany and Banerjee 1991; Wilson 2010 |
| - | - | - | - | - | - | - | - | 7.44 | 6.87 | 7.24 | 7.27 | Mekki et al. 2005 | |
| Adult body weight (kg) | - | - | - | - | - | - | - | - | 1.79 | 1.69 | 1.69 | 1.68 | Mekki et al. 2005 |
| Male adult live weight (kg) | 1.3 | 1.7 | 1.6 | 1.5 | 1.4 | - | - | - | - | - | - | - | Mebratu 1997; Dessie et al. 2000; Wilson\ 2010 |
| Female adult live weight (kg) | 1.0 | 1.2 | 1.2 | 1.1 | 1.1 | - | - | - | - | - | - | - | Mebratu 1997; Dessie et al. 2000; Wilson 2010 |
| - | - | - | - | - | 1.49 | 1.54 | 1.20 | - | - | - | - | Mekki et al. 2005 | |
| Feed intake (kg/bird/yr) | 50.9 | 53.2 | 37 | 36.4 | 39.1 | - | - | - | - | - | - | - | Shanawany and Banerjee 1991; Wilson 2010 |
| Feed conversion efficiency (g DM/g gain) | 4.17 | 3.57 | 3.45 | 4.00 | 3.70 | - | - | - | - | - | - | - | Shanawany and Banerjee 1991; Wilson 2010 |
| Carcass weight (kg) at 24 wk | 0.54 | 0.56 | 0.54 | 0.51 | 0.61 | - | - | - | - | - | - | - | Shanawany and Banerjee 1991; Wilson, 2010 |
| Dressing (%) | 56.4 | 56.0 | 57.8 | 53.8 | 51.5 | - | - | - | - | - | - | - | Shanawany and Banerjee 1991; Wilson 2010 |
| Eggs /hen/yr | 64 | 82 | 54 | 58 | 64 | - | - | - | - | - | - | - | Mebratu 1997; Dessie et al. 2000, Wilson 2010 |
| Egg weight (g) | 44 | 49 | 45 | 44 | 47 | - | - | - | - | - | - | - | Mebratu 1997; Dessie et al. 2000 |
| - | - | - | - | - | 38.46 | 39.89 | 37.95 | - | - | - | - | Mekki et al. 2005 | |
| Egg shell thickness (micron) | - | - | - | - | - | 34.32 | 36.21 | 36.21 | - | - | - | - | Mekki et al. 2005 |
Reproduction and Production Performance of Various Indigenous Chicken Populations
Sexual maturity was not similar among different indigenous ecotypes. This may be due to genetic variability and environmental conditions. In Ethiopia, Kei and Tikur are qualified as early ecotypes and lay their first eggs at 23.7 and 24.7 wk of age respectively, contrary to Melita, Netch, and Gebsima ecotypes, which seem to be later maturing (Abebe, 1992; Dessie et al., 2000; Wilson, 2010). Gebsima ecotype was characterized by low egg fertility (0.53) comparing to its counterparts while the high fertility is observed in the Melita ecotype with 0.60 (Mebratu, 1997; Dessie et al., 2000; Wilson, 2010). A low hatchability was present in the Netch ecotype at 9% followed by Gebsima 395 while other ecotypes does not show a difference varying from 42 to 44% (Mebratu, 1997; Dessie et al., 2000, Wilson, 2010).
After hatching, the weight of day-old chicks between Tikur, Kei, and Gebsima ecotypes were quite similar (around 29 g) while the day-old chicks of Netch and Melita weighed 32 and 35 g, respectively (Mebratu, 1997; Dessie et al., 2000). At 24 wk of age, Tikur, Kei and Gebsima ecotypes had a similar body weight of about 1.1 kg while Melita and Netch ecotypes had the same weight of about 1.4 kg (Forssido, 1986; Abebe, 1992; Dessie et al., 2000). In Tanzania, at the same age, a study showed that there was no difference between the bodyweight of Betwi, Normal feathered (nana), Crested-head, Fizzle ecotypes and Naked neck (Nana) crosses (Mekki et al., 2005) which is around 1.5 kg. This bodyweight is quite similar to that of Melita and Netch in Ethiopia. The growth rate among ecotypes did not greatly differ in each of these 2 countries. However, the Kenyan IC showed a higher growth potential than Ethiopian chickens. The growth rate of Ethiopian chickens was about 2 g per day less than that of Kenyan chickens (Shanawany and Banerjee, 1991; Mekki et al., 2005; Wilson, 2010). The adult body weight was also a little higher in Kenyan chickens with an average of 1.7 kg for males (Mekki et al., 2005) while that of Ethiopian chickens ranged gradually from 1.4 to 1.7 (Mebratu, 1997; Dessie et al., 2000; Wilson, 2010). The growth potential of Melata and Kei was not different from that of Kenyan ecotypes. Tikur was the lighter ecotype with male adult body weights at 1.3 kg. Females were lighter than males but they did not present the same weight among different ecotypes. Figures in Table 9 show that females in Ethiopia weighed 1.0 to 1.2 kg body weight (Mebratu, 1997; Dessie et al., 2000; Wilson, 2010) and 1.2 to 1.5 kg for Sudanese ecotypes (Mekki et al., 2005). The Sudanese females were heavier than females in Ethiopia. The feed intake was positively correlated with growth rate but the Gebsima and Tikur ecotypes were not efficient in feed utilization due to their high feed conversion coefficients of 4.0 and 4.2 g of DM feed/g of gain (Shanawany and Banerjee, 1991; Wilson, 2010). Therefore, the ecotypes did not greatly differ in carcass yield, which was approximately 0.54 kg. except Necth, which represented a little higher yield of 0.61 kg. For egg production, the Melata ecotype showed the capacity of laying 82 eggs and the Netch 54 eggs per hen per year (Mebratu, 1997; Dessie et al., 2000; Wilson, 2010). The egg weight was not different among the ecotypes in the same country but the eggs laid by Ethiopian ecotypes were heavier than those laid by Sudanese ecotypes while the eggshell thickness was similar in all ecotypes.
Health Management of Indigenous Chickens
According to Mwale and Masika (2009) and Kaingul et al. (2010), the management of poultry health was a key challenge of poultry development in smallholder farming. The high mortality rates and the decrease in the production performance of birds were the major consequences of poor health management as reported by Kusina et al. (2001) and Pedersen (2002). Infectious diseases, parasites, and predators were the main causes of high mortalities in IC. Poor quality and the low quantity of feeds, as well as poor farm management practices increased chicken mortality in the rural farming systems (Mbuza et al., 2016; Zemelak et al., 2016). Most of these causes were influenced by the climate conditions that favored or hindered the development of infectious agents (Kaingul et al., 2010). Evidence was also provided by these authors of the effect of temperature on the development of parasites (helminths). Internal parasites such as worms (helminths), protozoa (coccidia), viruses, and bacteria were the primary infectious diseases in poultry production. Mutinda et al. (2013) reported higher mortality in IC caused by Gumboro disease in Kenya compared to exotic varieties. This confirmed what was observed by Okoye and Aba-Adulugba, (1998) in Nigeria where the local chickens were more susceptible to this disease compared to exotic varieties of birds. Higher mortality of IC compared to the White Leghorn reared under intensive management was reported (Forssido, 1986; Abebe, 1992), where it was thought to be that local birds were not adapted to intensive conditions (Dessie et al., 2000). These authors also reported in their study the higher incidence of coccidiosis in IC under confinement compared to exotic varieties. The most prevalent diseases reported to be in the region were Newcastle (MoALD and Marketing, 1996; Njue et al., 2006; Babiker et al., 2009; Hunduma et al., 2010; Magothe et al., 2012; Zemelak et al., 2016; Mahoro et al., 2017), infectious bursal disease (IBD, Mutinda et al., 2013), coccidiosis caused by protozoa (Babiker et al., 2009; Kaingul et al. 2010; Magothe et al., 2012; Mahoro et al., 2017), gastroenteric parasites known as helminths (Kaingul et al. 2010; Magothe et al., 2012), fowl pox (Kingori et al., 2010), fowl typhoid (Magothe et al., 2012), salmonellosis (Babiker et al., 2009; Magothe et al., 2012; Mahoro et al., 2017) infectious coryza and pullorum (Magothe et al., 2012) and pests such as mites, fleas, and lice (Kingori et al., 2010) have been reported as other causes of high mortalities in IC. Predators were also reported to be the cause of high poultry losses especially in IC (Babiker et al., 2009; Mbuza et al., 2016). The management of each of the above health-compromising factors as noted below must be specific and systematic, as they occur in different seasons, and different management systems
Newcastle Disease
Newcastle disease (NCD) was the most prevalent disease that caused high mortality in IC in many countries (MoALD and Marketing, 1996; Sonaiya and Swan, 2004; Ssewannyana et al., 2008b; Nwanta et al., 2008; Dana et al. 2010; Moreki, 2010; Yakubu 2010; Magothe et al., 2012; Mahoro et al., 2017). This disease was considered severe and caused higher losses during the rainy season compared to the dry season. The seasonality of Newcastle disease was reported by Hunduma et al. (2010) with bird mortality rates as high as 80% in the rainy season. These results were confirmed by Nebiyu et al. (2013) who reported severe mortality rates of 75.4% in the rainy season and that of 24.6% in the dry season. In contrast, a situation was observed in Kenya where the NCD occurs prevalently in the dry season (Magothe et al., 2012). The reason given was that during this period, the dry conditions favor the spread of NCD virus and contamination by the higher movement of IC during a festival period (Nwanta et al., 2008). The spread NCD virus was transmitted from birds of one household to other birds of another when infected chickens were used in various parties. Vaccination was considered an effective measure used to control the NCD and the vaccines were widely available through veterinary services. However, the severity of the disease in poultry smallholder farming was influenced by the low number of farmers who did vaccinate their birds, and many who were not aware the vaccination was used to control the disease (Njue et al., 2006; Kingori et al., 2010; Okeno et al., 2012). Therefore, the vaccination programs should take into account the season, thereby vaccinating the chickens allowing them to develop the immune response prior to the disease outbreak season (Okeno et al., 2012). Vaccination programs should be backed by strong extension services to help farmers understand the importance of vaccination and facilitate them in finding vaccines more easily. When the disease appears in the flock, most farmers try to treat the infected birds while some at least try to isolate them (Nebiyu et al., 2013). Beyond vaccinations used as a conventional measure to control the NCD, some farmers used traditional medicine to cure the disease when birds were infected or showed the symptoms. In Ethiopia, the common traditional medicines used to treat the NCD were table oil, tobacco leaf, lemon juice, pepper, salt and onion, and alcoholic drink mixed with drinking water (Nebiyu et al., 2013; Zemelak et al., 2016). Other common plants used in NCD treatment were Aloe vera, milkweed, and croton as reported by Ndegwa et al. (1998) and Njenga (2005). The efficacy of these traditional medicines was largely unknown. The exception was the Aloe secundiflora, which was recommended to farmers after having been tested for its effect on internal parasites and revealed to have an inhibitor effect of larval development of Ascardia galli in IC (Kaingul et al., 2010). In Ethiopia, bleeding the wings of infected birds was used as a traditional treatment of NCD (Mengesha and Tamir, 2011). Two varieties of Aloe; Aloe secundiflora and Aloe ferox were used to treat NCD in Kenya, but were often used to treat any kind of diseases in rural poultry production (Okitoi et al., 2007; Mwale and Masika 2009; Kaingul et al. 2010; Okeno et al., 2012). In Rwanda 93% of the local farmers, during the NCD outbreaks, intervene with burning feathers on the head, bleeding from wing vein, and using green pepper to treat the disease while 7% only rely on conventional medicine (Mahoro et al., 2017). As the majority of small poultry farmers in all East African countries confirm the efficacy of ethnoveterinary medicine, further research is needed to explore the composition and efficacy of the traditional medicines on the disease treatment of NCD.
IBD or Gumboro Disease
Infectious bursal disease (IBD) commonly known as Gumboro was found to cause high mortality in IC. It was more severe in lighter varieties compared to heavier varieties (Bumstead et al., 1993; Abdul, 2004). Among exotic varieties, the White Leghorn was reported to be more susceptible to Gunboro disease (Bumstead et al., 1993). Okoye and Aba-Adulugba (1998) reported that ICs were more susceptible to IBD than exotic varieties. The susceptibility and high mortality of IC were confirmed in Egypt by Hassan et al. (2002) who reported a mortality rate of 20% caused by IBD in White Leghorn and 11 to 85.3% IC. In Bangladesh, the Fayoumi ecotype was reported to be much more susceptible than White Leghorn (Chakraborty et al., 2010). A survey conducted in Kenya by Mutinda et al. (2013) reported the high susceptibility of IC with mortality rates up to 100% in some outbreaks. In comparison between the IC and crosses, Okoye et al. (1999) and Oluwayelu et al. (2002) reported the equal susceptibility between the 2 genotypes, but the mortality rate was higher in IC. These observations show how the severity of this disease depended on the chicken genotype susceptibility and the virulence of the pathogen (Mutinda et al., 2013). Vaccination was the only measure that could be adopted for IBD control. However, the efficacy of this method presented a challenge in rural areas and often leads to nonsatisfying outcomes. The mortality rates observed in vaccinated birds differed due to various factors. Babiker et al. (2009) reported the preservation of vaccines, vaccine inocula preparation related-problems, timing, and efficacy of the vaccination procedures as the factors of vaccination failing against IBD.
Other Infectious Diseases
Other infectious diseases frequently seen in IC include salmonellosis, fowl pox, and fowl typhoid. All of these diseases occur during wet or rainy seasons when most of the chickens infected suffer great losses (Dana et al., 2010; Moreki, 2010; Yakubu, 2010; Magothe et al., 2012). Salmonellosis was the disease that was reported to have a high incidence in chicken production. A study by Babiker et al. (2009) reported that at least 4 of 10 flocks were infected by salmonellosis while, according to Mahoro et al. (2017), 15% of farmers reported the infection in their flocks. The high prevalence of this disease was influenced by poor hygiene and it was found in almost all premises and instruments used for different practices in poultry farms (Babiker et al., 2009). Sasipreeyajan et al. 1996 showed that 42% of salmonella can be isolated from chicken litter, 36% from drinking water, and 28% from feed troughs. Salmonella were not only horizontally transmitted but also vertically (Babiker et al., 2009). The control of salmonellosis and fowl typhoid could be based on improved management practices mainly promoting hygiene. The routine administration of pharmaceuticals such as antibiotics can serve as a preventive measure and these drugs can also be used when the birds are infected (Kingori et al., 2010). This author indicated that regarding fowl pox, vaccination was the chief measure of prevention. However, vaccination should be only one part of improved chicken management.
Parasitic Diseases
Among parasitic diseases, 2 types can be distinguished: endoparasite borne diseases (coccidiosis and helminthiasis) and ectoparasite borne disease (including lice and mites). Coccidiosis causes some of the highest economic loss in poultry production. The most common coccidiosis comprises the cecal or bloody coccidiosis caused by Eimeria tenella, bloody intestinal coccidiosis caused by E. necatrix, and chronic intestinal coccidiosis caused by E. acervulina (Murray, 2001). These protozoa can develop and complete its life cycle in the presence of a range of temperatures even at extreme temperatures (Kaingul et al., 2010). It was found to be serious diseases which cause, when affected the flock, with high mortality of birds leading to considerable economic loss in poultry production (Nakamura et al., 1990). Coccidiosis was found to be most severe under confinement conditions (Dessie et al., 2000) and more frequently in the wet season (Magothe et al., 2012) causing a more serious economic loss in IC compared to exotic varieties (Dessie et al., 2000). Coccidiosis is often associated with the other internal parasites such as helminths. Helminths were the other endoparasites causing enormous but silent economic losses. Helminthes with coccidia were reported as the most important internal parasites in poultry production in Kenya (Magothe et al., 2012). Mungube et al. (2008) reported the infestation of 93% of adult IC by at least one type of helminth in the semi-arid region of Kenya. The infestation in all agroecological zones (highlands, midlands, and lowlands) was reported by Kaingul et al. (2010). It was also revealed that helminths infest at a high level in all categories of chickens. Adult chicken, growers, and chicks were infested by the helminths at the rate of 93, 95, and 71%, respectively (Ondwassy et al., 2000). These parasites in poultry production cause severe losses, because most farmers do not take any control measure due to lack of their awareness of the existence of helminths in their flocks (Ndegwa et al., 1998). In scavenging systems, the preventive measures are relatively difficult, especially for coccidiosis. The long distances traveled by the birds and unlimited contacts with those from different flocks increase exposure. From this, we could say that the confinement system can reduce contamination and infestation. Even if the systems increase the chance of contamination among birds in the flock, the propagation and spreading of parasites could be decreased by the limitation of the movement. The management conditions must be improved and backed by good hygiene in the houses, application of biosecurity measures, and prophylactic measures such as regular and systematic deworming. These measures can also be taken to prevent external parasites. Most of them reported by farmers comprise pests. The common pests frequent in the region include fleas, mites, lice, and ticks (Njenga, 2005; Halima et al., 2007; Kingori et al., 2010; Magothe et al., 2012). Although there have been no studies conducted on the prevalence of external parasites and their effects on IC production (Magothe et al., 2012), there is no doubt that they may cause considerable economic loss due to the decrease in bird production performance. Magothe et al. (2012) reported the lack of effort of farmers in pest control although they are aware of the existence of external parasites. When the control measures applied especially when the birds are infested, they include insecticide spray (Kingori et al., 2010), smoke of leaves of Eucalyptus tree inside the chicken house (Zemelak et al., 2016), the use of ash, paraffin oil, and pesticides (Magothe et al., 2012). However, the control of external parasites could be easily accomplished through good sanitation in the chicken shed, and regular dusting with insecticide spraying (Kingori et al., 2010) providing an easy, and affordable measure for rural farmers.
Predators
Most farmers mentioned great losses of chickens to predators (Mahoro et al., 2017) and ranked them as the primary limitation in IC production (Mekonnen, 2007; Babiker et al., 2009; Nebiyu et al., 2013). The predators reported causing considerable losses in IC include; rats, dogs, wild cats (Babiker et al., 2009; Nebiyu et al., 2013), foxes, hawks (Nebiyu et al., 2013), snakes (Regassa et al., 2007), and eagles (Aberra, 2000). The presence of predators depends on the environmental conditions, including the seasons. It was reported that in Ethiopia, eagle and hawks caused the highest losses during the dry season, while during the rainy season the most dangerous predator was the wild cat (Aberra, 2000). Chicken confinement, scarecrows, and trap nets were the main strategies used for preventing predators (Mbuza et al., 2016). Farmers mentioned the effect of plumage color on the attraction of predators and easy tracking. However, during breeding, farmers selected against individuals with white plumage because it was thought to facilitate the predators ability to track the chickens and increase predation (Zemelak et al., 2016). Health management in IC production could be considered one of the important pillars for its development. Farmers could improve measures to prevent disease outbreaks and predators. The movement allowing contact with wild animals could be restricted as it makes it difficult to control the diseases and predators in small household farms (Mapiye et al., 2008). It was reported that collaboration and communication between rural poultry farmers and veterinary or extension services are scarce (Muchadeyi et al., 2004), however, the delivery of veterinary services to rural poultry farming could serve to prevent and treat diseases. Further research on ethnoveterinary medicine is required, in collaboration with pharmacists, to determine the composition and doses of traditional medicines, especially Aloe sp to be recommended to farmers for animal diseases treatment. Chaheuf (1990) suggested the development of the IC health program as the tool of a network that could be used to share reliable information between stakeholders about the disease epidemiology and reducing the outbreaks. The sensitization of farmers and their training on records keeping support this network, as it was noted that most farmers of IC do not keep and manage the farming record (Mapiye et al., 2008; Mbuza et al., 2016). The research on the role played by feather colors reported by farmers to help birds resistant to diseases such as greyish and reddish-brown (Dessie et al., 2000) and to reduce the risk at predation (Zemelak et al., 2016) is needed. Therefore, a breeding program with the breeding objective of plumage color should consider these qualitative and other quantitative traits of fitness (Falconer and Mackay, 1996). Note that, all these interventions are associated with the maintenance of good sanitation at farm level and hygiene of all materials and equipment as primordial attempting to further way of improving the IC productivity and genetic resources conservation.
CONCLUSIONS
The East African region hosts a high number of IC populations presenting great socioeconomic importance of small household farmers. These populations show a high phenotypic variability. This variability explains their different production characteristics and potentials but with the same quality of products (eggs and meat). All family members participate in their management but predominated by women. The development of these chickens is hampered by the system of their management predominated by scavenging systems with poor conditions including especially feeding, housing, and health. Traditional methods sometimes combined with conventional methods are mostly used to control diseases. Due to their potential, the exploration of strategies for improvement supported by the details on their genetic variability and adaptation to their different management conditions is needed.
Acknowledgments
ACKNOWLEDGMENTS
The authors wish to thank the Strengthen for Education in Agriculture (SEAD) project for their financial support and the staff from University of Rwanda (UR) and Kwame Nkrumah University of Science and Technology (KNUST) for their support along the drafting of this paper.
Authors’ contributions: Valentin Mujyambere: Conceptualization, Methodology, Data curation, Writing - Original Draft preparation. Kwaku Adomako: Validation, Review & Editing, Supervision. Simon O. Olympio: Validation, Supervision. Martin Ntawubizi: Validation, review. Laetitia Nyinawamwiza: Validation, review. Janvier Mahoro: Validation, review. Andrew Conroy: Validation, Review & Editing.
DISCLOSURES
The authors have nothing to disclose.
REFERENCES
- Abdul A. Royal Veterinary and Agricultural University; Frederiksberg, Denmark: 2004. MSc Thesis. [Google Scholar]
- Abebe H. Alemaya Univ. Agriculture; Dire Dawa, Ethiopia: 1992. Mimeographed Report. [Google Scholar]
- Aberra M. Martin-Luther Univ.; Halle- Wittenberg, Germany: 2000. Ph.D. Diss. [Google Scholar]
- Alders R.G., Pym R.A.E. Village poultry: still important to millions, eight thousand years after domestication. Worlds Poult. Sci. J. 2009;65:181–190. [Google Scholar]
- Babiker M.S., Kijora C., Abbas S.A., Danier J. Nutrient composition of main poultry feed ingredients used in sudan and their variations from local standard tables values. Int. J. Poult. Sci. 2009;8:355–358. [Google Scholar]
- Barua A., Yoshimura Y. Rural poultry keeping in Bangladesh. Worlds Poult. Sci. J. 1997;53:387–394. [Google Scholar]
- Birech E.K. Egerton University; Njoro Kenya: 2002. MSc. Thesis. [Google Scholar]
- Bumstead N., Reece R.L., Cook J.K.A. Genetic differences in susceptibility of chicken lines of infection with infectious bursal disease virus. Poult. Sci. 1993;72:403–410. doi: 10.3382/ps.0720403. [DOI] [PubMed] [Google Scholar]
- Butcher, G.D., and R.D. Miles. 2002. Interrelationship of nutrition and immunity, University of Florida IFAS Extension. Accessed Oct. 2018. http://edis.ifas.ufl.edu.
- Bwalya R. An analysis of the Value Chain for indigenous chickens in Zambia's Lusaka and Central Provinces. J. Agric. Stud. 2014;2:32–51. [Google Scholar]
- Chaheuf N. Pages 129–137 in Disease prevention in smallholder village poultry production in Africa. Proc. of CTA seminar on smallholder rural poultry production, 9-13 October 1990; Thessaloniki, Greece, Vol. 1; 1990. [Google Scholar]
- Chakraborty P., Nath B.D., Islam R.M., Das P.M. Comparative susceptibility of fayoumi, indigenous and white leghorn chicks to infectious bursal disease. ARPN J. Agric. Biol. Sci. 2010;5:27–34. [Google Scholar]
- Chemjor W. Egerton University; Njoro, Kenya: 1998. M.Sc. Thesis. [Google Scholar]
- Dana N., Van Der Waaij L.H., Dessie T., Van Arendonk J.A.M. Production objectives and trait preferences of village poultry producers of Ethiopia: implications for designing breeding schemes utilizing indigenous chicken genetic resources. Trop. Anim. Heal. Prod. 2010;42:1519–1529. doi: 10.1007/s11250-010-9602-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessie T. Swedish Univ. Agr. Sci.; Uppsala, Sweden: 1996. MSc. Thesis. [Google Scholar]
- Dessie T. Humboldt Univ. Berlin; Germany: 2003. Ph.D. thesis. [Google Scholar]
- Dessie T., Alemu Y., Peters K.J. Indigenous chicken in Ethiopia: genetic potential and attempts at improvement. Worlds Poult. Sci. J. 2000;56:45–54. [Google Scholar]
- Dessie T., Ogle B. Village poultry production system in the central highlands of Ethiopia. Trop. Anim. Health Prod. 2001;33:521–537. doi: 10.1023/a:1012740832558. [DOI] [PubMed] [Google Scholar]
- Ekue F.N., Pone K.D., Mafeni M.J., Nfi A.N., Njoya J. Pages 15–25 in Characteristics and Parameters of Family Poultry Production in Africa. IAEA.; Vienna: 2002. Survey of the traditional poultry production system in the Bamenda area, Cameroon.http://www-naweb.iaea.org/nafa/aph/public/aph-poultry-africa.html Accessed Aug. 2018. [Google Scholar]
- F.A.O . Rome; Italy: 2000. Statistical Database of Food and Agriculture Organization of the United Nations. [Google Scholar]
- Falconer D.S., Mackay T.F.C.F. 4th ed. Longman; Harlow, UK: 1996. Introduction to Quantitative Genetics. [Google Scholar]
- F.A.O. STAT . F.A.O; Rome, Italy: 2007. Food and Agriculture Organization Statistical Databases. [Google Scholar]
- Fisseha M., Aberra M., Dessie T. Assessment of village chicken production system and evaluation of the productive and reproductive performance of local chicken ecotype in Bure district North West Ethiopia. Afr. J. Agric. Res. 2010;5:1739–1748. [Google Scholar]
- Forssido T. J.L. University of Giessen; Germany: 1986. PhD Thesis. Studies on the meat production potential of some local strains of chickens in Ethiopia (cgiar.org). Accessed October 2018. [Google Scholar]
- Gondwe T.N., Wollny C.B.A. Local chicken production system in Malawi: household flock structure, dynamics, management, and health. Trop. Anim. Health Prod. 2007;39:103–113. doi: 10.1007/s11250-006-4293-8. [DOI] [PubMed] [Google Scholar]
- Goodger W.J., Bennett T.B., Dwinger R.H. Pages 33–37 in Characteristics and Parameters of Family Poultry Production in Africa. IAEA; Vienna: 2002. Comparative analysis of family poultry production in twelve African countries.http://www-naweb.iaea.org/nafa/aph/public/aph-poultry-africa.html Accessed Aug. 2018. [Google Scholar]
- Guèye E.F. Village egg and fowl meat production in Africa. Worlds Poult. Sci. J. 1998;54:73–86. [Google Scholar]
- Guèye E.F. Women and family poultry production in rural Africa. Dev. Pract. 2000;10:98–102. doi: 10.1080/09614520052565. [DOI] [PubMed] [Google Scholar]
- Hailu A., Hailu M., Zewdu W. Indigenous chicken production system and breeding practice in North Wollo, Amhara Region, Ethiopia. Poult. Fish Wildl. Sci. 2013;1:108. [Google Scholar]
- Halima H., Neser F.W.C., Van Marle-koster E., De Kock A. Phenotypic variation of native chicken populations in northwest Ethiopia. Trop. Anim. Health Prod. 2007;39:507–513. doi: 10.1007/s11250-007-9032-2. [DOI] [PubMed] [Google Scholar]
- Hassan M.K., Afify M., Aly M.M. Susceptibility of vaccinated and unvaccinated Egyptian chickens to very virulent infectious bursal disease virus. Avian. Pathol. 2002;31:149–156. doi: 10.1080/03079450120118630. [DOI] [PubMed] [Google Scholar]
- Hirwa C.D.A., Kugonza D.R., Kayitesi A., Murekezi T., Semahoro F., Uwimana G., Habimana R. Phenotypes, production systems and reproductive performance of indigenous chickens in contemporary Rwanda. Int. J. Livest. Prod. 2019;10:213–231. [Google Scholar]
- Hunduma D., Regassa C., Fufa D., Endale B., Samson L. Major constraints and health management of village poultry production in Rift Valley of Oromia, Ethiopia. Am-Eurasian J. Agric. Environ. Sci. 2010;9:529–533. [Google Scholar]
- Kaingul F.B., Kibor A.C., Shivairo R., Kutima H., Okeno T.O., Waihenya R., Kahi A.K. Prevalence of gastro-intestinal helminths and coccidia in indigenous chicken from different agro-climatic zones in Kenya. Afr. J. Agricu. Res. 2010;5:458–462. [Google Scholar]
- Katule A.M. The Sokoine Univ. Agriculture; Morogoro, Tanzania: 1990. Ph.D. Thesis. [Google Scholar]
- Kaudia, T.J., and A.J. Kitalyi. 2002. Commercializing rearing of village chicken in Kenya, E.F. Guéye, (Ed). The Second INFPD/FAO Electronic Conference on The Bangladesh Model and Other Experiences in Family Poultry Development. Accessed July 2018. http://www.fao.org/ag/AGAinfo/themes/en/infpd/documents/econf_bang/
- Khalafalla, A.I., S. Awad, and W. Hass. 2002. Village poultry production in Sudan. Pages 87–93 in Characteristics and Parameters of family Poultry Production in Africa. IAEA. Vienna Accessed Aug. 2018. http://www-naweb.iaea.org/nafa/aph/public/aph-poultry-africa.html
- Khobondo J.O., Muasya T.K., Miyumo S., Okeno T.O., Wasike C.B., Mwakubambanya R., Kingori A.M., Kahi A.K. Genetic and nutrition development of indigenous chicken in Africa. Livest. Res. Rural Dev. 2015;27 http://www.lrrd.org/lrrd27/7/khob27122.html Accessed July 2018. [Google Scholar]
- Kingori A.M., Tuitoek J.K., Muiruri H.K., Wachira A.M. Protein requirements of growing indigenous chicken during the 14-21 weeks growing period. S. Afr. J. Anim. Sci. 2003;33:78–82. [Google Scholar]
- Kingori A.M. Egerton Univ.; Kenya: 2004. Ph.D. thesis. [Google Scholar]
- Kingori A.M., Tuitoek J.K., Muiruri H.K., Wachira A.M., Birech E.K. Protein intake of growing indigenous chicken on free-range and their response to supplementation. Int. J. Poult. Sci. 2007;6:617–621. [Google Scholar]
- Kingori A.M., Wachira A.M., Tuitoek J.K. Indigenous chicken production in Kenya: a review. Int. J. Poult. Sci. 2010;9:309–316. [Google Scholar]
- Kingori A.M., Wachira A.M., Tuitoek J.K. Influence of energy intake on egg production and weight in indigenous chickens of Kenya. Int. J. Poult. Sci. 2014;13:151–155. [Google Scholar]
- Kirunda H., Muwereza N. Evaluation of options for improving hatchability in indigenous free-range chickens in Eastern Uganda. Livest. Res. Rural Dev. 2011;23 [Google Scholar]
- Kusina, J. F., and N.T. Kusina. 1999. Feasibility study of agricultural and household activities as they relate to livestock production in Guruve District of Mashonaland Central Province with emphasis on village chicken production. Page 129 in Report Prepared for Household Agricultural Support Programme, Harare, Zimbabwe.
- Kitalyi A.J. In: R. de Jong and E.A. Mukisira (Editors), P. 56-62 in the First INFPD/FAO Electronic Conference on family Poultry. KARI; Nairobi, Kenya: 1999. Family poultry management systems in Africa. [Google Scholar]
- Kusina J.F., Kusina N.T., Mhlanga F. Pages 148–155 in Proceedings of SADC planning workshop on Newcastle disease control in village chickens, Maputo, Mozambique, 6-9 March 2000, Australian Center for International Agricultural Research, Canberra Proceedings 103. 2001. A survey on village chicken losses: causes and solutions as perceived by farmers (Ed. R. G. Alders and P. B Spradbrow) [Google Scholar]
- Kyarishma C.C, Kugonza D.R., Twesigye C.K. The potential role of Ugandan indigenous chicken in poverty alleviation. Ugandan J. 2004;50:85–90. [Google Scholar]
- Lemlem A., Tesfay Y. Performance of exotic and indigenous poultry breeds managed by smallholder farmers in Northern Ethiopia. Livest. Res. Rural Dev. 2010;22 [Google Scholar]
- Lwelamira J., Kifaro G.S., Gwakisa P.S. On station and on-farm evaluation of two Tanzania chicken ecotypes for body weights at different ages and egg production. Afr. J. Agric. Res. 2008;3:843–851. [Google Scholar]
- Maciorowski K.G., Herrera P., Jones F.T., Pillai S.D., Ricke S.C. Effects on poultry and livestock of feed contamination with bacteria and fungi. Anim. Feed Sci. Technol. 2007;133:109–136. [Google Scholar]
- Magala H., Kugonza D.R., Kwizera H., Kyarisiima C.C. Influence of management system on growth and carcass characteristics of Ugandan local chickens. J. Anim. Sci. Adv. 2012;2:558–567. [Google Scholar]
- Magothe T.M., Okeno T.O., Muhuyi W., Kahi A.K. Indigenous chicken production in Kenya: I. Current status. Worlds Poult. Sci. J. 2012;68:119–132. [Google Scholar]
- Magothe, T.M., and A.K. Kahi. 2010. Indigenous chicken improvement in Kenya: past efforts and prospects. Animal Production Society of Kenya. Proceedings of the Annual Scientific Symposium 2010. From 20 to 22 April 2010.
- Mahoro J., Muasya T.K., Mbuza F., Habimana R., Kahi A.K. Characterization of indigenous chicken production systems in Rwanda. Poult. Sci. 2017;96:4245–4252. doi: 10.3382/ps/pex240. [DOI] [PubMed] [Google Scholar]
- MALF 2015. Ministry of agriculture, livestock and fisheries. Annual report 2015. Republic of Kenya.
- Maphosa T., Kusina J., Kusina N.T., Makuza S., Sibanda S. A monitoring study comparing production of village chickens between communal (Nharira) and small-scale commercial (Lancashire) farming areas in Zimbabwe. Livest. Res. Rural Dev. 2004;16 http://www.lrrd.org/lrrd16/7/maph16048.htm Art. #48. Accessed Jan. 2019. [Google Scholar]
- Mapiye C., Mwale M., Mupangwa J.F., Chimonyo M., Foti R., Mutenje M.J. A research review of village chicken production constraints and opportunities in Zimbabwe. Asian-Aust. J. Anim. Sci. 2008;21:1680–1688. [Google Scholar]
- Mapiye C., Sibanda S. Constraints and opportunities of village chicken production systems in the smallholder sector of Rushinga District of Zimbabwe. Livest. Res. Rural Dev. 2005;17 [Google Scholar]
- Marwa, L., and B. Lukuyu. 2015. Page 129 in Characterization and quantification of different indigenous chicken production, feeding and management systems in Babati district, Tanzania. International Livestock Research Institute (ILRI). Accessed Sept. 2018. www.africa-rising.net
- Mbuza F., Habimana R., Simbankabo T., Majyambere D. Characterization of layer poultry production in Rwanda. Int. J. Agr. Sci. 2016;6:1148–1156. [Google Scholar]
- Mebratu G.Y. Experiences from an FAO poultry development project in Ethiopia. Pages 57–65 in Sustainable Rural Poultry Production in Africa. Proceedings of an International Workshop held on 13-16 Iune 2995 at the lnternational Livestock Research lnstitute (Sonaiya, E.B., Ed.); Addis Ababa, Ethiopia; 1997. [Google Scholar]
- Mekki D.M., Yousif I.A., Abdelrahman M.K., Wang J., Musa H.H. Comparison of the egg characteristics of different Sudanese indigenous chicken types. Int. J. Poult. Sci. 2005;4:455–457. [Google Scholar]
- Mekonnen G. Hawassa Univ; Awasa, Ethiopia: 2007. MSc Thesis. [Google Scholar]
- Mekonnen H., Mulatu D., Kelay B., Berhan T. Assessment of the nutritional status of indigenous scavenging chicken in Ada'a district. Ethiopia. Trop. Anim. Health Prod. 2010;42:123–130. doi: 10.1007/s11250-009-9395-7. [DOI] [PubMed] [Google Scholar]
- Menge E.O., Kosgey I.S., Kahi A.K. Bio-economic model to support breeding of indigenous chicken in different production systems. Int. J. Poult. Sci. 2005;4:827–839. [Google Scholar]
- Ministry of Agriculture, Livestock Development (MoALD) and Marketing. 1996. Animal Production Division, Kenya. Annual Report.
- Mengesha M., Tamir B., Dessie Village chicken constraints and traditional management practices in Jamma District, South Wollo, Ethiopia. Livest. Res. Rural Dev. 2011;23 [Google Scholar]
- Mlambo T., Mbiriri D.T., Mutibvu T., Kashangura M.T. Village chicken production systems in Zhombe communal area of Zimbabwe. Livest. Rural Dev. 2011;23 [Google Scholar]
- Moreki J.C. Village poultry production in Serowe-Palapye subdistrict of Botswana. Livest. Res. Rural Dev. 2010;22 http://www.lrrd.org/lrrd22/3/more22046.htm Accessed July 2018. [Google Scholar]
- Muchadeyi F.C., Sibanda S., Kusina N.T., Kusina J., Makuza S.M. The village chicken production system in Rushinga District of Zimbabwe. Livest. Res. Rural Dev. 2004;16 http://www.cipav.org.co/lrrd/lrrd16/6/much16040.htm Accessed Oct. 2018. [Google Scholar]
- Muchadeyi F.C., Wollny C.B.A., Eding H., Weigend S., Makuza M., Simianer H. Variation in village chicken production systems among agro-ecological zones of Zimbabwe. Trop. Anim. Health Prod. 2007;39:453–461. doi: 10.1007/s11250-007-9050-0. [DOI] [PubMed] [Google Scholar]
- Muchenje, V., and S. Sibanda S. 1997. Pages 23–42 in informal survey report on poultry production systems in Chivhu and Sanyati farming areas. Crop-Livestock Farming Systems Research Methodologies Training Workshop. UZ/RVAU/DIAS/Danida Project Report.Unpublished. Zimbabwe.
- Mungube E.O., Bauni S.M., Tenhagen B.A., Wamae L.W., Nzioka S.M., Muhammed L., Nginyi J.M. Prevalence of parasites of the local scavenging chickens in a selected semi-arid zone of Eastern Kenya. Trop. Anim. Health Prod. 2008;40:101–109. doi: 10.1007/s11250-007-9068-3. [DOI] [PubMed] [Google Scholar]
- Mustafa R., Hakim A.S., Azizullah M., Rab N.S., Hidayatullah S., Nissar A.U. Effect of various protein source feed ingredient on the growth performance of broiler. Int. J. Med. Plant Res. 2012;1:38–44. [Google Scholar]
- Mutenje M.J., Mapiye C., Mavunganidze Z., Mwale M., Muringai V., Katsinde C.S., Gavumende I. Livestock as a buffer against HIV and AIDS income shocks in the rural households of Zimbabwe. Dev. S. Afr. 2008;25:75–82. [Google Scholar]
- Mutinda W.U., Nyaga P.N., Njagi L.W., Bebora L.C., Mbuthia P.G. Gumboro disease outbreaks cause high mortality rates in indigenous chickens in Kenya. Bull. Anim. Hlth. Prod. Afr. 2013;61:571–578. [Google Scholar]
- Mwale M., Masika P.J. Ethno-veterinary control of parasites, management and role of village chickens in rural households of Centane district in the Eastern Cape. S Afr. Trop. Anim. Health Prod. 2009;41:1685–1693. doi: 10.1007/s11250-009-9366-z. [DOI] [PubMed] [Google Scholar]
- Mwalusanya N.A. Productivity and nutritional status of local chickens under village management conditions. INFPD Newslett. 1999;9:19–21. doi: 10.1023/a:1020048327158. [DOI] [PubMed] [Google Scholar]
- Mwalusanya N.A., Katule A.M., Mutayoba S.K., Mtambo M.M.A., Olsene J.E., Minga U.M. Productivity of local chickens under village management conditions. Trop. Anim. Health Prod. 2002;34:405–416. doi: 10.1023/a:1020048327158. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Osebe T., Narita M. Dual infection of Eimeria tenella and Escherichia coli in chickens. Res. Vet. Sci. 1990;49:125–126. [PubMed] [Google Scholar]
- Ndegwa J.M., Kimani C.M. Proceedings of the fifth Kenya Agricultural Research Institute (KARI) Scientific Conference, (KARI, Nairobi) 1996. Rural poultry production in Kenya: research and development strategies. [Google Scholar]
- Ndegwa J.M., Kimani C.M., Siamba D.N., Mukisira E.A., De Jong R. Characteristics of rural poultry production in different agro-ecological zones in Kenya. Pages 540–547 in Proceedings of the 6th Kenya Agricultural Research Institute Biennial Scientific Conference; Nairobi, Kenya; 1998. [Google Scholar]
- Ndegwa J.M., Mead R., Norrish P., Kimani C.W., Washira A.M. The growth performance of indigenous Kenyan chickens fed diets containing different levels of protein during rearing. Trop. Anim. Health Prod. 2001;33:441–448. doi: 10.1023/a:1010552008639. [DOI] [PubMed] [Google Scholar]
- Nebiyu Y., Berhan T., Kelay B. Characterization of village chicken production performance under scavenging system in Halaba district of southern Ethiopia. Ethiop. Vet. J. 2013;17:69–80. [Google Scholar]
- NISR. 2012. Rwanda fourth population and housing census. Thematic Report: Population size, structure and distribution.
- NISR. 2016. National Institute of Statistics of Rwanda integrated household living conditions survey. Page 72 in Enquête Intégrale sur les Conditions de Vie des Ménages (EICV). Thematic report- Gender.
- NISR, Yearbook 2015, Statistical report, 2015, Ministry of Finance and Economic Planning; Kigali, Rwanda https://statistics.gov.rw/publication/statistical-yearbook-2015. Accessed Aug. 2018.
- Njenga S.K. Danish Institute of Agricultural Sciences; Tjele, Denmark: 2005. MSc Thesis. [Google Scholar]
- Njue, S. W., J.L. Kasiiti, and S.G. Gacheru. 2006. Assessing the economic impact of commercial poultry feeds supplementation and vaccination against Newcastle disease in local Chicken in Kenya. Proceedings of a final research coordination meeting organized by the Joint FAO/International Atomic Energy Agency (IAEA) held in Vienna, 24-28 May 2004.
- Njue, S.W., J.L. Kasiiti, M.J. Macharia, S.G. Gacheru, and H.C.W. Mbugua. 2002. Health and management improvements of family poultry production in Africa- Survey results from Kenya. Pages 39–45 in Characteristics and Parameters of family Poultry Production in Africa. IAEA. Vienna. Accessed Aug. 2018. http://www-naweb.iaea.org/nafa/aph/public/aph-poultry-africa.html
- Nkululeko J., Ndiweni Prudent poultry farming as a source of livelihood and food security in a changing climate: the case of Zhombe communal lands, Zimbabwe. Int. J. Sci. Res. Publications. 2013;3:557–561. [Google Scholar]
- Nqindi J. Pages 137–142 in Characteristics and Parameters of family Poultry Production in Africa. IAEA; Vienna: 2002. Improvement of health and management of family poultry production in Zimbabwe.http://www-naweb.iaea.org/nafa/aph/public/aph-poultry-africa.html Accessed Aug. 2018. [Google Scholar]
- Nwanta J.A., Abdu P.A., Ezema W.S. Epidemiology, challenges, and prospects for control of Newcastle disease in village poultry in Nigeria. Worlds Poult. Sci. J. 2008;64:119–127. [Google Scholar]
- Nzioka M. Indigenous poultry production in the Katumani mandate area. In: Kooijiman Margo, Mukisira E., editors. Netherlands support to the National Agricultural Research Project Phase II. Proceedings of the end of project conference; 29th Nov.-1st Dec. 2002. KARI Headquarters, Nairobi, Kenya; 2000. [Google Scholar]
- Ochieng J. Egerton Univ.; Kenya: 2010. Unpublished Thesis. [Google Scholar]
- Ochieng J., George O., Bockline O.B. Management practices and challenges in smallholder indigenous chicken production in Western Kenya. J. Agric. Rural Dev. Trop. Subtrop. 2013;114:51–58. [Google Scholar]
- Ochieng J., Owuor G., Bebe B.O, Ochieng D.O. Effect of management interventions on productive performance of indigenous chicken in Western Kenya. Livest. Res. Rural Dev. 2011;23 [Google Scholar]
- Okello J.J., Gitonga Z., Mutune J., Okello R.M., Afande M., Rich K.M. HPAI Working Paper 24. IFPRI; Washington, DC: 2010. Value chain analysis of the Kenyan poultry industry: The case of Kiambu, Kilifi, Vihiga, and Nakuru Districts.http://www.ifpri.org/publication/value-chain-analysis-kenyan-poultry-industry Accessed July 2017. [Google Scholar]
- Okeno T.O., Kahi A.K., Peters K.J. Characterization of indigenous chicken production systems in Kenya. Trop. Anim. Heal. Prod. 2012;44:601–608. doi: 10.1007/s11250-011-9942-x. [DOI] [PubMed] [Google Scholar]
- Okitoi L.O. Improvement of poultry production in western Kenya. Pages 227–236 in Proceedings of the end of Agricultural Research Project Phase II Conference; Nairobi, Kenya; 2000. [Google Scholar]
- Okitoi L.O., Ondwasy H.O., Obali M.P., Murekefu F. Gender issues in poultry production in rural households of Western Kenya. Livest. Res. Rural Dev. 2007;19:17. http://www.lrrd.org/lrrd19/2/okit19017.htm Accessed July 2018. [Google Scholar]
- Okoye J.O.A., Aba-adulugba E.P. Comparative study of the resistance or susceptibility of local Nigerian and exotic chickens. Avian Pathol. 1998;27:168–173. doi: 10.1080/03079459808419319. [DOI] [PubMed] [Google Scholar]
- Okoye J.O.A., Aba-adulugba E.P., Ezeokonkwo R.C., Udem S.C., Orajaka L.J.E. Susceptibility of local Nigerian and Exotic chickens to Infectious bursal disease by contact exposure. Trop. Anim. Health Prod. 1999;31:75–81. doi: 10.1023/a:1005159522203. [DOI] [PubMed] [Google Scholar]
- Oluwayelu D.O., Emikpe D.O., Ikheloa J.O., Fagbohun O.A., Adeniran G.A. The pathology of infectious bursal disease in crossbreeds of harco cocks and indigenous Nigerian hens. Afr. J. Clin. Exp. Microbiol. 2002;3:95–97. [Google Scholar]
- Olwande P.O., William O.O., Samwel O.O., Gerald M., Okoth E., Odindo M.O., Adhiambo R.F. Assessing the productivity of indigenous chickens in an extensive management system in southern Nyanza, Kenya. Trop. Anim. Health Prod. 2010;42:283–288. doi: 10.1007/s11250-009-9418-4. [DOI] [PubMed] [Google Scholar]
- Ondwassy H.O., Okitoi L.O., Obali M.P., Simwa S.M., Wakhusama S.W. Epidemiology of helminths in indigenous chicken in Western Kenya. Pages 407–412 in Proceedings of the 7th Kenya Agricultural Research Institute Biennial Scientific Conference; Nairobi, Kenya; 2000. [Google Scholar]
- Oswin F., Linuma, Kalista H.P. Contribution of indigenous chicken production to the household income and improvement of food; a case of same district, Tanzania. Int. J. Agricu. Environ. Res. 2017;3:2767–2783. [Google Scholar]
- Pedersen C.V. The Royal Veterinary and Agricultural University; Copenhagen, Denmark: 2002. PhD Thesis. [Google Scholar]
- Regassa C., Berhanu S., Fufa D., Hunduma D. Sero-prevalence of Newcastle Disease in backyard chickens in mid Rift Valley of Oromia, Ethiopia. Proceedings of the 12th International conference of the Association of Institutions of Tropical Veterinary Medicine; Montpellier, France; 2007. [Google Scholar]
- Sanka Y.D., Mbaga S.H. Evaluation of Tanzanian local chicken reared under intensive and semi-intensive systems: I. Growth performance and carcass characteristics. Livest. Res. Rural Dev. 2014;26 [Google Scholar]
- Sasipreeyajan J., Jemgklinchan J., Koowatananukul C., Saitanu K. Prevalence of Salmonellae in broiler and breeder flocks in Thailand. Trop. Anim. Health Prod. 1996;28:174–180. [PubMed] [Google Scholar]
- Sayda A.M.A. Family poultry as tool in alleviating environmental hazards in settled areas of transhumant families in gezira scheme Sudan. Asian J. Rural Dev. 2012;2:1–12. [Google Scholar]
- Shanawany M.M., Banerjee A.K. Indigenous chicken genotypes of Ethiopia. Anim. Gene. Resour. Inf. 1991;8:84–87. [Google Scholar]
- Siamba, D.N., J.M. Ndegwa, C.W. Kimani, J. Nampaso, J., S.N. Ole Sinkeet, E.A. Mukisira, R. De Jong. 2000. Rural poultry production: efforts to improve production and contribution to household economy. de Jong and Mukisira (Editors), Pages 141-156 in testing of livestock technologies on smallholder mixed farms in Kenya. Research-extension-farmer experiences in the National Dairy Cattle and Poultry Research Programme 1995-1999, Kenya.
- Simainga S., Banda F., Sakuya N., Moreki J.C. Health management in village poultry in Kalabo and Mongu districts in the western province of Zambia. Livest. Res. Rural Dev. 2010;22 [Google Scholar]
- Simainga S., Moreki J.C., Banda F., Sakuya N. Socio-economic study of family poultry in Mongu and Kalabo districts of Zambia. Livest. Res. Rural Dev. 2011;23 [Google Scholar]
- Smith V.H., Jones T.P., Smith M.S. Host nutrition and infectious disease: an ecological view. Front. Ecol. Environ. 2005;3:268–274. [Google Scholar]
- Solomon Z., Binyam K., Bilatu A., Ferede A. Village chicken production systems in Metekel zone, Northwest Ethiopia. Wudpecker J. Agricu. Res. 2013;2:256–262. [Google Scholar]
- Sonaiya E.B. Family poultry, food security and the impact of HPAI. Worlds Poult. Sci. J. 2007;63:132–138. [Google Scholar]
- Sonaiya E.B., Dazogbo J.S., Olukosi O.A. In: (Dwinger, R., Ed), P. 193-200 in characteristics and Parameters of Family Poultry Production in Africa Results of a FAO/IAEA Coordinated Research Project. IAEA; Vienna: 2002. Further assessment of scavenging feed resource base.https://inis.iaea.org/search/search.aspx?orig_q=RN:37062498 [Google Scholar]
- Sonaiya E.B., Swan S.E.J. FAO Animal Production and Health Manual. FAO; Rome, Italy: 2004. Small-scale poultry technical guide. [Google Scholar]
- Ssewannyana E., Onyait A.O., Masaba J. Strategies for improving meat and egg productivity of indigenous chickens in Kumi and Apac districts, Uganda. J. Appl. Biosci. 2008;12:661–664. [Google Scholar]
- Ssewannyana E., Ssali A., Kasadha T., Dhikusooka M., Kasoma P., Kalema J., Kwatotyo B.A., Aziku L. On-farm characterization of indigenous chickens in Uganda. J. Anim. Plant Sci. 2008;1:33–37. [Google Scholar]
- Swai E.S., Karimuribo E.D., Kyakaisho P.F., Mtui P.F. Free-range village chickens on the humid coastal belt of Tanga, Tanzania: their roles, husbandry and health status. Livest. Res. Rural Dev. 2007;19 [Google Scholar]
- Tadelle D., Ogle B. Swedish University of Agricultural Sciences; Sweden: 1996. MSc Thesis. [Google Scholar]
- Tadesse D., Harpal S., Ashenafi M., Wondimeneh E., Tadelle D. Study on management practices and marketing systems of village chicken in East Shewa. Ethiopia. Afr. J. Agric. Res. 2013;8:2696–2702. [Google Scholar]
- Wilson R.T. Poultry production and performance in the Federal Democratic Republic of Ethiopia. Worlds Poult. Sci. J. 2010;66:441–454. [Google Scholar]
- Wiener and Rouvier. L’amelioration genetique animale. Edutions Quae. 2009:275. doi: 10.35690/978-2-7592-0371-0. [DOI] [Google Scholar]
- Worku, M., Legessew, T. Berhanu, D. Girma, A. Girum, A. Wende, K. Tolera, B. Gezahegn, W. Dagne, and A. Solomon. 2012. Status and future direction of maize research and production in Ethiopia. In M. Worku, Twumasi-Afriyie, S., Wolde, L., Tadesse, B., Demisie G., Bogale, G., Wegary, D. and Prasanna, B.M. (Ed.), Page 77 in Meeting the Challenges of Global Climate Change and Food Security Through Innovative Maize Research, Addis, Ababa.
- Yakubu A. Indigenous chicken flocks of Nasarawa state, Nigeria: their characteristics, husbandry and productivity. Trop. Subtrop. Agro-ecosyst. 2010;12:69–76. [Google Scholar]
- Yami A. Poultry production in Ethiopia. World Poult. Sci. J. 1995;51:197–201. [Google Scholar]
- Zemelak G., Luizinho C., Cassio W., Gudrun A.B. Characterization of village chicken production systems and challenges across agro-climatic zones in Ethiopia. Int. J. Livest. Prod. 2016;7:94–105. [Google Scholar]
- Zewdu S., Kassa B., Bilatu A., Ferede A. Village chicken production systems in Metekel zone, Northwest Ethiopia. Wudpecker J. Agricu. Res. 2013;2:256–262. [Google Scholar]
- Murray J.K., 2001. Coccidiosis in chickens. Agri-facts. Agriculture, Food, and Rural Development (1992-2006), Edmonton, Canada. Accessed July 2018. https://open.alberta.ca/dataset/62aae9ea-776f-421a-965e-19354779b838/resource/4713a1e1-33fb-41c5-92d66bebe6d2f42e/download/2001-663-35.pdf.
- Fetsum, S., A. Solomon, D. Mikias, S. Yigremachew, M. Demeke, W. Gizachew, C. Yeshi. 2009. Gender-based participatory rural appraisal of farming systems in Wombera, Bullen, and Guba Woredas. Conference on Gender Differentials for Planning Agricultural Research, Addis Ababa, Ethiopia. Accessed Jan. 2021. https://www.researchgate.net/publication/313376973.