Abstract
Aim
We aimed to assess the ability of natural and modified polyunsaturated fatty acids to shorten QT interval in ex-vivo and in-vivo guinea pig hearts.
Methods
The effect of one natural (docosahexaenoic acid) and three modified (Lin-GLY, DHA-GLY, N-AT) polyunsaturated fatty acids on ventricular action potential duration (APD) and QT interval was studied in a E4031 drug-induced long QT2 model of ex-vivo guinea pig hearts. The effect of DHA-GLY on QT interval was also studied in in-vivo guinea pig hearts upon intravenous administration. The effect of modified polyunsaturated fatty acids on IKs was studied using Xenopus laevis oocytes expressing human KCNQ1 and KCNE1.
Results
All tested polyunsaturated fatty acids shortened ADP and QT interval in ex-vivo guinea pig hearts, however with different ability in restoring baseline APD/QT interval with specific modified polyunsaturated fatty acids being most efficacious. Despite comparable ability in activating the human KCNQ1/KCNE1 channel, Lin-GLY was not as effective in shortening APD/QT interval as DHA-GLY in ex-vivo hearts. By constructing a guinea pig-like KCNE1, we found Lin-GLY to induce less activating effect compared with DHA-GLY on human KCNQ1 co-expressed with guinea pig-like KCNE1. DHA-GLY was studied in more detail and was found to shorten QT interval in in-vivo guinea pig hearts.
Conclusion
Our results show that specific polyunsaturated fatty acids shorten QT interval in guinea pig hearts. The tendency of modified polyunsaturated fatty acids with pronounced IKs channel activating effect to better restore QT interval suggests that modifying polyunsaturated fatty acids to target the IKs channel is a means to improve the QT-shortening effect.
Keywords: Guinea pig heart, IKs channel, KCNQ1, Kv7.1, Long QT Syndrome, PUFA
Introduction
One of the major cardiac potassium currents, IKs, is generated by the slowly activating IKs potassium channel1 that is composed of 4 alpha (KV7.1, KCNQ1) subunits and 1 to 4 beta (KCNE1) subunits2–4. Over 300 different mutations in the genes encoding KCNQ1 and KCNE1 subunits are linked to a prolonged QT interval in the electrocardiogram (ECG), leading to a condition known as congenital Long QT Syndrome (LQTS)1. In the general population, one in 2000 has congenital LQTS5, where mutations in the alpha-subunit (KCNQ1) of the IKs channel complex is the most common form of LQTS (called LQT1)1. Such mutations decrease the current through the IKs channel, which impairs repolarization of the cardiac action potential and results in prolonged action potential duration. The prolonged ventricular action potential duration is seen in the surface ECG as a prolonged QT interval. LQTS is a risk factor for ventricular fibrillation and sudden cardiac death6–8. 4000 Americans die yearly from sudden cardiac death caused by congenital LQTS9, most of which occur in children and young adults. Additionally, QT-prolonging medications (such as specific anti-arrhythmics and antibiotics) may cause drug-induced LQTS, of which block of the delayed rectifier current IKr (i.e. drug-induced LQT2) is the most common cause10,11. At present, LQTS is treated using β blockers and implantable cardioverter-defibrillators12,13. Although these approaches reduce cardiac arrhythmias by reducing the occurrence of pro-arrhythmic sympathetic triggers (in the case of β blockers) or injecting currents to stop arrhythmias (in the case of defibrillators), neither of these approaches treat the underlying defect. In addition, β blockers do not work in all patients, are contraindicated in some patients, and have side effects, such as fatigue, diarrhea, vomiting, heart failure, and depression14–16, which may limit their utility. The cost of defibrillator implantation is high and implantation might cause damage to heart and lung tissue, and post-hospitalization infections, device related complications and most importantly in-appropriate shocks12,13,17. Another treatment strategy would be to restore physiological QT interval by directly targeting the underlying cause of the disease, which most commonly are defective IKs channels. However, there is no clinically approved activator of the IKs channel, highlighting the need to develop IKs channel activators whose QT-shortening potential are evaluated in different models. In this study we assess the ability of a set of IKs channel activators to shorten QT interval in guinea pig hearts.
Polyunsaturated fatty acids (PUFAs) have been put forward as anti-arrhythmic compounds18. The American Heart Association recommends intake of fish rich in PUFAs, particularly in individuals with cardiovascular problems19. However, results from both basic research and clinical trials have been mixed with some studies showing clear positive effects of PUFAs on cardiovascular parameters, while other trials showed no significant effect and some basic research showed even pro-arrhythmic effects19–26. These divergent results could be due to several factors, including the heterogeneity of the patients (or animal models), small patient populations in some studies, and potential differences in the efficacy of PUFAs for different types of arrhythmias (triggered versus re-entry)23,26. In addition, most of these studies did not measure the blood levels of PUFAs to determine their bioavailability19. A large prospective cohort study that measured blood levels of PUFAs found that there was a strong inverse correlation of mortality from cardiac arrhythmias and blood levels of PUFAs: the highest quintile had 45% less chance of dying from cardiac arrhythmias compared to the lowest quintile27.
It has been suggested that beneficial effects of PUFAs, such as docosahexaenoic acid (DHA, the most abundant PUFA in fish oils), are due to inhibition of voltage-gated Na+ and Ca2+ channels20,28–31. We and others have shown that natural PUFAs, such as DHA, have small or no effect on IKs channels32–34. In contrast, we recently showed that modified PUFAs (such as N-arachidonoyl taurine, N-AT, and DHA glycine, DHA-GLY) have large activating effects on IKs channels34 and restore the function of IKs channels with LQT1 mutations35. This suggests that modified PUFAs would be more effective at shortening the QT interval compared to natural PUFAs by adding an effect of PUFAs on IKs channels. In this study we assess the ability of natural and modified PUFAs to shorten the QT interval in ex-vivo and in-vivo guinea pig hearts. Guinea pigs are often used for cardiac studies because their cardiac action potential resembles the human, but still has the benefit of a relative small size as compared to rabbits and larger mammals36–41.
Our results show that specifically modified and natural PUFAs shorten the QT interval in ex-vivo guinea pig hearts. The most efficacious PUFA analogue, DHA-GLY, fully restored baseline QT interval in ex-vivo guinea hearts with drug-induced LQTS and significantly shortened QT interval in in-vivo guinea pig hearts. Our findings suggest that modified PUFAs, which directly increase the IKs currents, have a tendency towards increased QT-shortening efficacy compared with PUFAs that have no or very little effect on the IKs currents. We propose that these modified PUFAs could be developed into novel LQTS drugs that could potentially prevent prolonged QT intervals in LQTS patients and, thereby, reduce the risk of developing ventricular fibrillation and sudden cardiac deaths. Such developed modified PUFAs may offer a complement to the current treatments of LQTS, such as β blockers and implantable cardioverter-defibrillators.
Results
PUFA and PUFA analogues shorten QT interval in ex-vivo guinea pig hearts with drug-induced LQT2
We used the IKr blocker E4031 to induce LQTS in ex-vivo guinea pig hearts (Fig. 1a, see Methods for details). This model of drug-induced LQT2 gives the advantage of prolonging the QT interval by affecting another channel than the IKs channel. On average, 20 minutes of application of 0.03 μM of E4031 resulted in a prolongation of QT interval and action potential duration (APD90) of 18 ± 1 ms and 16 ± 1 ms (n = 23), respectively. The QT and APD prolongation induced by continuous application of E4031 was stable for a minimum of 30 minutes (Supplementary Fig. 1).
Figure 1. PUFAs shorten and partially or fully restore prolonged QT interval in isolated ex-vivo guinea pig hearts.
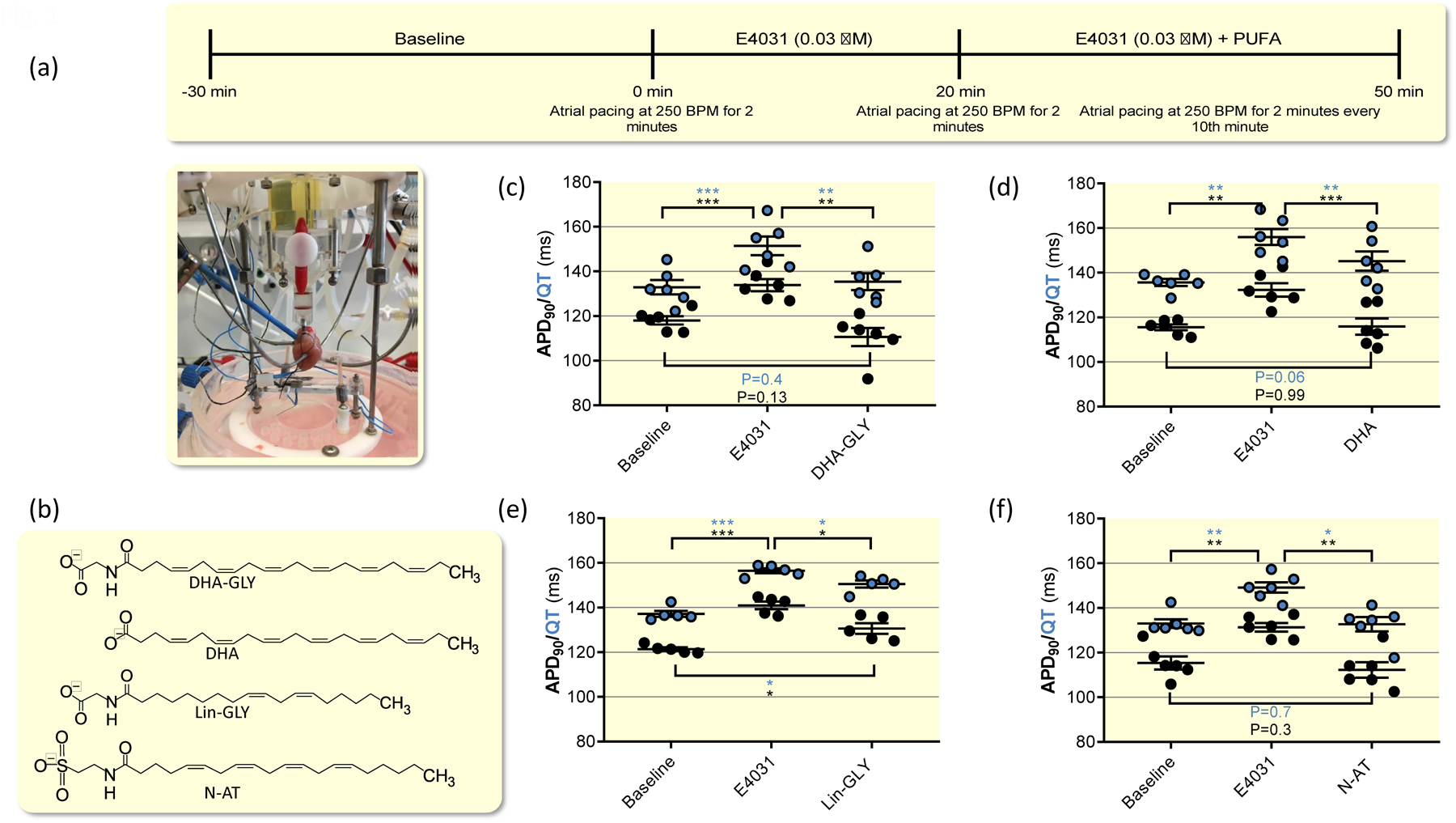
(a) Overview of study design for ex-vivo guinea pig heart experiments along with photo of experimental configuration. (b) Molecular structure of indicated PUFAs. (c) Effects of 0.03 μM E4031 and 10 μM DHA-GLY together with 0.03 μM E4031 on QT interval and action potential duration. Data shown as mean ± sem. Statistical analysis used was repeated measures one-way ANOVA, followed by Tukeýs multiple comparison test. n = 6. (d-f) Same as in C but for 10 μM DHA (d, n = 6), 10 μM Lin-GLY (e, n = 5), and 30 μM N-AT (f, n = 6).
We next assessed the ability of one PUFA (DHA) and three different PUFA analogues (DHA-GLY, Lin-GLY and N-AT) to restore baseline QT interval and APD in ex-vivo hearts with established drug-induced LQT2 (E4031 was continuously applied). Molecular structures of PUFA and PUFA analogues are provided in Figure 1b. We have previously described that DHA-GLY gradually shortens the QT interval and APD in ex-vivo guinea pig hearts with prolonged QT interval over an application period of 30 minutes34. In line with these previously published data, we here find that application of 10 μM of DHA-GLY for 30 minutes shortened the QT interval and APD90 compared to E4031 values and restored baseline QT interval (Fig. 1c, Table I). Application of 10 μM of DHA shortened QT interval and APD90 compared to E4031 values (Fig. 1d, Table I). However, the QT interval was not fully restored to baseline values with a trend towards statistically significant difference from baseline (P = 0.06) (Fig. 1d, Table I). The APD90 was restored to baseline values by 10 μM of DHA, but there was a trend towards larger effects of 10 μM of DHA-glycine on the APD90 as compared to DHA (P = 0.10) (Fig. 1d). The two PUFA analogues Lin-GLY and N-AT showed variable ability to shorten QT interval: Application of 10 μM of Lin-GLY for 30 minutes shortened the QT interval and APD90 compared to E4031 values but did not fully restore baseline QT interval or APD (Fig. 1e, Table I). Application of 30 μM of N-AT for 30 minutes significantly shortened the QT interval and APD90 compared to E4031 values and fully restored baseline QT interval and APD (Fig. 1f, Table I). The reason for the higher concentration of N-AT used in these experiments is that N-AT has an apparent lower affinity to the IKs channel compared with DHA-GLY and Lin-Gly42.
Table I.
Summary of PUFA effect on QT-intervals and APD90 in ex-vivo guinea pig hearts.
| Compound | Baseline (ms) | E4031 0.03 μM (ms) | ΔQT/APD90, compared with baseline (ms) | PUFA (ms) | ΔQT/APD90, compared with E4031 (ms) | ΔQT/APD90, compared with baseline (ms) | n | |
|---|---|---|---|---|---|---|---|---|
| DHA-GLY (10 μM) | QT | 132.9 ± 3.2 | 151.5 ± 4.1 | +18.6 ± 1.9 (P < 0.001) | 135.4 ± 3.7 | −16.2 ± 2.4 (P < 0.01) | +2.4 ± 1.7 (P = 0.4) | 6 |
| APD90 | 118.1 ± 1.9 | 133.8 ± 2.7 | +15.8 ± 1.7 (P < 0.001) | 110.6 ± 4.1 | −23.2 ± 3.6 (P < 0.01) | −7.4 ± 3.2 (P = 0.13) | 6 | |
| DHA (10 μM) | QT | 135.7 ± 1.5 | 156.0 ± 1.5 | +20.3 ± 2.7 (P < 0.01) | 145.2 ± 4.3 | −10.8 ± 1.4 (P < 0.01) | +9.6 ± 3.1 (P = 0.06) | 6 |
| APD90 | 115.5 ± 1.4 | 132.3 ± 3.0 | +16.8 ± 2.3 (P < 0.01) | 115.9 ± 3.7 | −16.4 ± 1.2 (P < 0.001) | +0.3 ± 3.2 (P = 0.99) | 6 | |
| Lin-GLY (10 μM) | QT | 134.6 ± 1.7 | 152.7 ± 1.0 | +18.1 ± 1.8 (P < 0.01) | 148.4 ± 1.3 | −4.3 ± 1.3 (P ≤ 0.05) | +13.8 ± 1.1 (P ≤ 0.05) | 5 |
| APD90 | 121.4 ± 0.7 | 141.0 ± 1.7 | +19.6 ± 1.7 (P < 0.001) | 132.4 ± 2.7 | −8.6 ± 1.5 (P ≤ 0.05) | +11.0 ± 3.0 (P ≤ 0.05) | 5 | |
| N-AT (30 μM) | QT | 133.0 ± 1.9 | 149.2 ± 2.3 | +16.2 ± 3.1 (P < 0.01) | 132.7 ± 3.2 | −16.4 ± 2.6 (P < 0.01) | −0.3 ± 2.7 (P = 0.7) | 6 |
| APD90 | 115.4 ± 2.9 | 131.3 ± 2.0 | +15.9 ± 2.5 (P < 0.01) | 112.3 ± 3.4 | −19.0 ± 3.0 (P < 0.01) | −3.1 ± 1.8 (P = 0.29) | 6 |
QT/APD90 determined during pacing at 250 BPM as described in Methods. Data shown as mean ± sem. Statistics denote repeated measures one-way ANOVA followed by Tukeýs multiple comparison test.
Because DHA-GLY was most efficacious in restoring the prolonged QT-interval to baseline values, we investigated DHA-GLY effects on other parameters of cardiac function. Application of 10 μM of DHA-GLY did not affect QRS-duration (Table II) or retrograde coronary flow (Table II). However, 10 μM of DHA-GLY significantly increased the PR interval (Table II).
Table II.
Summary of DHA-GLY effect on cardiovascular parameters in ex-vivo guinea pig hearts.
| Parameter | Baseline | E4031 0.03 μM | ΔE4031, compared with baseline | DHA-GLY 10 μM | ΔDHA-GLY, compared with E4031 | ΔDHA-GLY, compared with baseline | n |
|---|---|---|---|---|---|---|---|
| PR interval | 58.8 ± 3.6† | 61.2 ± 4.2† | +2.4 ± 0.9† (P = 0.09) | 69.4 ± 4.2† | +8.2 ± 2.2† (P ≤ 0.05) | +10.6 ± 2.7† (P ≤ 0.05) | 6 |
| QRS duration | 18.8 ± 0.6† | 18.8 ± 0.6† | +0.1 ± 0.2† (P = 0.9) | 19.1 ± 0.6† | + 0.3 ± 0.3† (P = 0.7) | + 0.4 ± 0.3† (P = 0.5) | 6 |
| Coronary flow | 16.2 ± 2.0‡ | 14.3 ± 2.0‡ | −1.8 ± 0.3‡ (P < 0.01) | 12.2 ± 1.8‡ | −2.0 ± 2.3‡ (P = 0.7) | −3.9 ± 2.2‡ (P = 0.3) | 6 |
Parameters determined during pacing at 250 BPM as described in Methods. Data shown as mean ± sem. Statistics denote repeated measures one-way ANOVA followed by Tukeýs multiple comparison test.
denotes ms.
denotes ml/min.
Altogether, data from ex-vivo guinea pig hearts show that all tested PUFA/PUFA analogues significantly shortened QT interval and APD (by about 4–16 ms and 9–23 ms, respectively) in ex-vivo guinea pig hearts with prolonged QT interval (Table I). However, in contrast to DHA-GLY and N-AT, Lin-GLY and DHA failed to fully restore baseline QT interval, while DHA, but not Lin-GLY, did normalize APD (Table I).
DHA-GLY shortens QT interval in in-vivo guinea pig hearts
To investigate whether the effects of DHA-GLY observed in ex-vivo hearts translate into in-vivo experiments, we utilised a novel closed chest cardiac electrophysiology model43, in which QT interval was monitored with custom-made ECG needle electrodes and cardiac parameter determined during intra-cardiac pacing at 300 BPM (Figure 2a, see Methods for details). QT interval, PR interval and QRS duration remained stable during 30 minutes of vehicle infusion (Fig. 2b–d, Table III). In contrast, infusion of 10 mg/ml of DHA-GLY (corresponding to 40 mg/kg/hr) gradually shortened QT interval over the 30 minutes DHA-GLY was infused (Fig. 2b). After 30 minutes of infusion of 10 mg/ml of DHA-GLY, the changes in QT interval was significantly larger in the DHA-GLY group as compared to the time-matched controls (−17.3 ± 2.8 ms vs 1.2 ± 1.6 ms) (Fig. 2b, Table III). No significant effect of DHA-GLY was observed on the QRS duration whereas the PR interval was prolonged by DHA-GLY (Fig. 2c–d, Table III).
Figure 2. DHA-GLY shortens QT interval in in-vivo guinea pig hearts.
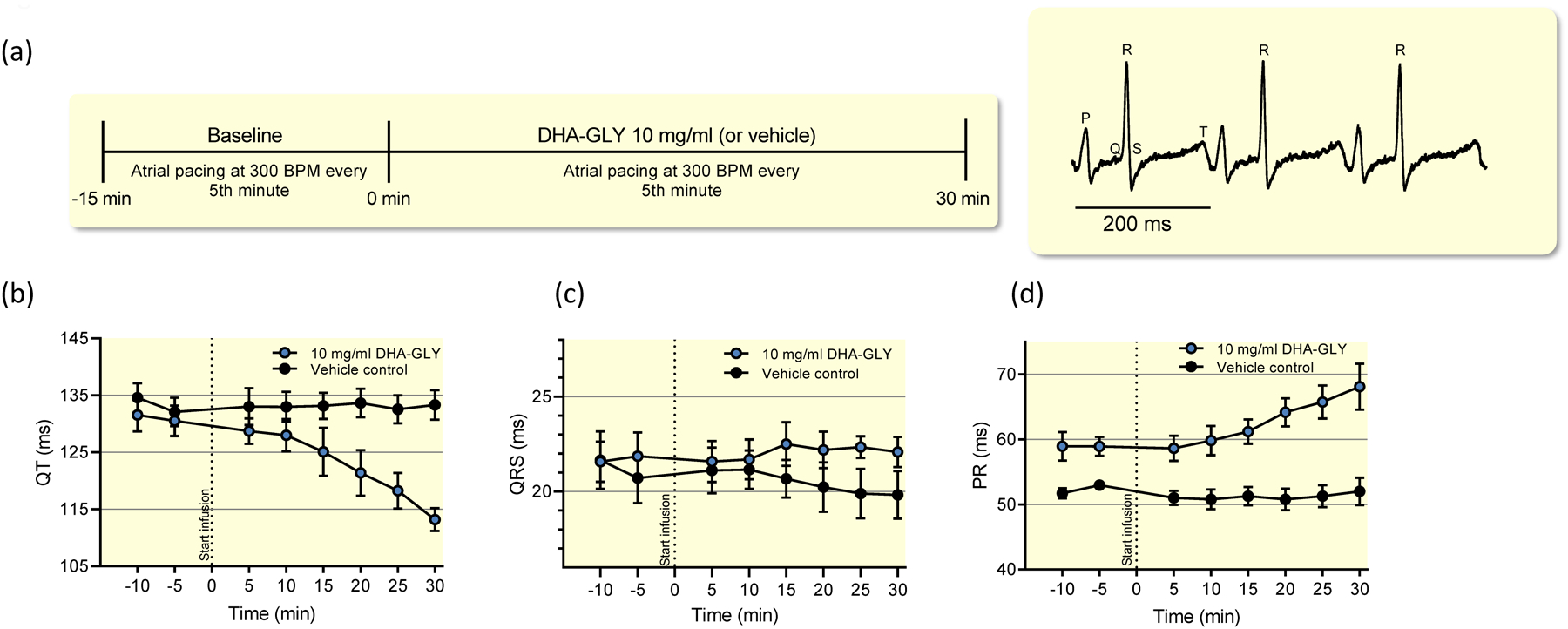
(a) Study design for in-vivo experiments in guinea pigs with representative ECG recording. (b) Effects of 10 mg/ml (40 mg/kg/hr) DHA-GLY and vehicle control on QT interval during intra-cardiac pacing. (c-d) same as (b) but for QRS-duration and PR-interval. Data shown as mean ± sem, n = 5. Data is summarized in Table III.
Table III.
Changes in PR interval, QRS duration and QT interval in in-vivo guinea pig experiments.
| Treatment | ΔPR (ms) | ΔQRS (ms) | ΔQT (ms) | n | |||
|---|---|---|---|---|---|---|---|
| TMC (vehicle) | −1.0 ± 2.3 | −0.9 ± 1.1 | +1.2 ± 1.6 | 5 | |||
| DHA-GLY (10 mg/ml) | +9.2 ± 3.0 | (P = 0.03†) | +0.2 ± 0.8 | (P = 0.561†) | −17.3 ± 2.8 | (P = 0.001†) | 5 |
Parameters determined during pacing at 300 BPM as described in Methods. TMC denotes time-matched controls receiving vehicle infusion. Δ-values refers to the absolute changes (test-baseline) in PR interval, QRS duration and QT interval between test values and baseline values. Test values (for vehicle or DHA-GLY) were determined after 30 minutes of infusion (time = +30 min). Baseline values were determined 5 minutes prior to DHA-GLY/vehicle infusion (time = −5 min). Data shown as mean ± sem.
P-values refer to the comparison of Δ-values between TMC and DHA-GLY groups using unpaired Student’s-t test..
Lin-GLY is less effective than DHA-GLY in activating a guinea-pig like IKs channel
Lin-GLY was as effective as DHA-GLY in activating the human IKs (hKCNQ1/hKCNE1) channel expressed in Xenopus oocytes (Fig. 3a): 20 μM of Lin-GLY or DHA-GLY shifted the midpoint for channel activation, V50, by about −34 mV (Fig. 3a, P > 0.05 for 20 μM using Student’s t-test). Because DHA-GLY was clearly effective in restoring the QT interval in guinea pig hearts, we were surprised that Lin-GLY shortened but did not restore the QT interval in guinea pig hearts. One hypothesis for the similar effect on human IKs channel but different effect in guinea pig heart is that DHA-GLY is not shortening the QT interval in guinea pig hearts by activating guinea pig IKs channels, but through some other mechanism that is specific for DHA-GLY, but not Lin-GLY. Another hypothesis is that DHA-GLY activates the guinea pig IKs channels, whereas Lin-GLY does not activate the guinea pig IKs channels. To test the second hypothesis, we looked for differences in the sequences of human and guinea pig KCNQ1 and KCNE1 subunits. The sequences of human and guinea pig KCNQ1 subunits are very similar in the region on which PUFAs have been suggested acting in IKs channels (external ends of S3, S4, and S6 of KCNQ134,44 (Supplementary Fig. 2). There are, however, three amino acids that are very different between human and guinea pig KCNE1 at the external end of the transmembrane region of KCNE1 (Fig. 3b), which are also located close to the putative PUFA binding site. We therefore mutated these three amino acids in hKCNE1 to the corresponding guinea pig amino acids (RSS to LRD), to create a guinea pig-like KCNE1 (referred to as gplKCNE1). Surprisingly, 20 μM of Lin-GLY had less effect compared with 20 μM of DHA-GLY on hKCNQ1 co-expressed with gplKCNE1 in Xenopus oocytes (Fig. 3c). Whereas DHA-GLY shifted V50 by about −24 mV Lin-GLY shifted V50 by only −17 mV (Fig. 3c, P < 0.05 for 20 μM using Student’s t-test). No significant difference in the effect was observed at 7 μM (P > 0.05 using Student’s t-test). However, because of challenges in exact translation of relevant concentrations between model systems, the different effects of the slightly higher concentrations of DHA-GLY and Lin-GLY on IKs current when using the guinea pig-like KCNE1 subunit may contribute to the different effects of DHA-GLY and Lin-GLY on shortening the QT interval in guinea pig hearts.
Figure 3. Lin-GLY effects depend on guinea pig KCNE1 residues.
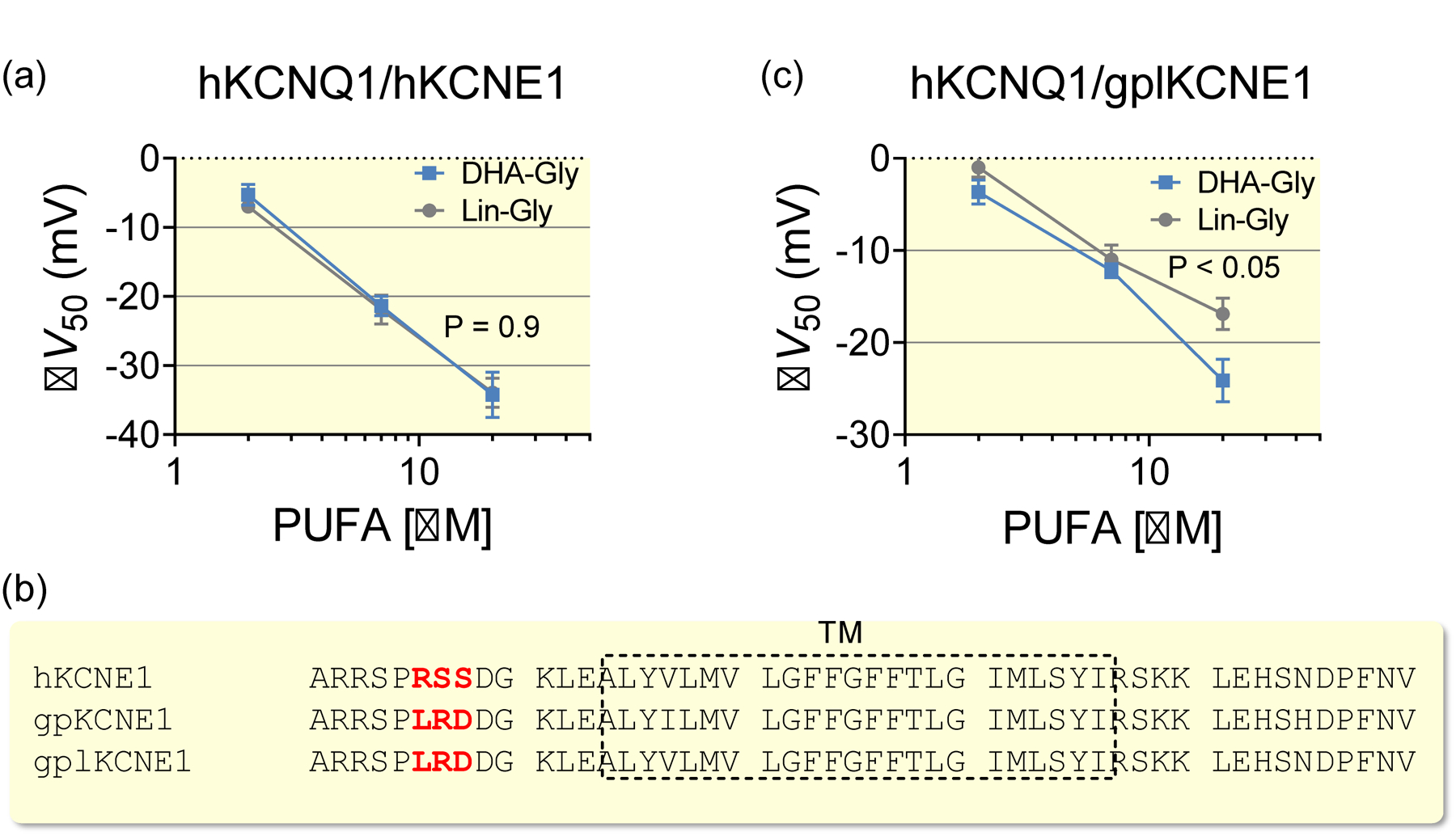
(a) Concentration-response relationship for the ability of DHA-GLY and Lin-GLY to shift the voltage dependence of activation (i.e. ΔV50) of hKCNQ1/hKCNE1 channels expressed in Xenopus oocytes. Data shown as mean ± sem, n = 8. Statistics denote Student’s t-test for the effect at 20 μM. (b) Sequence alignment of human KCNE1 (hKCNE1), guinea pig KCNE1 (gpKCNE1), and the constructed guinea pig like KCNE1 (gplKCNE1) subunits. Deviating residues in the extracellular terminal are marked in red. The transmembrane (TM) region is indicated by the dashed box. (c) Concentration-response relationship of the ability of DHA-GLY and Lin-GLY to shift V50 of hKCNQ1/gplKCNE1 channels expressed in Xenopus oocytes. Data shown as mean ± sem, n = 8–11. Statistics denote Student’s t-test for the effect at 20 μM.
Discussion
In this study, we assessed the ability of natural and modified PUFAs to shorten the QT interval in guinea pig hearts. DHA, DHA-GLY, Lin-GLY and N-AT all significantly shortened the QT interval in a drug-induced model of LQTS in ex-vivo guinea pig hearts. However, they restored baseline QT interval to different extent. In addition, for the first time we demonstrate that modified PUFAs shortened the QT interval in-vivo in guinea pig. Altogether, this study suggests that modifying PUFAs to target the IKs channel is a means to improve the QT-shortening effect of PUFA.
What may be the mechanistic basis underlying the variable ability of tested compounds to shorten the QT interval? We have previously suggested that natural and modified PUFAs activate the IKs channel by a lipoelectric mechanism, in which the PUFA inserts itself in the otherwise membrane-filled cleft between two voltage-sensing domains of KCNQ1 subunits (Supplementary Fig. 2)34,44. From this position, the negatively-charged head group of the PUFA would electrostatically interact with the positively charged residues in the KCNQ1 voltage sensor S4 and a positively charged lysine in S6 to activate the IKs channel by shifting the voltage-dependence of channel opening and increasing the maximal conductance, respectively44. We have previously shown that a low pKa of the PUFA head group is important to keep the head group negatively charged when bound to KCNQ1/KCNE1 channels33,34. For example, DHA has no significant effect on human KCNQ1/KCNE1 channels expressed in oocytes at pH 7.4, most likely because the carboxyl head group of DHA is protonated and uncharged33,34. In contrast, DHA-GLY has a much lower pKa and N-AT even a lower pKa34, such that DHA-GLY and N-AT would be partly and fully deprotonated, respectively, at physiological pH. A protonated DHA, with a limited ability to activate KCNQ1/KCNE1 channels in guinea pig hearts, could explain why DHA did not shorten the QT interval as much as DHA-GLY and N-AT in ex vivo guinea pig hearts. Even if DHA did restore the ADP90 to baseline values, there was a trend that DHA did not shorten the APD90 as much as DHA-GLY or N-AT, suggesting that these modified PUFAs would be more effective than regular PUFAs to treat Long QT Syndrome.
Surprisingly, although Lin-GLY and DHA-GLY had similar activating effects on human KCNQ1/KCNE1 (hKCNQ1/hKCNE1) channels expressed in Xenopus oocytes, we found that Lin-GLY shortened the QT interval and APD to a lesser extent than DHA-GLY. Based on our two-electrode voltage-clamp recordings, this is likely because Lin-GLY has less activating effect than DHA-GLY on “guinea pig-like” KCNQ1/KCNE1 (hKCNQ1/gplKCNE1) channels, and hence shortens the QT interval to a smaller extent. The three residues on the guinea pig KCNE1 subunit that when introduced in the human KCNE1 subunit make guinea pig like KCNQ1/KCNE1 channels less sensitive to Lin-GLY are located at the external end of the KCNE1 transmembrane segment (Fig. 3C). Why these three residues determine the DHA-GLY and Lin-GLY selectivity for KCNQ1/KCNE1 channels is not clear. However, in a recent model of KCNQ1/KCNE1 channels, these three KCNE1 residues would be located very close to our proposed PUFA binding site44 (Supplementary Fig. 2) and these residues could therefore interact with the PUFA. Further experiments are necessary to determine the molecular mechanism for the PUFA specificity of KCNQ1/KCNE1 channels. As importantly, our findings of species difference in the efficacy of PUFAs stresses the importance of thorough assessment of novel drug candidates in multiple species and in human cell systems prior to clinical testing, in order to approve the possibility for translation of preclinical findings to clinical efficacy.
Several observations suggest that natural and modified PUFAs target multiple ion channels. Firstly, although DHA in our hands does not activate KCNQ1/KCNE1 expressed in Xenopus oocytes33,34, we here observed that DHA shortened QT interval and APD in ex-vivo hearts. The QT shortening DHA effect could be caused by previously described inhibiting effects of DHA on cardiac voltage-gated Na+ and Ca2+ channels20,28–31. In line with this, DHA has been shown to shorten action potential duration in isolated guinea pig cardiomyocytes by inhibiting Na+ and Ca2+ currents, whereas the effect on delayed potassium current was limited45. We did observe a significant prolongation of PR-interval after DHA application (from 60 ± 2 to 65 ± 3 ms, P = 0.006 with one-way ANOVA followed by Tukeýs multiple comparison test), which could be caused by Ca2+ channel inhibition by DHA. However, as DHA has been shown to increase IKs in a fraction of guinea pig cardiomyocytes32, it is possible that DHA effects on guinea pig IKs also has a minor contribution to the QT shortening effect. We did also observe a significant prolongation of PR-interval after DHA-GLY application, which could be caused by Ca2+ channel inhibition by DHA-GLY. We have in a recent study shown that modified PUFAs inhibit Nav1.5 and Cav1.2 expressed in Xenopus oocytes, in addition to activating KCNQ1/KCNE1 channels42. Altogether, this suggests that natural and modified PUFAs shorten QT interval and APD in guinea pig hearts by targeting several ion channels. Notably though, the diverse effects of the different PUFAs on guinea pig hearts observed in this study mirror the effects of different PUFAs on KCNQ1/KCNE1 channels. This suggests that modified PUFAs shorten the QT interval, at least in part, by acting on IKs channels in guinea pig hearts and that the efficacy of PUFAs may be increased by engineering an activating effect on the IKs channel.
Regular PUFAs, such as DHA and EPA, have shown mixed results in clinical trials in preventing cardiac arrhythmia and sudden cardiac death. DHA did significantly reduce the prolonged QT interval and APD in our drug-induced model of LQTS in ex-vivo guinea pig hearts. There is a trend that two of the three modified PUFA analogues shorten the QT interval and APD more than DHA in our study, suggesting that these modified PUFAs would be more effective in treating LQTS than regular PUFAs. We tested DHA-GLY in-vivo and found it to significantly shorten the QT interval. Further studies are needed to test whether these modified PUFAs can prevent cardiac arrhythmia and sudden cardiac death in vivo.
Materials and Methods
The animal experiments followed the European Convention for the Protection of Vertebrate Animals used for experimental and other scientific purposes and were approved by The Ministry of Environment and Food of Denmark under License No. 2017-15-0201-01296 or The Linköping University Animal Ethics Committee (Ethical Permit 1659). Surgery of Xenopus laevis to isolate oocytes were approved by The Linköping University Animal Ethics Committee (Ethical Permit 1941). The study conforms with good publishing practice in physiology46.
Drugs
DHA (Docosahexaenoic acid), DHA-GLY (Docosahexaenoyl Glycine), Lin-GLY (Linoleoyl Glycine), N-AT (N-Arachidonoyl Taurine) were purchased from Cayman Chemical Company (Michigan, USA). E-4031 was purchased from Tocris (Bristol, UK). 10% albumin in 0.85% sodium chloride and 0.05% sodium azide was purchased from Sigma-Aldrich, St. Louis MO, USA. DHA-GLY was delivered as a stock solution of 130 mM in EtOH. DHA, Lin-GLY and N-AT were prepared as stock solutions of 30 mM (N-AT) or 100 mM (DHA and Lin-GLY) in EtOH and stored at −20°C. Test solutions were prepared at the day of experiments.
Isolated ex-vivo heart experiments
Female Dunkin Hartley guinea pigs (300–450 g. Charles River, France) were used for the ex-vivo, Langendorff, experiments. The guinea pigs were anesthetised by an intraperitoneal injection of 200 mg/ml pentobarbital and 20 mg/ml lidocaine hydrochloride (Glostrup Apotek, Denmark), 1.5 ml/kg. A tracheostomy was performed, and the guinea pigs were ventilated using a rodent ventilator, 60 strokes/minute and 5 ml volume, (Model 7025 Ugo Basile, Italy). The ribcage was cut open and hearts excised and cannulated in-situ by a small incision near the aortic arch and connected to the Langendorff apparatus (Hugo Sachs, Harvard Apparatus, Germany). The hearts were mounted on a Langendorff-perfusion apparatus and retrogradly perfused at a constant pressure of 60 mmHg with a 37°C modified Krebs’–Henseleit solution (in mmol L-1: NaCl 120, NaHCO3 25, KCl 4, MgCl 0.6, NaH2PO4 0.6, CaCl2 2.5, Glucose 11) saturated with 95% O2 and 5% CO2. The hearts were submerged into a temperature-controlled organ bath containing 37°C carbonated Krebs-Henseleit buffer. Perfusion pressure, coronary flow, ECG and MAP signals were continuously sampled at 2k/s and digitised by a Powerlab 16/30 (ADinstruments) and monitored using LabChart 7 software (ADinstruments). Volume conducted ECGs and monophasic action potentials (MAPs) were recorded throughout the experiments and the four MAP electrodes were placed on the right and left ventricle. The heart rate was controlled by electrical stimulation of the right atrial appendage using a bipolar pacing electrode. Atrial epicardial pacing was performed at 240 BCL/250 BPM with square pulses of 2 ms.
We previously showed that DHA-GLY successfully restored a prolonged QT-interval induced by hERG blocker E403134. Testing of PUFA and modified PUFA in this study followed the same procedure. In brief, after a stabilisation period of 30 minutes, baseline recordings were measured for 2 minutes during pacing at 250 BPM. This was followed by 20 minutes of perfusion of 0.03 μM E4031. At this concentration E4031 is believed to affect the IKr current exclusively47,48. After 20 minutes of perfusion with E4031, the hearts were paced for 2 minutes at 250 BPM. If the QT had prolonged by less than 10 ms the experiment was excluded from the study. This was followed by 30 minutes of perfusion with 10 μM of DHA, DHA-GLY, Lin-GLY or 30 μM of N-AT together with 0.03 μM of E4031 at intrinsic heart rate. Every 10th minute the hearts were paced for 2 minutes at 250 BPM. Left ventricular MAP recordings were used for the analysis of APD90 unless the signal was lost during the experiments. In this case the right ventricular APD signal was used.
In-vivo closed-chest experiments
Female Dunkin Hartley guinea pigs weighing 300–450 g were sedated with 5% isoflurane/oxygen in a sedation box and transferred to the procedure table. It should be noted that isoflurane is reported to mediate QT-prolongation through inhibition of the IKs and IKr current in guinea pigs49,50. The isoflurane level was reduced to a maintenance level of 2.5–3% and delivered through a mask. The body temperature was continuously monitored using a MicroTherma 2 rectal thermometer (ThermoWork Inc, UT, USA) and the guinea pigs were kept at 38–39°C using an (watts) infrared lamp. Custom-made ECG needle electrodes were attached to each limb and connected to an ADinstruments Octal Bio Amp and the signals were processed by an ADinstruments Powerlab 16/30 data acquisition system (Dundedin, New Zealand). An electrophysiological catheter for intracardiac pacing (EPR-802 Millar Inc. USA) was placed in the right atrium via a small surgical incision in the jugular vein. The pacing catheter was connected to a Digitimer DS3 isolated current stimulator (Settings; current amplitude × 100 μA, duration 100 μs × 4) and the hearts were paced with 2 × diastolic threshold. An intravenous catheter was also placed in the jugular vein (I.D. 0.5 mm O.D. 0.8 mm Natsume Seisakusho Japan) for drug administration and connected to a syringe pump (New Era Syringe Pump Systems NE300, Farmingdale, NY, USA).
The guinea pigs were randomised to receive either DHA-GLY 10 mg/ml (corresponds to 40 mg/kg/hr, n = 5) dissolved in vehicle or corresponding amount of vehicle (n = 4) (10% albumin in 0.85% sodium chloride and 0.05% sodium azide). The 15-minute baseline recording contained 1-minute trains with 200 BCL/300 BPM atrial stimulation every 5th minute. At time 0, the infusion pump was started and either vehicle (10% albumin in 0.85% sodium chloride and 0.05% sodium azide) or DHA-GLY was constantly infused over a 30-minute period. During infusion, 1-minute trains with 200 BCL/300 BPM atrial stimulation was performed every 5th minute.
Xenopus oocyte experiments
Xenopus oocytes were surgically isolated at Linköping University. KCNQ1 and KCNE1 channel cRNA were transcribed using the mMessage mMachine T7 kit (Ambion, Fischer Scientific, Sweden). Site-directed mutagenesis was performed using the Quickchange II XL Mutagenesis Kit (QuikChange II XL with 10 XL Gold cells, Agilent, CA, USA) for mutations in KCNE1 to construct the guinea pig like KCNE1 construct. 50 ng of cRNA was injected at a 3:1, weight:weight (KCNQ1:KCNE1) ratio into defolliculated Xenopus laevis oocytes for IKs channel expression. Injected oocytes were incubated at 16°C for two to five days before performing two-electrode voltage clamp experiments using a Dagan CA-1B Amplifier (Dagan, MN, USA). Currents were filtered at 500 Hz and sampled at 5 kHz. The holding voltage was set to −80 mV. Activation curves were generated in steps between −80 to +60 mV in increments of 20 mV (5 s duration). The tail voltage was set to −30 mV. The control solution contained (all in mmol L-1) 88, NaCl, 1 KCl, 15 HEPES, 0.4 CaCl2, and 0.8 MgCl2. pH was set to 7.4 using NaOH. All compounds were bought from Sigma-Aldrich (Stockholm, Sweden). Control or PUFA solution was applied using a Minipuls 3 peristaltic pump (Gilson, WI, USA) until the effect on current amplitude reached steady state (typically 3–8 minutes depending on concentration). The chamber was cleaned in-between each oocyte using ethanol-supplemented control solution.
Electrophysiological analysis was performed in GraphPad Prism 8 (GraphPad Software Inc., CA, USA). To quantify the voltage dependence for channel opening, tail currents were measured shortly after stepping to the tail voltage and plotted against the preceding activation voltage. A Boltzmann function was fitted to the data to generate the conductance versus voltage (G(V)) curve:
where Gmin is the minimal conductance, Gmax the maximal conductance, V50 the midpoint (i.e., the voltage at which the conductance is half the maximal conductance determined from the fit) and s the slope of the curve. The difference in V50 induced by DHA-GLY or Lin-GLY in each oocyte (i.e., ΔV50) was calculated to quantify the shift in the voltage dependence for channel opening.
Statistics
Average values are expressed as mean ± sem. Statistics were calculated using repeated measures one-way ANOVA followed by Tukeýs multiple comparison test, unpaired Student’s-t test followed by Holm-Sidak’s multiple comparisons method, or Student’s t-test. Used statistical tests for each calculation is indicated in the text or figure or table legend. P < 0.05 was considered statistically significant.
Supplementary Material
Supplementary Figure 1. E4031 stably prolongs QT interval in isolated ex-vivo guinea pig hearts. S1 left; Time matched control showing no effect of 0.03 μM E4031 on PR interval and QRS duration. S1 right; time matched control showing stable prolongation of action potential duration and QT interval when hearts were perfused with 0.03 μM E4031. Data shown as mean ± sem, n = 6.
Supplementary Figure 2. Putative PUFA interaction site in KCNQ1/KCNE1 channels responsible for V50 effect. Top view (left) and side view (right) of a homology model of human KCNQ1 based on the Cryo EM structure of Xenopus KCNQ151 with proposed localization of KCNE1 indicated in grey. Putative localization of PUFA near the voltage-sensing domain indicated in red44. The outermost arginines in S4 (green) have been previously shown to be important for the PUFA analogue induced shift in V5034,44. Mutagenesis of a lysine in the S3-S4 loop (yellow) has been previously shown to alter the apparent affinity of PUFA analogues34.
Acknowledgements
This work was supported by the National Institutes of Health (R01GM109762), The Carlsberg Foundation (CF17-0399 + CF18-0164), the Swedish Society for Medical Research, the Swedish Research Council (2017-02040), and travel grants awarded to Mark Skarsfeldt from Paula & Axel Nissens Legat, Ragna Rask-Nielsen Grundforskningsfond and Aase & Ejnar Danielsens Fond.
Footnotes
Conflict of Interest
A patent application based on these results has been submitted by the University of Miami with S.I.L. and H.P.L. identified as inventors.
References
- 1.Nerbonne JM & Kass RS Molecular physiology of cardiac repolarization. Physiol Rev 85, 1205–53 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Chen H, Kim LA, Rajan S, Xu S & Goldstein SA Charybdotoxin binding in the I(Ks) pore demonstrates two MinK subunits in each channel complex. Neuron 40, 15–23 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Morin TJ & Kobertz WR Counting membrane-embedded KCNE beta-subunits in functioning K+ channel complexes. Proc Natl Acad Sci U S A 105, 1478–82 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakajo K, Ulbrich MH, Kubo Y & Isacoff EY Stoichiometry of the KCNQ1 - KCNE1 ion channel complex. Proc Natl Acad Sci U S A 107, 18862–7 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz PJ et al. Prevalence of the congenital long-QT syndrome. Circulation 120, 1761–7 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Priori SG et al. Risk stratification in the long-QT syndrome. N Engl J Med 348, 1866–74 (2003). [DOI] [PubMed] [Google Scholar]
- 7.Schwartz PJ et al. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation 103, 89–95 (2001). [DOI] [PubMed] [Google Scholar]
- 8.Schwartz PJ et al. Left cardiac sympathetic denervation in the management of high-risk patients affected by the long-QT syndrome. Circulation 109, 1826–33 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Roden DM Clinical practice. Long-QT syndrome. N Engl J Med 358, 169–76 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Ayad RF, Assar MD, Simpson L, Garner JB & Schussler JM Causes and management of drug-induced long QT syndrome. Proc (Bayl Univ Med Cent) 23, 250–5 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee W, Windley MJ, Vandenberg JI & Hill AP In Vitro and In Silico Risk Assessment in Acquired Long QT Syndrome: The Devil Is in the Details. Front Physiol 8, 934 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldenberg I et al. Beta-blocker efficacy in high-risk patients with the congenital long-QT syndrome types 1 and 2: implications for patient management. J Cardiovasc Electrophysiol 21, 893–901 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldenberg I et al. Long-term benefit of primary prevention with an implantable cardioverter-defibrillator: an extended 8-year follow-up study of the Multicenter Automatic Defibrillator Implantation Trial II. Circulation 122, 1265–71 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Frishman WH Beta-adrenergic receptor blockers. Adverse effects and drug interactions. Hypertension 11, II21–9 (1988). [DOI] [PubMed] [Google Scholar]
- 15.Barron AJ et al. Systematic review of genuine versus spurious side-effects of beta-blockers in heart failure using placebo control: recommendations for patient information. Int J Cardiol 168, 3572–9 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lydtin H Side effects and contraindications of beta-receptor blocking agents. Klin Wochenschr 55, 415–22 (1977). [DOI] [PubMed] [Google Scholar]
- 17.Persson R, Earley A, Garlitski AC, Balk EM & Uhlig K Adverse events following implantable cardioverter defibrillator implantation: a systematic review. J Interv Card Electrophysiol 40, 191–205 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Den Ruijter HM et al. Pro- and antiarrhythmic properties of a diet rich in fish oil. Cardiovasc Res 73, 316–25 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Pepe M & Recchia FA Omega-3 fatty acids for the prevention of myocardial infarction and arrhythmias. Cardiovasc Ther 28, e1–4 (2010). [DOI] [PubMed] [Google Scholar]
- 20.London B et al. Omega-3 fatty acids and cardiac arrhythmias: prior studies and recommendations for future research: a report from the National Heart, Lung, and Blood Institute and Office Of Dietary Supplements Omega-3 Fatty Acids and their Role in Cardiac Arrhythmogenesis Workshop. Circulation 116, e320–35 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Saravanan P, Davidson NC, Schmidt EB & Calder PC Cardiovascular effects of marine omega-3 fatty acids. Lancet 376, 540–50 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Albert CM Omega-3 fatty acids, ventricular arrhythmias, and sudden cardiac death: antiarrhythmic, proarrhythmic, or neither. Circ Arrhythm Electrophysiol 5, 456–9 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Billman GE The effects of omega-3 polyunsaturated fatty acids on cardiac rhythm: a critical reassessment. Pharmacol Ther 140, 53–80 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Billman GE, Carnes CA, Adamson PB, Vanoli E & Schwartz PJ Dietary omega-3 fatty acids and susceptibility to ventricular fibrillation: lack of protection and a proarrhythmic effect. Circ Arrhythm Electrophysiol 5, 553–60 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belevych AE et al. Dietary omega-3 fatty acids promote arrhythmogenic remodeling of cellular Ca2+ handling in a postinfarction model of sudden cardiac death. PLoS One 8, e78414 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Schacky C Omega-3 Fatty acids: anti-arrhythmic, pro-arrhythmic, or both? Front Physiol 3, 88 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mozaffarian D et al. Plasma phospholipid long-chain omega-3 fatty acids and total and cause-specific mortality in older adults: a cohort study. Ann Intern Med 158, 515–25 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elinder F & Liin SI Actions and Mechanisms of Polyunsaturated Fatty Acids on Voltage-Gated Ion Channels. Front Physiol 8, 43 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang JX & Leaf A Evidence that free polyunsaturated fatty acids modify Na+ channels by directly binding to the channel proteins. Proc Natl Acad Sci U S A 93, 3542–6 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao YF, Gomez AM, Morgan JP, Lederer WJ & Leaf A Suppression of voltage-gated L-type Ca2+ currents by polyunsaturated fatty acids in adult and neonatal rat ventricular myocytes. Proc Natl Acad Sci U S A 94, 4182–7 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao YF, Kang JX, Morgan JP & Leaf A Blocking effects of polyunsaturated fatty acids on Na+ channels of neonatal rat ventricular myocytes. Proc Natl Acad Sci U S A 92, 11000–4 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreno C et al. Marine n-3 PUFAs modulate IKs gating, channel expression, and location in membrane microdomains. Cardiovasc Res 105, 223–32 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Larsson JE, Larsson HP & Liin SI KCNE1 tunes the sensitivity of KV7.1 to polyunsaturated fatty acids by moving turret residues close to the binding site. Elife 7(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liin SI et al. Polyunsaturated fatty acid analogs act antiarrhythmically on the cardiac IKs channel. Proc Natl Acad Sci U S A 112, 5714–5719 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liin SI, Larsson JE, Barro-Soria R, Bentzen BH & Larsson HP Fatty acid analogue N-arachidonoyl taurine restores function of IKs channels with diverse long QT mutations. Elife 5, e20272 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo L, Dong Z & Guthrie H Validation of a guinea pig Langendorff heart model for assessing potential cardiovascular liability of drug candidates. J Pharmacol Toxicol Methods 60, 130–51 (2009). [DOI] [PubMed] [Google Scholar]
- 37.Bunger R, Haddy FJ, Querengasser A & Gerlach E An isolated guinea pig heart preparation with in vivo like features. Pflugers Arch 353, 317–26 (1975). [DOI] [PubMed] [Google Scholar]
- 38.Busch AE et al. The novel class III antiarrhythmics NE-10064 and NE-10133 inhibit IsK channels expressed in Xenopus oocytes and IKs in guinea pig cardiac myocytes. Biochem Biophys Res Commun 202, 265–70 (1994). [DOI] [PubMed] [Google Scholar]
- 39.Carmeliet E & Zaman MY Comparative effects of lignocaine and lorcainide on conduction in the Langendorff-perfused guinea-pig heart. Cardiovasc Res 13, 439–49 (1979). [DOI] [PubMed] [Google Scholar]
- 40.Khalifa M et al. Block of potassium currents in guinea pig ventricular myocytes and lengthening of cardiac repolarization in man by the histamine H1 receptor antagonist diphenhydramine. J Pharmacol Exp Ther 288, 858–65 (1999). [PubMed] [Google Scholar]
- 41.Roden DM, Bennett PB, Snyders DJ, Balser JR & Hondeghem LM Quinidine delays IK activation in guinea pig ventricular myocytes. Circ Res 62, 1055–8 (1988). [DOI] [PubMed] [Google Scholar]
- 42.Bohannon BM, Wu X, Perez ME, Liin SI & Larsson HP Polyunsaturated fatty acids analogues differentially affect cardiac Nav, Cav, and Kv channels through unique mechanisms. Manuscript under revision. [DOI] [PMC free article] [PubMed]
- 43.Kirchhoff JE et al. The KCa2 Channel Inhibitor AP14145, But Not Dofetilide or Ondansetron, Provides Functional Atrial Selectivity in Guinea Pig Hearts. Front Pharmacol 10, 668 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liin SI, Yazdi S, Ramentol R, Barro-Soria R & Larsson HP Mechanisms Underlying the Dual Effect of Polyunsaturated Fatty Acid Analogs on Kv7.1. Cell Rep 24, 2908–2918 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Macleod JC, Macknight AD & Rodrigo GC The electrical and mechanical response of adult guinea pig and rat ventricular myocytes to omega3 polyunsaturated fatty acids. Eur J Pharmacol 356, 261–70 (1998). [DOI] [PubMed] [Google Scholar]
- 46.Persson PB Good publication practice in physiology 2019. Acta Physiol (Oxf) 227, e13405 (2019). [DOI] [PubMed] [Google Scholar]
- 47.Wettwer E, Scholtysik G, Schaad A, Himmel H & Ravens U Effects of the new class III antiarrhythmic drug E-4031 on myocardial contractility and electrophysiological parameters. J Cardiovasc Pharmacol 17, 480–7 (1991). [DOI] [PubMed] [Google Scholar]
- 48.Kugler P, Rast G & Guth BD Comparison of in vitro and computational experiments on the relation of inter-beat interval and duration of repolarization in a specific type of human induced pluripotent stem cell-derived cardiomyocytes. PLoS One 14, e0221763 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suzuki A, Bosnjak ZJ & Kwok WM The effects of isoflurane on the cardiac slowly activating delayed-rectifier potassium channel in Guinea pig ventricular myocytes. Anesth Analg 96, 1308–1315 (2003). [DOI] [PubMed] [Google Scholar]
- 50.Nagasawa Y et al. Sensitivity of inhalation anesthetics isoflurane and sevoflurane for the drug-induced QT-interval prolongation in guinea pigs. J Pharmacol Sci, 10.1016/j.jphs.2020.02.005 (2020). [DOI] [PubMed] [Google Scholar]
- 51.Sun J & MacKinnon R Cryo-EM Structure of a KCNQ1/CaM Complex Reveals Insights into Congenital Long QT Syndrome. Cell 169, 1042–1050 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. E4031 stably prolongs QT interval in isolated ex-vivo guinea pig hearts. S1 left; Time matched control showing no effect of 0.03 μM E4031 on PR interval and QRS duration. S1 right; time matched control showing stable prolongation of action potential duration and QT interval when hearts were perfused with 0.03 μM E4031. Data shown as mean ± sem, n = 6.
Supplementary Figure 2. Putative PUFA interaction site in KCNQ1/KCNE1 channels responsible for V50 effect. Top view (left) and side view (right) of a homology model of human KCNQ1 based on the Cryo EM structure of Xenopus KCNQ151 with proposed localization of KCNE1 indicated in grey. Putative localization of PUFA near the voltage-sensing domain indicated in red44. The outermost arginines in S4 (green) have been previously shown to be important for the PUFA analogue induced shift in V5034,44. Mutagenesis of a lysine in the S3-S4 loop (yellow) has been previously shown to alter the apparent affinity of PUFA analogues34.


