Abstract
Aims
To provide multi-national, multi-ethnic data on the clinical characteristics and prognosis of patients with microvascular angina (MVA).
Methods and results
The Coronary Vasomotor Disorders International Study Group proposed the diagnostic criteria for MVA. We prospectively evaluated the clinical characteristics of patients according to these criteria and their prognosis. The primary endpoint was the composite of major cardiovascular events (MACE), verified by institutional investigators, which included cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, and hospitalization due to heart failure or unstable angina. During the period from 1 July 2015 to 31 December 2018, 686 patients with MVA were registered from 14 institutes in 7 countries from 4 continents. Among them, 64% were female and the main ethnic groups were Caucasians (61%) and Asians (29%). During follow-up of a median of 398 days (IQR 365–744), 78 MACE occurred (6.4% in men vs. 8.6% in women, P = 0.19). Multivariable Cox proportional hazard analysis disclosed that hypertension and previous history of coronary artery disease (CAD), including acute coronary syndrome and stable angina pectoris, were independent predictors of MACE. There was no sex or ethnic difference in prognosis, although women had lower Seattle Angina Questionnaire scores than men (P < 0.05).
Conclusions
This first international study provides novel evidence that MVA is an important health problem regardless of sex or ethnicity that a diagnosis of MVA portends a substantial risk for MACE associated with hypertension and previous history of CAD, and that women have a lower quality of life than men despite the comparable prognosis.
Keywords: Microvascular angina, Coronary microvascular dysfunction, Prognosis
Graphical Abstract
See page 4601 for the editorial comment for this article ‘Microvascular angina: quo tendimus?’, by R. de Silva and K. Cheng,https://doi.org/10.1093/eurheartj/ehab534.
Introduction
Angina pectoris has been considered to be mainly caused by atherosclerotic obstructive coronary artery disease (CAD).1 However, up to 50% of patients undergoing diagnostic coronary angiography for typical chest pain have angiographically normal coronary arteries or non-obstructive CAD.2 In such cases, coronary functional abnormalities are implicated, including epicardial coronary artery spasm and coronary microvascular dysfunction (CMD).3 The latter is typically defined as increased susceptibility to vasoconstrictor stimuli resulting in microvascular spasm and/or impaired dilatation of coronary microvessels, with resultant inadequate increase in blood flow in response to stress.4–6 Thus, CMD may be the underlying mechanism in a large proportion of angina patients.
The term microvascular angina (MVA), originally proposed by Cannon and Epstein in 1988,7 is used for angina/myocardial ischaemia attributable to CMD. Recently, several studies with either invasive or non-invasive techniques for assessment of coronary physiology have provided extensive data, improving what is known about CMD and microvascular ischaemia.8 , 9 In addition, as the COronary VAsomotor Disorders International Study (COVADIS) Group, we have proposed the diagnostic criteria of MVA.10 Briefly, the diagnosis of MVA is established based upon symptoms suggestive of myocardial ischaemia in the absence of obstructive CAD (<50% diameter reduction and/or fractional flow reserve >0.80) associated with objective evidence of myocardial ischaemia and impaired coronary microvascular function defined by the following four findings: reduced coronary flow reserve (CFR), microvascular spasm, increased microvascular resistance, and/or coronary ‘slow flow phenomenon’.10
To date, clinical studies have mainly been the single centre. Given the lack of evidence from international multi-centre studies, the clinical characteristics, and prognosis of patients with MVA remain to be fully elucidated. Our first objective was to study the clinical characteristics and health outcomes of patients with MVA in a large, prospective, international registry. Our second objective was to assess for associations by sex and ethnicity. Thus, in the present study, we undertook a multinational, multi-centre, multi-ethnic, prospective, observational, and longitudinal cohort study.
Methods
Study population
Fourteen medical centres in seven countries from four continents participated in the present study. Data collection was performed via a standardized electronic case report system established by the Japanese Coronary Spasm Association.11 We enrolled patients who fulfilling the COVADIS diagnostic criteria for MVA as follows: (i) signs and/or symptoms of myocardial ischaemia, (ii) absence of obstructive CAD, (iii) objective evidence of myocardial ischaemia, and (iv) evidence of impaired coronary microvascular function, as determined by the clinical site (Figure 1, Supplementary material online, Table S1).10 Objective evidence of myocardial ischaemia, impaired coronary microvascular function, and ischaemic symptoms was determined by attending COVADIS site physicians using detailed criteria below. Clinical characteristics, cardiovascular risk factors (including body mass index, history of hypertension, dyslipidaemia, diabetes mellitus, and current smoking), diagnostic approaches, and the trend of medical therapies for contemporary MVA patients, particularly in terms of ethnic and sex differences were documented (Supplementary material online, Methods).
Figure 1.
Patient enrolment and follow-up.
Obstructive CAD was defined as any coronary stenosis of >50% reduction in diameter by conventional angiography or computed tomography angiography, and those patients with obstructive CAD were excluded. Objective evidence of myocardial ischaemia was evaluated using non-invasive stress testing. Evidence of myocardial ischaemia was obtained by rest/stress ECG and/or by means of non-invasive imaging by assessing either myocardial perfusion with single photon emission computed tomography (SPECT), positron emission tomography (PET), cardiac magnetic resonance (CMR), and left ventricular wall motion abnormality with stress echocardiography.10
Non-invasive assessment of CMD was obtained by measuring CFR for the whole left ventricle with PET, CMR, or by measuring Doppler flow velocity reserve on the left anterior descending coronary artery. The CFR cut-off was set at <2.5.4 , 6
Coronary microvascular dysfunction was assessed invasively by using coronary functional testing, including measurements of CFR and/or microvascular resistance and/or acetylcholine provocation testing for coronary microvascular spasm.10 The CFR cut-off was defined at <2.0 for invasive measurement.8–10 , 12 Abnormal microvascular resistance was defined as more than 25 units of index of microcirculatory resistance.8–10 Coronary slow flow phenomenon was defined as TIMI frame count >25 at invasive coronary angiography.8–10 Coronary microvascular spasm was defined as the reproduction of symptoms, ischaemic ECG changes, but no epicardial coronary spasm during acetylcholine provocation testing.10 In the present study, definitive MVA was diagnosed if all four criteria were present, while suspected MVA was diagnosed if symptoms of ischaemia were present with no obstructive CAD but one of the following only: objective evidence of myocardial ischaemia or evidence of CMD. Both definitive and suspected MVA were included in the present study. Seventy-five patients (11%) were diagnosed as having MVA by using non-invasive assessment alone, including exercise stress ECG (n = 34), SPECT (n = 18), PET (n = 16), stress echocardiography (n = 4), and CMR (n = 3). Study variables were obtained at enrolment, including patient demographic profiles, cardiovascular risk factors, past history of CAD including acute coronary syndrome and stable angina pectoris, non-invasive diagnostic modalities for myocardial ischaemia, invasive assessment of microvascular function, initial treatment after diagnosis, and assessment of quality of life (QOL) by the Seattle Angina Questionnaire (SAQ) (Supplementary material online, Methods).13
From 1 July 2015 to 31 December 2018, the participating centres prospectively enrolled 686 patients with MVA (Figure 1). All patients underwent clinical assessments and received usual medical care as determined by attending physicians. They did not receive any investigational treatments for MVA before study enrolment and during the follow-up period. Follow-up of each patient was conducted at least once from study entry to the end of December 2019 either by a telephone call or a site visit, depending on the approach considered most practical and effective.
The ethics committee of Tohoku University Graduate School of Medicine approved the study protocol (No. 2015-1-188) followed by the ethics committee and/or sponsors at each participating institute, in compliance with the Declaration of Helsinki (UMIN000035177). All patients provided written informed consent for research before study entry.
Study endpoints
The primary endpoint was the composite of major cardiovascular events (MACE), including cardiovascular death, non-fatal myocardial infarction (MI), non-fatal stroke, and hospitalization due to heart failure or unstable angina determined by the institutional investigators at each COVADIS site or an independent clinical event committee. Definition of MI was based on the third universal definition,14 and that of unstable angina was based on the presence of ischaemic chest pain and hospitalization within 24 h of most recent symptoms, without elevation in cardiac biomarkers but with ischaemic ECG changes.15 Stroke was defined as neurological deficit due to an ischaemic or haemorrhagic central nervous system event with residual systems >24 h after onset or leading to death.16 For each patient, a MACE was defined as the first occurrence of one of these events during the follow-up period. The associations between baseline characteristics, including sex and medical history, and MACE were evaluated.
Statistical methods
Statistical methods appropriate for epidemiological studies were used for the analysis of the collected data. Baseline continuous variables are presented as means ± SD or medians and interquartile range, depending on the distribution of the data that was tested by Shapiro–Wilk normality test. Categorical variables are presented as counts and percentages. We used the Wilcoxon rank-sum test to compare continuous variables and the Pearson chi-square test to compare categorical variables. Events were analysed as time from enrolment to the first occurrence of any event from the composite endpoint. We used the Kaplan–Meier method to provide survival estimates, which were assessed with a log-rank test. To reduce confounding effects, propensity score methods were used with potential confounding covariates, including age, sex, hypertension, dyslipidaemia, diabetes mellitus, current smoking, previous history of CAD, and previous percutaneous coronary intervention (PCI). Additional supportive analyses included time to the first occurrence of each component of the composite endpoint individually. Event rates of the composite endpoint and of each endpoint are reported separately at 1, 2, and 3 years since enrolment. To examine the determinants of the primary endpoint, we used a multivariable Cox proportional hazard model. A statistical analysis plan was pre-specified before each interim analysis. Multivariable analysis was conducted to analyse outcomes by study variables.
Results
Baseline clinical characteristics
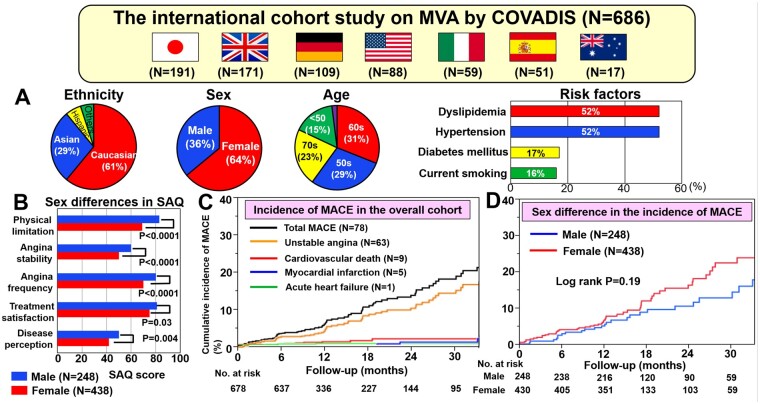
Patient enrolment and their follow-up are shown in Figure 1. From July 2015 to December 2018, a total of 686 patients with MVA (M/F 248/438, 61.2 ± 11.8 years) were finally enrolled, including 191 cases in Japan, 171 in the UK, 109 in Germany, 88 in the USA, 59 in Italy, 51 in Spain, and 17 in Australia. Follow-up rate was 97% (18 lost among 704 patients). Their pertinent clinical characteristics are summarized in Table 1. Approximately two-thirds (64%) were female and the main ethnic groups were Caucasians (61%), Asians (29%), and Hispanics (6%). More than half of them had hypertension (52%) and/or dyslipidaemia (52%), whereas relatively fewer patients had diabetes mellitus (17%) or were current smokers (16%). Although 233 patients (34%) had the previous history of CAD, including acute coronary syndrome and stable angina pectoris, only 9% had undergone PCI previously. Considering risk factors for coronary atherosclerosis, current smoking, previous history of CAD, vasospastic angina, and previous PCI were more prevalent among men, whereas the prevalence of other risk factors were comparable in both sexes (Table 1).
Table 1.
Baseline clinical characteristics of patients with MVA
| Characteristics | Total cohort (N = 686) | Male (N = 248) | Female (N = 438) | P-value |
|---|---|---|---|---|
| Age (years), mean ± SD | 61.7 ± 11.8 | 61.6 ± 12.7 | 60.9 ± 11.2 | 0.45 |
| Race or ethnic group, n (%) | <0.0001 | |||
| Caucasian | 419 (61) | 111 (45) | 308 (70) | |
| Asian | 199 (29) | 113 (46) | 86 (20) | |
| Hispanic | 40 (6) | 21 (8) | 19 (4) | |
| Black | 16 (2) | 1 (0.4) | 15 (3.4) | |
| Others | 12 (2) | 2 (0.8) | 10 (2) | |
| Body mass index (kg/m2), mean ± SD | 26.1 ± 5.9 | 25.9 ± 4.4 | 26.3 ± 6.7 | 0.48 |
| Hypertension, n (%) | 358 (52) | 139 (56) | 219 (50) | 0.13 |
| Dyslipidaemia, n (%) | 358 (52) | 119 (48) | 239 (55) | 0.09 |
| Diabetes mellitus, n (%) | 116 (17) | 51 (21) | 65 (15) | 0.06 |
| Current smoking, n (%) | 108 (16) | 49 (20) | 59 (13) | 0.03 |
| Previous history of CAD, n (%) | 233 (34) | 70 (28) | 163 (37) | 0.02 |
| Previous PCI, n (%) | 65 (9) | 46 (19) | 19 (4) | <0.0001 |
| LVEF (mean, %) | 65.6 ± 10.2 | 64.3 ± 11.3 | 66.6 ± 9.2 | 0.07 |
| Symptoms, n (%) | ||||
| Angina | 465 (68) | 154 (62) | 311 (71) | 0.0003 |
| Rest angina | 245 (36) | 94 (38) | 151 (34) | 0.37 |
| Effort angina | 99 (14) | 39 (16) | 60 (14) | 0.47 |
| Rest and effort angina | 121 (18) | 21 (8) | 100 (23) | <0.0001 |
| Shortness of breath | 125 (18) | 23 (9) | 102 (23) | <0.0001 |
| Other | 135 (19) | 55 (22) | 80 (18) | 0.0003 |
| Ischaemic ECG changes during angina attack, n (%) | 177 (26) | 94 (38) | 83 (19) | <0.0001 |
| ST-segment elevation, n (%) | 13 (2) | 12 (5) | 1 (0.2) | <0.0001 |
| ST-segment depression, n (%) | 164 (24) | 82 (33) | 82 (19) | <0.0001 |
| Seattle Angina Questionnaire score, median (IQR) | ||||
| Physical limitation | 75 (53-93) | 83 (64-97) | 69 (50-89) | <0.0001 |
| Angina stability | 50 (25-75) | 75 (50-100) | 50 (25-75) | <0.0001 |
| Angina frequency | 70 (50-90) | 80 (60-100) | 70 (50-80) | <0.0001 |
| Treatment satisfaction | 75 (63-88) | 81 (63-94) | 75 (56-88) | 0.01 |
| Disease perception | 50 (25-67) | 50 (33-67) | 42 (25-58) | 0.002 |
| Objective evidence of myocardial ischaemia in non-invasive tests, n (%) | 402 (59) | 129 (52) | 273 (62) | 0.009 |
| Exercise stress ECG, n (%) | 231 (34) | 79 (32) | 152 (35) | 0.45 |
| Doppler/stress echocardiography, n (%) | 86 (13) | 38 (15) | 48 (11) | 0.10 |
| CMR, n (%) | 68 (10) | 10 (4) | 58 (13) | <0.0001 |
| SPECT, n (%) | 42 (6) | 14 (6) | 28 (6) | 0.69 |
| PET, n (%) | 41 (6) | 5 (2) | 36 (8) | 0.0004 |
| Evidence of impaired microvascular function, n (%) | ||||
| Microvascular spasm | 288 (42) | 100 (40) | 188 (43) | 0.51 |
| Impaired coronary flow reserve | 241 (35) | 100 (40) | 141 (32) | 0.51 |
| Abnormal coronary microvascular resistance | 99 (14) | 46 (19) | 53 (12) | 0.03 |
| Slow flow/TIMI frame count abnormalities | 45 (6) | 20 (8) | 25 (6) | 0.24 |
| Initial treatment after diagnosis, n (%) | ||||
| Statin | 424 (62) | 141 (57) | 283 (65) | 0.04 |
| Nitrate | 295 (43) | 83 (33) | 212 (48) | 0.0001 |
| Calcium channel blocker | 249 (36) | 106 (43) | 143 (33) | 0.009 |
| Beta-blocker | 249 (36) | 83 (33) | 166 (38) | 0.25 |
| Angiotensin-converting enzyme inhibitor | 169 (25) | 57 (23) | 112 (26) | 0.49 |
| Angiotensin II receptor blocker | 117 (17) | 41 (17) | 76 (17) | 0.78 |
CAD, coronary artery disease; CMR cardiac magnetic resonance; ECG, electrocardiogram; IQR, interquartile range; LVEF, left ventricular ejection fraction; MVA, microvascular angina; PCI, percutaneous coronary intervention; PET, positron emission tomography; SD, standard deviation; SPECT, single photon emission computed tomography; TIMI, thrombolysis in myocardial infarction.
Predominant symptoms were chest pain or chest discomfort (68%), especially at rest (36%), and shortness of breath on exertion (18%). Ischaemic ECG changes during chest pain were documented in 26%, where the most frequent finding was ST-segment depression (24%). Of note, regarding SAQ scores, women had significantly lower scores compared with men in all items, indicating lower QOL in women (Table 1).
Of the 686 patients, 59% had objective evidence of myocardial ischaemia during non-invasive stress testing, including exercise stress ECG (34%), Doppler/stress echocardiography (13%), CMR (10%), SPECT (6%), and PET (6%). Invasive assessments of coronary microvascular function were performed in 611 patients (89%); these showed that microvascular spasm was most frequent (42%), followed by impaired CFR (35%), abnormal microvascular resistance (14%), and slow flow abnormalities (6%) (Table 1). In the present study, 261 patients (38%) had comorbid epicardial coronary spasm evaluated by acetylcholine provocation testing. For the initial treatment after the diagnosis of MVA was made, patients received oral treatment with statins (62%), nitrates (43%), calcium channel blockers (36%), and/or beta-blockers (36%), as determined by attending physicians (Table 1).
Clinical outcomes and prognostic factors
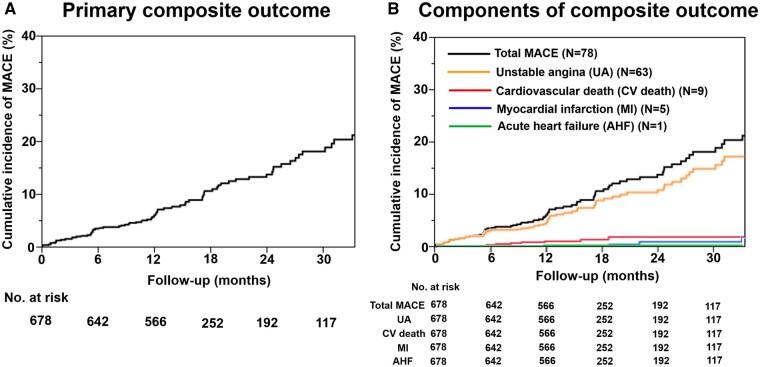
At a median follow-up of 398 days (interquartile range 365–744 days), there were 78 MACE in the overall cohort, including hospitalization for unstable angina (n = 63), cardiovascular death (n = 9), non-fatal MI (n = 5), and hospitalization for acute heart failure (n = 1). The annual incidence of the primary composite of MACE in the overall cohort was 7.7% per patient year (Figure 2A). Among these, the incidence of hospitalization for unstable angina (5.9% per patient year) was higher than that of the other adverse events, given that the rate of cardiovascular death was 1.0% per patient year and the rate of non-fatal MI was 0.5% per patient year (Figure 2B).
Figure 2.
Kaplan–Meier curves for MACE in the overall cohort. Kaplan–Meier curve for (A) the primary composite outcome and (B) each component of the composite outcome.
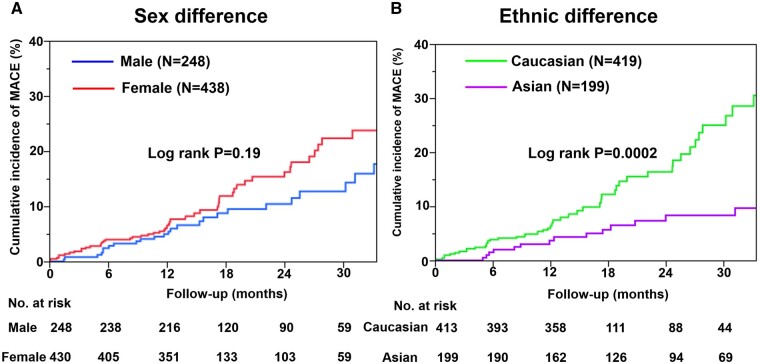
Importantly, there was no significant sex difference in the incidence of MACE (male 6.4% vs. female 8.6% per patient year, P = 0.19) (Figure 3A). Furthermore, the incidence of MACE was comparable even after propensity score matching (Supplementary material online, Figure S1). Considering MACE by ethnic group, the overall impact of race or ethnicity was significant, where Caucasians had a higher risk of MACE than Asians (9.3% vs. 4.5% per patient year, P = 0.0002) (Figure 3B). However, after propensity score matching, there was no significant difference in the incidence of MACE between the two ethnicities (Supplementary material online, Figure S2).
Figure 3.
Kaplan–Meier curves for MACE by patient group. (A) Sex difference in the incidence of MACE. (B) Ethnic difference in the incidence of MACE (Caucasian vs. Asian).
Furthermore, multivariable Cox proportional hazard analysis showed that hypertension and previous history of CAD, including acute coronary syndrome and stable angina pectoris, were independent predictors for the occurrence of MACE in patients with MVA (Table 2).
Table 2.
Prognostic factors for MACE in patients with MVA (Cox proportional hazard model)
| Univariable analysis |
Multivariable analysis |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Age | 0.987 | 0.970–1.004 | 0.14 | |||
| Female sex | 1.358 | 0.857–2.152 | 0.19 | |||
| Hypertension | 1.802 | 1.148–2.831 | 0.01 | 1.692 | 1.067–2.681 | 0.03 |
| Dyslipidaemia | 1.362 | 0.877–2.115 | 0.17 | |||
| Diabetes mellitus | 1.461 | 0.887–2.407 | 0.14 | |||
| Current smoking | 0.868 | 0.479–1.572 | 0.64 | |||
| Previous history of CAD | 2.233 | 1.448–3.442 | 0.005 | 2.032 | 1.312–3.147 | 0.001 |
| Family history of CAD | 1.700 | 1.093–2.645 | 0.02 | |||
CAD, coronary artery disease including acute coronary syndrome and stable angina pectoris; CI, confidence interval; HR, hazard ratio; MVA, microvascular angina.
Discussion
To the best of our knowledge, this is the first international and prospective study that focused on the clinical characteristics and prognosis of contemporary patients with MVA accurately diagnosed by established uniform criteria. We have found that patients with MVA are at substantial risk of MACE, especially hospitalization for unstable angina, and that hypertension and previous history of CAD were independent predictors of MACE, and that there were no sex differences in prognosis despite lower QOL in women. There were no differences in prognosis between ethnic groups.
Diagnosis of microvascular angina
Coronary microvascular dysfunction can develop in variable clinical settings and can be triggered by multiple pathogenetic mechanisms.5 Microvascular angina is attributable to varying degrees of disruption of normal coronary physiology, which may subsequently impair the capacity of myocardial blood flow to satisfy myocardial oxygen demand.4 In the present study, all patients were registered based on objective evidence of myocardial ischaemia and/or microvascular dysfunction according to the established diagnostic criteria for MVA by the COVADIS Group.10 Thus, employing the standardized criteria for MVA allowed us to utilize different diagnostic strategies, including non-invasive and invasive assessments in relation to their institutional feasibility and safety.
Clinical characteristics of patients with microvascular angina
Coronary microvascular dysfunction has been associated with cardiovascular risk factors, including age, hypertension, dyslipidaemia, and diabetes mellitus,17 although the prevalence of these conditions in patients with the MVA syndrome remains unknown. Moreover, CMD is also associated with clinical syndromes caused by cardiovascular disease, including left ventricular hypertrophy and heart failure with preserved ejection fraction.17 In the present study, more than half of the patients with MVA had hypertension (52%) and/or dyslipidaemia (52%), whereas relatively fewer patients had diabetes mellitus (17%) or were current smokers (16%). The prevalence of traditional coronary risk factors in our patients with MVA is consistent with a previous report that targeted patients with myocardial ischaemia and non-obstructive CAD.18
Prognosis of patients with microvascular angina
Previously, the prognosis of patients with MVA has been suggested to be good;19 however, the sample size was small, and a considerable proportion of patients lacked markers of potential worse outcome.20 In the present study, the incidence of the primary composite of MACE in the overall cohort (7.7% per patient year) was comparable to that reported by Pepine et al. 20 Although the prevalence of atherosclerotic risk factors was comparable with that of the previous studies of non-obstructive CAD, the incidence of subsequent acute MI in the present study was lower than that reported before.19 , 20
Sex and ethnic differences in microvascular angina
In previous studies, women, as compared with men, were more likely to have angina without significant coronary artery stenosis but had a comparable risk of cardiovascular events.18 , 20 Of note, in the present study, there was no significant sex-related difference in the incidence of cardiovascular events even after propensity score matching with potential confounding factors. Additionally, as demonstrated in Table 1, women had significantly lower SAQ scores than men, indicating worse QOL in the former. To date, a few studies addressed sex-related differences in QOL in patients with chronic coronary syndromes, but they did not address the underlying mechanisms of ischaemia.21 Recently, the CorMicA randomized controlled trial of stratified medicine reported improvements in anginal symptoms and QOL in patients with CMD in general, but not specifically sex-related differences.8 Female hormones are involved in sex differences in the perception of chest symptoms in patients with MVA.18 Furthermore, while no previous study has addressed ethnic differences in patients with MVA, in the present study, the incidence of adverse cardiovascular events was significantly higher in Caucasians than in Asians (Figure 3B), in part relating to differences in the burden of vascular risk factors since, after propensity score matching, the incidence of MACE was not different between the two ethnic groups (Supplementary material online, Table S2, Figure S2).
Treatment of microvascular angina
The management of MVA represents a major unmet need because the lack of large, randomized studies with homogeneous patient group makes it difficult to generate evidence-based recommendations.22 Two outcome trials are currently underway: the Women’s IschemiA TRial to Reduce Events In Non-ObstRuctive CAD (WARRIOR), a multicentre, prospective, randomized, blinded outcome evaluation (PROBE design) of a pragmatic strategy of intensive medical treatment vs. usual care in 4422 symptomatic women with ischaemia and no obstructive CAD (NCT 03417388); and the International Coronary Microvascular Angina Trial (iCorMicA: NCT04674449) of stratified medicine in angina. Furthermore, the treatment of MVA has been empirical because its pathophysiology appears to be multifactorial with overlapping phenotypes that often coexist. Recent reports discussed the management of MVA patients and suggested some potential treatment strategies.18 , 22 Anti-atherothrombotic treatments with statins, angiotensin-converting enzyme inhibitors (ACE-I) or angiotensin receptor blockers (ARB), and low-dose aspirin may improve symptoms and outcomes of MVA patients.23 , 24 Patients enrolled in the present study received oral treatment with statins (62%), nitrates (43%), ACE-I (25%), and ARB (17%) as determined by attending physicians after the diagnosis of MVA was made. Furthermore, conventional anti-anginal therapies, including beta-blockers, calcium channel blockers, and nitrates, are reasonable first-line regimens for MVA patients given the underlying pathophysiology.25 , 26 Regarding vasodilators, there was a sex difference in the present study as women received more frequently nitrates, whereas men received more frequently calcium channel blockers. More frequent use of nitrates in women might represent more frequent anginal attacks than in men, which is consistent with our observations of significantly lower SAQ scores in physical limitation and angina stability among women.
Study limitations
Although our study has multiple strengths (the first international study on MVA with multiple ethnicities and countries, large sample size, use of consensus diagnostic criteria for MVA, and high follow-up rate, etc.), several limitations should be mentioned. First, the present study was an observational study without a reference group. Second, the relatively small number of MACE during follow-up limits the statistical power of the present study and might have led to data overfitting. Third, the majority of MACE (90%) were hospitalization for unstable angina. However, the prevalence of hospitalization for unstable angina to total MACE was comparable with previous reports.9 , 27 Fourth, we excluded patients with obstructive CAD by conventional angiography or coronary computed tomography and have no data regarding functional relevance of coronary artery stenoses evaluated by physiological indices. Finally, we have no data regarding changes in or adherence to medical therapy, or symptoms and/or QOL (e.g. SAQ) during follow-up. These issues remain to be examined in future studies.
Conclusions
This first international study provides evidence on the prognostic impact of MVA and novel insights into sex and ethnicity. Female patients have lower QOL than male patients despite the comparable prognosis. Vascular risk factors are prevalent and a target for therapy. Further studies are needed to address knowledge gaps including risk stratification and effective treatments of patients with MVA.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
We thank all the staff who supported this study, including Chassidi Garrett, Sonoka Goto, Koichi Kaikita, Hideki Kawai, Sarah Long, Jenna Maughn, Valeria Martínez Pereyra, Sabine Nägele, Andreas Seitz, Chrisandra Shufelt, and Janet Wei.
Funding
The Japanese Coronary Spasm Association (funded by the Japan Heart Foundation). The British Heart Foundation (PG/17/2532884; RE/18/6134217 to. C.B.); The National Heart, Lung and Blood Institutes (N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, grants U0164829, U01 HL649141, U01 HL649241, K23HL105787, T32HL69751, R01 HL090957, 1R03AG032631) from the National Institute on Aging, GCRC (MO1-RR00425) from the National Center for Research Resources, the National Center for Advancing Translational Sciences (UL1TR000124 and UL1TR000064 to C.N.B.M. and C.J.P.); Funding from The Hospital Research Foundation to J.B.; the Berthold-Leibinger-Foundation, Germany to P.O. and U.S.
Conflict of interest: F.C. reports speaker fees from AstraZeneca, Amgen and Servier and institutional agreements between his employer, the Catholic University, and Biotronik, Boheringer Ingelheim. C.N.B.M. reports lecturer fees from Abbott Diagnostics, board director fees from iRhythm, consulting fees from Caladrius, and advisory board fees from Bayer. C.B. declares institutional agreements between his employer, the University of Glasgow, and AbbottVascular, AstraZeneca, Boehringer Ingelheim, Coroventis, DalCor, GSK, HeartFlow, Novartis, and Philips. P.G.C. reports speaking honoraria from Servier and Abbott. P.O. reports personal fees from Bayer Healthcare, Pfizer and Philips/Volcano. U.S. reports speaker and consulting fees from Amgen, Bristol-Myers Squibb, Boehringer- Ingelheim, Abbott, Servier, Astra-Zeneca, Bayer, and Pfizer. T.F. has acted as a speaker for Abbott Vascular, Boehringer Ingelheim and Novartis. None of the declared interests regard the submitted work. All other authors have nothing to disclose.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Contributor Information
Hiroaki Shimokawa, Department of Cardiovascular Medicine, Tohoku University Graduate School of Medicine, 1-1 Seiryo-machi, Aoba-ku, Sendai 980-8574, Sendai, Japan; International University of Health and Welfare, Narita, Japan.
Akira Suda, Department of Cardiovascular Medicine, Tohoku University Graduate School of Medicine, 1-1 Seiryo-machi, Aoba-ku, Sendai 980-8574, Sendai, Japan.
Jun Takahashi, Department of Cardiovascular Medicine, Tohoku University Graduate School of Medicine, 1-1 Seiryo-machi, Aoba-ku, Sendai 980-8574, Sendai, Japan.
Colin Berry, British Heart Foundation Glasgow Cardiovascular Research Centre, Institute of Cardiovascular and Medical Sciences, University of Glasgow, UK.
Paolo G Camici, Vita Salute University and San Raffaele Hospital, Milan, Italy.
Filippo Crea, Department of Cardiology, Fondazione Policlinico Universitario A. Gemelli IRCCS, Università Cattolica del Sacro Cuore, Rome, Italy.
Javier Escaned, Department of Cardiology, Hospital Clínico San Carlos IDISSC and Universidad Complutense de Madrid, Madrid, Spain.
Tom Ford, British Heart Foundation Glasgow Cardiovascular Research Centre, Institute of Cardiovascular and Medical Sciences, University of Glasgow, UK.
Eric Yii, British Heart Foundation Glasgow Cardiovascular Research Centre, Institute of Cardiovascular and Medical Sciences, University of Glasgow, UK.
Juan Carlos Kaski, Department of Cardiovascular Science, Cardiovascular and Cell Sciences Res Institute, St George’s, University of London, UK.
Takahiko Kiyooka, Department of Cardiology, Tokai University Oiso Hospital, Oiso, Japan.
Puja K Mehta, Department of Medicine, Division of Cardiology, Emory University, Atlanta, GA, USA.
Peter Ong, Department of Cardiology, Robert-Bosch-Krankenhaus, Stuttgart, Germany.
Yukio Ozaki, Department of Cardiology, Fujita Health University School of Medicine, Toyonaka, Aichi, Japan.
Carl Pepine, Division of Cardiovascular Medicine, University of Florida, College of Medicine, Gainesville, FL, USA.
Ornella Rimoldi, Institute of Molecular Bioimaging and Physiology, Consiglio Nazionale delle Ricerche, Segrate, Italy.
Basmah Safdar, Department of Emergency Medicine, Yale University, New Haven, CT, USA.
Udo Sechtem, Department of Cardiology, Robert-Bosch-Krankenhaus, Stuttgart, Germany.
Kenichi Tsujita, Department of Cardiovascular Medicine, Graduate School of Medical Sciences, Kumamoto University, Kumamoto, Japan.
Satoshi Yasuda, Department of Cardiovascular Medicine, Tohoku University Graduate School of Medicine, 1-1 Seiryo-machi, Aoba-ku, Sendai 980-8574, Sendai, Japan.
John F Beltrame, The Discipline of Medicine, University of Adelaide, Basil Hetzel Institute, Central Adelaide Local Health Network, Adelaide, South Australia, Australia.
C Noel Bairey Merz, Department of Cardiology, Cedars-Sinai Medical Center, Barbra Streisand Women's Heart Center, Smidt Heart Institute, Los Angeles, CA, USA.
References
- 1. Ohman EM. Chronic stable angina. N Engl J Med 2016;374:1167–1176. [DOI] [PubMed] [Google Scholar]
- 2. Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, Brindis RG, Douglas PS. Low diagnostic yield of elective coronary angiography. N Engl J Med 2010;362:886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shimokawa H. 2014 Williams Harvey Lecture: importance of coronary vasomotion abnormalities - from bench to bedside. Eur Heart J 2014;35:3180–3193. [DOI] [PubMed] [Google Scholar]
- 4. Camici PG, d'Amati G, Rimoldi O. Coronary microvascular dysfunction: mechanisms and functional assessment. Nat Rev Cardiol 2015;12:48–62. [DOI] [PubMed] [Google Scholar]
- 5. Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med 2007;356:830–840. [DOI] [PubMed] [Google Scholar]
- 6. Crea F, Camici PG, Merz CNB. Coronary microvascular dysfunction: an update. Eur Heart J 2014;35:1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cannon RO, Epstein SE. “Microvascular angina” as a cause of chest pain with angiographically normal coronary arteries. Am J Cardiol 1988;61:1338–1343. [DOI] [PubMed] [Google Scholar]
- 8. Ford TJ, Stanley B, Good R, Rocchiccioli P, McEntegart M, Watkins S, Eteiba H, Shaukat A, Lindsay M, Robertson K, Hood S, McGeoch R, McDade R, Yii E, Sidik N, McCartney P, Corcoran D, Collison D, Rush C, McConnachie A, Touyz RM, Oldroyd KG, Berry C. Stratified medical therapy using invasive coronary function testing in angina: the CorMicA Trial. J Am Coll Cardiol 2018;72:2841–2855. [DOI] [PubMed] [Google Scholar]
- 9. Suda A, Takahashi J, Hao K, Kikuchi Y, Shindo T, Ikeda S, Sato K, Sugisawa J, Matsumoto Y, Miyata S, Sakata Y, Shimokawa H. Coronary functional abnormalities in patients with angina and non-obstructive coronary artery disease. J Am Coll Cardiol 2019;74:2350–2360. [DOI] [PubMed] [Google Scholar]
- 10. Ong P, Camici PG, Beltrame JF, Crea F, Shimokawa H, Sechtem U, Kaski JC, Merz CNB. Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for microvascular angina. Int J Cardiol 2018;250:16–20. [DOI] [PubMed] [Google Scholar]
- 11. Suda A, Takahashi J, Beltrame JF, Berry C, Camici PG, Crea F, Escaned J, Ford T, Kaski JC, Kiyooka T, Metha PK, Ong P, Ozaki Y, Pepine C, Rimoldi O, Safdar B, Sechtem U, Tsujita K, Yii E, Merz CNB, Shimokawa H. Coronary Vasomotion Disorders International Study COVADIS Group. International prospective cohort study of microvascular angina -rationale and design. Int J Cardiol Heart Vasc 2020;31:100630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee JM, Choi KH, Hwang D, Park J, Jung JH, Kim HY, Jung HW, Cho YK, Yoon HJ, Song YB, Hahn JY, Doh JH, Nam CW, Shin ES, Hur SH, Koo BK. Prognostic implication of thermodilution coronary flow reserve in patients undergoing fractional flow reserve measurement. JACC Cardiovasc Interv 2018;11:1423–1433. [DOI] [PubMed] [Google Scholar]
- 13. Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonnell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol 1995;25:333–341. [DOI] [PubMed] [Google Scholar]
- 14. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. Writing Group on the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Eur Heart J 2012;33:2551–2567. [DOI] [PubMed] [Google Scholar]
- 15. Collet JP, Thiele H, Barbato E, Barthelemy O, Bauersachs J, Bhatt DL, Dandale P, Dorobantu M, Edvardsen T, Folliguet T, Gale CP, Gilard M, Jobs A, Juni P, Lambrinou E, Lewis BS, Mehilli J, Meliga E, Merkely B, Mueller C, Roffi M, Rutten FH, Sibbing D, Siontis GCM. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2021;42:1289–1367. [DOI] [PubMed] [Google Scholar]
- 16. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becher K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019;50:e344–e418. [DOI] [PubMed] [Google Scholar]
- 17. Camici PG, Tschöpe C, Di Carli MF, Rimoldi O, Van Linthout S. Coronary microvascular dysfunction in hypertrophy and heart failure. Cardiovasc Res 2020;116:806–816. [DOI] [PubMed] [Google Scholar]
- 18. Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL, Camici PG, Chilian WM, Clayton JA, Cooper LS, Crea F, Di Carli M, Douglas PS, Galis ZS, Gurbel P, Handberg EM, Hasan A, Hill JA, Hochman JS, Iturriaga E, Kirby R, Levine GN, Libby P, Lima J, Mehta P, Desvigne-Nickens P, Olive M, Pearson GD, Quyyumi AA, Reynolds H, Robinson B, Sopko G, Taqueti V, Wei J, Wenger N. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation 2017;135:1075–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaski JC, Rosano GMC, Collins P, Nihoyannopoulos P, Maseri A, Poole-Wilson PA. Cardiac syndrome X: clinical characteristics and left ventricular function. Long-term follow-up study. J Am Coll Cardiol 1995;25:807–814. [DOI] [PubMed] [Google Scholar]
- 20. Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson D, Sopko G, Merz CNB. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia. Results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) Study. J Am Coll Cardiol 2010;55:2825–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sheps DS, Kaufmann PG, Sheffield D, Light KC, McMahon RP, Bonsall R, Maixner W, Carney RM, Freedland KE, Cohen JD, Goldberg AD, Ketterer MW, Raczynski JM, Pepine CJ. Sex differences in chest pain in patients with documented coronary artery disease and exercise-induced ischemia: results from the PIMI study. Am Heart J 2001;142:864–871. [DOI] [PubMed] [Google Scholar]
- 22. Merz CNB, Pepine CJ, Shimokawa H, Berry C. Treatment of coronary microvascular dysfunction. Cardiovasc Res 2020;116:856–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pizzi C, Manfrini O, Fontana F, Bugiardini R. Angiotensin-converting enzyme inhibitors and 3-hydroxy-3-methylglutaryl coenzyme A reductase in cardiac syndrome X: role of superoxide dismutase activity. Circulation 2004;109:53–58. [DOI] [PubMed] [Google Scholar]
- 24. Kayikcioglu M, Payzin S, Yavuzgil O, Kultursay H, Can LH, Soydan I. Benefits of statin treatment in cardiac syndrome-X. Eur Heart J 2003;24:1999–2005. [DOI] [PubMed] [Google Scholar]
- 25. Sütsch G, Oechslin E, Mayer I, Hess OM. Effect of diltiazem on coronary flow reserve in patients with microvascular angina. Int J Cardiol 1995;52:135–143. [DOI] [PubMed] [Google Scholar]
- 26. Bugiardini R, Borghi A, Biagetti L, Puddu P. Comparison of verapamil versus propranolol therapy in syndrome X. Am J Cardiol 1989;63:286–290. [DOI] [PubMed] [Google Scholar]
- 27. Nihei T, Takahashi J, Hao K, Kikuchi Y, Odaka Y, Tsuburaya R, Nishimiya K, Matsumoto Y, Ito K, Miyata S, Sakata Y, Shimokawa H. Prognostic impacts of Rho-kinase activity in circulating leucocytes in patients with vasospastic angina. Eur Heart J 2018;39:952–959. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.