Abstract
Modeling of metastatic disease in animal models is a critical resource to study the complexity of this multi-step process in a relevant system. Available models of metastatic disease to the brain are still far from ideal but they allow to address specific aspects of the biology or mimic clinically relevant scenarios. We not only review experimental models and their potential improvements but also discuss specific answers that could be obtained from them on unsolved aspects of clinical management.
Keywords: brain metastasis, experimental models, therapy, treatment toxicity
Getting to Know the Enemy: The Need for Good Brain Metastasis Models
The complexity of the metastatic process has long been recognized and is comprised out of different dynamic steps: (a) Initially, carcinoma cells become invasive and leave the primary tumor. (b) Subsequently, they intravasate into the vasculature, where they have to evade immune attack and survive in the circulation. (c) A subset of circulating tumor cells will eventually extravasate into the foreign tissue. (d) Here, they are confronted with a potentially hostile microenvironment, forcing them to adapt and form a hospitable microenvironment, which may happen during a period of latency.1 Overall, this is a very inefficient process where the majority of cancer cells leaving the primary tumor will fail along the different steps. Even if considering the very last step of the cascade alone, organ colonization, the numbers of successful metastatic cells could get to 1 in 100 of the cells that completed extravasation in the brain.2 The brain microenvironment represents a unique niche: Metastatic cancer cells will encounter tissue-resident cell types such as microglia, oligodendrocytes, astrocytes, and neurons, and will have to cope with distinct metabolic and extracellular matrix characteristics.3 Additionally, the brain is shielded by the blood–brain barrier, providing a selective barrier for many molecules coming from the systemic circulation, including therapeutic agents.3 Even though the barrier gets transformed into a different entity termed blood–tumor barrier, it still keeps a selective permeability.4 Since cancer cells from diverse primary origins home to the brain, the complexity of the microenvironment is complemented by genetic and phenotypic heterogeneity of the brain metastatic cancer cells themselves.5 Thus, to decipher the formation of CNS metastasis and discover novel therapeutic options, it is paramount to use preclinical models that faithfully recapitulate the complexity of this multifaceted process. However, no model can cover the wide range of unresolved questions. Choosing the right model therefore depends on the scientific question asked by the investigator: Reductionist models can provide faster and more direct insights, while risking limited relevance in more complex settings, and are therefore more suited for gaining mechanistic insights into specific aspects of tumor biology. On the other hand, models recapitulating the heterogeneity and complexity of human disease and the clinical situation are needed for testing new therapeutics or biomarker approaches.
In this review, we introduce basic concepts of different experimental brain metastasis models and explore which questions they might help answer. While most work in the field has focused on murine models, other model organisms clearly have value in complementing this experimental tool and are therefore shortly summarized.
Generation of Brain Metastatic Cell Lines
The use of brain metastatic cell lines represents the most widely used approach. Here, cancer cells are routinely isolated from patient material and further propagated in vitro. These cells, termed parental (P), are usually transfected with reporters such as luciferase and a fluorescent protein (eg, GFP), compatible with evaluation of metastatic burden in vivo and by histology, respectively. Since inoculation of these parental cell lines into mice usually does not yield a high number of brain metastasis, brain-seeking clones can be enriched by repeated rounds of in vivo selection. For this, parental cells typically are injected into the arterial circulation (either into the left cardiac ventricle—intracardiac—or into the carotid artery) and few cells will follow the blood stream and colonize the brain. Subsequently, these cancer cells will be recovered from the animal’s brain, expanded in vitro and re-injected into mice. Repeating this selection process for 3–5 times increases the cancer cells ability to form lesions in the brain and eventually a brain-tropic cell line (BrM) can be established, that upon injection reliably generates brain metastasis.6 Of note, while more aggressively metastasizing derivatives are generated by dissecting bioluminescence-positive brains, dissection of bioluminescence-negative brains has been used to establish also indolent BrM cell lines.6 Utilizing this in vivo selection approach, a wide range of both human and murine BrM cell lines could be obtained, representing the most frequent primary origins and oncogenomic profiles.7 Most of these cell lines and their respective characteristics are summarized in the BrMPanel, a resource provided by a consortium of brain metastasis researchers.7
In order to generate brain metastasis in vivo, these cells are typically inoculated into animals in 2 different ways:
Systemic Inoculation
For this injection route, BrM cells are injected either into the left cardiac ventricle or, less common, into the carotid artery. Following intracardiac inoculation, BrM cells are distributed with the arterial blood flow into the brain, but also generate significant extracranial metastatic burden (eg, in the lungs, bone, liver). This can be particularly problematic in syngeneic hosts, in which intracardiacally injected mice usually reach their humane endpoint due to extensive visceral metastasis, before brain metastatic lesions reach a critical size. Extracranial metastasis can be avoided by inoculating cancer cells into the carotid artery. However, intra-carotid inoculation is a much more invasive procedure, which requires surgery and a bigger time investment, therefore may not be as readily applicable as intra-cardiac injection.8 While both ways include the strong selection step of extravasation, earlier steps of the metastatic cascade such as invasion and the possible formation of a premetastatic niche are neglected.9
Intracranial Inoculation
Here, BrM cells are injected directly into the brain, usually using a stereotactic apparatus. This provides the advantage of generating a singular, precisely located established lesion, making it suitable for questions in which the healthy hemisphere can be used as an endogenous control10 or for syngeneic models in which a longer period of time (eg, for treatment) after establishment of the lesion is needed.11 However, intracranial inoculation does not faithfully recapitulate the metastatic cascade, since cancer cells are not required to extravasate. Although it can be used to study the interaction of cancer cells with the brain microenvironment, attention should be paid to the fact that the injection itself already induces neuroinflammation.
Of note, by all of the listed routes of inoculation, BrM cells are injected into a tumor naïve animal without primary tumor, which does not reflect the clinical scenario. This might influence the response to therapy, in particular immunotherapy.12 One possibility to circumvent this challenge is the simultaneous co-implantation of orthotopic and brain metastatic tumors.12
Spontaneous Models of Brain Metastasis and Genetically Engineered Mouse Models
Ideally, an experimental model of brain metastasis would require cancer cells to undergo all steps of the metastatic cascade, either from orthotopically injected tumor cells, such as the mammary fat pad for breast cancer or subdermal for melanoma, or from genetically engineered mouse models, which spontaneously form tumors after genetic manipulation of oncogenes or tumor suppressors. Unfortunately, few cancer cell lines spontaneously form intracranial lesions from orthotopic injection (Table 1). Similar to brain metastasis in patients, their occurrence is rather late in the course of disease, often requiring surgical removal of the primary tumor in order to prevent mice from reaching the humane endpoint prematurely due to extensive extracranial disease.9 Additionally, the incidence of brain metastasis in these models is low, causing high experimental variability and therefore a need for bigger cohorts of mice. In spite of these drawbacks, these models are necessary to test therapeutic approaches aiming at the prevention of metastasis, the natural selection process of metastatic clones from the primary tumor, as well as the influence of the latter on the metastatic niche. While these models are scarce, for the common primary tumor types there are models available.
Table 1.
Spontaneous Models of Brain Metastasis
| Model | Cancer Type | Specie | Host | Primary Tumor/ Orthotopic Inoculation | Surgery of Primary Needed | Time to Brain Metastasis | Detection of Brain Metastasis | Incidence of Brain Metastasis | Reference |
|---|---|---|---|---|---|---|---|---|---|
| MDA-MB-453 | Breast cancer (Triple negative) | Human | Rag2-/-; Il2rg-/- | Subcutaneous and orthotopic inoculation (Fat pad) | Not described | Not described | Histology | Not described | Nanni et al. |
| BT-474 | Breast cancer | Human | Rag2-/-; Il2rg-/- | Subcutaneous and orthotopic inoculation (Fat pad) | Not described | Not described | Histology | Not described | Nanni et al. |
| MDA-231 | Breast cancer (Triple negative) | Human | NSG | Orthotopic inoculation | No | 2 months | Histology | 100% | Puchalapalli et al. |
| CN34BrM | Breast cancer (Triple negative) | Human | NSG | Orthotopic inoculation | No | 2 months | Histology | 90% | Puchalapalli et al. |
| SUM1315 | Breast cancer (Triple negative) | Human | NSG | Orthotopic inoculation | No | 3 months | Histology | 11% | Puchalapalli et al. |
| TBCP-1 | Breast cancer (Her2+) | Mouse | BALB/C | Orthotopic inoculation | Yes | 7 weeks | Histology | 60% | Nagpal et al. |
| Dct::TVA;BrafV600E; Cdkn2alox/lox; Ptenlox/ lox + RCAS-Cre and RCAS-myrAKT1 | Melanoma | Mouse | Mixed C57Bl/6 and FVB/N | GEMM (spontaneous primary tumor) | No | 6 weeks | Histology | 79% | Cho et al. |
| RMS; sBT-RMS | Melanoma | Mouse | C57Bl/6 | Orthotopic inoculation | Yes | RMS (3-6 months); RMS-BrM (1.5 months) | Histology and qRT-PCR | 23% (RMS); 64% (sBT- RMS) | Schwartz et al., Doron et al. |
| Rbnull/Trp53null | Small cell lung cancer | Mouse | Not reported | GEMM (spontaneous primary tumor) | No | 7 months | Histology | Not described | Meuwissen et al. |
Breast Cancer
Few breast cancer cell lines (MDA-MB-453, BT-474, MDA-231, CN34BrM, SUM1315) have been reported to spontaneously form brain metastasis after orthotopic injection, but not in commonly used host nude mice; Instead the use of more permissive hosts lacking NK-cells, such as NSG or Rag2-/-; Il2rg-/- mice, was necessary.13,14 In a immunocompetent host, Nagpal et al. recently reported a HER2+ breast cancer cell line derived from spontaneous BALB/C mammary tumors, which after orthotopic injection and subsequent resection of the primary tumor avidly metastasizes to the brain (60% of mice).15
Melanoma
Activation of Akt1 by either addition of a N-terminal myristoylation sequence16 or expression of constitutively active Akt1E17K17 in an autochthonous melanoma mouse model in the context mutated BrafV600E and loss of Pten and Cdkn2a leads to brain and extracranial metastasis in an otherwise nonmetastatic model. A second model has been described for the melanoma cell line RMS, derived from spontaneously arising skin tumors in Ret transgenic mice,18 which after subdermal injection yields brain metastasis 3–6 months after surgical removal of the primary tumor in 23% of injected mice.9 Isolation of and subsequent culture of cells from these spontaneous melanoma brain metastasis gave rise to a brain-trophic cell derivate of the RMS cell line, developing brain metastasis in 64% of mice upon subdermal re-injection.19
Small-Cell Lung Cancer
Inactivation of Rb1 and Trp53 are frequently found mutations in small-cell lung cancer and concomitant loss of Rb1 and Tp53 in mouse lungs leads to a high incidence of SCLC in these mice, recapitulating the aggressive phenotype observed in humans including the formation of extracranial and brain metastasis.20
Patient-derived Xenograft (PDX) Models
While the use of established cell lines has been the main methodical approach is brain metastasis research, it becomes increasingly recognized that these cell lines do not recapitulate the broad spectrum of genomic alterations in human cancers, particularly when it comes to the genomic evolution under different kind of therapies that primary tumor undergo before or simultaneously metastasizing to the brain or, in the case of lung cancer, the extensive tobacco-induced mutagenetic landscape.21,22 Furthermore, established cell lines often suffer from genomic drift during passage and acquire additional mutations in vitro over time.23
For PDX models, patient-derived tissue could be implanted directly into mice or patient-derived cells might be propagated in vitro for a very limited number of passages (usually < 10), during which they can be engineered with different reporters (Luciferase, GFP) before being injected into immunodeficient mice (NSG). Compatibility with in vitro passage is not granted and efficacy has been estimated between 24% and 48.8% depending on the amount of viable cancer cells in the surgical sample, ability of the cells to form colonies in the first culture, and the degree of senescence affecting cancer cells.24 Most brain metastatic PDX models are derived from either systemic25 or intracranial26 inoculation, but few models have been reported to spontaneously metastasize from orthotopic (subdermal for melanoma21 or mammary fat-pad for breast cancer27) injection. Of note, a brain-metastatic SCLC PDX model has recently been established from subcutaneous injection.22 This is of considerable importance, since human SCLC patients have an extremely high propensity to develop brain metastasis, but so far there are no human cell lines (and only one insufficient mouse model described above) that recapitulate this important feature of SCLC.
Remarkably, PDX-based brain metastasis accurately represent the histological, genomic and molecular features of their parental tumor,26 providing the opportunity to utilize these models as patient avatars to develop and validate more personalized therapies. The advantage of PDX models for testing of novel targeted therapies for rare, but potentially actionable genomic vulnerabilities has recently been illustrated for both ROS1 and MET mutated NSCLC.28,29 Both of these mutations represent distinct molecular subtypes of NSCLC that frequently metastasize to the brain,30,31 but have not been modeled yet by conventional cell lines. Treatment of experimental brain metastasis generated from orthotopically inoculated PDX from patients harboring these mutations with the targeted therapies repotrectinib29 or savolitinib,28 respectively, resulted in intracranial tumor growth inhibition and in the case of ROS1 mutated brain metastasis treated with repotrectinib even in doubled survival time of mice.
In contrast to established cancer cell lines, so far, the utility of PDX models for functional testing has been limited because of the inability to perform genetic targeting in these tumors. However, recent development of novel Crispr-Cas9 editing methods, that do not require in vitro culture for selection of transduced cells, enables targeted genome editing in PDX,32 augmenting their power.
Another approach to harness the utility of PDX in brain metastasis research is the derivation of PDX models from circulating tumor cells of brain metastasis patients (also CDX). These precursors of metastasis isolated from the blood of the patient have been shown to recapitulate their organ tropism to the brain when injected intracardiacally in mice,33 offering the unique opportunity to test preventative therapeutic approaches. This might be particularly interesting since a subset of brain metastasis patients is not eligible to undergo neurosurgery and therefore no surgical specimen for PDX derivation is available in these patients.
One limitation of PDX models is the necessary use of immunocompromised hosts, considering the growing interest in the use of various kinds of immunotherapies in brain metastasis.5 Humanized mouse models comprised a severely immune-suppressed host (eg, NSG or NOG mice) in which a functional human immune system is reconstituted by engraftment of either human peripheral blood mononuclear cells or human hematopoietic stem cells.34 While these models are constantly advancing and have been able to generate some insides into immunotherapy responses in other tumor entities,35 their generation remains labor-intensive and costly. Therefore, these models have yet to be introduced into brain metastasis research.
Other Animal Models of Brain Metastasis
While mouse models have been the most widely studied in the field of brain metastasis, other in vivo models may complement insights gained from mouse models or may even be better suited for certain research questions (Figure 1).
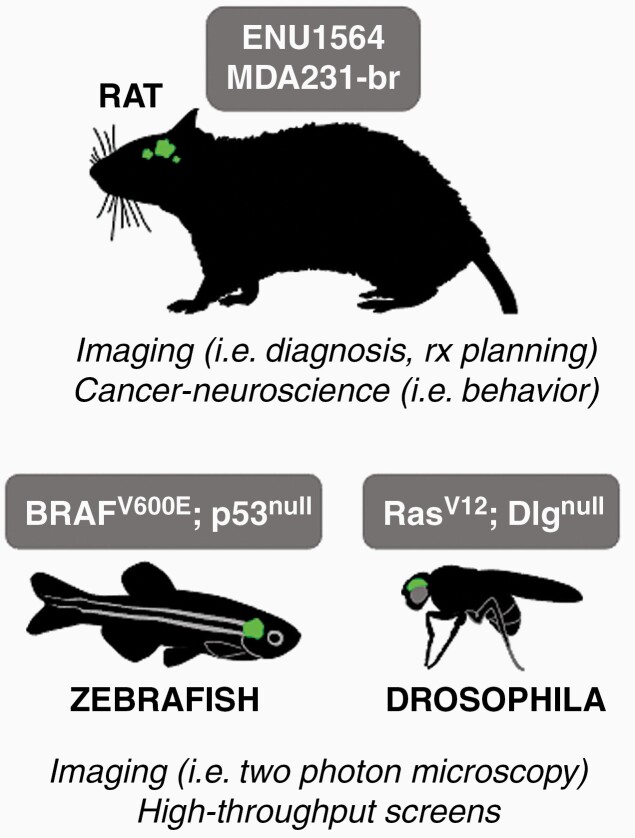
Figure 1.
Alternative brain metastasis models. Different models of brain metastasis reported in the literature are described including the cell line used (Rat, zebrafish) or the genetic modification leading to brain metastasis. Each of these organisms provide specific advantages for analyzing brain metastasis.
There has been at least 2 established brain-metastatic rat models: The syngeneic breast cancer cell line ENU1564, derived from a N-ethyl-N-nitrosourea–induced mammary adenocarcinoma in female Berlin-Druckrey IX (BD-IX) rats,10,36 which can be injected systemically or intracranially, as well as the human breast cancer cell line MDA-321br, which can be used in nude rats.37 These models have proven to be particularly superior for the study of imaging techniques, since the rat brain is larger than a mouse brain and therefore offers a better spatial resolution.36,37 Advanced imaging plays an essential role in the clinical management of brain metastasis patients, not only for establishing the diagnosis, but also for treatment planning of radio-therapy and accurate determination of treatment response. Particularly the latter purpose provides a considerable challenge for clinicians, as early recognition of treatment failure can be decisive to initiate salvage therapies before clinical onset of symptoms associated with tumor re-growth, but often is difficult to differentiate on conventional imaging platforms.38 Thus, the further improvement of brain metastasis rat models could serve as a platform for preclinical development of novel imaging techniques.
Another area in which rat brain metastasis models may prove as useful is the emerging field of cancer neuroscience39: There is a growing appreciation that brain tumors, including brain metastasis, are able to integrate into neuronal circuits of the CNS.40–42 While most studies so far have reported the influence of neuronal activity on cancer cell proliferation, the clinical observation of brain tumor patients experiencing different degrees and types of neurological symptoms, independent of location or size of their respective tumor, indicates that this cancer-neuronal crosstalk may be bi-directional and induces neuro-cognitive impairment.43 In order to dissect the impact of brain metastasis on cognition and behavior, specific task-related cognitive tests such as the morris water maze (assessing spatial learning and memory44) or attentional set-shifting task (assessing executive functioning45) need to be performed. Mice are much easier stressed by human contact than rats and this, as well as other “non-cognitive” distractors often cofound their performance in behavioral tests, while rats perform more stably over time.46 In conclusion, research into the cognitive impairment in brain metastasis patients could benefit from using rats as an additional model organism.
Of note, 2 nonrodent models have been used for brain metastasis research: The zebrafish (Danio rerio) has emerged as a powerful tool to study the metastatic cascade in vivo, owing to its optical transparency that in combination with fluorescently labeled cancer cells allows quantitative assessment of the spatial-temporal dynamics of metastasis on a single cell level.47 Since zebrafish are easy to breed and to genetically manipulate, they represent an ideal model organism to perform high-throughput genetic screening for possible mediators of metastasis.47 Using a spontaneous zebrafish melanoma model, generated by expression of mutant BRAFV600E under a melanocyte promoter in p53-deficient zebrafish,48 a transplantable metastatic cell line was established, enabling the functional manipulation of both cancer-cell intrinsic and microenvironmental determinants of the metastatic process.47 Also, Stoletov et al. used the zebrafish model in combination with the mouse breast cancer cell line 4T-1 to uncover the role of connexins during the early metastatic colonization of the brain.49
Another nonrodent model organism that was adapted for brain metastasis research is Drosophila melanogaster.50 Here, researchers made use of a fly model carrying overexpressed oncogenic RasV12, inactivated of the cell polarity gene Dlg and GFP in the imaginal eye disc, leading to tumor development in the Drosophila eye disc and consequent invasion into adjacent brain tissue.50,51 Similar to zebrafish, Drosophila offers an ideal platform for high-throughput genetic screening by simply crossing the above-described fly line with any RNAi fly line. Utilizing this approach to interrogate a 108-gene signature obtained from RNA-sequencing of small versus big mouse brain metastasis, Howe et al. identified Rab11b as a mediator of metastatic adaption in the brain,50 necessary for protein recycling of Integrin-ß1, which in turn enables the successful interaction of cancer cells with the brain microenvironment.
Overall, nonrodent model organisms offer attractive advantages compared with rodents, such as low maintenance effort and cost, simple ways to generate transgenics and, in the case of zebrafish, the unique ability for whole-body in vivo imaging. While most of the human protein-coding genome is conserved in these model organisms, rendering them ideal for genetic screening, it should be noted that they exhibit significant physiological and anatomical differences compared to human. Depending on the research question, these models could complement the research performed in rodent models.
Including Local Therapies Into Brain Metastasis Models
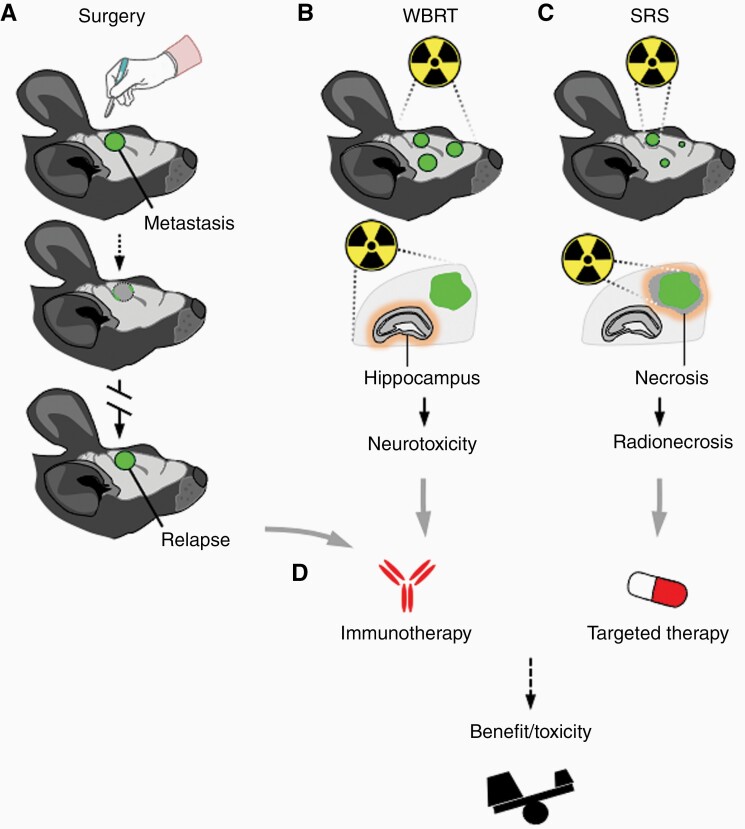
Neurosurgical resection is a cornerstone of the clinical management of brain metastasis patients, often providing immediate relief for neurological symptoms as well as a benefit in survival, at least for a subgroup of patients.38 However, local or distant intracranial recurrences are frequent and represent a considerable challenge.52 While neurosurgery and subsequent relapse has been established in mouse models of pediatric brain tumors53 and recently of glioblastoma,54 similar approaches mimicking this clinically relevant scenario in brain metastasis are lacking. To this end, our group recently has developed a novel in vivo model of local relapse after neurosurgical resection of a single brain metastasis (Figure 2), providing an experimental platform to test therapeutic approaches in a truly adjuvant setting as well as the experimental opportunity to study the biology of relapse.55 Given the recent clinical interest in immunotherapy in brain metastasis, it would be desirable to extent this approach to syngeneic models, in order to dissect the impact of neurosurgery and subsequent treatment with steroids on the immune-infiltrate of the brain.
Figure 2.
Experimental models incorporating local therapies. Both neurosurgery and radiotherapy have been applied to experimental brain metastasis models in vivo. These approaches could help to understand the biology of local relapse (a), neurotoxicity related to whole brain radiotherapy (WBRT) (b), and the radionecrosis that could be associated with stereotactic radiosurgery (SRS) (c). In addition, these models could be used to evaluate the benefit versus increased toxicity of combining local and systemic therapies (d).
In brain metastasis patients, neurosurgery is usually followed, or in some patients even completely replaced, by different radiotherapy modalities.38 Historically, whole-brain-radiotherapy (WBRT) used to be the gold standard in the management of brain metastasis, owing to its ability to control both local and distant intracranial disease. Recent clinical trials however have questioned this approach, given that compared to best supportive care no benefit in survival could be detected, at the cost of high neurotoxicity.56 Given this apparent resistance to irradiation, experimental models of brain metastasis also ought to incorporate this therapeutic modality, ideally in combination with neurosurgery, to faithfully recapitulate the clinical scenario. A small number of studies described the use of WBRT: Two studies have used a single dose of irradiation in breast and lung cancer brain metastasis models.57,58 While the first demonstrated an anti-tumoral effect when irradiation was applied to micrometastasis, both confirmed the clinically observed radio-resistance of established metastasis. In patients, WBRT is usually not given as a single-dose, but delivered using hypofractionated protocols.38 To this end, Smart et al. applied WBRT in 10 fractions of 3Gy to breast cancer brain metastasis in vivo, a schema closely mimicking the one given in the clinic.59 Here, fractionated irradiation failed to affect tumor growth of established brain metastasis.
Owing to the ambiguity of WBRT, clinical practice rapidly adapted stereotactic radiosurgery (SRS), a radio-therapeutic approach where a high dose of irradiation is delivered precisely to the metastatic lesion, while sparing healthy surrounding tissue, therefore avoiding neurocognitive toxicity.38 This mode of irradiation delivery has also been integrated into preclinical models of brain metastasis, using cone-beam computed tomography and magnetic resonance imaging for treatment planning and single arc radiation exposure to deliver doses raging from 18 to 40Gy.60 In this context, it was shown that tumor breakdown by SRS, rather than imprecise radiation, leads to cognitive decline after irradiation.60 Interestingly, the same authors were capable of achieving long term survival and eradication of brain tumors using a rat gliosarcoma model, enabling the study of late effects of irradiation on cognition in the context of a brain tumor.60
Overall, brain metastasis usually occurs late in the evolution of disease and therefore patients have undergone multiple lines of treatment for their primary tumors and subsequently will receive either neurosurgery, radiation, systemic treatment or a combination of these approaches for treatment of their brain metastasis. This complexity is not reflected in animal models of brain metastasis, which mostly are treatment naive. It is therefore imperative to include these modalities in preclinical models not only to mimic the clinical situation more closely, but also to study the biology of relapse after therapies such as neurosurgery or irradiation. Furthermore, animal models can serve as a preclinical discovery platform to dissect the impact of different treatment modalities on each other, such as radiotherapy and immunotherapy, which has recently been addressed in experimental models of brain metastasis.61
Systemic Therapies
Traditionally, systemic cytotoxic drugs only play a limited role in the clinical management of brain metastasis patients, due to their failure to penetrate the blood–brain barrier (BBB). Even though it has been demonstrated in experimental breast to brain metastasis models that the BBB is compromised in most metastatic lesions and these therefore enrich cytotoxic drugs more than an unaffected brain, these concentrations were not sufficient to show an effect on intracranial tumor growth. 62 Despite these discouraging results, few studies could show benefits from chemotherapeutic agents have shown promising results in preclinical models: When temozolomide, a BBB-permeable alkylating-agent frequently used in primary brain tumors, was given to mice 3 days after intracardiac inoculation of breast cancer cells, it completely prevented formation of brain metastatic lesions and enabled long-term survival of treated mice.63 This benefit however was not observed when Temozolomide was given to mice when metastases were already established or when O6-methylguanin-DNA-methyltransferase (MGMT), a DNA-repair enzyme known to confer resistance to alkylating-agent induced damage, was overexpressed. While in clinical trials, Temozolomide so far was not effective in brain metastasis patients, evaluation in a preventive setting is under ongoing investigation.64
Next to chemotherapy, considerable effort has been invested to develop targeted therapies which inhibit specific molecular drivers of cancer progression, providing distinct subsets of patients carrying these mutations with a significant benefit of survival.38 This has particularly important implications for the treatment of brain metastasis, since patients carrying some of these mutations have a higher propensity to develop brain metastasis (such as EGFR-mutant NSCLC or HER2+ breast cancer). While some of these agents have shown promising intracranial activity, many phase III randomized trials on the efficacy of targeted therapies still specifically exclude brain metastasis patients.65 Thus, the use of animal models provides an opportunity to test novel targeted therapies for their intracranial activity and if positive, promote the inclusion of brain metastasis patients in trials evaluating these agents. A wide range of targeted therapies has been tested in experimental models of brain metastasis; since this has recently been extensively reviewed elsewhere,66 here we will only give examples for the most common subtypes of brain metastatic cancers.
When experimental brain metastasis were established from intra-carotid injected human NSCLC cells harboring a EGFR exon 19 deletion, the third-generation EGFR inhibitor Osimertinib achieved sustained tumor regression and extended mice survival.67 This benefit of Osimertinib was later recapitulated in a randomized phase III trial, in which the compound showed good intracranial activity and was able to prolong disease-free survival in patients with CNS disease.68
HER2+ breast cancers can be targeted by a variety of different agents, with most of them showing a varying degree of intracranial activity.38 While monoclonal antibodies such as trastuzumab have low permeability across the BBB and therefore play a limited role in the management of brain metastasis, the more recently developed pan-HER kinase inhibitors tucatinib and neratinib have shown more promising intracranial response rates, particularly in combination with capecitabine.69 To this end, Nagpal et al. showed in a spontaneously metastasizing HER2+ breast cancer model that neoadjuvant neratinib monotherapy significantly reduces the incidence of brain metastasis, suggesting that this may be a more suitable clinical setting than late intervention.15
In melanoma, approximately half of advanced-stage patients display mutated BRAF, leading to hyperactivated BRAF kinase and enhanced MAPK signaling.70 Several MAPK inhibitors for targeting BRAFV600E-mutated melanomas, such as dabrafenib (targeting BRAF) or trametinib (targeting MEK), have been developed and already showed intracranial response rates as monotherapies, which was further improved by combining both dabrafenib and trametinib together.71 Despite the high frequency and therefore clinical importance of BRAF-mutated melanoma brain metastasis, preclinical models testing this treatment modalities are scarce. In a PDX brain metastasis model established from intracranially injected BRAFL597S-mutant melanoma cells, trametinib monotherapy was able to slow tumor growth, but not to extend survival. Combination of both trametinib and dabrafenib though significantly improved survival of mice, when compared with monotherapy or vehicle, confirming the clinically observed superior activity of combinational therapy.70
In summary, there is a wealth of preclinical evidence suggesting that testing both chemo- and targeted therapies in animal models of brain metastasis is able to recapitulate most of the clinical responses to these therapies and therefore can serve as a platform to test new agents as well as to design combinatorial treatments. Here, it is imperative to use approaches that model the most likely clinical setting, which is established, heavily pretreated brain metastasis. Looking at both the use of lapatinib in HER2+ breast to brain metastasis and vemurafenib in BRAF-mutant melanoma brain metastasis, trials have shown these agents to be less effective in previously irradiated patients.38 So far, this has not been addressed in preclinical studies modeling these therapies in experimental brain metastasis. Furthermore, in the landscape of available brain metastasis models, distinct genomic subsets, which are already treated with specific targeted therapy in the clinic, such as RET-mutated lung cancers, are currently still underrepresented or not available. In addition, taking into account the potential existence of genomic divergence between the primary tumor and derived metastases,72 including brain metastases,73,74 should be incorporated in experimental models to determine the contribution to differential responses to targeted treatments between the primary tumor and systemic disease. However, broadly used metastasis models (ie, organotropic cell lines) do not recapitulate this genomic evolution75 and, consequently, special attention should be paid to the analysis of other models that have a slower evolution from the development of spontaneous primary tumor and subsequently derived brain metastasis9,16,20 to evaluate whether they are compatible with such approaches.
Modeling Treatment-related Toxicity
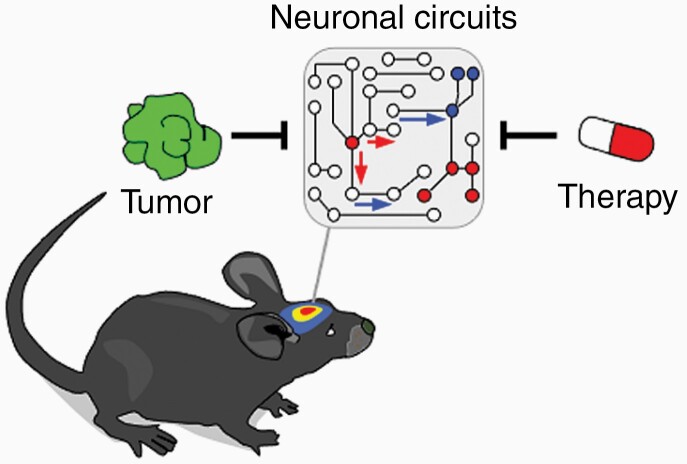
Given that modern therapeutic strategies increasingly enable improved cancer outcomes and long-term survivorship, treatment-related cognitive and behavioral impairments become more evident and contribute to suboptimal quality of life.76 While historically cognitive impairment was mostly associated with chemotherapy and whole-brain-radiotherapy, newer therapeutic modalities such as targeted- or immunotherapy have very different effects on cognition and brain function in general, and therefore represent new challenges in the management of neurotoxic side effects. Studying the interaction of therapeutic modalities and the CNS in preclinical models will enhance the understanding of molecular underpinnings of their neurotoxicity and facilitate the development of strategies to mitigate these symptoms (Figure 3).
Figure 3.
Impact of brain metastasis and their treatment in brain function. Both the tumor but also systemic and local treatments might have an impact in neuronal communication that could generate neurocognitive defects, which are highly prevalent among cancer patients with brain metastasis.
Whole-brain-radiotherapy is often followed by late-onset adverse effects like memory loss, confusion and leukencephalopathy.38 When tumor-naive mice were treated with 10Gy cranial irradiation, impairments of mice behavior and execution of cognitive tasks related hippocampal learning and memory were observed, which was accompanied by microglia activation.77 Cognitive deficits could be ameliorated by administration of either a CSF1R-inhibitor, depleting microglia, or microglia-specific deletion of C1q, together suggesting that radiotherapy-induced activated microglia contribute to cognitive dysfunction by complement-mediated synaptic loss.77,78 Other studies in rats have shown that treatment with 10Gy irradiation specifically inhibits the proliferation of hippocampal precursor cells by disrupting their neurogenic niche, ablating adult neurogenesis, which is thought to be important for normal hippocampal function.79 To this end, advances in radiation technology enable clinicians now to physically limit the radiation dose delivered to the hippocampus during whole-brain-radiotherapy, a technique called hippocampal sparing irradiation (HSI). When HSI was applied to mice and compared to regular WBRT, HSI-treated animals performed significantly better in behavioral tasks, consistent with recent clinical trials showing that HIS mitigates cognitive decline in brain metastasis patients receiving cranial irradiation.80
While stereotactic radiosurgery compared to WBRT does not cause a notable increase in neurocognitive toxicity, it can cause radionecrosis in 13%–14% of patients.81 Few murine models of radiation necrosis have been reported, although most of them with the purpose of developing novel imaging techniques to distinguish between tumor recurrence and radiation necrosis.82 Here, authors of one study treated the left hemisphere of mice with 50Gy radiation to develop radiation necrosis and subsequently administered bevacizumab, a VEGF antibody frequently used in the clinic to treat radiation necrosis.83 While bevacizumab significantly decreased necrotic lesion volume on imaging, histologically typical necrosis pathology was still present up to 12 weeks after irradiation.83 Of note, this and other studies utilized tumor-naive mice to study the effects of SRS-associated radiation necrosis; this may not fully represent the clinical phenotype, since another study observed that treating actual experimental brain tumors with SRS results in persistent cognitive deficits due to tumor disintegration and neuroinflammation, while SRS of healthy brain tissue only lead to transient effects.60 Clinical experience has shown that the risk of radionecrosis after SRS is increased when combined with immunotherapy.38 Unfortunately, this additive effect and its possible implications and adverse effects have not been addressed in the few preclinical studies that evaluated the combination of SRS and immunotherapy.84
In general, adverse effects of immunotherapy in brain metastasis specifically have not been considered in preclinical studies, even though the use of immune checkpoint inhibitors because increasingly popular in patients with brain metastasis.38 Clinically, about 4% to 6% of patients treated with one immunotherapy agent and up to 12% treated with 2 immunotherapeutic drugs experience neurological impairments, with symptoms ranging from headaches to meningitis.85 In experimental models of lung and colorectal cancer, although without brain metastasis, treated with a systemic CTLA-4 inhibitor and peripheral irradiation, changes in behavior and cognitive impairment were observed in tumor-bearing mice receiving therapy, compared with those mice without.86 These adverse events were accompanied by activation of microglia and general neuroinflammation, pointing to a similar mechanism as described for cranial irradiation-associated cognitive decline.86
Future Advances in Modeling Brain Metastasis
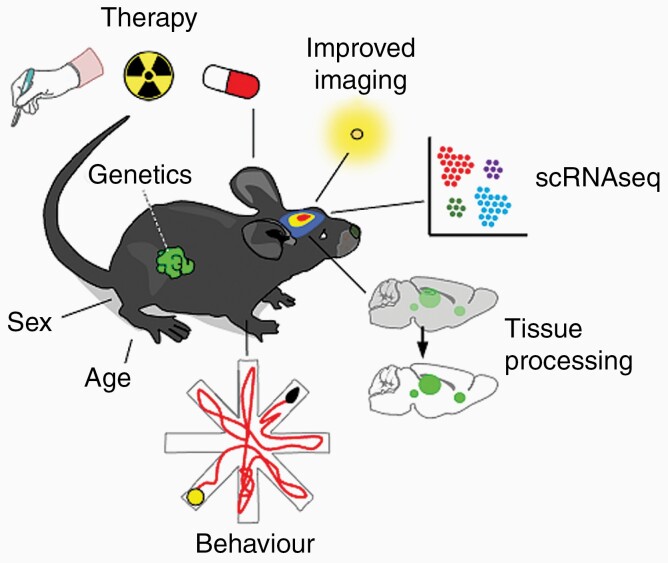
While our knowledge of the molecular underpinnings of brain metastasis is steadily increasing and this reflects in continuously improved preclinical models, new approaches are needed to more faithfully recapitulate human disease in its heterogeneity and evolution under treatment. Additionally, new technologies are emerging that allow to dissect tumor biology at unprecedented resolution, enabling the discovery of new druggable targets in both cancer and microenvironmental cells (Figure 4).
Figure 4.
Technical aspects to improve brain metastasis models. Incorporating therapies is highly necessary to recapitulate the disease in patients. In addition, the presence of a primary tumor with known genetics, considering sex and age as important variables, the use of improved molecular imaging resources (ie, next generation of luciferases), single-cell technologies to deconstruct the complexity of the brain metastasis associated microenvironment, advanced tissue processing technologies (ie, tissue clearing) so intact metastasis could be studied, and evaluate the impact of brain metastasis in the whole organisms (ie, behavior) will certainly increase the quality of the evaluation of metastatic disease in the brain.
Getting Closer to Reality
Systemic or local injection or brain-tropic established cell lines is still the most common method to study brain metastasis in a preclinical setting, however these approaches do not recapitulate the complete metastatic cascade, the heavily pretreated host or the genomic complexity of the human disease. All of these aspects should be incorporated in future models, ideally as spontaneously metastasizing mouse models that will be treated in a manner that closely relates to the clinical care—treating mice in mouse hospitals with neurosurgery, radiotherapy and different systemic therapies according to the mutational landscape of the tumor.
Another layer of complexion that recently has been acknowledged is the influence of host factors such as sex and age on various aspects of cancer and metastasis. Current in vivo models of experimental brain metastasis frequently neglect these aspects: Most studies in the field are conducted using only very young mice, not reflecting the clinical situation, in which brain metastasis occur rather in advanced stages of the disease, when cancer patients are usually older.38 In this context, experimentally it has been shown that the aging microenvironment can have a profound impact on the outgrowth of metastasis and therapy resistance.87 Similarly, recently it has been described how sex differences, particularly in the immune system, drive primary brain tumor growth and display distinct therapeutic vulnerabilities.88 Considering that the incidence of lung and melanoma brain metastasis is twice as high in men than it is in women,38 future models of brain metastasis should include both sexes and analyze the influence of perturbations separately to recognize these possible subtle differences.
Novel Toolbox
Emerging technologies such as single-cell sequencing or spatial transcriptomics allow to decipher the previously unseen heterogeneity in both cancer cells and their surrounding microenvironment and have started to be applied to brain metastasis.89 While these approaches are aimed at elucidating the complexity of brain metastasis on a genetic level, novel imaging techniques such as tissue clearing have been developed that make it possible to follow metastatic cells in intact whole organs at single-cell level and subcellular resolution.90 In order to also study the progression of cancer cells in the brain in alive animals, intravital imaging through a skull window using 2-photon imaging allows longitudinal follow-up of single cells within the brain, for instance to track the fate of tumor initiating cells during early stages of brain metastasis formation.91
In addition to these descriptive approaches, new functional read-outs are necessary to characterize the local and systemic effects that brain metastasis have on their host. To this end, the development of new luciferases and their respective substrates, which do not overlap with the commonly in brain metastatic cell lines used firefly luciferase, enable the tracking of 2 distinct biological processes or cell populations by bioluminescence in live animals, for instance separately engrafted T-cells and tumor cells.92 Furthermore, as discussed earlier, the emerging notion of brain metastasis influencing neuro-cognitive functions and behavior is still far from being included in current models or considered in commonly used assays. Normally, experimental read-outs include different measurements of tumor growth, phenotyping of different cell populations and basic physiological parameters such as weight. Thus, there is a need to expand the horizon of experimental read-outs of preclinical brain metastasis models to behavioral and cognitive measurements. Here, longitudinal tracking of movement and behavior of animals over the course of disease using methods such as CAPTURE, which combines continuous motion capture with deep learning to infer the behavior of rodents,93 could lead to new insights of how brain metastasis influence normal brain function.
Lastly, thinking of new tools to enhance brain metastasis research should not only consider technical advancements but also novel model organisms. In addition to the earlier discussed rats which may represent a superior model for cancer neuroscience related questions, even larger animals could support mouse studies and function as an additional step between rodents and humans. Compared with humans or larger animals, the rodents brain outer cortex is smooth and lacks sulci, which improves drug delivery and therefore offers one explanation why many compounds which were successfully tested in small animals later fail in clinical trials.94 The immunocompromised strain of the Yucatan minipig has been previously used for engraftment of human glioblastoma xenografts; With the porcine brain gyrification as well as BBB physiology resembling much closer the human brain, it should have greater translational quality when it comes to preclinical testing of novel compounds.94
Concluding Remarks
There is a wealth of human and murine models of brain metastasis, which are well characterized and constantly evolving, driven by a community-wide effort to improve this fatal disease. Novel tools and interdisciplinary collaboration with fields such as neuroscience, clinical research and genetics will help to generate a new generation of clinically relevant brain metastasis models, opening the door for the discovery of novel therapeutic targets.
Acknowledgments
We apologize to all authors whose contributions to the field could not be cited due to citation limitations.
Funding
Work in the Brain Metastasis Group is supported by Ministerio de Ciencia e Innovación (SAF2017-89643-R) (M.V.), Fundació La Marató de TV3 (141) (M.V.), Fundación Ramón Areces (CIVP19S8163) (M.V.), Worldwide Cancer Research (19-0177) (M.V.), H2020-FETOPEN (828972) (M.V.), Cancer Research Institute (Clinic and Laboratory Integration Program CRI Award 2018 (54545)) (M.V.), Asociación Española Contra el Cáncer (GCTRA16015SEOA, LABAE19002VALI) (M.V.), European Research Council (ERC CoG 864759) (M.V.), and a Boehringer Ingelheim Fonds MD fellowship (L.M.). M.V. is an EMBO YIP member (4053).
Conflict of interest statement.
None declared.
References
- 1. Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168(4):670–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kienast Y, von Baumgarten L, Fuhrmann M, et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med. 2010;16(1):116–122. [DOI] [PubMed] [Google Scholar]
- 3. Quail DF, Joyce JA. The microenvironmental landscape of brain tumors. Cancer Cell. 2017;31(3):326–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arvanitis CD, Ferraro GB, Jain RK. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat Rev Cancer. 2020;20(1):26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Valiente M, Ahluwalia MS, Boire A, et al. The evolving landscape of brain metastasis. Trends Cancer. 2018;4(3):176–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nguyen DX, Chiang AC, Zhang XH, et al. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell. 2009;138(1):51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Valiente M, Van Swearingen AED, Anders CK, et al. Brain metastasis cell lines panel: a public resource of organotropic cell lines. Cancer Res. 2020;80(20):4314–4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lorger M, Felding-Habermann B. Capturing changes in the brain microenvironment during initial steps of breast cancer brain metastasis. Am J Pathol. 2010;176(6):2958–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schwartz H, Blacher E, Amer M, et al. Incipient melanoma brain metastases instigate astrogliosis and neuroinflammation. Cancer Res. 2016;76(15):4359–4371. [DOI] [PubMed] [Google Scholar]
- 10. Sarmiento Soto M, Larkin JR, Martin C, et al. STAT3-mediated astrocyte reactivity associated with brain metastasis contributes to neurovascular dysfunction. Cancer Res. 2020;80(24):5642–5655. [DOI] [PubMed] [Google Scholar]
- 11. Priego N, Zhu L, Monteiro C, et al. STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis. Nat Med. 2018;24(7):1024–1035. [DOI] [PubMed] [Google Scholar]
- 12. Taggart D, Andreou T, Scott KJ, et al. Anti-PD-1/anti-CTLA-4 efficacy in melanoma brain metastases depends on extracranial disease and augmentation of CD8+ T cell trafficking. Proc Natl Acad Sci USA. 2018;115(7):E1540–E1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nanni P, Nicoletti G, Palladini A, et al. Multiorgan metastasis of human HER-2+ breast cancer in Rag2-/-;Il2rg-/- mice and treatment with PI3K inhibitor. PLoS One. 2012;7(6):e39626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Puchalapalli M, Zeng X, Mu L, et al. NSG mice provide a better spontaneous model of breast cancer metastasis than athymic (nude) mice. PLoS One. 2016;11(9):e0163521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nagpal A, Redvers RP, Ling X, et al. Neoadjuvant neratinib promotes ferroptosis and inhibits brain metastasis in a novel syngeneic model of spontaneous HER2+ve breast cancer metastasis. Breast Cancer Res. 2019;21(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cho JH, Robinson JP, Arave RA, et al. AKT1 activation promotes development of melanoma metastases. Cell Rep. 2015;13(5):898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kircher DA, Trombetti KA, Silvis MR, et al. AKT1E17K activates focal adhesion kinase and promotes melanoma brain metastasis. Mol Cancer Res. 2019;17(9):1787–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kato M, Liu W, Yi H, et al. The herbal medicine Sho-saiko-to inhibits growth and metastasis of malignant melanoma primarily developed in ret-transgenic mice. J Invest Dermatol. 1998;111(4):640–644. [DOI] [PubMed] [Google Scholar]
- 19. Doron H, Amer M, Ershaid N, et al. Inflammatory activation of astrocytes facilitates melanoma brain tropism via the CXCL10-CXCR3 signaling axis. Cell Rep. 2019;28(7):1785–1798.e6. [DOI] [PubMed] [Google Scholar]
- 20. Meuwissen R, Linn SC, Linnoila RI, Zevenhoven J, Mooi WJ, Berns A. Induction of small cell lung cancer by somatic inactivation of both Trp53 and Rb1 in a conditional mouse model. Cancer Cell. 2003;4(3):181–189. [DOI] [PubMed] [Google Scholar]
- 21. Krepler C, Sproesser K, Brafford P, et al. A comprehensive patient-derived xenograft collection representing the heterogeneity of melanoma. Cell Rep. 2017;21(7):1953–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Simpson KL, Stoney R, Frese KK, et al. A biobank of small cell lung cancer CDX models elucidates inter- and intratumoral phenotypic heterogeneity. Nat Cancer. 2020;1(4):437–451. [DOI] [PubMed] [Google Scholar]
- 23. Daniel VC, Marchionni L, Hierman JS, et al. A primary xenograft model of small-cell lung cancer reveals irreversible changes in gene expression imposed by culture in vitro. Cancer Res. 2009;69(8):3364–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim SY, Lee JY, Kim DH, et al. Patient-derived cells to guide targeted therapy for advanced lung adenocarcinoma. Sci Rep. 2019;9(1):19909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shih DJH, Nayyar N, Bihun I, et al. Genomic characterization of human brain metastases identifies drivers of metastatic lung adenocarcinoma. Nat Genet. 2020;52(4):371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dankner M, Caron M, Al-Saadi T, et al. Invasive growth associated with Cold-Inducible RNA-Binding Protein expression drives recurrence of surgically resected brain metastases. Neuro Oncol. 2021;23(9):1470–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Malladi S, Macalinao DG, Jin X, et al. Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell. 2016;165(1):45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baschnagel AM, Kaushik S, Durmaz A, et al. Development and characterization of patient-derived xenografts from non-small cell lung cancer brain metastases. Sci Rep. 2021;11(1):2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yun MR, Kim DH, Kim SY, et al. Repotrectinib exhibits potent antitumor activity in treatment-naïve and solvent-front-mutant ROS1-rearranged non-small cell lung cancer. Clin Cancer Res. 2020;26(13):3287–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Patil T, Smith DE, Bunn PA, et al. The incidence of brain metastases in stage IV ROS1-rearranged non-small cell lung cancer and rate of central nervous system progression on crizotinib. J Thorac Oncol. 2018;13(11):1717–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Offin M, Luo J, Guo R, et al. CNS metastases in patients with MET exon 14-altered lung cancers and outcomes with crizotinib. Jco Precis Oncol. 2020;4:871–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hulton CH, Costa EA, Shah NS, et al. Direct genome editing of patient-derived xenografts using CRISPR-Cas9 enables rapid in vivo functional genomics. Nat Cancer. 2020;1(3):359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Klotz R, Thomas A, Teng T, et al. Circulating tumor cells exhibit metastatic tropism and reveal brain metastasis drivers. Cancer Discov. 2020;10(1):86–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guil-Luna S, Sedlik C, Piaggio E. Humanized mouse models to evaluate cancer immunotherapeutics. Annu Rev Cancer Biol. 2021;5(1):119–136. [Google Scholar]
- 35. Ny L, Rizzo LY, Belgrano V, et al. Supporting clinical decision making in advanced melanoma by preclinical testing in personalized immune-humanized xenograft mouse models. Ann Oncol. 2020;31(2):266–273. [DOI] [PubMed] [Google Scholar]
- 36. Larkin JR, Simard MA, de Bernardi A, Johanssen VA, Perez-Balderas F, Sibson NR. Improving delineation of true tumor volume with multimodal MRI in a rat model of brain metastasis. Int J Radiat Oncol Biol Phys. 2020;106(5):1028–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. De Meulenaere V, Descamps B, De Wever O, Vanhove C, Deblaere K. In vivo selection of the MDA-MB-231br/eGFP cancer cell line to obtain a clinically relevant rat model for triple negative breast cancer brain metastasis. PLoS One. 2020;15(12):e0243156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Suh JH, Kotecha R, Chao ST, Ahluwalia MS, Sahgal A, Chang EL. Current approaches to the management of brain metastases. Nat Rev Clin Oncol. 2020;17(5):279–299. [DOI] [PubMed] [Google Scholar]
- 39. Monje M, Borniger JC, D’Silva NJ, et al. Roadmap for the emerging field of cancer neuroscience. Cell. 2020;181(2):219–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Venkatesh HS, Morishita W, Geraghty AC, et al. Electrical and synaptic integration of glioma into neural circuits. Nature. 2019;573(7775):539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zeng Q, Michael IP, Zhang P, et al. Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature. 2019;573(7775):526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Neman J, Termini J, Wilczynski S, et al. Human breast cancer metastases to the brain display GABAergic properties in the neural niche. Proc Natl Acad Sci USA. 2014;111(3):984–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jena A, Taneja S, Talwar V, Sharma JB. Magnetic resonance (MR) patterns of brain metastasis in lung cancer patients: correlation of imaging findings with symptom. J Thorac Oncol. 2008;3(2):140–144. [DOI] [PubMed] [Google Scholar]
- 44. Morris RGM. Spatial localization does not require the presence of local cues. Learn Motiv. 1981;12(2):239–260. [Google Scholar]
- 45. Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20(11):4320–4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ellenbroek B, Youn J. Rodent models in neuroscience research: is it a rat race? Dis Model Mech. 2016;9(10):1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Heilmann S, Ratnakumar K, Langdon E, et al. A quantitative system for studying metastasis using transparent Zebrafish. Cancer Res. 2015;75(20):4272–4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Patton EE, Widlund HR, Kutok JL, et al. BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Curr Biol. 2005;15(3):249–254. [DOI] [PubMed] [Google Scholar]
- 49. Stoletov K, Strnadel J, Zardouzian E, et al. Role of connexins in metastatic breast cancer and melanoma brain colonization. J Cell Sci. 2013;126(Pt 4):904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Howe EN, Burnette MD, Justice ME, et al. Rab11b-mediated integrin recycling promotes brain metastatic adaptation and outgrowth. Nat Commun. 2020;11(1):3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pagliarini RA, Xu T. A genetic screen in Drosophila for metastatic behavior. Science. 2003;302(5648):1227–1231. [DOI] [PubMed] [Google Scholar]
- 52. Kavouridis VK, Harary M, Hulsbergen AFC, et al. Survival and prognostic factors in surgically treated brain metastases. J Neurooncol. 2019;143(2):359–367. [DOI] [PubMed] [Google Scholar]
- 53. Morrissy AS, Garzia L, Shih DJ, et al. Divergent clonal selection dominates medulloblastoma at recurrence. Nature. 2016;529(7586):351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Otvos B, Alban TJ, Grabowski MM, et al. Preclinical modeling of surgery and steroid therapy for glioblastoma reveals changes in immunophenotype that are associated with tumor growth and outcome. Clin Cancer Res. 2021;27(7):2038–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhu L, Yebra N, Miarka L, et al. A drug-screening platform based on organotypic cultures identifies vulnerabilities to prevent local relapse and treat established brain metastasis. Biorxiv. October 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mulvenna P, Nankivell M, Barton R, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet. 2016;388(10055):2004–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Smith DL, Debeb BG, Thames HD, Woodward WA. Computational modeling of Micrometastatic breast cancer radiation dose response. Int J Radiat Oncol Biol Phys. 2016;96(1):179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Choi SH, Yang H, Lee SH, Ki JH, Nam DH, Yoo HY. TopBP1 and Claspin contribute to the radioresistance of lung cancer brain metastases. Mol Cancer. 2014;13:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Smart D, Garcia-Glaessner A, Palmieri D, et al. Analysis of radiation therapy in a model of triple-negative breast cancer brain metastasis. Clin Exp Metastasis. 2015;32(7):717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chu C, Davis CM, Lan X, et al. Neuroinflammation after stereotactic radiosurgery-induced brain tumor disintegration is linked to persistent cognitive decline in a mouse model of metastatic disease. Int J Radiat Oncol Biol Phys. 2020;108(3):745–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Niesel K, Schulz M, Anthes J, et al. The immune suppressive microenvironment affects efficacy of radio-immunotherapy in brain metastasis. EMBO Mol Med. 2021;13(5):e13412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lockman PR, Mittapalli RK, Taskar KS, et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res. 2010;16(23):5664–5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Palmieri D, Duchnowska R, Woditschka S, et al. Profound prevention of experimental brain metastases of breast cancer by temozolomide in an MGMT-dependent manner. Clin Cancer Res. 2014;20(10):2727–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zimmer AS, Steinberg SM, Smart DD, et al. Temozolomide in secondary prevention of HER2-positive breast cancer brain metastases. Future Oncol. 2020;16(14):899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Camidge DR, Lee EQ, Lin NU, et al. Clinical trial design for systemic agents in patients with brain metastases from solid tumours: a guideline by the Response Assessment in Neuro-Oncology Brain Metastases working group. Lancet Oncol. 2018;19(1):e20–e32. [DOI] [PubMed] [Google Scholar]
- 66. Masmudi-Martín M, Zhu L, Sanchez-Navarro M, et al. Brain metastasis models: What should we aim to achieve better treatments? Adv Drug Deliv Rev. 2021;169:79–99. [DOI] [PubMed] [Google Scholar]
- 67. Ballard P, Yates JW, Yang Z, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res. 2016;22(20):5130–5140. [DOI] [PubMed] [Google Scholar]
- 68. Mok TS, Wu Y-L, Ahn M-J, et al. ; AURA3 Investigators . Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376(7):629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Freedman RA, Gelman RS, Anders CK, et al. ; Translational Breast Cancer Research Consortium . TBCRC 022: a phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J Clin Oncol. 2019;37(13):1081–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dankner M, Lajoie M, Moldoveanu D, et al. Dual MAPK inhibition is an effective therapeutic strategy for a subset of class II BRAF mutant melanomas. Clin Cancer Res. 2018;24(24):6483–6494. [DOI] [PubMed] [Google Scholar]
- 71. Davies MA, Saiag P, Robert C, et al. Dabrafenib plus trametinib in patients with BRAFV600-mutant melanoma brain metastases (COMBI-MB): a multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017;18(7):863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hu Z, Li Z, Ma Z, Curtis C. Multi-cancer analysis of clonality and the timing of systemic spread in paired primary tumors and metastases. Nat Genet. 2020;52(7):701–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Brastianos PK, Carter SL, Santagata S, et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 2015;5(11):1164–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kotecha R, Tonse R, Rubens M, et al. Systematic review and meta-analysis of breast cancer brain metastasis and primary tumor receptor expression discordance. Neurooncol Adv. 2021;3(1):vdab010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jacob LS, Vanharanta S, Obenauf AC, et al. Metastatic competence can emerge with selection of preexisting oncogenic alleles without a need of new mutations. Cancer Res. 2015;75(18):3713–3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gibson EM, Monje M. Microglia in cancer therapy-related cognitive impairment. Trends Neurosci. 2021;44(6):441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Acharya MM, Green KN, Allen BD, et al. Elimination of microglia improves cognitive function following cranial irradiation. Sci Rep. 2016;6:31545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Markarian M, Krattli RP Jr, Baddour JD, et al. Glia-selective deletion of complement c1q prevents radiation-induced cognitive deficits and Neuroinflammation. Cancer Res. 2021;81(7):1732–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8(9):955–962. [DOI] [PubMed] [Google Scholar]
- 80. Tomé WA, Gökhan Ş, Brodin NP, et al. A mouse model replicating hippocampal sparing cranial irradiation in humans: a tool for identifying new strategies to limit neurocognitive decline. Sci Rep. 2015;5:14384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Le Rhun E, Dhermain F, Vogin G, Reyns N, Metellus P. Radionecrosis after stereotactic radiotherapy for brain metastases. Expert Rev Neurother. 2016;16(8):903–914. [DOI] [PubMed] [Google Scholar]
- 82. Jost SC, Hope A, Kiehl E, Perry A, Travers S, Garbow JR. A novel murine model for localized radiation necrosis and its characterization using advanced magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2009;75(2):527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Duan C, Perez-Torres CJ, Yuan L, et al. Can anti-vascular endothelial growth factor antibody reverse radiation necrosis? A preclinical investigation. J Neurooncol. 2017;133(1):9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Pfannenstiel LW, McNeilly C, Xiang C, et al. Combination PD-1 blockade and irradiation of brain metastasis induces an effective abscopal effect in melanoma. Oncoimmunology. 2019;8(1):e1507669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Joly F, Castel H, Tron L, Lange M, Vardy J. Potential effect of immunotherapy agents on cognitive function in cancer patients. J Natl Cancer Inst. 2020;112(2):123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. McGinnis GJ, Friedman D, Young KH, et al. Neuroinflammatory and cognitive consequences of combined radiation and immunotherapy in a novel preclinical model. Oncotarget. 2017;8(6):9155–9173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Fane M, Weeraratna AT. How the ageing microenvironment influences tumour progression. Nat Rev Cancer. 2020;20(2):89–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bayik D, Zhou Y, Park C, et al. Myeloid-derived suppressor cell subsets drive Glioblastoma growth in a sex-specific manner. Cancer Discov. 2020;10(8):1210–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Rubio-Perez C, Planas-Rigol E, Trincado JL, et al. Immune cell profiling of the cerebrospinal fluid enables the characterization of the brain metastasis microenvironment. Nat Commun. 2021;12(1):1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Pan C, Schoppe O, Parra-Damas A, et al. Deep learning reveals cancer metastasis and therapeutic antibody targeting in the entire body. Cell. 2019;179(7):1661–1676.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Berghoff AS, Liao Y, Karreman MA, et al. Identification and characterization of cancer cells that initiate metastases to the brain and other organs. Mol Cancer Res. 2021;19(4):688–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Su Y, Walker JR, Park Y, et al. Novel NanoLuc substrates enable bright two-population bioluminescence imaging in animals. Nat Methods. 2020;17(8):852–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Marshall JD, Aldarondo DE, Dunn TW, Wang WL, Berman GJ, Ölveczky BP. Continuous whole-body 3D kinematic recordings across the rodent behavioral repertoire. Neuron. 2021;109(3):420–437.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Khoshnevis M, Carozzo C, Bonnefont-Rebeix C, et al. Development of induced glioblastoma by implantation of a human xenograft in Yucatan minipig as a large animal model. J Neurosci Methods. 2017;282:61–68. [DOI] [PubMed] [Google Scholar]