Abstract
Objective
This study evaluated the association between pain outcomes and post-traumatic stress disorder (PTSD) symptom trajectories after combat-related injury, while adjusting for receipt of regional anesthesia (RA) soon after injury.
Methods
The PTSD symptom trajectories of N = 288 combat-injured service members were examined from within a month of injury up to two-years after. Linear mixed-effects models evaluated the association between PTSD symptom trajectories and average pain and pain interference outcomes while adjusting for receipt of RA during combat casualty care.
Results
Four PTSD trajectories were characterized: resilient, recovering, worsening, and chronic. Differential pain presentations were associated with PTSD symptom trajectories, even after adjusting for receipt of RA. Compared to those with a resilient PTSD symptom trajectory, individuals presenting with chronic PTSD trajectories were estimated to experience average pain scores 2.61 points higher (95% CI: 1.71, 3.14). Participants presenting with worsening (β = 1.42; 95% CI: 0.77, 1.78) and recovering PTSD trajectories (β = 0.65; 95% CI: 0.09, 1.08) were estimated to experience higher average pain scores than participants with resilient PTSD trajectories. Significant differences in pain interference scores were observed across PTSD trajectories. Receiving RA was associated with improved pain up to two years after injury (β = -0.31; 95% CI: -0.90, -0.04), however no statistically significant association was detected between RA and PTSD trajectories.
Conclusions
Chronic and worsening PTSD trajectories were associated with greater pain intensity and interference following combat injury even when accounting for receipt of early RA for pain management. These findings underscore the need to jointly assess pain and PTSD symptoms across the trauma care continuum.
Keywords: Injury, Pain, PTSD, Regional Anesthesia, Patient-Reported Outcomes
Background
Significant advancements in combat casualty care have increased survival rates of American service members sustaining complex combat injuries during Operation Enduring Freedom and Operation Iraqi Freedom (OEF/OIF) [1]. The severity of many of these combat-related injuries warranted early and sustained pain management [2]. However, there is a dearth of research examining how early multimodal pain management interventions, delivered in the immediate aftermath of injury and throughout the military evacuation chain, impact long-term pain and mental health. Evidence suggests that hyperstimulation of central neuronal pathways and unrelieved acute pain induces neural plasticity in the central nervous system and leads to maladaptive neuropathological remodeling after injury [3,4]. This rewiring may result in chronic pain and elevate subsequent risk for mental health conditions, such as post-traumatic stress disorder (PTSD), for patients who have experienced unrelieved severe pain after traumatic injury [5,6].
The mutual presence of PTSD and pain in OEF/OIF veterans has been investigated since the onset of the conflicts. Estimates show 30%–60% of combat-injured veterans are diagnosed with PTSD, compared to 11%–20% in the general OEF/OIF veteran population [7,8]. PTSD is a risk factor for transitioning from acute to chronic pain [9]. To date, the relationship of pain and PTSD in OEF/OIF combat-injured populations, and their subsequent care needs, has primarily been examined using cross-sectional or retrospective studies conducted long after the initial injury without evaluating symptom severity or symptom trajectories [10–12]. Longitudinal research is needed to understand how the mutual maintenance of conditions impacts changes in PTSD and pain symptom severity over time. The few longitudinal investigations of pain and PTSD symptom severity in OEF/OIF service members and veterans are limited to one year follow up periods, rarely report time since injury, and depend on self-reported injury status rather than objective measures [10].
Few longitudinal studies examining PTSD symptom trajectories after combat account for the potential effects of pain management interventions. Regional anesthesia (RA) has been shown to provide adequate acute pain management for modern combat casualties in the austere environment and throughout evacuation [2]. Benefits of RA over systemic analgesics and anesthetics include the avoidance of intubation for mechanical ventilation, lower risk of hemorrhage, and better postoperative analgesia [13–15]. Metanalyses have consistently shown that RA may reduce the development of chronic pain postoperatively, however less is known on how the use of RA may impact long-term pain outcomes after combat-related injury [16]. Moreover, observational studies have suggested early opioid based pain management after combat-injury is protective against the development of PTSD [17]. This indicates that early pain management approaches, such as RA, delivered soon after being injured in combat could potentially influence not only pain outcomes but also PTSD symptoms. Therefore, the objective of this secondary analysis was to evaluate the association between pain outcomes across PTSD symptom trajectories while adjusting for the administration of RA for early pain management after combat injury. We hypothesized that participants with chronic or worsening PTSD symptom trajectories would experience higher pain intensity and interference, even after adjusting for receipt of early RA. An exploratory aim of this study was to examine if early RA moderated the relationship between PTSD trajectories and pain outcomes. This study’s longitudinal nature, ability to capture patient-reported outcomes from within a time frame that was close in proximity to injury causing events, and the incorporation of pain interference measures, rather than pain intensity alone, provides a unique opportunity to study the long-term relationships between RA, PTSD trajectories, and pain outcomes in combat-injured populations.
Methods
Study Design
This was a secondary analysis of a prospective observational cohort study that investigated the benefits of early RA following combat-related limb injuries sustained in the OEF/OIF conflicts by United States (U.S.) service members and the subsequent patient-reported pain outcomes (NCT00431847) [18]. Enrollment began in 2007 and data collection concluded in 2015. The research team at Corporal Michael J. Crescenz Veterans Affairs Medical Center (Philadelphia, PA) collected participants’ patient-reported outcomes via telephone. Attempts were made to collect patient-reported outcomes monthly for individuals joining the study within the first 6 months after injury and every 3 months thereafter, for up to 24 months after combat injury. Individuals could join the study at any time within 24 months of injury, resulting in variation in the number of follow-up interviews. Data on pain intensity and interference, and mental health symptom severities, including PTSD were collected at each time point. Medical record review from The Department of Defense’s (DoD) Joint Theater Trauma Registry, now known as the DoD Trauma Registry, provided clinical and military career information, including date of injury, pain management and treatment status, and Injury Severity Score (ISS). IRB approval was provided by both the Department of Veterans Affairs Medical Center Research & Development Committee and the University of Pennsylvania. This study adheres to the STROBE guidelines.
Participants
Participants were recruited from the former Walter Reed Army Medical Center (Washington, DC), the current Walter Reed National Military Medical Center (Bethesda, MD), and the U.S. Army Institute of Surgical Research at Brooke Army Medical Center (San Antonio, TX). Service members with a combat-related extremity injury requiring hospitalization were eligible for enrollment. Those with a moderate or severe traumatic brain injury (TBI) were excluded. For this analysis all individuals with 2 or more pain and Post-Traumatic Stress Disorder Checklist (PCL) scores were included (Supplementary Data 1).
Measures
The PCL is a 17-item validated PTSD assessment instrument [19]. Respondents rate the extent to which they have experienced each of the diagnostic symptoms for PTSD outlined in the Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV) since the time of their combat injury. Total scores are computed by adding the 17 items, with each item scored based on the degree to which participants experienced symptoms over the past month from 1, “not at all,” to 5, “extremely.” PCL scores range from 17 to 85. The VA National Center for PTSD recommends a minimum threshold of 30 for total symptom severity score on the PCL be considered meeting PTSD diagnostic criteria [20]. PTSD trajectories were utilized as the independent variables in this analysis.
The validated Brief Pain Inventory (BPI) measures pain intensity and pain interference [21]. The BPI measures the multiple dimensions of pain, including asking respondents to reflect on their pain experience to better contextualize the pain they are reporting at time of assessment. Respondents rate their average pain intensity over the past week on a scale of 0–10, with 0 being “no pain,” and 10 being “pain as bad as you could imagine.” Participants rate the degree to which pain interferes with general activity, mood, walking, work, relationships, sleep, and enjoyment of life from 0, “pain does not interfere,” to 10, “interferes completely,” collectively known as pain interference. BPI average pain and pain interference scores were the primary outcomes of this analysis.
Early RA is a unique definition used for the purposes of this study to reflect the use of continuous peripheral nerve blocks placed early on in transitions in care combat-injured services members typically experienced prior to arriving in a continental U.S. military facility. RA refers to analgesics delivered under ultrasound imaging to a specific body region via a catheter, thereby allowing for the localized delivery of pain management to an injured extremity. Early RA was only possible based on an injured service member’s proximity to a forward operating base with a trained military anesthesia provider and acute pain service deployed at time of injury, and the availability of these services upon arrival in a U.S. military hospital. RA receipt was confirmed in the medical and surgical records upon enrollment. Participants not receiving early RA still received standard pain management including systemic multimodal analgesia throughout transportation and acute care at U.S. military medical facilities or received RA beyond 7 days if clinicians and patients determined additional pain management approaches were warranted (e.g., during wound debridement). Data on battlefield medication administered using regional anesthesia and discharge medication were not available.
At baseline, research nurses collected participants’ sociodemographic characteristics (e.g., age, gender, race, ethnicity, education, marital status). The length of hospitalization (in days), number of deployments, and Injury Severity Score (ISS) were obtained from the health record. The ISS is a scale used to evaluate anatomic injury severity that ranges from 0 to 75, with 75 indicating the greatest severity and incompatible with life [22]. The ISS is based on the Abbreviated Injury Scale (AIS) score allocated to the six body regions (i.e., Head, Face, Chest, Abdomen, Extremities, and External). The highest AIS score in each body region was used. The three most severely injured body regions scores were squared and summed to produce the ISS.
Statistical Analysis
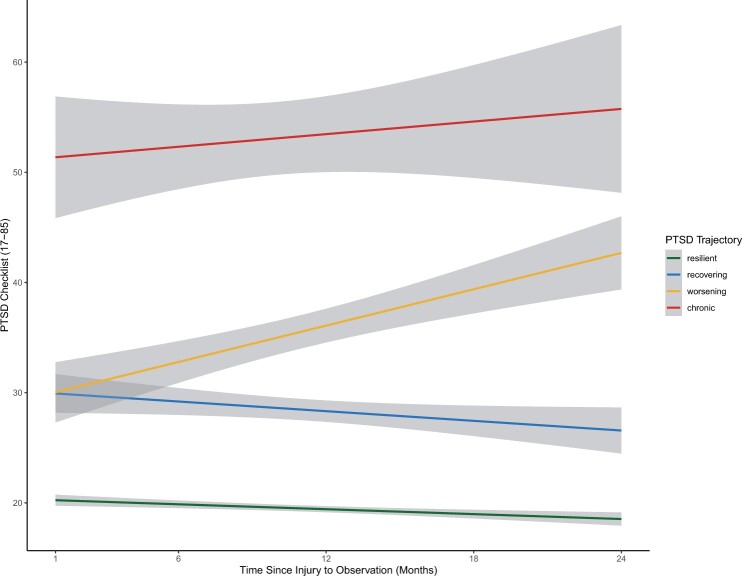
Univariate descriptive statistics (e.g., mean, standard deviation (±)) for both cohorts were calculated to characterize the sample. Differences in demographics and clinical characteristics between PTSD symptom trajectory groups were compared using ANOVA and chi-square tests. For this analysis, PTSD symptom severity was examined using PCL total scores. Trajectories were identified using an exploratory k-means algorithm interpolating PCL scores over each time point [23]. Average PCL scores per trajectory were examined with an ANOVA and post hoc pairwise comparisons were computed using a Tukey’s Test. After characterizing trajectories, PTSD symptom trajectories were transformed from a continuous variable into an indicator term: (1) individuals with a resilient trajectory, or low stable scores; (2) individuals with a recovering trajectory (e.g., PTSD symptoms decrease over time); (3) individuals with worsening trajectory (e.g., PTSD symptoms increase over time); and (4) individuals with chronic trajectories (e.g., consistently high, unremitting scores) (Figure 1). These groupings reflect four symptom trajectories commonly reported in the literature [24–26].
Figure 1.
PTSD symptom trajectories estimated from average PTSD checklist scores over 2 years after injury, with 95% confidence intervals.
Time was defined as the number of months since injury that a pain outcome measure was observed (i.e., months 1 to 24). When used as a continuous random-effect in the model, time accounts for both within and between subject variability in point estimates. Therefore, a random intercept unique to each individual was used in the model parameter estimates. Indicator variable for time since injury and time to first observation in the study, known as the study entry cohort, were included in the model to account for potential selection bias. The study entry cohorts were categorized as ≤6 months of injury or >6 months after injury.
Linear mixed effects models examined whether longitudinal progression of the outcomes, pain intensity and pain interference, were associated with a participant’s PTSD symptom trajectory, while adjusting for receipt of RA. First, bivariate models were fit to assess the association of each fixed effect (e.g., RA receipt, ISS, length of hospitalization) to pain outcomes, using a forward stepwise variable selection process. Goodness of fit for each model was assessed using Akaike information criterion (AIC). Each model included a random intercept and slope to account for individual differences in the time between injury to observation (i.e., the number of months after injury that the outcome measure was recorded per person). Both a model with random intercept only, and a model with random slope only were assessed for goodness of fit and compared to the combined random intercept and slope model. The combined intercept and slope model were determined to be best fitting based on AIC to account for time since injury and time of observation per person.
Mixed effects models were constructed to evaluate the association between PTSD trajectories and pain intensity and interference, separately. Sensitivity analyses included generating models after controlling for baseline pain intensity and interference but did not improve overall fit. Interaction terms between RA and time (month since injury) were not significant and did not improve model fits compared to models without interaction terms, based on AIC. Similarly, interaction terms between RA and PTSD trajectory were not significant, thus indicating RA did not moderate the association between PTSD trajectories and pain outcomes (Supplementary Data 2). Model parameters were estimated using maximum likelihood with degrees of freedom derived using the Satterthwaite method, after comparing against full restricted maximum likelihood estimation (REML) for parameters [27,28]. Post hoc power analyses indicated that the present sample size achieved sufficient power, 81%, to detect a difference as small as -0.25 in the observed differences of the repeated outcomes of interest (e.g., average pain) between the slopes of the PTSD trajectories at an alpha level of 0.05 (PASS V.16, NCSS, LLC, Kaysville, Utah, USA). Statistical significance was set to P < 0.05. All analyses were conducted with R Studio.
Results
This sample of 288 combat-injured service members was predominately young, with a mean age of 28 years (±7), White (77%), and almost exclusively male (99%) (Table 1). There was an average of 7 (±2.6) study visits per participant. Over a third of participants (36%) received RA within seven days of injury. The mean ISS score was 18.2 (10.5), with blast-related injuries being the most frequent mechanism of injury (75%). The majority of the sample sustained leg (84%) or arm injuries (56%), with approximately 38% of participants requiring amputation. The median length of initial hospitalization was 26 days.
Table 1.
Sample characteristics at baseline
| Total | Resilient | Recovery | Worsening | Chronic | P | |
|---|---|---|---|---|---|---|
| N = 288 | N = 152 | N = 57 | N = 53 | N = 26 | ||
| Age, mean (SD)† | 28.0 (7.0) | 28.1 (6.6) | 27.7 (7.2) | 28.7 (8.8) | 29.1 (6.7) | 0.807 |
| Male, n (%)* | 285 (99.0) | 151 (99.3) | 56 (98.2) | 53 (100.0) | 25 (96.2) | 0.386 |
| Marital status, n (%)* | 0.882 | |||||
| Single, Never Married | 136 (47.2) | 75 (49.3) | 28 (49.1) | 21 (39.6) | 12 (46.2) | |
| Married/Partnered | 139 (48.3) | 71 (46.7) | 27 (47.4) | 29 (54.7) | 12 (46.2) | |
| Separated/Divorced | 13 (4.5) | 6 (3.9) | 2 (3.5) | 3 (5.7) | 2 (7.7) | |
| Race, n (%)* | 0.343 | |||||
| Caucasian/White | 223 (77.4) | 123 (80.9) | 44 (77.2) | 39 (73.6) | 17 (65.4) | |
| African American/Black | 14 (4.9) | 4 (2.6) | 5 (8.8) | 3 (5.7) | 2 (7.7) | |
|
Asian American/Pacific Islander/Native American/Other |
51 (17.7) | 25 (16.4) | 8 (14.0) | 11 (20.8) | 7 (26.9) | |
| Non-Caucasian Hispanic, n (%)* | 40 (13.9) | 21 (13.8) | 5 (8.8) | 9 (17.0) | 5 (19.2) | 0.514 |
| Education, n (%)* | 0.327 | |||||
| High School/GED | 113 (39.2) | 54 (35.5) | 24 (42.1) | 26 (49.1) | 9 (34.6) | |
| Any College | 175 (60.8) | 98 (64.5) | 33 (57.9) | 27 (50.9) | 17 (65.4) | |
| Military Service, n (%)* | 0.851 | |||||
| Army | 216 (75.0) | 117 (77.0) | 39 (68.4) | 40 (75.5) | 20 (76.9) | |
| Marine Corps | 55 (19.1) | 28 (18.4) | 13 (22.8) | 9 (17.0) | 5 (19.2) | |
| Other | 17 (5.9) | 7 (4.6) | 5 (8.8) | 4 (7.5) | 1 (3.8) | |
| Number of study visits, mean (SD) † | 7.1 (2.6) | 7.2 (2.6) | 7.4 (2.3) | 6.7 (2.9) | 6.2 (2.4) | 0.795 |
| Months from injury to enrollment, n (%)* | 0.370 | |||||
| ≤ 6 months | 228 (79.2) | 120 (78.9) | 48 (84.2) | 38 (71.7) | 22 (84.6) | |
| > 6 months | 60 (20.8) | 32 (21.1) | 9 (15.8) | 15 (28.3) | 4 (15.4) | |
| Number of deployments, median [IQR]‡ | 2 [1, 3] | 2 [1, 3] | 2.00 [1, 2] | 1 [1, 3] | 2 [1, 3] | 0.652 |
| Baseline PTSD Checklist, mean (SD) † | 28.7 (13.8) | 19.3 (3.2) | 27.9 (5.8) | 37.3 (10.3) | 59.8 (9.2) | <0.001 |
| Early regional anesthesia, n (%)* | 104 (36.1) | 51 (33.6) | 21 (36.8) | 20 (37.7) | 12 (46.2) | 0.650 |
| Length of Stay, Days, median, [IQR]‡ | 26 [13, 49] | 28 [15, 50] | 31 [10, 50] | 21 [10, 52] | 23 [17, 34] | 0.370 |
| Injury Severity Score, mean (SD) † | 18.2 (10.5) | 18.9 (10.8) | 18.1 (11.6) | 17.0 (9.6) | 16.9 (7.5) | 0.603 |
| Injury resulting in amputation, n (%)* | 108 (37.6) | 65 (42.8) | 22 (39.3) | 16 (30.2) | 5 (19.2) | 0.081 |
| Arm injury, n (%)* | 160 (55.7) | 86 (56.6) | 32 (57.1) | 29 (54.7) | 13 (50.0) | 0.063 |
| Leg injury, n (%)* | 241 (84.0) | 132 (86.8) | 46 (82.1) | 40 (75.5) | 23 (88.5) | 0.999 |
| Head injury, n (%)* | 113 (39.4) | 57 (37.5) | 22 (39.3) | 22 (41.5) | 12 (46.2) | 0.910 |
| Chest injury, n (%)* | 129 (44.9) | 66 (43.4) | 23 (41.1) | 27 (50.9) | 13 (50.0) | 0.123 |
| Explosive/Blast-related injury, n (%)* | 216 (75.0) | 117 (77.0) | 38 (66.7) | 43 (81.1) | 18 (69.2) | 0.066 |
| Gunshot injury, n (%)* | 46 (16.0) | 20 (13.2) | 13 (22.8) | 7 (13.2) | 6 (23.1) | 0.297 |
| Other mechanism of injury, n (%)* | 16 (5.6) | 12 (7.9) | 2 (3.5) | 1 (1.9) | 1 (3.8) | 0.494 |
Chi-square test.
ANOVA.
Kruskal-Wallis.
PTSD = posttraumatic stress disorder; IQR = interquartile range.
PTSD Trajectories
ANOVA results indicated that the average PCL scores differed across trajectories (P < 0.001). Participants’ with resilient trajectories had the lowest average PCL score over the duration of the study, 19.3 (±3.2), followed by the recovery trajectory, 27.9 (±5.8), the worsening trajectory, 37.3 (±10.3), and the individuals experiencing a chronic trajectory, 59.8 (±9.2). Post hoc pairwise comparisons indicated that the mean difference in PCL scores between resilient and chronic trajectories significantly differed by 33.0 points (95% CI: 30.7, 35.3). Additionally, individuals with the worsening trajectory were estimated to have a mean PCL score 7.1 points higher than the recovery trajectory (95% CI: 5.1, 9.0). There were no significant differences in the mean PCL score based on time of entry into the study (≤6 vs. >6 months after injury). Neither mean PCL scores nor the proportion of participants in each PTSD trajectory statistically significantly differed based on receipt of early RA (Table 1).
Mixed-Effects Models: Association between PTSD Trajectories and Pain Outcomes
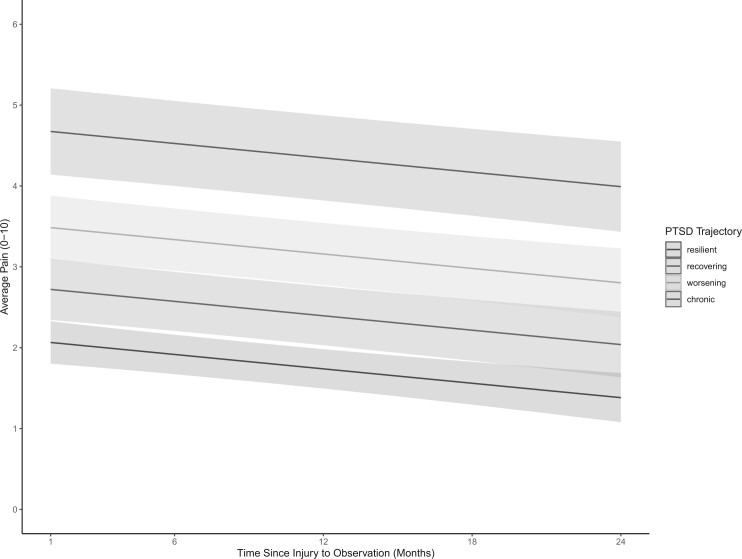
There was a statistically significant difference in pain outcomes based on PTSD symptom trajectories (Table 2). Compared to individuals experiencing resilient trajectories, those with recovering, worsening, and chronic PTSD symptoms were associated with average higher pain scores. For example, individuals presenting with recovering (β = 0.65; 95% CI: 0.09, 1.08) or worsening PTSD symptom trajectories (β = 1.42; 95% CI: 0.77, 1.78) were estimated to experience higher average pain scores compared to those with resilient trajectories, controlling for all other covariates and accounting for RA receipt (Figure 2). Similarly, participants experiencing chronic PTSD were found to report average pain scores over 2 points higher compared to participants experiencing resilient PTSD trajectories (β = 2.61; 95% CI: 1.71, 3.14).
Table 2.
Linear mixed effects models estimating pain outcomes on the Brief Pain Inventory
| Average Pain |
Pain Interference |
|||||
|---|---|---|---|---|---|---|
| β | (95% CI) | P | β | (95% CI) | P | |
| Intercept | 1.90 | (1.57, 2.36) | <0.001 | 1.15 | (0.74, 1.55) | <0.001 |
| PTSD Trajectories | ||||||
| Resilient Reference | ||||||
| Recovery | 0.65 | (0.09, 1.08) | <0.001 | 0.83 | (0.43, 1.24) | <0.001 |
| Worsening | 1.42 | (0.77, 1.78) | <0.001 | 1.85 | (1.43, 2.27) | <0.001 |
| Chronic | 2.61 | (1.71, 3.14) | <0.001 | 3.35 | (2.80, 3.89) | <0.001 |
| Early Regional Anesthesia | ||||||
| No Reference | ||||||
| Yes | −0.31 | (-0.90, -0.04) | 0.048 | −0.26 | (-0.59, 0.05) | 0.102 |
| Time Since Injury (Months) | −0.03 | (-0.04. –0.01) | 0.001 | −0.04 | (-0.05, -0.02) | <0.001 |
| Length of Hospitalization (Days) | 0.01 | (0.01, 0.01) | <0.001 | 0.01 | (0.01, 0.01) | <0.001 |
| Injury Severity Score | 0.01 | (-0.01, 0.01) | 0.374 | 0.01 | (-0.01, -0.01) | 0.644 |
| Entry Cohort | ||||||
| ≤6 Months Reference | ||||||
| >6 Months | 0.05 | (-0.35, 0.44) | 0.118 | −0.04 | (-0.44, 0.36) | 0.847 |
β = coefficient; 95% CI = 95% confidence interval of coefficient.
Figure 2.
Plotted linear mixed effects model coefficients estimating Brief Pain Inventory Average Pain from 1 to 24 months after injury. A differential pain response is observed based on PTSD symptom trajectories. Individuals presenting with chronic PTSD symptom trajectories (red line) are estimated to experience average pain 2.61 points higher compared to those on a resilient trajectory (green line) (95% CI: 1.71, 3.14). Individuals presenting with a worsening PTSD symptom trajectory (yellow line) are estimated to experience average pain scores 1.42 points higher than participants presenting with resilient PTSD trajectories (95% CI: 0.77, 1.78). Individuals experiencing a recovering PTSD trajectory (blue line) were associated with average pain scores 0.65 points higher than those with resilient trajectories (95% CI 0.09, 1.08).
This differential response in pain experiences by PTSD symptom trajectory was also observed for pain interference scores. Participants’ presenting with recovering trajectories reported interference scores nearly a point higher than that of individuals with resilient trajectories (β = 0.85; 95% CI: 0.43, 1.24). Similarly, participants experiencing worsening trajectories were estimated to have interference scores nearly 2-points higher compared to individuals reporting resilient trajectories (β = 1.85; 95% CI: 1.43, 2.27). Chronic PTSD symptom trajectories were associated with experiencing pain interference scores over 3-points higher than participants’ presenting with resilient PTSD trajectories (β = 3.35; 95% CI: 2.80, 3.89). The difference in interference scores observed between participants experiencing resilient compared to chronic trajectories were double that of the difference observed between other PTSD trajectory groups.
Additionally, participants receiving early pain management interventions were observed reporting improved pain outcomes over the 24-month study period. Individuals receiving early RA were estimated to report lower average pain scores than those who did not receive early RA, across all study time points. For example, when adjusting for PTSD trajectory, time since injury, length of hospitalization, ISS, and entry to study, those participants receiving early RA were estimated to experience lower average pain scores up to 24-months (β=-0.31; 95% CI: -0.90, -0.04). However, no statistically significant association was observed between receiving early RA and pain interference scores (β = -0.26; 95% CI: -0.59, 0.05).
Time since injury, measured in months, remained statistically significant in models for average pain and pain interference, indicating that over the course of the study, patient-reported pain intensity and inference decreased monthly. While statistically significant, the association between longer initial length of hospitalization after injury and increases in patient-reported pain outcome scores was marginal. Time to entry in the study and ISS were not statistically significant in any model estimating pain outcomes, indicating that there is no statistical difference in pain intensity and interference estimates by injury severity or delayed participation in the study. Both ISS and length of hospitalization were included in the multivariable model to account for severity of injury and adjust for possible confounding.
Discussion
In this study we observed that an association between pain outcomes and PTSD symptom trajectories persists up to two years following combat-related injury, even after adjusting for the administration of early pain management interventions delivered as part of the combat casualty care. A differential pain response was observed across PTSD symptom trajectories in regard to average pain and pain interference over the two years following initial injury. Compared to individuals with resilient PTSD symptom trajectories, participants experiencing worsening, recovering, and chronic PTSD trajectories reported poorer pain outcomes long after initial injury. These differences in pain scores across PTSD trajectories surpass clinically important differences typically seen in chronic pain research [29]. Therefore, mitigating the development of poor PTSD symptom trajectories after combat-related injures may help mitigate the exacerbation of pain. Despite receipt of RA not being associated with any of the PTSD symptom trajectories, participants who received RA experienced lower average pain across all four PTSD symptom trajectories in this sample of combat-injured service members. Improvements in pain scores observed after receiving RA, while small, reflect the incremental benefits active pain treatments delivered as part of trauma care can have even years after injury [18, 29].
Considering the significant impairment PTSD symptoms and pain can have on physical and social functioning, ongoing joint evaluation of PTSD symptoms and pain in combat injured populations is warranted, even for individuals whose PTSD symptoms do not meet diagnostic criteria. Changes in PTSD symptoms, even at early stages after combat injury, have the potential to inform future care planning and monitoring worsening symptom severity. The observed association between PTSD symptoms and pain outcomes supports previous findings that PTSD symptoms are significantly associated with worsening pain after injury [30–32]. There is a well-established relationship between the mutual presentations and maintenance of PTSD and pain after injury [33,34]. Civilian and military trauma researchers have long posited that pain and PTSD influence one another in a mutually maintaining way so that PTSD exacerbates pain, and vice versa [9]. The present study expands upon other investigations that have shown combat exposure and PTSD to be among the strongest statistical predictors of chronic pain [35,36]. For example, our study examined the link between PTSD symptom severity and pain, utilizing a validated multi-item patient-reported pain scale, rather than the presence of pain and PTSD alone. Furthermore, our study differs from other longitudinal investigations examining PTSD symptoms and pain, which were often limited to a one year follow up period or not specific to OEF/OIF combat-injured American service members.
The present study reflects findings from earlier investigations of PTSD and pain conducted in injured civilian patient populations. Both Stratton et al., as well as Jenewein et al., identified that PTSD symptoms significantly impacted pain intensity up to a year after enrollment in their respective studies, but pain did not exert the same strength on predicting the development of PTSD [31, 37]. While the present investigation did not examine the bidirectional nature of PTSD and pain as these earlier researcher did, findings from our longitudinal study do support that PTSD symptoms are correlated with pain outcomes both in close proximity to severe injury and out to years later. Vaughan and colleagues demonstrated that pain intensity influences exacerbations of specific PTSD symptoms while Alschuler et al. found that PTSD symptom severity may cause variability in pain outcomes [30, 38]. This juxtaposition in the literature warrants further investigation examining the role of PTSD on not only pain intensity, but also functional outcomes (e.g., pain interference), after combat injury given unidimensional ratings of pain intensity can vary greatly depending upon contextual details of the assessment whereas functional outcomes may not be as variable as pain transitions from acute to chronic [39, 40]. While the proportion of participants seen in each trajectory was similar to other investigators’ findings, the average PCL scores seen in this study’s PTSD symptom trajectories were slightly lower than those seen in larger cohorts reported by both Bonanno et al.’s and Berntsen et al.’s studies of non-combat injured OEF/OIF personnel [24,25].
This work builds upon observational research that found RA to be an effective acute care pain management intervention for controlling pain following combat-related injuries. Other investigations report that RA improves the pain experience after combat injury, however, these studies are often limited to showing the short-term advantages of RA [2, 14, 41,42]. Often study periods fall short of capturing the lasting benefits of early aggressive pain management delivered using RA, as accomplished in this study [10]. As such, our study demonstrates the potential long-term benefits of early RA delivered as part of combat casualty care, on subsequent pain outcomes, but not PTSD symptom trajectories evident by the lack of significant interaction terms between RA and PTSD. The lack of significance indicates that RA receipt does not alter long-term pain outcomes by influencing PTSD symptom trajectories. Future research in more controlled environments than the combat theater will need to evaluate which combination of multimodal analgesics delivered using RA may influence PTSD symptom trajectories and subsequently pain outcomes after serious injury. For example, researchers have observed that the administration of ketamine, a key component of multimodal pain management, soon after combat-related injury was associated with a lower prevalence of PTSD, however pain outcomes were not examined [43].
Advancing current understanding of symptom trajectories after serious injury will enable trauma researchers and clinicians to better plan for the long-term health care requirements of combat-injured service members. Introduced more than two decades ago, multimodal analgesia is currently recommended for managing both acute and, to a lesser degree, chronic pain [44]. The synergy created when multimodal regimens are used to target discrete components of the peripheral and central pain pathways provides effective analgesia at lower opioid dosing, reducing related risk and producing fewer adverse effects [14,15]. Advanced training of anesthesiologists and certified registered nurse anesthetists with training in RA is critical to ensuring that optimal pain management approaches are available to aid in the recovery of all injured persons beginning in the acute post-injury period.
Importantly, trauma providers do not exist in silos, and combat-injured veterans depend on the support of interdisciplinary care providers, including pain and mental health providers. Patients, families, and clinicians recognize the physical and behavioral health needs of individuals with serious injuries continue long after acute care [45]. Therefore, engaging acute pain services, capable of delivering RA, early on in trauma care may help improve pain trajectories. Sustaining those positive trajectories in a multimodal stepped model of chronic pain management that incorporates both pain management and mental health care, such as established in the Veterans Health Administration in collaboration with the Department of Defense, will be important to improving longer-term outcomes of trauma survivors in their communities [46]. Given the significant longitudinal associations between pain and PTSD symptom trajectories identified in this analysis, clinicians caring for service members and veterans during their prolonged rehabilitative care should consider evaluating patient-reported pain outcomes and PTSD symptoms jointly. Due to the co-occurring nature of these conditions, and the potential for individuals to underreport PTSD symptom severity, integrated pain and mental health symptom assessments are key to connecting patients to treatment for underlying psychological contributions of pain.
Strengths and Limitations
Given the challenges of recruiting and retaining a sample of combat-injured participants, there was a high degree of variability as to when participants entered the study and duration of participation. For recruitment, every effort was made to enroll participants at the time of discharge from acute care at the only two military hospitals in the US capable of providing acute post-injury care. Extended follow-up of these patients was challenged by the dispersion of subjects across the U.S. following discharge. This created considerable challenges in finding and following subjects, and thus, patient-reported outcome data was available for an average of 7 time points for each subject and data were not available on all subjects at all-time points. Unlike other methods used to assess the relationship between PTSD symptom severity and pain, mixed-effects modeling approaches in this analysis are not as sensitive to data missing at random and can adjust for disparate data points in the analysis. The small number of individuals entering the study six months after injury did not report higher pain intensity or interference, on average, compared to those enrolled closer to time of injury. While the inclusion of these participants could introduce sample bias, this small number of participants was evenly distributed between RA and No RA cohorts and across PTSD trajectories. Participants’ PTSD total symptom severity was measured using the DSM-IV criteria, which has been found to identify similar prevalence rates of PTSD as the more recent DSM-5 criteria in OEF/OIF veteran populations [47]. Both the PCL and the pain interference component of the BPI capture similar domains related to sleep, enjoyment of life, and relationships with other individuals, which may partially account for the correlation observed between PTSD trajectories and pain interference scores. The limited number of individuals presenting with chronic PTSD trajectories indicates that these hypothesis generating findings should be interpreted with caution and be used to inform future larger cohort studies. For example, the small number of participants in the chronic PTSD trajectory may have limited our statistical power to detect a moderating relationship between RA and PTSD trajectories on pain outcomes. Strengths of the current study include the ability to account for time since traumatic combat injury to observation in order to provide longitudinal estimates of outcomes, and the modeling adjustment of both early pain management and behavioral health symptom trajectories in an effort to isolate the associations between either on pain outcomes over time.
Conclusions
A differential pain response was observed across PTSD symptom trajectories. Participants reporting chronic, worsening, or recovering PTSD symptom trajectories were observed experiencing greater pain intensity and interference following combat injury, compared to individuals presenting with resilient PTSD trajectories. Continued evaluation of both pain and PTSD symptoms have the potential to inform the development and implementation of comprehensive rehabilitative trauma services to mitigate poor symptom trajectories. It is imperative to integrate psychological assessment and treatment throughout the continuum of trauma care. Doing so can enable clinicians to stratify patients at risk of experiencing poor symptom trajectories. This in turn can inform the implementation of multimodal PTSD and pain management interventions earlier on in the care continuum following serious injuries.
Supplementary Material
Acknowledgements:
The authors thank the entire Regional Anesthesia Military Battlefield Pain Outcome Study team including Drs. Wensheng Guo and Lynne Taylor. The authors appreciate the insightful feedback provided by Drs. Amy Sawyer, Peggy Compton, and Sydney Axson. Additionally, the authors would like to thank the service members who participated in the study for both their contributions to this research and their military service.
Supplementary Data
Supplementary data are available at Pain Medicine online.
Funding sources: Dr. Giordano received a grant from the National Institutes of Health (1F31NR017151-01A1) and The Rita & Alex Hillman Foundation’s Hillman Scholars in Nursing Innovation program.
Conflicts of interest: Dr. Giordano received a grant from the National Institutes of Health (1F31NR017151-01A1) and The Rita & Alex Hillman Foundation’s Hillman Scholars in Nursing Innovation program. Dr. Gallagher received funding for the original study from the U.S. Department of Veterans Affairs (VA RRD D45064-1). For the remaining authors, no conflicts were declared. The views expressed in this manuscript are those of the authors and do not reflect the official policy of the Department of Veterans Affairs, Uniformed Services University, the Department of the Army/Navy/Air Force, the Department of Defense, or the United States Government.
References
- 1. Clifford JL, Fowler M, Hansen JJ, et al. State of the science review: Advances in pain management in wounded service members over a decade at war. J Trauma Acute Care Surg 2014;77(3 Suppl 2):S228–S236. [DOI] [PubMed] [Google Scholar]
- 2. Buckenmaier IIC, Mahoney PF, Anton T, Kwon N, Polomano RC.. Impact of an acute pain service on pain outcomes with combat-injured soldiers at Camp Bastion, Afghanistan. Pain Med 2012;13(7):919–26. [DOI] [PubMed] [Google Scholar]
- 3. Milligan ED, Watkins LR.. Pathological and protective roles of glia in chronic pain. Nat Rev Neuro 2009;10(1):23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Glare P, Aubrey KR, Myles PS.. Transition from acute to chronic pain after surgery. Lancet 2019;393(10180):1537–46. [DOI] [PubMed] [Google Scholar]
- 5. Norman SB, Stein MB, Dimsdale JE, Hoyt DB.. Pain in the aftermath of trauma is a risk factor for post-traumatic stress disorder. Psychol Med 2008;38(4):533–42. [DOI] [PubMed] [Google Scholar]
- 6. Liedl A, O'donnell M, Creamer M, et al. Support for the mutual maintenance of pain and post-traumatic stress disorder symptoms. Psychol Med 2010;40(7):1215–23. [DOI] [PubMed] [Google Scholar]
- 7.US Department of Veterans Affairs. How common is PTSD? https://www.ptsd.va.gov/public/PTSD-overview/basics/how-common-is-ptsd.asp Web site. Updated 2016. (accessed March, 5 2020).
- 8. Lew HL, Otis JD, Tun C, Kerns RD, Clark ME, Cifu DX.. Prevalence of chronic pain, posttraumatic stress disorder, and persistent postconcussive symptoms in OIF/OEF veterans: Polytrauma clinical triad. J Rehabil Res Dev Clin 2009;46(6):697–702. [DOI] [PubMed] [Google Scholar]
- 9. Edwards RR, Dworkin RH, Sullivan MD, Turk DC, Wasan AD.. The role of psychosocial processes in the development and maintenance of chronic pain. J Pain 2016;17(9 Suppl):T70–T92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giordano NA, Bader C, Richmond TS, Polomano RC.. Complexity of the relationships of pain, posttraumatic stress, and depression in Combat‐Injured populations: An integrative review to inform Evidence‐Based practice. Worldviews Evid Based Nurs 2018;15(2):113–26.. [DOI] [PubMed] [Google Scholar]
- 11. Pugh MJV, Finley EP, Copeland LA, et al. Complex comorbidity clusters in OEF/OIF veterans: The polytrauma clinical triad and beyond. Med Care 2014;52(2):172–81. [DOI] [PubMed] [Google Scholar]
- 12. Lee SY, Finkelstein-Fox L, Park CL, Mazure CM, Huedo-Medina TB, Hoff R.. Bidirectionality of pain interference and PTSD symptoms in military veterans: Does injury status moderate effects? Pain Med 2019;20(5):934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gabriel RA, Ilfeld BM.. Use of regional anesthesia for outpatient surgery within the united states: A prevalence study using a nationwide database. Anesth Analg 2018;126(6):2078–84. [DOI] [PubMed] [Google Scholar]
- 14. Ilfeld BM. Continuous peripheral nerve blocks: A review of the published evidence. Anesth Analg 2011;113(4):904–25. [DOI] [PubMed] [Google Scholar]
- 15. Neuman MD, Rosenbaum PR, Ludwig JM, Zubizarreta JR, Silber JH.. Anesthesia technique, mortality, and length of stay after hip fracture surgery. JAMA 2014;311(24):2508–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weinstein EJ, Levene JL, Cohen MS, Andreae DA, et al. Local anaesthetics and regional anaesthesia versus conventional analgesia for preventing persistent postoperative pain in adults and children. Coch Data Sys Rev 2018;(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holbrook TL, Galarneau MR, Dye JL, Quinn K, Dougherty AL.. Morphine use after combat injury in Iraq and post-traumatic stress disorder. N Engl J Med 2010;362(2):110–7. [DOI] [PubMed] [Google Scholar]
- 18. Gallagher RM, Polomano RC, Giordano NA, et al. Prospective cohort study examining the use of regional anesthesia for early pain management after combat-related extremity injury. Reg Anesth Pain Med 2019;44(12):1045–52. [DOI] [PubMed] [Google Scholar]
- 19. Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA.. Psychometric properties of the PTSD checklist (PCL). Behav Res Ther 1996;34(8):669–73. [DOI] [PubMed] [Google Scholar]
- 20.National Center for PTSD. Using the PTSD checklist for DSM-IV (PCL). United States Veterans Affairs. Available at: https://sph.umd.edu/sites/default/files/files/PTSDChecklistScoring.pdf.
- 21. Cleeland CS, Ryan KM.. Pain assessment: Global use of the brief pain inventory. Ann Acad Med Singapore 1994. [PubMed] [Google Scholar]
- 22. BAKER SP, O'NEILL B, HADDON W, LONG WB.. The injury severity score: A method for describing patients with multiple injuries and evaluating emergency care. J Trauma Acute Care Surg 1974;14(3):187–96. [PubMed] [Google Scholar]
- 23. Genolini C, Pingault JB, Driss T, et al. KmL3D: A non-parametric algorithm for clustering joint trajectories. Comput Methods Programs Biomed 2013;109(1):104–11. [DOI] [PubMed] [Google Scholar]
- 24. Bonanno GA, Mancini AD, Horton JL, Millennium Cohort Study Team, et al. Trajectories of trauma symptoms and resilience in deployed US military service members: Prospective cohort study. Br J Psychiatry 2012;200(4):317–23. [DOI] [PubMed] [Google Scholar]
- 25. Berntsen D, Johannessen KB, Thomsen YD, Bertelsen M, Hoyle RH, Rubin DC.. Peace and war: Trajectories of posttraumatic stress disorder symptoms before, during, and after military deployment in Afghanistan. Psychol Sci 2012;23(12):1557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bryant RA, Nickerson A, Creamer M, et al. Trajectory of post-traumatic stress following traumatic injury: 6-year follow-up. Br J Psych 2015;206(5):417–23. [DOI] [PubMed] [Google Scholar]
- 27. Laird NM, Ware JH.. Random-effects models for longitudinal data. Biometrics 1982;38(4):963–74. [PubMed] [Google Scholar]
- 28. Satterthwaite FE. An approximate distribution of estimates of variance components. Biometrics Bulletin 1946;2(6):110–4. [PubMed] [Google Scholar]
- 29. Dworkin RH, Turk DC, McDermott MP, et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain 2009;146(3):238–44. [DOI] [PubMed] [Google Scholar]
- 30. Vaughan CA, Miles JN, Eisenman DP, Meredith LS.. Longitudinal associations among pain, posttraumatic stress disorder symptoms, and stress appraisals. J Trauma Stress 2016;29(2):176–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stratton KJ, Clark SL, Hawn SE, Amstadter AB, Cifu DX, Walker WC.. Longitudinal interactions of pain and posttraumatic stress disorder symptoms in US military service members following blast exposure. J Pain 2014;15(10):1023–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Osenbach JE, Lewis C, Rosenfeld B, et al. Exploring the longitudinal trajectories of posttraumatic stress disorder in injured trauma survivors. Psychiatry 2014;77(4):386–97. [DOI] [PubMed] [Google Scholar]
- 33. Outcalt SD, Kroenke K, Krebs EE, et al. Chronic pain and comorbid mental health conditions: Independent associations of posttraumatic stress disorder and depression with pain, disability, and quality of life. J Behav Med 2015;38(3):535–43. [DOI] [PubMed] [Google Scholar]
- 34. MacGregor AJ, Tang JJ, Dougherty AL, Galarneau MR.. Deployment-related injury and posttraumatic stress disorder in US military personnel. Injury 2013;44(11):1458–64. [DOI] [PubMed] [Google Scholar]
- 35. Afari N, Ahumada SM, Wright LJ, et al. Psychological trauma and functional somatic syndromes: A systematic review and meta-analysis. Psychosom Med 2014;76(1):2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brennstuhl M, Tarquinio C, Montel S.. Chronic pain and PTSD: Evolving views on their comorbidity. Perspect Psychiatr Care 2015;51(4):295–304. [DOI] [PubMed] [Google Scholar]
- 37. Jenewein J, Wittmann L, Moergeli H, Creutzig J, Schnyder U.. Mutual influence of posttraumatic stress disorder symptoms and chronic pain among injured accident survivors: A longitudinal study. J Trauma Stress 2009;22(6):540–8. [DOI] [PubMed] [Google Scholar]
- 38. Alschuler KN, Otis JD.. Coping strategies and beliefs about pain in veterans with comorbid chronic pain and significant levels of posttraumatic stress disorder symptoms. Eur J Pain 2012;16(2):312–9. [DOI] [PubMed] [Google Scholar]
- 39. Dansie EJ, , Turk DC.. Assessment of patients with chronic pain. Br J Anaesth 2013;111(1):19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Smith SM, Dworkin RH, Turk DC, et al. Interpretation of chronic pain clinical trial outcomes: IMMPACT recommended considerations. Pain 2020;161(11):2446–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Buckenmaier CC III, McKnight GM, Winkley JV, et al. Continuous peripheral nerve block for battlefield anesthesia and evacuation. Reg Anesth Pain Med 2005;30(2):202–5. [DOI] [PubMed] [Google Scholar]
- 42. Fleming I, Egeler C.. Regional anaesthesia for trauma: An update. BJA Educ 2014;14(3):136–41. [Google Scholar]
- 43. McGhee LL, Maani CV, Garza TH, Gaylord KM, Black IH.. The correlation between ketamine and posttraumatic stress disorder in burned service members. J Trauma Acute Surg 2008;64(2 Suppl):S195–S199. [DOI] [PubMed] [Google Scholar]
- 44. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: A clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain 2016;17(2):131–57. [DOI] [PubMed] [Google Scholar]
- 45. Berwick DM, Downey AS, Cornett EA.. A national trauma care system to achieve zero preventable deaths after injury: Recommendations from a national academies of sciences, engineering, and medicine report. JAMA 2016;316(9):927–8. [DOI] [PubMed] [Google Scholar]
- 46. Gallagher RM. Advancing the pain agenda in the veteran population. Anesthesiol Clin 2016;34(2):357–78. [DOI] [PubMed] [Google Scholar]
- 47. Hoge CW, Riviere LA, Wilk JE, Herrell RK, Weathers FW.. The prevalence of post-traumatic stress disorder (PTSD) in US Combat soldiers: A head-to-head comparison of DSM-5 versus DSM-IV-TR symptom criteria with the PTSD checklist. Lancet Psych 2014;1(4):269–77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.