Abstract
The incidence of astrovirus infection in children less than 5 years of age hospitalized with acute gastroenteritis in Melbourne, Australia, from 1995 to 1998 was determined. Astrovirus was detected in 40 of 449 specimens tested by Northern hybridization, and astrovirus infection was confirmed by reverse transcription-PCR with or without culture in CaCO-2 cells. This represented 3.0% (40 of 1,327) of all children tested for enteric pathogens, including viral, bacterial, and parasitic pathogens, over the survey period. The incidences of astrovirus infection in each year were 4.4% (1995), 2.2% (1996), 3.9% (1997), and 1.4% (1998). In 1995 and 1997, the incidences of astrovirus infection were greater than the incidence of infection with all individual bacterial pathogens and were either greater than or equal to the incidence of adenovirus infection. Astrovirus exhibited an unusual biennial winter peak of incidence that correlated with a greater incidence of serotype 1 virus and an increased rate of hospitalization of children aged 6 to 12 months. Uncommon (serotype 2 and 4) and rare (serotype 8) serotypes were detected during the survey period. Genetic analysis of ORF2 (which encodes the astrovirus capsid precursor) of Melbourne isolates showed nucleotide sequence variation from year to year. This was not accompanied by significant amino acid substitutions. However, geographical variation was apparent by comparison of Melbourne astrovirus isolates with prototype strains identified in the United Kingdom.
Human astroviruses are members of the family Astroviridae which are recognized as a common cause of infantile gastroenteritis worldwide (13). Initially associated with an outbreak of diarrhea in infants in a maternity unit, these viruses were given the name astrovirus because of the characteristic five- or six-point star shape they display when viewed by electron microscopy after negative staining of fecal extracts (1, 11). The medical importance of human astrovirus infection has been established by reports which have shown that in some settings astrovirus is the second most common cause of diarrhea in children (5). A recent report from Mexico found astrovirus in the stools of 61% of all children and 26% of children with diarrhea (12).
The astrovirus virion is composed of a single nonenveloped capsid layer of between 27 and 34 nm in diameter (7). The genome consists of a single-stranded, positive-sense, polyadenylated RNA of 6.8 to 7.2 kb in length. Three open reading frames (ORFs) designated ORF1a, ORF1b, and ORF2 have been identified (8). The first two ORFs contain amino acid motifs homologous to protease and polymerase proteins, respectively (10). ORF2 (∼2.4 kb in length) is found at the 3′ end of the genome and encodes the capsid protein precursor.
Astrovirus infections have been associated with sporadic diarrhea in children in the community as well as focal outbreaks. The main symptom of infection is watery diarrhea, which is often associated with vomiting, fever, and abdominal pain (13). Settings in which outbreaks among children have occurred include children's wards, day-care centers, kindergartens, and schools (5). Diarrheal outbreaks have been described in nursing homes for the elderly and among military recruits (2, 5).
Astroviruses can be classified into serotypes according to the reactivities of the capsid proteins with polyclonal sera and monoclonal antibodies (13). Astroviruses are also classified into genotypes on the basis of the nucleotide sequence of a 348-bp region of ORF2 (16). There is a good correlation between genotype and serotype (16). There are currently seven established serotypes of human astrovirus that correlate with seven genotypes. The existence of an eighth genotype is suggested by the sequence of a putative serotype 8 astrovirus deposited in GenBank. Previous studies have shown that serotype 1 is the predominant disease-causing type, followed by serotypes 2, 3, 4, and 5, which are less common (9). Serotypes 6, 7, and 8 have rarely been detected.
We report here on a 4-year study (1995 to 1998) of astrovirus infection in children admitted to the Royal Children's Hospital, Melbourne, Australia, with acute gastroenteritis. The monthly distribution, serotype distribution, and extent of genetic variation of clinical isolates were investigated.
MATERIALS AND METHODS
Sample selection.
Stool specimens were collected from children under the age of 5 years who were admitted to the Royal Children's Hospital, Melbourne, with acute gastroenteritis between January 1995 and December 1998. Routine diagnostic tests for rotavirus, adenovirus, and common bacterial pathogens were carried out with all specimens. Specimens negative for rotavirus were tested for the presence of astrovirus. In addition, samples were collected from children under 2 years of age involved in an outbreak of gastroenteritis at the Royal Children's Hospital staff creche.
Astrovirus detection.
Astroviruses were detected by a Northern hybridization method as described previously (18). Briefly, RNA was isolated from 10% (wt/vol) fecal homogenates by phenol-chloroform extraction, purified by adsorption to hydroxyapatite, and eluted in potassium phosphate buffer (6). The RNA was blotted onto a nylon membrane and fixed by UV cross-linking. Hybridization was carried out under stringent conditions (5× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 50% formamide, 50°C) with a digoxigenin (DIG)-labeled cDNA probe specific for astrovirus. After posthybridization washes, bound probe was detected with anti-DIG antibody conjugated to alkaline phosphatase (Roche Biochemicals, Mannheim, Germany) and the chemiluminescent substrate CDP-Star (Roche Biochemicals).
RT-PCR and tissue culture adaptation of astrovirus-containing clinical samples.
All specimens that tested positive for astrovirus by Northern hybridization were also tested in an astrovirus-specific reverse transcription-PCR (RT-PCR) assay with the primer pair Mon269 and Mon270 (16). This primer pair amplifies a 449-bp region of ORF2. Specimens that failed to generate an RT-PCR product were cultured in CaCO-2 cells (15) and were retested as described above.
Astrovirus genotyping and sequence analysis.
RT-PCR cDNA products were purified from agarose gels prepared in 1× TAE (Tris-acetate-EDTA) buffer by using the Bresaclean DNA purification kit (Geneworks, Adelaide, Australia). The nucleotide sequence of a 348-bp region of the cDNA was determined by direct cycle sequencing with the fmol DNA Cycle Sequencing System (Promega, Madison, Wis.) and primers Mon269 and Mon270. The nucleotide sequence of each individual isolate was compared to those of reference strains in order to assign a genotype and, hence, a serotype by proxy. Sequence analysis was carried out with the extended GCG package available through the Australian National Genomic Information Service (University of Sydney). Sequence alignments were carried out by using the E-CLUSTALW program. Replicate data sets (n = 1,000) were generated by bootstrap resampling with the E-SEQBOOT program and were analyzed by the neighbor-joining method with E-NEIGHBOR software. The phylogenetic tree was drawn by using TreeView software (17). All major branches satisfied the 100% confidence level.
Nucleotide sequence accession numbers.
The nucleotide sequences determined in this study have been deposited in the GenBank database and assigned the accession nos. AF175253 to AF175261.
RESULTS
Epidemiology of astrovirus infection in hospitalized children.
Over a 4-year period spanning January 1995 to December 1998, a total of 1,327 stool specimens collected from children admitted to the Royal Children's Hospital in Melbourne with acute gastroenteritis were tested for enteric pathogens (Table 1). As expected, rotavirus was the most common pathogen during these 4 years and was detected overall in 65.2% of specimens. Bacterial pathogens were detected overall in 6.3% of specimens, and enteric adenoviruses were detected overall in 4.1% of specimens. Of the 462 specimens negative for rotavirus, 449 (97.2%) were tested for astrovirus. The remaining 13 samples were not tested due to the lack of availability of sufficient fecal material.
TABLE 1.
Incidence of pathogens detected in stool specimens from children hospitalized with acute gastroenteritis in Melbourne, 1995 to 1998
| Pathogen | No. (%) of specimensa
|
|||
|---|---|---|---|---|
| 1995 (n = 360) | 1996 (n = 368) | 1997 (n = 309) | 1998 (n = 290) | |
| Rotavirus | 243 (67.5) | 247 (67.1) | 203 (65.7) | 172 (59.3) |
| Astrovirus | 16 (4.4) | 8 (2.2) | 12 (3.9) | 4 (1.4) |
| Adenovirus | 14 (3.9) | 18 (4.9) | 12 (3.9) | 10 (3.4) |
| Salmonella | 5 (1.4) | 8 (2.2) | 8 (2.6) | 9 (3.1) |
| Campylobacter | 5 (1.4) | 4 (1.1) | 5 (1.6) | 8 (2.7) |
| Clostridium | 4 (1.1) | 0 (0) | 2 (0.6) | 5 (1.7) |
| Escherichia coli | 0 (0) | 1 (0.3) | 0 (0) | 0 (0) |
| Yersinia | 0 (0) | 0 (0) | 0 (0) | 1 (0.3) |
| Shigella | 0 (0) | 0 (0) | 1 (0.3) | 0 (0) |
| total | 287 (79.7) | 286 (77.7) | 243 (78.6) | 209 (72.1) |
The data for astrovirus are in boldface. n is the number of specimens tested.
The overall incidence of astrovirus infection was found to be 3.0% (40 of 1,327 total samples). The incidences for each year were 4.4% (1995), 2.2% (1996), 3.9% (1997), and 1.4% (1998). The results for 1995 have been corrected and extended from those described previously (18). In general, the incidence of astroviruses either equaled or was slightly less than the incidence of enteric adenoviruses and exceeded those of Salmonella and/or Campylobacter in most years. Astroviruses appeared to show biennial peaks of incidence, being most common in 1995 and 1997.
In 1998, the first isolate detected was collected from a child cared for by the hospital creche. This child was involved in an outbreak of astrovirus infection that occurred in the creche between 7 March and 19 March and was subsequently admitted for treatment of severe gastroenteritis. The outbreak was characterized by symptoms of diarrhea and vomiting that affected at least nine children and four staff members. Samples were collected from six children, and serotype 1 astrovirus was detected in four of these. Sequence analysis indicated that all children were infected with the same virus, but the virus differed from the serotype 1 strains isolated from other patients in the same year (see below).
The monthly distribution of astrovirus detection is shown in Fig. 1. Astrovirus was not detected in February or September in any of the years studied. In 1995 and 1997, the majority of astrovirus infections occurred between May and August, which correspond to late autumn and winter, respectively, in the Southern Hemisphere. This seasonal peak was not evident in 1996 and 1998. Although differences in the seasonal patterns between the years were evident, these differences were not significant when analyzed by a t test.
FIG. 1.
Monthly distribution of astrovirus identified in the stools of children admitted to the Royal Children's Hospital, Melbourne, from 1995 to 1998 with acute gastroenteritis.
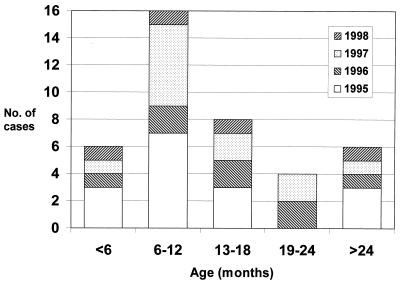
The age distribution of astrovirus infection is shown in Fig. 2. The mean age of infected children was 14.7 months, and the median age was 14 months. The majority of infected children were less than 2 years of age. In 1995 and 1997, children in the 6- to 12-month age group were the most susceptible population, making up 44 and 50% of infected children, respectively. The mean number of children in the 6- to 12-month age group in 1995 and 1997 was 6.5 ± 0.71, while the mean number of children in this age group in 1996 and 1998 was 1.5 ± 0.71. When analyzed by a t test, the difference between the means was statistically significant (P < 0.02). In 1996 and 1998, the numbers of children in each age group were roughly equivalent.
FIG. 2.
Age distribution of children admitted to the Royal Children's Hospital, Melbourne, from 1995 to 1998 with astrovirus gastroenteritis.
Astrovirus serotypes (genotypes).
The serotype (deduced from the genotype) of each astrovirus isolate was determined. The incidence of each serotype is shown in Fig. 3. Isolates of serotype 1 were found in every year, and this serotype was the predominant type both overall and in each individual year. However, a difference in the pattern of serotype 1 astrovirus occurrence was observed. The mean number of serotype 1 isolates in 1995 and 1997 was 11.5 ± 0.71, whereas in 1996 and 1998 the mean number was 3.5 ± 0.71. This difference was significant (t test; P < 0.01). Serotype 4 was isolated in 3 of the 4 years with a diminishing frequency. In 1996, two isolates of the same serotype 2 strain were identified. An isolate which did not resemble serotypes 1 to 7 was detected in 1997. This isolate was found to have 95% nucleotide identity to the sequence of a putative serotype 8 astrovirus deposited in GenBank (accession no. Z66541).
FIG. 3.
Genotype (serotype) distribution of astroviruses identified in this study for the years 1995 to 1998.
Genetic variation of astrovirus isolates.
Variation in nucleotide sequence was observed between isolates of serotypes 1 and 4. However, phylogenetic analysis showed that all serotype 1 isolates clustered into one major lineage containing a number of minor branches (Fig. 4). One branch was represented by strains isolated in 1998, including the outbreak strain (Melb1I) described above, which differed from other strains from the same and different years. Overall, limited variation was observed in Melbourne serotype 1 and 4 isolates, which displayed a maximum of 2.0% nucleotide sequence divergence and 0.9% amino acid sequence divergence. All but one of the nucleotide sequence changes within the Melbourne isolates were silent with respect to amino acid coding. Only one serotype 1 isolate (Melb1G) contained a Gly→Cys change at amino acid 98 of the capsid protein. This suggested that, with the exception of isolate Melb1G, the different Melbourne isolates may represent variants of the same strain within each serotype.
FIG. 4.
Phylogenetic tree of a 348-bp region of ORF2 of astrovirus isolates collected in Melbourne from 1995 to 1998 and standard human astrovirus strains (HAstV-1 to HAstV-8) isolated in the United Kingdom. Melb1A-1D and Melb4A-4B correspond to previously reported strains RCH1A-1D and RCH4A-4B, respectively (18). The scale bar is proportional to genetic distance. All major branches satisfied the 100% bootstrap confidence level. Numbers on the major branch divisions indicate the genotype (serotype) of the isolates found on that branch.
The degree of variation was more evident between Melbourne isolates and prototype strains from the United Kingdom used to derive Fig. 4. Melbourne serotype 1 and 4 isolates displayed up to 8.9% nucleotide sequence variation and 4.3% amino acid sequence variation with respect to the nucleotide and amino acid sequences of the prototype United Kingdom strains. Similarly, the Melbourne serotype 2 isolates displayed 14.0% nucleotide sequence variation and 2.6% amino acid sequence variation from the prototype serotype 2 astrovirus, while the Melbourne serotype 8 and prototype serotype 8 strain displayed 4.9% nucleotide sequence variation and 7.8% amino acid sequence variation.
DISCUSSION
This is the first study to use molecular techniques (Northern hybridization and RT-PCR) in a long-term prospective study of astrovirus infection in children hospitalized with acute gastroenteritis. Previous epidemiological studies of astrovirus infection in children have been carried out in a variety of settings, including hospitals and outpatient clinics, and through community-based studies by using electron microscopy and enzyme immunoassay. Surveys of the incidence of hospitalization due to astrovirus-induced severe gastroenteritis in developed countries have reported rates of 2 to 3% (3). The incidence of astrovirus infection reported in this study is consistent with those findings. In contrast, a recent study from Chile found that astroviruses were responsible for up to 20% of hospital admissions (4). This suggests that the burden of astrovirus disease may be greater in developing countries.
The finding of biennial peaks of astrovirus infection was unusual since previous studies conducted in temperate climates have generally found an annual winter peak of infection similar to that observed for rotavirus (13). An exception was the study by Lee and Kurtz (9) in the United Kingdom, which found a biennial peak of incidence over the period from 1988 to 1992. We found an association of the biennial pattern with three other observations. First, in the 2 years in which a winter peak was observed, a large proportion of infected children were aged 6 to 12 months and, conversely, this age group was underrepresented in years without a winter peak. Second, the incidence of serotype 1 isolation was higher in years with a winter peak. Third, the total incidence of astrovirus infection was higher in years that contained a winter peak. The association between these observations is unclear. One hypothesis is that the high rates of serotype 1 isolation in certain years reflect an increased incidence of this serotype across all age groups in the community. This could result in short-term herd immunity that protects the subsequent “at-risk” population from the major circulating serotype during the following 12 to 18 months.
As with previous surveys, serotype 1 was found to be the most prevalent serotype. However, we also detected samples with viruses classified into less common (serotypes 2 and 4) and rare (serotype 8) serotypes. There was minimal genetic variation in serotype 1 over the 4 years studied and potential antigenic variation in only 1 of 10 strains during the same period. By comparison, the degree of sequence variation between the Melbourne serotype 2 strain and the prototype serotype 2 strain suggests that this serotype may be composed of distinct subtypes, even though the degree of amino acid divergence was minimal. Similarly, the degree of amino acid variation between the Melbourne serotype 8 isolate and the prototype serotype 8 strain may also indicate the existence of subtypes within this serotype. The previous survey carried out in the Royal Children's Hospital identified astroviruses belonging to the uncommon serotype 5 in samples collected in the early 1980s (18). Given that this serotype has not been identified in the current study and that new types have emerged since then, it is suggested that, with the exception of serotype 1, the serotype distribution of astrovirus in Melbourne is not static. This has implications if future development of astrovirus vaccines is considered important.
Although variation in nucleotide sequence was observed, this translated to minimal intratypic amino acid variation. However, the region of the capsid gene analyzed is in a relatively conserved part of this gene so that further analysis of ORF2, in particular, the more variable 3′ end (19), may yield more information about the extent of genetic diversity. Furthermore, no immunoreactive epitopes have been identified in the 348-bp region studied here so that no information about the potential for antigenic diversity is available. Nevertheless, geographical diversity was evident such that Melbourne isolates and prototype strains from the United Kingdom showed significant variation. We have also observed geographical variation within Australia, with isolates from Sydney differing from those isolated in Melbourne (data not shown).
We highlight the detection of a serotype 8 astrovirus, only the fourth documented isolation of this rare type and the first documented isolation from a hospitalized child. Other serotype 8 astroviruses have been identified in the United Kingdom (GenBank accession no. Z66541), Africa, and the Middle East (14). Why this type should be found in such diverse geographical locations is unknown. Perhaps this serotype is more common than surveillance studies indicate but rarely causes severe disease. Alternatively, this serotype may represent an emerging astrovirus type worldwide.
We also note that, although this study has established astrovirus as an important cause of severe gastroenteritis in Melbourne, an etiologic agent remained unidentified in a large proportion of fecal samples (up to 28%; Table 1). Hence, further investigation of other recognized agents (e.g., calicivirus) or unrecognized agents is required.
The results of this study provide further epidemiological and molecular information about astrovirus strains that cause severe disease in children. This information is relevant to the development of vaccines and other preventative therapies, should they be considered worthwhile.
ACKNOWLEDGMENTS
We thank Paul Masendycz, Helen Bugg, and Nada Bogdanovich-Sakran for identification of rotavirus-negative fecal specimens and the Department of Microbiology and Infectious Diseases, Royal Children's Hospital, for providing fecal specimens.
This study was supported by a postgraduate fellowship to H.M. and project grants to E.A.P from the Royal Children's Hospital Research Institute.
REFERENCES
- 1.Appleton H, Higgins P G. Viruses and gastroenteritis in infants. Lancet. 1975;i:1297. doi: 10.1016/s0140-6736(75)92581-7. [DOI] [PubMed] [Google Scholar]
- 2.Belliot G, Laveran H, Monroe S S. Outbreak of gastroenteritis in military recruits associated with serotype 3 astrovirus infection. J Med Virol. 1997;51:101–106. doi: 10.1002/(sici)1096-9071(199702)51:2<101::aid-jmv3>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 3.Carter M J, Willcocks M M. The molecular biology of astroviruses. Arch Virol Suppl. 1996;12:277–285. doi: 10.1007/978-3-7091-6553-9_30. [DOI] [PubMed] [Google Scholar]
- 4.Gaggero A, O'Ryan M, Noel J S, Glass R I, Monroe S S, Mamani N, Prado V, Avendano L F. Prevalence of astrovirus infection among Chilean children with acute gastroenteritis. J Clin Microbiol. 1998;36:3691–3693. doi: 10.1128/jcm.36.12.3691-3693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glass R I, Noel J, Mitchell D, Herrmann J E, Blacklow N R, Pickering L K, Dennehy P, Ruiz-Palacios G, de Guerrero M L, Monroe S S. The changing epidemiology of astrovirus-associated gastroenteritis: a review. Arch Virol Suppl. 1996;12:287–300. doi: 10.1007/978-3-7091-6553-9_31. [DOI] [PubMed] [Google Scholar]
- 6.Gouvea V, Allen J R, Glass R I, Fang Z-Y, Bremont M, Cohen J, McCrae M A, Saif L J, Sinarachatanant P, Caul E O. Detection of group B and C rotaviruses by polymerase chain reaction. J Clin Microbiol. 1991;29:519–523. doi: 10.1128/jcm.29.3.519-523.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenberg H B, Matsui S M. Astroviruses and caliciviruses: emerging enteric pathogens. Infect Agents Dis. 1992;1:71–91. [PubMed] [Google Scholar]
- 8.Jiang B, Monroe S S, Koonin E V, Stine S E, Glass R I. RNA sequence of astrovirus: distinctive genomic organization and a putative retrovirus-like ribosomal frameshifting signal that directs the viral replicase synthesis. Proc Natl Acad Sci USA. 1993;90:10539–10543. doi: 10.1073/pnas.90.22.10539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee T W, Kurtz J B. Prevalence of human astrovirus serotypes in the Oxford region 1976–92, with evidence for two new serotypes. Epidemiol Infect. 1994;112:187–193. doi: 10.1017/s0950268800057551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis T L, Greenberg H B, Herrmann J E, Smith L S, Matsui S M. Analysis of astrovirus serotype 1 RNA, identification of the viral RNA-dependent RNA polymerase motif, and expression of a viral structural protein. J Virol. 1994;68:77–83. doi: 10.1128/jvi.68.1.77-83.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madeley C R, Cosgrove B P. 28 nm particles in faeces in infantile gastroenteritis. Lancet. 1975;ii:451–452. doi: 10.1016/s0140-6736(75)90858-2. [DOI] [PubMed] [Google Scholar]
- 12.Maldonado Y, Cantwell M, Old M, Hill D, Sanchez M L, Logan L, Millan-Velasco F, Valdespino J L, Sepulveda J, Matsui S. Population-based prevalence of symptomatic and asymptomatic astrovirus infection in rural Mayan infants. J Infect Dis. 1998;178:334–339. doi: 10.1086/515625. [DOI] [PubMed] [Google Scholar]
- 13.Matsui S M, Greenberg H B. Astroviruses. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 811–824. [Google Scholar]
- 14.Monceyron C, Grinde B, Jonassen T O. Molecular characterisation of the 3′-end of the astrovirus genome. Arch Virol. 1997;142:699–706. doi: 10.1007/s007050050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mustafa H, Palombo E A, Bishop R F. Improved sensitivity of astrovirus-specific RT-PCR following culture of stool samples in CaCO-2 cells. J Clin Virol. 1998;11:103–107. doi: 10.1016/s1386-6532(98)00049-x. [DOI] [PubMed] [Google Scholar]
- 16.Noel J S, Lee T W, Kurtz J B, Glass R I, Monroe S S. Typing of human astroviruses from clinical isolates by enzyme immunoassay and nucleotide sequencing. J Clin Microbiol. 1995;33:797–801. doi: 10.1128/jcm.33.4.797-801.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Page R D M. TREEVIEW: an application to display phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- 18.Palombo E A, Bishop R F. Annual incidence, serotype distribution, and genetic diversity of human astrovirus isolates from hospitalized children in Melbourne, Australia. J Clin Microbiol. 1996;34:1750–1753. doi: 10.1128/jcm.34.7.1750-1753.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willcocks M M, Kurtz J B, Lee T W, Carter M J. Prevalence of human astrovirus serotype 4: capsid protein sequence and comparison with other strains. Epidemiol Infect. 1995;114:385–391. doi: 10.1017/s0950268800058015. [DOI] [PMC free article] [PubMed] [Google Scholar]