Abstract
Objective
To retrospectively evaluate the safety and efficacy of percutaneous image-guided mediastinal mass core-needle biopsy.
Patients and Methods
Retrospective review of an institutionally maintained biopsy registry identified 337 computed tomography– or ultrasound-guided percutaneous mediastinal mass core needle biopsies between October 2002 and August 2017 in a single quaternary referral center. Mean patient age was 51 (range, 18 to 93) years. Procedural techniques, anticoagulation/antiplatelet therapy, and tumor anatomical characteristics were reviewed. Classification and gradation of complications was based on the Clavien-Dindo system. Diagnostic yield was defined as the ratio of diagnostic biopsy to all biopsies performed.
Results
Mean tumor size was 59.2 (range, 10 to 180) mm with 89.9% (n=303) of lesions located in the prevascular (anterior) mediastinum. There was a single major complication (0.3%) of a symptomatic pneumothorax requiring intervention. There were seven (2.1%) minor complications, including three bleeding complications. A transpleural approach was the only variable associated with an increased complication rate (P<.01). Forty-one (12.2%) patients had a biopsy performed while taking an antiplatelet/anticoagulant agent within the therapeutic window, with a single case (0.3%) associated with a minor bleeding complication. Of 18 (5.3%) procedures performed without cessation of anticoagulant/antiplatelet therapy, there were no bleeding complications. Of all 337 biopsies, 322 (95.5%) were diagnostic. None of the analyzed variables were significantly associated with a nondiagnostic biopsy.
Conclusion
Image-guided percutaneous core-needle biopsy of mediastinal masses is a safe procedure with high diagnostic yield. Further prospective studies are required to assess the complication profile in higher risk patients.
Abbreviations and Acronyms: CT, computed tomography; INR, international normalized ratio; SIR, Society of Interventional Radiology; US, ultrasound
The mediastinum is a soft tissue division between the two pleural sacs, densely populated with many vascular and neurological structures. Masses, when they arise within this space, may relate to malignant conditions such as lymphoma, invading lung cancer, thymic neoplasm, metastatic disease, or nonmalignant conditions such as infectious or granulomatous disease and benign neoplastic lesions.1 Image-guided percutaneous mediastinal biopsy allows three-dimensional appreciation of the biopsy field and real-time or near real-time confirmation of biopsy tract. Computed tomography (CT)– and ultrasound (US)–guided approaches have both demonstrated excellent efficacy and safety profiles in attaining adequate tissue volume.1, 2, 3, 4, 5
Percutaneous image-guided biopsy of mediastinal masses shows high diagnostic accuracy combined with minimal patient sedation and low cost when compared to other more invasive approaches.6,7 Reported complications include pneumomediastinum, pneumothorax, and bleeding complications such as hemoptysis, hemothorax, and hematoma formation.1,6,8 Complication rates in CT-guided procedures range from 4% to 17% with a diagnostic yield ranging from 74% to 96%.1,4,9, 10, 11, 12 In studies assessing the US-guided approach, there were no reported complications with a diagnostic yield of 80% to 89%.3,13,14
The management of anticoagulant and antiplatelet medications, in particular aspirin, when considering percutaneous biopsy is widely debated. It is claimed that cessation may be unnecessary for many percutaneous organ biopsies15,16; however, there are conflicting studies demonstrating an increase in bleeding complications related to periprocedural use.17 The Society of Interventional Radiology (SIR) offers guidance for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions with varying recommendations depending on the type of anticoagulant or antiplatelet agent and the procedure involved.18 However, this bleeding risk must be balanced with the possible implications of medication withdrawal — in particular, the risk of cardiovascular events and peripheral ischemia.19
Our aim was to evaluate US- and CT-guided mediastinal biopsies performed in a single center over a 15-year period and to assess the factors which may alter the complication rate and diagnostic yield.
Patients and Methods
Ethical approval was attained from the Institutional Review Board with consent waived. This single-center study was compliant with the Health Insurance Portability and Accountability Act.
Patients
This was a retrospective study, assessing percutaneous image-guided mediastinal core-needle biopsies (CNBs) performed between October 2002 and August 2017 in a single academic quaternary referral center, reviewing data from an institutional biopsy database and review of patient medical records. Adult patients (≥18 years old) were included who had undergone a percutaneous US-guided or CT-guided biopsy of a mediastinal mass or lymph node. Only patients who had consented to the retrospective use of medical records for research purposes were included. Exclusion criteria also included patients who only underwent lesion fine-needle aspiration without CNB or biopsy performed in pediatric patients (<18 years old).
Data were entered into the radiology department biopsy database at the time of biopsy and included information on patient demographics, biopsy technique, periprocedural medications, standard laboratory values, and presence of postprocedure complications. In particular, antiplatelet and anticoagulant medications such as aspirin (81 mg and 325 mg, respectively), warfarin, clopidogrel, intravenous heparin, and subcutaneous heparin were included in the database. The novel anticoagulants were not included during data accrual. The anatomical location of the lesion was based on the three-compartment CT model developed by the International Thymic Malignancy Interest Group which divides the mediastinum into prevascular (anterior), visceral (middle), and paravertebral (posterior) compartments.20 The final study cohort comprised 318 patients who underwent 337 biopsy procedures during the study period. Demographic information is included in Table 1. Mean age of the cohort was 50.6 (range, 18 to 93) years with almost identical female-to-male ratio of 166:171 (49.3%:50.7%).
Table 1.
| Mean age (range), years | 51±18.9 (18 to 93) |
| Sex | |
| Male | 171 (50.7) |
| Female | 166 (49.3) |
| Site of tumor | |
| Anterior | 303 (89.9) |
| Middle | 9 (2.7) |
| Posterior | 25 (7.4) |
| Mean tumor size (range), mm | 59.2±33.8 (10 to 180) |
| Tumor pathology | |
| Benign | 63 (18.7) |
| Primary mediastinal malignancy | 26 (7.7) |
| Lymphoma | 136 (40.4) |
| Metastatic | 97 (28.8) |
| Nondiagnostic | 15 (4.5) |
| Modality | |
| CT | 323 (95.5) |
| US | 14 (4.5) |
| Route | |
| Parasternal | 267 (79.2) |
| Trans-sternal | 22 (6.5) |
| Transpleural | 19 (5.6) |
| Paraspinal | 29 (8.6) |
| Mean (range) number of passes | 5±2.5 (1 - 14) |
| Introducer needle | |
| Yes | 325 (96.4) |
| No | 12 (3.6) |
| Gauge | |
| 16G | 11 (3.3) |
| 18G | 236 (70.0) |
| 20G | 90 (26.7) |
CT, computed tomography; US, ultrasound.
Values shown are n (%) or mean (range) as appropriate.
Biopsy Technique
Image-guided biopsy was either performed or supervised by a fellowship-trained staff radiologist. The median experience of the performing radiologist was 10 years with a range of 3 to 30 years. Image guidance technique, biopsy route, and biopsy device were selected at the discretion of the performing radiologist.
Preprocedural coagulation profile consisting of an international normalized ratio (INR) and platelet count was obtained based on patient screening criteria as per institutional protocol. Standard prebiopsy target parameters included an INR of less than or equal to 1.6 and platelet count greater than 50×109/L. Patients were advised to remain off antiplatelet and anticoagulation medication before biopsy for a defined length based on SIR guidelines.21,22 Deviation from protocol was not a contraindication to biopsy as the ultimate decision was made following discussion between the referring provider and the radiologist performing the procedure.
Seventy-nine patients were on some form of anticoagulation or antiplatelet medication in the 30 days before biopsy (23.4%), with 16 (4.7%) patients on multiple medications. This comprised 29 (8.6%) patients on 81-mg aspirin dose, 20 on 325-mg aspirin dose (5.9%), 16 on warfarin (4.7%), two on clopidogrel (0.6%), 13 on intravenous Heparin (3.9%), and 11 on subcutaneous heparin (3.3%). All medications were stopped within the recommended therapeutic efficacy time frame before biopsy except for 41 patients (12.2%). Of these 41 patients, 23 (56.1%) were taking aspirin 81 mg, 16 (39.0%) were taking aspirin 325 mg, and two (4.9%) were taking a prophylactic dose of subcutaneous heparin. Of this subgroup, 18 procedures were performed in patients who had not stopped taking their medication with 6 (33.3%) taking 81 mg aspirin, 10 (55.6%) taking 325 mg aspirin, and 2 (11.1%) taking subcutaneous heparin at the time of biopsy procedure.
Biopsies were performed using sterile technique either under fluoroscopic CT guidance (GE LightSpeed 16-slice scanner GE Healthcare, Waukesha, Wisconsin) or ultrasound guidance (GE Logiq E9 system, GE Healthcare, Waukesha, WI). Local anesthesia in the form of 1% lidocaine was invariably used, with or without monitored moderate sedation with fentanyl and midazolam. A coaxial technique was used to perform all CT-guided biopsies. An introducer needle was not typically used for US-guided biopsies as the introduction of air can possibly lead to decreased target lesion visibility. A spring-loaded, side-cutting biopsy device was used, offered in a variety of gauges (Bard Monopty, Bard Medical, Covington, GA). Biopsy route was classified as either parasternal, paraspinal, trans-sternal, or transpleural if a portion of lung parenchyma was transgressed. The use of tract embolization was not standard practice in the department. A cytopathologist was not routinely present for the biopsy, with samples submitted for both cytology and pathology analysis as per institution protocol. Postbiopsy, patients were observed for a minimum of 2 hours postprocedure in the outpatient radiology recovery area. As per departmental protocol, a chest radiograph was requested for 1-hour postbiopsy to assess for potential complications. Additional imaging or investigations were performed depending on postprocedure symptoms.
Complication Rate
Complications were identified and classified as minor or major based on the definitions of the Clavien-Dindo system.23 This scale consists of seven grades with minor complications classified as grade I (any deviation from the normal postoperative course not requiring surgical, endoscopic, or radiological intervention), grade II (complications requiring drug treatments other than those allowed for grade I complications), and grade IIIa/b (major complications requiring surgical, endoscopic, or radiological intervention), grade IVa/b (life-threatening complications), or grade V (death).
Information on postprocedure complications was accrued via telephone conversations from a dedicated radiology registered nurse in the 24 to 72 hours following biopsy using a standardized format. Further information to identify any delayed complication was obtained by review of the patient’s electronic medical record by one of two investigators. If a discrepancy arose as to the presence of a postbiopsy complication, consensus was achieved by review of the patient’s electronic medical record by an additional investigator.
Diagnostic Yield
Based on the findings of a fellowship-trained pathologist, each biopsy was classified as either diagnostic for malignancy, benign, or nondiagnostic. Patients were followed for 1 year to ensure a benign biopsy result was correct. Nondiagnostic studies were classified as such based on the absence of adequate tissue for a diagnosis or if a prior benign result was deemed inaccurate on follow-up imaging or biopsy. Diagnostic yield was defined as the ratio of a diagnostic biopsy to all biopsies performed.
Statistical Analysis
Descriptive statistics are reported for subject demographics, diagnostic yield, and complication rate as numbers and percentages with means and standard deviations. All continuous variables were compared between the bleeding and nonbleeding groups by the Kruskal-Wallis test. Categorical variables were compared using the χ2 test and Fisher exact test for any n less than 5 variables. A P less than .05 was considered statistically significant. A P less than .05 was considered statistically significant. All statistical analyses were completed using Jmp software (JMP, version 13, SAS institute Inc, Cary, NC).
Results
Of the 337 mediastinal mass biopsies, the targeted mass was most commonly located in the prevascular (anterior) mediastinum (n=303 procedures; 89.9%), with CT imaging guidance most frequently used (n=322 procedures; 95.5%). Mean tumor axial diameter was 59.2 (range, 10 to 180) mm. A parasternal approach without crossing pleura was most used, occurring in 267 (79.2%) procedures. There were 106 patients classified as hypertensive during the periprocedural period (31.5%). Of the diagnostic biopsies (n=322), pathology revealed 63 patients (19.5%) with benign tissue, 26 patients (8.1%) with primary mediastinal malignancy, 136 patients (42.2%) with lymphoma, and 97 patients (30.1%) with metastatic disease (Table 1).
Complication Rate
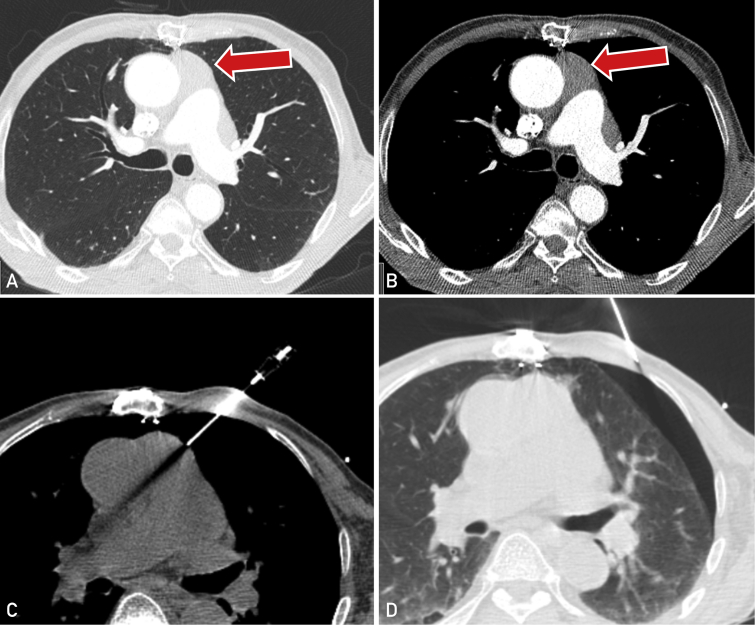
There were eight patients (2.4 %) who encountered a complication following biopsy, comprising seven (2.1%) minor complications and a single (0.3%) major complication (Tables 2 and 3). Of these, five patients (1.5%) had a pneumothorax and three patients (0.9%) had a minor bleeding complication. No subject encountered a pneumomediastinum. The single major complication occurred in a 79-year-old male subject who developed a symptomatic moderate-sized pneumothorax following biopsy of a prevascular (anterior) mediastinal mass using a transpleural approach (Figure). This required the insertion of a chest tube and a 2-day admission for observation and management. The other four pneumothoraces were small, requiring no further intervention, and patients were discharged on the same day as the procedure.
Table 2.
| Variable | Complication (8) | No complication (329) | P | |
|---|---|---|---|---|
| Age, years | 60.6±17.9 | 50.6±18.8 | .13 | |
| Platelets, ×109/L | 337.3±163.1 | 282.0±110.7 | .41 | |
| INR | 1.1±0.1 | 1.1±0.1 | .26 | |
| Number of passes | 4.0±1.5 | 5.3±2.5 | .15 | |
| Dimensions, mm | 56.0±36.9 | 59.3±33.8 | .71 | |
| Hypertension, 105 (31.2) | 4 (1.2) | 101 (29.9) | .22 | |
| Modality | ||||
| US | 14 (4.2) | 0 | 14 | .71 |
| CT | 323 (95.8) | 8 | 315 | |
| Route | ||||
| Parasternal | 267 (79.2) | 4 | 263 | <.01 |
| Trans-sternal | 22 (6.5) | 0 | 22 | |
| Transpleural | 19 (5.6) | 4 | 15 | |
| Paraspinal | 29 (8.6) | 0 | 29 | |
| Introducer needle, 325 (96) | 8 | 317 | .75 | |
| Cutting needle size, G | .85 | |||
| 16 | 11 (3.3) | 0 | 11 | |
| 18 | 236 (70.0) | 6 | 230 | |
| 20 | 90 (26.7) | 2 | 88 | |
| Anticoagulation in 1 month prior, 79 (23.4) | 1 | 78 | .89 | |
| Discontinued as per protocol, 38 (11.3) | 0 | 38 | 1.00 | |
| Discontinued in therapeutic window, 23 (6.8) | 1 | 22 | .44 | |
| Not discontinued, 18 (5.3) | 0 | 18 | 1.00 | |
CT, computed tomography; INR, international normalized ratio; US, ultrasound.
Seven minor and one major complication.
Values presented are mean ± SD or n (%) as appropriate.
Table 3.
Characteristics of Subjects With Complicationsa
| Complication | Subject | Age | Sex | Pathology | Location | Greatest mass dimension, mm | Biopsy route | Complication type | Anticoagulant | INR | Platelets, ×109/L | Needle size, G | No. of passes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Major | 1 | 79 | M | Malignant thymoma | Prevascular | 24 | Transpleural | Pneumothorax | None | 1.2 | 241 | 20 | 4 |
| Minor | 1 | 80 | M | Benign tissue | Prevascular | 19 | Parasternal | Bleeding | Aspirin 81mg, warfarin | 1.2 | 139 | 18 | 5 |
| 2 | 41 | M | Lymphoma | Prevascular | 99 | Parasternal | Bleeding | None | 1 | 608 | 18 | 2 | |
| 3 | 41 | M | Nondiagnostic | Prevascular | 99 | Parasternal | Bleeding | None | 1 | 512 | 18 | 3 | |
| 4 | 78 | F | Benign | Prevascular | 30 | Parasternal | Pneumothorax | None | 1 | 199 | 18 | 5 | |
| 5 | 49 | F | Benign | Prevascular | 17 | Transpleural | Pneumothorax | None | 1.1 | 273 | 20 | 2 | |
| 6 | 71 | M | Lymphoma | Prevascular | 88 | Transpleural | Pneumothorax | None | 1.2 | 295 | 18 | 6 | |
| 7 | 46 | F | Lymphoma | Prevascular | 72 | Transpleural | Pneumothorax | None | 1 | 431 | 18 | 5 |
F, female; INR, international normalized ratio; M, male.
Figure.
Major complication of a large pneumothorax following biopsy of a prevascular mass (arrow) in a 78-year-old male patient with subsequent diagnosis of thymoma. A,B Contrast enhanced computed tomography (CT) showing infiltrative prevascular mediastinal mass in both lung and soft tissue windows in patient status post median sternotomy. C, CT image showing transpleural pathway of biopsy needle. D, CT shows postbiopsy symptomatic moderate-sized pneumothorax requiring chest tube insertion.
The only variable associated with increased risk of a complication was transpleural approach (P<.01). Of the five pneumothoraces, four were using transpleural approach, with the other using the parasternal approach.
Three patients developed minor bleeding complications, none of whom required radiologic or surgical intervention. A single 80-year-old patient required overnight admission for observation and analgesia after developing a hematoma subsequent to biopsy of a 19-mm pericardiophrenic lymph node. The patient was discharged the following day with a stable hemoglobin level. This patient had discontinued aspirin (325 mg) therapy 4 days before biopsy. The patient had also been receiving warfarin therapy, which was also discontinued 4 days before biopsy with an INR of 1.2 at the time of biopsy.
All patients who had a history of antiplatelet/anticoagulation use did not show a significantly higher complication rate (n=79, P=.89) (Table 2). Of the 41 (12.2%) patients who were taking an anticoagulant/antiplatelet agent within the therapeutic window, there was only a single minor bleeding complication. Of 18 (5%) patients for whom a biopsy was performed without cessation of this agent, there were no bleeding complications.
Mean INR of the patients with bleeding complications was 1.07 (SD, 0.09) versus 1.05 (SD, 0.13) in patients without. There were only two patients with a supratherapeutic INR at the time of procedure (both with values of 1.7); neither of these patients encountered a complication. Only two patients required an intervention to improve coagulation profile; with the administration of platelets and vitamin K before the procedure, neither of these patients encountered a complication.
Diagnostic Yield
There were 322 patients (95.5%) with an adequate diagnostic biopsy. Of the 15 nondiagnostic biopsies, 10 were ultimately found to have a malignant etiology on repeat biopsy, surgical removal, or clinical progression. Subject age, lesion size, pathology of the lesion, modality used, route of biopsy, use of an introducer needle, needle gauge, and number of passes were all not statistically significant in attaining a nondiagnostic biopsy (Table 4).
Table 4.
Effect of Subject and Procedure Variables on Diagnostic Yielda
| Diagnostic (322) | Nondiagnostic (15) | P | |
|---|---|---|---|
| Age, years | 51±19.1 | 44±12.4 | .12 |
| Dimensions, mm | 59.5±34.2 | 52.1±25.9 | .41 |
| Modality | |||
| US | 14 | 0 | .41 |
| CT | 308 | 15 | |
| Route | |||
| Parasternal | 253 | 14 | .42 |
| Trans-sternal | 22 | 0 | |
| Transpleural | 18 | 1 | |
| Paraspinal | 29 | 0 | |
| Introducer needle | |||
| Yes | 310 | 15 | .45 |
| No | 12 | 0 | |
| Cutting needle size, G | |||
| 16 | 11 | 0 | .18 |
| 18 | 228 | 8 | |
| 20 | 83 | 7 | |
| Number of passes | 5.3±2.5 | 4.7±1.8 | .36 |
CT, computed tomography; US, ultrasound.
Discussion
Image-guided biopsy is often used to obtain tissue for oncologic, genetic, and immunologic testing of cancer mutations, with the intention to improve diagnosis and treatment of patients undergoing targeted therapy. The current study shows that image-guided percutaneous biopsy of mediastinal lesions is an efficacious and safe technique. Such a technique provides a favorable alternative to surgical or endoscopic techniques.
The diagnostic yield of the current study compares favorably with similar studies. In a recent meta-analysis, pooled diagnostic yield of CT-guided percutaneous biopsy measured 92%, compared to 96% in this current study.6 The range of reported diagnostic yield in each study included in the meta-analysis ranged from 74% to 96%.1,4,9, 10, 11, 12 Possible reasons for this variability may reflect technical advances in pathology, imaging, and biopsy equipment along with the volume of procedures performed in each institution. The experience represented in the current study is based in a large quaternary referral center including radiologists with subspecialty training with image-guided biopsy.
Similar to prior studies, there was no statistically significant variable which affected the diagnostic yield of the biopsy technique.1,4,9, 10, 11, 12 However, biopsy needle gauge has shown significance in one study assessing CT fluoroscopy–guided biopsy of anterior mediastinal masses.24 In that study it was found that using a 20-gauge needle was a significant risk factor for diagnostic failure. In the current study, although 7 of 15 diagnostic failures were performed using a 20-gauge biopsy needle (46.7%), this finding was not statistically significant (P=.18).
The overall and major complication rate in the current study was lower compared to other similar studies at 2% and 0.3%, respectively. In the same meta-analysis,6 the pooled complication rate was 13% with a major complication rate of 2%. However, the definition of a major complication in this meta-analysis may not be identical to the definition used in the current study. The complication rate in the current study is also lower compared to the prior largest sample, where they encountered an overall complication rate of 7%.1 However, they did not differentiate between major and minor complications. In the current study, the majority of complications were minor (n=7 of 8) and consisted of pneumothorax (n=5 of 8) and bleeding (n=3 of 8) complications.
The only variable associated with an increased rate of a complication was the transpleural approach. The rate of pneumothorax in transpleural mediastinal mass biopsy in this study (n=4 of 19, 21.1%) is slightly higher than the rate of pneumothorax in percutaneous lung mass biopsy,25,26 likely related to the crossing of two separate pleural interfaces. The choice of approach is often tailored to each individual mediastinal mass. Certain techniques may decrease the likelihood of pleural involvement such as hydrodisplacement or trans-sternal approach. These techniques may not be appropriate in certain scenarios; however, the transpleural approach is the safest option likely to lead to a diagnostic result.
The most appropriate management of patients on anticoagulant or antiplatelet medications before percutaneous biopsy is cause for much discussion. The rate of major complications in this current study of 0.3% is low and the clinical significance of the complication was relatively minimal. Guidance on performing biopsies varies between departments. Local protocols dictate that anticoagulation medication should be held for a predetermined time frame based on SIR guidelines,21,22 that INR should be less than 1.6, and that platelets should be greater than 50×109/L. In this study, 12.2% (n=41 patients) were on anticoagulant/antiplatelet therapy within the therapeutic period before biopsy, all of them taking aspirin therapy. There was only a single minor bleeding complication in this group. No bleeding complications occurred in the 5.3% (n=18) of patients who had not stopped aspirin therapy at time of biopsy. This observation supports that seen in prior studies.15,27 Application of these protocols varies depending on the clinical situation and institution. However, the balance of benefit to risk in this decision is sometimes biased towards the bleeding risk from the anticoagulant medication and neglects the risk of cessation of this medication. For instance, a figure of approximately 1% absolute risk of an embolic event when warfarin is discontinued for 4 to 7 days is quoted in the literature.28, 29, 30, 31 When compared to the 0.3% major complication risk encountered in the current study, it may lead more to question the validity of unmitigated anticoagulation medication discontinuation.
There are a number of limitations to the current study. There is inherent selection bias given the retrospective nature. Only patients who were deemed suitable for biopsy underwent the procedure, and the technique (including approach and biopsy gauge) was chosen by an experienced proceduralist on a case-by-case basis. As a result, there is a dearth of patients with elevated INR (>1.6) or on continued anticoagulation. Also, some parameters such as liver function or thromboplastin time were not available. Given that these biopsies were performed in a quaternary referral center with specialized staff encountering a high volume of mediastinal biopsies, this study may not be representative of other procedural practices.
Conclusion
In conclusion, the current study shows that image-guided percutaneous CNB of mediastinal masses is a safe procedure with high diagnostic yield, and that biopsy in these cases can be achieved with patients on recent anticoagulant/antiplatelet therapy. Further prospective studies are required to assess the complication profile in higher risk patients.
Footnotes
Potential Competing Interests: The authors report no potential competing interests.
References
- 1.de Margerie-Mellon C., de Bazelaire C., Amorim S., et al. Diagnostic yield and safety of computed tomography-guided mediastinal core needle biopsies. J Thorac Imaging. 2015;30(5):319–327. doi: 10.1097/RTI.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 2.Westcott J.L. Transthoracic needle biopsy of the hilum and mediastinum. J Thorac Imaging. 1987;2(2):41–48. doi: 10.1097/00005382-198704000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Yang P.C., Chang D.B., Lee Y.C., Yu C.J., Kuo S.H., Luh K.T. Mediastinal malignancy: ultrasound guided biopsy through the supraclavicular approach. Thorax. 1992;47(5):377–380. doi: 10.1136/thx.47.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bressler E.L., Kirkham J.A. Mediastinal masses: alternative approaches to CT-guided needle biopsy. Radiology. 1994;191(2):391–396. doi: 10.1148/radiology.191.2.8153311. [DOI] [PubMed] [Google Scholar]
- 5.Protopapas Z., Westcott J.L. Transthoracic hilar and mediastinal biopsy. Radiol Clin North Am. 2000;38(2):281–291. doi: 10.1016/s0033-8389(05)70163-9. [DOI] [PubMed] [Google Scholar]
- 6.Lee H.N., Yun S.J., Kim J.I., Ryu C.-W. Diagnostic outcome and safety of CT-guided core needle biopsy for mediastinal masses: a systematic review and meta-analysis. Eur Radiol. 2020;30(1):588–599. doi: 10.1007/s00330-019-06377-4. [DOI] [PubMed] [Google Scholar]
- 7.Date H. Diagnostic strategies for mediastinal tumors and cysts. Thorac Surg Clin. 2009;19(1):29–35. doi: 10.1016/j.thorsurg.2008.09.001. vi. [DOI] [PubMed] [Google Scholar]
- 8.Gupta S., Seaberg K., Wallace M.J., et al. Imaging-guided percutaneous biopsy of mediastinal lesions: different approaches and anatomic considerations. Radiographics. 2005;25(3):763–786. doi: 10.1148/rg.253045030. discussion 86-88. [DOI] [PubMed] [Google Scholar]
- 9.Kulkarni S., Kulkarni A., Roy D., Thakur M.H. Percutaneous computed tomography-guided core biopsy for the diagnosis of mediastinal masses. Ann Thorac Med. 2008;3(1):13–17. doi: 10.4103/1817-1737.37948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petranovic M., Gilman M.D., Muniappan A., et al. Diagnostic yield of CT-guided percutaneous transthoracic needle biopsy for diagnosis of anterior mediastinal masses. AJR Am J Roentgenol. 2015;205(4):774–779. doi: 10.2214/AJR.15.14442. [DOI] [PubMed] [Google Scholar]
- 11.Neyaz Z., Lal H., Thakral A., Nath A., Rao R.N., Verma R. Percutaneous computed tomography-guided aspiration and biopsy of intrathoracic lesions: results of 265 procedures. Lung India. 2016;33(6):620–625. doi: 10.4103/0970-2113.192863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rabbani M., Sarrami A.H. Computed tomography-guided percutaneous core needle biopsy for diagnosis of mediastinal mass lesions: experience with 110 cases in two university hospitals in Isfahan, Iran. Adv Biomed Res. 2016;5:152. doi: 10.4103/2277-9175.188939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yi D., Feng M., Wen Ping W., Zheng Biao J., Fan P.L. Contrast-enhanced US-guided percutaneous biopsy of anterior mediastinal lesions. Diagn Interv Radiol. 2017;23(1):43–48. doi: 10.5152/dir.2016.15590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otani Y., Yoshida I., Ishikawa S., et al. Use of ultrasound-guided percutaneous needle biopsy in the diagnosis of mediastinal tumors. Surg Today. 1996;26(12):990–992. doi: 10.1007/BF00309959. [DOI] [PubMed] [Google Scholar]
- 15.Potretzke T.A., Harvey J.A., Gunderson T.M., et al. Frequency of bleeding complications after percutaneous core needle biopsy and the association with aspirin usage and length of aspirin discontinuation. AJR Ame J Roentgenol. 2019;213:1–5. doi: 10.2214/AJR.18.20366. [DOI] [PubMed] [Google Scholar]
- 16.Monahan H., Gunderson T., Greene E., Schmit G., Atwell T., Schmitz J. Risk factors associated with significant bleeding events after ultrasound-guided percutaneous native renal biopsies: a review of 2204 cases. Abdom Radiol (NY) 2019;44(6):2316–2322. doi: 10.1007/s00261-019-01962-z. [DOI] [PubMed] [Google Scholar]
- 17.Burger W., Chemnitius J.M., Kneissl G.D., Rucker G. Low-dose aspirin for secondary cardiovascular prevention — cardiovascular risks after its perioperative withdrawal versus bleeding risks with its continuation — review and meta-analysis. J Intern Med. 2005;257(5):399–414. doi: 10.1111/j.1365-2796.2005.01477.x. [DOI] [PubMed] [Google Scholar]
- 18.Davidson J.C., Rahim S., Hanks S.E., et al. Society of Interventional Radiology consensus guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions — Part I: review of anticoagulation agents and clinical considerations: endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J Vasc Interv Radiol. 2019;30(8):1155–1167. doi: 10.1016/j.jvir.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Ferrari E., Benhamou M., Cerboni P., Marcel B. Coronary syndromes following aspirin withdrawal: a special risk for late stent thrombosis. J Am Coll Cardiol. 2005;45(3):456–459. doi: 10.1016/j.jacc.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 20.Carter B.W., Benveniste M.F., Madan R., et al. ITMIG classification of mediastinal compartments and multidisciplinary approach to mediastinal masses. Radiographics. 2017;37(2):413–436. doi: 10.1148/rg.2017160095. [DOI] [PubMed] [Google Scholar]
- 21.Patel I.J., Davidson J.C., Nikolic B., et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012;23(6):727–736. doi: 10.1016/j.jvir.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Patel I.J., Davidson J.C., Nikolic B., et al. Addendum of newer anticoagulants to the SIR consensus guideline. J Vasc Interv Radiol. 2013;24(5):641–645. doi: 10.1016/j.jvir.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iguchi T., Hiraki T., Matsui Y., et al. CT fluoroscopy-guided core needle biopsy of anterior mediastinal masses. Diagn Interv Imaging. 2018;99(2):91–97. doi: 10.1016/j.diii.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Lorenz J., Blum M. Complications of percutaneous chest biopsy. Semin Intervent Radiol. 2006;23(2):188–193. doi: 10.1055/s-2006-941449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boskovic T., Stanic J., Pena-Karan S., et al. Pneumothorax after transthoracic needle biopsy of lung lesions under CT guidance. J Thorac Dis. 2014;6(suppl 1):S99–S107. doi: 10.3978/j.issn.2072-1439.2013.12.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atwell T.D., Spanbauer J.C., McMenomy B.P., et al. The timing and presentation of major hemorrhage after 18,947 image-guided percutaneous biopsies. AJR Am J Roentgenol. 2015;205(1):190–195. doi: 10.2214/AJR.14.13002. [DOI] [PubMed] [Google Scholar]
- 28.Garcia D.A., Regan S., Henault L.E., et al. Risk of thromboembolism with short-term interruption of warfarin therapy. Arch Intern Med. 2008;168(1):63–69. doi: 10.1001/archinternmed.2007.23. [DOI] [PubMed] [Google Scholar]
- 29.Wahl M.J., Pinto A., Kilham J., Lalla R.V. Dental surgery in anticoagulated patients — stop the interruption. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119(2):136–157. doi: 10.1016/j.oooo.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Blacker D.J., Wijdicks E.F., McClelland R.L. Stroke risk in anticoagulated patients with atrial fibrillation undergoing endoscopy. Neurology. 2003;61(7):964–968. doi: 10.1212/01.wnl.0000086817.54076.eb. [DOI] [PubMed] [Google Scholar]
- 31.Wysokinski W.E., McBane R.D., Daniels P.R., et al. Periprocedural anticoagulation management of patients with nonvalvular atrial fibrillation. Mayo Clinic Proc. 2008;83(6):639–645. doi: 10.4065/83.6.639. [DOI] [PubMed] [Google Scholar]