Abstract
The Oricult-N semiquantitative dipslide (Orion Diagnostica, Espoo, Finland) was evaluated for the laboratory diagnosis of vaginal candidiasis. It was compared with broth culture (Vagicult; Orion Diagnostica). Oricult-N was positive for 14.5% of 124 symptomatic patients and 12% of 50 asymptomatic controls. The results for broth cultures were 17 and 22%, respectively. Thus, the test group and the control group did not differ significantly by either method. High vaginal yeast counts (≥105 CFU/ml) were detected by Oricult-N in 7% of patients and in 0% of controls, but both groups harbored low numbers of yeasts. An accurate quantitative cutoff point separating a level of yeast associated with infection from vaginal yeast carriage could not be defined in the study. Nevertheless, the easy semiquantitation allowed by the Oricult-N method could be helpful because, especially in low-count carriers of Candida, other potential causes of vaginal symptoms should be considered. The Oricult-N method was technically simple and could be applied in primary health care. Further studies are required, however, before Oricult-N can be recommended as a routine diagnostic tool.
Vaginal candidiasis is one of the most common infections seen in general practice. Forty to 75% of sexually active women have experienced symptomatic vaginal candidiasis (3). Symptoms of vaginal candidiasis are itching, burning, soreness, abnormal vaginal discharge, and dyspareunia. Signs include vaginal and vulvar erythema and edema (16). The most common causative agent is Candida albicans, but the proportion of other species, especially Candida glabrata, is increasing (17, 19).
The vaginal Candida carriage rate for asymptomatic nonpregnant women is around 20% (10). In pregnancy, the rate tends to be higher. There is little definitive quantitative data on the amount of vaginal Candida in apparently healthy carriers. The numbers of yeasts are usually low in asymptomatic women, but thousandfold differences in the quantities of yeasts recovered from these asymptomatic women may exist (9, 12). Why carriers of Candida develop an episode of symptomatic disease is at present poorly understood, but it is assumed that both host- and pathogen-related factors are involved.
The clinical diagnosis of vaginal candidiasis is unreliable (6, 11, 17), and laboratory confirmation is needed. Microscopy, yeast cultures, and latex agglutination tests have been used (5). Wet mounts prepared in saline or 10% KOH, as well as Gram-stained smears, can be microscopically examined. The sensitivity of microscopy compared to culture is about 30 to 45% (5, 18).
Sabouraud dextrose agar plates and, more infrequently, broth media or dipslides have been used in vaginal yeast cultures. Substantial controversy exists about the interpretation of a positive culture result. Traditionally, any growth of Candida has been considered a pathological finding. More recently, however, it has been shown that symptomatic candidiasis is usually associated with higher numbers of vaginal yeasts than those found in asymptomatic carriers (6, 8, 12). Unfortunately, conventional mycological laboratory techniques for yeast quantitation involve serial dilutions (8), and dipslides or broth media do not allow for quantitation (4, 13, 14, 20).
The Oricult-N semiquantitative dipslide method (Orion Diagnostica, Espoo, Finland) was developed for the enumeration of yeasts in clinical samples. In particular, it has been applied to the quantitation of Candida species in the oral cavity (1). In the present study, we evaluated the Oricult-N method in the laboratory diagnosis of Candida vaginitis. It was compared with microscopy and broth culture. Both symptomatic women and a group of healthy volunteers were studied.
MATERIALS AND METHODS
Patients.
The study population consisted of 124 consecutive nonpregnant women attending the gynecological outpatient clinic of the University Central Hospital, Helsinki, Finland, for suspected vaginitis. The mean age of the patients was 37 years, with a range of 19 to 81 years. Women who had received any antibacterial or antifungal agents during the preceeding 14 days were excluded from the study. The patients had had an average of 0.8 deliveries (range, 0 to 6), 18 (15%) used oral contraceptives, and 2 (2%) had an intrauterine device. Gynecological speculum examination was performed by one of us (J.P.). Symptoms of itching and soreness, signs of vulvovaginitis, mucosal edema, and the presence and nature of vaginal discharge were noted. Fifty gynecologically asymptomatic female health care professionals (mean age, 40 years; range, 19 to 58 years) formed the control group.
Specimen collection and laboratory techniques.
Two swabs were taken high in the vagina of each patient and control subject. A wet smear in physiological saline was immediately prepared from the first swab. The smears were examined by microscopy for the presence of characteristic yeast and hyphal forms of Candida species. The second swab was first rubbed thoroughly on the surface of an Oricult-N dipslide and then immersed into a liquid medium (Vagicult; Orion Diagnostica). The Oricult-N slide consists of Nickerson's medium containing chloramphenicol and gentamicin to control bacterial overgrowth. Candida species grow on it as characteristic brown-pigmented, smooth colonies (1, 15). The slides were incubated at 37°C for 2 days before examination, as recommended by the manufacturer. The numbers of colonies per square centimeter of agar surface were then counted. The number of yeasts per milliliter (range, <103 to 106 CFU/ml) in the original vaginal secretion was estimated by comparing the colony densities to color charts provided by the manufacturer. The Vagicult tubes were incubated for 2 days at 37°C before microscopic examination for yeasts.
The data were analyzed using the two-tailed Fisher's exact test.
RESULTS
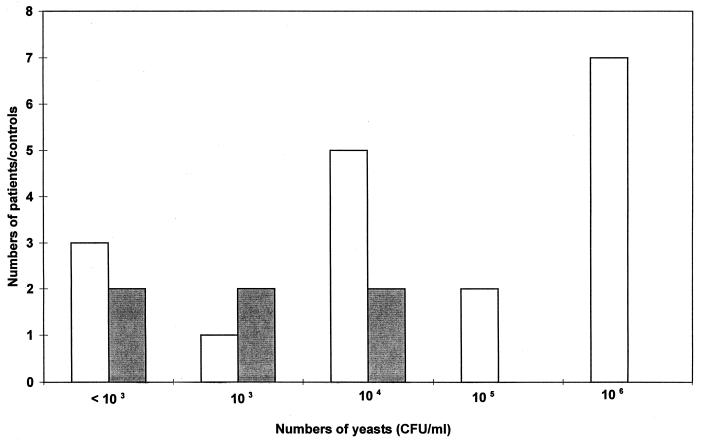
The wet mount microscopy and qualitative Vagicult and Oricult-N culture results are given in Table 1. The microscopy was positive for yeast for 14 of the 124 patients (11%). However, only eight (57%) of these could be verified by culture. Two of 50 controls (4%) had a positive result in microscopy, and both were also culture positive. The results of both culture methods were easy to interpret. Bacterial overgrowth did not interfere with reading of Oricult-N slides. Eighteen of the patients (14.5%) and six controls (12%) had positive Oricult-N cultures, while the Vagicult cultures were positive in 21 (17%) versus 11 (22%) subjects, respectively. The patient and control groups did not differ statistically by either of the culture methods. The sensitivity, specificity, and positive and negative predictive values of the qualitative Oricult-N culture compared to the Vagicult broth culture in the patient group were 86, 100, 100, and 100%, respectively. The quantitative results given by the Oricult-N method are given in Table 2. Nine of the patients (7%) but none of the controls had high counts of yeasts (≥105 CFU/ml) in their vaginas, while there was low-grade carriage of yeasts (≤104 CFU/ml) in both patients (9 of 124 [7%]) and controls (6 of 50 [12%]). When groups of women with either no yeasts or only low numbers of yeasts (≤104 CFU/ml) in their vaginas were combined and then compared to subjects with high vaginal yeast counts (≥105 CFU/ml) (see Table 2 for details), patients and controls differed, but not statistically significantly (P = 0.089). As can be seen in Fig. 1, the distribution of yeast counts was bimodal in the culture-positive patient group but unimodal in the corresponding control group.
TABLE 1.
Proportions of vaginal swabs that were microscopy and culture positive in vaginitis patients and control subjects
| Group | Total no. of samples | No. (%) of samples qualitatively positive by:
|
||
|---|---|---|---|---|
| Microscopy | Oricult-N | Vagicult | ||
| Patients | 124 | 14 (11) | 18 (14.5) | 21 (17) |
| Controls | 50 | 2 (4) | 6 (12) | 11 (22) |
TABLE 2.
Quantitative results of Oricult-N cultures
| Group | Total no. of samples | No. (%) of samples with yeast count ofa:
|
||
|---|---|---|---|---|
| Negative | ≤104 CFU/ml | ≥105 CFU/ml | ||
| Patients | 124 | 106 (85) | 9 (7) | 9 (7) |
| Controls | 50 | 44 (88) | 6 (12) | 0 (0) |
Four groups were analyzed by Fisher's exact test as follows: (i) patients with negative plus low-count (≤104 CFU/ml) yeast cultures (n = 115), (ii) patients with high-count (≥105 CFU/ml) yeast cultures (n = 9), (iii) controls with negative plus low-count yeast cultures (n = 50), and (iv) controls with high-count yeast cultures (n = 0).
FIG. 1.
Distribution of Oricult-N results for culture-positive patients (n = 18) (□) and controls (n = 6) (■).
The diagnoses of the 103 symptomatic women who remained culture negative included trichomoniasis in one patient, bacterial vaginosis in one patient, condylomatous vaginitis in one patient, and desquamative inflammatory vaginitis in five patients. In addition, noninfectious conditions were diagnosed in three cases (lichen sclerosus in two cases and lichen planus in one case).
DISCUSSION
Candida carriage in the vagina has often been studied with both healthy women and those suffering from vaginitis. However, attention has rarely been paid to the quantities of yeasts in these groups. The present study has shown that it is possible to obtain semiquantitative estimates of vaginal yeast amounts using a simple and practical dipslide method. The technique could be particularly applicable to primary health care, because special mycological laboratory facilities are not required. The Oricult-N slide can be referred to a reference laboratory for further testing if necessary.
In the present study it was clearly shown that qualitative yeast cultures do not separate patient and control groups. As has been shown previously by others, broth culture methods like Vagicult do not discriminate between patients with disease and symptom-free carriers (14). Microscopy may be better in this respect, because its sensitivity is probably lower than that of culture. The fact that 6 of 14 patients with positive microscopic examinations were negative by culture may be due to false-positive microscopy results or because not all yeast cells seen were viable. Patients harboring vaginal yeasts can be divided into two groups, i.e., those with either low or high yeast counts. Carriers with low counts may well represent women having vaginal symptoms caused by etiologic factors other than Candida. We emphasize that all vaginitis patients should be carefully evaluated for other etiologies besides Candida. When Candida vaginitis is suspected, a wet mount for direct microscopy is usually first prepared. If the result is negative for yeasts, a swab for a qualitative yeast culture is sometimes taken. However, the indiscriminate use and interpretation of qualitative Candida cultures may lead to overdiagnosis and excessive antifungal treatment.
Most of the earlier studies have shown some degree of correlation between the numbers of vaginal yeasts and symptoms and signs of vaginitis (6, 8, 11, 12), although two studies gave opposite results (2, 21). The control groups in earlier studies have consisted either exclusively or at least in part of women suspected to have a sexually transmitted disease or some other gynecological disorder (2, 6, 8, 11, 12, 21). According to Odds (10), a higher proportion of these females than healthy volunteers tend to carry vaginal yeasts. For this reason, we used healthy volunteers as controls.
The most accurate sampling technique for suspected vaginitis is a vaginal washing (8). Odds and coworkers (9, 12) and Hopwood et al. (8) have shown, however, that vaginal swabs are a sufficiently sensitive and reproducible substitute. The methods of quantitation used in the past have usually been quite imprecise. The range of quantitation of the Oricult-N method used in the present study extended from <103 to 106 CFU of yeasts per ml in the original sample, a range broader than in most previous studies. Simple swabbing of a Sabouraud dextrose plate allows counting of colonies from 1 to 100. According to Odds (9), these colony counts correspond to a range of 103 to at most 105 CFU/ml in the original vaginal secretion. In this study, we somewhat arbitrarily set a cutoff point of 105 CFU/ml as a pathological vaginal yeast count. In two previous studies (6, 9), 10 colonies growing on a swabbed culture plate has been proposed as a limit between a pathological and a physiological amount of Candida in the vagina. This corresponds to about 104 CFU/ml in the original vaginal fluid. Hopwood et al. (8) proposed a broader range, i.e., one between 103 and 105 CFU/ml. As can be seen in Fig. 1, both patients and healthy controls had yeast contents of 104 CFU/ml in their vaginas; this may represent an intermediate zone between the groups.
An accurate cutoff point between a physiological and a pathological vaginal yeast content cannot yet be defined based on the present or previous studies. More patients need to be studied and more clinical experience needs to be gained before any firmer conclusions can be drawn. In selected cases, women may become sensitized to Candida (7) and then even small amounts of yeasts may elicit intense symptoms.
The Oricult-N method was easy to use and easy to interpret. It provided a semiquantitative estimate of the vaginal yeast count, which could be useful to clinicians in evaluating patients with suspected vaginal candidiasis. Further studies are required, however, before the product can be recommended as a routine diagnostic tool.
ACKNOWLEDGMENT
This work was supported in part by Pfizer, Finland.
REFERENCES
- 1.Axéll T, Simonsson T, Birkhed D, Rosenborg J, Edwardsson S. Evaluation of a simplified diagnostic aid (Oricult-N) for detection of oral candidoses. Scand J Dent Res. 1985;93:52–55. doi: 10.1111/j.1600-0722.1985.tb01308.x. [DOI] [PubMed] [Google Scholar]
- 2.Bro F. The diagnosis of Candida vaginitis in general practice. Scand J Prim Health Care. 1989;7:19–22. doi: 10.3109/02813438909103665. [DOI] [PubMed] [Google Scholar]
- 3.Eckert L O, Hawes S E, Stevens C E, Koutsy L A, Eschenbach D A, Holmes K K. Vulvovaginal candidiasis: clinical manifestations, risk factors, management algorithm. Obstet Gynecol. 1998;92:757–765. doi: 10.1016/s0029-7844(98)00264-6. [DOI] [PubMed] [Google Scholar]
- 4.Erdman Y J, Holton J M, Baker A. Growth of Candida species in liquid culture medium for Trichomonas vaginalis. Br J Vener Dis. 1984;60:39–41. doi: 10.1136/sti.60.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans E G V. Diagnostic laboratory techniques in vaginal candidosis. Br J Clin Pract Suppl. 1990;71:70–72. [PubMed] [Google Scholar]
- 6.Evans E G V, Lacey C J N, Carney J A. Criteria for the diagnosis of vaginal candidosis: evaluation of a new latex agglutination test. Eur J Obstet Gynecol Reprod Biol. 1986;22:365–371. doi: 10.1016/0028-2243(86)90127-9. [DOI] [PubMed] [Google Scholar]
- 7.Fidel P L, Sobel J D. Immunopathogenesis of recurrent vulvovaginal candidiasis. Clin Microbiol Rev. 1996;9:335–348. doi: 10.1128/cmr.9.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hopwood V, Crowley T, Horrocks C T, Milne J D, Taylor P K, Warnock D W. Vaginal candidosis: relation between yeast counts and symptoms and clinical signs in non-pregnant women. Genitourin Med. 1988;64:331–334. doi: 10.1136/sti.64.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Odds F C. Genital candidosis. Clin Exp Dermatol. 1982;7:345–354. doi: 10.1111/j.1365-2230.1982.tb02441.x. [DOI] [PubMed] [Google Scholar]
- 10.Odds F C. Candida and candidosis. 2nd ed. London, England: Baillière Tindall; 1988. pp. 68–92. [Google Scholar]
- 11.Odds F C, Webster C E, Mayuranathan P, Simmons P D. Candida concentrations in the vagina and their association with signs and symptoms of vaginal candidosis. J Med Vet Mycol. 1988;26:277–283. doi: 10.1080/02681218880000391. [DOI] [PubMed] [Google Scholar]
- 12.Odds F C, Webster C E, Riley V C, Fisk P G. Epidemiology of vaginal Candida infection: significance of numbers of vaginal yeasts and their biotypes. Eur J Obstet Gynecol Reprod Biol. 1987;25:53–66. doi: 10.1016/0028-2243(87)90092-x. [DOI] [PubMed] [Google Scholar]
- 13.Pattman R S, Sprott M S, Moss T R. Evaluation of a culture slide in the diagnosis of vaginal candidosis. Br J Vener Dis. 1981;57:69. doi: 10.1136/sti.57.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Räisänen S, Eskelinen S, Merilä M, Kaartinen M. Diagnosis of candidal colpitis using a semiliquid culture medium. Ann Chir Gynaecol. 1982;71:340–343. [PubMed] [Google Scholar]
- 15.Roser H. Bakteriologische diagnostik von Candida-erkrankungen auf Nickersons Medium. Med Welt. 1970;17:792–793. [PubMed] [Google Scholar]
- 16.Sobel J D. Vaginal infections in adult women. Sex Transm Dis. 1990;74:1573–1601. doi: 10.1016/s0025-7125(16)30496-5. [DOI] [PubMed] [Google Scholar]
- 17.Sobel J D. Current concepts: vaginitis. N Engl J Med. 1997;337:1896–1903. doi: 10.1056/NEJM199712253372607. [DOI] [PubMed] [Google Scholar]
- 18.Sobel J D, Faro S, Force R W, Foxman B, Ledger W J, Nyirjesy P R, Reed B D, Summers P R. Vulvoginal candidiasis: epidemiological, diagnostic and therapeutic considerations. Am J Obstet Gynecol. 1998;178:203–211. doi: 10.1016/s0002-9378(98)80001-x. [DOI] [PubMed] [Google Scholar]
- 19.Spinillo A, Capuzzo E, Egbe T O, Baltaro F, Nicola S, Piazzi G. Torulopsis glabrata vaginitis. Obstet Gynecol. 1995;85:993–998. doi: 10.1016/0029-7844(95)00047-U. [DOI] [PubMed] [Google Scholar]
- 20.Weissberg S M. Evaluation of a dipstick for Candida. Obstet Gynecol. 1978;52:506–509. [PubMed] [Google Scholar]
- 21.Zdolsek B, Hellberg D, Fróman G, Nilsson S, Mårdh P E. Culture and wet smear microscopy in the diagnosis of low-symptomatic vulvovaginal candidosis. Eur J Obstet Gynecol Reprod Biol. 1995;58:47–51. doi: 10.1016/0028-2243(94)01981-c. [DOI] [PubMed] [Google Scholar]