Abstract
Placental hypoxia and increased levels of maternal blood anti-angiogenic protein, soluble fms-like tyrosine kinase-1 (sFLT1), are associated with the pathogenesis of pre-eclampsia. We have demonstrated that hypoxia-inducible factor (HIF)-2α mediates the upregulation of the hypoxia-induced FLT1 gene in trophoblasts and their cell lines. Here, we investigated the involvement of HIF-1β, which acts as a dimerization partner for HIF-α, in the upregulation of the FLT1 gene via hypoxia. We confirmed the interactions between HIF-1β and HIF-2α in the nuclei of BeWo, JAR and JEG-3 cells under hypoxia via co-immunoprecipitation. We found that hypoxia-induced upregulation of the FLT1 gene in BeWo cells and secretion of sFLT1 in human primary trophoblasts were significantly reduced by siRNAs targeting HIF-1β. Moreover, the upregulation of the FLT1 gene in BeWo cells induced by dimethyloxaloylglycine (DMOG) was also inhibited by silencing either HIF-2α or HIF-1β mRNA. It was recently shown that DNA demethylation increases both basal and hypoxia-induced expression levels of the FLT1 gene in three trophoblast-derived cell lines. In the demethylated BeWo cells, siRNAs targeting HIF-2α and HIF-1β suppressed the further increase in the expression levels of the FLT1 gene due to hypoxia or treatment with DMOG. However, luciferase reporter assays and bisulfite sequencing revealed that a hypoxia response element (−966 to −962) of the FLT1 gene is not involved in hypoxia or DMOG-induced upregulation of the FLT1 gene. These findings suggest that HIF-1β is essential for the elevated production of sFLT1 in the hypoxic trophoblasts and that the HIF-2α/HIF-1β complex may be a crucial therapeutic target for pre-eclampsia.
Keywords: trophoblast, hypoxia, soluble fms-like tyrosine kinase-1, hypoxia-inducible factor-1β, hypoxia-inducible factor-2α
Introduction
The placenta is a transient organ composed of trophoblasts that contribute to the transport of nutrients, gases and waste products between the maternal and fetal blood (Gude et al., 2004). Placental hypoxia/ischemia observed during gestation involves the improper regulation of oxygen levels, resulting in abnormal placentation and placental vascular diseases, which can lead to pregnancy-related complications, including pre-eclampsia (Hutter et al., 2010; Thompson et al., 2015; Soares et al., 2017). Pre-eclampsia is clinically characterized by the development of hypertension and proteinuria and is one of the leading causes of maternal and fetal death (Uzan et al., 2011). In particular, one of the common characteristics of this disease is the increase in the levels of maternal blood soluble fms-like tyrosine kinase-1 (sFLT1) protein, an anti-angiogenic factor (Koga et al., 2003; Maynard SE et al., 2003; Levine RJ et al., 2004).
sFLT1 is composed of the extracellular domain of the fms-like tyrosine kinase-1/vascular endothelial growth factor (VEGF) receptor-1 (FLT1/VEGFR1), which tightly binds to VEGF (VEGF-A) and the placental growth factor (PlGF) (Shibuya, 2011). It acts as a decoy receptor that sequesters VEGF and prevents the initiation of intracellular signal transduction. Therefore, it is widely accepted that increased levels of circulating sFLT1 may contribute to the development and progression of pre-eclampsia by antagonizing the activity of VEGF and PlGF, resulting in hypertension and proteinuria due to maternal endothelial dysfunction. However, the molecular mechanism underlying the upregulation of sFLT1 in pre-eclampsia has not yet been fully elucidated.
sFLT1 mRNA is generated by the alternative splicing of FLT1 pre-mRNA (Kendall and Thomas, 1993; Shibuya, 2011). In humans, four splice variants of sFLT1 have been reported so far (Heydarian et al., 2009). Among them, in placental tissues, most of sFLT1-i13 and sFLT1-e15a mRNAs are localized within the trophoblasts (Jebbink et al., 2011). In addition, the expression levels of the placental mRNA as well as the serum protein levels of both sFLT1-i13 and sFLT1-e15a increased in preeclamptic pregnant women compared to normal pregnant women (Rajakumar et al., 2009; Whitehead et al., 2011; Souders et al., 2015). Therefore, the excess sFLT1 proteins in the maternal blood are considered to be derived from the trophoblasts.
Hypoxia-inducible factor (HIF) is a member of the basic helix–loop–helix-Per-Arnt-Sim (bHLH–PAS) protein family and an important mediator of the cellular response to hypoxia. Under hypoxic conditions, an alpha subunit with two isoforms (HIF-1α and HIF-2α) forms a heterodimeric transcription factor complex with a beta subunit (HIF-1β), resulting in the transcriptional activation of numerous target genes involved in the adaptation to hypoxia (Wenger et al., 2005; Pringle et al., 2010). During this process, the HIF-α/HIF-1β heterodimer interacts directly with the consensus hypoxia response element (HRE) sequence 5′-(A/G)CGTG-3′ that is located upstream or downstream of the target genes (Wenger et al., 2005; Pringle et al., 2010). We have previously reported that HIF-2α, but not HIF-1α, mediates the hypoxia-induced upregulation of the FLT1 gene expression in the human trophoblast-derived cell lines (BeWo, JAR and JEG-3) and human primary trophoblasts in vitro (Sasagawa et al., 2018), however, the associated molecule(s) of HIF-2α that help upregulate the expression of the FLT1 gene in these cells under exposure to hypoxia are unknown. Therefore, in this study, we investigated whether HIF-1β is involved in the upregulation of the FLT1 gene in trophoblast-derived cell lines and primary trophoblasts under hypoxic conditions.
Materials and methods
Trophoblast-derived choriocarcinoma cell lines and cell culture
Three trophoblast-derived choriocarcinoma cell lines: BeWo (JCRB9111; Japanese Collection of Research Bioresources (JCRB) Cell Bank, Tokyo, Japan), JAR (HTB-144; American Tissue Culture Collection (ATCC), Manassas, VA, USA) and JEG-3 (HTB-36; ATCC) were maintained in Ham’s F-12 medium (Nacalai Tesque, Inc., Kyoto, Japan) containing 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin. All cells were cultured at 37°C in a humidified atmosphere with 5% CO2. This cell culture condition was designated as ‘ambient condition’.
For hypoxic exposure, the cells were seeded on cell culture plates or dishes (Sumitomo Bakelite Co., Ltd, Tokyo, Japan) at a density of 4.35 × 104 cells/cm2. After 24 h of incubation under ambient conditions, the medium was replaced with fresh medium and the cells were maintained under hypoxic conditions (2% O2, 5% CO2 and 93% N2) for 24 h in a Multigas Incubator (WAKEN 9000EX; WAKEN B TECH Co., Ltd, Kyoto, Japan).
To induce chemically mimicked hypoxia, the cells were treated with dimethyloxaloylglycine (DMOG; Enzo Life Sciences, Farmingdale, NY, USA) at different doses (1–200 μM) for 24 h. Dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA) was used as the vehicle control.
For DNA demethylation, the cells were treated with 10 µM 5′-aza-2′-deoxycytidine (5azadC; Sigma-Aldrich) or DMSO as the vehicle control for 3 days. The culture media were changed daily to maintain the stability of 5azadC during treatment.
Isolation and culture of human primary trophoblasts
All procedures involving the purification of cytotrophoblasts from the normal placental tissues and their cultivation for several experiments were approved by the Ethical Committee of the Jobu University (No. 20-H03) and the institutional review board of Faculty of Medicine, the University of Tokyo (IRB number: 10979-3), respectively. Human villous cytotrophoblasts were isolated from the normal term placentas and cryopreserved at −80°C, as previously reported (Sasagawa et al., 2018). Thawed trophoblasts were suspended in a 1:1 mixture of the Dullbecco’s modified Eagle medium (Nacalai Tesque)/Ham’s F-12 containing 10% FBS and antibiotics and then seeded into type I collagen-coated 12-well culture plates (Sumilon Celltight; Sumitomo Bakelite Co., Ltd) at a density of ∼1.2 × 106 cells per well. After incubation for 16 h, siRNA transfection was performed.
Quantitative reverse transcription-polymerase chain reaction
RNA was extracted and the mRNA expression levels were assessed using quantitative reverse transcription-polymerase chain reaction (qRT-PCR) as previously described (Sasagawa et al., 2018). All data were normalized to β-actin expression. Each value was obtained from the mean of three independent experiments. The oligonucleotide primer sequences are listed in Supplementary Table S1.
Western blotting analysis
Preparation of whole cell lysates and nuclear extracts, gel electrophoresis and electroblotting were performed as previously reported (Sasagawa et al., 2018). The following primary antibodies were used: anti-HIF-1α (1:500, #3716; Cell Signaling Technology, Beverly, MA, USA), anti-HIF-2α (1:1000, NB100-122; Novus Biologicals, Littleton, CO, USA), anti-HIF-1β (1:2000, NB100-110; Novus Biologicals), anti-β-actin (1:1000, #4967; Cell Signaling Technology), anti-TATA binding protein (1:200, sc-421; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-human FLT1 N-terminal region (rabbit polyclonal) antibody (1:1000) (Tanaka et al., 1997; Sasagawa et al., 2018; Sasagawa et al., 2020).
Immunoprecipitation
The nuclear extracts (200 µg protein) of hypoxic cells were subjected to immunoprecipitation using Dynabeads Protein G (Invitrogen, Carlsbad, CA, USA) bound with normal rabbit IgG (#2729; Cell Signaling Technology), anti-HIF-1β (NB100-110; Novus Biologicals) or anti-HIF-2α (NB100-122; Novus Biologicals) antibodies, according to the manufacturer’s instructions. The precipitated proteins were then subjected to western blotting analysis.
Heparin-affinity pull-down for the concentration of sFLT1 proteins
As previously reported (Sasagawa et al., 2018), the secreted sFLT1 isoforms within the conditioned medium from trophoblasts were concentrated using heparin-immobilized beads (Heparin Sepharose 6 Fast Flow; GE Healthcare, Uppsala, Sweden) and the bound proteins were eluted from these beads. The eluted proteins were then subjected to western blotting analysis.
Immunofluorescence staining
Three choriocarcinoma cell lines were cultured on FBS-coated coverslips. After incubation for 24 h under ambient or hypoxic conditions, these cells were fixed and permeabilized as previously reported (Sasagawa et al., 2018). For immunostaining of HIF-1β, the specimens were treated with the anti-HIF-1β monoclonal antibody (1:100, NB100-124; Novus Biologicals) for 1 h and then visualized using the Alexa Fluor 488-conjugated secondary antibody (1:500, A-11029; Invitrogen). The nuclei were counterstained with 4',6-diamidino-2-phenylindole (1:500, Wako Pure Chemicals, Osaka, Japan). The cell images were obtained using a fluorescence microscope (Biozero BZ-8000; Keyence, Osaka, Japan).
Transfection with siRNA
Silencer Select Negative Control No.1 siRNA (4390843) and Silencer select siRNAs targeting HIF-1α (s6541), HIF-2α (s4699) and HIF-1β (#1; s1613 and #2; s1614) were purchased from Ambion (Austin, TX, USA). BeWo cells and human primary trophoblasts were transfected with siRNAs using the Lipofectamine RNAiMAX transfection reagent (Invitrogen) as described previously (Sasagawa et al., 2018). The siRNA transfection time for each cell was optimized based on the knockdown efficiency of over 70% for the target gene, and the time was set to 72 h for the BeWo cells and 48 h for the trophoblasts. After transfection, the medium was replaced with fresh medium and the cells were exposed to hypoxic or chemically mimicked hypoxic experimental conditions for an additional 24 h.
Treatment with the HIF-2α-specific inhibitor TC-S7009
BeWo cells were cultured for 24 h. Then, the medium was replaced with fresh medium containing 0.1% DMSO as a vehicle control or 30 μM TC-S7009 (Tocris Biosciences, Bristol, UK). After incubation for 24 h under chemically mimicked hypoxic conditions, the cells were subjected to cell viability assays or qRT-PCR analysis.
Cell viability assay
Cell viability was assessed using the Cell Counting Kit-8 (CCK-8; Dojindo, Kumamoto, Japan) as described previously (Sasagawa et al., 2018).
Luciferase reporter assay
To generate a luciferase reporter construct (pFLT1-Luc), the FLT1 promoter region (−1629/+8) fragment obtained by digesting the pPS00CAT vector (Ikeda et al., 1996) with HindIII and HincII was inserted into the EcoRV and HindIII sites of the promoterless firefly luciferase vector pGL4.10 (Promega, Madison, WI, USA). The position of the transcription start site (TSS) (+1) of the human FLT1 gene is as per our previous report (Ikeda et al., 1996).
For the FLT1 promoter assay, the BeWo cells were seeded into 12-well cell culture plates (Sumitomo Bakelite Co., Ltd). After incubation for 24 h, the cells were co-transfected with 1 μg of the pFLT1-Luc vector and 20 ng of the control renilla luciferase vector pGL4.74 (hRuc/TK) (Promega) together with 3 μl of the Viafect Transfection Reagent (Promega) for 24 h according to the manufacturer’s instructions. The cells were then incubated for 24 h under hypoxic or chemically mimicked hypoxic experimental conditions after the medium was replaced with fresh medium, followed by qRT-PCR analysis or luciferase reporter assay. Luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega) with a luminometer (Gene Light 210A; Microtech Nichion, Chiba, Japan). The firefly luciferase activity of the reporter plasmid was normalized to the renilla luciferase activity.
Bisulfite sequencing
Genomic DNA extraction and bisulfite modification were carried out as previously reported (Sasagawa et al., 2020). Amplification was performed using EpiTaq HS (Takara Bio, Shiga, Japan) and PCR primers for the detection of an HRE between −966 and −962 bp in the FLT1 gene (forward 5′ -GGGGAGGTAGTTTAGTTTTTTA-3′ and reverse 5′-AAAACACTTAAACTTTATTCCCTAA-3′). The thermal cycling conditions consisted of an initial activation cycle (98°C for 20 s), followed by 40 cycles of denaturation (98°C for 10 s), annealing (55°C for 30 s) and amplification (72°C for 30 s). The PCR products were then cloned into pGEM-T easy vectors (Promega) and DNA sequencing was performed on 19 clones from each sample.
Statistical analysis
Data are expressed as the mean ± SD and the parametric data were analysed using an unpaired t-test. Statistical analyses were performed using Excel 2011 (Microsoft, Seattle, WA, USA) with the Statcel4 (OMS, Tokyo, Japan) ‘add-in’ software. A P-value <0.05 was considered to be statistically significant.
Results
Increased mRNA and protein expression levels of HIF-1β and the interactions of HIF-1β with HIF-2α in the nuclei of three trophoblast-derived choriocarcinoma cell lines exposed to hypoxic conditions
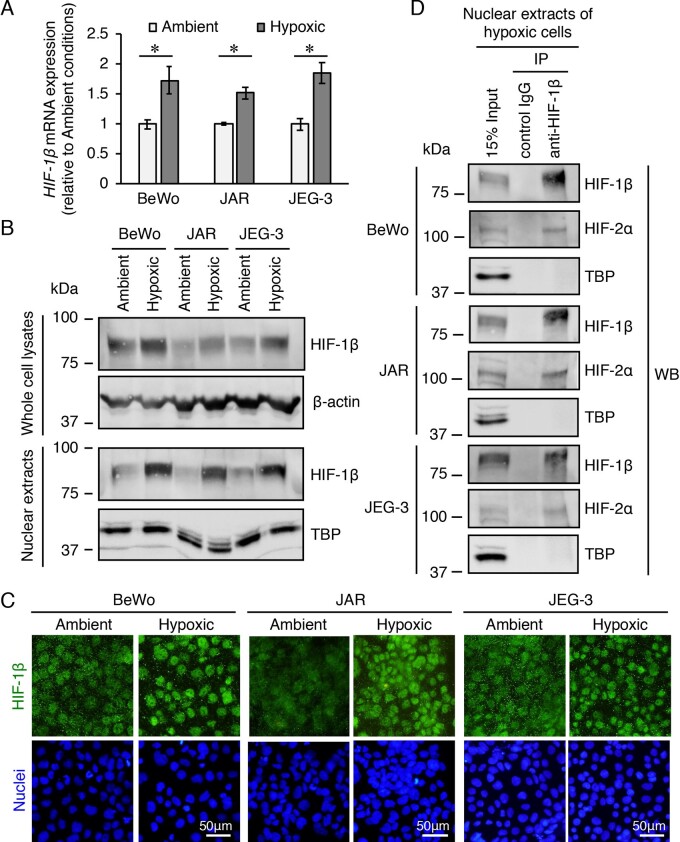
We investigated the expression levels of HIF-1β in the trophoblast-derived choriocarcinoma cell lines cultured under ambient (atmosphere with 5% CO2) or hypoxic (2% O2, 5% CO2 and 93% N2) conditions. Unexpectedly, the levels of HIF-1β mRNA were significantly increased in hypoxic conditions compared to those in ambient conditions in all the three cell lines (Fig. 1A). Western blotting analysis of whole cell lysates also showed that HIF-1β was constitutively expressed under ambient conditions and its expression was increased by exposure to hypoxia in all the three cell lines (Fig. 1B). In addition, HIF-1β levels were significantly increased in the nuclear extract fractions by hypoxia stimulation (Fig. 1B). The increase in levels of HIF-1β in the nucleus under hypoxia was also shown by immunofluorescence staining of these cell lines (Fig. 1C). After observing the nuclear accumulation of HIF-2α in these cell lines under hypoxia (Sasagawa et al., 2018), we examined the heterodimer formation of HIF-2α and HIF-1β in the nuclei of these cells by co-immunoprecipitation using the nuclear extracts of the hypoxic cells. The HIF-2α protein was immunoprecipitated using the antibody against HIF-1β in the nuclear extracts of the three hypoxic cell lines (Fig. 1D). Similarly, this heterodimer formation was also confirmed by immunoprecipitation of the HIF-1β protein using the antibody against HIF-2α (Supplementary Fig. S1). These results suggest that HIF-1β forms a heterodimeric transcription factor complex with HIF-2α in the nuclei of cells from all three trophoblast-derived cell lines under hypoxia.
Figure 1.
mRNA and protein expression levels of hypoxia-inducible factor (HIF)-1β and the interactions between HIF-1β and HIF-2α in three trophoblast-derived choriocarcinoma cell lines exposed to hypoxia. BeWo, JAR and JEG-3 cells were incubated under ambient (atmosphere with 5% CO2) or hypoxic (2% O2, 5% CO2 and 93% N2) conditions for 24 h. (A) The mRNA expression levels of HIF-1β were measured by quantitative reverse transcription-polymerase chain reaction using β-actin mRNA as a reference. Results are expressed as the fold change relative to the cells under ambient conditions. All values are represented as the mean ± SD (n=3). Asterisks indicate the significant difference (P<0.05). (B) The expression levels of HIF-1β in the whole cell lysates and nuclear extracts of the three cell lines were assessed by western blotting analysis. β-Actin and the nuclear protein, TATA binding protein, were used as the loading controls. (C) Cellular localization of HIF-1β in the three cell lines was determined by immunofluorescence staining. (D) Immunoprecipitation of HIF-2α protein using an antibody against HIF-1β in nuclear extracts of the three hypoxic cell lines. The immunoprecipitates and nuclear extracts (input) were subjected to western blotting analysis. Uncropped images of the western blots are presented in Supplementary Fig. S4.
Inhibition of HIF-1β reduces hypoxia-induced upregulation of the FLT1 gene in BeWo cells and hypoxia-elevated sFLT1 secretion in human primary trophoblasts
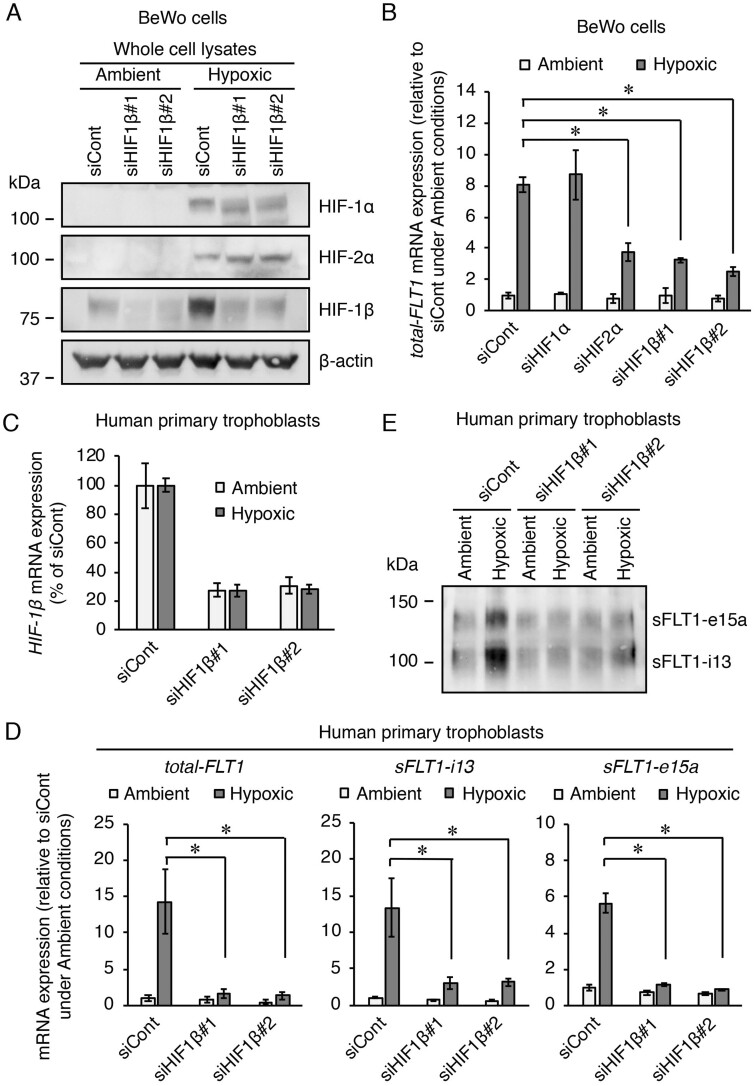
We further examined the involvement of HIF-1β in the upregulation of the FLT1 gene under hypoxia. BeWo cells were transfected with the indicated siRNAs targeting HIF-1α, HIF-2α or HIF-1β for 72 h prior to exposure to either ambient or hypoxic conditions for 24 h. Two HIF-1β siRNAs (siHIF1β#1 and siHIF1β#2) reduced HIF-1β protein expression without affecting either HIF-1α or HIF-2α protein expressions under ambient or hypoxic conditions (Fig. 2A). FLT1 gene expression was measured using qRT-PCR with primers to cover all FLT1 transcript variants, including full-length transmembrane FLT1 (tmFLT1) and sFLT1 mRNAs. The tmFLT1 plus sFLT1s amplicon was designated as total-FLT1. As we previously reported (Sasagawa et al., 2018), the hypoxia-induced upregulation of total-FLT1 mRNA expression was significantly inhibited when transfected with HIF-2α siRNA, but not HIF-1α siRNA (Fig. 2B). In addition, HIF-1β siRNA transfection also significantly inhibited the total-FLT1 mRNA upregulation induced by hypoxia (Fig. 2B).
Figure 2.
Silencing of HIF-1β via transfection with a siRNA reduces the hypoxia-induced upregulation of the FLT1 gene in both BeWo cells and human primary trophoblasts. BeWo cells and primary trophoblasts were each transfected with a siRNA at 10 nM for 72 h and 48 h, respectively, after which the cells were incubated under ambient or hypoxic conditions for 24 h. (A) The protein expression levels of HIFs in the BeWo cells were assessed by western blotting analysis. (B) The mRNA expression levels of all FLT1 transcript variants (total-FLT1) in the BeWo cells were measured by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) using β-actin mRNA as a reference. Results are expressed as the fold change relative to the control siRNA (siCont)-transfected cells under ambient conditions. (C) Evaluation of the knockdown of the HIF-1β mRNA via transfection with siRNA in the primary trophoblasts. Results are expressed as a percentage relative to the control siCont-transfected cells under ambient or hypoxic conditions. (D) The mRNA expression levels of total-FLT1, sFLT1-i13 and sFLT1-e15a in the primary trophoblasts were measured by qRT-PCR using β-actin mRNA as a reference. Results are expressed as the fold change relative to the siCont-transfected cells under ambient conditions. (E) Western blotting of the sFLT1 proteins secreted by primary trophoblasts into the conditioned media. Uncropped images of the western blots are presented in Supplementary Fig. S4. All values are represented as the mean ± SD (n=3). Asterisks indicate the significant difference (P<0.05).
Next, we investigated whether HIF-1β siRNAs were also able to suppress the hypoxia-induced upregulation FLT1 gene in human primary trophoblasts. Primary trophoblasts were transfected with each HIF-1β-specific siRNA for 48 h and further incubated for 24 h under ambient and hypoxic conditions. As a result, more than 70% knockdown of HIF-1β mRNA by the respective siRNAs was achieved under both ambient and hypoxic conditions (Fig. 2C). In addition, each HIF-1β-specific siRNA significantly inhibited the hypoxia-induced upregulation of the mRNA expression levels of total-FLT1, sFLT1-i13 and sFLT1-e15a (Fig. 2D). Western blotting analysis showed an increase in sFLT1-i13 and sFLT1-e15a secretion in the presence of control siRNA in trophoblasts exposed to hypoxia (Fig. 2E). On the other hand, transfection with each HIF-1β siRNA inhibited the hypoxia-induced elevation of sFLT1 production in trophoblasts (Fig. 2E). Even in the trophoblasts derived from another donor, the siHIF1β#1 also achieved more than 70% knockdown of HIF-1β mRNA expression and specifically inhibited the hypoxia-induced elevation of sFLT1 production (Supplementary Fig. S2). These results indicate that HIF-1β contributes to the hypoxia-induced upregulation of the FLT1 gene in not only the trophoblast-derived cell lines but also in the human primary trophoblasts.
HIF-2α and HIF-1β, but not HIF-1α, regulate upregulation of the FLT1 gene induced by DMOG in BeWo cells
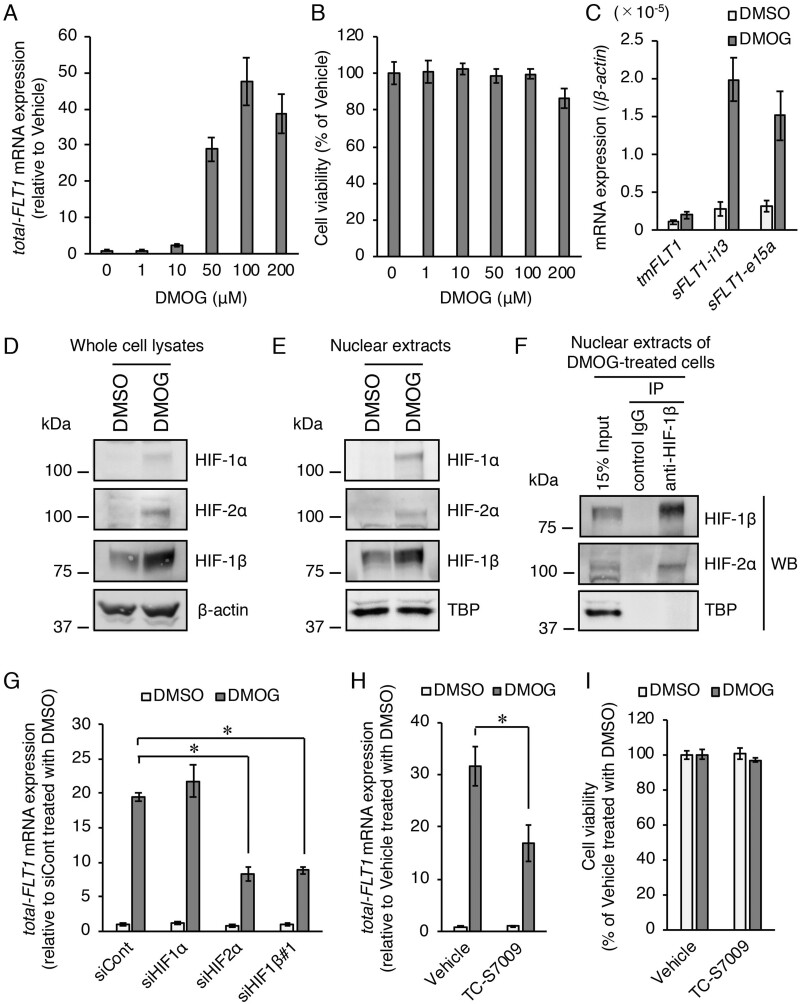
DMOG acts as a cell-permeable prolyl-4-hydroxylase inhibitor and mimics hypoxic conditions by stabilizing HIF-α subunits. We first evaluated the dose-dependent effects of DMOG on the FLT1 gene expression by exposing BeWo cells to different concentrations of DMOG for 24 h. As a result, the levels of total-FLT1 mRNA expression increased in a dose-dependent manner, with a DMOG concentration of 100 µM being sufficient to achieve optimal induction levels (Fig. 3A). No cytotoxicity was observed in the treatment with DMOG at a concentration of 100 µM (Fig. 3B). In addition, the effect of DMOG on the mRNA expression levels of three FLT1 splicing variants (tmFLT1, sFLT1-i13 and sFLT1-e15a) was also investigated. DMOG treatment predominantly upregulated the mRNA levels of both sFLT1s (Fig. 3C). Next, the protein expression levels of HIFs were examined by western blotting analysis. HIF-1α and HIF-2α proteins were detected and an increase in HIF-1β protein was observed in both whole cell lysates and nuclear extracts after DMOG treatment (Fig. 3D and E). Similar to the results of hypoxic treatment in three trophoblast-derived choriocarcinoma cell lines (Fig.1A), DMOG treatment in BeWo cells also significantly increased the HIF-1β mRNA levels (Supplementary Fig. S3). In addition, co-immunoprecipitation of the HIF-2α protein by the antibody against HIF-1β was also shown in nuclear extracts from DMOG-treated cells (Fig. 3F). We further examined the roles of HIFs in the FLT1 gene upregulation induced by DMOG using siRNAs. The DMOG-induced upregulation of total-FLT1 mRNA expression was also significantly inhibited by siRNAs targeting HIF-2α and HIF-1β, but not HIF-1α (Fig. 5G). Furthermore, an HIF-2α-specific inhibitor TC-S7009 that antagonizes HIF-2α heterodimerization and DNA-binding activity also significantly decreased upregulation of FLT1 gene by DMOG treatment (Fig. 3H). Treatment with TC-S7009 had no toxic effects on the cells (Fig. 3I). These results indicate that DMOG-induced upregulation of the FLT1 gene in BeWo cells is also mediated by HIF-2α and HIF-1β.
Figure 3.
Role of HIFs in the upregulation of the FLT1 gene under chemically mimicked hypoxic conditions using the HIF-α stabilizer dimethyloxalylglycine (DMOG) in BeWo cells. (A) Effect of various doses of DMOG on the mRNA expression levels of all FLT1 transcript variants (total-FLT1). The cells were treated with various doses of DMOG for 24 h. Results are expressed as the fold change relative to the vehicle (dimethyl sulfoxide (DMSO))-treated cells. (B) Cell viability was assessed to determine the potential toxicity of DMOG. Results are expressed as the percentage relative to the vehicle-treated cells. (C) The mRNA expression levels of the three FLT1 splice variants in the presence of 100 µM DMOG or 0.1% DMSO (vehicle control). (D, E) Western blotting analysis of the expression levels of HIF proteins in the whole cell lysates and nuclear extracts of the vehicle- or 100 µM DMOG-treated cells. β-Actin and TATA binding protein were used as the loading controls. (F) Immunoprecipitation of HIF-2α protein using an antibody against HIF-1β in the nuclear extracts of the 100 µM DMOG-treated cells. The immunoprecipitates and nuclear extracts (input) were subjected to western blotting analysis. Uncropped images of the western blots are presented in Supplementary Fig. S4. (G) Effects of the gene silencing of each HIF on DMOG-induced upregulation of the FLT1 gene. Cells were transfected with the indicated siRNAs targeting HIFs for 72 h prior to treatment with either DMSO or DMOG for 24 h. Results are expressed as the fold change relative to the siCont-transfected cells in the presence of the vehicle. (H) Effect of the HIF-2α inhibitor, TC-S7009, on DMOG-induced upregulation of the FLT1 gene. The cells were cultured for 24 h in the presence of 100 µM DMOG or the vehicle in the presence of 0.1% DMSO (vehicle control) or 30 µM TC-S7009. Results are expressed as the fold change relative to the DMSO-treated cells in the presence of the vehicle. (I) Cell viability was assessed to determine the potential toxicity of TC-S7009. Results are expressed as the percentage relative to the DMSO-treated cells in the presence of the vehicle. All values are represented as the mean ± SD (n=3). Asterisks indicate the significant difference (P<0.05).
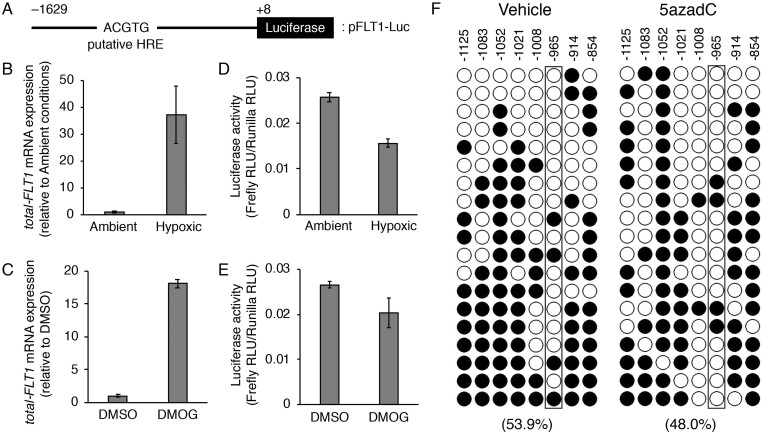
Figure 5.
The hypoxia response element (HRE) located ∼1 kb upstream of the transcription start site (TSS) in the FLT1 gene is not involved in the hypoxia- or DMOG-induced upregulation of the FLT1 gene in BeWo cells. (A) Schematic representation of the luciferase reporter construct, pFLT1-Luc. Numbering refers to the regions of the FLT1 promoter inserted into the parental pGL4.10 vector. The putative HRE is indicated within the −966/−962 promoter region. (B, C) The cells were transiently co-transfected with 1 μg of the pFLT1-Luc vector and 20 ng of the control renilla luciferase vector pGL4.74 (hRuc/TK). After 24 h of transfection, the cells were subjected to hypoxic or chemically mimicked hypoxic conditions for 24 h and the mRNA expression levels of all FLT1 transcript variants (total-FLT1) were measured by quantitative reverse transcription-polymerase chain reaction using β-actin mRNA as a reference. (D, E) Luciferase activity was measured under the same conditions as in (B) and (C). The firefly luciferase activity of the pFLT1-Luc reporter plasmid was normalized to that of renilla. The luciferase activity was measured in relative light units. (F) Methylation patterns in the promoter region of the FLT1 gene. The eight cytosine-phosphate-guanine (CpG) sites of FLT1 were analysed by bisulfite sequencing. The DNA methylation data were analysed by the QUantification tool for Methylation Analysis (http://quma.cdb.riken.jp/). Open circles and closed circles represent the unmethylated and methylated CpG sites, respectively. Numbers indicate the position relative to the TSS. Numbers in parentheses represent the overall percentage of methylated CpG sites. Boxed areas indicate the CpG site in the putative HRE.
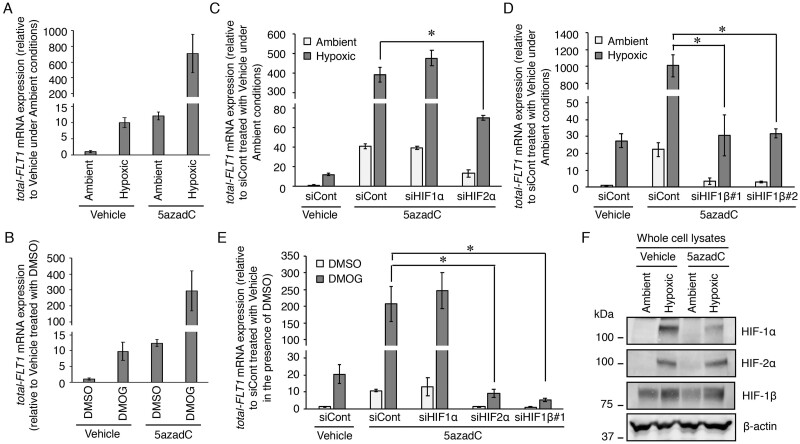
Inhibition of HIF-2α and HIF-1β, but not HIF-1α, reduces the further increase in FLT1 gene expression by hypoxia or DMOG in demethylated BeWo cells
Treatment with a DNA methyltransferase inhibitor, 5azadC, for 120 h can upregulate both the basal and hypoxia-induced expression levels of the FLT1 gene in three trophoblast-derived choriocarcinoma cell lines (BeWo, JAR and JEG-3 cells) (Sasagawa et al., 2020). To understand the role of HIFs in the further increase in the FLT1 gene expression by hypoxia, we attempted experiments in which both demethylation and siRNA transfection were performed simultaneously. First, we examined whether 72 h of demethylation could further increase the hypoxia-induced FLT1 gene expression in BeWo cells because the siRNA transfection time has been optimized to 72 h. BeWo cells were incubated in the presence of 0.1% DMSO (vehicle control) or 10 μM 5azadC and the culture media were changed daily. After 3 days of culture, the culture media were changed with fresh growth media without 5azadC. The cells were treated under hypoxia for 24 h and then the mRNA expression levels of all FLT1 transcript variants (total-FLT1) were measured by qRT-PCR. The expression level of total-FLT1 mRNA in 5azadC-treated BeWo cells under hypoxia was ∼70-fold higher than that in vehicle-treated cells under hypoxia (Fig. 4A). Therefore, the further increase in hypoxia-induced FLT1 gene expression was observed even at 72 h of demethylation treatment time. Next, both demethylation and siRNA transfection were performed simultaneously in BeWo cells for 72 h, followed by a 24-h incubation under ambient or hypoxic conditions. As a result, the further increase in the FLT1 expression induced by hypoxic stimulation was significantly inhibited by siRNAs targeting HIF-2α and HIF-1β, not HIF-1α (Fig. 4C and D). In addition, the effect of DMOG on FLT1 gene expression in 5azadC-treated BeWo cells was also investigated. Under DMOG-treated conditions, the total-FLT1 mRNA expression levels in 5azadC-treated BeWo cells were ∼30-fold higher than those in vehicle-treated cells (Fig. 4B). The further increase in FLT1 gene expression induced by DMOG treatment was also significantly inhibited by siRNAs targeting HIF-2α and HIF-1β rather than HIF-1α (Fig. 4E). Noteworthy, western blotting analysis revealed that HIF protein expression levels did not change with or without 5azadC treatment (Fig. 4F). These results indicate that the further increases in the FLT1 gene expression induced by hypoxia or treatment with DMOG after demethylation are also regulated by the HIF-2α/HIF-1β complex and are due to an increase in the number of HREs to which the heterodimers can bind.
Figure 4.
Both HIF-2α and HIF-1β regulate the further increase in the expression levels of the FLT1 gene induced by hypoxia or DMOG in the BeWo cells treated with a DNA methyltransferase inhibitor 5′-aza-2′-deoxycytidine (5azadC). (A, B) The cells were incubated in the presence of 0.1% DMSO (vehicle control) or 10 μM 5azadC and the culture media were changed daily. After 3 days of culture, the culture media were changed with fresh growth media without 5azadC. The cells were treated under hypoxia or with DMOG for 24 h and then the mRNA expression levels of all FLT1 transcript variants (total-FLT1) were measured by quantitative reverse transcription-polymerase chain reaction using β-actin mRNA as a reference. Results are expressed as the fold change relative to the control vehicle-treated cells under ambient conditions and in the presence of DMSO. (C–E) The cells that underwent demethylation and siRNA transfection simultaneously for 72 h were subjected to hypoxic or chemically mimicked hypoxic conditions for 24 h, after which the mRNA expression levels of total-FLT1 were determined. Results are expressed as the fold change relative to the siCont-transfected cells treated with the vehicle under ambient conditions and in the presence of DMSO. All values represent the mean ± SD (n=3). Asterisks indicate significant differences (P<0.05). (F) Western blotting analysis of the expression levels of HIF proteins in the whole cell lysates of the BeWo cells, with or without 5azadC treatment, under ambient and hypoxic conditions. β-Actin was used as the loading control. Uncropped images of the western blots are presented in Supplementary Fig. S4.
A HRE between −966 and −962 bp in the FLT1 gene is not involved in hypoxia or DMOG-induced upregulation of the FLT1 gene in BeWo cells
Previously, a putative HRE was reported to locate ∼1 kb upstream of the TSS of the mouse and human FLT1 genes (Gerber et al., 1997). To elucidate whether this HRE contributes to the hypoxia or DMOG-induced upregulation of the FLT1 gene, a luciferase reporter construct pFLT1-Luc (as shown in Fig. 5A) was transfected in BeWo cells. After transfection for 24 h, the cells were treated with hypoxia or DMOG for 24 h for qRT-PCR analysis or luciferase reporter assay. Upregulation of endogenous total-FLT1 gene expression was induced by hypoxia or DMOG (Fig. 5B and C). However, an increase in luciferase activity was not observed in the cells treated with hypoxia or DMOG (Fig. 5D and E). We then further investigated the cytosine-phosphate-guanine (CpG) methylation level of an HRE located between −966 and −962 bp in the FLT1 gene promoter in BeWo cells with or without 5azadC treatment. The overall percentage of methylated CpG sites in the HRE near region of the FLT1 promoter decreased from 53.9% to 48.0% compared to those before 5azadC treatment, but there was no difference in CpG methylation level in the HRE sequence itself (Fig. 5F). These results strongly suggest that an HRE located ∼1 kb upstream of the TSS in FLT1 gene is not involved in hypoxia- or DMOG-induced upregulation of the FLT1 gene in BeWo cells.
Discussion
In this study, we showed that HIF-1β interacted with HIF-2α in the nuclei of hypoxic human trophoblast-derived choriocarcinoma cell lines (BeWo, JAR and JEG-3 cells). We also demonstrated that specific siRNAs targeting HIF-1β reduced the hypoxia-induced upregulation of the FLT1 gene in BeWo cells and hypoxia-elevated secretion of sFLT1 in the human primary trophoblasts. These results indicate that HIF-1β is a partner molecule of HIF-2α that regulates the hypoxia-induced upregulation of the FLT1 gene in the placental trophoblasts. We previously reported that HIF-2α, but not HIF-1α, mediates the hypoxia-induced upregulation of the FLT1 gene in human primary trophoblasts and its three cell lines (BeWo, JAR and JEG-3 cells) (Sasagawa et al., 2018). The data from this study along with the findings from previous study strongly suggest that the HIF-2α/HIF-1β heterodimer transcription factor is a critical mediator responsible for the production of abnormal sFLT1 from the trophoblasts during pre-eclampsia.
The expression of HIF-1β is generally considered to be ubiquitous, constitutive, abundant and unaffected by environmental conditions, such as hypoxia (Mandl and Depping, 2014). However, in this study, we found that hypoxia and the hypoxia-mimetic agent, DMOG, increased both the mRNA and protein levels of HIF-1β in all three human trophoblast-derived choriocarcinoma cell lines (Figs 1A and B and 3D and Supplementary Fig. S4). Hypoxia and hypoxia-mimetics increase the expression levels of HIF-1β in several human cancer cell lines, including the prostate cancer (PC-3), melanoma (518A2), breast carcinoma (MCF-7), hepatocellular carcinoma (Hep3B), cervix adenocarcinoma (HeLa) and multiple myeloma (H929) cell lines (Zhong et al., 2001; Mandl et al., 2013; Wolff et al., 2013; Wu et al., 2018). Interestingly, in some of these cancer cell lines overexpression of HIF-1β results in drug or radiation resistance, while the knockdown of HIF-1β exerts the opposite effects (Mandl et al., 2015; Wu et al., 2018). From these findings, it is considered that the increased expression of HIF-1β due to hypoxia plays an important role in the survival of cancer cells. As the three human trophoblast cell-derived choriocarcinoma cell lines consist of cancer cells, it is possible that the increase in the expression levels of HIF-1β under hypoxic and chemically mimicked hypoxic conditions was due to cellular transformation. Only a few mechanisms of hypoxia-dependent increase in the expression levels of HIF-1β in cancer cells have been reported so far. Wu et al. (2018) reported that the hypoxia-induced upregulation of HIF-1β may depend on the activation of the nuclear factor-kappa B pathway in the human multiple myeloma cells. Mandl and Depping (2017) showed that HIF-1α directly induces the expression of HIF-1β by binding to the promoter of the HIF-1β gene in Hep3B cells. Extensive investigations have to be performed to understand whether hypoxia also increases the expression levels of HIF-1β in human trophoblasts and to determine if hypoxia-dependent upregulation of HIF-1β is a cellular response characteristic of the placental trophoblasts.
Immunohistochemistry of the sFLT1 protein in the placentas obtained from preeclamptic patients showed that sFLT1 expression is significantly enhanced in syncytiotrophoblasts, which form the outermost layer of the placenta, when compared to those in the normal placentas (Helske et al., 2001; Nevo et al., 2006; Rajakumar et al., 2012). Thus, the increased sFLT1 expression observed in the maternal blood during pre-eclampsia could be attributed largely to syncytiotrophoblasts. Human primary cytotrophoblasts have been shown to differentiate into syncytiotrophoblasts via spontaneous cell fusion (Li and Schust, 2015). This study also observed syncytialized trophoblasts during siRNA transfection (data not shown). Therefore, our culture conditions in which both cytotrophoblasts and syncytiotrophoblasts are present might reflect the human placental villi.
Under hypoxic conditions, the protein levels of HIF-α are stabilized and it translocates to the nucleus, where it forms a heterodimeric transcription factor complex with HIF-1β to activate the transcription of its downstream target genes. In this process, the HIF-α/HIF-1β heterodimer interacts directly with the consensus HRE sequence 5′-(A/G)CGTG-3′ of the target genes (Wenger et al., 2005; Pringle et al., 2010). Gerber et al. (1997) reported that a putative HRE motif was located ∼1 kb upstream of the TSS in the mouse and human FLT1 genes. However, Camenisch et al. (2001) used an electrophoretic mobility shift assay to demonstrate that the in vitro-translated HIF-α/HIF-β1 heterodimers did not bind to the oligonucleotide probe containing this FLT1 HRE. Moreover, they also showed that treatment under hypoxia or with the HIF-induced iron chelator, desferrioxamine, did not induce the luciferase activity of the reporter plasmid containing this FLT1 HRE candidate sequence. In this study as well, treatment under hypoxia or with DMOG did not increase the luciferase activity in the BeWo cells transfected with the pFLT1-Luc plasmid containing the FLT1 HRE sequence (Fig. 5D and E). The HRE motif contains a single CpG dinucleotide and the CpG methylation within this sequence interferes with the binding of HIF-1α or HIF-2α followed by the HIF-mediated transcription of HRE-containing genes (Robinson et al., 2018). However, the CpG methylation levels of the FLT1 HRE in the BeWo cells did not decrease after demethylation (Fig. 5F). We have previously reported that three human trophoblast-derived choriocarcinoma cell lines are hypermethylated in the FLT1 gene promoter containing 23 CpG sites from positions −272 to −80 relative to the TSS. The overall percentage of methylated CpG sites in this FLT1 promoter region was 91.5% in BeWo cells, 96.7% in JAR cells and 85.0% in JEG-3 cells (Sasagawa et al., 2020). By contrast, the region containing eight CpG sites near the FLT1 HRE in BeWo cells showed a moderate methylation (53.9%) (Fig. 5F). In addition, the decrease in the overall percentage of methylated CpG sites in this region after demethylation was only 5.9% (Fig. 5F), indicating that this region might not be the main target for DNA methylation in the first place. Therefore, we conclude that there are other functional HRE(s) that can respond to hypoxic stimulation. Whilst most HREs are located proximal to the promoter, these sites have also been identified in intergenic, 5′-untranslated and 3′-untranslated regions as well as the intronic and exonic regions (Schödel et al., 2011). To date, a few intronic HREs to which HIF-2α binds have also been reported, including those that regulate the genes TWIST1 (intron 1) (Gort et al., 2008) and WT1 (intron 3) (Krueger et al., 2019). Since the further increases in the FLT1 gene expression induced by hypoxia or treatment with DMOG after demethylation are thought to be due to an increase in the number of HREs to which the HIF-2α/HIF-1β complex can bind, the functional FLT1 HRE will be revealed by chromatin immunoprecipitation sequencing analysis using demethylated BeWo cells under hypoxia.
Although low-dose aspirin has been shown to prevent the development of pre-eclampsia (Dutta et al., 2019), there is currently no therapeutic drug that is completely effective for the treatment of the pregnant women with pre-eclampsia and there is no cure other than preterm delivery in severe cases. Previously, we have demonstrated that the HIF-2α-specific inhibitor, TC-S7009, which inhibits the heterodimerization of HIF-2α and HIF-1β, significantly reduces the hypoxia-induced upregulation of the FLT1 gene in BeWo, JAR and JEG-3 cells (Sasagawa et al., 2018). Likewise, DMOG-induced upregulation of the FLT1 gene in BeWo cells was also decreased by this inhibitor (Fig. 3H). In addition to elevated production of sFLT1 from hypoxic trophoblasts, HIF-2α is also involved in the reduced production of the trophoblastic PlGF (Fujii et al., 2017) and the formation of impaired cytotrophoblast syncytium (Colson et al., 2020) that contribute to the development and progression of pre-eclampsia. As HIF-1β is also presumed to be involved in these two HIF-2α-dependent adverse events, antagonists that inhibit the HIF-2α/HIF-1β heterodimerization may be used as novel therapeutic agents for pre-eclampsia. PT2385 and PT2977 (also known as Belzutifan) are orally-administrable HIF-2α-specific small molecule inhibitors used in clinical trials and recently reported to be effective in treating patients with von Hippel–Lindau tumor suppressor inactivated renal cell carcinoma that caused inappropriate stabilization of HIF-2α (Courtney et al., 2018; Choueiri et al., 2021). These inhibitors are also considered as candidate drugs for preeclamptic patients. However, small molecule drugs are generally known to be transported from the mother to the fetus via the placenta, which can cause fetal side effects (Gedeon and Koren, 2006). For this reason, further studies are required to determine the adverse effects of these HIF-2α inhibitors on fetation. Minimizing the fetal drug exposure is one of the major challenges in developing safer drugs for the treatment of several diseases during pregnancy, not just pre-eclampsia. To address this issue, Zhang et al. (2018) developed nanoparticles for targeted delivery to the placental trophoblasts using a synthetic chondroitin sulfate A-binding peptide derived from the malarial protein, VAR2CSA, and demonstrated that there was no accumulation of drugs filled in these nanoparticles in the fetal tissues when administered to pregnant mice. Thus, by combining this technology and the HIF-2α inhibitors, a novel molecular targeting therapy for the treatment of pre-eclampsia could be developed in the future.
In conclusion, our study suggests that the HIF-2α/HIF-1β heterodimer is a critical regulator involved in the hypoxia-induced upregulation of the FLT1 gene in placental trophoblasts. Therefore, our study provides a novel therapeutic approach for the treatment of pre-eclampsia by targeting the dissociation of the HIF2α/HIF-1β complex.
Supplementary data
Supplementary data are available at Molecular Human Reproduction online.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Authors’ roles
T.S. and M.S. were involved in experimental design. M.Y., T.N. and T.F. contributed to human trophoblast isolation. T.S. was responsible for acquisition of data. T.S. and M.S. analysed the data. All authors were involved in data interpretations. T.S. and M.S. wrote the manuscript. All authors read and approved the final manuscript.
Funding
This work was partly supported by Japan Society for the Promotion of Science KAKENHI [grant number JP20K09675] to T.S.
Conflict of interest
All authors state no conflict of interest for this research.
Supplementary Material
References
- Camenisch G, Stroka DM, Gassmann M, Wenger RH.. Attenuation of HIF-1 DNA-binding activity limits hypoxia-inducible endothelin-1 expression. Pflugers Arch Eur Arch 2001;443:240–249. [DOI] [PubMed] [Google Scholar]
- Choueiri TK, Bauer TM, Papadopoulos KP, Plimack ER, Merchan JR, McDermott DF, Dror Michaelson M, Appleman LJ, Thamake S, Perini RF. et al. Inhibition of hypoxia-inducible factor-2α in renal cell carcinoma with belzutifan: a phase 1 trial and biomarker analysis. Nat Med 2021;27:802–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colson A, Depoix CL, Baldin P, Hubinont C, Sonveaux P, Debiève F.. Hypoxia-inducible factor 2 alpha impairs human cytotrophoblast syncytialization: new insights into placental dysfunction and fetal growth restriction. FASEB J 2020;34:15222–15235. [DOI] [PubMed] [Google Scholar]
- Courtney KD, Infante JR, Lam ET, Figlin RA, Rini BI, Brugarolas J, Zojwalla NJ, Lowe AM, Wang K, Wallace EM. et al. Phase I dose-escalation trial of PT2385, a first-in-class hypoxia-inducible factor-2α antagonist in patients with previously treated advanced clear cell renal cell carcinoma. J Clin Oncol 2018;36:867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S, Kumar S, Hyett J, Salomon C.. Molecular targets of aspirin and prevention of preeclampsia and their potential association with circulating extracellular vesicles during pregnancy. Int J Mol Sci 2019;20:4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T, Nagamatsu T, Morita K, Schust DJ, Iriyama T, Komatsu A, Osuga Y, Fujii T.. Enhanced HIF2α expression during human trophoblast differentiation into syncytiotrophoblast suppresses transcription of placental growth factor. Sci Rep 2017;7:12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gedeon C, Koren G.. Designing pregnancy centered medications: drugs which do not cross the human placenta. Placenta 2006;27:861–868. [DOI] [PubMed] [Google Scholar]
- Gerber HP, Condorelli F, Park J, Ferrara N.. Differential transcriptional regulation of the two vascular endothelialgrowth factor receptor genes. Flt-1, but not Flk-1/KDR, is up-regulated by hypoxia. J Biol Chem 1997;272:23659–23667. [DOI] [PubMed] [Google Scholar]
- Gort EH, van Haaften G, Verlaan I, Groot AJ, Plasterk RHA, Shvarts A, Suijkerbuijk KPM, van Laar T, van der Wall E, Raman V. et al. The TWIST1 oncogene is a direct target of hypoxia-inducible factor-2α. Oncogene 2008;27:1501–1510. [DOI] [PubMed] [Google Scholar]
- Gude NM, Roberts CT, Kalionis B, King RG.. Growth and function of the normal human placenta. Thromb Res 2004;114:397–407. [DOI] [PubMed] [Google Scholar]
- Helske S, Vuorela P, Carpen O, Hornig C, Weich H, Halmesmäki E.. Expression of vascular endothelial growth factor receptors 1, 2 and 3 in placentas from normal and complicated pregnancies. Mol Hum Reprod 2001;7:205–210. [DOI] [PubMed] [Google Scholar]
- Heydarian M, McCaffrey T, Florea L, Yang Z, Ross MM, Zhou W, Maynard SE.. Novel splice variants of sFlt1 are upregulated in preeclampsia. Placenta 2009;30:250–255. [DOI] [PubMed] [Google Scholar]
- Hutter D, Kingdom J, Jaeggi E.. Causes and mechanisms of intrauterine hypoxia and its impact on the fetal cardiovascular system: a review. Int J Pediatr 2010;2010:401323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T, Wakiya K, Shibuya M.. Characterization of the promoter region for flt-1 tyrosine kinase gene, a receptor for vascular endothelial growth factor. Growth Factors 1996;13:151–162. [DOI] [PubMed] [Google Scholar]
- Jebbink J, Keijser R, Veenboer G, van der Post J, Ris-Stalpers C, Afink G.. Expression of placental FLT1 transcript variants relates to both gestational hypertensive disease and fetal growth. Hypertension 2011;58:70–76. [DOI] [PubMed] [Google Scholar]
- Kendall RL, Thomas KA.. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci USA 1993;90:10705–10709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga K, Osuga Y, Yoshino O, Hirota Y, Ruimeng X, Hirata T, Takeda S, Yano T, Tsutsumi O, Taketani Y.. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab 2003;88:2348–2351. [DOI] [PubMed] [Google Scholar]
- Krueger K, Catanese L, Sciesielski LK, Kirschner KM, Scholz H.. Deletion of an intronic HIF-2α binding site suppresses hypoxia-induced WT1 expression. Biochim Biophys Acta Gene Regul Mech 2019;1862:71–83. [DOI] [PubMed] [Google Scholar]
- Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH. et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 2004;350:672–683. [DOI] [PubMed] [Google Scholar]
- Li L, Schust DJ.. Isolation, purification and in vitro differentiation of cytotrophoblast cells from human term placenta. Reprod Biol Endocrinol 2015;13:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandl M, Depping R.. ARNT is a potential direct HIF-1 target gene in human Hep3B hepatocellular carcinoma cells. Cancer Cell Int 2017;17:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandl M, Depping R.. Hypoxia-inducible aryl hydrocarbon receptor nuclear translocator (ARNT) (HIF-1beta): is it a rare exception? Mol Med 2014;20:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandl M, Kapeller B, Lieber R, Macfelda K.. Hypoxia‐inducible factor‐1beta (HIF‐1beta) is upregulated in a HIF‐1alpha‐dependent manner in 518A2 human melanoma cells under hypoxic conditions. Biochem Biophys Res Commun 2013;434:166–172. [DOI] [PubMed] [Google Scholar]
- Mandl M, Lieberum MK, Dunst J, Depping R.. The expression level of the transcription factor aryl hydrocarbon receptor nuclear translocator (ARNT) determines cellular survival after radiation treatment. Radiat Oncol 2015;10:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE. et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 2003;111:649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevo O, Soleymanlou N, Wu Y, Xu J, Kingdom J, Many A, Zamudio S, Caniggia I.. Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am J Physiol Regul Integr Comp Physiol 2006;291:R1085–R1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle KG, Kind KL, Sferruzzi-Perri AN, Thompson JG, Roberts CT.. Beyond oxygen: complex regulation and activity of hypoxia inducible factors in pregnancy. Hum Reprod Update 2010;16:415–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakumar A, Cerdeira AS, Rana S, Zsengeller Z, Edmunds L, Jeyabalan A, Hubel CA, Stillman IE, Parikh SM, Karumanchi SA.. Transcriptionally active syncytial aggregates in the maternal circulation may contribute to circulating soluble fms-like tyrosine kinase 1 in preeclampsia. Hypertension 2012;59:256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakumar A, Powers RW, Hubel CA, Shibata E, von Versen-Höynck F, Plymire D, Jeyabalan A.. Novel soluble Flt-1 isoforms in plasma and cultured placental explants from normotensive pregnant and preeclamptic women. Pacenta 2009;30:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CM, Lefebvre F, Poon BP, Bousard A, Fan X, Lathrop M, Tost J, Kim WY, Riazalhosseini Y, Ohh M.. Consequences of VHL loss on global DNA methylome. Sci Rep 2018;8:3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasagawa T, Jinno-Oue A, Nagamatsu T, Morita K, Tsuruga T, Mori-Uchino M, Fujii T, Shibuya M.. Production of an anti-angiogenic factor sFLT1 is suppressed via promoter hypermethylation of FLT1 gene in choriocarcinoma cells. BMC Cancer 2020;20:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasagawa T, Nagamatsu T, Morita K, Mimura N, Iriyama T, Fujii T, Shibuya M.. HIF-2α, but not HIF-1α, mediates hypoxia-induced up-regulation of Flt-1 gene expression in placental trophoblasts. Sci Rep 2018;8:17375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schödel J, Oikonomopoulos S, Ragoussis J, Pugh CW, Ratcliffe PJ, Mole DR.. High-resolution genome-wide mapping of HIF-binding sites by ChIP-seq. Blood 2011;117:e207–e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M. Involvement of Flt-1 (VEGF receptor-1) in cancer and preeclampsia. Proc Jpn Acad Ser B Phys Biol Sci 2011;87:167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares MJ, Iqbal K, Kozai K.. Hypoxia and placental development. Birth Defects Res 2017;109:1309–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souders CA, Maynard SE, Yan J, Wang Y, Boatright NK, Sedan J, Balyozian D, Cheslock PS, Molrine DC, Simas TA.. Circulating levels of sFlt1 splice variants as predictive markers for the development of preeclampsia. Int J Mol Sci 2015;16:12436–12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Yamaguchi S, Sawano A, Shibuya M.. Characterization of the extracellular domain in vascular endothelial growth factor receptor-1 (Flt-1 tyrosine kinase). Jpn J Cancer Res 1997;88:867–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LP, Crimmins S, Telugu BP, Turan S.. Intrauterine hypoxia: clinical consequences and therapeutic perspectives. RRN 2015;5:79–89. [Google Scholar]
- Uzan J, Carbonnel M, Piconne O, Asmar R, Ayoubi JM.. Pre-eclampsia: pathophysiology, diagnosis, and management. Vasc Health Risk Manag 2011;7:467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger RH, Stiehl DP, Camenisch G.. Integration of oxygen signaling at the consensus HRE. Sci STKE 2005;2005:re12. [DOI] [PubMed] [Google Scholar]
- Whitehead CL, Palmer KR, Nilsson U, Gao Y, Saglam B, Lappas M, Tong S.. Placental expression of a novel primate-specific splice variant of sFlt-1 is upregulated in pregnancies complicated by severe early onset pre-eclampsia. BJOG 2011;118:1268–1271. [DOI] [PubMed] [Google Scholar]
- Wolff M, Jelkmann W, Dunst J, Depping R.. The aryl hydrocarbon receptor nuclear translocator (ARNT/HIF-1beta) is influenced by hypoxia and hypoxia-mimetics. Cell Physiol Biochem 2013;32:849–858. [DOI] [PubMed] [Google Scholar]
- Wu C, Yang T, Liu Y, Lu Y, Yang Y, Liu X, Liu X, Ye L, Sun Y, Wang X. et al. ARNT/HIF-1beta links high-risk 1q21 gain and microenvironmental hypoxia to drug resistance and poor prognosis in multiple myeloma. Cancer Med 2018;7:3899–3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Tan L, Yu Y, Wang B, Chen Z, Han J, Li M, Chen J, Xiao T, Ambati BK. et al. Placenta-specific drug delivery by trophoblast-targeted nanoparticles in mice. Theranostics 2018;8:2765–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Hanrahan C, van der Poel H, Simons JW.. Hypoxia-inducible factor 1alpha and 1beta proteins share common signaling pathways in human prostate cancer cells. Biochem Biophys Res Commun 2001;284:352–356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.