Abstract
Objectives
Irritability is a transdiagnostic symptom in developmental psychopathology, conceptualized as a low threshold for frustration and increased proneness to anger. While central to emotion regulation, there is a vital need for empirical studies to explore the relationship between irritability and underlying physiological mechanisms of cardiovascular arousal.
Methods
We examined the relationship between irritability and cardiovascular arousal (i.e., heart rate [HR] and heart rate variability [HRV]) in a transdiagnostic sample of 51 youth (M = 12.63 years, SD = 2.25; 62.7% male). Data was collected using the Empatica E4 during a laboratory stop‐signal task. In addition, the impact of motion activity, age, medication, and sleep on cardiovascular responses was explored.
Results
Main findings showed that irritability was associated with increased HR and decreased HRV during task performance.
Conclusions
Findings support the role of peripheral physiological dysregulation in youth with emotion regulation problems and suggest the potential use of available wearable consumer electronics as an objective measure of irritability and physiological arousal in a transdiagnostic sample of youth.
Keywords: arousal, child/adolescence, heart rate, heart rate variability, irritability, sleep
1. INTRODUCTION
Irritability is a multifaceted clinical construct conceptualized as a low threshold for frustration and proneness to anger. Irritability is an impairing symptom in various childhood psychopathologies and a risk factor for later depression, anxiety, and suicidality (Brotman, Kircanski, et al., 2017). The clinical presentation of irritability, as formulated in the related diagnosis of Disruptive Mood Dysregulation Disorder (DMDD), includes both a behavioral component of aggression/temper outbursts and a mood component of frustration, grouchiness, or annoyance (Avenevoli et al., 2015; Brotman, Kircanski, & Leibenluft, 2017; Cardinale et al., in press; Copeland et al., 2013; Moore et al., 2019; Silver et al., 2021).
While commonly measured using either clinician‐ or parent‐ and youth‐reports (Haller et al., 2020; Stringaris et al., 2012), there is a need for more ecologically valid, objective measures of irritability in situ (Murray‐Close et al., 2017; Smith et al., 2019). Ambulatory peripheral physiological assessments offer the possibility to provide naturalistic data on mood‐related symptoms, with the potential to evaluate the external validity of more stringently controlled laboratory findings (Wilhelm & Grossman, 2010).
Heart rate (HR) and heart rate variability (HRV) are two standard indices of peripheral physiological arousal mediated by the autonomic nervous system (Appelhans & Luecken, 2006; Kreibig, 2010). HR, the number of heartbeats per minute, typically increases under stress; HRV, variation in heartbeats within a specific timeframe, typically decreases under stress. Thus, HRV can be conceptualized as a measure of autonomic flexibility, with some researchers in the field suggesting it indexes an individual's ability to regulate emotional arousal in response to environmental demands (Beauchaine & Thayer, 2015).
Easily acquired cardiovascular measures have the potential facilitate unique insights into emotional regulation in youth (Deutz et al., 2019). Lower resting HR at baseline (e.g., Herpertz et al., 2001; Van Goozen et al., 2000) and increased HR during emotional arousal (e.g., Gatzke‐Kopp et al., 2015; Woltering et al., 2016) have been demonstrated to predict externalizing behaviors in youth who engage in aggressive, anti‐social, and conduct behaviors. Lower HRV at baseline (e.g., Koenig et al., 2016; Monk et al., 2001), large reductions in HRV in response to stressors (e.g., Monk et al., 2001; Rozenman et al., 2017), and lower HRV during post‐stress recovery (e.g., Rozenman et al., 2017) are associated with both internalizing and externalizing psychopathology in youth such as anxiety, depression, or anti‐social behaviors. Moreover, a recent study demonstrated that increased HR during rest is associated with dysregulation rather than uniquely associated with either externalizing or internalizing symptomology (Deutz et al., 2019) and a review by Pinna and Edwards (2020) reported that increased HRV was associated with effective emotional regulation strategies and better acceptance of emotions across multiple cognitive tasks in adult participants.
Cardiovascular arousal has been primarily studied in anger/aggression in the context of samples with dysregulation problems (Bimmel et al., 2008; Fairchild et al., 2008; Fanti, 2019; Popma et al., 2006; Sijtsema et al., 2013; Tonacci et al., 2019; Vidal‐Ribas et al., 2016; Williams et al., 2003). Both constructs of aggression and dysregulation relate to irritability (Appelhans & Luecken, 2006; Brotman, Kircanski, & Leibenluft, 2017; Gatzke‐Kopp et al., 2015; Grabell et al., 2018; Murray‐Close et al., 2017); however, irritability is a complex transdiagnostic symptom in developmental psychopathology that spans a continuum of severity (Vidal‐Ribas et al., 2016), and its association with physiological arousal has yet to be explored. Only a few studies have examined psychophysiological arousal in youth with irritability, and results are mixed. Two studies (Leno et al., 2020; Mikita et al., 2015) demonstrate diminished cardiovascular reactivity patterns in response to emotional arousal in irritable youth with high‐functioning autism. Other work has found no cardiovascular differences between irritable and other subtypes of youth with mild and severe attention‐deficit/hyperactivity disorder (ADHD; Karalunas et al., 2014). Notably, these are the only studies we are aware of that examine cardiovascular arousal in irritable youth; however, irritability was not an explicit target in those studies, and cardiovascular arousal in youth whose primary clinical concern is chronic irritability has yet to be examined.
Taken together, early evidence suggests that increased irritability may be associated with disrupted physiological arousal patterns; a relationship that is still poorly understood. Exploring cardiovascular activity as a potential mechanism for irritability may have salient mechanistic and treatment implications (Brotman, Kircanski, et al., 2017; Phillips et al., 2008). To the best of our knowledge, there are no previous studies investigating this association in the context of irritability as a transdiagnostic symptom.
In the present study, we explore the link between irritability and cardiovascular arousal (i.e., HR and HRV) during an in‐lab standardized stop‐signal task (SST; Verbruggen & Logan, 2008) in a transdiagnostic sample of youth. The SST measures inhibitory control, which has been found to be deficit in youth with irritability (e.g., Fishburn et al., 2019; Liuzzi et al., 2020), particularly in the context of frustration (Deveney et al., 2013). SST adapts to individual performance to maintain an adequate level of difficultly and has also been used in other paradigms to elicit frustration (Deveney et al., 2013; Tseng et al., 2019). Thus, we anticipated SST will elicit physiological arousal and frustration and that performance on this task would be affected by this, particularly in irritable youth who are characterized by over reactivity to frustration (Brotman, Kircanski, et al., 2017).
Based on prior work (Deutz et al., 2019; Mikita et al., 2015; Pinna & Edwards, 2020), we hypothesized that higher levels of irritability in youth would be associated with blunted HRV responses and greater HR during the SST compared to a resting baseline condition. Our secondary aim was to investigate potential associations among irritability, task performance of inhibitory control, sleep, and cardiovascular arousal, as well as links between disrupted sleep (e.g., less sleep time and more awakening during the night) and emotion dysregulation across different clinical diagnoses (e.g., Delaplace et al., 2018; Faedda et al., 2016; Huỳnh et al., 2016; Tobaldini, Cogliati, et al., 2013; Tobaldini, Nobili, et al., 2013; Tobaldini et al., 2014). Previous research indicates a bidirectional relationship between sleep and cardiovascular regulation. Disrupted sleep can alter the cardiovascular system, leading to increased cardiovascular risk, while aberrant cardiovascular function is often characterized by altered sleep. Furthermore, associations between sleep deprivation and increased cardiovascular arousal during stressors have been reported (Zhong et al., 2005). Based on this existing data, we anticipated less sleep time and worse task performance to be associated with increased cardiovascular arousal during SST. In an exploratory manner, we tested the potential effect of irritability on these associations.
2. METHODS
2.1. Participants
Table 1 presents participant demographic, clinical, medication, and sleep information. Fifty‐four youth were recruited from the community to participate in research at the National Institute of Mental Health (NIMH). Participants were informed that they could stop the task or withdraw from the study at any time and received monetary compensation for their participation. Three participants dropped out of the study before completion and were excluded from the final analyses.
TABLE 1.
Demographic and clinical characteristics
| Characteristic (N = 51) | |
|---|---|
| Sex, % female | 37.3% |
| Age (years) | |
| Mean (SD) | 12.63 (2.25) |
| Range | 8.12–17.76 |
| Race, N (%) | |
| White | 33 (64.74) |
| Multiracial | 10 (19.59) |
| Black or African American | 6 (11.81) |
| Unknown | 2 (3.90) |
| Ethnicity, N (%) | |
| Not Hispanic or Latino | 41 (80.42) |
| Hispanic or Latino | 6 (11.83) |
| Unknown | 4 (7.83) |
| Youth‐reported ARI | |
| Mean (SD) | 3.49 (3.02) |
| Range | 0.00–11. 00 |
| Parent‐reported ARI | |
| Mean (SD) | 5.02 (3.90) |
| Range | 0.00–12.00 |
| Medication, N (%) | |
| None | 35 (68.6) |
| Stimulants | 15 (29.4) |
| SSRI (anti‐depressant) | 5 (9.80) |
| SGA (anti‐psychotic) | 2 (3.92) |
| No information | 1 (1.96) |
| Primary diagnosis, N (%) | |
| ADHD | 16 (31.37) |
| DMDD | 16 (31.37) |
| Anxiety disorder | 10 (19.61) |
| No diagnosis | 9 (17.65) |
| Stop‐signal reaction time, mean (SD) | 290.18 (69.26) |
| Sleep duration, mean (SD) | 8.41 (1.74) |
| HR, experimental blocks, mean (range) | 77.46 (51.80–107.54) |
| RMSSD, experimental blocks, mean (range) | 165.49 (18.35–475.80) |
Note: Anxiety disorder includes generalized anxiety disorder, separation anxiety disorder, social anxiety disorder, and specific phobia. Experimental blocks represent the five blocks included in the SST. Primary diagnosis refers to the primary diagnosis for which participants were recruited for research purposes. Participants could have comorbid/multiple diagnoses and could use multiple medications.
Abbreviations: ADHD, attention‐deficit/hyperactivity disorder; ARI, affective reactivity index; DMDD, disruptive mood dysregulation disorder; HR, heart rate; RMSSD, root mean square of the successive difference; SGA, second‐generation antipsychotic; SSRI, selective serotonin reuptake inhibitor; SST, stop signal task.
The final sample consisted of 51 youth, aged 8 to 18 years (M = 12.63 years, SD = 2.25; 62.7% males), with primary diagnoses of DMDD (N = 16), ADHD (N = 16), anxiety disorders (generalized, social, or separation anxiety disorder; N = 10), or no diagnosis (N = 9). Diagnoses were established prior to participation using the Kiddie Schedule for Affective Disorders and Schizophrenia (K‐SADS‐PL; Kaufman et al., 1997), including an additional extended DMDD module (Wiggins et al., 2016).
Participants were recruited through the NIMH Emotion and Development Branch for characterization and intervention research, through online postings, mailed postcards, and community talks. All caregivers and youth provided consent and assent to the study protocol, respectively, in accordance with the NIMH Institutional Review Board procedures. The initial evaluation visit included a diagnostic interview conducted by a doctoral‐ or master's‐level clinician using the Schedule for Affective Disorders and Schizophrenia for School‐Age Children‐Present and Lifetime version (K‐SADS‐PL; Kaufman et al., 1997) to evaluate primary diagnosis and to determine eligibility for the study protocol. Exclusionary criteria included: cardinal bipolar disorder symptoms, diagnosis of schizophrenia, schizoaffective disorders, posttraumatic stress disorder, substance abuse within the last 3 months, and an intelligence quotient below 70, as assessed by the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999).
2.2. Materials
2.2.1. The stop‐signal task
The SST has been widely used to assess response inhibition in youth (Alderson et al., 2007; Lipszyc & Schachar, 2010; Verbruggen & Logan, 2008). In the current study, participants completed five experimental blocks of the task for a total of 440 trials. The task includes simple “go” trials as well as “stop‐signal” trials. In the simple “go” trials, participants are presented with a standard two‐choice reaction time task (i.e., the participant is presented with an “X” or “O”) and instructed to press the right arrow key if an X appears on the computer screen and the left arrow key if an O appears. A stop‐signal (1000 Hz auditory tone) randomly occurs in 25% of the total trials (110 trials across the five experimental blocks). Participants are instructed not to press an arrow key in response to an X or O if the auditory stop‐signal occurs. The onset of the stop‐signal is presented after a 250 ms delay relative to the presentation of the go‐stimuli (X or O).
Throughout the SST task, the stop‐signal delay was adjusted based on performance to titrate accuracy on the inhibition trials as close to 50% as possible across all participants. This was accomplished by increasing or decreasing the stop‐signal delay by 50 ms following a correct or incorrect inhibition trial, respectively.
Prior to completing the task, participants completed two practice blocks. First, participants learned the go‐response by responding only to go‐trials. During this practice block, participants received feedback indicating if their response was correct or not to ensure that they learned the simple go‐response options. Next, participants completed an abridged version of the experimental task during which they had the opportunity to hear the auditory stop‐cue and learn about the stop‐trials. Performance on the task was measured using the stop‐signal reaction time (SSRT). SSRT is a measure of motor inhibition calculated as mean stop‐signal delay time minus mean reaction time to the go trials. Higher values represent worse task performance and reflect less efficient inhibitory motor control. After each of the five experimental blocks, participants rated their subjective level of frustration on a 9‐point Likert scale (1 = “not at all”; 9 = “extremely”).
2.2.2. Self‐report and clinical measures
2.2.2.1. Affective reactivity index
The affective reactivity index (ARI) is a 7‐item parallel parent‐report and youth‐report questionnaire that probes for irritable mood and outbursts over the past 6 months (Stringaris et al., 2012). The first six items are related to irritable mood and behaviors, with a total score that ranges between 0 and 12. Higher scores indicating higher levels of irritability. The last item assesses levels of impairment due to irritability. In the present study, both parents and their youth completed the ARI questionnaire within three months of completing the SST. In the current study, parent and youth assessments were significantly correlated (r (51) = 0.57, p < 0.001).
2.2.2.2. Medication and sleep form
Medication usage and sleep report were used to collect information that could potentially impact cardiovascular response. Specifically, youth reported the number of hours they slept the night before completing the SST. In addition, medications taken by participants in the past 24 h and the time of day they were taken were reported by youth and their parents.
2.2.2.3. Task experience of frustration
As described above, participants rated their subjective level of frustration on a nine‐point Likert scale after each experimental block of the SST.
2.2.3. Cardiovascular recording
Cardiovascular arousal and motion activity were collected wirelessly during the SST using the E4 by Empatica, Inc. The E4 uses photoplethysmography (PPG; Allen, 2007) to record blood volume pulse (BVP) data at 64 Hz, from which HR and HRV can be derived. Although this sampling rate is relatively low (Berntson et al., 1997), recent studies comparing waveform features acquired from PPG measurement across varying sampling rates indicate that 64 Hz is comparable to higher sampling rates and can measure cardiac measures of HR and HRV accurately (Fujita & Suzuki, 2019).
E4 device firmware includes motion artifact removal and beat detection algorithms that automatically discard non‐prototypical beats without smoothing inter‐beat interval sequences. The PPG sensing technology in the E4 has been validated against gold‐standard laboratory‐based and clinical equipment in typically developing individuals (McCarthy et al., 2016; van Lier et al., 2019). The E4 records motion‐based activity up to ±8 g at 32 Hz using three‐axis accelerometry and has an event marking button that places a time‐stamped annotation in the data collection output file when pressed.
2.3. Procedure
Eligible participants were invited on‐site to the National Institutes of Health in Bethesda, Maryland. At the beginning of the visit, participants completed self‐report clinical questionnaires. In preparation for the SST task, an experimenter introduced participants to the E4, placed it on the wrist of their non‐dominant hand, and initiated a practice run of the task. As mentioned above, participants were instructed that they would be presented with an X or an O and to press the right arrow key if an X appeared on the computer screen and the left arrow key if an O appeared. Additionally, if they heard a beep, they were instructed not to press any buttons. E4 data were recorded continuously throughout the whole assessment period, with timing tags set up to measure a 3‐min resting phase (i.e., baseline), five experimental blocks, and a 3‐min resting phase 10 min after completing the task to capture recovery rates (i.e., follow‐up). Participants were prompted to press the event marking button on the E4 at the start and end of each phase. Experimenters were present at the time of data acquisition to monitor and validate these event markings. During the two resting phase intervals, participants were instructed to sit quietly and avoid moving. Data were then downloaded, pre‐processed, and plotted for signal quality evaluation before analysis.
2.4. Data analysis
We pre‐processed our cardiovascular data at the individual level using Kubios HRV 3.3.1 premium software, a common commercial package (Tarvainen et al., 2014), which has been applied in previous studies during dynamic tasks (e.g., Weippert et al., 2013). The E4 BVP trace was used to compute HR and HRV. Kubios HRV Premium software includes an adaptive QRS detection algorithm, pulse wave detectors, and tools for automatic and threshold‐based artifact correction and trend removal (Tarvainen et al., 2014). We used the automatic correction method offered in Kubios (Lipponen & Tarvainen, 2019) to separate ectopic (i.e. skipped or extra heartbeats) or outliers from typical beats by comparing current RR values with the median of the surrounding 10 RR interval values. This algorithm uses a correction default of 5% to avoid significant distortions or suppression of variability in statistical analyses. For each beat, the 90 surrounding beats' quartile deviation was calculated and multiplied by a factor of 5.2. Missed or extra beats were detected by comparing the current RR value with the median of the surrounding 10 RR interval values (Lipponen & Tarvainen, 2019). Ectopic beats were corrected by replacing corrupted RR times with interpolated RR values, using a piecewise cubic spline interpolation method. Beats that were too long or too short were corrected by interpolating new values to the RR time series. Missed beats were corrected by adding a new R‐wave occurrence time. Extra beats were corrected by removing erroneously detected R‐waves and recalculating the RR interval series. A total beat correction ratio for the percentage of ectopic, extreme, missed, or extra corrected beats were generated by the software for each participant. Overall, the mean of the beat correction ratios in the current sample was 7.77% (SD = 4.45, range 0%–24.8%). There were no differences in correction ratios across the different time points (χ 2 = 56, p = 0.233) or across diagnostic groups at any of the time points (ps > 0.070).
We explored potential outliers exceeding 3 SD from the mean HR/HRV scores at the individual level (Barnett & Lewis, 1994). No outliers were observed. After pre‐processing the cardiovascular data, we ran multilevel mixed‐effects regression with robust standard errors to analyze within‐subject time‐series data using Hierarchical Linear Modeling (HLM software, version 8.0, Raudenbush et al., 2019). Primary analyses examined associations between parent/youth‐reported irritability and trajectories of cardiovascular response throughout the task using HR and Root Mean Square of the Successive Difference (RMSSD; a time‐domain index used to assess HRV, the successive difference between neighboring heartbeats) as dependent variables. RMSSD was selected as it exhibits high reliability among youth (Weiner & McGrath, 2017) and high accuracy capturing cardiovascular reactivity during short periods of recording time (Salahuddin et al., 2007). HR measures were computed in beats per minute. Motion activity and age were included as covariates.
To test these associations, primary HLM included HR/RMSSD changes as outcome measures, Level‐1 included within‐subject repeated data (i.e., time, motion activity), and Level‐2 included between‐subject variables (ARI scores and age). Level‐1 continuous variables were person‐centered, and Level‐2 continuous predictors were grand‐mean centered before analysis to enable meaningful interpretation of the conditional main effects (Kraemer & Blasey, 2004). Outcome variables were specified as continuous. All models included a random intercept. We used the Maximum Likelihood estimation procedure and considered a p‐value < 0.05 to be statistically significant. Following HLM, we ran generalize linear model repeated measures models with post hoc tests to evaluate differences among specific means driving observed effects.
Additional similar series of HLM analyses were performed to investigate our secondary questions, examining associations between: (1) behavioral task performance of inhibitory control (i.e., SSRT), ARI, and the interaction between these variables in predicting cardiac changes in HR/HRV; (2) sleep duration, ARI, and the interaction between these variables in predicting cardiac changes in HR/HRV; and (3) task experience of frustration, ARI, and the interaction between these variables in predicting cardiac changes in HR/HRV.
Analyses were performed separately for parent‐ and youth‐report measures. Given a reported cross‐informant discrepancy in youth with behavioral and emotional problems (Achenbach et al., 1987), as well as with irritability (Vidal‐Ribas et al., 2016), separating analyses across informants allowed us to explore whether this factor should be considered when linking irritability and physiological arousal. Models with HR and HRV were conducted independently.
Finally, we ran regression models to test associations between irritability and HR/HRV levels at different time points (baseline, during the task, post‐task, and at follow‐up) and between irritability and change in HR/HRV from post‐task to follow‐up to assess whether irritability predicts recovery rate and return to baseline after the task. Change scores for HR were computed by subtracting HR levels at follow‐up from HR levels at the final experimental block (i.e., larger difference represents a greater change between these time points). Positive values represent higher HR levels after the final experimental block relative to follow‐up. Change scores for HRV were computed by subtracting HRV levels at the final experimental block from HRV levels at follow‐up (i.e., larger difference represents a greater change between these time points). Positive values represent higher HRV levels at follow‐up relative to the final experimental block.
3. RESULTS
3.1. Cardiac changes over the course of the task
Bivariate Pearson correlation testing between HR and HRV yielded a strong negative association between the two indices (r = −0.78, p < 0.001). The main effects of time testing changes in cardiac measures during the task showed that both HR (β = 0.96, t (50) = 1.60, SE = 0.16, p = 0.01) and HRV (β = −4.86, t (50) = −2.58, SE = 1.88, p = 0.01) changed significantly during the course of the task (see Figure 1). Post hoc tests revealed that the observed time effects associated with increased HR (observed delta range: 4.01–5.34, SE range: 1.38–1.69) and decreased HRV (observed delta range: 30.6–101.59, SE range: 16.64–21.12) during the task compared to baseline‐ and to follow‐up‐ resting phase measurements (all ps < 0.010).
FIGURE 1.
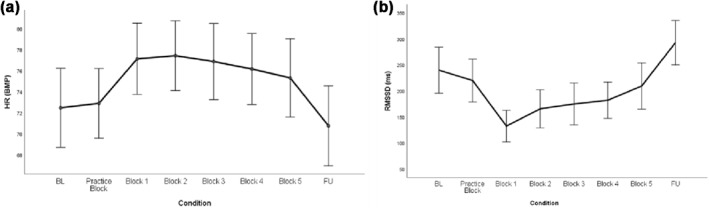
Changes in cardiac measures during SST; (a) HR levels across time and (b) RMSSD levels across time. BL, baseline resting phase; Blocks 1–5, experimental blocks of the SST; FU, follow‐up; HR, heart rate; RMSSD, root mean square of successive difference between normal heartbeats; SST, stop signal task
3.2. Cardiac reactivity as a function of irritability levels
Adjusting for motion activity and age, the time‐by‐ARI interaction was a significant predictor of HR. Specifically, parent‐reported irritability, as measured by ARI (β = 1.08, t (48) = 2.99, SE = 0.36, p = 0.004), was significantly associated with HR, such that higher reported irritability predicted greater increased HR over time. No significant findings for youth‐reported irritability were found.
Parallel findings were observed for HRV. Adjusting for motion activity and age, the time‐by‐ARI interaction was a significant predictor of HRV. Specifically, parent‐reported irritability, as measured by ARI (β = −8.46, t (48) = −2.54, SE = 3.33, p = 0.014), was significantly associated with HRV, such that higher reported irritability predicted a larger decrease of HRV over time. No significant findings were found for youth‐reported irritability.
Regression models were conducted to further explore the relationship between parent‐rated irritability and HR/HRV levels across the different time points (baseline, during the task, post‐task, and follow‐up). Adjusting for age and motion activity, results showed that higher levels of parent‐rated irritability predicted elevated HR at baseline (r = 0.28, b = 0.98, t (49) = 2.03, p = 0.048). Notably, parent‐rated irritability predicted elevated HR at the beginning of the task (r = 0.29, b = 0.98, t (49) = 2.12, p = 0.04), post‐task (r = 0.29, b = 1.25, t (49) = 2.12, p = 0.04) and at follow‐up (i.e., recovery phase) (r = 0.39, b = 1.38, t (49) = 2.88, p = 0.006) (see Figure 2). Additionally, irritability was associated with less positive change from post‐task to follow‐up (r = −0.32, b = −0.98, t (49) = −2.26, p = 0.029) (see Figure 3). This negative association between irritability and post‐task to follow‐up changes in HR suggests lower rates of recovery for youth with higher irritability. No significant associations for youth‐reported irritability were observed.
FIGURE 2.
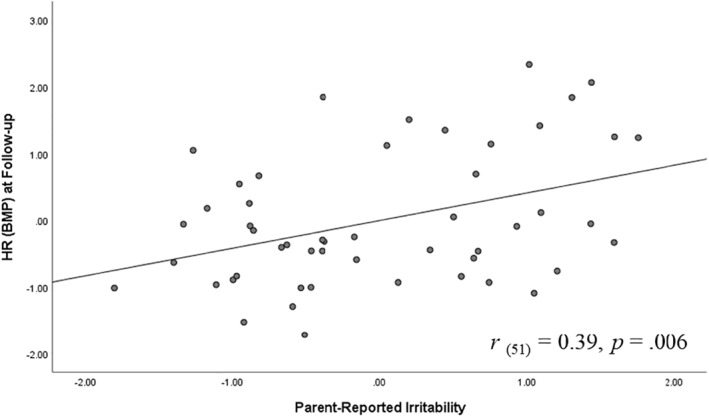
Association between parent‐reported irritability and heart rate (HR) during recovery phase. Graph reflects model in which variables were standardized
FIGURE 3.
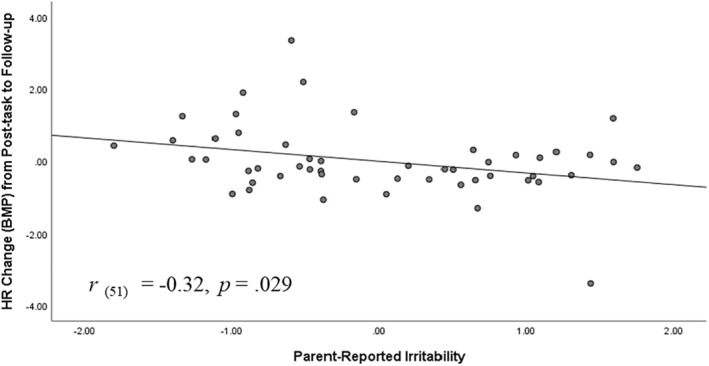
Heart rate (HR) change from post‐task to follow‐up as a function of parent‐reported irritability. Graph reflects model in which variables were standardized
Models for HRV revealed a similar pattern of results, wherein higher levels of parent‐rated irritability associated with lower RMSSD at follow‐up (i.e., recovery phase) (r = −0.39, b = −15.14, t (49) = −2.91, p = 0.005) and lower recovery from post‐task to follow‐up (r = −0.35, b = −14.93, t (49) = −2.55, p = 0.014) (see Figures 4 and 5).
FIGURE 4.
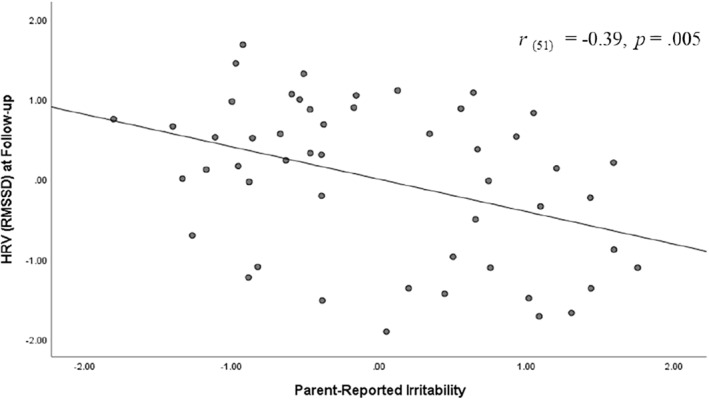
Association between parent‐reported irritability and heart rate variability (HRV)–root mean square of the successive difference (RMSSD) during recovery phase. Graph reflects model in which variables were standardized
FIGURE 5.
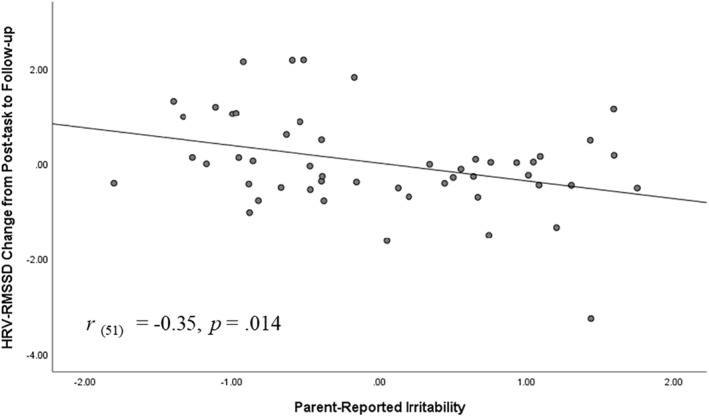
Heart rate variability (HRV)–root mean square of the successive difference (RMSSD) change from post‐task to follow‐up as a function of parent‐reported irritability. Graph reflects model in which variables were standardized
Finally, changes in HR during the task were marginally predicted by experienced frustration during the task (β = 0.51, t (50) = 1.92, SE = 0.27, p = 0.057), suggesting that elevated frustration was associated with elevated HR during the task. However, this association was not moderated by levels of irritability (ps > 0.478). No association was found between frustration ratings during the task and changes in HRV.
3.3. Behavioral performance during the task
Our mixed model analysis showed a main effect of SSRT scores on both HR (β = −0.10, t (45) = −4.10, SE = 0.02, p < 0.001) and HRV (β = 0.66, t (45) = 3.43, SE = 0.19, p = 0.001). These effects suggest that better performance on the task, as indicated by lower SSRT scores, associate with higher levels of cardiovascular arousal (i.e., higher HR and lower HRV). No interaction effects were found with irritability for this model. Additionally, SSRT was not correlated with irritability in the current study (rs < 0.177, ps > 0.219), suggesting that irritability did not mediate the relationship between cardiovascular arousal and task performance.
3.4. Sleep measure
Our model showed that longer sleep duration the night before was a significant predictor of lower HR (β = −2.65, t (45) = − 2.89, SE = 0.92, p = 0.006) and higher HRV (β = 30.62, t (45) = 3.99, SE = 7.68, p < 0.001) throughout the task. More importantly, higher‐order interaction effects for sleep duration by parent‐reported irritability were found for both HR (β = −0.46, t (45) = −2.11, SE = 0.22, p = 0.040) and HRV (β = 3.30, t (45) = 1.96, SE = 1.69, p = 0.050). No other significant associations were found for sleep.
To further explore these interaction effects, we used the median split method to divide the sample into two sub‐groups of high and low irritability, based on parent‐reported ARI scores (Mdn = 4.00), and modeled the effect of sleep duration within each of the sub‐groups. Results suggest that the negative association between sleep duration and HR was stronger in high levels of irritability (β = −3.04, t (23) = −1.74, SE = 1.74, p = 0.078) compared to low levels (β = −0.99, t (22) = −1.02, SE = 0.98, p = 0.320). The positive association between sleep duration and HRV was also stronger for youth with high levels of irritability (β = 37.2, t (23) = 2.36, SE = 15.82, p = 0.027) compared to low levels of irritability (β = 17.2, t (22) = 2.42, SE = 7.09, p = 0.031), as reported by the parent.
3.5. Covariates
Age was negatively and significantly correlated with irritability, as observed by the ARI measures (Youth‐reported ARI: r (51) = −0.44, p = 0.001; Parent reported‐ARI: r (51) = −0.32, p = 0.022). Therefore, models were analyzed with age as a covariate to explore potential mediation effects and rule out the possible confounding effect of age on associations between irritability and HR/HRV change. All findings survived this inclusion. Age did not emerge as a significant predictor for either HR or HRV change (ps ≥ 0.050).
Motion activity, as measured within‐individuals across the different time points, did not significantly correlate with ARI scores (rs ≤ 0.13, ps ≥ 0.360). All findings survived after including within‐individual motion activity as a covariate.
As shown in Table 1, participants in the current study differed in psychotropic medication exposure. There is some indication in the child psychiatry literature that medication usage may potentially impact physiological measures, specifically elevated HR levels at rest (for review, see Hennissen et al., 2017). Therefore, post hoc analyses were conducted adding medication usage as a categorical covariate to the model (uncentred) using dummy‐coding (0 = participant not medicated; 1 = participant medicated). All findings survived this inclusion.
4. DISCUSSION
We found that HR increased during the SST task and HRV decreased, compared to baseline and follow‐up, across the entire sample. Specifically, we found cardiovascular reactivity to be associated with higher reported irritability levels, an association that remained significant after adjusting for potential confounds such as motion activity and age. These findings speak to the putative benefit of HR and HRV as peripheral physiological biomarkers of irritability and points to the potential effectiveness of the SST in inducing physiological arousal and evoking regulation demands.
Relatedly, a trend was found between elevated frustration as experienced by the participants during the task, and increased HR. Frustration is a construct associated with irritability, as irritable symptoms are often triggered by increasing task difficulty or blocked goals (Brotman, Kircanski, et al., 2017; Kircanski et al., 2019). Taken together, our findings support the role of peripheral dysregulation in youth with emotion regulation problems. Parent‐reported irritability predicted the magnitude of task‐evoked cardiac reactivity. Though peripheral reactivity during stress or frustration‐provoking tasks might be expected and adaptive to some extent, the current findings support accumulating evidence that enhanced and prolonged reactivity might be maladaptive and associated with lower emotional regulation and increased symptoms severity (Gatzke‐Kopp et al., 2015; Murray‐Close et al., 2017), as reflected by increased levels of irritability in the current study. Increased irritability was also predictive of increased cardiovascular reactivity at follow‐up and with minor change in peripheral activity from task to follow‐up. Collectively, these findings suggest lower levels of autonomic recovery for youth reported to be highly irritable and align with previous studies demonstrating lower post‐stress recovery in youth with internalizing and externalizing psychopathology (e.g., Kircanski et al., 2016; Mikita et al., 2015; Rozenman et al., 2017). Future studies aiming to replicate this study in other samples or contexts, and those that intervene (i.e., try to change physiological reactivity and assess the effect on irritability), are needed to evaluate the robustness of the current findings that cardiovascular reactivity is a potential mechanism for, or peripheral biomarker of, irritability.
Our findings are in line with previous research suggesting that cardiovascular responding may serve as a potential physiological mechanism associated with negative emotional arousal states and related clinical phenotypes. An accumulative body of research demonstrates the role cardiovascular activity plays in regulation processes and adaptation to changing circumstances (Thayer et al., 2009; Thayer & Lane, 2000). Numerous studies observe maladaptive cardiovascular activity in dysregulated youth across diagnoses such as ADHD (Musser et al., 2011; Rash & Aguirre‐Camacho, 2012), conduct problems (Garralda et al., 1991; Lorber, 2004; Van Goozen et al., 2000, 2007), and anxiety and depression (Kircanski et al., 2016) compared to healthy controls. Findings show aberrant cardiovascular reactivity and recovery during stress inducing task or provocation in emotional disorders. However, to our knowledge, no previous studies have examined cardiac reactivity in the context of irritability, a construct which is strongly and by definition concomitant with dysregulation and over‐reactivity (Avenevoli et al., 2015). Some previous studies focus on related constructs such as aggression, mainly in the context of conduct behaviors such as in anti‐social disorder, and find predictive value in HR pre‐ and post‐stress inducing task or provocation (Bimmel et al., 2008; Fairchild et al., 2008; Popma et al., 2006; Sijtsema et al., 2013; Williams et al., 2003). Other studies shows that cardiovascular responses to psychosocial stress are reduced in youth diagnosed with conduct or anti‐social disorders compared to control subjects (e.g., Fairchild et al., 2008). This is the gap we sought to address in the current study.
While our primary aim was to examine associations between multiple cardiac measures and irritability, we also explored the potential role of sleep as a moderator, as disrupted sleep has been linked to emotional over‐reactivity and autonomic dysregulation (e.g., Delaplace et al., 2018). As expected, more hours of sleep the night before predicted less cardiovascular reactivity during tasking. Interestingly, this effect of sleep on decreased physiological arousal was more pronounced in youth with higher irritability levels than youth with lower irritability levels. Based on existing findings linking poor sleep to autonomic dysregulation in youth with irritability (e.g., Delaplace et al., 2018), the current results putatively suggest that improved sleep hygiene, specifically sleep duration, might inoculate against the cardiovascular over‐reactivity we observed in more irritable youth.
Inconsistent with previous studies, we found no association between irritability and task performance. The SST used in the present study is a canonical assessment of inhibitory control. Inhibitory control reflects the ability to modify prepotent responses (Buzzell et al., 2017; Cardinale et al., 2019; Nigg, 2017) and is perturbed in youth with irritability‐related diagnoses (Fishburn et al., 2019; Lipszyc & Schachar, 2010). Previous studies indicate that irritability is associated with reduced performance on inhibitory control tasks, with corresponding neural dysfunction in prefrontal regions (Fishburn et al., 2019; Perlman et al., 2015). However, in the current study, youth with higher cardiovascular reactivity and reported irritability did not exhibit diminished inhibitory control. Notably, SSRT served as a predictor of cardiovascular arousal, wherein better task performance was associated with higher HR and lower HRV levels. One possible interpretation of this potentially counterintuitive finding could be explained by the well‐established inverted U‐shaped relationship between emotional arousal and performance (Yerkes & Dodson, 1908), whereby task performance is improved when levels of emotional arousal are elevated but not extreme. We speculate that performance on the SST might be similarly related to physiological arousal. It may be that the SST, although including elements of increased difficulty that might elicit frustration, still evokes emotional arousal only to a certain degree, but not in an extreme way. Thus, performance improves without reaching the point of the U‐shape carve in which arousal is too highly elevated, leading to a decrease in performance. We propose that SST in the current study is only capturing the first half of the inverted U‐shaped curve. This is a putative mechanism that could not be tested more directly given the structure of the current study. Future studies could explore our hypothesis by using other tasks perceived to be more provocative or emotionally arousing. This would allow comparison to the current results and elucidate our potential interpretation regarding the way physiological arousal might affect performance.
While our results are informative, it is important to note several limitations. First, whereas our findings on parent‐reported irritability were significant and consistent, youth‐reported irritability was not statistically significant across analyses. This pattern is in line with well‐known informant discrepancies in developmental psychopathology (De Los Reyes & Kazdin, 2005), particularly in youth with externalizing problems (Salbach‐Andrae et al., 2009). Specifically, studies have shown moderate parent and child agreement for the ARI measurement (Pan & Yeh, 2019; Stoddard et al., 2014; Stringaris et al., 2012), suggesting youth may underreport levels of symptoms relative to parents. Indeed, in the current sample, youth‐reported irritability scores were lower than parent‐reported scores across the different ARI measures (see Table 1), which might serve as a potential explanation of the null findings associated with youth‐reported measures.
Second, these findings are preliminary. Extant research does not indicate robust or consistent positive associations between cardiac and other physical measures among youth without distinguished physical problems (e.g., obesity; Martins et al., 2002). While research suggests RMSSD is less susceptible to such effects (e.g., Penttilä et al., 2001; Quintana et al., 2016), the autonomic measures we used may have been influenced by blood pressure status, respiration rate, body mass index, and overall physical health, as well as by individual differences at the time of assessment. Although assessment times were comparable throughout the sample (generally during early afternoon hours), we did not have detailed records to formally rule out the assessment time's influence and other physical parameters on our findings.
Third, future research should include other and larger diagnostic groups such as depression or oppositional defiant disorder to further explore the transdiagnostic aspect of cardiac reactivity in irritability. Similarly, future studies could include more objective measures of sleep (e.g., actigraphy) as the current study relied on self‐reported sleep and it has been suggested that individuals cannot accurately report how much they sleep on a single night (e.g., Lauderdale et al., 2009). Finally, 31.4% of the sample was medicated at the time of the study. While post hoc analyses did not indicate differences in HR or HRV by medication status, future studies should include a larger sample of nonmedicated youth to evaluate this possible interaction effect more sensitively.
In sum, this study demonstrates higher HR and lower HRV as a function of irritability during a standard cognitive inhibition task in a transdiagnostic sample of youth. These findings indicate that youth with high irritability may have greater sensitivity to environmental demands and maladaptive physiological reactions in the context of a frustration‐inducing cognitive task. Additionally, sleep duration might play a putative protective role in regulating physiological arousal, especially for youth with high levels of irritability. The present study is the first we are aware of to examine potential psychophysiological mechanisms of physiological arousal and its effects on task performance in a sample including highly irritable youth. These effects are of theoretical and clinical interest, potentially aiding our understanding of the psychophysiological mechanisms involved in irritability and how to address them.
At a broader level, our findings provide a preliminary foundation for potential future applications exploring in situ use of commercially available wearable consumer electronics as an objective measure of irritability in transdiagnostic youth. Future studies examining the usage of wearable devices during other emotional states would advance understanding of their ability to also differentiate between irritability and other clinical constructs and phenotypes.
CONFLICT OF INTEREST
Matthew S. Goodwin is a Scientific Advisory Board member at Empatica Inc and a Scientific Consultant for the National Institute of Mental Health . All other authors declare that they have no conflicts of interest regarding this manuscript.
AUTHOR CONTRIBUTIONS
Melissa A. Brotman is the principal investigator for this project. Melissa A. Brotman and Reut Naim conceptualized the initial study design. Reut Naim wrote the manuscript. All authors contributed to writing and revising the manuscript. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
The study described in this study protocol was supported by the National Institute of Mental Health (NIMH) Intramural Research Program (ZIAMH002786‐15, ZIAMH002778‐17). ClinicalTrials.gov identifier: NCT00025935. The funding body had no role in the study design, writing the manuscript, or submitting the paper for publication. The study has not received specific, external funding.
Naim, R. , Goodwin, M. S. , Dombek, K. , Revzina, O. , Agorsor, C. , Lee, K. , Zapp, C. , Freitag, G. F. , Haller, S. P. , Cardinale, E. , Jangraw, D. , & Brotman, M. A. (2021). Cardiovascular reactivity as a measure of irritability in a transdiagnostic sample of youth: Preliminary associations. International Journal of Methods in Psychiatric Research, 30(4), e1890. 10.1002/mpr.1890
DATA AVAILABILITY STATEMENT
Data will be available in compliance with study specific ethics protocol.
References
- Achenbach, T. M. , McConaughy, S. H. , & Howell, C. T. (1987). Child/adolescent behavioral and emotional problems: Implications of cross‐informant correlations for situational specificity. Psychological Bulletin, 101(2), 213–232. 10.1037/0033-2909.101.2.213 [DOI] [PubMed] [Google Scholar]
- Alderson, R. M. , Rapport, M. D. , & Kofler, M. J. (2007). Attention‐deficit/hyperactivity disorder and behavioral inhibition: A meta‐analytic review of the stop‐signal paradigm. Journal of Abnormal Child Psychology, 35(5), 745–758. 10.1007/s10802-007-9131-6 [DOI] [PubMed] [Google Scholar]
- Allen, J. (2007). Photoplethysmography and its application in clinical physiological measurement. Physiological Measurement, 28(3), R1–R39. 10.1088/0967-3334/28/3/R01 [DOI] [PubMed] [Google Scholar]
- Appelhans, B. M. , & Luecken, L. J. (2006). Heart rate variability as an index of regulated emotional responding. Review of General Psychology, 10(3), 229–240. 10.1037/1089-2680.10.3.229 [DOI] [Google Scholar]
- Avenevoli, S. , Blader, J. C. , & Leibenluft, E. (2015). Irritability in youth: An update. Journal of the American Academy of Child & Adolescent Psychiatry, 54(11), 881–883. 10.1016/j.jaac.2015.08.012 [DOI] [PubMed] [Google Scholar]
- Barnett, V. , & Lewis, T. (1994). Outliers in statistical data (3rd ed.). New York: Wiley. [Google Scholar]
- Beauchaine, T. P. , & Thayer, J. F. (2015). Heart rate variability as a transdiagnostic biomarker of psychopathology. International Journal of Psychophysiology, 98(2), 338–350. 10.1016/j.ijpsycho.2015.08.004 [DOI] [PubMed] [Google Scholar]
- Berntson, G. G. , Bigger, J. T. J. , Eckberg, D. L. , Grossman, P. , Kaufmann, P. G. , Malik, M. , Nagaraja, H. N. , Porges, S. W. , Saul, J. P. , Stone, P. H. , & van Der Molen, M. W. (1997). Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology, 34(6), 623–648. [DOI] [PubMed] [Google Scholar]
- Bimmel, N. , Van Ijzendoorn, M. H. , Bakermans‐Kranenburg, M. J. , Juffer, F. , & De Geus, E. J. C. (2008). Problem behavior and heart rate reactivity in adopted adolescents: Longitudinal and concurrent relations. Journal of Research on Adolescence, 18(2), 201–214. 10.1111/j.1532-7795.2008.00557.x [DOI] [Google Scholar]
- Brotman, M. A. , Kircanski, K. , & Leibenluft, E. (2017). Irritability in children and adolescents. Annual Review of Clinical Psychology, 13, 317–341. 10.1146/annurev-clinpsy-032816-044941 [DOI] [PubMed] [Google Scholar]
- Brotman, M. A. , Kircanski, K. , Stringaris, A. , Pine, D. S. , & Leibenluft, E. (2017). Irritability in youths: A translational model. American Journal of Psychiatry, 174(6), 520–532. 10.1176/appi.ajp.2016.16070839 [DOI] [PubMed] [Google Scholar]
- Buzzell, G. A. , Troller‐Renfree, S. V. , Barker, T. V. , Bowman, L. C. , Chronis‐Tuscano, A. , Henderson, H. A. , Kagan, J. , Pine, D. S. , & Fox, N. A. (2017). A neurobehavioral mechanism linking behaviorally inhibited temperament and later adolescent social anxiety. Journal of the American Academy of Child & Adolescent Psychiatry, 56(12), 1097–1105. 10.1016/j.jaac.2017.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale, E. M , Freitag, G. F , Brotman, M. A , Pine, D. S , Leibenluft, E , & Kircanski, K . Phasic versus tonic irritability: Differential associations with attention‐deficit/hyperactivity disorder symptoms. Journal of the American Academy of Child & Adolescent Psychiatry. 10.1016/j.jaac.2020.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale, E. M. , Subar, A. R. , Brotman, M. A. , Leibenluft, E. , Kircanski, K. , & Pine, D. S. (2019). Inhibitory control and emotion dysregulation: A framework for research on anxiety. Development and Psychopathology, 31(3), 859–869. 10.1017/S0954579419000300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland, W. E. , Angold, A. , Costello, J. E. , & Egger, H. (2013). Prevalence, comorbidity, and correlates of DSM‐5 proposed disruptive mood dysregulation disorder. American Journal of Psychiatry, 170(2), 173–179. 10.1176/appi.ajp.2012.12010132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaplace, R. , Garny de La Rivière, S. , Bon Saint Come, M. , Lahaye, H. , Popov, I. , Rey, N. , Visticot, A. , & Guilé, J.‐M. (2018). Sleep and disruptive mood dysregulation disorder: A pilot actigraphy study. Archives de Pédiatrie, 25(5), 303–308. 10.1016/j.arcped.2018.05.003 [DOI] [PubMed] [Google Scholar]
- De Los Reyes, A. , & Kazdin, A. E. (2005). Informant discrepancies in the assessment of childhood psychopathology: A critical review, theoretical framework, and recommendations for further study. Psychological Bulletin, 131(4), 483–509. 10.1037/0033-2909.131.4.483 [DOI] [PubMed] [Google Scholar]
- Deutz, M. H. F. , Woltering, S. , Vossen, H. G. M. , Deković, M. , van Baar, A. L. , & Prinzie, P. (2019). Underlying psychophysiology of dysregulation: Resting heart rate and heart rate reactivity in relation to childhood dysregulation. Journal of the American Academy of Child & Adolescent Psychiatry, 58(6), 589–599. 10.1016/j.jaac.2018.09.434 [DOI] [PubMed] [Google Scholar]
- Deveney, C. M. , Connolly, M. E. , Haring, C. T. , Bones, B. L. , Reynolds, R. C. , Kim, P. , Pine, D. S. , & Leibenluft, E. (2013). Neural mechanisms of frustration in chronically irritable children. American Journal of Psychiatry, 170(10), 1186–1194. 10.1176/appi.ajp.2013.12070917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faedda, G. L. , Ohashi, K. , Hernandez, M. , McGreenery, C. E. , Grant, M. C. , Baroni, A. , Polcari, A. , & Teicher, M. H. (2016). Actigraph measures discriminate pediatric bipolar disorder from attention‐deficit/hyperactivity disorder and typically developing controls. The Journal of Child Psychology and Psychiatry and Allied Disciplines, 57(6), 706–716. 10.1111/jcpp.12520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild, G. , van Goozen, S. H. M. , Stollery, S. J. , Brown, J. , Gardiner, J. , Herbert, J. , & Goodyer, I. M. (2008). Cortisol diurnal rhythm and stress reactivity in male adolescents with early‐onset or adolescence‐onset conduct disorder. Biological Psychiatry, 64(7), 599–606. 10.1016/j.biopsych.2008.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanti, K. A. (2019). Editorial: Heart rate as a biomarker for co‐occurring externalizing and internalizing problems. Journal of the American Academy of Child & Adolescent Psychiatry, 58(6), 569–571. 10.1016/j.jaac.2018.11.009 [DOI] [PubMed] [Google Scholar]
- Fishburn, F. A. , Hlutkowsky, C. O. , Bemis, L. M. , Huppert, T. J. , Wakschlag, L. S. , & Perlman, S. B. (2019). Irritability uniquely predicts prefrontal cortex activation during preschool inhibitory control among all temperament domains: A LASSO approach. NeuroImage, 184, 68–77. 10.1016/j.neuroimage.2018.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita, D. , & Suzuki, A. (2019). Evaluation of the possible use of PPG waveform features measured at low sampling rate. IEEE Access, 7, 58361–58367. 10.1109/ACCESS.2019.2914498 [DOI] [Google Scholar]
- Garralda, M. E. , Connell, J. , & Taylor, D. C. (1991). Psychophysiological anomalies in children with emotional and conduct disorders. Psychological Medicine, 21(4), 947–957. 10.1017/S0033291700029937 [DOI] [PubMed] [Google Scholar]
- Gatzke‐Kopp, L. M. , Willner, C. J. , Jetha, M. K. , Abenavoli, R. M. , DuPuis, D. , & Segalowitz, S. J. (2015). How does reactivity to frustrative non‐reward increase risk for externalizing symptoms? International Journal of Psychophysiology, 98(2), 300–309. 10.1016/j.ijpsycho.2015.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabell, A. S. , Li, Y. , Barker, J. W. , Wakschlag, L. S. , Huppert, T. J. , & Perlman, S. B. (2018). Evidence of non‐linear associations between frustration‐related prefrontal cortex activation and the normal:Abnormal spectrum of irritability in young children. Journal of Abnormal Child Psychology, 46(1), 137–147. 10.1007/s10802-017-0286-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller, S. P. , Kircanski, K. , Stringaris, A. , Clayton, M. , Bui, H. , Agorsor, C. , Cardenas, S. I. , Towbin, K. E. , Pine, D. S. , Leibenluft, E. , & Brotman, M. A. (2020). The clinician affective reactivity index: Validity and reliability of a clinician‐rated assessment of irritability. Behavior Therapy, 51(2), 283–293. 10.1016/j.beth.2019.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennissen, L. , Bakker, M. J. , Banaschewski, T. , Carucci, S. , Coghill, D. , Danckaerts, M. , Dittmann, R. W. , Hollis, C. , Kovshoff, H. , McCarthy, S. , Nagy, P. , Sonuga‐Barke, E. , Wong, I. C. K. , Zuddas, A. , Rosenthal, E. , & Buitelaar, J. K. (2017). Cardiovascular effects of stimulant and non‐stimulant medication for children and adolescents with ADHD: A systematic review and meta‐analysis of trials of methylphenidate, amphetamines and atomoxetine. CNS Drugs, 31(3), 199–215. 10.1007/s40263-017-0410-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herpertz, S. C. , Wenning, B. , Mueller, B. , Qunaibi, M. , Sass, H. , & Herpertz‐Dahlmann, B. (2001). Psychophysiological responses in ADHD boys with and without conduct disorder: Implications for adult antisocial behavior. Journal of the American Academy of Child & Adolescent Psychiatry, 40(10), 1222–1230. 10.1097/00004583-200110000-00017 [DOI] [PubMed] [Google Scholar]
- Huỳnh, C. , Guilé, J. M. , Breton, J. J. , & Godbout, R. (2016). Sleep‐wake patterns of adolescents with borderline personality disorder and bipolar disorder. Child Psychiatry and Human Development, 47(2), 202–214. 10.1007/s10578-015-0557-8 [DOI] [PubMed] [Google Scholar]
- Karalunas, S. L. , Fair, D. , Musser, E. D. , Aykes, K. , Iyer, S. P. , & Nigg, J. T. (2014). Subtyping attention‐deficit/hyperactivity disorder using temperament dimensions: Toward biologically based nosologic criteria. JAMA Psychiatry, 71(9), 1015–1024. 10.1001/jamapsychiatry.2014.763 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kaufman, J. , Birmaher, B. , Brent, D. , Rao, U. , Flynn, C. , Moreci, P. , Williamson, D. , & Ryan, N. (1997). Schedule for affective disorders and schizophrenia for school‐age children‐present and lifetime version (K‐SADS‐PL): Initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry, 36(7), 980–988. 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- Kircanski, K. , Craske, M. G. , Averbeck, B. B. , Pine, D. S. , Leibenluft, E. , & Brotman, M. A. (2019). Exposure therapy for pediatric irritability: Theory and potential mechanisms. Behaviour Research and Therapy, 118, 141–149. 10.1016/j.brat.2019.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircanski, K. , Waugh, C. E. , Camacho, M. C. , & Gotlib, I. H. (2016). Aberrant parasympathetic stress responsivity in pure and co‐occurring major depressive disorder and generalized anxiety disorder. Journal of Psychopathology and Behavioral Assessment, 38(1), 5–19. 10.1007/s10862-015-9493-y [DOI] [Google Scholar]
- Koenig, J. , Kemp, A. H. , Beauchaine, T. P. , Thayer, J. F. , & Kaess, M. (2016). Depression and resting state heart rate variability in children and adolescents—A systematic review and meta‐analysis. Clinical Psychology Review, 46, 136–150. 10.1016/j.cpr.2016.04.013 [DOI] [PubMed] [Google Scholar]
- Kraemer, H. C. , & Blasey, C. M. (2004). Centering in regression analyses: A strategy to prevent errors in statistical inference. International Journal of Methods in Psychiatric Research, 13(3), 141–151. 10.1016/j.jad.2020.04.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreibig, S. D. (2010). Autonomic nervous system activity in emotion: A review. Biological Psychology, 84(3), 394–421. 10.1016/j.biopsycho.2010.03.010 [DOI] [PubMed] [Google Scholar]
- Lauderdale, D. S. , Knutson, K. L. , Rathouz, P. J. , Yan, L. L. , Hulley, S. B. , & Liu, K. (2009). Cross‐sectional and longitudinal associations between objectively measured sleep duration and body mass index. American Journal of Epidemiology, 170(7), 805–813. 10.1093/aje/kwp230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leno, V. C. , Forth, G. , Chandler, S. , White, P. , Yorke, I. , Charman, T. , Pickles, A. , & Simonoff, E. (2020). Behavioural and physiological response to frustration in autistic youth: Associations with irritability. Research Square. 10.21203/rs.3.rs-96188/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipponen, J. A. , & Tarvainen, M. P. (2019). A robust algorithm for heart rate variability time series artefact correction using novel beat classification. Journal of Medical Engineering & Technology, 43(3), 173–181. 10.1080/03091902.2019.1640306 [DOI] [PubMed] [Google Scholar]
- Lipszyc, J. , & Schachar, R. (2010). Inhibitory control and psychopathology: A meta‐analysis of studies using the stop signal task. Journal of the International Neuropsychological Society, 16(6), 1064–1076. 10.1017/S1355617710000895 [DOI] [PubMed] [Google Scholar]
- Liuzzi, M. T. , Kryza‐Lacombe, M. , Christian, I. R. , Palumbo, D. E. , Amir, N. , & Wiggins, J. L. (2020). Neural and behavioral correlates of inhibitory control in youths with varying levels of irritability. Journal of Affective Disorders, 273, 567–575. 10.1016/j.jad.2020.04.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorber, M. F. (2004). Psychophysiology of aggression, psychopathy, and conduct problems: A meta‐analysis. Psychological Bulletin, 130(4), 531–552. 10.1037/0033-2909.130.4.531 [DOI] [PubMed] [Google Scholar]
- McCarthy, C. , Pradhan, N. , Redpath, C. , & Adler, A. (2016, May 29–31). Validation of the Empatica E4 wristband. 2016 IEEE EMBS International Student Conference: Expanding the Boundaries of Biomedical Engineering and Healthcare, ISC 2016—Proceedings, Ottawa, ON, Canada, 17–20. IEEE. 10.1109/EMBSISC.2016.7508621 [DOI] [Google Scholar]
- Martins, D. , Tareen, N. , Pan, D. , & Norris, K. (2002). The relationship between body mass index and pulse pressure in older adults with isolated systolic hypertension. American Journal of Hypertension, 15, 538–543. 10.1016/S0895-7061(02)02269-0 [DOI] [PubMed] [Google Scholar]
- Mikita, N. , Hollocks, M. J. , Papadopoulos, A. S. , Aslani, A. , Harrison, S. , Leibenluft, E. , Simonoff, E. , & Stringaris, A. (2015). Irritability in boys with autism spectrum disorders: An investigation of physiological reactivity. The Journal of Child Psychology and Psychiatry and Allied Disciplines, 56(10), 1118–1126. 10.1111/jcpp.12382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk, C. , Kovelenko, P. , Ellman, L. M. , Sloan, R. P. , Bagiella, E. , Gorman, J. M. , & Pine, D. S. (2001). Enhanced stress reactivity in paediatric anxiety disorders: Implications for future cardiovascular health. International Journal of Neuropsychopharmacology, 4(2), 199–206. 10.1017/S146114570100236X [DOI] [PubMed] [Google Scholar]
- Moore, A. A. , Lapato, D. M. , Brotman, M. A. , Leibenluft, E. , Aggen, S. H. , Hettema, J. M. , York, T. P. , Silberg, J. L. , & Roberson‐Nay, R. (2019). Heritability, stability, and prevalence of tonic and phasic irritability as indicators of disruptive mood dysregulation disorder. The Journal of Child Psychology and Psychiatry and Allied Disciplines, 60(9), 1032–1041. 10.1111/jcpp.13062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray‐Close, D. , Holterman, L. A. , Breslend, N. L. , & Sullivan, A. (2017). Psychophysiology of proactive and reactive relational aggression. Biological Psychology, 130, 77–85. 10.1016/j.biopsycho.2017.10.005 [DOI] [PubMed] [Google Scholar]
- Musser, E. D. , Backs, R. W. , Schmitt, C. F. , Ablow, J. C. , Measelle, J. R. , & Nigg, J. T. (2011). Emotion regulation via the autonomic nervous system in children with attention‐deficit/hyperactivity disorder (ADHD). Journal of Abnormal Child Psychology, 39(6), 841–852. 10.1007/s10802-011-9499-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg, J. T. (2017). Annual Research Review: On the relations among self‐regulation, self‐control, executive functioning, effortful control, cognitive control, impulsivity, risk‐taking, and inhibition for developmental psychopathology. The Journal of Child Psychology and Psychiatry and Allied Disciplines, 58(4), 361–383. 10.1111/jcpp.12675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, P.‐Y. , & Yeh, C.‐B. (2019). Irritability and maladaptation among children: The utility of Chinese versions of the affective reactivity index and aberrant behavior checklist‐irritability subscale. Journal of Child and Adolescent Psychopharmacology, 29(3), 213–219. 10.1089/cap.2018.0070 [DOI] [PubMed] [Google Scholar]
- Penttilä, J. , Helminen, A. , Jartti, T. , Kuusela, T. , Huikuri, H. V. , Tulppo, M. P. , Coffeng, R. , & Scheinin, H. (2001). Time domain, geometrical and frequency domain analysis of cardiac vagal outflow: Effects of various respiratory patterns. Clinical Physiology, 21(3), 365–376. 10.1046/j.1365-2281.2001.00337.x [DOI] [PubMed] [Google Scholar]
- Perlman, S. B. , Jones, B. M. , Wakschlag, L. S. , Axelson, D. , Birmaher, B. , & Phillips, M. L. (2015). Neural substrates of child irritability in typically developing and psychiatric populations. Developmental Cognitive Neuroscience, 14, 71–80. 10.1016/j.dcn.2015.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, M. L. , Ladouceur, C. D. , & Drevets, W. C. (2008). A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry, 13(9), 833–857. 10.1038/mp.2008.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna, T. , & Edwards, D. J. (2020). A systematic review of associations between interoception, vagal tone, and emotional regulation: Potential applications for mental health, wellbeing, psychological flexibility, and chronic conditions. Frontiers in Psychology, 11, 1–15. 10.3389/fpsyg.2020.01792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popma, A. , Jansen, L. M. C. , Vermeiren, R. , Steiner, H. , Raine, A. , Van Goozen, S. H. M. , van Engelande, H. , & Doreleijers, T. A. H. (2006). Hypothalamus pituitary adrenal axis and autonomic activity during stress in delinquent male adolescents and controls. Psychoneuroendocrinology, 31(8), 948–957. 10.1016/j.psyneuen.2006.05.005 [DOI] [PubMed] [Google Scholar]
- Quintana, D. S. , Elstad, M. , Kaufmann, T. , Brandt, C. L. , Haatveit, B. , Haram, M. , Nerhus, M. , Westlye, L. T. , & Andreassen, O. A. (2016). Resting‐state high‐frequency heart rate variability is related to respiratory frequency in individuals with severe mental illness but not healthy controls. Scientific Reports, 6, 1–8. 10.1038/srep37212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash, J. A. , & Aguirre‐Camacho, A. (2012). Attention‐deficit hyperactivity disorder and cardiac vagal control: A systematic review. ADHD Attention Deficit and Hyperactivity Disorders, 4(4), 167–177. 10.1007/s12402-012-0087-1 [DOI] [PubMed] [Google Scholar]
- Raudenbush, S. W. , Bryk, A. S. , Cheong, Y. F. , & Congdon, R. (2019). HLM 8 for Windows. Scientific Software International, Inc. [Google Scholar]
- Rozenman, M. , Sturm, A. , McCracken, J. T. , & Piacentini, J. (2017). Autonomic arousal in anxious and typically developing youth during a stressor involving error feedback. European Child & Adolescent Psychiatry, 26(12), 1423–1432. 10.1007/s00787-017-1001-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahuddin, L. , Cho, J. , Jeong, M. G. , & Kim, D. (2007, August 22–26). Ultra short term analysis of heart rate variability for monitoring mental stress in mobile settings. Annual International Conference of the IEEE Engineering in Medicine and Biology—Proceedings, Lyon, France, 4656–4659. IEEE. 10.1109/IEMBS.2007.4353378 [DOI] [PubMed] [Google Scholar]
- Salbach‐Andrae, H. , Lenz, K. , & Lehmkuhl, U. (2009). Patterns of agreement among parent, teacher and youth ratings in a referred sample. European Psychiatry, 24(5), 345–351. 10.1016/j.eurpsy.2008.07.008 [DOI] [PubMed] [Google Scholar]
- Sijtsema, J. J. , Nederhof, E. , Veenstra, R. , Ormel, J. , Oldehinkel, A. J. , & Ellis, B. J. (2013). Effects of family cohesion and heart rate reactivity on aggressive/rule‐breaking behavior and prosocial behavior in adolescence: The Tracking Adolescents’ Individual Lives Survey study. Development and Psychopathology, 25(3), 699–712. 10.1017/S0954579413000114 [DOI] [PubMed] [Google Scholar]
- Silver, J. , Carlson, G. A. , Olino, T. M. , Perlman, G. , Mackin, D. , Kotov, R. , & Klein, D. N. (2021). Differential outcomes of tonic and phasic irritability in adolescent girls. The Journal of Child Psychology and Psychiatry and Allied Disciplines. 10.1111/jcpp.13402 [DOI] [PubMed] [Google Scholar]
- Smith, A. R. , Kircanski, K. , Brotman, M. A. , Do, Q. B. , Subar, A. R. , Silk, J. S. , Engel, S. , Crosby, R. D. , Harrewijn, A. , White, L. K. , Haller, S. P. , Cardinale, E. M. , Buzzell, G. A. , Barker, T. , Leibenluft, E. , & Pine, D. S. (2019). Advancing clinical neuroscience through enhanced tools: Pediatric social anxiety as an example. Depression and Anxiety, 36(8), 701–711. 10.1002/da.22937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard, J. , Stringaris, A. , Brotman, M. A. , Montville, D. , Pine, D. S. , & Leibenluft, E. (2014). Irritability in child and adolescent anxiety disorders. Depression and Anxiety, 31(7), 566–573. 10.1002/da.22151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris, A. , Goodman, R. , Ferdinando, S. , Razdan, V. , Muhrer, E. , Leibenluft, E. , & Brotman, M. A. (2012). The affective reactivity index: A concise irritability scale for clinical and research settings. The Journal of Child Psychology and Psychiatry and Allied Disciplines, 53(11), 1109–1117. 10.1111/j.1469-7610.2012.02561.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarvainen, M. P. , Niskanen, J. P. , Lipponen, J. A. , Ranta‐aho, P. O. , & Karjalainen, P. A. (2014). Kubios HRV—Heart rate variability analysis software. Computer Methods and Programs in Biomedicine, 113(1), 210–220. 10.1016/j.cmpb.2013.07.024 [DOI] [PubMed] [Google Scholar]
- Thayer, J. F. , Hansen, A. L. , Saus‐Rose, E. , & Johnsen, B. H. (2009). Heart rate variability, prefrontal neural function, and cognitive performance: The neurovisceral integration perspective on self‐regulation, adaptation, and health. Annals of Behavioral Medicine, 37(2), 141–153. 10.1007/s12160-009-9101-z [DOI] [PubMed] [Google Scholar]
- Thayer, J. F. , & Lane, R. D. (2000). A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders, 61(3), 201–216. 10.1016/S0165-0327(00)00338-4 [DOI] [PubMed] [Google Scholar]
- Tobaldini, E. , Cogliati, C. , Fiorelli, E. M. , Nunziata, V. , Wu, M. A. , Prado, M. , Bevilacquab, D. , Trabattoni, D. , Porta, A. , & Montano, N. (2013). One night on‐call: Sleep deprivation affects cardiac autonomic control and inflammation in physicians. European Journal of Internal Medicine, 24(7), 664–670. 10.1016/j.ejim.2013.03.011 [DOI] [PubMed] [Google Scholar]
- Tobaldini, E. , Nobili, L. , Strada, S. , Casali, K. R. , Braghiroli, A. , & Montano, N. (2013). Heart rate variability in normal and pathological sleep. Frontiers in Physiology, 4, 1–11. 10.3389/fphys.2013.00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobaldini, E. , Pecis, M. , & Montano, N. (2014). Effects of acute and chronic sleep deprivation on cardiovascular regulation. Archives Italiennes de Biologie, 152(2–3), 103–110. 10.12871/000298292014235 [DOI] [PubMed] [Google Scholar]
- Tonacci, A. , Billeci, L. , Calderoni, S. , Levantini, V. , Masi, G. , Milone, A. , Pisano, S. , & Muratori, P. (2019). Sympathetic arousal in children with oppositional defiant disorder and its relation to emotional dysregulation. Journal of Affective Disorders, 257, 207–213. 10.1016/j.jad.2019.07.046 [DOI] [PubMed] [Google Scholar]
- Tseng, W.‐L. , Deveney, C. M. , Stoddard, J. , Kircanski, K. , Frackman, A. E. , Yi, J. Y. , Hsu, D. , Moroney, E. , Machlin, L. , Donahue, L. , Roule, A. , Perhamus, G. , Reynolds, R. C. , Roberson‐Nay, R. , Hettema, J. M. , Towbin, K. E. , Stringaris, A. , Pine, D. S. , Brotman, M. A. , & Leibenluft, E. (2019). Brain mechanisms of attention orienting following frustration: Associations with irritability and age in youths. American Journal of Psychiatry, 176(1), 67–76. 10.1176/appi.ajp.2018.18040491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Goozen, S. H. M. , Fairchild, G. , Snoek, H. , & Harold, G. T. (2007). The evidence for a neurobiological model of childhood antisocial behavior. Psychological Bulletin, 133(1), 149–182. 10.1037/0033-2909.133.1.149 [DOI] [PubMed] [Google Scholar]
- Van Goozen, S. H. M. , Matthys, W. , Cohen‐Kettenis, P. T. , Buitelaar, J. K. , & Van Engeland, H. (2000). Hypothalamic‐pituitary‐adrenal axis and autonomic nervous system activity in disruptive children and matched controls. Journal of the American Academy of Child & Adolescent Psychiatry, 39(11), 1438–1445. 10.1097/00004583-200011000-00019 [DOI] [PubMed] [Google Scholar]
- van Lier, H. G. , Pieterse, M. E. , Garde, A. , Postel, M. G. , de Haan, H. A. , Vollenbroek‐Hutten, M. M. R. , Schraagen, J. M. , & Noordzij, M. L. (2019). A standardized validity assessment protocol for physiological signals from wearable technology: Methodological underpinnings and an application to the E4 biosensor. Behavior Research Methods, 52(2), 607–629. 10.3758/s13428-019-01263-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen, F. , & Logan, G. D. (2008). Response inhibition in the stop‐signal paradigm successful stopping: Inhibition and performance monitoring. Trends in Cognitive Sciences, 12(11), 418–424. 10.1016/j.tics.2008.07.005.Response [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal‐Ribas, P. , Brotman, M. A. , Valdivieso, I. , Leibenluft, E. , & Stringaris, A. (2016). The status of irritability in psychiatry: A conceptual and quantitative review. Journal of the American Academy of Child & Adolescent Psychiatry, 55(7), 556–570. 10.1016/j.jaac.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler, D. (1999). Wechsler abbreviated scale of intelligence. The Psychological Corporation. [Google Scholar]
- Weiner, O. M. , & McGrath, J. J. (2017). Test‐retest reliability of pediatric heart rate variability: A meta‐analysis. Journal of Psychophysiology, 31(1), 6–28. 10.1027/0269-8803/a000161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weippert, M. , Behrens, K. , Rieger, A. , Stoll, R. , & Kreuzfeld, S. (2013). Heart rate variability and blood pressure during dynamic and static exercise at similar heart rate levels. PLoS One, 8(12), 1–8. 10.1371/journal.pone.0083690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins, J. L. , Brotman, M. A. , Adleman, N. E. , Kim, P. , Oakes, A. H. , Reynolds, R. C. , Chen, G. , Pine, D. S. , & Leibenluft, E. (2016). Neural correlates of irritability in disruptive mood dysregulation and bipolar disorders. American Journal of Psychiatry, 173(7), 722–730. 10.1176/appi.ajp.2015.15060833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm, F. H. , & Grossman, P. (2010). Emotions beyond the laboratory: Theoretical fundaments, study design, and analytic strategies for advanced ambulatory assessment. Biological Psychology, 84(3), 552–569. 10.1016/j.biopsycho.2010.01.017 [DOI] [PubMed] [Google Scholar]
- Williams, S. C. , Lochman, J. E. , Phillips, N. C. , & Barry, T. D. (2003). Aggressive and nonaggressive boys’ physiological and cognitive processes in response to peer provocations. Journal of Clinical Child and Adolescent Psychology, 32(4), 568–576. 10.1207/S15374424JCCP3204_9 [DOI] [PubMed] [Google Scholar]
- Woltering, S. , Lishak, V. , Hodgson, N. , Granic, I. , & Zelazo, P. D. (2016). Executive function in children with externalizing and comorbid internalizing behavior problems. The Journal of Child Psychology and Psychiatry and Allied Disciplines, 57(1), 30–38. 10.1111/jcpp.12428 [DOI] [PubMed] [Google Scholar]
- Yerkes, R. M. , & Dodson, J. D. (1908). The relation of strength of stimulus to rapidity of habit‐formation. Journal of Comparative Neurology and Psychology, 18, 459–482. 10.1002/cne.92018050 [DOI] [Google Scholar]
- Zhong, X. , Hilton, H. J. , Gates, G. J. , Jelic, S. , Stern, Y. , Bartels, M. N. , DeMeersman, R. E. , & Basner, R. C. (2005). Increased sympathetic and decreased parasympathetic cardiovascular modulation in normal humans with acute sleep deprivation. Journal of Applied Physiology, 98(6), 2024–2032. 10.1152/japplphysiol.00620.2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be available in compliance with study specific ethics protocol.


