Abstract
Hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs) is a prominent substrate for activated tyrosine kinase receptors that has been proposed to play a role in endosomal membrane trafficking. The protein contains a FYVE domain, which specifically binds to the lipid phosphatidylinositol (PI) 3-phosphate (PI 3-P). We show that this interaction is required both for correct localization of the protein to endosomes that only partially coincides with early endosomal autoantigen 1 and for efficient tyrosine phosphorylation of the protein in response to epidermal growth factor stimulation. Treatment with wortmannin reveals that Hrs phosphorylation also requires PI 3-kinase activity, which is necessary to generate the PI 3-P required for localization. We have used both hypertonic media and expression of a dominant-negative form of dynamin (K44A) to inhibit endocytosis; under which conditions, receptor stimulation fails to elicit phosphorylation of Hrs. Our results provide a clear example of the coupling of a signal transduction pathway to endocytosis, from which we propose that activated receptor (or associated factor) must be delivered to the appropriate endocytic compartment in order for Hrs phosphorylation to occur.
Hepatocyte growth factor (HGF)-regulated tyrosine kinase substrate (Hrs) is a prominent target for tyrosine phosphorylation following the activation of tyrosine kinase receptors (11). It was initially shown to lie downstream of the HGF (scatter factor) receptor c-met, but activation of other tyrosine kinase receptors and by cytokines such as interleukin-2 and granulocyte-macrophage colony-stimulating factor also results in phosphorylation of Hrs (1). It is localized to transferrin receptor-containing endosomes (12) and bears significant similarity (including a FYVE-finger motif and a VHS domain) to the Saccharomyces cerevisiae protein Vps27. Vps27 belongs to the class E set of Vps mutants which are defective in transport from the sorting endosome to the vacuole (2, 22). A highly related protein, Hrs-2, has been shown to interact with SNAP-25 and SNAP-23, homologous proteins which are involved in regulated exocytosis and other intracellular fusion events, respectively (3).
Two proteins that bind to Hrs have been identified which have been named STAM (for signal-transducing adapter molecule) and Hrs binding protein (Hbp) (1, 31). They show 53% sequence identity, each bears an SH3 domain, and both are also tyrosine phosphorylated. In T cells, overexpression of Hrs leads to suppression of cytokine-mediated DNA synthesis, while a mutant unable to bind STAM is without effect (1). NIH 3T3 cells stably transfected with mutants of Hbp that lack the SH3 domain or the binding site for Hrs are impaired in degrading internalized platelet-derived growth factor (31). This latter result is consistent with a role for Hrs in regulating transport from early to late endosomes (or multivesicular bodies) proposed by analogy to Vps27 function in yeast.
The FYVE domain is a double zinc finger domain that has been shown to specifically bind the lipid phosphatidylinositol (PI) 3-phosphate (PI 3-P) (8, 19, 29). The domain can be recognized in five proteins from baker's yeast, Saccharomyces cerevisiae, and in several mammalian proteins including early endosomal autoantigen 1 (EEA1), Hrs, and SARA (Smad anchor for receptor activation) (30, 38). In yeast, three of these proteins have been implicated in the regulation of endocytic trafficking events (Vps27, Fab1, and Vac1 [4]), while in mammalian cells EEA1 has been shown to regulate early endosome fusion (16, 27). The function of the FYVE domain may be to localize proteins to specific membranes, as in the case of EEA1, or to allosterically regulate the function of membrane-associated protein (7).
Localization studies of FYVE domain proteins in mammalian cells are so far limited. As its name implies, EEA1 is probably now considered the classical marker for early endosomes (18). On the basis of colocalization with transferring receptor (12), Hrs may be expected to overlap with EEA1, while SARA displays a punctate immunofluorescence-labeling pattern also reminiscent of endosomes (33). If the FYVE–PI 3-P interaction specifies localization, then the distribution of all FYVE proteins should overlap. However, other membrane-associated factors may also influence protein distribution and perhaps override this localization signal. In this paper, we show that localization of epitope-tagged Hrs (Hrs-HA) only partially overlaps with EEA1 at relatively low levels of expression.
We have proceeded to investigate the circuitry required for Hrs phosphorylation. Our data lead us to propose that this requires both endosomal localization by the Hrs FYVE domain interacting with PI 3-P and vesicular trafficking to the same compartment.
MATERIALS AND METHODS
Cell culture, plasmids, and transfections.
Baby hamster kidney (BHK) cells and HeLa cells were incubated in a 5% CO2 atmosphere in Glasgow minimal essential medium supplemented with 5% fetal bovine serum and 10% tryptose broth or Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum and 1% nonessential amino acids, respectively. K44A HeLa cells (a generous gift of S. Schmid) were cultured in HeLa medium supplemented with Geneticin (G418 sulfate; 400 μg/ml), puromycin (200 ng/ml), and tetracycline (1 μg/ml). For induction of K44A dynamin, tetracycline was withdrawn for 48 h before the experiment. Expression of the HA-tagged dynamin mutant was monitored by Western blotting and immunofluorescence. Typically, >95% of cells expressed the K44A mutant, and the corresponding cells showed inhibition of biotinylated epidermal growth factor (EGF) and transferrin uptake as judged by labeling with streptavidin-Oregon Green 488.
For overexpression of HA-tagged Hrs, the previously described construct, pmiw-Hrs-HA, was used (12). The deletion mutant used was pmiw-ΔZF-HA, in which amino acids 166 to 215 have been removed (12). A 1.4-kb PmlI-NheI fragment (nucleotides 175 to 1587 of the mouse Hrs open reading frame) of the point mutant C215S and the point mutant Y216F was subcloned from pGEM-C215S and pGEM-Y216F (B. Bremnes and H. Stenmark, unpublished data) into pmiw. The point mutants C190S-HA and C190/215S-HA were generated by site-directed mutagenesis of pmiw-Hrs-HA and pmiw-C215S-HA, respectively, using the primers 5′-GTGGGCAGATCTTCTCTGGCAAGTGCTCCTC-3′ and 5′-GAGGAGCACTTGCCAGAGAAGATCTGCCCAC-3′. The point mutant Y197F was generated by site-directed mutagenisis of pmiw-Hrs-HA using the primers 5′-CAAGTGCTCCTCCAAGTTCTCCACCATCCCCAAG-3′ and 5′-CTTGGGGATGGTGGAGAACTTGGAGGAGCACTTG-3′. The GFP-NAGTI construct was a gift from D. Shima (ICRF, London, United Kingdom). HeLa cells were transfected using standard calcium phosphate precipitation. Typically, 30 to 50% of cells expressed Hrs-HA 22 h posttransfection.
Antibodies and other reagents.
Hrs polyclonal antibody generated against a glutathione S-transferase–Hrs fusion protein has been previously described (11). All HA antibodies were obtained from Babco. The polyclonal EEA1 antibody against a His-tagged fusion protein encompassing amino acids 1098 to 1411 of human EEA1 has been previously described (16). Complete overlap by immunofluorescence was obtained with this antibody judged against monoclonal anti-EEA1 (obtained from Transduction Laboratories). Monoclonal CD63 antibody (CLB-gran12) was obtained from BIODESIGN International. ci-M6PR antibody was a gift from Paul Luzio, Cambridge, United Kingdom (23). The anti-phosphotyrosine monoclonal antibody, PY20, was obtained from Transduction Laboratories. Purified human EGF was obtained from J. Smith, Liverpool, United Kingdom. Biotinylated EGF, transferrin, fluorescent streptavidin, and secondary antibodies were from Molecular Probes.
Immunofluorescence.
Transfected cells grown on coverslips were either first extracted with 0.05% saponin in piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES) buffer (80 mM PIPES–KOH [pH 7.0], 5 mM EGTA, 1 mM MgCl2) or fixed immediately with 3% paraformaldehyde (PFA; TAAB Laboratories, Aldermaston, United Kingdom) in phosphate-buffered saline (PBS). Residual PFA was quenched with 50 mM NH4Cl–PBS. Cells were permeabilized with either 0.05% saponin–PBS or 0.2% Triton X-100–PBS and were blocked with 10% goat serum in PBS. All antibody dilutions were in 5% goat serum, and incubation times were 20 to 30 min. Coverslips were mounted using Mowiol, and cells were viewed using a Bio-Rad LaserSharp confocal microscope. Z sections were taken at 260-nm steps and analyzed with the accompanying software.
EGF stimulation and detection of phosphorylated Hrs and Hrs-HA.
Cells were starved for 16 h in serum-free medium and then stimulated with 100 ng of EGF per ml. When indicated, cells were preincubated for 15 min with 100 nM wortmannin and then stimulated in the presence of 100 nM wortmannin. The cells were washed three times with ice-cold PBS and lysed for 20 min on ice in lysis buffer (25 mM Tris–HCl [pH 7.5], 100 mM NaCl, 0.5% NP-40, 50 mM NaF), supplemented with mammalian protease inhibitor cocktail and phosphatase inhibitor cocktail II (Sigma). The lysate was precleared by centrifugation, and 0.6 to 1 mg of protein at 1 mg/ml was incubated with 5 μl of anti-Hrs or anti-HA and protein A-Sepharose (Pharmacia). Immunoprecipitates were washed three times with 25 mM Tris–HCl (pH 7.5) and 150 mM NaCl supplemented with phosphatase inhibitor cocktail II and then once in 10 mM Tris (pH 7.5) before preparation for sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (with 8% polyacrylamide gels unless indicated otherwise). Following SDS-PAGE, proteins were transferred to polyvinylidene difluoride membranes (0.45-μm pore size; Millipore) which were blocked overnight with blocking buffer (1% bovine serum albumin–0.1% Tween in 10 mM Tris [pH 7.5], 100 mM NaCl). Primary and secondary antibody incubations were for 2 and 1 h, respectively, in blocking buffer. Development of Western blots was by enhanced chemiluminescence with Pierce Supersignal. Blots were routinely stripped and reprobed to assess that equal amounts of protein had been immunoprecipitated in each sample.
Preparation of membrane and cytosolic fractions.
Cells were homogenized in homogenization buffer (10 mM HEPES–3 mM imidazole–HCl [pH 7.2], 250 mM sucrose, mammalian protease inhibitor cocktail, phosphatase inhibitor cocktail II) by repeated passage through a 23-gauge needle at 4°C and then were immediately supplemented with 10 mM NaF. Membrane-particulate and cytosolic fractions were prepared from postnuclear supernatants by ultracentrifugation for 15 min at 65,000 rpm in a Beckman TLA 100.2 rotor.
RESULTS
Hrs localizes to endosomal compartments.
We expressed epitope-tagged Hrs-HA in HeLa cells. At early time points posttransfection (i.e., after 22 h), many cells exhibited a punctate staining pattern that partially colocalized with the early endosomal markers EEA1 and internalized transferrin, as well as the late endosomal marker cation-independent mannose 6-phosphate receptor (M6PR) (Fig. 1). No colocalization with the late endosomal marker CD63 was observed. When protein expression levels are higher, most notably at later time points posttransfection (Fig. 1B), large structures are formed, which contain the majority of Hrs, EEA1, M6PR, and transferrin receptor. These structures which may represent either aggregates or fused endocytic compartments are completely distinct from CD63-positive late endosomes and/or lysosomes (Fig. 1E). Note, however, that EEA1 and M6PR in nontransfected cells localize to different compartments (data not shown). A Golgi marker, GFP-NAGTI, is not included in these large structures and Golgi morphology is maintained, indicating that the perturbation is specific for elements of the endocytic pathway (Fig. 1F). The anti-Hrs polyclonal antibody did not provide specific labeling under our fixation conditions (using PFA). We have therefore confined our localization analysis to epitope-tagged protein. The fact that we see excellent colocalization of Hrs-HA with internalized transferrin (Fig. 1C) fits well with previous studies of endogenous protein using methanol fixation (12) and provides an indication that the distribution of Hrs-HA at low levels of expression is a reliable reflection of native protein.
FIG. 1.
Immunolocalization of Hrs-HA. HeLa cells were transfected with Hrs-HA and processed for immunofluorescence at 22 (A, C, D, E, and F) or 40 (B) h posttransfection. The cells were either saponin permeabilized before fixation (A, B, D, and F) or fixed immediately and permeabilized with Triton X-100 (C and E) and costained with anti-HA (shown in red) and either anti-EEA1 (A and B), anti-M6PR (D), or anti-CD63 (E) (all shown in green). Cells shown in panel C were incubated for 15 min with biotinylated transferrin (25 μg/ml in Dulbecco's modified Eagle medium) prior to fixation and costained with Oregon Green 488-labeled streptavidin. Cells shown in panel F were also cotransfected with GFP-NAGTI. All panels show a composite of a Z series taken at 260-nm intervals. Large bodies were apparent in cells expressing large amounts of Hrs, which were more frequent at longer times posttransfection (B) but could also be seen in a minority cells at earlier times (E and F). These structures contain EEA1 (B), M6PR, and transferrin receptor (data not shown) but exclude lysosomal and Golgi markers (E and F).
We next analyzed the distribution and effects of expressed Hrs-HA mutants (Fig. 2). Two expression vectors were prepared that bear point mutations in cysteines (C190S and C215S) located in the FYVE domain of Hrs. These cysteines are required to coordinate distinct zinc atoms which are necessary for FYVE finger conformation and consequently PI 3-P binding (17). We also prepared the corresponding double mutant and used a previously characterized mutant in which the entire FYVE domain (Δfyve = ΔZF) has been deleted (12). None of these mutants recapitulated the wild-type phenotype. They were proportionately more cytosolic in appearance, and the membrane-associated fraction decorated discrete structures lacking in EEA1, M6PR, transferrin receptor, and CD63 (Fig. 2 and results not shown). For all of the cysteine point mutants, at high levels of expression these frequently presented as distinctive ring structures (Fig. 2D and J). The Δfyve deletion mutant did not produce these structures. At longer time points posttransfection, this mutant exhibited some overlap with EEA1 and transferrin receptor, particularly in some larger structures that are formed (12).
FIG. 2.
FYVE domain mutants of Hrs do not colocalize with EEA1. HeLa cells were transfected with Hrs-HA (A to C), C215S-HA (D to F), ΔZF-HA (G to I), or C190/215S-HA (J to L) and processed for immunofluorescence at 22 h posttransfection. The cells were either saponin permeabilized before fixation to highlight the particulate structures (A to F) or fixed immediately and permeabilized with Triton X-100 (G to L) and costained with anti-HA (shown in red) and anti-EEA1 (shown in green). The areas indicated with a star are shown enlarged in the insets (A through F). All panels show a single confocal section.
PI 3-kinase activity is required for Hrs phosphorylation.
FYVE domains in proteins such as EEA1 and Hrs bind to PI 3-P, a product of some PI 3-kinase enzymes, most notably hVPS34 (36). Treatment of HeLa or BHK cells with the PI 3-kinase inhibitor, wortmannin, leads to an almost complete redistribution of EEA1 from membranes to the cytosol (16, 20). We similarly measured Hrs distribution between particulate and cytosolic fractions, prepared from cells treated or untreated with wortmannin. The majority of Hrs is cytosolic (11), but as with EEA1, there is a reduction of particulate-associated Hrs following wortmannin treatment of both BHK and HeLa cells (Fig. 3A). We also prepared an early-endosome-enriched fraction from BHK cells with a well-established flotation gradient as described by Gorvel et al. (9). Again, Hrs association with this fraction was wortmannin sensitive (Fig. 3A).
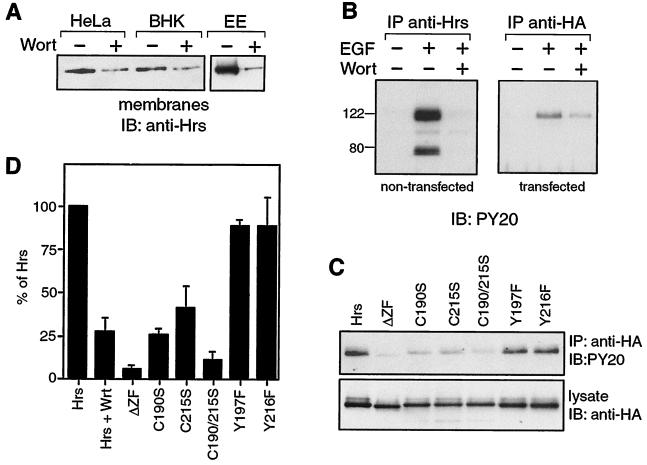
FIG. 3.
PI 3-kinase activity together with an intact FYVE domain is required for EGF-dependent tyrosine phosphorylation of Hrs. (A) Wortmannin sensitivity of membrane association. (Left) Membrane fractions were prepared from HeLa and BHK cells that had been preincubated for 15 min with (+) or without (−) 100 nM wortmannin (Wort). (Right) A fraction enriched in early endosomes (EE) by separation on a flotation gradient as described by Gorvel et al. (9) was prepared from BHK cells pretreated for 15 min with (+) or without (−) 100 nM wortmannin. In each case, 10 μg of protein was analyzed by SDS–12% PAGE, followed by transfer to nitrocellulose and blotting with anti-Hrs antibody. (B) Hrs phosphorylation is sensitive to wortmannin. Nontransfected or Hrs-HA-transfected HeLa cells were starved for 16 h in serum-free medium, then preincubated for 22 h posttransfection for 15 min with (+) or without (−) wortmannin, and stimulated for 8 min with EGF (100 ng/ml) with (+) or without (−) wortmannin (100 nM). A lysate was prepared from the nontransfected and transfected cells and subjected to immunoprecipitation with anti-Hrs or anti-HA antibodies, respectively. Phosphorylation was assessed by immunoblotting with PY20 antibody. Molecular weight markers are indicated. (C and D) An intact FYVE domain is required for efficient Hrs phosphorylation. (C) HeLa cells were transfected with either Hrs-HA, ΔZF-HA, Y197F-HA, Y216F-HA, C215S-HA, C190S-HA, or C190/215S-HA. Cells were starved for 12 h before the experiment. The cells were stimulated at 22 h posttransfection with 100 ng of EGF per ml, then lysed, and processed as described in Materials and Methods. The lysates were subjected to immunoprecipitation with anti-HA antibody, and the immunoprecipitated proteins were analyzed by immunoblotting with PY20 antibody (top). Levels of expression of the different mutants were compared by immunoblotting 25 μg of lysate with anti-HA antibody (bottom). Quantitation of collected experiments (n ≥ 3, for each mutant) is shown in panel D. Mutation of the FYVE domain reduces phosphorylation to levels similar to those seen in the presence of wortmannin (Hrs + Wrt, n = 4).
We considered the hypothesis that PI 3-P-dependent localization to membranes may be required for Hrs phosphorylation. As a first test, serum-starved HeLa cells were pretreated with wortmannin or left untreated for 15 min prior to stimulation with EGF for a further 8 min with or without wortmannin. We observed a phosphotyrosine signal associated with immunoprecipitated Hrs that was entirely dependent on EGF and could be completely inhibited by wortmannin at concentrations where it acts as a selective inhibitor of PI 3-kinase enzymes (37) (Fig. 3B). A protein of approximately 72 kDa, for which tyrosine phosphorylation was likewise EGF dependent and wortmannin sensitive, coimmunoprecipitated with Hrs. We consider that this protein is likely to be STAM, Hbp, or a related protein. When we immunoprecipitate Hrs-HA from transfected cells with an anti-HA antibody, we obtain similar results with respect to Hrs but do not pull down the associated protein (Fig. 3B). We also repeated this experiment using HGF to stimulate the cells and obtained equivalent results (data not shown). This indicates that PI 3-kinase activity is likely to be a general requirement for Hrs phosphorylation and not specific to a given receptor.
An intact FYVE domain is required for efficient Hrs phosphorylation.
One consequence of treating cells with wortmannin is to inhibit the hVPS34 PI 3-kinase enzyme (36), which solely and constitutively produces PI 3-P, the lipid that accumulates on endosomes and binds to FYVE domains. If this interaction is necessary for phosphorylation, then removal or mutagenesis of the FYVE domain should similarly ablate phosphorylation.
Overexpressed Hrs-HA in HeLa cells is phosphorylated following EGF stimulation, similar to the endogenous protein. This phosphorylation is also greatly reduced by wortmannin treatment (73% ± 8% inhibition; n = 4 (Fig. 3B and D). When FYVE domain mutants (C215S, C190S, C190/215S, and Δfyve) of Hrs-HA are expressed at the same levels, the corresponding level of phosphorylation is also greatly reduced (Fig. 3C), in fact to a level similar to that achieved by wortmannin treatment (Fig. 3D). Out of 30 total tyrosine residues in Hrs, there is one tyrosine (Y197) within the FYVE domain and one adjacent to the final cysteine (Y216). It was therefore important to assess their contribution to the phosphotyrosine pool in case their ability to act as substrates was directly perturbed by the FYVE domain mutations that we introduced. Mutation of either of these tyrosines to phenylalanine did not significantly affect the intensity of Hrs-associated phosphotyrosine and therefore cannot account for the large reduction in phosphorylation observed with FYVE mutations (Fig. 3C and D).
Endocytosis is required for Hrs phosphorylation.
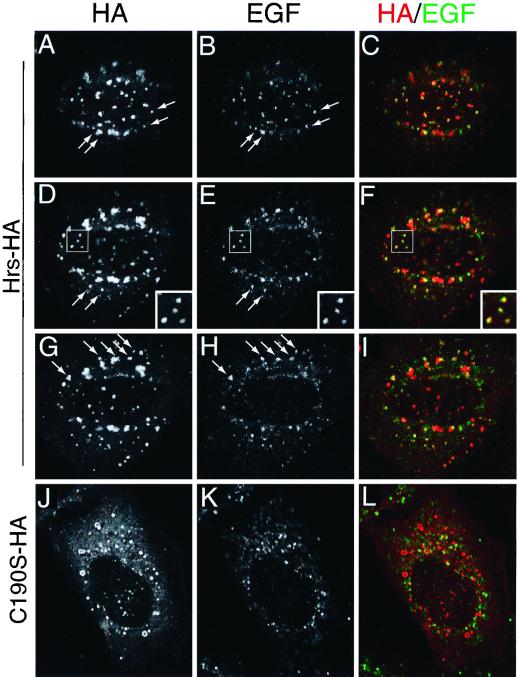
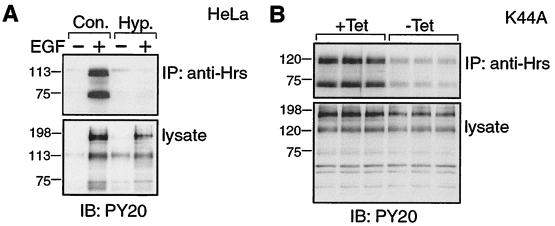
Hrs partially localizes to early endosomes, where it can specifically bind the PI 3-kinase product PI 3-P. We wondered if it might be necessary for the EGF receptor (or a downstream effector) to be delivered to this location by endocytic vesicle transport in order for phosphorylation to occur. Immunofluorescence studies demonstrated that a substantial fraction of internalized EGF reaches an Hrs-HA-positive compartment following an 8-min incubation pulse, mimicking our stimulation conditions (Fig. 4A through I). We could not detect internalized EGF in compartments labeled with mutant forms of Hrs-HA even after 30 min of internalization (see, for example, Figure 4J through L for 8-min internalization; 30-min internalization not shown). We first used a rather crude method of inhibiting clathrin-coated vesicle-mediated internalization, that of incubating cells in hyperosmotic medium (10). This condition completely abrogated EGF-dependent tyrosine phosphorylation of Hrs (Fig. 5A). We also obtained similar results when we depleted the plasma membrane of cholesterol using β-methylcyclodextrin (data not shown), another treatment which has been shown to inhibit endocytosis (25). We required a more specific intervention and turned to a stably transfected HeLa cell line, which when cultured in the absence of tetracycline expresses a dominant-negative form of dynamin (K44A), a protein essential for clathrin-coated vesicle-mediated endocytosis (34). In these experiments, more than 95% of cells in culture were able to express HA epitope-tagged K44A dynamin upon tetracycline withdrawal. Expression of K44A dynamin inhibited internalization of transferrin and EGF as judged by immunofluorescence (data not shown) and correspondingly inhibited EGF-dependent phosphorylation of Hrs and its associated 72-kDa polypeptide (Fig. 5B).
FIG. 4.
Internalized EGF colocalizes with Hrs-HA but not with C190S-HA. HeLa cells were transfected with Hrs-HA (A through I) or C190S-HA (J through L) and starved in serum-free medium for 12 h. Biotinylated EGF (50 ng/ml) was internalized for 8 min, and the cells were fixed with PFA and processed for immunofluorescence. The cells were labeled with anti-HA antibody, followed by a Texas red-coupled secondary antibody (A, D, G, and J; red in overlays). Internalized EGF was labeled with streptavidin coupled to Oregon Green 488 (B, E, H, and K; green in overlays). Panels A to C, D to F, and G to I show three consecutive sections taken at 260-nm intervals. Note the corresponding labeling pattern for HA and EGF in single channels (first and second columns). Arrows have been used to highlight examples of punctae associated with both labels. In panels D to F, a constellation of double-labeled punctae bounded by a box is also shown at higher magnification. Panels J to L show a single confocal section.
FIG. 5.
Clathrin-mediated endocytosis is required for EGF-dependent tyrosine phosphorylation of Hrs. (A) HeLa cells were starved for 16 h in serum-free medium and stimulated for 8 min with (+) or without (−) EGF (100 ng/ml) in the absence (Con.) or presence (Hyp [hypertonic medium]) of 450 mM sucrose. The cells were lysed, and proteins were immunoprecipitated with anti-Hrs antibody. Immunoprecipitated and total proteins (lysate) were analyzed by immunoblotting with PY20 antibody. (B) Stably transfected K44A cells (+Tet) were induced to express a dominant negative dynamin mutant by tetracycline withdrawal (−Tet). Cells were then starved for 16 h in serum-free medium, stimulated for 8 min with EGF (100 ng/ml), and lysed. Tyrosine-phosphorylated proteins immunoprecipitated with anti-Hrs antibody, and total proteins from triplicate experiments were analyzed as described in the legend to panel A. No phosphotyrosine signal was immunoprecipitated in the absence of EGF (data not shown). The tyrosine-phosphorylated band in the lysate just below the 198-kDa marker corresponds to EGF receptor and is decreased in the absence of clathrin-mediated endocytosis in the results shown in panels A and B.
Phosphorylated Hrs is predominantly cytosolic.
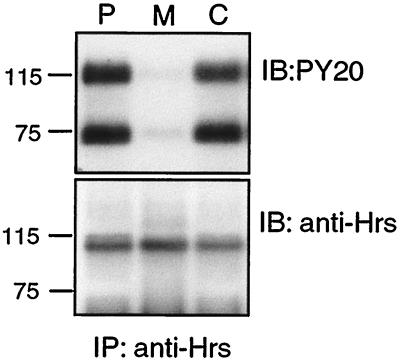
The simplest model to explain the foregoing data is that recruitment of Hrs to the early endosome via PI 3-P interaction with the FYVE domain is required for Hrs phosphorylation, by or downstream of an internalized factor. We examined the distribution of endogenous Hrs between particulate-membrane and cytosolic constituents of a HeLa cell postnuclear supernatant. Immunoblot analyses performed on total proteins from membranes and cytosol of cells treated or untreated with EGF did not reveal any redistribution of bulk Hrs following stimulation (data not shown). We found the majority of Hrs to be cytosolic and most surprisingly that the phosphorylated form is proportionately enriched in the cytosol (Fig. 6). In fact, it was difficult to detect the tyrosine-phosphorylated form associated with the membrane fraction.
FIG. 6.
Phosphorylated Hrs is predominantly cytosolic. HeLa cells were starved for 16 h in serum-free medium and stimulated for 8 min with 100 ng of EGF per ml. Postnuclear supernatant (P), membranes (M), and cytosol (C) were prepared as described in Materials and Methods. Equal amounts of protein were then subjected to immunoprecipitation with anti-Hrs antibody and analyzed by immunoblotting with PY20 antibody (top) or anti-Hrs (bottom). Molecular weight markers (prestained) are indicated.
DISCUSSION
The discovery of the FYVE domain as a PI 3-P binding motif has encouraged some general questions about its cellular utilization. In the best-studied case of EEA1, it is required for membrane association through PI 3-P binding, although the picture is somewhat complicated by the ability of this domain to interact with other membrane-associated factors such as rab5 and syntaxin 6/13 (15, 26, 27). Does the distribution of FYVE domain proteins therefore reflect the subcellular distribution of PI 3-P, or does it tend to reflect statistical cooperativity with more-specific interacting factors? It is also possible to imagine that the interaction with PI 3-P may be used to allosterically regulate a protein while membrane localization is conferred by other interactions.
We have chosen to study the protein Hrs which contains a classical FYVE domain that has been shown to bind PI 3-P (5, 8). It is a candidate regulator of endocytic membrane traffic, and its prominent tyrosine phosphorylation provides us with an easily measurable output for probing the influence of the FYVE domain–PI 3-P interaction. Hrs has previously been localized to endocytic compartments on the basis of colocalization with transferrin receptor (12). We have now examined the overlap with EEA1 in some detail and find this is by no means complete. Using expression of epitope-tagged Hrs-HA, we find that at relatively low levels of overexpression there is considerable overlap with EEA1-labeled punctae but that there remain many punctae which label with only one of either marker (Fig. 1A and 2A through C). This demonstrates for the first time that possession of a FYVE domain need not dictate localization to a shared compartment. High levels of Hrs-HA expression lead to the creation of large structures which show a high degree of colocalization between Hrs, EEA1, transferrin receptor, and the late endosomal marker M6PR, although they remain distinct from Golgi markers and the late endosome-lysosome marker CD63 (Fig. 1). We interpret this to indicate that Hrs specifically influences the dynamics of multiple endocytic compartments which merge when the protein is overexpressed, perhaps due to promotion of vesicle aggregation or of vesicle fusion. A related protein, Hrs2, is proposed to negatively regulate exocytosis by competing with VAMP for SNAP-25 binding, thereby inhibiting SNARE complex formation (32). If function is conserved, then it may be that the large structures represent aggregates of vesicles that accumulate via a tethering step, prior to SNARE complex formation and commitment to fusion (6). It is also possible that large endocytic vacuoles may form if multivesicular body formation at the sorting endosome is blocked by Hrs expression, similar to those which accumulate with class E mutants in yeast (28). As the M6PR traverses the early endosome en route to late endosomes where it accumulates (14), the observed colocalization of M6PR with early endosomal markers is consistent with this model.
What then specifies Hrs compartmental localization? Our data are most consistent with a multiplicity of signals. When we disrupt FYVE domain structure by mutation of cysteines that coordinate zinc, we shift the distribution towards the cytosol, and the membrane fraction that remains no longer overlaps with EEA1 or other markers we have studied. Instead, we find the expressed proteins confined to distinct structures which frequently adopt a unique ring appearance. When we overexpress Hrs-HA, it is less sensitive to wortmannin than endogenous protein, in that the membrane-bound fraction does not fall off to the same degree. Perhaps, in this case, high levels of expression allow efficient interaction with an accessory factor through mass action. Thus, we propose that FYVE–PI 3-P interactions may cooperate with a second interaction located elsewhere in the protein to specify the localization of wild-type protein. When the whole FYVE domain is removed, membrane interaction reverts to the second interaction utilized by the wild-type protein. In the absence of statistical cooperativity with PI 3-P binding, the overall affinity for membranes is reduced, and there is now no overlap with EEA1 unless the protein is expressed at very high levels (data not shown). Although these data are striking, we regard the formation of ring structures by the cysteine mutants as an epiphenomenon at present.
EGF reaches Hrs-HA-positive compartments within our 8-min stimulation period (Fig. 4). Importantly, it fails to reach the compartments labeled by any of the FYVE domain mutants even after a 30-min incubation when overlap with wild-type protein is even more striking, as assessed by immunofluorescence. We hypothesized that Hrs phosphorylation may reflect the coincidence of PI 3-P-determined localization of Hrs to an endosomal compartment together with vesicle-mediated internalization of activated receptor (or effector) to that same compartment. This model requires that depletion of PI 3-P, disruption of the PI 3-P-interacting FYVE domain, or inhibition of endocytosis should abrogate EGF-dependent phosphorylation of Hrs. Our data show that each of these requirements is met. Depletion of PI 3-P by wortmannin, disruption of the FYVE domain by mutation and inhibition of endocytosis by hypertonic medium, or overexpression of dominant-negative mutant dynamin leads to failure of phosphorylation.
There are of course caveats to some of our experiments. We have used wortmannin to inhibit PI 3-kinase activity; this will undoubtedly inhibit the hVps34 enzyme believed to generate PI 3-P that accumulates on endosomes but will also inhibit other members of the PI 3-kinase family, including the p110 catalytic subunit which associates with activated growth factor receptors via a p85 adapter subunit (21). We cannot therefore completely discount a supplementary role for class 1 PI 3-kinase activity in Hrs phosphorylation although the effects of mutations in the FYVE domain argue strongly for a role for hVps34. Expression of dominant-negative dynamin has been shown to change the binding affinity of EGF receptors at the plasma membrane (24). With regard to this latter point, we have used saturating conditions of ligand and while EGF receptor phosphorylation is itself somewhat reduced (Fig. 5) as previously described (35), many other EGF-dependent tyrosine phosphorylations occur as efficiently as in control cells. Although it is simplest to assume that the requirement for endocytosis reflects a requirement for receptor internalization, it is also possible that internalization of a downstream effector is the relevant event. In COS-7 cells, expression of mutant dynamin K44A inhibits mitogen-activated protein kinase activation following EGF stimulation, but it appears that endocytosis of activated mitogen-activated protein kinase kinase is required rather than that of the receptor itself (13).
We have used tyrosine phosphorylation as our measurable output to provide a vivid example that combines several contemporary themes in discussion of signal transduction pathways: localization, coincidence detection, and dynamic regulation resulting from membrane traffic. As yet, the function of Hrs tyrosine phosphorylation is unclear. Although we believe phosphorylation occurs at the endosomal membrane, the phosphorylated protein is proportionately more cytosolic. We propose, then, that phosphorylation is used as a switch for translocation to the cytosol where it may carry out signaling functions together with associated proteins such as STAM or Hbp. If we assume that membrane-associated Hrs influences endocytic trafficking (and this is certainly true following overexpression) (Fig. 1), this leaves us with the attractive notion that an incoming receptor can govern its own fate by stimulating Hrs phosphorylation on endosomes. Further experiments will be needed to decide exactly which aspect of trafficking Hrs regulates and whether it acts as a clamp or a stimulator.
ACKNOWLEDGMENTS
S.U. is supported by the North West Cancer Research Fund and I.G.M. is the recipient of a Wellcome Trust Prize studentship.
We gratefully acknowledge members of the Haematology Department, University of Liverpool, for the use of their confocal microscope. We also thank S. Schmid for dynamin (K44A) cells, B. Reaves and P. Luzio for gifts of antibodies, D. Shima for the GFP-NAGTI construct, and D. Fernig and J. Smith for EGF.
REFERENCES
- 1.Asao H, Sasaki Y, Arita T, Tanaka N, Endo K, Kasai H, Takeshita T, Endo Y, Fujita T, Sugamura K. Hrs is associated with STAM, a signal-transducing adaptor molecule. J Biol Chem. 1997;272:32785–32791. doi: 10.1074/jbc.272.52.32785. [DOI] [PubMed] [Google Scholar]
- 2.Bankaitis V A, Johnson L M, Emr S D. Isolation of yeast mutants defective in protein targeting to the vacuole. Proc Natl Acad Sci USA. 1986;83:9075–9079. doi: 10.1073/pnas.83.23.9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bean A J, Seifert R, Chen Y A, Sacks R, Scheller R H. Hrs-2 is an ATPase implicated in calcium-regulated secretion. Nature. 1997;385:826–829. doi: 10.1038/385826a0. [DOI] [PubMed] [Google Scholar]
- 4.Burd C G, Babst M, Emr S D. Novel pathways, membrane coats and PI kinase regulation in yeast lysosomal trafficking. Semin Cell Dev Biol. 1998;9:527–533. doi: 10.1006/scdb.1998.0255. [DOI] [PubMed] [Google Scholar]
- 5.Burd C G, Emr S D. Phosphatidylinositol(3)-phosphate signaling mediated by specific binding to RING FYVE domains. Mol Cell. 1998;2:157–162. doi: 10.1016/s1097-2765(00)80125-2. [DOI] [PubMed] [Google Scholar]
- 6.Clague M J. Membrane transport: take your fusion partners. Curr Biol. 1999;9:R258–R260. doi: 10.1016/s0960-9822(99)80158-4. [DOI] [PubMed] [Google Scholar]
- 7.Gaullier J-M, Simonsen A, D'Arrigo A, Bremnes B, Stenmark H. FYVE finger proteins as effectors of phosphatidylinositol 3-phosphate. Chem Phys Lipids. 1999;98:87–94. doi: 10.1016/s0009-3084(99)00021-3. [DOI] [PubMed] [Google Scholar]
- 8.Gaullier J-M, Simonsen A, D'Arrigo A, Bremnes B, Stenmark H. FYVE fingers bind PtdIns(3)P. Nature. 1998;394:432–433. doi: 10.1038/28767. [DOI] [PubMed] [Google Scholar]
- 9.Gorvel J P, Chavrier P, Zerial M, Gruenberg J. Rab 5 controls early endosome fusion in vitro. Cell. 1991;64:915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- 10.Heuser J, Anderson R G W. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J Cell Biol. 1989;108:389–400. doi: 10.1083/jcb.108.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Komada M, Kitamura N. Growth factor-induced tyrosine phosphorylation of Hrs, a novel 115-kilodalton protein with a structurally conserved putative zinc finger domain. Mol Cell Biol. 1995;15:6213–6221. doi: 10.1128/mcb.15.11.6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Komada M, Masaki R, Yamamoto A, Kitamura N. Hrs, a tyrosine kinase substrate with a conserved double zinc finger domain, is localized to the cytoplasmic surface of early endosomes. J Biol Chem. 1997;272:20538–20544. doi: 10.1074/jbc.272.33.20538. [DOI] [PubMed] [Google Scholar]
- 13.Kranenburg O, Verlaan I, Moolenar W H. Dynamin is required for the activation of mitogen-activated protein (MAP) kinase by MAP kinase kinase. J Biol Chem. 1999;274:35301–35304. doi: 10.1074/jbc.274.50.35301. [DOI] [PubMed] [Google Scholar]
- 14.Ludwig T, Griffiths G, Hoflack B. Distribution of newly synthesized lysosomal enzymes in the endocytic pathway of normal rat kidney cells. J Cell Biol. 1991;115:1561–1572. doi: 10.1083/jcb.115.6.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McBride H M, Rybin V, Murphy C, Giner A, Teasdale R, Zerial M. Oligomeric complexes link Rab5 effectors with NSF and drive membrane fusion via interactions between EEA1 and syntaxin 13. Cell. 1999;98:377–386. doi: 10.1016/s0092-8674(00)81966-2. [DOI] [PubMed] [Google Scholar]
- 16.Mills I G, Jones A T, Clague M J. Involvement of the endosomal autoantigen EEA1 in homotypic fusion of early endosomes. Curr Biol. 1998;8:881–884. doi: 10.1016/s0960-9822(07)00351-x. [DOI] [PubMed] [Google Scholar]
- 17.Misra S, Hurley J H. Crystal structure of a phosphatidylinositol 3-phosphate-specific membrane-targeting motif, the FYVE domain of Vps27p. Cell. 1999;97:657–666. doi: 10.1016/s0092-8674(00)80776-x. [DOI] [PubMed] [Google Scholar]
- 18.Mu F-T, Callaghan J M, Steele-Mortimer O, Stenmark H, Parton R G, Campbell P L, McCluskey J, Yeo J-P, Tock E P C, Toh B-H. EEA1, an early endosome-associated protein. J Biol Chem. 1995;270:13503–13511. doi: 10.1074/jbc.270.22.13503. [DOI] [PubMed] [Google Scholar]
- 19.Patki V, Lawe D C, Corvera S, Virbasius J V, Chawla A. A functional PtdIns(3)P-binding motif. Nature. 1998;394:433–434. doi: 10.1038/28771. [DOI] [PubMed] [Google Scholar]
- 20.Patki V, Virbasius J, Lane W S, Toh B-H, Shpetner H S, Corvera S. Identification of an early endosomal protein regulated by phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 1997;94:7326–7330. doi: 10.1073/pnas.94.14.7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rameh L E, Cantley L C. The role of phosphoinositide 3-kinase lipid products in cell function. J Biol Chem. 1999;274:8347–8350. doi: 10.1074/jbc.274.13.8347. [DOI] [PubMed] [Google Scholar]
- 22.Raymond C K, Howald-Stevenson I, Vater C A, Stevens T H. Morphological classification of the yeast vacuolar protein sorting mutants: evidence for a prevacuolar compartment in class E vps mutants. Mol Biol Cell. 1992;3:1389–1402. doi: 10.1091/mbc.3.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reaves B J, Bright N A, Mullock B M, Luzio J P. The effect of wortmannin on the localisation of lysosomal type 1 integral membrane glycoproteins suggests a role for phosphoinositide 3-kinase activity in regulating membrane traffic late in the endocytic pathway. J Cell Sci. 1996;109:749–762. doi: 10.1242/jcs.109.4.749. [DOI] [PubMed] [Google Scholar]
- 24.Ringerike T, Stang E, Johannessen L E, Sandness D, Levy F O, Madshus I H. High-affinity binding of epidermal growth factor (EGF) to EGF receptor is disrupted by overexpression of mutant dynamin. J Biol Chem. 1998;273:16639–16642. doi: 10.1074/jbc.273.27.16639. [DOI] [PubMed] [Google Scholar]
- 25.Rodal S K, Skretting G, Garred O, Vilhardt F, van Deurs B, Sandvig K. Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formation of clathrin coated vesicles. Mol Biol Cell. 1999;10:961–974. doi: 10.1091/mbc.10.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simonsen A, Gaullier J M, D'Arrigo A, Stenmark H. The rab5 effector EEA1 interacts directly with syntaxin-6. J Biol Chem. 1999;274:28857–28860. doi: 10.1074/jbc.274.41.28857. [DOI] [PubMed] [Google Scholar]
- 27.Simonsen A, Lippé R, Christoforidis S, Gaullier J-M, Brech A, Callaghan J, Toh B-H, Murphy C, Zerial M, Stenmark H. EEA1 links PI(3)K function to rab5 regulation of endosome fusion. Nature. 1998;394:494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- 28.Stack J H, Horazdovsky B, Emr S D. Receptor-mediated protein sorting to the vacuole in yeast: roles for a protein kinase, a lipid kinase and GTP binding proteins. Annu Rev Cell Dev Biol. 1995;11:1–33. doi: 10.1146/annurev.cb.11.110195.000245. [DOI] [PubMed] [Google Scholar]
- 29.Stenmark H, Aasland R. FYVE-finger proteins—effectors of an inositol lipid. J Cell Sci. 1999;112:4175–4183. doi: 10.1242/jcs.112.23.4175. [DOI] [PubMed] [Google Scholar]
- 30.Stenmark H, Vitale G, Ullrich O, Zerial M. Rabaptin-5 is a direct effector of the small GTPase rab5 in endocytic membrane fusion. Cell. 1995;83:423–432. doi: 10.1016/0092-8674(95)90120-5. [DOI] [PubMed] [Google Scholar]
- 31.Takata H, Kato M, Denda K, Kitamura N. A hrs protein having a Src homology 3 domain is involved in intracellular degradation of growth factors and their receptors. Genes Cells. 2000;5:57–69. doi: 10.1046/j.1365-2443.2000.00303.x. [DOI] [PubMed] [Google Scholar]
- 32.Tsujimoto S, Bean A J. Distinct protein domains are responsible for the interaction of Hrs-2 with SNAP-25. J Biol Chem. 2000;275:2938–2942. doi: 10.1074/jbc.275.4.2938. [DOI] [PubMed] [Google Scholar]
- 33.Tsukazaki T, Chiang T A, Davison A F, Attisano L, Wrana J L. SARA, a FYVE domain protein that recruits Smad2 to the TGFb receptor. Cell. 1998;95:779–791. doi: 10.1016/s0092-8674(00)81701-8. [DOI] [PubMed] [Google Scholar]
- 34.van der Bliek A, Redelmeier T E, Damke H, Tisdale E J, Meyerowitz E M, Schmid S L. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J Cell Biol. 1993;122:553–563. doi: 10.1083/jcb.122.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vieria A V, Lamaze C, Schmid S L. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- 36.Volinia S, Dhand R, Vanhaesebroeck B, Macdougall L K, Stein R, Zvelebil M J, Domin J, Panaretou C, Waterfield M D. A human phosphatidylinositol 3-kinase complex related to the yeast Vps34p-Vps15p protein sorting system. EMBO J. 1995;14:3339–3348. doi: 10.1002/j.1460-2075.1995.tb07340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woscholski R, Kodaki T, McKinnon M, Waterfield M D, Parker P J. A comparison of demethoxyviridin and wortmannin as inhibitors of phosphatidylinositol 3-kinase. FEBS Lett. 1994;342:109–114. doi: 10.1016/0014-5793(94)80482-6. [DOI] [PubMed] [Google Scholar]
- 38.Wurmser A E, Gary J D, Emr S D. Phosphoinositide 3-kinases and their FYVE domain-containing effectors as regulators of vacuolar/lysosomal membrane trafficking pathways. J Biol Chem. 1999;274:9129–9132. doi: 10.1074/jbc.274.14.9129. [DOI] [PubMed] [Google Scholar]