Abstract
OBJECTIVE
To identify the geographic distribution of exhibition swine in the Midwestern United States, characterize management practices used for exhibition swine, and identify associations between those practices and influenza A virus (IAV) detection in exhibition swine arriving at county or state agricultural fairs.
DESIGN
Cross-sectional survey.
SAMPLE
480 swine exhibitors and 641 exhibition swine.
PROCEDURES
Inventories of swine exhibited at fairs in 6 selected Midwestern states during 2013 and of the total swine population (including commercial swine) in these regions in 2012 were obtained and mapped. In 2014, snout wipe samples were collected from swine on arrival at 9 selected fairs in Indiana (n = 5) and Ohio (4) and tested for the presence of IAV. Also at fair arrival, swine exhibitors completed a survey regarding swine management practices.
RESULTS
Contrary to the total swine population, the exhibition swine population was heavily concentrated in Indiana and Ohio. Many swine exhibitors reported attending multiple exhibitions within a season (median number, 2; range, 0 to 50), with exhibited swine often returned to their farm of origin. Rearing of commercial and exhibition swine on the same premises was reported by 13.3% (56/422) of exhibitors. Hosting an on-farm open house or sale was associated with an increased odds of IAV detection in snout wipe samples.
CONCLUSIONS AND CLINICAL RELEVANCE
The exhibition swine population was highly variable and differed from the commercial swine population in terms of pig density across geographic locations, population integrity, and on-farm management practices. Exhibition swine may be important in IAV transmission, and identified biosecurity deficiencies may have important public and animal health consequences.
Influenza A virus, a member of the Orthomyxoviridae family, is characterized by an enveloped virion containing a single-stranded, negative-sense RNA genome.1 Mutations accumulating within the IAV genome and reassortment of viral genomic segments allow for ongoing evolution of the virus to rapidly occur.2 Although many animal species serve as hosts for IAV,3 swine have been designated the so-called mixing vessel for IAV, and reassortant IAV strains have been routinely identified within that population.4,5 Surveillance of IAV infections in US swine herds has recently revealed rapid expansion of viral diversity.6 Bidirectional transmission of IAV between swine and human populations has resulted in an increase in IAV diversity and detrimental health outcomes for both species.7–12
Zoonotic transmission of swine-lineage IAV to humans (referred to as a variant IAV infection) has recently gained considerable attention from public health officials.13 Since 2005, 378 human cases of variant IAV infection have been reported in the United States,14 with many additional infections likely unreported.15 Individuals most likely to have severe consequences from seasonal and variant IAV infections include those in high-risk groups, such as children < 5 years of age, people > 65 years of age, pregnant women, and the immunocompromised.16 Zoonotic transmission of IAV between swine and humans has been most commonly reported to occur at agricultural fairs.10,17–19
Exhibition swine represent a unique population in the swine industry, given that such swine may be shown multiple times across wide geographic expanses, over a fairly short time span, in exhibitions that can include swine of any age. Although the exhibition swine industry accounts for only an estimated 1.5% of the total swine population in the United States,a agricultural fairs provide an opportunity for exhibition swine to come into contact with the general public as well as other exhibition swine from multiple locations, providing a permissive environment for IAV transmission. For example, at a large state fair, 97% of exhibitors reportedly later returned to their farm with their animals,20 and 39% of youths registered to exhibit pigs at another state fair reportedly raised commercial and exhibition swine at the same location.21 These practices could have promoted IAV transmission between the 2 swine populations.
The National Association of State Public Health Veterinarians, National Assembly of State Animal Health Officials, CDC, and National 4-H Headquarters have put forth several mitigation strategies and suggestions for controlling the movement of IAV in exhibition swine populations and decreasing the risk of human exposure to the virus.22 Addressing transmission to humans, they advise that people at high risk of serious IAV complications avoid contact with swine or swine environments.23 Individuals not at high risk are generally advised to avoid or minimize contact with ill swine, practice good hand hygiene, and use personal protection if coming into direct contact with ill swine.24 The CDC also recommends that swine caretakers receive seasonal influenza vaccinations.25
With respect to managing swine health, owners of exhibition swine are encouraged to vaccinate swine against IAV infection and to contact a veterinarian if swine are noticed to have influenza-like illness.22 Other suggestions include the implementation of biosecurity measures, isolation and observation of swine for indications of influenza-like illness for 7 days after exhibition, cleaning and disinfection of swine equipment, and maintenance of adequate ventilation within the swine barns.22,25 Although implementation of these general measures is expected to decrease the likelihood of swine-to-human IAV transmission, no more specific recommendations for controlling the transmission of IAV have been made because of the paucity of data regarding the exhibition swine population and the animal management practices used within this niche industry.
Eighteen states have reported variant IAV infections since 2005, with > 70% of cases occurring in Indiana (n = 154) and Ohio (113),14 which are states that rank fifth and tenth, respectively, in total swine inventory.26 Interestingly, the number of reported cases of variant IAV infection does not strongly correspond geographically with the larger commercial swine populations in Iowa, North Carolina, and Minnesota (ranked first, second, and third, respectively, in total swine inventory).
The purpose of the study reported here was to identify the geographic distribution of the exhibition swine industry in 6 Midwestern states, characterize swine management practices used at exhibition swine farms in 2 states (Indiana and Ohio), and identify associations between these practices and IAV detection in exhibition swine arriving at agricultural fairs.
Materials and Methods
Swine inventories
State animal health officials, county extension educators, or local fair organizers in Illinois, Indiana, Iowa, Michigan, Missouri, and Ohio were contacted to determine the number of swine exhibitions and swine exhibited at county or local agricultural fairs during 2013. State fairs and other swine exhibitions were intentionally excluded. Total swine population data for each county were obtained from the 2012 US Agricultural Census report.26 The reported total number of swine and number of exhibition swine per county were interpolated to the geometric centroid of each county. A continuous spatial distribution for each population was developed by use of the inverse distance weighting based on 15 neighbors.b An 8-tier geometric interval color scale was used to generate a visual heat-map, reflecting geographic swine densities.
Survey development and administration
Adult people accompanying the exhibition swine to 9 selected agricultural fairs (A through I) across Indiana (n = 5) and Ohio (4) during July and August 2014 were approached in person, informed about the study, and recruited with oral consent. A 24-question paper survey containing closed-ended questions was administered in English to consenting participants (Supplementary Appendix S1, available at avmajournals.avma.org/doi/suppl/10.2460/javma.249.6.706). Surveys were modeled after a survey administered to fair officials in another study.27 No personal identifying information was collected, but participants were asked to provide the individual identification numbers of their swine, allowing survey and swine IAV surveillance data to be linked. Investigators had no owner information associated with the swine identification numbers and therefore could not trace the results to a specific person or farm. Participants were asked to complete only 1 survey for each exhibition swine–rearing premises (ie, 1 completed survey could have represented multiple swine exhibitors and several exhibition swine).
Because of differences in individual management practices at the fairs, surveys were administered in 1 of 2 ways. At exhibition A, surveys were administered in person while samples were being collected from swine. At exhibitions B through I, surveys were distributed to participants at the beginning of the fair and collected via a centrally located drop box throughout the exhibition period. Survey research was conducted with the approval of The Ohio State University Institutional Review Board (protocol No. 2014E0141).
Sample collection at fairs
Snout wipe samples were collected from exhibition swine arriving at all 9 agricultural fairs as part of a previously reported prevalence study,28 for which the methods of sample collection have been reported in detail. Samples were tested for IAV by means of a real-time reverse-transcription PCR assay, and samples with cycle threshold values ≤ 35 were classified as IAV positive.29 Virus isolation was attempted on samples with positive results.28 The IAV test results were then linked to the corresponding survey. The protocol for this portion of the study was approved by The Ohio State University Institutional Animal Care and Use Committee (protocol No. 2009A0134-R1).
Statistical analysis
Survey responses were entered into a statistical programc and screened for data-entry errors, and obviously spurious results were dropped from analyses. Descriptive statistics were calculated for all variables. Data from surveys for which respondents provided no swine identification were used for descriptive analyses of swine management practices but were excluded from analyses of risk factors for IAV detection. Univariate exact logistic regression was used to examine reported individual swine management practices for associations with IAV detection. Odds ratios and 95% CIs were calculated. Values of P < 0.05 were considered significant.
Results
Exhibition swine inventories
Inventories of county fair exhibition swine were received from 553 of the 578 (95.7%) counties in 6 surveyed Midwestern states (Table 1). The mean number of swine exhibited per county fair ranged from 53.5 in Missouri to 226.8 in Indiana. Mapping of these data revealed that the exhibition industry was concentrated in Indiana and Ohio, whereas of the 6 states evaluated, the largest concentration of commercial swine was in Iowa (Figure 1).
Table 1—
Distribution of local or county agricultural fairs and exhibition swine per fair in 6 Midwestern states in 2013.
| State | No. of local fairs | Median (range) No. of exhibition swine per fair |
|---|---|---|
| Illinois | 103 | 50 (0–346) |
| Indiana | 96 | 223.5 (13–539) |
| Iowa | 100 | 106.5 (21–405) |
| Michigan | 86 | 84.5 (0–450) |
| Missouri | 123 | 36 (0–350) |
| Ohio | 95 | 175 (0–768) |
State fairs were deliberately excluded.
Figure 1—
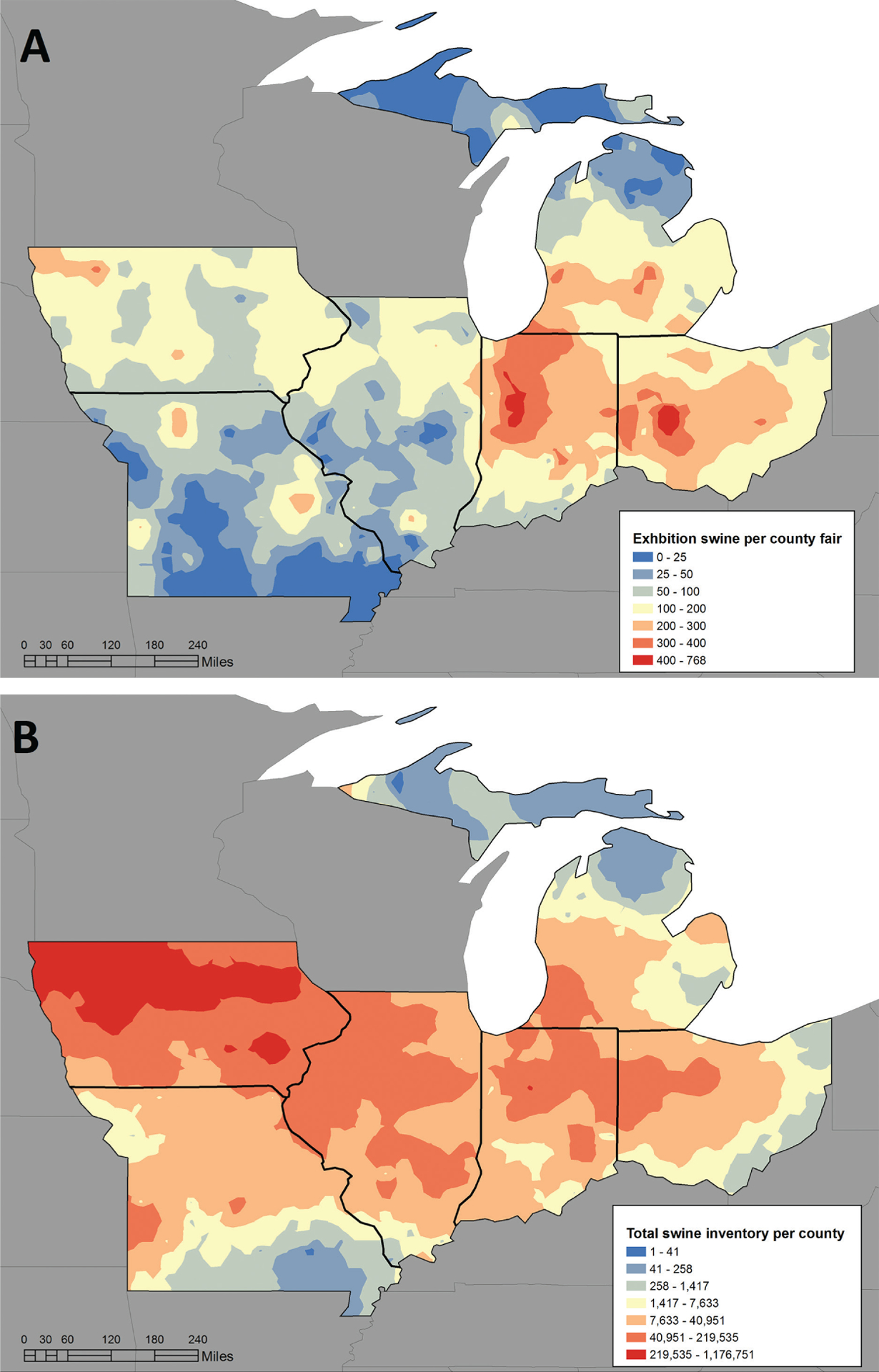
Reported numbers (heat maps) of exhibition and commercial swine in the Midwestern United States by geographic region in 2013. A—Distributions of exhibition swine at county fairs during 2013 in Illinois, Indiana, Iowa, Michigan, Missouri, and Ohio. B—Total swine (exhibition and commercial) distributions in each county in 2012, per the USDA 2012 Census of Agriculture. In both panels, dark red indicates the highest density of swine, and dark blue represents the lowest density of swine.
Swine exhibitor survey
Completed surveys were collected from swine exhibitors at all 9 fairs in Indiana and Ohio. Fair organizers reported a total of 3,662 swine exhibitors in attendance; 480 surveys were collected. Because multiple exhibitors could be represented per survey, a true response rate could not be calculated. Fifty-two (10.8%) of the collected surveys were excluded from the study because of illegibility or unusable responses. Data from the remaining 428 (89.2%) were included in analyses.
Survey responses were summarized (Tables 1 and 2). The median number of swine on the respondents’ farms on January 1, 2014, was 0, reflecting that swine were not housed year-round at most farms. Respondents reported showing swine at a mean of 3.1 exhibitions during the year of the fair where they completed the survey (ie, the study fair; median, 2 exhibitions; range, 0 to 50 exhibitions). Swine were reportedly returned to their farm of origin from prior exhibitions a mean of 2.9 times during calendar year 2014 (median, 2 times; range, 0 to 40 times). Of those who returned home from an exhibition with their swine, 48.6% (186/383) implemented some form of isolation for returning swine from other swine.
Table 2—
Summary of continuous data responses to a survey of swine management practices for 428 swine exhibitors at 9 selected agricultural fairs in Indiana (n = 5) and Ohio (4) during July and August 2014.
| Variable | No. of responses received | Median | Range | Mean | SD |
|---|---|---|---|---|---|
| No. of swine on farm on January 1 | 424 | 0 | 0–6,500 | 87.4 | 487.8 |
| Maximum No. of swine on farm in 2014 | 410 | 6 | 0–6,500 | 103.3 | 508.3 |
| No. of swine on farm the day before the fair | 424 | 4 | 0–6,500 | 75.1 | 465.3 |
| No. of exhibitions attended by farm representatives in 2014 | 425 | 2 | 0–50 | 3.1 | 4.8 |
| No. of exhibitions from which swine were returned to the farm of origin in 2014 | 426 | 2 | 0–40 | 2.9 | 4.1 |
| No. of exhibitions attended by individual swine | 542 | 1 | 0–30 | 2.2 | 3.2 |
| No. of exhibitions that individual swine were planned to attend after survey completion | 537 | 0 | 0–12 | 0.5 | 1.1 |
| Duration of isolation (d) from other farm animals for swine returning to the farm of origin after exhibition* | 23 | 14 | 0.08–30 | 14.7 | 11.1 |
| Estimated travel time (min) from loading to exhibition | 414 | 120 | 5–4,440 | 200.9 | 281.9 |
Responses pertained only to exhibitors from farms that included isolation as part of their farm management practices. The minimum duration of 0.08 days represents 2 hours.
Respondents could have included > 1 swine/survey.
Swine were obtained from an off-farm source by 75.4% of respondents, with 84.3% of respondents reporting their pig purchases occurred during March and April. Exhibition swine were raised in small herds (median maximum number of swine on farm during the calendar year prior to arrival at the study fair, 6; Table 2) and typically housed in an open building with natural ventilation and no outside access (201/423 [47.5%]) or an open building with outside access (166/423 [39.2%]). Additionally, 45.2% of the respondents reported that exhibitors or household members had contact with swine or a swine environment other than their own on at least a weekly basis. Commercial swine managed on the same farm as exhibition swine was reported by 13.3% of respondents. Twenty-one (37.5%) of the represented farms with commercial swine production and 41 (11.4%) of the represented farms without commercial swine production were reported as hosting an on-farm open house or sale, to which farm visitors were welcomed.
For farms where new swine were not directly mixed into the existing swine population, swine were placed in a separate pen that was not cleaned or disinfected (25/277 [9.0%]), a separate cleaned pen (69/277 [24.9%]), or a separate pen that was cleaned and disinfected (183/277 [66.1%]). For farms where other livestock were raised at the same location as exhibition swine, other reported species included cattle (53.5%), goats (32.4%), poultry (32.6%), horses (27.3%), sheep (24.8%), and other (eg, llamas or rabbits; 11.7%).
IAV detection
Of the 314 swine for which a vaccination status was reported in the survey at fair entry, 297 (94.6%) were sufficiently identified to be linked with an IAV test result. Vaccination against IAV infection was reported for 62.7% (197/314) of swine with a reported vaccination status. Of the 193 IAV-vaccinated swine that could be matched to an IAV sample, 15 (7.8%) had positive results of real-time reverse-transcription PCR assay for IAV, and IAV was recovered from snout wipe samples of 5 (2.6%) of these swine. The prevalence of IAV detection in the 104 swine reported not to have been vaccinated against IAV was 2.9% (n = 3), and IAV isolates were recovered from only 1 (1.0%) of these swine.
Hosting (vs not hosting) an open house or sale on the farm of origin was the only management practice significantly associated with an increased odds of IAV detection in snout wipe specimens at fair entry (OR, 3.9; 95% CI, 1.1 to 13.1). In addition, respondents who reported such activities also reported attending significantly (Student t test; P = 0.02) more exhibitions (mean number of exhibitions, 5.5) than did those who did not (mean number of exhibitions, 2.7).
Discussion
Findings of the present study indicated that exhibition swine in 6 Midwestern states differed in geographic concentration relative to commercial swine and that frequency of commingling as well as on-farm management practices used in the exhibition swine sector varied greatly. Although still a poorly defined interface, both commercial and exhibition swine were reportedly managed on a considerable number (13.3%) of the farms represented by surveyed swine exhibitors at local or county agricultural fairs in Indiana and Ohio.
The swine density maps constructed for the 6 states in the present study, which to our knowledge represented the first of their kind, revealed apparently greater numbers of exhibition swine in areas that maintained lower concentrations of commercial swine, as indicated by 2012 USDA swine census data. Given that exhibition swine account for only an estimated 1.5% of the US swine population, the total swine inventory represents a reliable estimate of commercial swine location by county. To maintain confidentiality, a small proportion of county data were omitted if individual operations could be identified in the 2012 USDA census (ie, only 1 producer in the county); therefore, the potential for bias in the density mapping was possible but not likely.
In a previous study,18 cases of variant IAV infection were linked to commercial swine density; however, visual examination of the geographic distribution of variant IAV cases suggests the locations are more closely aligned with the exhibition swine–dense areas detailed in the present study than with commercial swine populations. State fairs were deliberately excluded from the swine exhibitor survey and IAV detection portion of the present study because of our desire to more closely characterize the geographic locations where exhibition swine were raised. In the authors’ experience, swine exhibited at county fairs are raised closer to those fairs than swine exhibited at state fairs. Additionally, in instances in which county-exhibited swine were eligible for exhibition at a state fair, recruitment of subjects at the county level eliminated the chance of testing individual swine twice.
In the present study, most exhibition swine in the Midwestern United States were concentrated in Indiana and Ohio, corresponding to the area where > 70% of American cases of variant IAV infection have been reported and suggesting that exhibition swine density may play a role in the incidence of variant IAV infection. Considering the human population densities of counties in Indiana and Ohio, compared with those in other major swine production states, and the herein reported high number of swine exhibited at the county fairs in Ohio and Indiana, one could predict a greater opportunity for human-swine interactions in these 2 states than in others. Previous research27 has shown that the odds of having IAV-infected swine at fairs increases by 27% (OR, 1.27; 95% CI, 1.0 to 1.7) for every 20-pig increase in the size of the swine exhibition.
Results of the present study indicated that exhibition swine were generally raised on small farms where an assortment of management practices was used. Nearly half of swine exhibitors reported having direct contact with swine owned by others or with the environment of those swine at least weekly, indicating that these caretakers commonly moved between different groups of swine. Access to, or interaction of people or swine between, swine production premises is a practice that is strongly discouraged and is often against biosecurity policies in commercial swine production because of the increased risk of disease transmission associated with these behaviors. Consequently, the exhibition swine–rearing practices, relaxed biosecurity practices, and generally accepted movement of people or swine to multiple exhibitions identified in the present study may have resulted in greater opportunity for transmission of IAV. Given that most exhibition swine are reared as part of 4-H or FFA activities during the spring and summer months, these youth educational programs should consider emphasizing infectious disease control practices in their curricula.
With the successful elimination of pseudorabies virus from US swine population,30 laws requiring all exhibited swine to proceed directly to harvest following the show (ie, terminal shows) have been lifted and nonterminal shows (ie, pigs can return to farms) have become common, creating new challenges for disease control in the country. Because most swine exhibited in the Midwestern United States are obtained in late winter or spring, and the survey of the present study was conducted in midsummer, participating swine exhibitors had only a 3- to 4-month period to attend the fairly large number of exhibitions that they reported attending. Astoundingly, 1 respondent reported showing swine at 50 exhibitions in the 7 months preceding the study fair.
Exhibition-to-exhibition movement of swine creates a pathway for the rapid dissemination of many pathogens, including IAV. The detection of highly identical IAV among exhibition swine across Ohio during 201217 was likely the result of such interexhibition swine movement. Exhibition swine in Ohio and Indiana commonly share related IAV strains,9 suggesting frequent viral movement within this swine-dense exhibition region. Additionally, return of swine to farms of origin after exhibition may threaten future exhibitions because IAV introduced to naïve swine on those farms can further perpetuate IAV transmission.
Generally accepted disease control practices, including limiting swine movement and performing the suggested 7-day on-farm isolation of returning swine, are important for controlling the transmission of IAV between exhibitions.22 If swine have signs of influenza-like illness during their isolation period, owners should consult with a veterinarian to determine the best course of action. Whereas almost half of the respondents in the present study had already implemented some form of isolation for returning swine, there was a wide range in the reported isolation period (2 hours to 30 days), highlighting the need for better education of exhibitors about effective biosecurity measures.
With 62.7% of swine identified in the survey having been vaccinated against IAV infection, it appeared that exhibitors were following the vaccination protocols suggested by officials22; however, vaccination was not associated with lower risk of IAV detection. Indeed, results of the present study suggested that vaccination against IAV infection might be associated with IAV shedding, given that the prevalence of IAV detection was 7.8% in vaccinated swine but 2.9% in unvaccinated swine. Yet, it is possible that swine brought to multiple exhibitions were more likely to be vaccinated than those brought to only 1 or 2 exhibitions. This possibility was not examined in the present study. Other possible explanations for the apparent lack of vaccine protection include improper timing of vaccine administration, poor cross-protection against currently circulating IAV strains, or response bias (respondents were aware they were participating in an IAV study). Although IAV vaccination can eliminate clinical signs of disease in swine, it is not completely effective at blocking infection and pathogen transmission.31 Therefore, the subclinical nature of IAV infections identified in Ohio and Minnesota exhibition swine populations32,33 may be due to suppression of the clinical signs by vaccination.
The high degree of commingling and movement of exhibition swine is distinctly different from that of commercial swine. Commercial swine are typically raised together in large herd sizes, with a mean of 1,044 swine/farm.26 Commercial swine herds typically maintain a high degree of population integrity in both the breeding and postweaning phases, remaining essentially stable with few, if any, swine entering, exiting, and reentering the system. In all-in–all-out management models, commercial swine are typically moved 2 to 3 times during their lifetime, corresponding with movement to a new location at weaning (to a nursery or directly to a finisher) or transfer from the nursery phase directly to the finisher phase as well as transfer from the finisher phase directly to the final market destination. The contrast with reported movement activities of exhibition swine and frequent subsequent reintroduction of these animals to their place of origin is cause for concern to the swine industry as a whole, owing to the tremendous economic losses that can occur in severe disease outbreaks.
Although both commercial and exhibition swine have contact with people, people attending agricultural fairs may have little to no previous exposure to swine. Children generally represent an immunologically naïve population, with little to no preexisting antibody against common swine-lineage IAV.34 Most exhibition swine in the present study were raised on small farms, so young swine exhibitors likely had limited prior exposure to swine-lineage IAV. A large number of young (8- to 18-year-old) swine exhibitors attend agricultural fairs, and during these fairs, these youths are in prolonged, close contact with exhibition swine, including those of other exhibitors. If IAV were transmitted through the swine population during a fair, young exhibitors would likely have repeated opportunities for zoonotic IAV transmission.
Interestingly, 13.3% of swine exhibitors in the present study reported commercial swine production on the same premises as exhibition swine. This is lower than the 39% previously identified in Minnesota.21 The difference may be reflective of the large exhibition swine population in Indiana and Ohio and the relatively smaller commercial swine populations, or it may be indicative of the larger population base to which young exhibitors belong, leading to added interest and opportunity for youths with no farm background to raise livestock in a small scale, rural setting on a temporary basis.
In the study reported here, 37.5% of represented farms with commercial swine production and 11.4% of represented farms without commercial swine production were reported to host an on-farm open house or sale to which visitors were welcomed. Although such activities (vs no such activities) were associated with a significantly greater odds of IAV detection in snout wipe samples obtained at fair arrival from tested exhibition swine, we do not believe the actual act of hosting an open house or sale was responsible for this increased risk. Rather, we believe this variable represented a surrogate indicator of professional swine exhibitors (ie, people who, beyond youth education programs, breed, raise, and sell exhibition swine for hobby or income purposes). As additional analysis revealed, such exhibitors and their swine attended a significantly larger number of exhibitions during the year than other exhibitors, providing their swine with additional opportunities for IAV exposure.
The present study had several limitations, one of which was the self-reported nature of the survey data. Participating swine exhibitors received no assistance or guidance from the investigators; therefore, some questions may have been misunderstood, introducing bias. Other potential sources of bias included response bias, recall bias, and misclassification bias. It should be noted that 52 completed surveys were excluded from the analysis because of incomplete or unusable responses that brought into question the integrity of the data.
Although exhibition swine represent only a small percentage of the total swine population, they can serve as the face of the swine industry to the general public. Exhibitions allow a physical interface between farm animals and the general public that provides not only an excellent opportunity to showcase agriculture but also unique challenges in zoonotic disease control. Results of the present study highlighted unique management areas within the exhibition swine sector that posed risks and opportunities in relation to the general public. The findings also supported the need for increasing educational efforts for young and adult swine exhibitors with respect to biosecurity and strategies for the prevention of IAV transmission among exhibition swine and people.
Supplementary Material
Table 3—
Summary of categorical data responses to a survey of swine management practices for 428 swine exhibitors at 9 selected agricultural fairs in Indiana (n = 5) and Ohio (4) during July and August 2014.
| Variable | Yes | No |
|---|---|---|
| Commercial swine managed at same location as exhibition swine | 13.3 (56/422) | 86.7 (366/422) |
| Hosted an open house or sale where attending people came into contact with swine during 2014 | 14.6 (62/425) | 85.4 (363/425) |
| New swine were mixed directly into existing swine population on farm | 22.7 (93/410) | 77.3 (317/410) |
| Exhibition swine were obtained from an off-farm source | 75.4 (315/418) | 24.6 (103/418) |
| Other livestock raised at the same location as exhibition swine | 66.5 (282/424) | 33.5 (142/424) |
| Swine from multiple farms were transported to the fair together | 18.1 (76/421) | 82.0 (345/421) |
| Other species were hauled to the fair with swine | 7.4 (31/422) | 92.7 (391/422) |
| Household members or respondents have contact with swine or a swine environment other than their own at least once per week | 45.2 (191/423) | 54.8 (232/423) |
Data are reported as percentage (proportion).
Acknowledgments
This manuscript represents a portion of a thesis submitted by Ms. Bliss to The Ohio State University as partial fulfillment of the requirements for a Master of Science degree.
Supported in part by the National Pork Checkoff; the CDC Department of Health and Human Services (cooperative agreement No. U38OT000143); the Centers of Excellence for Influenza Research and Surveillance, National Institute of Allergy and Infectious Diseases; and the Department of Health and Human Services, National Institutes of Health (contract No. HHSN272201400006C).
Presented in abstract form at the 46th Annual Meeting of the American Association of Swine Veterinarians, Orlando, Fla, February 2015; and the 3rd International Symposium on Neglected Influenza Viruses, Athens, Ga, April 2015.
The authors thank Jacqueline Nolting, Jody Edwards, Alexa Edmunson, Elise Gerken, Amber Kihm, Sarah Nelson, Grant Price, Christine Szablewski, Jeffrey Workman, Michele Zentkovich, Bret Marsh, Tony Forshey, and Sue Trock for technical assistance and support.
ABBREVIATIONS
- CI
Confidence interval
- IAV
Influenza A virus
Footnotes
Paul M, National Swine Registry, West Lafayette, Ind: Personal communication, 2015.
ArcMap, version 9.3.1, ESRI, Redlands, Calif.
Stata, version 11.1, StataCorp, College Station, Tex.
References
- 1.Palese P, Shaw ML. Othromyxoviridae: the viruses and their replication. In: Knipe DM, Howley PM, eds. Fields virology. 5th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2007;1647–1740. [Google Scholar]
- 2.Webster RG, Laver WG, Air GM, et al. Molecular mechanisms of variation in influenza viruses. Nature 1982;296:115–121. [DOI] [PubMed] [Google Scholar]
- 3.Webster RG, Bean WJ, Gorman OT, et al. Evolution and ecology of influenza A viruses. Microbiol Rev 1992;56:152–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scholtissek C Pigs as “mixing vessels” for the creation of new pandemic influenza A viruses. Med Princ Pract 1990;2:65–71. [Google Scholar]
- 5.Ma W, Kahn RE, Richt JA. The pig as a mixing vessel for influenza viruses: human and veterinary implications. J Mol Genet Med 2008;3:158–166. [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson TK, Nelson MI, Kitikoon P, et al. Population dynamics of cocirculating swine influenza A viruses in the United States from 2009 to 2012. Influenza Other Respir Viruses 2013;7(suppl 4):42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson MI, Stratton J, Killian ML, et al. Continual reintroduction of human pandemic H1N1 influenza A viruses into swine in the United States, 2009 to 2014. J Virol 2015;89:6218–6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson MI, Viboud C, Vincent AL, et al. Global migration of influenza A viruses in swine. Nat Commun 2015;6:6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson MI, Wentworth DE, Das SR, et al. Evolutionary dynamics of influenza A viruses in US exhibition swine. J Infect Dis 2016;213:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myers KP, Olsen CW, Gray GC. Cases of swine influenza in humans: a review of the literature. Clin Infect Dis 2007;44:1084–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawood FS, Jain S, Finelli L, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 2009;360:2605–2615. [DOI] [PubMed] [Google Scholar]
- 12.Shinde V, Bridges CB, Uyeki TM, et al. Triple-reassortant swine influenza A (H1) in humans in the United States, 2005–2009. N Engl J Med 2009;360:2616–2625. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Standardization of terminology for the variant A(H3N2) virus recently infecting humans: update. World Health Organization, 2014. Available at: www.who.int/influenza/gisrs_laboratory/terminology_variant/en/. Accessed Feb 16, 2015. [Google Scholar]
- 14.CDC. Reported infections with variant influenza viruses in the United States since 2005. Available at: www.cdc.gov/flu/swineflu/variant-cases-us.htm. Accessed Dec 28, 2015.
- 15.Biggerstaff M, Reed C, Epperson S, et al. Estimates of the number of human infections with influenza A (H3N2) variant virus, United States, August 2011–April 2012. Clin Infect Dis 2013;57(suppl 1):S12–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gambhir M, Swerdlow DL, Finelli L, et al. Multiple contributory factors to the age distribution of disease cases: a modeling study in the context of influenza A (H3N2v). Clin Infect Dis 2013;57(suppl 1):S23–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowman AS, Nelson SW, Page SL, et al. Swine-to-human transmission of influenza A (H3N2) virus at agricultural fairs, Ohio, USA, 2012. Emerg Infect Dis 2014;20:1472–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jhung MA, Epperson S, Biggerstaff M. Outbreak of variant influenza A (H3N2) virus in the United States. Clin Infect Dis 2013;57:1703–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Killian ML, Swenson SL, Vincent AL, et al. Simultaneous infection of pigs and people with triple-reassortant swine influenza virus H1N1 at a US county fair. Zoonoses Public Health 2013;60:196–201. [DOI] [PubMed] [Google Scholar]
- 20.Thunes C, Carpenter TE. Biosecurity practices and travel history of individuals exhibiting livestock at the 2005 California State Fair. J Am Vet Med Assoc 2007;231:581–585. [DOI] [PubMed] [Google Scholar]
- 21.Wayne SR, Morrison RB, Odland CA, et al. Potential role of noncommercial swine populations in the epidemiology and control of porcine reproductive and respiratory syndrome virus. J Am Vet Med Assoc 2012;240:876–882. [DOI] [PubMed] [Google Scholar]
- 22.National Assembly of State Animal Health Officials, National Association of State Public Health Veterinarians. Measures to minimize influenza transmission in swine exhinbitions, 2014. National Assembly of State Animal Health Officals and National Association of State Public Health Veterinarians, 2014. Available at: www.usaha.org/Portals/6/news/SwineExhibitions2014.pdf. Accessed Feb 3, 2015. [Google Scholar]
- 23.CDC. 4-H. Key facts for people exhibiting pigs at fairs. CDC, 4-H National Headquarters, 2013. Available at: www.cdc.gov/flu/pdf/swineflu/fair_exhibitor_factsheet.pdf. Accessed Nov 1, 2014. [Google Scholar]
- 24.CDC. Take action to prevent the spread of flu between people and pigs at fairs. CDC, 2014. Available at: www.cdc.gov/flu/swineflu/h3n2v-fairs-factsheet.htm. Accessed Feb 16, 2015. [Google Scholar]
- 25.CDC. What people who raise pigs need to know about influenza (flu). CDC, 2014. Available at: www.cdc.gov/flu/swineflu/people-raise-pigs-flu.htm. Accessed Nov 19, 2014. [Google Scholar]
- 26.USDA National Agricultural Statistics Service. 2012 census of agriculture. Washington, DC: USDA National Agricultural Statistics Service, 2014. [Google Scholar]
- 27.Bowman AS, Workman JD, Nolting JM, et al. Exploration of risk factors contributing to the presence of influenza A virus in swine at agricultural fairs. Emerg Microbes Infect 2014;3:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bliss N, Nelson SW, Nolting JM, et al. Prevalence of influenza A virus in exhibition swine during arrival at agricultural fairs. Zoonoses Public Health 2016;63:477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nolting JM, Szablewski CM, Edwards JL, et al. Nasal wipes for influenza A virus detection and isolation from swine. J Vis Exp 2015;106:e53313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson L, Black N, Hagerty T, et al. Pseudorabies (Aujeszky’s disease) and its eradication: a review of the U.S. experience. Technical Bulletin No. 1923. Washington, DC: USDA APHIS, 2008. [Google Scholar]
- 31.Loving CL, Lager KM, Vincent AL, et al. Efficacy in pigs of inactivated and live attenuated influenza virus vaccines against infection and transmission of an emerging H3N2 similar to the 2011–2012 H3N2v. J Virol 2013;87:9895–9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bowman AS, Nolting JM, Nelson SW, et al. Subclinical influenza virus a infections in pigs exhibited at agricultural fairs, Ohio, USA, 2009–2011. Emerg Infect Dis 2012;18:1945–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gray GC, Bender JB, Bridges CB, et al. Influenza A (H1N1) pdm09 virus among healthy show pigs, United States. Emerg Infect Dis 2012;18:1519–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skowronski DM, Janjua NZ, De Serres G, et al. Cross-reactive and vaccine-induced antibody to an emerging swine-origin variant of influenza A virus subtype H3N2 (H3N2v). J Infect Dis 2012;206:1852–1861. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


