Abstract
Background
Recombinant protein subunit vaccination is considered to be a safe, fast and reliable technique when combating emerging and re-emerging diseases such as coronavirus disease 2019 (COVID-19). Typically, such subunit vaccines require the addition of adjuvants to attain adequate immunogenicity. AS01, which contains adjuvants MPL and saponin QS21, is a liposome-based vaccine adjuvant system that is one of the leading candidates. However, the adjuvant effect of AS01 in COVID-19 vaccines is not well described yet.
Methods
In this study, we utilized a mixture of AS01 as the adjuvant for an S1 protein-based COVID-19 vaccine.
Results
The adjuvanted vaccine induced robust immunoglobulin G (IgG) binding antibody and virus-neutralizing antibody responses. Importantly, two doses induced similar levels of IgG binding antibody and neutralizing antibody responses compared with three doses and the antibody responses weakened only slightly over time up to six weeks after immunization.
Conclusion
These results suggested that two doses may be enough for a clinical vaccine strategy design using MPL & QS21 adjuvanted recombinant protein, especially in consideration of the limited production capacity of COVID-19 vaccine in a public health emergency.
Keywords: COVID-19, Protein vaccine, Dose optimum, Adjuvant
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first isolated from patients and identified at the end of 2019. It emerged to cause a major pandemic, spreading globally as coronavirus disease 2019 (COVID-19). The infection progresses to cause respiratory failure and systemic inflammatory disease, with the elderly, the immunocompromised, and people with co-morbidities being at greatest risk of death (Zhou et al., 2020). Vaccination is one of the most effective strategies to control such pandemics and vaccine candidates that are easily scalable at high speed are required (Operation Warp Speed: imp, 2021; van Riel and de Wit, 2020). Up to March of 2021, 83 and 184 vaccine candidates based on diverse platform technologies are being evaluated in clinical and preclinical stages, respectively (Who. Draft landscape and, 2021).
The use of recombinant protein sub-units of the infective agent as the candidate antigens for vaccine production is considered to be a safe and reliable technique. However, low immunogenicity, often due to poor presentation to the immune system or incorrect antigen folding, is one of the most important challenges for the development of recombinant protein vaccines (Khalaj-Hedayati, 2020). In response to this challenge, such vaccines usually incorporate adjuvants to enhance the immune system processes and result in greater vaccine efficacy.
Aluminum adjuvant was first used in clinical trials in the 1930s and is still used in approximately 80% of the vaccines that are delivered with adjuvants (Bouazzaoui et al., 2021). Recently, it was reported that three doses of a dimeric RBD-based protein subunit vaccine adjuvanted with aluminum hydroxide were well-tolerated and immunogenic against COVID-19 in phase 1 and 2 trials (Yang et al., 2021). An alternative adjuvant, AS01 that contains MPL (3-O-desacyl–monophosphoryl lipid A) and saponin QS21 (Quillaja Saponaria Molina, fraction 21), is an effective liposome-based vaccine adjuvant system which was developed 20 years ago. However, the use of MPL & QS21 in COVID-19 protein vaccines has not been well described.
In this study, we investigated vaccination schedules incorporating MPL & QS21 for induction of robust antibody responses to SARS-CoV-2 antigen S1 in mice. We showed that two doses two weeks apart were sufficient to induce a strong neutralizing antibody response and antigen-specific IgG response that persisted for at least 10 weeks. These data might provide a useful guide for clinical strategies incorporating AS01 adjuvanted vaccines.
2. Materials and methods
Immunization of mice. Specific pathogen-free BALB/c female mice, aged six weeks, were randomly divided into groups. S1 protein was obtained from Sino Biological Inc. (Beijing, China) as a purified protein from recombinant HEK293 cells and carried a polyhistidine tag at the C-terminus. One, two, or three doses of 10 μg recombinant S1 protein which was suspended into MPL (5 μg/dose) and QS21 (5 μg/dose) were injected intramuscularly in each hind leg at 2-week intervals. Single doses of adjuvant, S1 protein without adjuvant, PBS were used as controls. To compare the adjuvant effect between alum adjuvant and MPL/QS21, 1 μg recombinant protein was mixed with alum adjuvant (20 μL/dose, Aluminium hydroxide gel, InvivoGen) and the mice were immunized with two doses. The IgG antibody levels were detected 2, 4, 6, 8, and 10 weeks after the final immunization.
IgG antibody assay. Recombinant S1 protein at a concentration of 0.5 μg/mL was coated onto 96-well plates at 4°C overnight. The plates were washed and blocked before use. Whole blood was collected from immunized mice and incubated at 37°C for 30 min then kept at 4°C for 1 h. The separated serum was serially diluted 10-fold in PBS and incubated at 37°C for 1 h. After washing, the plates were incubated with HRP-labeled anti-mouse IgG at 37°C for 1 h. TMB substrate solution was added and incubated for 10 min. The reaction was stopped by adding 2 M H2SO4. The OD450 was measured on a microplate reader. The cut-off value was defined as twice the OD450 value of the buffer control.
Neutralizing antibody assay. The virus-neutralizing antibody titers were determined according to the kit instructions (SARS-CoV-2 Neutralizing Antibody Titer Assay Kit, ACROBiosystems). Briefly, the serum was serially diluted 2-fold and added into 96-well plates that were pre-coated with human ACE2 protein. HRP-SARS-CoV-2 Spike RBD was added and incubated at 37°C for 1 h. After washing, the substrate solution was added and incubated at 37°C for 20 min. Stop solution was added and the OD450 was measured. Percent inhibition was calculated as (1 - Sample OD450/Negative control OD450) × 100%. The cut-off value was 20% signal inhibition.
3. Results
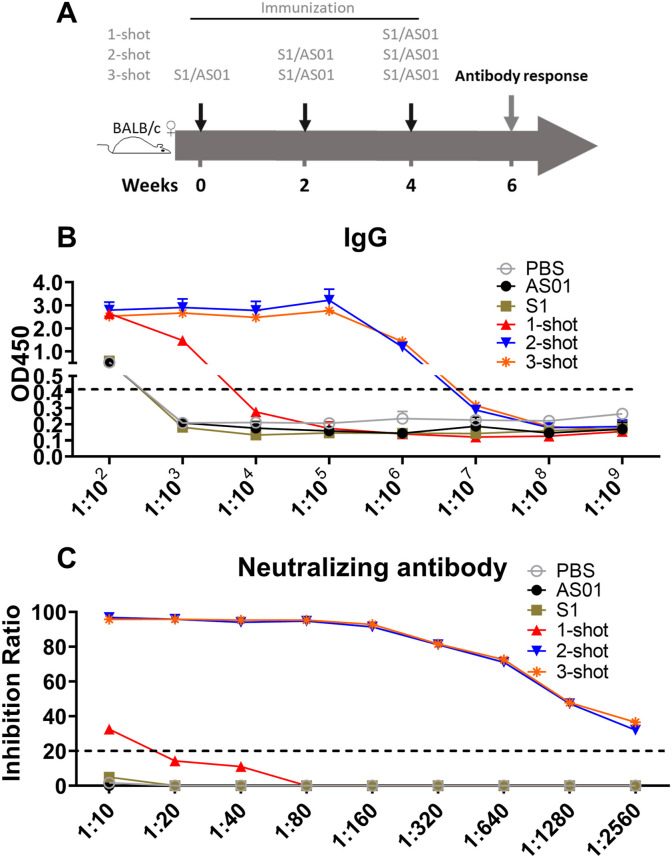
To optimize a short-duration protocol of immunization for the recombinant S1 protein vaccine delivered with adjuvant MPL & QS21 (AS01), BALB/c mice were vaccinated with single, double or triple doses of recombinant S1 protein with adjuvant. Two weeks after the last dose, the mice were sacrificed to test S1-specific antibody responses and neutralizing-antibody titers against SARS-CoV-2 ACE2 protein (Fig. 1 A). As shown in Fig. 1B, immunization induced S1-specific IgG in the serum that was detectable in the assay at dilutions down to 1:106 if the mice had received either 2 or 3 doses. In contrast, the specific IgG response to one dose was weak and undetectable at dilutions below 1:103 (Fig. 1B). The associated neutralizing antibody titer was also minimal after a single vaccination (1:10) whereas there were strong neutralization antibody titers after double or triple immunizations. A positive neutralization score was obtained even after dilution to 1:2560 as shown in Fig. 1C. These data suggested that two doses would be enough for strong antibody responses with this recombinant protein/adjuvant combination.
Fig. 1.
The optimization of dosage schedule. (A) The vaccination schedule. BALB/c mice were vaccinated intramuscularly with vaccines in groups of six as indicated, then the mice were sacrificed at 2 weeks after the final vaccination for assays of IgG binding antibody and neutralizing antibody responses. (B) The S1-specific antibody responses. (C) The neutralizing antibody titer. Data are shown as mean values (mean ± SD) for each group of six mice.
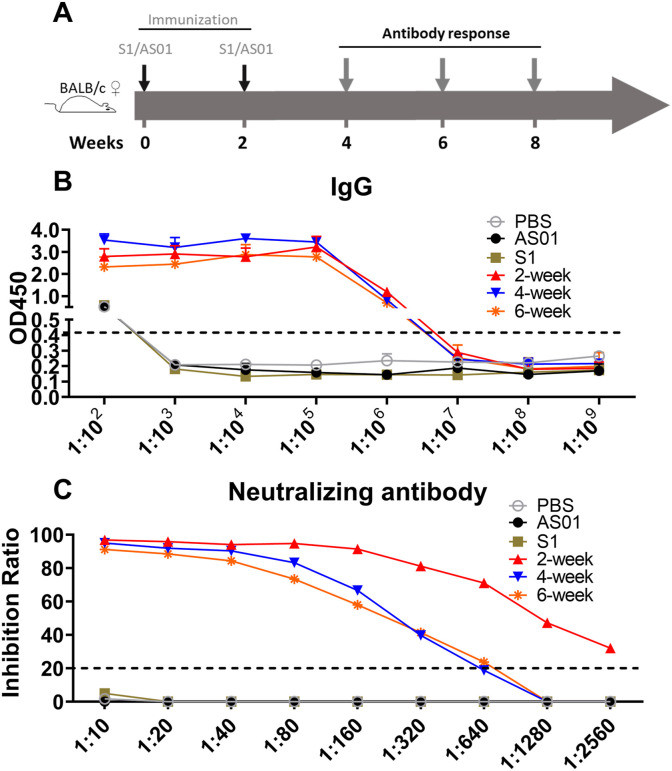
In a separate experiment, the persistence of the responses was measured at intervals up to 6 weeks after a two-dose immunization (Fig. 2 A). The S1-specific IgG responses remained at high levels for at least six weeks (Fig. 2B). Although the neutralizing titers were lower at four weeks compared to two weeks post-vaccination (1:320 and 1:2560 respectively, Fig. 2C), they had not declined any further at six weeks (1:320).
Fig. 2.
Duration of responses after two-dose vaccination. (A) The vaccination schedule. BALB/c mice were vaccinated intramuscularly with vaccines as indicated, then the mice were sacrificed at 2 weeks, 4 weeks, and 6 weeks after the final vaccination for assays of IgG binding antibody and neutralizing antibody responses. (B) S1-specific antibody responses. (C) The virus-neutralizing antibody titer. Data are shown as mean values (mean ± SD) for each group of six mice.
To compare the adjuvants effect between AS01 and alum, a separate experiment was conducted using a lower concentration of antigen (1 μg/dose) adjuvanted with either AS01 or alum, and using adjuvant alone as control. As expected, the adjuvants alone showed negligible effects (data not shown). Importantly, the AS01 adjuvant induced slightly lower levels of IgG antibody responses at 2, 4, 6, 8, and 10 weeks after the final immunization (Table 1 ), supporting the conclusion that AS01 adjuvant could be used as an alternative adjuvant in the SARS-CoV-2 protein vaccine.
Table 1.
The comparison of antibody responses with different adjuvanted S1 vaccination.
| Serum IgG binding endpoint titers |
|||||
|---|---|---|---|---|---|
| Week 4 | Week 6 | Week 8 | Week 10 | Week 12 | |
| S1/AS01 | 1:210 | 1:213 | 1:213 | 1:213 | 1:213 |
| S1/Alum | 1:210 | 1:214 | 1:214 | 1:214 | 1:214 |
BALB/c mice were immunized with 1 μg recombinant protein mixed with adjuvant with two doses two weeks apart. The adjuvants without antigen were used as controls. The plasma was collected at weeks 4, 6, 8, 10, and 12. The serum IgG binding endpoint titers are shown as mean values (mean ± SD) for each group of six mice.
4. Discussion
In a public health emergency, vaccines that are recombinant protein, nucleic acid-based or viral-vectored tend to be favored because of shorter vaccine production timeframes (Operation Warp Speed: imp, 2021; van Riel and de Wit, 2020). Although the production technology of inactive virus vaccines is mature, low amplification speeds and bio-safety concerns may impede emergency use to combat emerging and re-emerging diseases such as COVID-19. In contrast, the construction of recombinant protein vaccines is faster but may need the addition of an adjuvant as described here.
AS01 is an advanced adjuvant that contains both MPL and QS21 adjuvants which act synergistically to enhance the antigen presentation ability of antigen-presenting cells and induce antigen-specific adaptive responses (Didierlaurent et al., 2017). AS01 was employed in the leading candidate malaria vaccine RTS,S/AS01 that was shown in a phase 3 trial to induce robust IgA responses in peripheral blood against the vaccine antigens (Suau et al., 2021). AS01 was also included in the M72/AS01 tuberculosis subunit vaccine that gave high protection against disease progression in a phase 2b trial (Tait et al., 2019). Recently, it was shown that liposomes containing MPL and QS21 potently enhanced the antibody response to recombinant SARS-CoV-2 RBD antigen in mice and rabbits (Huang et al., 2020) and the addition of QS21 to a liposomal adjuvant (GLA-LS) formulated with tuberculosis recombinant poly-antigen ID93 enhanced IgG responses in mice (Baldwin et al., 2021).
Coronavirus SARS-CoV-2 antigen S1 is widely accepted as a key target for vaccines against COVID-19. Indeed, it is the key antigen in vaccines that are currently achieving some success in vaccine trials and roll-outs (Samrat et al., 2020; Arashkia et al., 2020). Herein, we show that when the recombinant S1 protein is adjuvanted in an MPL & QS21 vaccine strategy design, two doses are sufficient to induce robust and sustained S1-specific IgG responses and neutralizing-antibody titers. These data provide some guidance for the use of AS01 adjuvant (MPL & QS21) in vaccine design.
The main limitations of this study are i) the dosing interval between prime and boost is relatively short. At the time of designing the original experiment, the dosing interval between prime and boost in clinical use with inactivated COVID-19 vaccine was 14 days in China. Now the interval has been changed to 21 days. Longer dosing intervals might be needed to guide the AS01-based COVID-19 vaccine design. ii) A competitive ELISA assay kit was used to detect the levels of neutralizing-antibody against human ACE2 protein in the serum, which is only an indirect indicator of neutralizing activity in vivo.
Funding
This work was supported by Grants from the National Natural and Science Foundation of China (82171815, 82171739, 81873884, 31771004), Shanghai Science and Technology Commission (19XD1403100, 20Y11903400), and Shanghai Municipal Medical and Health Excellent Young Talents Training Program (GWV-10.2-YQ01).
CRediT authorship contribution statement
Zhidong Hu: Methodology, Investigation, Writing – original draft, Funding acquisition. Jian-Ping Chen: Methodology, Resources. Jin-Chuan Xu: Methodology, Investigation. Zhen-Yan Chen: Methodology, Investigation. Rong Qu: Methodology, Investigation. Ling Zhang: Resources. Wenrong Yao: Resources. Juan Wu: Methodology, Investigation. Heng Yang: Methodology, Investigation. Douglas B. Lowrie: Writing – review & editing. Yong Liu: Conceptualization, Resources. Xiao-Yong Fan: Conceptualization, Writing – review & editing, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
References
- Arashkia A., Jalilvand S., Mohajel N., et al. Severe acute respiratory syndrome-coronavirus-2 spike (S) protein based vaccine candidates: state of the art and future prospects. Rev. Med. Virol. 2020 doi: 10.1002/rmv.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin S.L., Reese V.A., Larsen S.E., Beebe E., Guderian J., Orr M.T., et al. Prophylactic efficacy against Mycobacterium tuberculosis using ID93 and lipid-based adjuvant formulations in the mouse model. PLoS One. 2021;16(3) doi: 10.1371/journal.pone.0247990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouazzaoui A., Abdellatif A., Al-Allaf F.A., et al. Strategies for vaccination: conventional vaccine approaches versus new-generation strategies in combination with adjuvants. Pharmaceutics. 2021;13(2) doi: 10.3390/pharmaceutics13020140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didierlaurent A.M., Laupeze B., Di Pasquale A., et al. Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expert Rev. Vaccines. 2017;16(1):55–63. doi: 10.1080/14760584.2016.1213632. [DOI] [PubMed] [Google Scholar]
- Huang W.C., Zhou S., He X., et al. SARS-CoV-2 RBD neutralizing antibody induction is enhanced by particulate vaccination. Adv. Mater. 2020;32(50) doi: 10.1002/adma.202005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalaj-Hedayati A. Protective immunity against SARS subunit vaccine candidates based on spike protein: lessons for coronavirus vaccine development. J Immunol Res. 2020;2020 doi: 10.1155/2020/7201752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Operation Warp Speed: implications for global vaccine security. Lancet Glob Health. 2021 doi: 10.1016/S2214-109X(21)00140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samrat S.K., Tharappel A.M., Li Z., et al. Prospect of SARS-CoV-2 spike protein: potential role in vaccine and therapeutic development. Virus Res. 2020;288 doi: 10.1016/j.virusres.2020.198141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suau R., Vidal M., Aguilar R., et al. RTS,S/AS01E malaria vaccine induces IgA responses against CSP and vaccine-unrelated antigens in African children in the phase 3 trial. Vaccine. 2021;39(4):687–698. doi: 10.1016/j.vaccine.2020.12.038. [DOI] [PubMed] [Google Scholar]
- Tait D.R., Hatherill M., Van Der Meeren O., et al. Final analysis of a trial of M72/as01e vaccine to prevent tuberculosis. N. Engl. J. Med. 2019;381(25):2429–2439. doi: 10.1056/NEJMoa1909953. [DOI] [PubMed] [Google Scholar]
- van Riel D., de Wit E. Next-generation vaccine platforms for COVID-19. Nat. Mater. 2020;19(8):810–812. doi: 10.1038/s41563-020-0746-0. [DOI] [PubMed] [Google Scholar]
- Who Draft Landscape and Tracker of COVID-19 Candidate Vaccines. 2021. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines Available from:
- Yang S., Li Y., Dai L., et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect. Dis. 2021 doi: 10.1016/S1473-3099(21)00127-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.