Abstract
Recent studies in cognitively unimpaired elderly individuals suggest that the APOE ε4 allele exerts a dosage-dependent effect on brain tau deposition. The aim of this study was to investigate sex differences in APOE ε4 gene dosage effects on brain tau deposition in cognitively impaired individuals using quantitative 18F-flortaucipir PET.
Preprocessed 18F-flortaucipir tau PET images, T1-weighted structural MRI, demographic information, global cortical amyloid-β burden measured by 18F-florbetapir PET, CSF total tau and phosphorylated tau measurements were obtained from the Alzheimer’s Disease Neuroimaging Initiative database. Two hundred and sixty-eight cognitively impaired individuals with 146 APOE ε4 non-carriers and 122 carriers (85 heterozygotes and 37 homozygotes) were included in the study. An iterative reblurred Van Cittert iteration partial volume correction method was applied to all downloaded PET images. Magnetic resonance images were used for PET spatial normalization. Twelve regional standardized uptake value ratios relative to the cerebellum were computed in standard space. APOE ε4 dosage × sex interaction effect on 18F-flortaucipir standardized uptake value ratios was assessed using generalized linear models and sex-stratified analysis.
We observed a significant APOE ε4 dosage × sex interaction effect on tau deposition in the lateral temporal, posterior cingulate, medial temporal, inferior temporal, entorhinal cortex, amygdala, parahippocampal gyrus regions after adjusting for age and education level (P < 0.05). The medial temporal, entorhinal cortex, amygdala and parahippocampal gyrus regions retained a significant APOE ε4 dosage × sex interaction effect on tau deposition after adjusting for global cortical amyloid-β (P < 0.05). In sex-stratified analysis, there was no significant difference in tau deposition between female homozygotes and heterozygotes (P > 0.05). In contrast, male homozygotes standardized uptake value ratios were significantly greater than heterozygotes or non-carriers throughout all 12 regions of interest (P < 0.05). Female heterozygotes exhibited significantly increased tau deposition compared to male heterozygotes in the orbitofrontal, posterior cingulate, lateral temporal, inferior temporal, entorhinal cortex, amygdala and parahippocampal gyrus (P < 0.05). Results from voxel-wise analysis were similar to the ones obtained from regions of interest analysis.
Our findings indicate that an APOE ε4 dosage effect on brain region-specific tau deposition exists in males, but not females. These results have important clinical implications towards developing sex and genotype-guided therapeutics in Alzheimer’s disease and uncovers a potential explanation underlying differential APOE ε4-associated Alzheimer’s risk in males and females.
Keywords: Alzheimer’s disease, 18F-flortaucipir PET, sex, apolipoprotein E, dose effect
Recent studies in cognitively unimpaired elderly individuals suggest that the APOE ε4 allele exerts a dose-dependent effect on brain tau deposition. Using 18F-flortaucipir PET, Yan et al. investigate whether there are sex differences in this dose effect in cognitively impaired individuals.
Introduction
Alzheimer’s disease is the most common cause of dementia in elderly individuals. The strongest genetic modifier of late-onset Alzheimer’s disease is the apolipoprotein E (APOE) ε4 allele.1 The APOE ε4 allele is associated with a heightened risk of developing Alzheimer’s disease, earlier age of onset and worse cognitive performance in a dose-dependent manner (i.e. the number of ε4 alleles in a person’s APOE genotype).2-4 In spite of these epidemiological and cognitive data, the dose effects of APOE ε4 on brain tau pathology remain unclear, especially in cognitively impaired cohorts.
18F-flortaucipir (also called 18F-T807 or 18F-AV-1451) is the first drug approved by the Food and Drug Administration to image tau pathology in patients with Alzheimer’s disease. 18F-flortaucipir PET has been used to examine the effects of APOE ε4 in brain tau deposition. Analysis from genome-wide association studies, histopathology, CSF and tau PET imaging of the brain have consistently found a relationship between APOE ε4 and elevated tau pathology in cognitively unimpaired elderly individuals with mild cognitive impairment and Alzheimer’s disease.5–10 Similarly, APOE ε4 carriers show accelerated brain amyloid-β accumulation relative to non-carriers. Furthermore, a recent study detected that the APOE ε4 allele is associated with increased entorhinal cortex tau standardized uptake value ratio (SUVR) in younger cognitively unimpaired individuals (47–70 years) in a genotype dosage-dependent manner.11 Similarly in another study involving patients with Alzheimer’s disease, APOE ε4 homozygotes had significantly more neurofibrillary tangles in the midfrontal, inferior parietal, superior temporal and hippocampus regions compared to either APOE ε4 heterozygotes or non-carriers.12 Another 18F-FDG PET study in patients with Alzheimer’s disease found that APOE ε4 dosage is associated with glucose hypometabolism in the precuneus, posterior cingulate, parietotemporal and frontal regions.13
Previous studies have suggested that APOE ε4 confers a greater risk for Alzheimer’s disease, tau pathology, glucose hypometabolism and amyloid-β burden in females compared to males. Among APOE ε4 heterozygotes, the risk of developing Alzheimer’s disease for females is approximately 1.5 times greater than that of males.2 In regard to APOE ε4 and sex effects on tau pathology, CSF studies have demonstrated that APOE ε4 is more strongly associated with CSF tau in females compared with males.14–16 A PET study found that while APOE ε4 is associated with hypometabolism and greater amyloid-β burden across sex in individuals with mild cognitive impairment, it is associated with greater amyloid-β burden only in males and not females among patients with Alzheimer’s disease.17 Several studies have investigated how sex modulates the effects of APOE ε4 on brain tau deposition measured by tau PET. A study in cognitively unimpaired participants found that the association between CSF amyloid-β and tau accumulation measured by PET was strongest in female APOE ε4 carriers compared to other groups.18 Further, we have previously elucidated a sex × APOE ε4 carrier status interaction effect on tau deposition in the entorhinal cortex, amygdala, parahippocampal gyrus in individuals with mild cognitive impairment.5 Importantly, in all of these studies, the APOE ε4 genotype was analysed as binary carrier/non-carrier variable, preventing the analysis of APOE ε4 dosage effects on brain tau deposition. A recent PET study has shown an APOE ε4 dosage effect on increased tau deposition in the entorhinal cortex in younger cognitively unimpaired individuals (47–70 years).11 In light of overwhelming data supporting a sex × APOE ε4 carrier status interaction effect on tau pathology, a sex × APOE ε4 dosage interaction effect on brain tau deposition should be explored.
The main aim of this study was to investigate sex differences in APOE ε4 dosage effect on brain tau deposition using data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI). We hypothesized that APOE ε4 dosage effect on brain tau deposition are different in cognitively impaired females and males.
Materials and methods
Participants
Data in the study were obtained from the ADNI database. The ADNI study was launched in 2003 as a public–private partnership, led by Principal Investigator, Michael W. Weiner, MD. Written informed consent was obtained from all individuals.
From the ADNI database, 268 cognitively impaired individuals with either clinically diagnosed mild cognitive impairment (n = 197) or Alzheimer’s disease (n = 71) who underwent 18F-flortaucipir PET imaging were included in the study. Among the study participants, 188 individuals underwent 18F-florbetapir amyloid-β PET imaging and were used in subsequent analyses that controlled for global cortex amyloid-β burden. Global cortical SUVR values were downloaded from the ADNI-LONI database (adni.loni.usc.edu/methods/pet-analysis-method/pet-analysis/). Individuals with the APOE ε2/ε4 allele, a putative protective allele for Alzheimer’s disease, were excluded.19 In total, 146 APOE ε4 non-carriers and 122 carriers (85 heterozygotes and 37 homozygotes) were included in the study. Performance on the Mini-Mental Status Examination (MMSE), Clinical Dementia Rating (CDR), Alzheimer's Disease Assessment Scale score (13 items; ADAS13), CSF total tau (t-tau), phosphorylated tau (p-tau) and amyloid-β42 levels were also obtained (Table 1). A full list of inclusion/exclusion criteria for ADNI study can be found at https://adni.loni.usc.edu/wp-content/uploads/2008/07/adni2-procedures-manual.pdf.
Table 1.
Clinical characteristics of the study cohort
| Parameter (±SD) | Female |
Male |
||||
|---|---|---|---|---|---|---|
|
Non-carriers
(n = 54) |
Heterozygotes
(APOE ε3 ε4) (n = 38) |
Homozygotes
(APOE ε4 ε4) (n = 15) |
Non-carriers
(n = 92) |
Heterozygotes
(APOE ε3 ε4) (n = 47) |
Homozygotes
(APOE ε4 ε4) (n = 22) |
|
| Alzheimer’s disease/MCI, n | 12/42 | 11/27 | 5/10 | 17/75 | 17/30 | 9/13 |
| Age, years ± SD (range) | 76.62 ± 9.23 (56–93) | 73.70 ± 7.26 (61–88) | 69.86 ± 6.75b (57–82) | 78.13 ± 8.06 (57–94) | 77.49 ± 7.57 (59–90) | 73.38 ± 9.77a (56–91) |
| Education, years ± SD | 15.19 ± 2.37 | 14.89 ± 2.22 | 15.67 ± 2.58 | 16.61 ± 2.66 | 16.51 ± 2.76 | 16.86 ± 3.17 |
| MMSE, score ± SD | 26.17 ± 4.55 | 25.50 ± 3.94 | 25.67 ± 4.53 | 27.04 ± 3.60 | 25.81 ± 3.88 | 25.00 ± 3.83a |
| CDR, score ± SD | 0.62 ± 0.49 | 0.63 ± 0.40 | 0.70 ± 0.41 | 0.53 ± 0.28 | 0.60 ± 0.46 | 0.66 ± 0.36 |
| ADAS13, score ± SD | 21.52 ± 11.11 | 17.59 ± 9.51 | 19.38 ± 7.62 | 18.09 ± 8.21 | 19.29 ± 8.66 | 17.30 ± 11.86 |
| 18F-florbetapir PET, n | 44 | 23 | 9 | 66 | 30 | 16 |
| Aβ positive (%) | 25/54 (46.30) | 32/38 (84.21) | 15/15 (100.00) | 35/92 (32.61) | 37/47 (78.72) | 21/22 (95.45) |
| APOE ε2 ε4, n | 5 | 5 | ||||
| CSF | n = 43 | n = 29 | n = 13 | n = 71 | n = 30 | n = 19 |
| CSF Aβ42 (pg/ml ± SD) | 1379.30 ± 728.11 | 845.80 ± 459.71b | 613.70 ± 217.87b | 1314.27 ± 763.47 | 786.86 ± 426.06b | 499.36 ± 124.55b |
| CSF t-tau (pg/ml ± SD) | 265.65 ± 128.30 | 370.47 ± 148.29b | 406.11 ± 250.91b | 261.42 ± 121.17 | 305.03 ± 105.58 | 280.17 ± 97.14 |
| CSF p-tau (pg/ml ± SD) | 23.99 ± 13.78 | 37.06 ± 18.71b | 43.18 ± 30.94b | 24.22 ± 13.37 | 30.06 ± 12.07a | 28.19 ± 11.07 |
ADAS = Alzheimer's Disease Assessment Scale; Aβ = amyloid-β; CDR = Clinical Dementia Rating Scale; MMSE = Mini-Mental State Examination. Amyloid-β status was positive (negative) if amyloid-β load was higher (lower) than 18F-florbetaben cortical SUVR based on the whole cerebellum reference = 1.08 and 18F-florbetapir = 1.11 (adni.loni.usc.edu/methods).
P < 0.05 compared to non-carriers.
P < 0.01 compared to non-carriers.
APOE ε4 genotyping and gene dose
Peripheral blood from study individuals was previously obtained by ADNI study investigators to be used for APOE ε4 genotyping. Restriction enzyme isoform genotyping was applied on DNA extracts to test for the presence of the APOE ε4 genotype.20APOE ε4 dosage was defined as the number of APOE ε4 alleles (0, 1 or 2) carried by a participant.
PET data acquisition and processing
Raw T1-weighted structural MRI and preprocessed 18F-flortaucipir PET images were downloaded from the ADNI database (http://adni.loni.usc.edu/). The preprocessed PET images had been aligned, averaged, reoriented, interpolated into a standard space and smoothed with an 8-mm in full-width at half-maximum (FWHM) 3D Gaussian filter by the ADNI consortium. The details of tau PET acquisition parameters can be found at adni.loni.usc.edu/methods/pet-analysis-method/pet-analysis/. As described in our previous studies,5,21,22 we further processed the PET images with partial volume correction (PVC) and spatial normalization using Statistical Parametric Mapping (SPM12, Wellcome Department of Imaging Neuroscience, Institute of Neurology, London, UK) and MATLAB R2019b (The MathWorks Inc.). PVC was applied to the processed PET images to correct or minimize potential underestimation in PET measurement. In brief, an iterative reblurred Van Cittert method was used for PVC on the individual PET images, where a 3D Gaussian kernel of 8 mm FWHM was used for the spatial smoothing function with a step length α of 1.5. The iteration was stopped if the relative percentage change of PVC images was <1%.23 All the PET images were then coregistered to the individuals structural MRI images, which were normalized to standard Montreal Neurologic Institute (MNI) space using an MRI template (image volume: 121 × 145 × 121, voxel size: 1.5 × 1.5 × 1.5 mm in x, y, z). The transformation parameters determined by MRI spatial normalization were then applied to the co-registered PET images for PET spatial normalization. SUVR images were calculated relative to the middle-inferior cerebellar grey matter reference region, which was drawn on 11 consecutive slices from z = 27 to z = 17 mm in the axial view from top of the head, as demonstrated in our previous studies.5,24–26 For reference, SUVR images calculated from PET images without PVC were also analysed.
A total of 12 cortical regions of interest were defined in the entorhinal cortex, parahippocampus, amygdala, inferior temporal, medial temporal, lateral temporal, posterior cingulate, posterior precuneus, parietal, orbitofrontal, superior frontal and prefrontal cortex.5,21,25 These region of interest were previously proposed by the Johns Hopkins Department of Radiology and manually drawn on the MRI template using PMOD (PMOD Technologies Ltd, Zürich, Switzerland) in the standard MNI space.21,24,26 To minimize variance resulting from the variability of region of interest volume and shape in native space, the region of interest SUVRs were calculated on the SUVR images in standard MNI space.5,21,27,28
Statistical analysis
All statistical analyses were performed using Statistical Analysis System (SAS v.9.4, SAS Institute, Inc.) and SPM12. A generalized linear model was used to evaluate group differences among APOE ε4 non-carriers, heterozygotes and homozygotes in age, MMSE, CDR, ADAS13 and CSF measurements, separately for females and males.
A generalized linear model was used to assess APOE ε4 × sex interaction effects on global cortical 18F-florbetapir amyloid-β SUVR after controlling for age and years of education. This analysis revealed no significant APOE ε4 × sex interaction effect (P = 0.72) or sex main effect (P = 0.27) on global cortical 18F-florbetapir SUVR.
Two generalized linear models with and without controlling for global cortical 18F-florbetapir SUVR were fit to investigate APOE ε4 dosage × sex interaction effects on regional brain tau deposition as described earlier on the region of interest level:
Model 1: ROI_SUVR (18F-flortaucipir) ∼ age + educational level + sex: APOE ε4 dosage
Model 2: ROI_SUVR (18F-flortaucipir) ∼ age + educational level + global cortex_SUVR (18F-florbetapir) + sex: APOE ε4 dosage
The APOE ε4 dosage × sex interaction effects on brain tau deposition was further evaluated by sex-stratified analysis. Specifically, group differences (i.e. APOE ε4 non-carriers, heterozygotes and homozygotes) in 18F-flortaucipir SUVR at the region of interest and voxel-wise levels were assessed for male and female, separately. In addition, we also evaluated differences in 18F-flortaucipir SUVR between males and females in APOE ε4 dosage groups. SAS was used for region of interest analyses and SPM12 (P < 0.001, cluster size > 100 voxels) was used for voxel-wise analysis as described previously.5,21,25 The Benjamini–Hochberg method was used to control the false discovery rate (FDR) using the 12 study regions of interest at both region of interest-based and voxel-wise levels. For region of interest-based analyses, an FDR corrected P-value <0.05 was defined as significant. For voxel-wise based analyses, an FDR corrected value P < 0.05 and cluster size > 100 voxels were defined as significant.
Data availability
All data used in the current study were obtained from the ADNI database (available at https://adni.loni.usc.edu).
Results
Demographics
Two hundred and sixty-eight individuals with cognitive impairment with 18F-flortaucipir PET imaging were included in our study. A full list of measures of demographic variables, Alzheimer’s disease cognition assessment, and measures of pathology including amyloid-β positivity, CSF amyloid-β42, CSF tau and CSF p-tau with statistical comparisons between APOE ε4 genotype groups is presented in Table 1. The overall APOE ε4 non-carrier, heterozygote and homozygote frequencies were 54%, 33% and 14%, respectively.
In the female cohort, compared to non-carriers, APOE ε4 heterozygotes and homozygotes had decreased CSF amyloid-β42 (P < 0.01), increased CSF t-tau (heterozygotes: P < 0.01; homozygotes: P < 0.01) and increased p-tau (heterozygotes: P < 0.01; homozygotes: P < 0.01); APOE ε4 non-carriers, heterozygotes and homozygotes groups did not differ in MMSE score (P = 0.76), years of education (P = 0.55), CDR (P = 0.83) and ADAS13 (P = 0.20). In the male cohort, compared to non-carriers, APOE ε4 homozygotes were younger (P = 0.02) and with a lower MMSE score (P = 0.02); APOE ε4 heterozygotes and homozygotes had decreased CSF amyloid-β42 levels (P < 0.01); APOE ε4 heterozygotes had increased p-tau (P = 0.04). We observed no significant differences among male non-carriers, heterozygotes and homozygotes in years of education (P = 0.88), CDR (P = 0.26), ADAS13 (P = 0.64) and CSF t-tau (P = 0.21).
APOE ε4 dosage effect on regional 18F-flortaucipir SUVR in overall study cohort
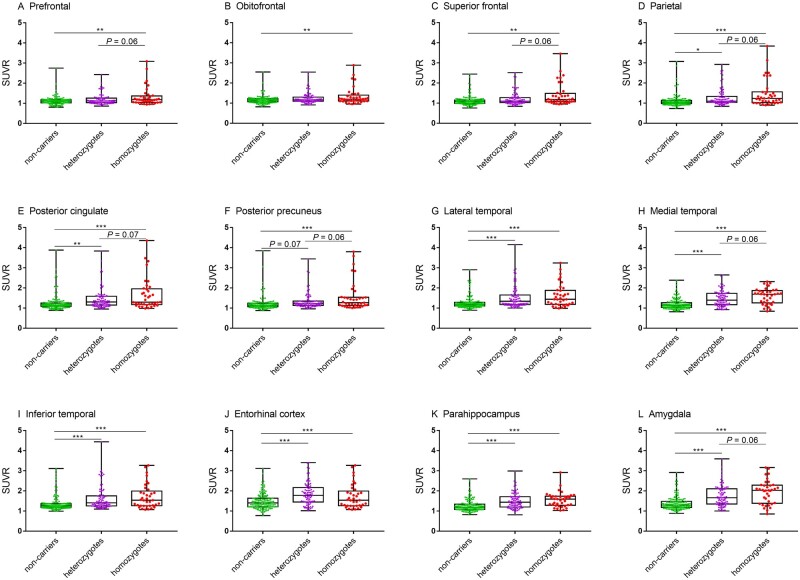
We first investigated APOE ε4 dosage effects on regional tau deposition in the overall study cohort, including both males and females. There was a positive association between APOE ε4 dosage and region of interest 18F-flortaucipir SUVRs in the prefrontal, superior frontal, parietal, posterior cingulate, posterior precuneus, medial temporal and amygdala (Fig. 1).
Figure 1.
APOE ε4 dose effect on region of interest 18F-flortaucipir SUVRs in the study cohort of cognitively impaired individuals. Mean (± SD) of SUVR for APOE ε4 non-carriers (green), heterozygotes (purple) and homozygotes (red) are depicted. P-value was defined using a generalized linear model, adjusting for age, years of education and sex. SUVR: Standardized uptake value ratio. *P < 0.05, **P < 0.01, ***P < 0.001.
APOE ε4 dosage × sex interaction effect on regional 18F-flortaucipir SUVR
Regions of interest with significant APOE ε4 dosage × sex interaction on regional 18F-flortaucipir SUVR were identified with and without controlling for global cortical 18F-florbetapir SUVR (Table 2) using the 12 regions of interest described in the ‘Materials and methods' section. We observed a significant APOE ε4 dosage × sex interaction effect on 18F-flortaucipir tau deposition in the lateral temporal, posterior cingulate, medial temporal, inferior temporal, entorhinal cortex, amygdala, parahippocampal gyrus regions without adjusting for global cortical 18F-florbetapir SUVR (FDR P < 0.05; Table 2). The medial temporal, entorhinal cortex, amygdala and parahippocampal gyrus regions of interest retained significant APOE ε4 dosage × sex interaction effect on tau deposition after adjusting for global cortical 18F-florbetapir SUVR (FDR P < 0.05; Table 2).
Table 2.
APOE ε4 dosage × sex interaction effect on tau deposition in cognitively impaired participants
| Region of interest | Not adjusted for global cortical amyloid level |
Adjusted for global cortical amyloid level |
||||
|---|---|---|---|---|---|---|
|
Standardized β
(95% CI) |
APOE ε4 × Sex | FDR |
Standardized β
(95% CI) |
APOE ε4 × Sex | FDR | |
| P-value | P-value | P-value | P-value | |||
| Orbital frontal | −0.08 (−0.13 to 0.05) | 0.04 | 0.06 | −0.02 (−0.11 to 0.10) | 0.82 | 0.95 |
| Prefrontal | −0.07 (−0.13 to 0.06) | 0.12 | 0.14 | −0.01 (−0.11 to 0.09) | 0.88 | 0.95 |
| Superior frontal | −0.03 (−0.13 to 0.09) | 0.38 | 0.38 | −0.01 (−0.13 to 0.12) | 0.80 | 0.95 |
| Lateral temporal | −0.03 (−0.12 to 0.18) | 0.02 | 0.04 | 0.09 (−0.09 to 0.25) | 0.20 | 0.43 |
| Parietal | 0.00 (−0.14 to 0.14) | 0.18 | 0.20 | 0.03 (−0.13 to 0.18) | 0.57 | 0.93 |
| Posterior precuneus | −0.06 (−0.20 to 0.09) | 0.05 | 0.07 | −0.03 (−0.18 to 0.14) | 0.96 | 0.96 |
| Posterior cingulate | 0.01 (−0.16 to 0.18) | 0.02 | 0.04 | 0.06 (−0.13 to 0.25) | 0.30 | 0.56 |
| Medial temporal | 0.15 (−0.01 to 0.21) | 0.002 | 0.01 | 0.22 (0.04 to 0.28) | 0.006 | 0.03 |
| Inferior temporal | 0.03 (−0.14 to 0.19) | 0.03 | 0.05 | 0.10 (−0.08 to 0.29) | 0.16 | 0.42 |
| Entorhinal cortex | 0.21 (0.03 to 0.33) | <0.001 | <0.001 | 0.30 (0.12 to 0.47) | <0.001 | 0.001 |
| Amygdala | 0.12 (−0.04 to 0.26) | 0.008 | 0.03 | 0.19 (0.02 to 0.37) | 0.02 | 0.07 |
| Parahippocampal | 0.16 (−0.01 to 0.21) | <0.001 | <0.001 | 0.23 (0.04 to 0.29) | 0.002 | 0.01 |
P-value was defined using a generalized linear model to detect significant APOE ε4 dosage × sex interaction effect in cognitively impaired individuals. Age and education were included as covariates. Global cortical amyloid SUVR was also included as a covariate in the right column using 188 individuals with 18F-florbetapir PET data. FDR P-value was defined using Benjamini–Hochberg procedure to control the FDR; 95% CI represents the 95% confidence interval of the APOE ε4 dosage by sex coefficient.
Sex and APOE ε4 dosage-stratified analysis on regional 18F-flortaucipir SUVR
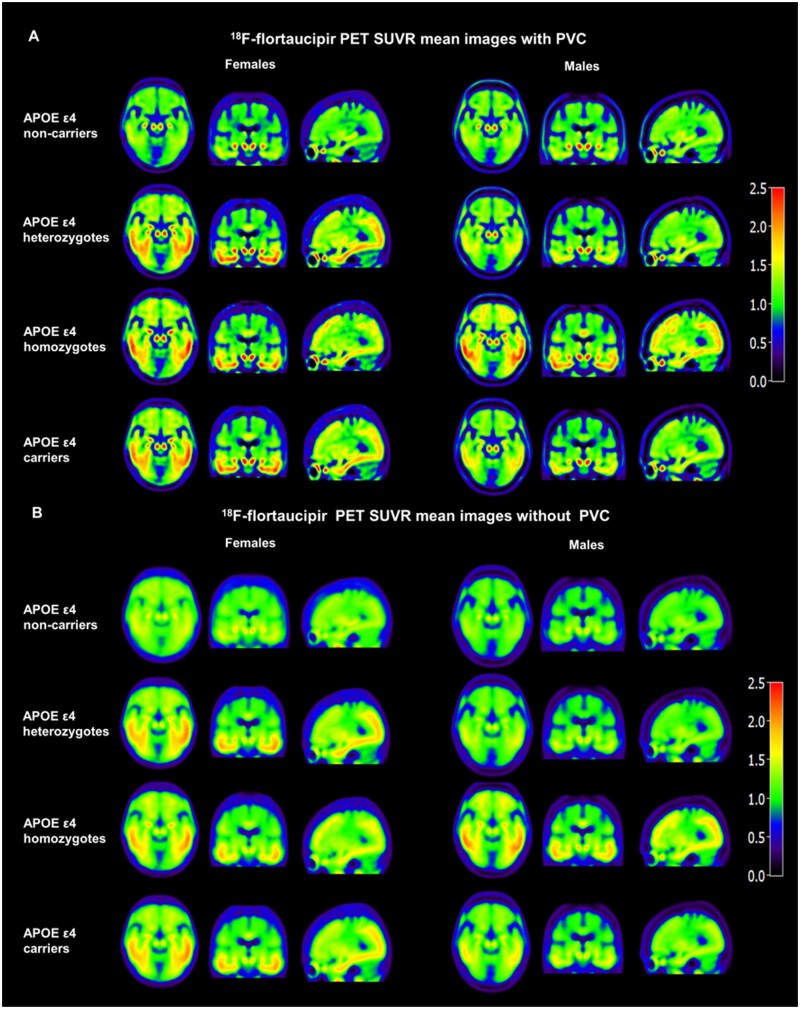
The 18F-flortaucipir SUVR images with PVC (Fig. 2A) demonstrated increased contrast among APOE ε4 non-carriers, heterozygotes and homozygotes compared to the SUVR images without PVC (Fig. 2B) as reported previously in other cohorts.5,21
Figure 2.
Mean images with and without PVC illustrate that sex modulates the APOE ε4 dose effect on 18F-flortaucipir SUVR in cognitively impaired participants. PVC images show (A) increased contrast and spatial resolution compared to images without PVC (B). Both PVC and non-PVC images visually demonstrate an interaction effect between sex and APOE ε4 status.
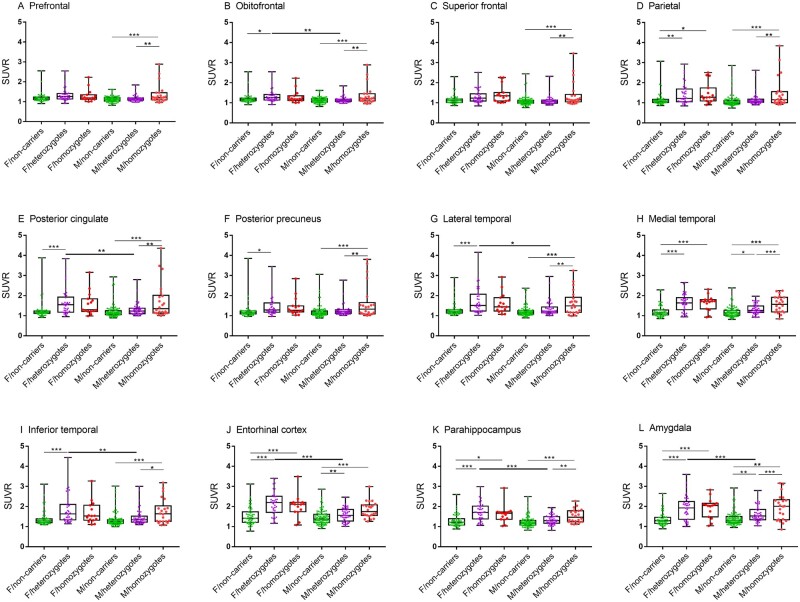
The region of interest-based SUVRs of female APOE ε4 homozygotes were significantly higher than non-carriers in the medial temporal, entorhinal cortex, parahippocampus and amygdala after adjusting for age and years of education (FDR P < 0.05; Fig. 3). The regions of interest of medial temporal, entorhinal cortex and amygdala retained a significant difference between homozygotes and non-carriers after adjusting for global cortical 18F-florbetapir SUVR. Similarly, female heterozygotes had higher 18F-flortaucipir SUVR in the orbitofrontal, parietal, posterior cingulate and posterior precuneus, lateral temporal, medial temporal, inferior temporal, entorhinal cortex, parahippocampus and amygdala (FDR P < 0.05; Fig. 3) compared to female non-carriers. The regions of interest of medial temporal, entorhinal cortex, amygdala and parahippocampal gyrus retained a significant difference between heterozygotes and non-carriers after adjusting for global cortical 18F-florbetapir SUVR (FDR P < 0.05). Strikingly, there were no significant differences in any of the 12 regions of interest SUVRs between female homozygotes and female heterozygotes with or without adjusting for global cortical 18F-florbetapir SUVR (FDR P > 0.05).
Figure 3.
Sex modifies the APOE ε4 dosage effect on region of interest-based analysis of 18F-flortaucipir PET in cognitively impaired individuals. Box plots depict median value and the interquartile ranges of regional SUVRs for APOE ε4 non-carriers (green), heterozygotes (purple) and homozygotes (red) in females and males. P-value was defined using a generalized linear model, adjusting for age and years of education. F = female; M = male. Bold significance lines indicate comparison between APOE ε4 dosage groups. *P < 0.05, **P < 0.01, ***P < 0.001.
Among males, APOE ε4 homozygotes exhibited a marked increase in 18F-flortaucipir SUVR compared to both heterozygotes and non-carriers in all 12 regions of interest. The entorhinal cortex, amygdala and parahippocampal gyrus retained a significant APOE ε4 dosage effect on tau deposition after adjusting for global cortical 18F-florbetapir SUVR. In addition, male heterozygotes had higher 18F-flortaucipir SUVR than male non-carriers in the medial temporal cortex, entorhinal cortex and amygdala after adjusting for age and years of education (FDR P < 0.05; Fig. 3). No regions of interest exhibited a significant difference between male heterozygotes and male non-carriers after adjusting for global cortical 18F-florbetapir SUVR (FDR P > 0.05).
Female heterozygotes exhibited significantly increased 18F-flortaucipir SUVR compared to male heterozygotes in the orbitofrontal, posterior cingulate, lateral temporal, inferior temporal, entorhinal cortex, amygdala and parahippocampal gyrus (P < 0.05; Fig. 3). There were no significant differences in region of interest 18F-flortaucipir SUVRs between males and females for both homozygotes and non-carriers (P > 0.05).
Sex-stratified effect of APOE ε4 dosage on voxel-wise 18F-flortaucipir SUVR analysis
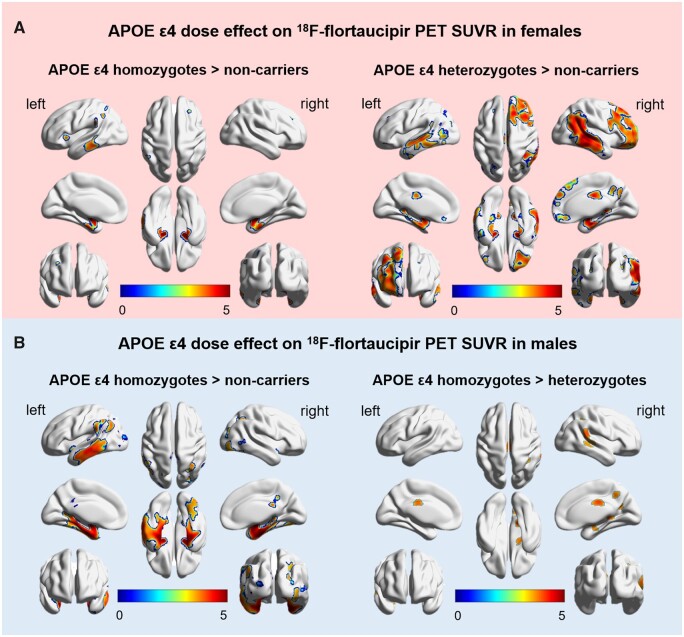
Female APOE ε4 homozygotes had significantly higher 18F-flortaucipir SUVR than non-carriers in the clusters corresponding to the left middle temporal, inferior temporal, superior parietal; right middle frontal, superior frontal, bilateral parahippocampus, amygdala, entorhinal cortex and inferior parietal regions. Female heterozygotes had higher 18F-flortaucipir SUVR than non-carriers in an even greater number of clusters involving the temporal cortex, middle cingulate and precuneus locations (FDR P < 0.05). No significant differences between female homozygotes and heterozygotes were found (Fig. 4A and Table 3).
Figure 4.
Sex modifies the APOE ε4 dose effect on voxel-wise analysis of 18F-flortaucipir PET in cognitively impaired participants. APOE ε4 dose effect on voxel-wise SUVR is depicted in (A) females and (B) males. Female heterozygotes (A, left) and homozygotes (A, right) display increased 18F-flortaucipir SUVR compared to non-carriers. No significant differences in SUVR were observed between female heterozygotes and homozygotes. Male homozygotes demonstrate increased 18F-flortaucipir SUVR compared to male heterozygotes (B, left) and male non-carriers (B, right). No significant differences in SUVR were observed between male heterozygotes and male non-carriers. T-values are expressed on blue–red scale from 0 to 5 depicting voxels level with P < 0.001 (adjusted for age and years of education).
Table 3.
Clusters with significant association between 18F-flortaucipir SUVR and APOE ε4 dosage
| Clusters | Female |
Male |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Homozygotes >
non-carriers |
Heterozygotes >
non-carriers |
Homozygotes >
non-carriers |
Homozygotes >heterozygotes |
|||||||||
| x | y | z | x | y | z | x | y | z | x | y | z | |
| Temporal_Mid_L Temporal_Inf_L |
−63 | −28.5 | −21 | 55 | −66 | 0 | −54 | −26 | −5 | − | − | − |
| Temporal_Mid_R Temporal_Inf_R |
− | − | − | 55 | −21 | −21 | 58 | −21 | −7 | 60 | −30 | 0 |
| Temporal_Sup_R | − | − | − | 64.5 | −33 | 16.5 | 57 | −6 | 2 | − | − | − |
| ParaHippocampal_L Amygdala_L |
−18 | −9 | −18 | −22 | −12 | −25 | −18 | −6 | −21 | − | − | − |
| ParaHippocampal_R Amygdala_R |
18 | −7.5 | −18 | 18 | −7.5 | −18 | 18 | −4 | −22 | − | − | − |
| Cingulum_Mid_L Precuneus_L |
− | − | − | -6 | −43 | 20 | 9 | −27 | 36 | −6 | −22 | 36 |
| − | − | − | ||||||||||
| Cingulum_Mid_R Precuneus_R |
− | − | − | 9 | −43 | 26 | 9 | −24 | 41 | 10.5 | −51 | 45 |
| Parietal_Inf_L | −52.5 | −52.5 | 42 | −44 | −48 | 40 | −42 | −48 | 43 | 9 | −57 | 58.5 |
| Parietal_Inf_R | 34.5 | −52.5 | 46.5 | − | − | − | 45 | −53 | 53 | 33 | −54 | 54 |
| Parietal_Sup_L | −31.5 | −63 | 60 | −30 | −67.5 | 57 | −24 | −67 | 51 | − | − | − |
| Frontal_Mid_L Frontal_Sup_L |
− | − | − | − | − | − | −4 | 59 | 17 | − | − | − |
| Frontal_Mid_R Frontal_Sup_R |
28.5 | 34.5 | 36 | − | − | − | 13.5 | 27 | 57 | − | − | − |
Data extracted from SPM12 analysis showing voxels with significantly increased 18F-flortaucipir SUVR in cognitively impaired female APOE ε4 homozygotes versus non-carriers, female heterozygotes versus female non-carriers, male APOE ε4 homozygotes versus male non-carriers, male homozygotes versus male heterozygotes, adjusted for age and years of education. There were no significant differences between female homozygotes and female heterozygotes or between male heterozygotes and male non-carriers (not listed in Table 3). Cluster locations correspond to the brain maps shown in Fig. 3. Atlas coordinates were obtained from Automated Anatomical Labelling (AAL).40
Male APOE ε4 homozygotes showed significantly higher 18F-flortaucipir SUVR than non-carriers in the bilateral middle temporal, inferior temporal, parahippocampus, amygdala, fusiform, entorhinal cortex, middle cingulate, precuneus, inferior parietal, middle frontal, superior frontal and left superior parietal regions. Furthermore, male homozygotes showed higher 18F-flortaucipir SUVR than heterozygotes in right middle temporal, inferior temporal, precuneus, bilateral middle cingulate and inferior parietal locations (FDR P < 0.05). No significant differences in 18F-flortaucipir SUVR between male heterozygotes and male non-carriers were found (Fig. 4B and Table 3). Further, there were no cerebral locations where SUVR was higher in the male non-carriers compared to either the male heterozygotes or male homozygotes.
Discussion
The main finding from this study is that females and males show different patterns of APOE ε4 dosage-related tau deposition in cognitively impaired elderly individuals. Specifically, in females, increased tau deposition was observed in both APOE ε4 heterozygotes and homozygotes compared to non-carriers. But, in males, only the APOE ε4 homozygotes (and not the heterozygotes) had increased tau deposition compared to non-carriers. Together, these results suggest that only one APOE ε4 allele is sufficient to increase tau accumulation in females, while two APOE ε4 alleles are needed to cause a similar effect in males. Consistent with previous studies,29,30 we found that region of interest analysis of entorhinal cortex, amygdala and parahippocampal gyrus showed an amyloid-β-independent association between the APOE ε4 gene dosage and tau deposition in males but not females after controlling for global cortex 18F-florbetapir SUVR. These findings have important clinical implications towards developing sex and genotype-guided therapeutics in Alzheimer’s disease and uncover a possible explanation underlying differential APOE ε4-associated Alzheimer’s risk in males and females.
Our findings indicate that the three levels of APOE ε4 alleles exert different effects on tau deposition in males and females. In males, a significant APOE ε4 dose effect on tau deposition was observed throughout the cortex, especially in medial temporal and amygdala (homozygotes > heterozygotes > non-carriers), but no APOE ε4 dosage effect was observed among females. These results were consistent across region of interest-based and voxel-wise analyses. Our findings are similar to the recent 18F-flortaucipir PET study, which demonstrated an APOE ε4 dose-dependent effect on entorhinal cortex tau deposition in cognitively unimpaired individuals.11 The current findings are in line with our group’s previous results that female APOE ε4 carriers had greater tau deposition than males in the entorhinal cortex, amygdala and parahippocampal gyrus.5 Our findings are also in line with the previous studies, which showed that APOE ε4 heterozygosity is sufficient to increase Alzheimer’s disease risk in females, while in males, APOE ε4 homozygosity is required to increase Alzheimer’s disease risk.1,2,31,32 Taken together, our data indicate that females may have higher susceptibility to the APOE ε4 allele than males. These findings are also consistent with work from Payami et al.32 showing that the age of Alzheimer’s disease onset among female APOE ε4 heterozygotes was similar to homozygotes, but younger than non-carriers. In contrast, in males, the age of Alzheimer’s disease onset among APOE ε4 heterozygotes was similar to non-carriers, but younger than homozygotes.32 Another study suggested that females have a higher risk of Alzheimer’s disease than males not because of their greater longevity,33 but probably due to the female-specific susceptibility among heterozygotes.2,32 Combined with these previous findings and results for the study, we provide a potential genetic-informed explanation underlying increased susceptibility to Alzheimer’s disease in females.34
Alzheimer’s disease pathology is characterized by the accumulation of amyloid-β plaques and neurofibrillary tangles in the brain. Our study found an amyloid-β-independent association between APOE ε4 dosage and tau deposition only in regions of early tau deposition (medial temporal, entorhinal cortex, amygdala and parahippocampal gyrus). This suggests that APOE ε4 dosage × sex interaction effect on tau deposition were partially mediated by amyloid-β. In line with this, a biochemical study found that accumulations of tau and amyloid-β occur independently in the entorhinal cortex.35 Two recent cross-sectional tau PET studies indicated that APOE ε4 is associated with tau deposition in the medial temporal cortex independent of amyloid-β status.30,36 The neocortex amyloid-β-dependent tau deposition maybe explained by the dual-cascade hypothesis.37 However, our results are in contrast with a recent longitudinal tau PET study, which showed that the accumulation rate of tau in temporal meta- and neocortical regions of interest is increased in females amyloid-β-positive individuals.38 It should be noted that the analyses with controlling for global cortical 18F-florbetapir SUVR were performed in a subset of the cohort of 188 individuals. Therefore, the reduced regions of interest of APOE ε4 dosage × sex interaction effect after controlling for 18F-florbetapir SUVR may be partly explained by differences in sample size. We realized that the time interval between the 18F-florbetapir and 18F-flortaucipir PET scans was 4.35 months (SD: 5.68; range: 0–27.53 months), which were unlikely to be a source of error as confirmed by analyses with and without controlling the time interval of the two scans.
Past reports have demonstrated that the effect of APOE ε4 on CSF t-tau and p-tau levels is stronger in females than males.14 Sundermann et al.17 showed that APOE ε4 is associated with greater amyloid-β burden across males and females in individuals with mild cognitive impairment, but only in males for Alzheimer’s disease individuals. These findings are in line with the previous work of our group and others showing that both t-tau and p-tau increased more in female APOE ε4 carriers compared to males.5,16 This lack of concordance of APOE ε4 dose effects between brain 18F-flortaucipir SUVR and CSF tau is probably due to the limited number of samples with CSF information, reducing statistical power in CSF analysis. In the present study, the interval between tau PET and CSF biomarkers was 21.07 months (SD: 30.29; range: 0–128 months), which may reduce the statistical power on the analysis of the APOE ε4 dose effect on CSF t-tau and p-tau. The inconsistency between tau PET and CSF tau may be due to the increased tau in CSF reflects not regional tau deposition, but neuronal damage and disease progression. Compared to CSF tau assessments, brain tau PET provides quantitative brain tau measurements with higher sensitivity and specificity in Alzheimer’s disease study.39
Nevertheless, there were some limitations to this study. Even in our large study cohort of 268 cognitively impaired participants, only 37 individuals were homozygous for the APOE ε4 allele. This limits our ability to accurately model the effects of APOE ε4 dosage across the spectrum of Alzheimer’s disease severity and age ranges. As reported in a previous study, APOE ε4 homozygotes may have an increased risk of Alzheimer’s disease before their seventies, but resilience to Alzheimer’s disease beyond their seventies.11 Further, our study involved a relatively small number of APOE ε4 carriers (69 male APOE ε4 carriers and 53 female APOE ε4 carriers), potentially limiting the generalizability of our results, although a generalized linear model to assess group differences in tau deposition was used for its robustness with smaller sample size. The statistical power for 18F-flortaucipir SUVR to distinguish between APOE ε4 heterozygotes and homozygotes ranged from 0.97 to 0.999 in all 12 regions of interest among males, and 0.05 to 0.69 among females. The results of this study will be evaluated further in the near future on the going ADNI projects and other larger cohort studies.
In conclusion, this quantitative 18F-flortaucipir PET study in individuals with cognitive impairment showed that an APOE ε4 gene dose-dependent effect on brain region-specific tau deposition exists in males, but not in females. This work highlights the importance of considering sex and APOE ε4 dose effect in biomarker development and mechanistic studies in Alzheimer’s disease using quantitative 18F-flortaucipir PET.
Glossary
- ADNI
Alzheimer’s Disease Neuroimaging Initiative
- PVC
partial volume correction
- SUVR
standardized uptake value ratio
Funding
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). The ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
References
- 1. Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261(5123):921–923. [DOI] [PubMed] [Google Scholar]
- 2. Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: A meta-analysis. JAMA. 1997;278(16):1349–1356. [PubMed] [Google Scholar]
- 3. Cosentino S, Scarmeas N, Helzner E, et al. APOE ε4 allele predicts faster cognitive decline in mild Alzheimer disease. Neurology. 2008;70(19 Part 2):1842–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Neu SC, Pa J, Kukull W, et al. Apolipoprotein E genotype and sex risk factors for Alzheimer disease: A meta-analysis. JAMA Neurol. 2017;74(10):1178–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu M, Paranjpe MD, Zhou X, et al. Sex modulates the ApoE epsilon4 effect on brain tau deposition measured by 18F-AV-1451 PET in individuals with mild cognitive impairment. Theranostics. 2019;9(17):4959–4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Risacher SL, Kim S, Nho K, et al. APOE effect on Alzheimer's disease biomarkers in older adults with significant memory concern. Alzheimers Dement. 2015;11(12):1417–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deming Y, Li Z, Kapoor M, et al. Genome-wide association study identifies four novel loci associated with Alzheimer’s endophenotypes and disease modifiers. Acta Neuropathol. 2017;133(5):839–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mattsson N, Zetterberg H, Hansson O, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA. 2009;302(4):385–393. [DOI] [PubMed] [Google Scholar]
- 9. Liu Y, Tan L, Wang H-F, et al. Multiple effect of APOE genotype on clinical and neuroimaging biomarkers across Alzheimer’s disease spectrum. Mol Neurobiol. 2016;53(7):4539–4547. [DOI] [PubMed] [Google Scholar]
- 10. Buerger K, Teipel SJ, Zinkowski R, et al. Increased levels of CSF phosphorylated tau in apolipoprotein E ɛ4 carriers with mild cognitive impairment. Neurosci Lett. 2005;391(1-2):48–50. [DOI] [PubMed] [Google Scholar]
- 11. Ghisays V, Goradia DD, Protas H, et al. Brain imaging measurements of fibrillar amyloid-β burden, paired helical filament tau burden, and atrophy in cognitively unimpaired persons with two, one, and no copies of the APOE ε4 allele. Alzheimers Dement 2019;16(4):598-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tiraboschi P, Hansen L, Masliah E, Alford M, Thal L, Corey-Bloom J.. Impact of APOE genotype on neuropathologic and neurochemical markers of Alzheimer disease. Neurology. 2004;62(11):1977–1983. [DOI] [PubMed] [Google Scholar]
- 13. Reiman EM, Chen K, Alexander GE, et al. Correlations between apolipoprotein E ε4 gene dose and brain-imaging measurements of regional hypometabolism. Proc Natl Acad Sci U S A. 2005;102(23):8299–8302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hohman TJ, Dumitrescu L, Barnes LL, et al. ; for the Alzheimer’s Disease Genetics Consortium and the Alzheimer’s Disease Neuroimaging Initiative. Sex-specific association of Apolipoprotein E with cerebrospinal fluid levels of tau. JAMA Neurol. 2018;75(8):989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Damoiseaux JS, Seeley WW, Zhou J, et al. ; for the Alzheimer's Disease Neuroimaging Initiative. Gender modulates the APOE epsilon4 effect in healthy older adults: Convergent evidence from functional brain connectivity and spinal fluid tau levels. J Neurosci. 2012;32(24):8254–8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Altmann A, Tian L, Henderson VW, Greicius MD., Alzheimer's Disease Neuroimaging Initiative Investigators. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol. 2014;75(4):563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sundermann EE, Tran M, Maki PM, et al. Sex differences in the association between apolipoprotein E ε4 allele and Alzheimer's disease markers. Alzheimers Dement. 2018;10:438–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buckley RF, Mormino EC, Rabin JS, et al. Sex differences in the association of global amyloid and regional tau deposition measured by positron emission tomography in clinically normal older adults. JAMA Neurol. 2019;76(5):542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Suri S, Heise V, Trachtenberg AJ, Mackay CE.. The forgotten APOE allele: A review of the evidence and suggested mechanisms for the protective effect of APOE ɛ2. Neurosci Biobehav Rev. 2013;37(10):2878–2886. [DOI] [PubMed] [Google Scholar]
- 20. Hixson JE, Vernier DT.. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31(3):545–548. [PubMed] [Google Scholar]
- 21. Paranjpe MD, Chen X, Liu M, et al. The effect of ApoE epsilon4 on longitudinal brain region-specific glucose metabolism in patients with mild cognitive impairment: A FDG-PET study. Neuroimage Clin. 2019;22:101795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yan S, Zheng C, Cui B, et al. Multiparametric imaging hippocampal neurodegeneration and functional connectivity with simultaneous PET/MRI in Alzheimer's disease. Eur J Nucl Med Mol Imaging. 2020;47(10):2440–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tohka J, Reilhac A.. Deconvolution-based partial volume correction in Raclopride-PET and Monte Carlo comparison to MR-based method. Neuroimage. 2008;39(4):1570–1584. [DOI] [PubMed] [Google Scholar]
- 24. Gottesman RF, Schneider AL, Zhou Y, et al. The ARIC-PET amyloid imaging study: brain amyloid differences by age, race, sex, and APOE. Neurology. 2016;87(5):473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhao Q, Liu M, Ha L, Zhou Y.. Quantitative 18F-AV1451 brain tau PET imaging in cognitively normal older adults, mild cognitive impairment, and Alzheimer's disease patients. Front Neurol. 2019;10:486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhou Y, Resnick SM, Ye W, et al. Using a reference tissue model with spatial constraint to quantify [11C]Pittsburgh compound B PET for early diagnosis of Alzheimer's disease. Neuroimage. 2007;36(2):298–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tudorascu DL, Minhas DS, Lao PJ, et al. The use of Centiloids for applying [11C] PiB classification cutoffs across region-of-interest delineation methods. Alzheimers Dement. 2018;10:332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gottesman RF, Schneider AL, Zhou Y, et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA. 2017;317(14):1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Salvadó G, Grothe MJ, Groot C, et al. Differential associations of APOE-ε2 and APOE-ε4 alleles with PET-measured amyloid-β and tau deposition in older individuals without dementia. Eur J Nucl Med Mol Imaging. 2021;48(7):2212–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Therriault J, Benedet AL, Pascoal TA, et al. Association of apolipoprotein E ε4 with medial temporal tau independent of amyloid-β. JAMA Neurol. 2020;77(4):470–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim S, Kim MJ, Kim S, et al. Gender differences in risk factors for transition from mild cognitive impairment to Alzheimer’s disease: A CREDOS study. Compr Psychiatry. 2015;62:114–122. [DOI] [PubMed] [Google Scholar]
- 32. Payami H, Montee KR, Kaye JA, et al. Alzheimer's disease, apolipoprotein E4, and gender. JAMA. 1994;271(17):1316–1317. [PubMed] [Google Scholar]
- 33. Cannon-Albright LA, Foster NL, Schliep K, et al. Relative risk for Alzheimer disease based on complete family history. Neurology. 2019;92(15):e1745–e1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Riedel BC, Thompson PM, Brinton R.. Age, APOE and sex: Triad of risk of Alzheimer’s disease. J Steroid Biochem Mol Biol. 2016;160:134–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Katsuno T, Morishima-Kawashima M, Saito Y, et al. Independent accumulations of tau and amyloid beta-protein in the human entorhinal cortex. Neurology. 2005;64(4):687–692. [DOI] [PubMed] [Google Scholar]
- 36. Mattsson N, Ossenkoppele R, Smith R, et al. Greater tau load and reduced cortical thickness in APOE ε4-negative Alzheimer's disease: a cohort study. Alzheimers Res Ther. 2018;10(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Small SA, Duff K.. Linking Abeta and tau in late-onset Alzheimer's disease: A dual pathway hypothesis. Neuron. 2008;60(4):534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smith R, Strandberg O, Mattsson-Carlgren N, et al. The accumulation rate of tau aggregates is higher in females and younger amyloid-positive subjects. Brain. 2020;143(12):3805–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Holtzman DM, Carrillo MC, Hendrix JA, et al. Tau: From research to clinical development. Alzheimers Dement. 2016;12(10):1033–1039. [DOI] [PubMed] [Google Scholar]
- 40. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used in the current study were obtained from the ADNI database (available at https://adni.loni.usc.edu).