Abstract
Background and Aims
To investigate the significance of 9 amino acids as risk factors for incident cardiovascular disease events in 9584 Finnish men.
Materials and Methods
A total of 9584 men (age 57.4 ± 7.0 years, body mass index 27.2 ± 4.2 kg/m2) from the Metabolic Syndrome in Men study without cardiovascular disease and type 1 diabetes at baseline were included in this study. A total of 662 coronary artery disease (CAD) events, 394 ischemic stroke events, and 966 cardiovascular disease (CVD; CAD and stroke combined) events were recorded in a 12.3-year follow-up. Amino acids were measured using nuclear magnetic resonance platform.
Results
In Cox regression analysis, phenylalanine and tyrosine were significantly associated with increased risk of CAD and CVD events, and phenylalanine with increased risk of ischemic stroke after the adjustment for confounding factors. Glutamine was significantly associated with decreased risk of stroke and CVD events and nominally with CAD events. Alanine was nominally associated with CAD events.
Conclusion
We identified alanine as a new amino acid associated with increased risk of CAD and glutamine as a new amino acid associated with decreased risk of ischemic stroke. We also confirmed that phenylalanine and tyrosine were associated with CAD, ischemic stroke, and CVD events.
Keywords: amino acids, coronary artery disease, ischemic stroke, cardiovascular disease
Cardiovascular disease (CVD), including coronary artery disease (CAD) and ischemic stroke, is the leading cause of mortality worldwide (1). Multiple risk factors accelerate the development of CVD, including age, dyslipidemia, hypertension, smoking, hyperglycemia, and inflammatory factors (2-6). However, overall predictive value of the conventional cardiovascular risk factors is limited, and therefore there is an increasing need to identify novel risk factors for senility CVD (5, 6).
Several previous studies on amino acids have especially focused on their associations with obesity (7) and type 2 diabetes (T2D), a major risk factor for CVD (8-10). Previously published studies on the association of amino acids with CAD, ischemic stroke, and CVD (11-19) have been heterogeneous with respect to size of the study (small, large), study design (cross-sectional case-control studies, prospective studies), endpoints (CAD, ischemic stroke, or combination of both).
T2D, CAD, and ischemic stroke share several risk factors, but the role of amino acids remains unclear in the development of cardiovascular events. Previous prospective studies have not systematically compared the significance of amino acids as predictors of CAD, stroke, and CVD in a large prospective population-based study. Therefore, the aim of our study was to investigate the association of serum concentrations of 9 amino acids (alanine, glutamine, glycine, histidine, isoleucine, leucine, phenylalanine, tyrosine, and valine), with the risk CAD, ischemic stroke, and CVD in a large longitudinal population-based Metabolic Syndrome in Men (METSIM) study (20).
Study Population
The METSIM study includes 10 197 men, aged from 45 to 73 years, and randomly selected from the population register of Kuopio, Eastern Finland. At baseline, each participant had an outpatient visit to the Clinical Research Unit at the University of Kuopio. The design and methods of the METSIM study have been previously described in detail (21).
A total of 9584 men including 1412 men who had T2D were included in the current study. Participants with type 1 diabetes (n = 25) were excluded from current analyses. Altogether, 602 CVD events before the baseline study were recorded, including 404 CAD and 198 ischemic stroke events. These participants were excluded from all statistical analyses. We measured height and weight to the nearest 0.5 cm and 0.1 kg, respectively. Body mass index (BMI) was calculated as weight (kilograms) divided by height (meters) squared. We measured waist circumference at the midpoint between the lateral iliac crest and lowest rib. Smoking status was defined as current smoking (yes vs no). Diagnosis of hypertension was based on the use of antihypertensive medication. All participants underwent a 2-hour oral glucose tolerance test (75 g of glucose), and samples for plasma glucose and insulin were drawn at 0, 30, and 120 minutes. Diabetes was defined as fasting plasma glucose ≥7.0 mmol/L and/or 2-hour plasma glucose ≥11.1 mmol/L, and/or hemoglobin A1c ≥6.5%) and/or medication for diabetes according to the American Diabetes Association 2003 criteria (22).
Calculations
We validated the best oral glucose tolerance test-derived index of insulin sensitivity in a separate sample of 287 nondiabetic individuals from the region of Kuopio against the euglycemic-hyperinsulinemic clamp, a gold standard measurement of insulin sensitivity (21). Among 6 different indices tested the Matsuda insulin sensitivity index (Matsuda ISI) had the highest correlation 0.776 with the M value from the clamp. We calculated Matsuda ISI for insulin sensitivity based on glucose and insulin measurements at 0, 30, and 120 minutes as previously reported (21).
Laboratory Measurements
We measured plasma glucose, insulin, total triglycerides, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and serum high-sensitivity C-reactive protein (hs-CRP), as previously described (23). We measured serum metabolites and lipoprotein lipids using a high-throughput serum nuclear magnetic resonance (NMR) platform operating at 500 MHz. Fasting serum samples collected at the baseline study were stored at -80°C and thawed overnight in a refrigerator before sample preparation. Aliquots of each sample (300 μL) were mixed with sodium phosphate buffer (300 μL). Details of the NMR experimentation have been described previously (24, 25).
Diagnosis of CAD and Ischemic Stroke Events
We identified nonfatal and fatal CAD and ischemic stroke events from the hospital discharge registry of Kuopio University Hospital, which is the only hospital and cardiology outpatient clinic treating patients in the Kuopio region. Thereafter, 2 cardiologists (R.J., J.K.) reviewed medical records of all subjects with diagnosis of CAD based on the codes I22-I24 in the International Classification of Diseases according to internationally accepted criteria (26). Causes of CAD death were confirmed from death certificates obtained from the National Causes-of-Death Register of Statistics Finland. We identified 662 nonfatal or fatal CAD events, 394 nonfatal or fatal ischemic stroke events based on the codes I63-I69 in the International Classification of Diseases, and 966 nonfatal or fatal CVD events (CAD and stroke events combined). Incident cases of CVD events were recorded from the baseline study visit until February 28, 2021, with a mean follow-up period of 12.3 years.
Statistical Analyses
We performed statistical analyses using SPSS version 27 (SPSS, Chicago, IL). We log-transformed all continuous variables for statistical analyses, except for age and LDL-C. We used independent samples t test and χ 2 test to assess statistical significance between the 2 groups. We applied Cox regression analysis to evaluate baseline concentrations of the nine amino acids as predictors for CAD, ischemic stroke, and CVD events. Results of Cox regression analyses are presented without and with adjustment for confounding factors (age, diabetes status, fasting insulin, smoking, BMI, LDL-C, HDL-C, statin treatment, drug treatment for hypertension, and hs-CRP). Hazard ratio (HR) values are shown as per 1 SD increase in amino acid concentrations. The association with outcomes could be reasonably fit by a linear model. We applied a C-index (27) that is a standard measure of the predictive accuracy of a logistic regression model. To calculate C-index we first included confounding factors listed above in the Cox model (model 1) and then we added in the Cox model 3 amino acids (phenylalanine, tyrosine, alanine) (model 2). Correlations were calculated by Pearson correlations. Bonferroni-corrected limit for statistically significance was P < 0.0056 given 9 amino acids included in statistical analyses. P < 0.05 was considered as nominally significant.
Ethics Statement
The study protocol was approved by the Ethics Committee of University of Eastern Finland and Kuopio University Hospital. The study was conducted in accordance with the Helsinki Declaration. All study participants gave written informed consent.
Results
Clinical and laboratory characteristics of the METSIM study participants at baseline are shown in Table 1. Participants with CAD, stroke, or CVD had more often CVD risk factors, including smoking, type 2 diabetes, hyperglycemia, obesity, medication for hypertension, elevated LDL-C, and elevated hs-CRP compared with participants without CVD. Participants with CAD or CVD had elevated concentrations of alanine, isoleucine, leucine, phenylalanine, and tyrosine, and lower concentrations of glutamine than participants without CVD. Supplementary Table 1 shows the correlations of amino acids with clinical and laboratory parameters (28). Alanine (except for LDL-C), isoleucine, leucine, phenylalanine, tyrosine (except for LDL-C), and valine (except for systolic blood pressure and LDL-C) correlated significantly with BMI, waist circumference, systolic blood pressure, LDL-C, total triglycerides, fasting plasma glucose and 2-hour plasma glucose, fasting plasma insulin, 2-hour plasma insulin, hs-CRP, and inversely with Matsuda ISI insulin sensitivity index.
Table 1.
Clinical and laboratory characteristics including amino acid measurements of 9548 participants at the baseline study of the METSIM cohort
| Variables | No CVD (N = 8578) | CAD (N = 666) | Stroke (N = 394) | CVD (N = 970) |
|---|---|---|---|---|
| Age, y | 57.0 ± 7.0 | 60.6 ± 6.7a | 61.8 ± 6.7a | 60.8 ± 6.8a |
| Type 2 diabetes, % of total | 5.9 | 14.0a | 15.2a | 13.3a |
| Current smokers, % of total | 17.6 | 24.9a | 20.1 | 23.5a |
| Medication for hypertension, % | 19.3 | 31.0a | 30.7a | 29.6a |
| Body mass index, kg/m2 | 27.2 ± 4.1 | 28.2 ± 4.5a | 27.9 ± 4.6 | 28.0 ± 4.5a |
| Waist, cm | 98.4 ± 11.4 | 101.7 ± 12.3a | 100.9 ± 12.0a | 101.17 ± 12.1a |
| Systolic blood pressure, mmHg | 137.6 ± 16.4 | 143.5 ± 18.0a | 144.0 ± 19.1a | 143.5 ± 18.2a |
| Diastolic blood pressure, mmHg | 87.4 ± 9.3 | 88.3 ± 10.5 | 87.5 ± 10.2 | 88.25 ± 10.4 |
| HDL cholesterol, mmol/L | 1.45 ± 0.39 | 1.36 ± 0.39a | 1.40 ± 0.40 | 1.38 ± 0.40a |
| LDL cholesterol, mmol/L | 3.36 ± 0.88 | 3.38 ± 0.94 | 3.25 ± 0.99 | 3.36 ± 0.94 |
| Total triglycerides, mmol/L | 1.44 ± 0.87 | 1.70 ± 1.17a | 1.51 ± 0.88 | 1.60 ± 1.03a |
| Blood HbA1c, % | 5.8 ± 0.6 | 6.0 ± 0.8a | 6.0 ± 0.7a | 6.0 ± 0.8a |
| Fasting plasma glucose, mmol/L | 5.92 ± 0.98 | 6.27 ± 1.57a | 6.17 ± 1.31a | 6.19 ± 1.42a |
| 2-h plasma glucose, mmol/L | 6.37 ± 2.34 | 7.04 ± 2.88a | 7.05 ± 2.69a | 6.96 ± 2.75a |
| Fasting plasma insulin, mU/L | 9.2 ± 9.1 | 13.4 ± 24.9a | 12.5 ± 19.8a | 12.4 ± 21.7a |
| 2-hour plasma insulin, mU/L | 53.9 ± 56.7 | 66.6 ± 64.6a | 65.0 ± 59.3a | 64.2 ± 60.6a |
| C-reactive protein, mg/L | 2.07 ± 4.19 | 2.75 ± 4.18a | 3.16 ± 5.58a | 2.90 ± 4.87a |
| Alanine (mmol/L) | 0.416 ± 0.064 | 0.433 ± 0.066a | 0.428 ± 0.064 | 0.429 ± 0.065a |
| Glutamine (mmol/L) | 0.514 ± 0.075 | 0.502 ± 0.073a | 0.499 ± 0.075 | 0.502 ± 0.074a |
| Glycine (mmol/L) | 0.277 ± 0.071 | 0.279 ± 0.072 | 0.278 ± 0.076 | 0.279 ± 0.075 |
| Histidine (mmol/L) | 0.064 ± 0.009 | 0.063 ± 0.009 | 0.062 ± 0.009a | 0.063 ± 0.009a |
| Isoleucine (mmol/L) | 0.060 ± 0.017 | 0.064 ± 0.019a | 0.062 ± 0.017 | 0.063 ± 0.018a |
| Leucine (mmol/L) | 0.090 ± 0.017 | 0.093 ± 0.02a | 0.091 ± 0.017 | 0.092 ± 0.019a |
| Phenylalanine (mmol/L) | 0.075 ± 0.012 | 0.080 ± 0.012a | 0.080 ± 0.014a | 0.080 ± 0.013a |
| Tyrosine (mmol/L) | 0.057 ± 0.012 | 0.060 ± 0.012a | 0.059 ± 0.011 | 0.059 ± 0.012a |
| Valine (mmol/L) | 0.217 ± 0.037 | 0.221 ± 0.04 | 0.220 ± 0.037 | 0.220 ± 0.038 |
Values shown as mean ± SD for each category.
Abbreviations: CAD, coronary artery disease; CVD, cardiovascular disease; HDL, high-density lipoprotein; LDL, low-density lipoprotein; METSIM, Metabolic Syndrome in Men.
a Statistically significant differences (P < 0.002) between CAD, stroke, and CVD and control group (no CVD).
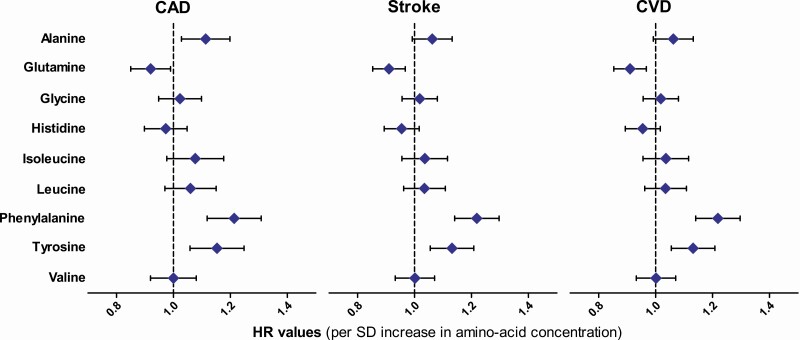
Figure 1 and Supplementary Table 2 give the results for Cox regression analyses of the associations of amino acids with incident CAD, ischemic stroke, and CVD events (28). In unadjusted Cox regression analysis, alanine, isoleucine, leucine, phenylalanine, and tyrosine were significantly associated with increased risk of CAD, and glutamine and histidine with decreased risk of CAD. When adjusted for conventional cardiovascular risk factors, phenylalanine (HR 1.21; 95% CI, 1.12-1.31; P = 5.0-E06) and tyrosine (HR 1.15; 95% CI, 1.06-1.25; P = 0.002) remained significantly associated, and alanine nominally associated (HR 1.11; 95% CI, 1.03-1.20; P = 0.021) with an increase in CAD events, and glutamine nominally associated (0.92; 95% CI, 0.85-0.99; P = 0.026) with a decreased risk of CAD events.
Figure 1.
Hazards ratios (HR) and their 95% CIs for the associations of 9 amino acids with the risk of coronary artery disease (CAD), ischemic stroke, and cardiovascular disease (CVD) events (N = 9584). HR values were adjusted for age, diabetes status, fasting insulin, smoking, body mass index, low-density lipoprotein cholesterol, drug treatment for hypertension, high-sensitivity C-reactive protein, high-density lipoprotein cholesterol, and statin treatment.
In nonadjusted Cox regression analyses, alanine, phenylalanine, and tyrosine were significantly associated with increased risk of ischemic stroke, and glutamine and histidine significantly associated with decreased risk of ischemic stroke events (Fig. 1, 28). After the adjustment for confounding factors phenylalanine (HR 1.24; 95% CI, 1.13-1.37; P = 1.5E-05) was significantly associated with increased risk of ischemic stroke, and glutamine (HR 0.86; 95% CI, 0.78-0.95; P = 0.003) significantly associated with decreased risk of stroke (28). We also calculated the phenylalanine to tyrosine ratio reflecting phenylalanine hydroxylase activity. This ratio has been suggested to be a risk factor for acute ischemic stroke (29). Phenylalanine to tyrosine ratio was nominally associated with incident ischemic stroke in unadjusted model (HR 1.14; 95% CI, 1.04-1.25; P = 0.008) but lost its statistical significance when adjusted for confounding factors.
In unadjusted Cox regression analysis, alanine, isoleucine, leucine, phenylalanine, and tyrosine were significantly associated with increased risk of CVD, and glutamine and histidine with decreased risk of CVD. When adjusted for confounding factors (Fig. 1, 28) phenylalanine (HR 1.22; 95% CI, 1.14-1.30; P = 4.5E-09) and tyrosine (HR 1.13; 95% CI, 1.06-1.21; P = 0.001) remained significantly associated with an increase in CVD events, and glutamine significantly associated with a decrease in CVD events (HR 0.91; 95% CI, 0.85-0.97; P = 0.004) (28).
We calculated the C-index first for model 1 including conventional risk factors for CVD events, and then we added amino acids phenylalanine, tyrosine, and alanine in model 2. C-index increased nonsignificantly from 0.694 to 0.697 for CAD (model 2). In corresponding models, C-index increased from 0.705 to 0.710 (P = 0.032) for stroke, and from 0.692 to 0.696 (P = 0.017) for CVD.
Discussion
Our 12.3-year follow-up study including 9584 participants of the METSIM study showed that alanine, glutamine, histidine, isoleucine, leucine, phenylalanine, tyrosine, and valine were significantly or nominally associated with CAD, ischemic stroke, or CVD events.
Previous studies have shown that conventional risk factors, such as elevated LDL-C concentration, hypertension, smoking, diabetes, obesity, central obesity, and inflammation are associated with an increase in CVD events (30). However, these established risk factors explain approximately only one-half of the risk of incident CAD events, and therefore new risk markers for CVD are needed.
Our study shows that a nonessential amino acid alanine nominally increased the risk of CAD by 11% after the adjustment for confounding factors. This is a novel finding not reported in previous studies. Alanine has been shown to be associated with elevated blood pressure (31), insulin resistance (9), nonalcoholic fatty liver disease (32), and type 2 diabetes (9), which may explain at least in part its role as a risk factor for cardiometabolic diseases. In our study, alanine correlated positively and significantly with several other cardiovascular risk factors, including BMI, waist, systolic blood pressure, fasting and 2-hour glucose and insulin, hs-CRP, and decreased insulin sensitivity.
Branched-chain amino acids isoleucine, leucine, and valine are essential amino acids in humans and play an important role in the pathogenesis of metabolic disorders, including obesity and diabetes (33). In our study, isoleucine and leucine were significantly associated with increased risk of CAD in an unadjusted model but after adjustment for confounding factors the associations lost their statistical significance. None of the branched-chain amino acids was significantly associated with ischemic stroke.
Several studies have been previously published about the role of branched-chain amino acids as risk factors for CVD. These studies have had differences in the design of the study (case-control vs prospective), the size of the study, and the definition of cardiovascular events (CAD vs combination of CAD and ischemic stroke). Almost all studies reported an association of branched-chain amino acids with CAD or CVD (11-17), but 1 previous study from Finland (18) failed to show that branched-chain amino acids are associated with increased risk of CVD events.
Phenylalanine is an essential aromatic amino acid, and the precursor for tyrosine and dopamine-related neurotransmitters. In our study, phenylalanine was the only amino acid significantly associated with CAD, ischemic stroke, and CVD events after the adjustment for confounding factors, confirming the results of a previous large prospective study comprising 3 population-based cohorts (18). Furthermore, phenylalanine has been shown to be associated with incident heart failure (34). In our study, phenylalanine correlated with elevated BMI, waist circumference, systolic blood pressure, LDL-C, total triglycerides, fasting and 2-hour glucose, insulin, and hs-CRP (35), and inversely with Matsuda ISI, all important cardiovascular risk factors. Additionally, we have previously shown that phenylalanine increased the risk of T2D by impairing both insulin secretion and insulin sensitivity (9). Interestingly, a recent study reported that a downstream gut microbial metabolite of phenylalanine, phenylacetylglutamine, was also associated with an increased risk of CVD especially in individuals with T2D (36).
Tyrosine is a nonessential amino acid and a precursor of the catecholamines. In our study, tyrosine was associated with increased risk of CAD by 15% and CVD by 13% after the adjustment for confounding factors. Tyrosine has been previously associated with an increase of carotid intima-media thickness in the Cardiovascular Risk in Young Finns Study (37). In our study, tyrosine was not associated with ischemic stroke. In our previous study, tyrosine was significantly associated also with an increase in the risk of T2D (9).
Histidine was significantly associated with a decreased risk of CAD, ischemic stroke, and CVD in an unadjusted Cox model in our study but after adjustment for confounding factors these associations were not statistically significant. Previous studies have reported that histidine is associated with a decreased risk of CAD (14, 15, 38) and ischemic stroke (38).
Glutamine is a nonessential amino acid and is among the most abundant amino acids in humans. Diet is the main source of nutrients involved in glutamine metabolism (39). In our study, glutamine decreased significantly ischemic stroke by 14% and CVD events by 9% after adjustment for confounding factors. Glutamine has a cardioprotective effect against CAD and CVD, as shown by a study including 2 cohorts, the Nurse’s Health Study and Health Professionals Follow-up study. This study showed that glutamine was inversely and significantly associated with cardiovascular mortality (40). As far as we know, the preventive effect of glutamine for ischemic stroke has not been previously published.
Our study demonstrates that metabolic risk factors (diabetes, fasting insulin, BMI, LDL-C, HDL-C) are important to take into account when analyzing the associations of amino acids with the risk of cardiovascular complications. Including metabolic risk factors in statistical analyses decreased significantly statistical significance between amino acids and CAD, stroke, and CVD suggesting that not only classic risk factors, but also metabolic factors play an important role in the risk of cardiovascular complications.
We calculated the C-index first for conventional risk factors for CVD events, and then added amino acids phenylalanine, tyrosine, and alanine in the models. C-index increased nonsignificantly for CAD events, but for stroke and CVD an increase in C-index was nominally significant. These results suggest that these amino acids increased the prediction for stroke and CAD significantly, but the improvement was relatively small.
The strengths of our study include the large and homogeneous METSIM study population. We had detailed phenotype at baseline, carefully defined diagnoses for CAD, ischemic stroke and CVD events, and a long follow-up period. Additionally, we adjusted our results for multiple confounding factors and used a very conservative threshold for statistical significance to increase credibility of our study. The limitation of our study is that it included only middle-aged and elderly Finnish men, and that our results do not imply causality between amino acids and cardiovascular events. We could not include all 20 amino acids in our statistical analyses because of limitations of the proton NMR method. Further studies in other populations are warranted to confirm our results and to assess their applicability to women and other populations and age groups.
In summary, our 12.3-year follow-up study of the METSIM cohort identified alanine as a new amino acid associated with increased risk of CAD and glutamine as a new amino acid associated with decreased risk of ischemic stroke. We also confirmed that phenylalanine and tyrosine were associated with CAD, ischemic stroke, and CVD events.
Acknowledgments
Funding: The study was supported by the Kuopio University Hospital and the Finnish Heart Research Foundation (J.K.) and the Academy of Finland (M.L.).
Glossary
Abbreviations
- BMI
body mass index
- CAD
coronary artery disease
- CVD
cardiovascular disease
- HR
hazard ratio
- HDL-C
high-density lipoprotein cholesterol
- hs-CRP
high-sensitivity C-reactive protein
- ISI
insulin sensitivity index
- LDL-C
low-density lipoprotein
- METSIM
Metabolic Syndrome in Men
- NMR
nuclear magnetic resonance
- T2D
type 2 diabetes
Additional Information
Disclosure Statement: The authors have nothing to disclose.
Data Availability
Restrictions apply to the availability of data generated or analyzed during this study to preserve the confidentiality of the participants. The corresponding author will, on request, detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685-1695. [DOI] [PubMed] [Google Scholar]
- 2. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e563-e595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837-1847. [DOI] [PubMed] [Google Scholar]
- 4. Gensini GF, Comeglio M, Colella A. Classical risk factors and emerging elements in the risk profile for coronary artery disease. Eur Heart J. 1998;19(Suppl A):A53-A61. [PubMed] [Google Scholar]
- 5. CARDIoGRAMplusC4D Consortium, Deloukas P, Kanoni S, Willenborg C, et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. 2013;45:25-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473(7347):317-325. [DOI] [PubMed] [Google Scholar]
- 7. Siddik MAB, Shin AC. Recent progress on branched-chain amino acids in obesity, diabetes, and beyond. Endocrinol Metab (Seoul). 2019;34(3):234-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laakso M. Biomarkers for type 2 diabetes. Mol Metab. 2019;27S: S139-S146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vangipurapu J, Stancáková A, Smith U, Kuusisto J, Laakso M. Nine amino acids are associated with decreased insulin secretion and elevated glucose levels in a 7.4-year follow-up study of 5181 Finnish men. Diabetes. 2019;68(6):1353-1358. [DOI] [PubMed] [Google Scholar]
- 10. Sun Y, Gao HY, Fan ZY, et al. Metabolomics signatures in type 2 diabetes: a systematic review and integrative analysis. J Clin Endocrinol Metab. 2020;105:dgz240. [DOI] [PubMed] [Google Scholar]
- 11. Ruiz-Canela M, Toledo E, Clish CB, et al. Plasma branched-chain amino acids and incident cardiovascular disease in the PREDIMED trial. Clin Chem. 2016;62(4):582-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tobias DK, Lawler PR, Harada PH, et al. Circulating branched-chain amino acids and incident cardiovascular disease in a prospective cohort of US women. Circ Genom Precis Med. 2018;11(4):e002157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shah SH, Bain JR, Muehlbauer MJ, et al. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ Cardiovasc Genet. 2010;3(2):207-214. [DOI] [PubMed] [Google Scholar]
- 14. Bhattacharya S, Granger CB, Craig D, et al. Validation of the association between a branched chain amino acid metabolite profile and extremes of coronary artery disease in patients referred for cardiac catheterization. Atherosclerosis. 2014;232(1):191-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Magnusson M, Lewis GD, Ericson U, et al. A diabetes-predictive amino acid score and future cardiovascular disease. Eur Heart J. 2013;34(26):1982-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ottosson F, Smith E, Melander O, Fernandez C. Altered asparagine and glutamate homeostasis precede coronary artery disease and type 2 diabetes. J Clin Endocrinol Metab. 2018;103(8):3060-3069. [DOI] [PubMed] [Google Scholar]
- 17. Du X, You H, Li Y, et al. Relationships between circulating branched chain amino acid concentrations and risk of adverse cardiovascular events in patients with STEMI treated with PCI. Scient Rep. 2018;8:15809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Würtz P, Havulinna AS, Soininen P, et al. Metabolite profiling and cardiovascular event risk: a prospective study of 3 population-based cohorts. Circulation. 2015;131(9):774-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vojinovic D, Kalaoja M, Trompet S, et al. Association of circulating metabolites in plasma or serum and risk of stroke: meta-analysis from seven prospective cohorts. Neurology. 2020;96:e1110-e1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Laakso M, Kuusisto J, Stančáková A, et al. The Metabolic Syndrome in Men study: a resource for studies of metabolic and cardiovascular diseases. J Lipid Res. 2017;58(3):481-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stancáková A, Javorský M, Kuulasmaa T, Haffner SM, Kuusisto J, Laakso M. Changes in insulin sensitivity and insulin release in relation to glycemia and glucose tolerance in 6414 Finnish men. Diabetes. 2009;58(5):1212-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Genuth S, Alberti KG, Bennett P, et al. ; Expert Committee on the Diagnosis and Classification of Diabetes Mellitus . Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26(11):3160-3167. [DOI] [PubMed] [Google Scholar]
- 23. Fizelova M, Jauhiainen R, Kangas AJ, et al. Differential associations of inflammatory markers with insulin sensitivity and secretion: the prospective METSIM study. J Clin Endocrinol Metab. 2017;102(9):3600-3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Soininen P, Kangas AJ, Würtz P, et al. High-throughput serum NMR metabonomics for cost-effective holistic studies on systemic metabolism. Analyst. 2009;134(9):1781-1785. [DOI] [PubMed] [Google Scholar]
- 25. Soininen P, Kangas AJ, Würtz P, Suna T, Ala-Korpela M. Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circ Cardiovasc Genet. 2015;8(1):192-206. [DOI] [PubMed] [Google Scholar]
- 26. Thygesen K, Alpert JS, Jaffe AS, et al. ; Joint ESC/ACCF/AHA/WHF Task Force for Universal Definition of Myocardial Infarction; Authors/Task Force Members Chairpersons; Biomarker Subcommittee; ECG Subcommittee; Imaging Subcommittee; Classification Subcommittee; Intervention Subcommittee; Trials & Registries Subcommittee; Trials & Registries Subcommittee; Trials & Registries Subcommittee; Trials & Registries Subcommittee; ESC Committee for Practice Guidelines (CPG); Document Reviewers. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60(16):1581-1598. [DOI] [PubMed] [Google Scholar]
- 27. Kang L, Chen W, Petrick NA, Gallas BD. Comparing two correlated C indices with right-censored survival outcome: a one-shot nonparametric approach. Stat Med. 2015;34(4):685-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jauhiainen R, Vangipurapu J, Laakso A, Kuulasmaa T, Kuusisto J, Laakso M. Supplementary tables for the association of nine amino acids with cardiovascular events in Finnish men in a 12-year follow-up study. Dryad, Dataset. Deposited 3 June 2021. doi: 10.5061/dryad.0cfxpnw21 [DOI] [PMC free article] [PubMed]
- 29. Ormstad H, Verkerk R, Sandvik L. Serum phenylalanine, tyrosine, and their ratio in acute ischemic stroke: on the trail of a biomarker? J Mol Neurosci. 2016;58(1):102-108. [DOI] [PubMed] [Google Scholar]
- 30. Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10(5):293-302. [DOI] [PubMed] [Google Scholar]
- 31. Su J, Pan C-W, Ke C. Identification of biomarkers for essential hypertension based on metabolomics. Nutrition, Metabolism and Cardiovascular Diseases. 2021;31:382-395. [DOI] [PubMed] [Google Scholar]
- 32. Tricò D, Biancalana E, Solini A. Protein and amino acids in nonalcoholic fatty liver disease. Curr Opin Clin Nutr Metab Care. 2021;24(1):96-101. [DOI] [PubMed] [Google Scholar]
- 33. Simonson M, Boirie Y, Guillet C. Protein, amino acids and obesity treatment. Rev Endocr Metab Disord. 2020;21(3):341-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Delles C, Rankin NJ, Boachie C, et al. Nuclear magnetic resonance-based metabolomics identifies phenylalanine as a novel predictor of incident heart failure hospitalisation: results from PROSPER and FINRISK 1997. Eur J Heart Fail. 2018;20(4):663-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109(21 Suppl 1):II2-I10. [DOI] [PubMed] [Google Scholar]
- 36. Nemet I, Saha PP, Gupta N, et al. A Cardiovascular disease-linked gut microbial metabolite acts via adrenergic receptors. Cell. 2020;180(5):862-877.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Würtz P, Raiko JR, Magnussen CG, et al. High-throughput quantification of circulating metabolites improves prediction of subclinical atherosclerosis. Eur Heart J. 2012;33(18):2307-2316. [DOI] [PubMed] [Google Scholar]
- 38. Yu B, Li AH, Muzny D, et al. Association of rare loss-of-function alleles in HAL, serum histidine: levels and incident coronary heart disease. Circ Cardiovasc Genet. 2015;8(2):351-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Khogali SEO, Pringle SD, Weryk BV, et al. Is glutamine beneficial in ischemic heart disease? Nutrition. 2002;18:123-126. [DOI] [PubMed] [Google Scholar]
- 40. Ma W, Heianza Y, Huang T, et al. Dietary glutamine, glutamate and mortality: two large prospective studies in US men and women. Int J Epidemiol. 2018;47(1):311-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of data generated or analyzed during this study to preserve the confidentiality of the participants. The corresponding author will, on request, detail the restrictions and any conditions under which access to some data may be provided.