Abstract
Multiple sclerosis is a highly heterogeneous disease, and the detection of neuroaxonal damage as well as its quantification is a critical step for patients. Blood-based serum neurofilament light chain (sNfL) is currently under close investigation as an easily accessible biomarker of prognosis and treatment response in patients with multiple sclerosis. There is abundant evidence that sNfL levels reflect ongoing inflammatory-driven neuroaxonal damage (e.g. relapses or MRI disease activity) and that sNfL levels predict disease activity over the next few years. In contrast, the association of sNfL with long-term clinical outcomes or its ability to reflect slow, diffuse neurodegenerative damage in multiple sclerosis is less clear. However, early results from real-world cohorts and clinical trials using sNfL as a marker of treatment response in multiple sclerosis are encouraging. Importantly, clinical algorithms should now be developed that incorporate the routine use of sNfL to guide individualized clinical decision-making in people with multiple sclerosis, together with additional fluid biomarkers and clinical and MRI measures. Here, we propose specific clinical scenarios where implementing sNfL measures may be of utility, including, among others: initial diagnosis, first treatment choice, surveillance of subclinical disease activity and guidance of therapy selection.
Keywords: biomarkers, neurofilament, prognosis, therapy response, multiple sclerosis
Bittner et al. discuss the association of emerging biomarker serum neurofilament light chain (sNfL) with clinical activity, disease progression, MRI measures and therapeutic interventions in multiple sclerosis. They propose specific scenarios implementing sNfL for individualized clinical decision-making.
Introduction
Multiple sclerosis is a chronic inflammatory CNS disorder in which neuroaxonal damage is closely related to clinical and MRI events and prognostication.1,2 Neurofilament light chain (NfL) is a major component of neuronal and axonal cytoskeleton proteins, providing structural support in the central and peripheral nervous systems. Apart from inflammatory diseases, elevated NfL has among others been reported in neurodegenerative, traumatic and ischaemic brain diseases.3-5 A comparison of CSF NfL levels across more than 30 different neurological disorders revealed increased levels compared with healthy controls in most cases.6 High NfL levels are therefore a general reflection of axonal damage, independent of underlying aetiology. However, the absolute values and/or temporal dynamics seem to reflect different competing aetiologies. In patients with multiple sclerosis, especially in cases where serum NfL (sNfL) values are higher than expected in otherwise stable patients, alternative causes and comorbidities such as head trauma, polyneuropathy or microvascular CNS lesions need to be considered. Thus far, neuropsychiatric or cognitive symptoms are not related to sNfL levels according to results from a smaller cohort.7 With axonal damage, NfL proteins are not only released into the CSF compartment, but also subsequently in low amounts (∼2%) into the peripheral blood.8,9 Research on neurofilament proteins has been performed for more than 20 years,10,11 but was initially limited with respect to translational suitability due to the necessity of obtaining CSF samples. However, because of novel highly sensitive analytic methods (namely single molecule array, SIMOA, technology),12 minimal concentrations in the single-digit picogram/millilitre range can now be detected in serum or plasma samples by specialized laboratories13 and, since c. 2017, are increasingly being used in multiple sclerosis research8 (Box 1). Correlations between CSF and blood values of NfL are high,14,15 and thus assessment in the peripheral blood is presumed sufficient, although a certain amount of uncertainty still exists as to whether this assumption can be generalized to all concentrations and patient subgroups.16 The minimally invasive detection in blood samples can be considered a breakthrough for the potential broader application of this marker in clinical practice. Herein, we will critically discuss the current progress and view regarding the role of sNfL in answering essential clinical questions in patients with multiple sclerosis (Box 2).
Box 1.
Neurofilament in a nutshell
Blood levels of sNfL, a neuron-specific cytoskeletal protein, have emerged as a biomarker able to capture neuronal damage in multiple sclerosis and a wide variety of neurological conditions. Following neuronal damage, sNfL is released into the CSF and subsequently into the blood, where it can be measured with current ultrasensitive assays (SIMOA), overcoming the problem of the inherently invasive lumbar punctures needed for CSF-based markers. This simple approach to assessing the degree of ongoing neuronal damage in the peripheral blood during standard patient care could greatly enhance clinical decision-making. One major advantage of sNfL is that it shows high stability at room temperature and in frozen blood samples, and it is not affected by thawing cycles or storage time,8,17,18 opening the door for broad application. Although many candidate biomarkers were in the past found to correspond to existing clinical information, to add no additional information to MRI19,20 or to be too technically challenging to implement in clinical practice, sNfL is not hampered by these issues. It is important to note when comparing different studies that the commercially available SIMOA assay21,22 is known to produce lower absolute sNfL concentrations (by about 50%) compared to previously used assays with different protocols in earlier studies.23,24 Importantly, a recent multicentre study analysing identical serum samples across 17 different international sites reported excellent inter-assay (<6%) and inter-site (<9%) coefficients of variation for the most widely used commercial NF-lightTM assay.25–29 Inter- and intra-batch assay variability, as well as variability across different newly emerging technical platforms (e.g. the ELLA system)30 are issues that still need to be addressed. Further international efforts to standardize sNfL measures are ongoing.27,31 See Table 2 for recommendations on quality controls to be reported in publications. Furthermore, sNfL has thus far been investigated on a group level, whereas prospective use on an individual patient level has not yet been established (see Fig. 1 for proposed clinical algorithms).
Box 2.
Overview on the role of sNfL in multiple sclerosis
Role in preclinical multiple sclerosis? Serum NfL is increased up to 6 years prior to first clinical symptoms and indicates a risk for a first clinical event in patients with radiologically isolated syndrome, when it is increased.
Role in diagnosis? At a group level, sNfL is higher in patients with RRMS than in healthy controls. sNfL thus indicates disease versus functional symptoms and might improve differentiation between CIS and RRMS patients when included in current diagnostic criteria.
Role for prognosis? Elevated sNfL levels have predictive value for future relapses, new gadolinium-enhancing or T2 lesions and future brain and spinal cord atrophy. With regard to clinical outcome, long-term predictions according to high sNfL levels is still controversial, while the predictive value of short-term EDSS-deterioration is undisputed.
Role for monitoring of disease activity? Levels of sNfL are associated with clinical and MRI parameters that indicate inflammatory disease activity. Low or stable sNfL levels can exclude clinical or subclinical disease activity. Small increases in sNfL levels may indicate progression in relapse-free phases.
Treatment response? Levels of sNfL are decreased by effective treatment initiation in both clinical trials and real-world cohorts. First studies suggest that sNfL levels might be able to differentiate between low and high efficacy treatments.
Serum NfL as a diagnostic biomarker in multiple sclerosis: distinct diagnostic situations
Without considering any additional clinical context, sNfL alone is insufficient for a diagnosis of multiple sclerosis or for differentiating multiple sclerosis from other neuroinflammatory disorders with neuroaxonal damage and elevated sNfL levels,32 such as neuromyelitis optica spectrum disorders or myelin oligodendrocyte glycoprotein (MOG)-encephalomyelitis.33–35 However, in specific clinical situations, sNfL can contribute to differential diagnostics. As one example, patients developing progressive multifocal leukoencephalopathy (PML) on natalizumab treatment had a steeper sNfL increase than those with multiple sclerosis-related relapses.36 A recent study took advantage of a unique prospective longitudinal serum biobank effort with detailed clinical and demographic data in the US Department of Defense Serum Repository. Sixty individuals were identified who subsequently developed multiple sclerosis and had previous serum collection in the years preceding diagnosis. sNfL levels were already elevated 6 years (range: 4–10 years) prior to disease onset and showed a further increase in the years leading up to the first clinical symptoms.37 Other studies have investigated patients with radiologically isolated syndrome, who are clinically asymptomatic patients undergoing MRI examinations for other reasons with incidental multiple sclerosis-like CNS lesions. In patients with radiologically isolated syndrome, elevated sNfL levels indicate a higher risk for developing either clinically isolated syndrome (CIS) or multiple sclerosis in the future.38 These studies from presymptomatic patients with multiple sclerosis underscore the long prodromal phase of this disease prior to the first clinical relapse with evident ongoing neurodegenerative processes.
MRI lesions and oligoclonal bands in the CSF are established risk factors for a diagnosis of multiple sclerosis.39 In several studies performed in patients with CIS, elevated NfL values in the CSF40–42 or serum43,44 were an additional predictor of future relapses. Importantly, studies mostly corrected for other known risk factors (such as age, oligoclonal bands and T2 lesion numbers), highlighting the added value of sNfL as an independent risk factor. In a cohort of more than 800 patients of the German Clinical Competence Network for Multiple Sclerosis (KKNMS), the role of early sNfL values for the diagnostic evaluation of patients was assessed.21 The inclusion of sNfL levels as an additional parameter into the current 2017 version of the McDonald criteria45 increased the sensitivity and specificity of differentiating patients with CIS and relapsing-remitting multiple sclerosis (RRMS).21
Serum NfL as a biomarker of disease activity in multiple sclerosis
Serum NfL, clinical activity and MRI: short-term
A broad spectrum of clinical and MRI parameters linked to inflammatory processes have demonstrated correlations to concurrently assessed sNfL levels (Table 1). Large studies in 814 and 607 patients clarified that the current Expanded Disability Status Scale (EDSS) score and sNfL levels are weakly, yet significantly, associated (12% and 8% sNfL increase per EDSS step, respectively).21,23 Furthermore, sNfL levels were shown to correlate with concurrent relapses,8,21,23 the presence of gadolinium-enhancing lesions,8,9,21,43 the number of gadolinium-enhancing lesions,14,15,23,47 the occurrence of new T2-weighted lesions,9 the number of new T2-weighted lesions,21,23,43,46 the number and volume of cortical lesions,57 the presence of T1-hypointense lesions in patients with CIS41 and T1 lesion volume46 as well as normalized brain volume, a cross-sectional measure of brain atrophy.23 Here it should be noted that sNfL levels are significantly increased after a relapse or detection of a gadolinium-enhancing lesion and can persist for some time (a few weeks to several months).9,15,23,58 Other parameters thus far have demonstrated mixed results [e.g. (i) sNfL with T2 lesion volumes with significant8,47 and not significant23 correlations; and (ii) sNfL and deep grey-matter structures with significant14,44,51 and not significant correlations] or showed no correlation with sNfL values (e.g. the presence of oligoclonal bands or vitamin D3 levels).43,47 See also Table 1 for a summary of the relationships between sNfL and different clinical/MRI parameters. Despite differences in methodological approaches across studies, all findings support that sNfL levels provide a good reflection of ongoing inflammation-driven neuroaxonal damage. This is in line with positive correlations between the inflammatory activity of multiple sclerosis lesions and axonal damage.59
Table 1.
Current evidence on the correlation and prediction of clinical and MRI parameters by sNfL levels
| Parameter | Level of evidence | Key results |
|---|---|---|
| Cross-section correlation | ||
| Relapses and T1-gadolinium enhancing lesions | +++ | Relapses and gadolinium-enhancing lesions causing acute neuronal damage are the most important driver of sNfL peaks. It is currently unclear whether blood-brain barrier damage in acute lesions facilitates efflux of sNfL proteins into the peripheral blood thereby resulting in higher absolute levels.8,9,15,21,23,43,44 |
| EDSS | ++ | Large well-powered studies have clarified that sNfL and current EDSS scores are weakly, yet significantly correlated. Furthermore, multiple studies have confirmed higher levels at later disease stages compared to earlier stable patients. Studies showing no correlation are most likely underpowered.8,21,23 |
| New T2 lesions | ++ | Both the occurrence and number of new T2-weighted lesions raise sNfL levels.9,21,23,43 |
| T1-hypointense lesions | + | Not as well studied, but was positively correlated in a few smaller studies.9,46 |
| Existing T2 lesion load | + | sNfL and number or volume of existing T2 lesions were significantly correlated in some studies, whereas no correlation was found in others. As sNfL indicates acute ongoing axonal damage, existing lesions without ongoing pathology are less likely to contribute to sNfL level increase.8,23,47 |
| Relapses and EDSS increase in the next 1–3 years | +++ | High sNfL levels were consistently associated with an increased risk for relapses in the next years. Some studies indicate that the sNfL percentile category reflects the strength of this prediction.21,23,48–50 |
| Prediction | ||
| Brain and spinal cord volume loss in the next 2–5 years | ++ | High sNfL levels are associated with future brain and spinal cord volume loss on a group level. It is plausible that high sNfL levels precede visual structural alterations in MRI, while exact time frames are still unclear.46,49,51 |
| Long-term EDSS progression (>5 years) and SPMS conversion | + | The long-term predictive value of sNfL values is so far not consistent in all studies. While it is likely that investigations from further studies will bring more clarity, sNfL will probably be more useful in clinical situations with regards to prediction of the next 1–3 years.24,48,51–56 |
= non-replicated observations that require further study or conflicting evidence.
= observations that have been replicated and/or supported by independent methods.
= high level of evidence from larger studies, consistently replicated.
Over a relatively short period, high sNfL levels were associated with an increased risk for relapses and/or EDSS deterioration over the next 1–3 years.8,21,23,48,49 In a study of patients with multiple sclerosis and healthy controls (n = 259 each), sNfL levels above the 90th percentile of healthy controls predicted EDSS worsening in the subsequent year [odds ratio (OR) 2.8, confidence interval (CI) 1.61–4.83],23 which confirmed findings from an earlier study in 241 patients with repeated serum sampling (OR 2.1, CI 1.03–4.29).8 The probability of EDSS deterioration gradually increased with each category of higher sNfL level percentile.23 Notably, in a multivariable model, only sNfL predicted future brain volume loss in contrast with other parameters (T2 lesion volume, baseline normalized brain volume and contrast-enhancing lesions).23 Importantly, the central messages of these studies only apply to a relatively small portion of patients with multiple sclerosis who have the highest sNfL levels. Of note, studies reported 46% of samples23 and 49% of samples8 from patients with multiple sclerosis were above the 80th percentile and showed significantly more EDSS worsening in the following year.
Serum NfL, clinical activity and MRI: long-term
A number of studies have confirmed that high sNfL levels have predictive value for future MRI-based brain atrophy over the next 2–5 years23,46,49,51 and two studies have found predictive value for brain atrophy in the longer-term at 10 and 12 years.24,52 In contrast, data concerning the longer-term predictive value of sNfL for disability progression are thus far less convincing. sNfL levels predicted the transition from RRMS to secondary progressive multiple sclerosis (SPMS) in two 5-year follow-up studies,51,53 whereas other studies did not find a significant relationship between sNfL and the risk of SPMS conversion.48,54 In three studies, sNfL was not associated with EDSS-progression over 5 years48 and 10 years24,53, whereas a correlation was observed in other 5-year studies.53,60 In a study of more than 120 patients, initial sNfL levels were not correlated with EDSS values after 10 years, but did predict T2 lesion load and brain atrophy rates.52 The patient cohort in this study, however, was rather benign, with only 11% of patients with multiple sclerosis reaching an EDSS score of 3.0 or more by 10 years (mean disease duration at first visit: 1.6 years). In contrast, another study with a more aggressive disease cohort recruited in the era before modern disease-modifying drugs (43% of patients reaching an EDSS score of 3.0 by 10 years; mean disease duration at first visit: 3.1 years) showed that patients with the highest sNfL levels progressed most rapidly with an annual rate of increase in the EDSS score of 0.16 over a median follow-up of 19 years.55,56 In one large study in >4000 patients and a median follow-up of 5 years, high sNfL levels were associated with the risk of reaching an EDSS score of 3.0 and 4.0, but not 6.0.54 One plausible explanation for the discrepancies observed across these studies might be the fact that sNfL levels strongly reflect acute, focal inflammatory neuronal injury due to relapses or subclinical MRI lesions in RRMS, and that this might mask slowly progressing neurodegenerative processes. At this point, our assessment is based on the size of the cohort studies and comes with the knowledge that technical differences in laboratories may still influence results (Box 1). Precisely defined progression states in large patient cohorts may clarify the exact value of sNfL, reflecting gradual degenerative processes, since even patients classified as having RRMS can suffer from disability increase in relapse-free phases of the disease (so-called ‘progression independent of relapse activity’, PIRA61). Overall, sNfL is most likely a predictor for brain atrophy and a milder predictor for long-term EDSS development over several years; however, so far, it is not an irrefutable predictor for conversion to SPMS.
Relationship between serum NfL and cognitive impairment
A limited number of studies thus far have investigated the association between NfL and cognition in multiple sclerosis. A small study in 27 patients did not find correlations between sNfL levels and symbol digit modalities test (SDMT) scores after 1 and 10 years, while other studies demonstrated an association between serum and CSF NfL levels and lower verbal fluency performance or SDMT scores.52,62,63 In one larger study using BICAMS (Brief International Cognitive Assessment for Multiple Sclerosis), cognitively impaired patients with multiple sclerosis had higher sNfL levels and a greater longitudinal sNfL increase compared with non-cognitively impaired patients.51 Limitations of this study include the heterogeneous study cohort (mixed CIS, RRMS and SPMS patients) with quite low inflammatory activity, the use of binary categorizations of both BICAMS and sNfL based on cut-off values (‘normal’ versus ‘not-normal’), and the fact that cognitively impaired and non-cognitively impaired patients varied in a number of parameters that potentially impact sNfL levels (e.g. age and EDSS). Nevertheless, another study confirmed the main finding that CSF NfL is higher in multiple sclerosis patients with cognitive impairment and especially in those with impaired information processing speed and verbal fluency, assessed by the Brief Repeatable Battery of Neuropsychological Tests (BRBN).64 Another interesting concept is that high sNfL might (probably due to relapses or new gadolinium-enhancing lesions) precede short-term changes in cognitive parameters: data from the phase III EXPAND trial (siponimod versus placebo in patients with SPMS) showed that patients with high baseline sNfL levels had a ∼40% greater risk of 6-month SDMT worsening than patients with low sNfL values.65 Overall, while initial data do indeed point towards an association between sNfL and measures of cognitive impairment, sNfL as a marker of axonal damage will probably not be useful as a singular specific marker for cognitive damage in patients with multiple sclerosis. This notion is further supported by pathological concepts preferentially linking grey matter damage and network dysfunction to cognitive impairment.66,67 Further studies should therefore focus on developing multi-modal approaches integrating sNfL levels with MRI data and other molecular biomarkers indicative of grey matter damage (see also below).
Combination of serum NfL with other markers
As sNfL specifically reflects neuroaxonal damage, the addition of one or several other markers might give a broader view of the pathophysiological processes in multiple sclerosis. Indeed, a number of studies have started to investigate whether sNfL composite scores are able to outperform single biomarkers.
Glial fibrillary acidic protein (GFAP) is the major cytoskeleton protein in astrocytes and released upon changes in cellular integrity. GFAP is drawing increased research interest as a second major blood biomarker that can reliably be measured in serum samples and that is moderately correlated with sNfL.68,69 Early studies in patients with multiple sclerosis suggested that GFAP is not elevated in association with acute relapses and focal inflammatory infiltrates, and hence could be used to elucidate the ongoing glial-driven neurodegenerative pathology.68,70 Indeed, using diffusion tensor imaging as a means to assess diffuse neuroaxonal damage not visible in conventional MRI, it has recently been shown that both T2 lesions and diffuse damage contribute to sNfL levels, and that the latter was preferentially found in older patients with more advanced disease course.71 These concepts were further expanded by efforts combining sNfL with GFAP, emphasizing that a combination of both markers might be useful in differentiating RRMS from SPMS patients.69 This notion is supported by an independent report that assessed GFAP and chitinase-3-like protein 1 (CHI3L1) as markers of astrocytic and microglial activation. A simplified ‘glia score’ (GFAP × CHI3L1 / sNfL) was higher in SPMS versus RRMS patients and correlated with EDSS values only in SPMS patients.72 These studies are also interesting as they are in line with the concept that glia activation is closely linked with axonal damage and disability progression in multiple sclerosis. Assessing both sNfL and GFAP simultaneously might be useful for differentiating multiple sclerosis activity across different stages of the disease.
Other approaches have assessed the combination of sNfL and markers of B cell activity in light of the recent appreciation of (intrathecal) B cells as drivers of multiple sclerosis pathology.73 Patients that are positive for oligoclonal bands have higher serum and CSF NfL levels in comparison with oligoclonal band-negative patients.74,75 In one study of 142 patients, sNfL values were associated with CSF total CD80+ (i.e. B cells and myeloid cells) as well as CD80+CD19+ (i.e. B cells) frequency.47 Patients with early multiple sclerosis were stratified into probable benign and aggressive disease courses based on MRI criteria, and the investigators found that combining sNfL with CD20+/CD14+ ratios in the CSF (i.e. increased B cell frequency) considerably improved distinction between the groups compared to sNfL alone.76
As elaborated above, sNfL strongly reflects (focal) acute inflammatory axonal damage of the white matter and its clinical value might be extended by combination with other biomarkers indicating gradual grey matter damage. Parvalbumin is a protein expressed in GABAergic interneurons and has been proposed as a marker of cortical grey matter neurodegeneration in patients with multiple sclerosis.77,78 A reduction in CSF parvalbumin correlated with meningeal inflammation, cortical lesion load, cortical thickness and cognitive impairment; all of which possibly showed a better correlation of gradual degenerative pathology than NfL levels. Extending these findings in larger, multicentre longitudinal studies is warranted to assess a possible combination of both biomarkers.
In summary, combining sNfL with other biomarkers reflecting glial activation, intrathecal inflammation or grey matter pathology is highly promising. The integration of a multi-modal biomarker assessment of multiple sclerosis pathology must still be investigated in large cohorts and in international efforts.
Serum NfL in multiple sclerosis: considerations of age as a potential confounder
An important point that needs to be considered when implementing the use of sNfL in clinical practice is the difficulty in developing normative values. Reassuringly, consistent reports have demonstrated that sNfL is not impacted by sex in healthy cohorts or in patients with multiple sclerosis.25 However, the physiological increase in sNfL levels seen with ageing in healthy individuals54,79,80 needs to be taken into consideration when interpreting sNfL data. The strength of the association between age and sNfL levels seems to be dependent on the specific age of the investigated cohort as well as the underlying disease. In both a meta-analysis and independent cohort studies in multiple sclerosis, no clear association between CSF NfL levels and age was observed for patients with multiple sclerosis, in contrast with healthy controls or people with most neurodegenerative disorders.6,21,47,54 It is plausible that the increase in sNfL levels observed in healthy controls (e.g. due to age-related neuronal loss or preclinical age-related disorders) is masked by the higher baseline inflammatory activity regularly observed in younger patients with multiple sclerosis. Thus, compared with inflammatory-associated elevations, the age-dependency of sNfL may not be a relevant confounder in clinical practice in younger patients without comorbidities. Some studies took the approach of modelling the ‘normal’ sNfL distribution across different ages based on a matched healthy donor control cohort,8,23,79 but unfortunately, different normative sNfL values were reached in each study despite being from the same laboratory.
Serum NfL as a biomarker of treatment response in multiple sclerosis: towards personalized immunotherapy
In an early study, CSF NfL levels in patients with multiple sclerosis were reduced to the levels of healthy controls 6 to 12 months after treatment initiation with natalizumab, providing for the first time evidence that that: (i) NfL levels increase upon acute inflammatory attacks; and (ii) a subsequent control of inflammatory disease activity with an immunomodulatory drug reduces NfL levels back to baseline levels.81 Since then, a broad number of studies have confirmed that sNfL values from patients receiving immunomodulatory drugs are generally lower than untreated patients and that initiation of any treatment is associated with a decrease in sNfL levels.8,9,15,21,24,82,83
Early studies suggest that sNfL levels might be able to differentiate between different treatments at a patient group level. In one study, patients who changed treatment between disease-modifying therapies with similar efficacy had stable sNfL concentrations, while patients who escalated to therapies with higher efficacies had decreased sNfL concentrations after a median follow-up of 12 months.15 However, it should be noted that this study cohort of multiple sclerosis patients was quite heterogeneous and that no data on T2 lesions was available. Confirming and extending these findings, patients starting highly active immunotherapies have higher sNfL levels at treatment initiation than those starting on mild/moderate therapies, leading to a larger relative decrease after commencing therapy.21,24,63 The number of future therapy changes, as well as treatment escalations were predicted by baseline sNfL levels.21,24 Another well-powered study assessed registry data from 1261 patients on different disease-modifying therapies and after using inverse propensity score weighting to correct for differences in baseline factors, confirmed a similar picture: patients starting alemtuzumab displayed the highest reduction and lowest on-treatment sNfL levels, while patients on teriflunomide started from lower levels, had a smaller decrease in sNfL and higher on-treatment levels.63 These studies underscore that repetitive measurements and evaluating longitudinal changes in sNfL will likely be an important part of supporting and managing therapy decisions.
After broadly introducing SIMOA assays, sNfL levels were analysed retrospectively from stored samples of completed randomized clinical trials. In phase 3 trials of fingolimod, natalizumab and alemtuzumab, sNfL reflected the same benefit with therapy initiation as clinical and MRI parameters.56,84 Subsequently, a recent study simulated whether sNfL could serve as an end point in phase 2 studies in patients with multiple sclerosis. Assuming typical features of a phase 2 trial in RRMS (6 months, 90% power, 5% significance level) and taking into account sNfL data from the FREEDOMS trial, between 28 and 143 subjects per arm would have been needed to show a 20–40% reduction in sNfL levels.85 Although the calculated numbers sound realistic, no trials utilizing such a design have been performed to date, but this may change in the near future. In the ASCLEPIOS trial (ofatumumab versus teriflunomide), sNfL was included for the first time prospectively as a secondary end point in a phase 3 multiple sclerosis trial. Interestingly, sNfL levels were significantly different between both groups, whereas brain atrophy rates were not, raising questions about the underlying causes for this discrepancy.86 It is plausible that short-term sNfL changes might rather reflect inflammatory processes in multiple sclerosis and changes are therefore evident earlier than MRI-based brain atrophy. As sNfL is included in most upcoming larger clinical trials, more insight will be obtained in the future.
Conclusions and future directions
In recent years, numerous studies have linked sNfL with outcomes related to disease activity, disability progression, treatment response and prognosis in patients with multiple sclerosis, generating convincing evidence that sNfL may soon be broadly used as the first blood-based biomarker monitoring disease activity and treatment responses in clinical practice. One major advantage of sNfL is that it is stable in fresh and frozen blood samples and is not affected by thawing cycles or storage time, opening the door for a broad range of applications. From a technical viewpoint, the next challenges in the sNfL field are to establish age- and comorbidity-adjusted normative values and to ensure methodological harmonization across different laboratories (see Box 3 and Table 2 for recommendations on quality controls). From a clinical point of view, the two questions ‘When’ and ‘How’ are of utmost relevance: When should sNfL be measured and how should findings be integrated into clinical decision-making? Because of the high inter-individual distribution of sNfL levels, we postulate longitudinal intra-individual changes being the most appropriate application for assessing clinical activity and treatment responses. Given the ongoing expansion of the therapeutic landscape in multiple sclerosis, sNfL could support individualized decision-making. From a clinical standpoint, longitudinal sNfL assessments of patients with RRMS can support therapeutic decisions in key areas including: (i) initial classification of CIS versus RRMS; (ii) choice of initial treatment; (iii) evaluation of subclinical disease activity in parallel with MRI measurements; (iv) treatment escalation in clinically active patients; and (v) treatment de-escalation or treatment cessation (Fig. 1 and Table 3). Importantly, sNfL measurement needs to be considered in a comprehensive and context-specific manner together with clinical information and other MRI markers of disease activity. While sNfL may indeed become the first blood biomarker with relevance in multiple sclerosis monitoring, multimodal composite indices integrating existing or other emerging markers could enable increasingly precise individualized treatment decisions.
Box 3.
Key challenges
To clarify when and how often sNfL should be measured to assess subclinical disease activity and guide therapeutic decisions.
To define the threshold that constitutes a clinically meaningful change in longitudinal measurements.
To clarify whether absolute values are comparable in standardized investigations.
To implement the standardization of neurofilament measures and values across different assays and laboratories.
To take confounding factors (e.g. age and other comorbidities) into account. It should be clarified whether it is sufficient to consider age exclusively in elderly cohorts (>60 years) as the association with sNfL is weak in younger patients with RRMS.
Table 2.
Suggested quality criteria to support the validity of measurements
| Checkpoint | Quality criteria |
|---|---|
| Replicate measurements | Calibrators and samples should be measured at least in duplicates. Samples with a missing result for a replicate or a CV of duplicate determination >20% should be measured again. The number of samples with repeated measurements due to quality criteria should be reported in the method section. |
| Intra-assay precision | Mean CV of duplicate determinations should be reported. Intra-assay CVs below 10% can usually be achieved. |
| Control samples | Three (pre-characterized) control samples with low, medium and high NfL concentrations should be included in each run to monitor any matrix effects and to determine the inter-assay CV. Control samples should preferably be derived from the same material as samples (e.g. serum, plasma or CSF). |
| Inter-assay precision | Inter-assay CV should be reported. Values below 10% can usually be achieved and may reduce the risk of reporting plate effects instead of true group effects. |
| Different LOTs or assay versions | Inter-LOT effects should be negligible. However, caution is advised with different assay versions. If different LOTs were used this should be announced in the method section and the inter-LOT CV should be reported. |
| Blinding | Individuals performing the NfL measurements should be blinded to clinical data. |
Note that the recommendations apply to the first broadly used commercially available platform (NfL-lightTM assays, Quanterix, HD-1/HD-X). CV = coefficient of variation.
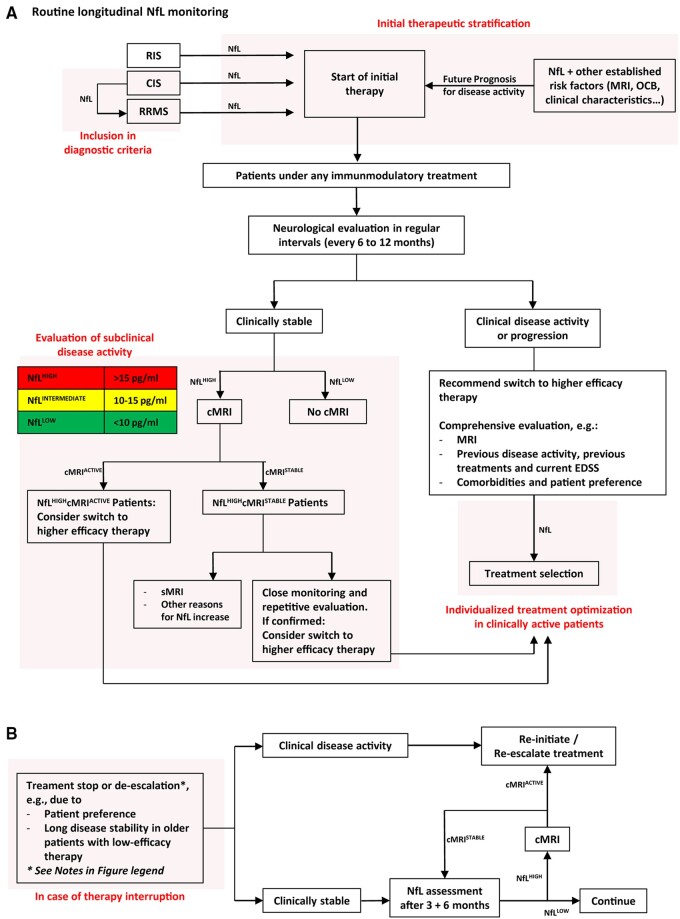
Figure 1.
Potential sNfL decision concepts in clinical practice. (A) Longitudinal sNfL assessment algorithm guiding treatment optimization in RRMS. Red fields mark four areas using sNfL for guiding decisions in (i) initial diagnosis of multiple sclerosis; (ii) choice of initial treatment; (iii) evaluation of subclinical disease activity; and (iv) treatment optimization in clinically active patients. (B) An sNfL assessment algorithm in patients undergoing treatment cessation or de-escalation, stratifying patients with stable disease course and those in need of therapy reinitiation or re-escalation. Note that First, no randomized controlled trials have directly addressed the question of whether or when to discontinue treatment in RRMS patients who have no evidence of relapses, no disability progression and stable MRI parameters. Especially in patients on higher-efficacy therapies (e.g. natalizumab or fingolimod), the risk of return of disease activity or rebound when stopping treatment has been well documented. In agreement with the European and American Academy of Neurology (AAN) guidelines,87 this algorithm is not suggesting treatment cessation in specific patient cohorts, but rather is an approach to implement sNfL in ongoing follow-ups and periodic re-evaluations when treatment cessation occurs for clinical reasons. Second, the suggested differentiation into NfLHIGH, NfLINTERMEDIATE and NfLLOW is a rough estimation based on our datasets and data from Table 3. These values apply to RRMS patients (age 18 to ∼40–50 years) without comorbidities and are currently only partially validated within international efforts. Older age groups still have to be compared to normal cohorts, since the age-associated sNfL increase seems to be markedly steeper beyond about 50 years of age and is less studied up to date. cMRI = cranial MRI; NEDA = no evidence of disease activity; OCB = oligoclonal bands; RIS = radiologically isolated syndrome; sMRI = spinal cord MRI.
Table 3.
Corrected reference values for sNfL levels for two clinically relevant scenarios
| Parameter | Reported values | Corrected values |
|---|---|---|
| MS versus healthy controls | 9.7 pg/ml (age 18–40 years; sNfL >95th percentile of healthy cohort)26 | 9.7 pg/ml |
| 29.3 pg/ml (age 30 years, sNfL > 95th percentile of healthy cohort)23 | 14.7 pg/mla | |
| 27.9 pg/ml (age 30 years, sNfL > 95th percentile of healthy cohort)8 | 14.0 pg/mla | |
| 14.4 pg/ml versus 8.5 pg/ml (pooled study data patients versus healthy controls)84 | 14.4 pg/ml versus 8.5 pg/ml | |
| 11.4 pg/ml versus 7.5 pg/ml (MS patients versus healthy controls)54 | 14.3 pg/ml versus 9.4 pg/mlb | |
| 17.0 pg/ml versus 8.2 pg/ml (MS patients versus healthy controls)88 | 17.0 pg/ml versus 8.2 pg/ml | |
| 10.1 pg/ml versus 7.3 pg/ml (MS patients versus healthy controls)55 | 10.1 pg/ml versus 7.3 pg/ml | |
| Serum NfL comparisons indicating disease activity in MS patients | 25.0–45.1 pg/ml (median; presymptomatic to symptomatic)37 | 12.5–22.5 pg/mla |
| 29.6–43.4 pg/ml (median; no Gd+ lesion to Gd+ lesion)8 | 14.8–21.7 pg/mla | |
| 28.9–39.3 pg/ml (median; no relapse to recent relapse <60 days)8 | 14.5–20.0 pg/mla | |
| 9.9–16.1 pg/ml (median; no Gd+ lesion to Gd+ lesion)21 | 9.9–16.1 pg/ml | |
| 28.1–63.2 pg/ml (median, no Gd+ lesion to Gd+ lesion)44 | 14.1–31.6 pg/mla |
Note that when considering published data, there is a significant variation in data analysis procedures, making it difficult to systematically compare different studies. To name a few challenges: published datasets have used mean, median, geomean, different parametric or non-parametric tests and cross-sectional or longitudinal analyses. Analyses are performed either on raw data (non-parametric tests) or in log-transformed data in order to use parametric tests. While age-adjusted z-scores of log-transformed data might indeed be the optimal statistical approach,26 this is challenging to implement in a broad clinical setting. Therefore, only reports publishing absolute values with cut-offs were included. The presented data are only meant as a simplified approach for a rough range of expected values in two clinically relevant scenarios and not as validated cut-offs. Gd+ = gadolinium-enhancing lesions; MS = multiple sclerosis.
Because of technical differences between different protocols, values are reduced by 50% to give a rough estimation. For full values and more details (e.g. interquartile range), see the original publications.
Plasma concentrations are ∼25% lower than serum concentration (Thebault et al.26 and own experience), values were increased accordingly.
Search strategy and selection criteria
We searched PubMed and Web of Science for articles on neurofilament published between 1 January 2018 and 15 May 2021. Search terms were ‘neurofilament’, ‘neurofilaments’, ‘neurofilament light chain’, ‘NfL’, ‘sNfL’, ‘PML’ and all combinations of these phrases with ‘multiple sclerosis’, ‘MS’ and ‘neuroinflammation’. The final reference list was generated based on novelty and relevance to this review.
Acknowledgements
The authors thank Dr Cheryl Ernest for proofreading and editing the manuscript. The thumbnail image was created with BioRender.com.
Funding
This work was supported by the German Research Council (DFG, CRC-TR-128 to F.Z. and S.B.), Hertie-Stiftung (myLab to S.B.), the Progressive Multiple Sclerosis Alliance (PMSA, BRAVEinMS PA-1604-08492 to F.Z.), and the German Federal Ministry of Education and Research (BMBF, VIP+ HaltMS to F.Z.).
Competing interests
S.B. has received honoraria and compensation for travel from Biogen Idec, Merck Serono, Novartis, Sanofi-Genzyme and Roche. J.O. has received funding from the MS Society of Canada, the National MS Society, Brain Canada, EMD-Serono, Roche and Biogen-Idec. J.O. has also received compensation for consulting or speaking engagements from Alexion, EMD-Serono, Sanofi-Genzyme, Biogen-Idec, Novartis, BMS, and Roche. E.K.H. has received honoraria/research support from Biogen, Merck Serono, Novartis, Roche and Teva; has served as a member of advisory boards for Actelion, Biogen, Celgene, Merck Serono, Novartis and Sanofi Genzyme; and has been supported by the Czech Ministry of Education research, project PROGRES Q27/LF1. M.T. has received compensation for consulting services and speaking honoraria from Almirall, Bayer Schering Pharma, Biogen-Idec, Genzyme, Merck-Serono, Novartis, Roche, Sanofi-Aventis, Viela Bio and Teva Pharmaceuticals. M.T. is co-editor of Multiple Sclerosis Journal-ETC. F.Z. has recently received research grants and/or consultation funds from DFG, BMBF, PMSA, MPG, Genzyme, Merck Serono, Roche, Novartis, Sanofi-Aventis, Celgene, ONO and Octapharma.
Glossary
- CIS
clinically isolated syndrome
- EDSS
Expanded Disability Status Scale
- RRMS
relapsing-remitting multiple sclerosis
- sNfL
serum neurofilament light chain
- SPMS
secondary progressive multiple sclerosis
References
- 1. Reich DS, Lucchinetti CF, Calabresi PA.. Multiple sclerosis. N Engl J Med. 2018;378(2):169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Suthiphosuwan S, Kim D, Bharatha A, Oh J.. Imaging markers for monitoring disease activity in multiple sclerosis. Curr Treat Options Neurol. 2017;19(5):18. [DOI] [PubMed] [Google Scholar]
- 3. Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14(10):577–589. [DOI] [PubMed] [Google Scholar]
- 4. Uphaus T, Bittner S, Groschel S, et al. NfL (neurofilament light chain) levels as a predictive marker for long-term outcome after ischemic stroke. Stroke. 2019;50(11):3077–3084. [DOI] [PubMed] [Google Scholar]
- 5. Moseby-Knappe M, Mattsson N, Nielsen N, et al. Serum neurofilament light chain for prognosis of outcome after cardiac arrest. JAMA Neurol. 2019;76(1):64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bridel C, van Wieringen WN, Zetterberg H, et al. ; the NFL Group. Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: A systematic review and meta-analysis. JAMA Neurol. 2019;76(9):1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aktas O, Renner A, Huss A, et al. Serum neurofilament light chain: No clear relation to cognition and neuropsychiatric symptoms in stable MS. Neurol Neuroimmunol Neuroinflamm. 2020;7(6):e885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Disanto G, Barro C, Benkert P, et al. ; the Swiss Multiple Sclerosis Cohort Study Group. Serum neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann Neurol. 2017;81(6):857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Varhaug KN, Barro C, Bjornevik K, et al. Neurofilament light chain predicts disease activity in relapsing-remitting MS. Neurol Neuroimmunol Neuroinflamm. 2018;5(1):e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rosengren LE, Karlsson JE, Karlsson JO, Persson LI, Wikkelso C.. Patients with amyotrophic lateral sclerosis and other neurodegenerative diseases have increased levels of neurofilament protein in CSF. J Neurochem. 1996;67(5):2013–2018. [DOI] [PubMed] [Google Scholar]
- 11. Lycke JN, Karlsson JE, Andersen O, Rosengren LE.. Neurofilament protein in cerebrospinal fluid: potential marker of activity in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1998;64(3):402–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rissin DM, Kan CW, Campbell TG, et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat Biotechnol. 2010;28(6):595–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuhle J, Barro C, Andreasson U, et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med. 2016;54(10):1655–1661. [DOI] [PubMed] [Google Scholar]
- 14. Kuhle J, Barro C, Disanto G, et al. Serum neurofilament light chain in early relapsing remitting MS is increased and correlates with CSF levels and with MRI measures of disease severity. Mult Scler. 2016;22(12):1550–1559. [DOI] [PubMed] [Google Scholar]
- 15. Novakova L, Zetterberg H, Sundstrom P, et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology. 2017;89(22):2230–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Myhr KM, Torkildsen O.. Serum NFL levels should be used to monitor multiple sclerosis evolution-No. Mult Scler. 2020;26(1):19–21. [DOI] [PubMed] [Google Scholar]
- 17. Altmann P, Leutmezer F, Zach H, et al. Serum neurofilament light chain withstands delayed freezing and repeated thawing. Sci Rep. 2020;10(1):19982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keshavan A, Heslegrave A, Zetterberg H, Schott JM.. Stability of blood-based biomarkers of Alzheimer’s disease over multiple freeze-thaw cycles. Alzheimers Dement (Amst). 2018;10:448–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Comabella M, Montalban X.. Body fluid biomarkers in multiple sclerosis. Lancet Neurol. 2014;13(1):113–126. [DOI] [PubMed] [Google Scholar]
- 20. Housley WJ, Pitt D, Hafler DA.. Biomarkers in multiple sclerosis. Clin Immunol. 2015;161(1):51–58. [DOI] [PubMed] [Google Scholar]
- 21. Bittner S, Steffen F, Uphaus T, et al. ; KKNMS Consortium. Clinical implications of serum neurofilament in newly diagnosed MS patients: A longitudinal multicentre cohort study. EBioMedicine. 2020;56:102807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bsteh G, Berek K, Hegen H, et al. Serum neurofilament levels correlate with retinal nerve fiber layer thinning in multiple sclerosis. Mult Scler. 2019;26(13):1682–1690. [DOI] [PubMed] [Google Scholar]
- 23. Barro C, Benkert P, Disanto G, et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain. 2018;141(8):2382–2391. [DOI] [PubMed] [Google Scholar]
- 24. Canto E, Barro C, Zhao C, et al. Association between serum neurofilament light chain levels and long-term disease course among patients with multiple sclerosis followed up for 12 years. JAMA Neurol. 2019;76(11):1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barro C, Chitnis T, Weiner HL.. Blood neurofilament light: a critical review of its application to neurologic disease. Ann Clin Transl Neurol. 2020;7(12):2508–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thebault S, Booth RA, Rush CA, MacLean H, Freedman MS.. Serum neurofilament light chain measurement in MS: hurdles to clinical translation. Front Neurosci. 2021;15:654942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kapoor R, Smith KE, Allegretta M, et al. Serum neurofilament light as a biomarker in progressive multiple sclerosis. Neurology. 2020;95(10):436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kuhle J, Barro C, Hrusovsky K, Chang L, Jeromin A.. International multi-site analytical validation of the Simoa NF-light assay in human serum samples from multiple sclerosis patients. Mult Scler. 2018;24:249–251. [Google Scholar]
- 29. Sharma A, Petrillo M, Zhao G, Unkown A, Unkown A.. Strategic platform selection and validation of biomarker assays to measure serum neurofilament light and heavy chain in multiple sclerosis. Mult Scler. 2018;24:660–661. [Google Scholar]
- 30. Gauthier A, Viel S, Perret M, et al. ; OFSEP Investigators. Comparison of Simoa(TM) and Ella(TM) to assess serum neurofilament-light chain in multiple sclerosis. Ann Clin Transl Neurol. 2021;8(5):1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Freedman M, Gnanapavan S.. Letter to the Editor: Consensus statement on neurofilament proteins in multiple sclerosis under development by Consortium of Multiple Sclerosis Centers (CMSC) expert panel. Int J MS Care. 2020;22(6):294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chitnis T. Serum NFL levels should be used to monitor multiple sclerosis evolution-Commentary. Mult Scler. 2020;26(1):21–22. [DOI] [PubMed] [Google Scholar]
- 33. Kim H, Lee EJ, Kim S, et al. Serum biomarkers in myelin oligodendrocyte glycoprotein antibody-associated disease. Neurol Neuroimmunol Neuroinflamm. 2020;7(3):e708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peng L, Bi C, Xia D, Mao L, Qian H.. Increased cerebrospinal fluid neurofilament light chain in central nervous system inflammatory demyelinating disease. Mult Scler Relat Disord. 2019;30:123–128. [DOI] [PubMed] [Google Scholar]
- 35. Watanabe M, Nakamura Y, Michalak Z, et al. Serum GFAP and neurofilament light as biomarkers of disease activity and disability in NMOSD. Neurology. 2019;93(13):e1299–e1311. [DOI] [PubMed] [Google Scholar]
- 36. Dalla Costa G, Martinelli V, Moiola L, et al. Serum neurofilaments increase at progressive multifocal leukoencephalopathy onset in natalizumab-treated multiple sclerosis patients. Ann Neurol. 2019;85(4):606–610. [DOI] [PubMed] [Google Scholar]
- 37. Bjornevik K, Munger KL, Cortese M, et al. Serum neurofilament light chain levels in patients with presymptomatic multiple sclerosis. JAMA Neurol. 2019;77(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Matute-Blanch C, Villar LM, Alvarez-Cermeno JC, et al. Neurofilament light chain and oligoclonal bands are prognostic biomarkers in radiologically isolated syndrome. Brain. 2018;141(4):1085–1093. [DOI] [PubMed] [Google Scholar]
- 39. Miller DH, Chard DT, Ciccarelli O.. Clinically isolated syndromes. Lancet Neurol. 2012;11(2):157–169. [DOI] [PubMed] [Google Scholar]
- 40. Arrambide G, Espejo C, Eixarch H, et al. Neurofilament light chain level is a weak risk factor for the development of MS. Neurology. 2016;87(11):1076–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van der Vuurst de Vries RM, Wong YYM, Mescheriakova JY, et al. High neurofilament levels are associated with clinically definite multiple sclerosis in children and adults with clinically isolated syndrome. Mult Scler. 2019;25(7):958–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Teunissen CE, Iacobaeus E, Khademi M, et al. Combination of CSF N-acetylaspartate and neurofilaments in multiple sclerosis. Neurology. 2009;72(15):1322–1329. [DOI] [PubMed] [Google Scholar]
- 43. Dalla Costa G, Martinelli V, Sangalli F, et al. Prognostic value of serum neurofilaments in patients with clinically isolated syndromes. Neurology. 2019;92(7):e733–e741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Siller N, Kuhle J, Muthuraman M, et al. Serum neurofilament light chain is a biomarker of acute and chronic neuronal damage in early multiple sclerosis. Mult Scler. 2019;25(5):678–686. [DOI] [PubMed] [Google Scholar]
- 45. Zipp F, Oh J, Fragoso YD, Waubant E.. Implementing the 2017 McDonald criteria for the diagnosis of multiple sclerosis. Nat Rev Neurol. 2019;15(8):441–445. [DOI] [PubMed] [Google Scholar]
- 46. Srpova B, Uher T, Hrnciarova T, et al. Serum neurofilament light chain reflects inflammation-driven neurodegeneration and predicts delayed brain volume loss in early stage of multiple sclerosis. Mult Scler. 2021;27(1):52–60. [DOI] [PubMed] [Google Scholar]
- 47. Uher T, McComb M, Galkin S, et al. Neurofilament levels are associated with blood-brain barrier integrity, lymphocyte extravasation, and risk factors following the first demyelinating event in multiple sclerosis. Mult Scler. 2020;27(2):220–231. [DOI] [PubMed] [Google Scholar]
- 48. Sellebjerg F, Royen L, Soelberg Sorensen P, Oturai AB, Jensen PEH.. Prognostic value of cerebrospinal fluid neurofilament light chain and chitinase-3-like-1 in newly diagnosed patients with multiple sclerosis. Mult Scler. 2019;25(11):1444–1451. [DOI] [PubMed] [Google Scholar]
- 49. Kuhle J, Nourbakhsh B, Grant D, et al. Serum neurofilament is associated with progression of brain atrophy and disability in early MS. Neurology. 2017;88(9):826–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Disanto G, Adiutori R, Dobson R, et al. ; International Clinically Isolated Syndrome Study Group. Serum neurofilament light chain levels are increased in patients with a clinically isolated syndrome. J Neurol Neurosurg Psychiatry. 2016;87(2):126–129. [DOI] [PubMed] [Google Scholar]
- 51. Jakimovski D, Kuhle J, Ramanathan M, et al. Serum neurofilament light chain levels associations with gray matter pathology: a 5-year longitudinal study. Ann Clin Transl Neurol. 2019;6(9):1757–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chitnis T, Gonzalez C, Healy BC, et al. Neurofilament light chain serum levels correlate with 10-year MRI outcomes in multiple sclerosis. Ann Clin Transl Neur. 2018;5(12):1478–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bhan A, Jacobsen C, Myhr KM, Dalen I, Lode K, Farbu E.. Neurofilaments and 10-year follow-up in multiple sclerosis. Mult Scler. 2018;24(10):1301–1307. [DOI] [PubMed] [Google Scholar]
- 54. Manouchehrinia A, Stridh P, Khademi M, et al. Plasma neurofilament light levels are associated with risk of disability in multiple sclerosis. Neurology. 2020;94(23):e2457–e2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Thebault S, Abdoli M, Fereshtehnejad SM, Tessier D, Tabard-Cossa V, Freedman MS.. Serum neurofilament light chain predicts long term clinical outcomes in multiple sclerosis. Sci Rep. 2020;10(1):10381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Leppert D, Kuhle J.. Blood neurofilament light chain at the doorstep of clinical application. Neurol Neuroimmunol Neuroinflamm. 2019;6(5):e599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Magliozzi R, Howell OW, Nicholas R, et al. Inflammatory intrathecal profiles and cortical damage in multiple sclerosis. Ann Neurol. 2018;83(4):739–755. [DOI] [PubMed] [Google Scholar]
- 58. Rosso M, Gonzalez CT, Healy BC, et al. Temporal association of sNfL and gad-enhancing lesions in multiple sclerosis. Ann Clin Transl Neurol. 2020;7(6):945–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dutta R, Trapp BD.. Mechanisms of neuronal dysfunction and degeneration in multiple sclerosis. Prog Neurobiol. 2011;93(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jakimovski D, Zivadinov R, Ramanthan M, et al. Serum neurofilament light chain level associations with clinical and cognitive performance in multiple sclerosis: A longitudinal retrospective 5-year study. Mult Scler. 2019;26(13):1670–1681. [DOI] [PubMed] [Google Scholar]
- 61. Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77(9):1132–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Quintana E, Coll C, Salavedra-Pont J, et al. Cognitive impairment in early stages of multiple sclerosis is associated with high cerebrospinal fluid levels of chitinase 3-like 1 and neurofilament light chain. Eur J Neurol. 2018;25(9):1189–1191. [DOI] [PubMed] [Google Scholar]
- 63. Delcoigne B, Manouchehrinia A, Barro C, et al. Blood neurofilament light levels segregate treatment effects in multiple sclerosis. Neurology. 2020;94(11):e1201–e1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gaetani L, Salvadori N, Lisetti V, et al. Cerebrospinal fluid neurofilament light chain tracks cognitive impairment in multiple sclerosis. J Neurol. 2019;266(9):2157–2163. [DOI] [PubMed] [Google Scholar]
- 65. Kuhle J, Kropshofer H, Barro C, Unkown A, Unkown A.. The predictive value of neurofilament light chain levels in blood for cognitive impairment in patients with secondary progressive multiple sclerosis (S12.009). Neurology. 2019;92:S12.009 [Google Scholar]
- 66. Fleischer V, Muthuraman M, Anwar AR, et al. Continuous reorganization of cortical information flow in multiple sclerosis: A longitudinal fMRI effective connectivity study. Sci Rep. 2020;10(1):806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Geurts JJ, Calabrese M, Fisher E, Rudick RA.. Measurement and clinical effect of grey matter pathology in multiple sclerosis. Lancet Neurol. 2012;11(12):1082–1092. [DOI] [PubMed] [Google Scholar]
- 68. Abdelhak A, Huss A, Kassubek J, Tumani H, Otto M.. Serum GFAP as a biomarker for disease severity in multiple sclerosis. Sci Rep. 2018;8(1):14798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hogel H, Rissanen E, Barro C, et al. Serum glial fibrillary acidic protein correlates with multiple sclerosis disease severity. Mult Scler. 2020;26(2):210–219. [DOI] [PubMed] [Google Scholar]
- 70. Norgren N, Sundstrom P, Svenningsson A, Rosengren L, Stigbrand T, Gunnarsson M.. Neurofilament and glial fibrillary acidic protein in multiple sclerosis. Neurology. 2004;63(9):1586–1590. [DOI] [PubMed] [Google Scholar]
- 71. Saraste M, Bezukladova S, Matilainen M, et al. High serum neurofilament associates with diffuse white matter damage in MS. Neurol Neuroimmunol Neuroinflamm. 2021;8(1):e926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Huss A, Otto M, Senel M, Ludolph AC, Abdelhak A, Tumani H.. A score based on NfL and glial markers may differentiate between relapsing-remitting and progressive MS course. Front Neurol. 2020;11:608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bittner S, Ruck T, Wiendl H, Grauer OM, Meuth SG.. Targeting B cells in relapsing-remitting multiple sclerosis: from pathophysiology to optimal clinical management. Ther Adv Neurol Disord. 2017;10(1):51–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Engel S, Steffen F, Uphaus T, et al. Association of intrathecal pleocytosis and IgG synthesis with axonal damage in early MS. Neurol Neuroimmunol Neuroinflamm. 2020;7(3):e679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Farina G, Magliozzi R, Pitteri M, et al. Increased cortical lesion load and intrathecal inflammation is associated with oligoclonal bands in multiple sclerosis patients: a combined CSF and MRI study. J Neuroinflammation. 2017;14(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Engel S, Friedrich M, Muthuraman M, et al. Intrathecal B-cell accumulation and axonal damage distinguish MRI-based benign from aggressive onset in MS. Neurol Neuroimmunol Neuroinflamm. 2019;6(5):e595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Magliozzi R, Howell OW, Durrenberger P, et al. Meningeal inflammation changes the balance of TNF signalling in cortical grey matter in multiple sclerosis. J Neuroinflammation. 2019;16(1):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Magliozzi R, Pitteri M, Ziccardi S, et al. CSF parvalbumin levels reflect interneuron loss linked with cortical pathology in multiple sclerosis. Ann Clin Transl Neurol. 2021;8(3):534–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Khalil M, Pirpamer L, Hofer E, et al. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat Commun. 2020;11(1):812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Vagberg M, Norgren N, Dring A, et al. Levels and age dependency of neurofilament light and glial fibrillary acidic protein in healthy individuals and their relation to the brain parenchymal fraction. PLoS One. 2015;10(8):e0135886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gunnarsson M, Malmestrom C, Axelsson M, et al. Axonal damage in relapsing multiple sclerosis is markedly reduced by natalizumab. Ann Neurol. 2011;69(1):83–89. [DOI] [PubMed] [Google Scholar]
- 82. Sejbaek T, Nielsen HH, Penner N, et al. Dimethyl fumarate decreases neurofilament light chain in CSF and blood of treatment naive relapsing MS patients. J Neurol Neurosurg Psychiatry. 2019;90(12):1324–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Preziosa P, Rocca MA, Filippi M.. Current state-of-art of the application of serum neurofilaments in multiple sclerosis diagnosis and monitoring. Expert Rev Neurother. 2020;20(8):747–769. [DOI] [PubMed] [Google Scholar]
- 84. Kuhle J, Kropshofer H, Haering DA, et al. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology. 2019;92(10):e1007–e1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sormani MP, Haering DA, Kropshofer H, et al. Blood neurofilament light as a potential endpoint in phase 2 studies in MS. Ann Clin Transl Neurol. 2019;6(6):1081–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Fyfe I. Trials take MS treatment forward. Nat Rev Neurol. 2019;15(11):620. [DOI] [PubMed] [Google Scholar]
- 87. Bittner S, Zipp F.. AAN unveils new guidelines for MS disease-modifying therapy. Nat Rev Neurol. 2018;14(7):384–386. [DOI] [PubMed] [Google Scholar]
- 88. Piehl F, Kockum I, Khademi M, et al. Plasma neurofilament light chain levels in patients with MS switching from injectable therapies to fingolimod. Mult Scler. 2018;24(8):1046–1054. [DOI] [PubMed] [Google Scholar]