Abstract
Continuous translation elongation, irrespective of amino acid sequences, is a prerequisite for living organisms to produce their proteomes. However, nascent polypeptide products bear an inherent risk of elongation abortion. For example, negatively charged sequences with occasional intermittent prolines, termed intrinsic ribosome destabilization (IRD) sequences, weaken the translating ribosomal complex, causing certain nascent chain sequences to prematurely terminate translation. Here, we show that most potential IRD sequences in the middle of open reading frames remain cryptic and do not interrupt translation, due to two features of the nascent polypeptide. Firstly, the nascent polypeptide itself spans the exit tunnel, and secondly, its bulky amino acid residues occupy the tunnel entrance region, thereby serving as a bridge and protecting the large and small ribosomal subunits from dissociation. Thus, nascent polypeptide products have an inbuilt ability to ensure elongation continuity.
Keywords: cell‐free translation, nascent polypeptide chain, polypeptidyl‐tRNA, ribosomal exit tunnel, ribosome
Subject Categories: Translation & Protein Quality
Translation elongation continuity in spite of intrinsic ribosome destabilization sequences is ensured by interaction of the nascent polypeptide with the exit tunnel.
Introduction
The ribosome is the central player in translation, at the heart of the central dogma, in all organisms. Accurate and continuous peptide bond formation by the ribosome ensures the uniformity and quality of cellular proteins. Otherwise, aberrant polypeptides would lead to undesired outcomes such as impaired or dysregulated protein functions and aggregation, which perturb cellular homeostasis. Thus, living organisms have multilayered surveillance systems over ribosomes and translation, including proofreading of codon–anticodon recognition (Rodnina & Wintermeyer, 2001; Ieong et al, 2016; Noel & Whitford, 2016), quality control pathways to sense unusual translation reactions, and degradation of premature products (Keiler, 2008; Buskirk & Green, 2017; Inada, 2017).
During the elongation process, a growing nascent polypeptide travels from the peptidyl transferase center (PTC) through the ribosomal exit tunnel (Wilson & Beckmann, 2011), which accommodates 30–40 amino acid‐long segments of tRNA‐linked nascent polypeptides (Jenni & Ban, 2003; Voss et al, 2006). The tunnel is composed of the large ribosomal RNA and the tips of ribosomal proteins, such as uL4, uL22, and uL23 (Nissen et al, 2000). In contrast to the initial suggestion that the tunnel interior is inert in terms of interactions with the nascent polypeptide (Nissen et al, 2000), in reality, the tunnel interacts with nascent polypeptide chains, thus contributing to biological regulation (Gong & Yanofsky, 2002; Nakatogawa & Ito, 2002; Onouchi et al, 2005; Chiba et al, 2009; Yanagitani et al, 2011; Ito & Chiba, 2013; Ishii et al, 2015). Cryo‐EM structures of ribosomes stalled by ribosome arresting peptides have revealed a range of interactions between the nascent chains and the ribosomal interior components at the PTC and/or the exit tunnel, such as the uL22/uL4 constriction site (Seidelt et al, 2009; Bhushan et al, 2011; Sohmen et al, 2015; Su et al, 2017; Shanmuganathan et al, 2019). Recent analyses using ribosome profiling (Sabi & Tuller, 2015; Requião et al, 2016; Dao Duc & Song, 2018; Han et al, 2020) and direct detection of accumulating peptidyl‐tRNAs (Chadani et al, 2016) have suggested the widespread occurrence of translation pausing, to which nascent polypeptides may contribute. Thus, nascent polypeptides are not simple intermediates in translation; in contrast, they actively regulate the ribosomal functions.
Nascent polypeptides within the ribosome can even destabilize the translating ribosome, termed intrinsic ribosome destabilization (IRD), leading to a premature cessation of translation (Chadani et al, 2017). When the Escherichia coli ribosome translates a sequence with consecutive negatively charged residues or intermittent with prolines (DE/P motif), its subunit structure becomes destabilized. Thus, translation is aborted stochastically within the DE/P motif. IRD is independent from release factors and ribosome recycling factor, showing that IRD is distinct from so‐called “peptidyl‐tRNA drop off”, which takes place at the initial stage of the translation (Heurgué‐Hamard et al, 1998). The peptidyl‐tRNA within destabilized or completely dissociated ribosome is resolved by peptidyl‐tRNA hydrolase (Pth) (Das & Varshney, 2006; Sharma et al, 2014) to avoid the accumulation of nonproductive complex. IRD is enhanced by deleting bL31, which bridges the large and small subunits, indicating that specific amino acid sequences within a nascent polypeptide can compromise the ribosome's structural integrity and translation continuity. Remarkably, IRD contributes to gene regulation. MgtL, the leader peptide of MgtA (Park et al, 2010; Zhao et al, 2011; Gall et al, 2016), an Mg2+ transporter, contains a potential IRD‐inducing motif, EPDP, at its N terminus. The IRD in MgtL is exaggerated under ribosome‐destabilizing conditions, including lowered Mg2+ concentrations, triggering the MgtA‐upregulating genetic scheme. Whereas MgtL provides an example of programmed IRD in cells, our knowledge about the roles of nascent peptides in regulating the stability, in either direction, of the ribosomes in action remains incomplete.
Here, we report our proteome‐wide profiling of the potential IRD‐inducing sequences (hereafter, called “IRD sequences”) in E. coli and our analyses of how this organism maintains robust translation continuity by overcoming IRD. We found two IRD‐counteracting elements in nascent polypeptides: their lengths that occupy the tunnel and the bulkiness of the amino acid side chains in the PTC‐adjacent region. The “length” and “bulkiness” of nascent chains affect the genome‐wide amino acid distribution in ORFs to minimize the risk of nonproductive translation discontinuation. These results shed new light on the pivotal roles played by nascent chains within the ribosomal exit tunnel in ensuring the efficient synthesis of every possible peptide with any sequence context required by living organisms.
Results
N‐terminally adjacent nascent polypeptide segments in the exit tunnel counteract IRD in a length‐dependent manner
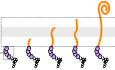
The translation of E. coli MgtL aborts within the first six amino acid residues (MEPDPT) of MgtL (Chadani et al, 2017). We searched other E. coli proteins with MgtL‐like N‐terminal DE/P‐enriched sequences and found that at least six ORFs also induce stochastic premature translation abortion in vivo and in vitro (Fig EV1A–D), indicating a widespread occurrence of IRD.
Figure EV1. N‐terminal IRD sequence induces frequent translation abortion.
-
AThe N‐terminal 10 amino acids of the IRD‐candidate ORFs are fused to lacZ (Nt10). To evaluate the translation continuity of the DE/P clusters in the ORFs (DE/P+ ), D, E, and P in the N‐terminal regions were replaced with N, Q, and A, respectively (no DEP).
-
BN‐terminal DE/P cluster‐dependent translation attenuation in vivo. Each of the Nt10 constructs with or without DE/P residues were expressed in E. coli, and β‐galactosidase activities were determined (Miller, 1972). The downstream translation frequency corresponding to IRD was calculated as the LacZ activity ratio [DE/P+ / no DEP], termed the translation continuation (T.C.) index. Asterisk in YhhS*: The high TC index in YhhS is due to the impaired translation initiation in the no‐DEP mutant of YhhS, leading to an apparent high TC value based on the equation to calculate the TC index. The mean values ± SE estimated from three independent biological and technical replicates are shown.
-
CPeptidyl‐tRNA accumulation during the mgtL translation. The Nt10 mgtL‐lacZ construct and its variant with the DE/P replacement (no DEP) were translated by the PURE system in the presence of 35S‐methionine, treated with peptidyl‐tRNA hydrolase (Pth) as indicated, and separated by neutral pH SDS–PAGE with an optional RNase A (RN) pretreatment. Peptidyl‐tRNAs are indicated by arrows with a schematic label. Radioactive formyl methionyl‐tRNA and stop codon‐terminated product are indicated as “M” and “FL” (translation‐completed chain), respectively.
-
DPeptidyl‐tRNA accumulation during the translation of other Nt10 constructs.
-
EProperties of the “30 aa‐EPDP” nascent chain during the translation of EPDP motif. The properties of the nascent chain were calculated per 10 aa from 2nd to 31st residue. Then, we divided the dataset (in vitro, n = 26) into half and plotted the average values for the higher half and the lower half. The probability of alpha‐helix formation (predicted by PSIPRED, panel 1), appearance of positively charged residues (panel 2), molecular radii (panel 3), and hydrophilicity/hydrophobicity (panel 4) are presented.
-
FCorrelation between in vivo reporter and in vitro translation assays. In vivo and in vitro results of the 5 aa‐EPDP constructs in Fig 1G were compared by two‐dimensional plot. Spearman's correlation coefficients are also shown.
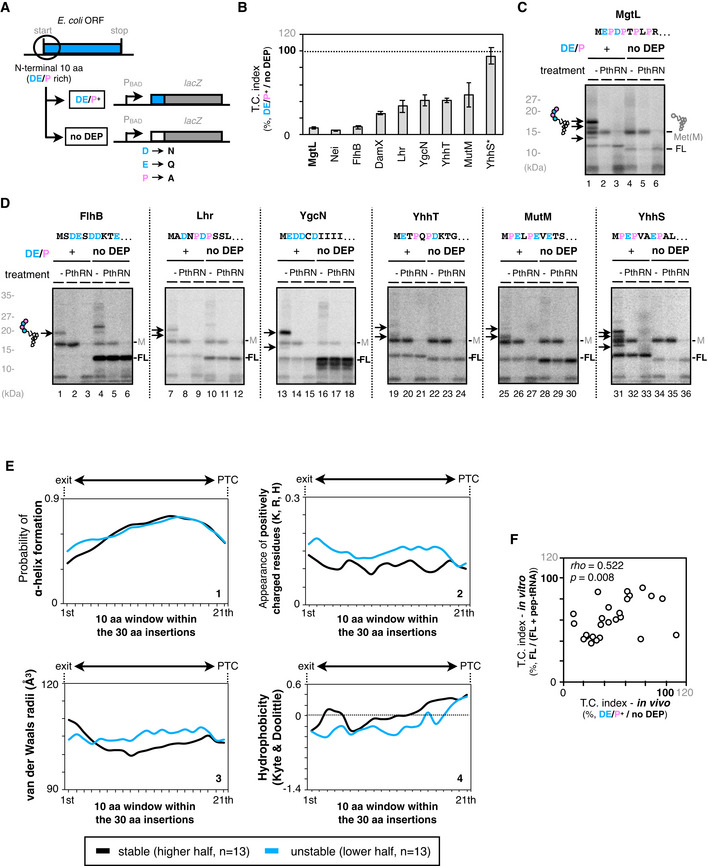
The translation abortion by the N‐terminal DE/P‐enriched sequences (Fig EV1A–D) led us to investigate whether IRD occurs similarly when a DE/P sequence resides elsewhere, such as internal positions, in a polypeptide chain. To study IRD induction by internal DE/P motifs, we surveyed the E. coli genome and found 2,110 genes, out of the annotated 4,187 ORFs, that contain one or more DE/P‐rich segment(s) (having four residues that are either D, E, or P in a six‐amino acid window). In most cases, the motifs were present at internal locations. We engineered the MgtL sequences with the EPDP sequence at various positions relative to the N terminus, by inserting GFP‐derived sequences between the initiator methionine and the EPDP motif. As shown in Fig 1A, the insertion of 238 amino acid residues counteracted the IRD‐like translation anomalies at the repositioned EPDP motifs (Fig 1A).
Figure 1. Exit tunnel‐occupying nascent polypeptides minimize the risk of IRD in their length‐dependent manners.
-
AIRD‐dependent translation attenuation by mgtL at the early (no insert, upper schematic) or middle (GFP+ , lower schematic) stage of translation elongation in vivo. Each of constructs with or without DE/P residues were expressed in E. coli, and β‐galactosidase activities were determined (Miller, 1972). The downstream translation frequency corresponding to IRD was calculated as the LacZ activity ratio [DE/P+ / no DEP], termed the translation continuation (TC) index. The mean values ± SE estimated from three independent biological and technical replicates are shown.
-
BSchematic of the working hypothesis that a tunnel‐occupying preceding nascent polypeptide stabilizes the translating ribosome to prevent IRD.
-
CSchematic of the mgtL‐lacZ variants, in which various lengths of the N‐terminal portions of GFP or other ORFs were inserted before the mgtL sequence.
-
DInserted amino acid length‐dependent IRD counteraction. The counteracting effects were expressed as the downstream translation continuities [DE/P+/no DEP] of each mgtL‐lacZ variant. The N‐terminal regions of E. coli GFP (panel 1), DnaK (panel 2), or DHFR (panel 3) were utilized as the preceding nascent polypeptides. Results of wild‐type (black line) and IRD‐prone ∆bL31 (dot line) cells are shown. The mean values ± SE estimated from three independent biological and technical replicates are shown.
-
EIRD‐counteracting effects of the N‐terminal insertions in a reconstituted in vitro translation system (PUREfrex). The ratio of the full‐length chain (FL) per aborted peptidyl‐tRNA (pep‐tRNA) accumulated in the PUREfrex reaction was calculated as the downstream translation continuation of each mgtL‐lacZ variant. The mean values ±SE estimated from three independent technical replicates are shown.
-
FIRD‐counteracting effect of short and long nascent polypeptides within the tunnel. Downstream translation continuation of mgtL‐lacZ variants with a 5 aa or 30 aa insertion (5 or 30 aa‐EPDP) in the in vivo reporter (left, n = 37) or the in vitro translation assay (right, n =21) are represented by boxplots using R software. Box portion and central band are described according to the 25th percentile and the median, respectively. ***P‐value < 0.001 (Welch’s t test).
-
GIndividual values of in vivo downstream translation continuation of the mgtL‐lacZ variants with the 5 aa‐ (white dots) or 30 aa‐EPDP constructs (black dots). N‐terminally inserted amino acid sequences are summarized in Appendix Table S4. The mean values ± SE estimated from three independent biological and technical replicates are shown.
When translating the wild‐type mgtL, the ribosome accommodates at most a six amino acid‐long nascent product in its exit tunnel before IRD occurs, leaving the tunnel almost unoccupied. By contrast, the exit tunnel of the ribosome translating the GFP‐inserted variant of MgtL is fully occupied by the GFP part of the nascent chain when it is translating the repositioned EPDP motif. Thus, we hypothesized that the tunnel‐spanning nascent polypeptide preceding the IRD sequence stabilizes the translating ribosome and thus counteracts IRD (Fig 1B). We tested this hypothesis by inserting various lengths of sequences from a variety of origins before the EPDP motif of MgtL, as shown in Fig 1C. We chose various lengths of the N‐terminal regions of GFP, E. coli DnaK, and DHFR and evaluated their effects on the EPDP‐mediated IRD in living cells. All of the nascent polypeptide segments preceding EPDP lowered the IRD efficiency in length‐dependent manners (black line in Fig 1D). It is noteworthy that the IRD‐counteracting effects of the inserted segments reached plateaus at a length of ˜30 amino acids. Interestingly, this 30 amino acid length coincides with the length of the nascent polypeptide that spans the exit tunnel in an extended form (Jenni & Ban, 2003; Voss et al, 2006). When the same proteins were expressed in the bL31 deletion strain, the IRD effect was more pronounced than that in the wild‐type host strain. These results suggest that the possession of an upstream polypeptide sequence exerts an IRD‐counteracting effect by stabilizing the translating ribosome. Since the in vivo reporter assay might be affected by not only IRD but also the post‐translational events such as degradation, we also translated them with a reconstituted in vitro translation system, PUREfrex (Shimizu et al, 2001). Consistent with the interpretation from the in vivo assays, we were able to reproduce the polypeptide insertion‐dependent IRD alleviation by in vitro assays (Fig 1E), indicating that the IRD counteraction does not require any factors other than the essential translation factors in this reconstituted system.
From these results, we propose that the nascent polypeptide chain within the ribosomal interior space generally contributes to the stability of the translating ribosome and the continuity of translation. To examine if any sequence specificity exists in the ribosome stabilizing ability of the tunnel‐occupying polypeptide, we randomly chose 40 E. coli genes (Appendix Table S4), and inserted 5 aa and 30 aa parts of their N‐terminal regions into the mgtL‐lacZ reporter, as described above. We successfully assessed the impacts of the sequences derived from 37 and 21 proteins by an in vivo reporter assay and in vitro translation, respectively (Fig 1F). As shown in the boxplots representation, the 30 aa insertions prevented IRD more effectively than the 5 aa insertions, both in vivo and in vitro. The polypeptide segments preceding the IRD sequence counteract the IRD in a largely sequence‐independent manner (Appendix Table S4). Also, the effects to prevent IRD seem to be not correlated with several physicochemical parameters such as α‐helical propensity and hydrophobicity (Fig EV1E), suggesting the polypeptides length to span the exit tunnel (˜30 amino acids or longer) is critical probably due to the stabilization by multiple contact with the tunnel interior.
Bulky amino acids near the PTC also counteract IRD
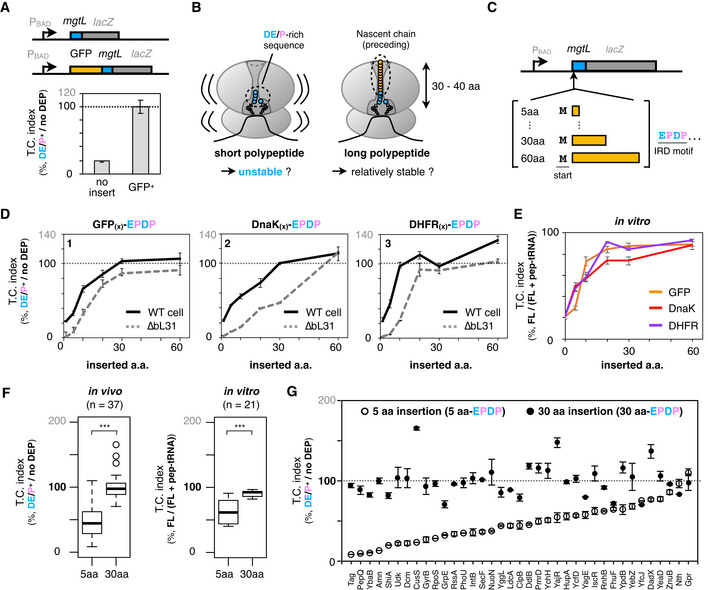
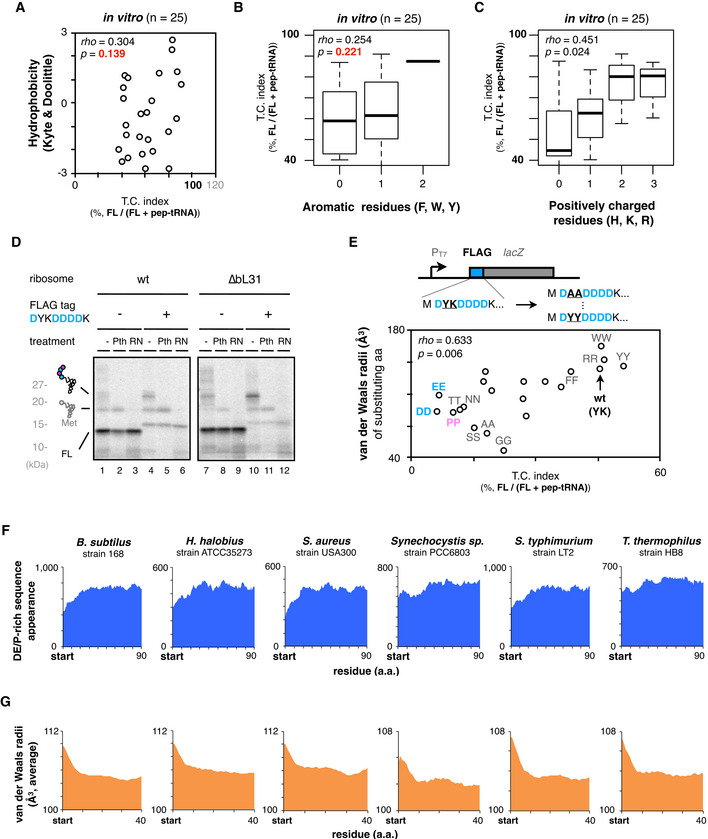
We noticed that the 5 aa insertions had a rather broad spectrum of effects on IRD compared to the 30 aa insertions (Fig 1F). Indeed, the individual in vivo data shown in Fig 1G indicate that the effects of the 5 aa insertions varied from nearly no effect to almost complete suppression of IRD (Fig 1G). A similar tendency was also observed in the PUREfrex analysis in vitro (Fig EV1F). Thus, not only the nascent chain length but also some property of the amino acids in the immediate N‐terminal vicinity of an IRD sequence can alleviate IRD, probably by stabilizing the 70S ribosome. We surveyed various properties of the inserted amino acids and found a positive correlation between the sum of the van der Waals radii of the five inserted amino acids and the IRD‐counteracting effects in vivo and in vitro (Fig 2A). Residues with bulkier side chains prevented IRD more effectively than those with smaller ones (Fig 2B). By contrast, the averaged hydrophobicity, appearance of aromatic, or positively charged residues showed a poor correlation with the effects compared to the molecular radii (Fig EV2A–C).
Figure 2. Genome‐wide biases in amino acids distributions and van der Waals radii.
-
ATwo‐dimensional plots of downstream translation continuation of the 5 aa‐EPDP constructs and averaged van der Waals molecular radii of inserted sequences. Both in vivo (left) and in vitro (right) experiments are represented with Spearman's rho and P‐values calculated by Spearman's rank correlation tests.
-
BSchematic of the ribosomal tunnel occupancy by the bulkiness of the nascent polypeptide adjacent to the PTC.
-
CEffect of the substitution of bulky (K and Y) residues of YeaD_5aa‐EPDP to smaller one (G) on IRD. The mean values ± SE estimated from three independent technical replicates are shown.
-
DEffect of the substitution of small (G) residues of YbaB_5aa‐EPDP to bulkier one (Y) on IRD. The mean values ± SE estimated from three independent technical replicates are shown.
-
EDistributions of the DE/P‐rich sequence (more than 3 DE/P in a 10‐residue moving window) appearance at the N‐terminal regions (left) and in the middle (right) of entire E. coli ORFs.
-
FAveraged van der Waals molecular radii of every 5‐aa window at the N‐terminal regions (left) and in the middle (right) of entire E. coli ORFs.
Figure EV2. IRD‐counteracting bulky amino acids near the PTC and genome‐wide biases in amino acids distributions.
-
ATwo‐dimensional plots of downstream translation continuation of the 5 aa‐EPDP constructs (TC index) and the average hydrophobicity of inserted amino acid sequences. The result of Spearman's correlation test is also shown.
-
B, CBoxplot of the TC index and the appearance of aromatic (B) or positively charged residues (C) of inserted amino acid sequences. The result of Spearman's correlation test is also shown.
-
DTranslation of the N‐terminal FLAG tag‐lacZ construct induces the accumulation of abortive peptidyl‐tRNAs. In vitro translation was performed using standard (lane 1–6) or a customized PURE system equipped with ∆bL31 ribosome (lane 7–12) in the presence of 35S‐methionine, and polypeptide products were separated by neutral pH SDS–PAGE.
-
EDependency of the molecular radii of amino acids on the translation continuation using FLAG tag as a model IRD‐prone sequence. Second tyrosine and third lysine residues of the N‐terminal FLAG tag‐lacZ were replaced with 20 kinds of dipeptide motif as described in the upper schematic. FLAG tag variants were translated in vitro, and the correlation between the downstream translation continuation and the van der Waals molecular radii of substituted amino acids are plotted in two‐dimensional. The result of Spearman's correlation test is also shown.
-
F, GAppearance of the DE/P‐rich sequences (F) or average van der Waals molecular radii of 5‐aa moving window (G) at the N‐terminal regions in the total ORFs of various bacterial species.
To examine whether the bulkiness of side chains affects IRD, we substituted the bulky 5aa insertion from YeaD (IKKIF) to smaller one (IGGIG) and found that the substitution enhanced IRD (Fig 2C). On the contrary, the substitution of small residues in YbaB to bulkier residues weakened IRD (Fig 2D). We also tested the effects of bulky amino acids in the D‐rich FLAG tag sequence (DYKDDDK), which exhibited significant IRD (Fig EV2D) in the PUREfrex translation. We mutated its YK part to pairs of identical amino acids and found that the IRD propensities correlated with the bulkiness of the dipeptides at this position (Fig EV2E). Taken together, bulky amino acid residues just preceding an IRD sequence counteract IRD. Our results suggest that the occupation of the exit tunnel by a nascent chain stabilizes the translating ribosome, by either the “length principle”, in which polypeptide chains with diverse sequences of ˜30 residues can work, or the “size principle”, in which a short stretch of bulky residues can work.
Proteome‐wide amino acid distribution bias that minimizes the IRD risk
The features of the non‐uniform translation revealed above might have affected the amino acid composition of proteins during the evolution of ORFs. Specifically, the N‐terminal regions of ORFs might have avoided the enrichment of D, E, and P and preferred amino acid residues with bulky side chains to allow for robustly continuous translation elongation. We addressed this question by bioinformatics. The appearance of more than three D, E, or P residues in ten‐residue moving windows is lower for the N‐terminal 20–30 positions of the E. coli ORFs than the following positions (Fig 2E). A previous bioinformatics analysis also showed a similar decline of negative charges at the beginning of the ORFs (Dao Duc & Song, 2018). We also observed that the van der Waals radii of amino acids in the N‐terminal regions are larger than those in the other parts of ORFs (Fig 2F). Such biased amino acid composition features of the N‐terminal regions are widely conserved among bacterial species, including Bacillus subtilis, Staphylococcus aureus, and Thermus thermophilus (Fig EV2F and G). We suggest that the amino acid distribution biases were acquired during evolution as IRD‐counteracting measures.
Proteome‐wide IRD and its counteraction revealed by ribosome profiling
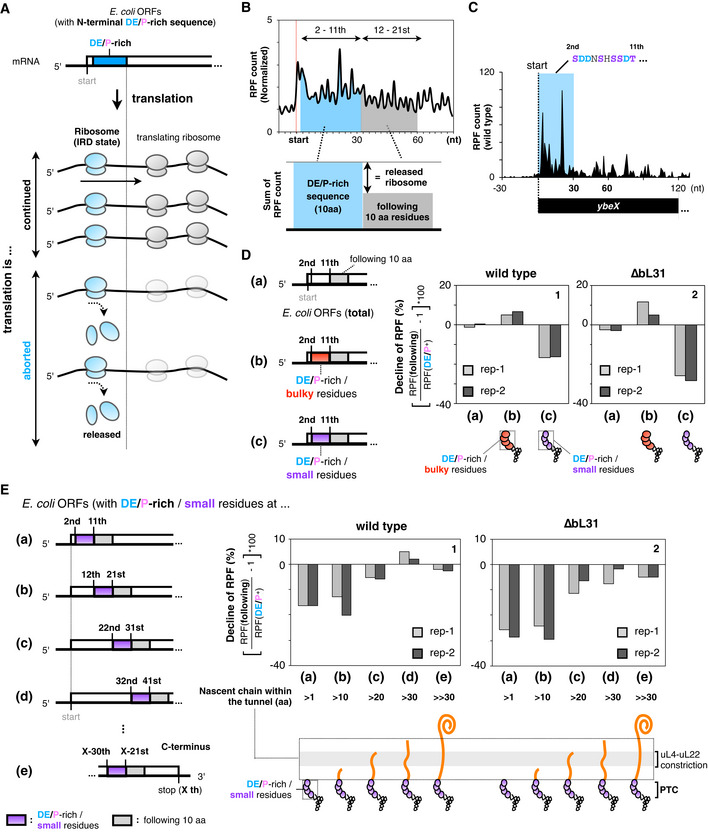
To capture a global translation status associated with IRD and the counteracting measures, we performed ribosome profiling (Ribo‐seq, Ingolia et al, 2009) and monitored the ribosomal occupancy along mRNAs. We expected that the translation of ORFs with a DE/P‐enriched sequence in the N‐terminal regions would cause a decline in the ribosome occupancy (the number of ribosome‐protected RNA fragments, RPFs) in the following 3' region, by the stochastic release of the ribosomes from the mRNA due to IRD (Fig 3A). To survey ORFs with the above‐mentioned characteristic patterns of ribosome distribution, we compared the sum of the RPF counts at the first 10 codons with those at the following 10 codons (Fig 3B). We first focused on ybeX as one of the ORFs that exhibited the above‐mentioned Ribo‐seq patterns. The N‐terminal segment (MSDDNSHSSDT) of ybeX is enriched in Asp and small‐sized amino acids (Ser and Thr). The RPFs from the ybeX mRNA decreased after the 11th codon (Fig 3C). A biochemical analysis showed that an abortive peptidyl‐tRNA accumulated in an Asp‐dependent manner during the in vitro translation of ybeX (Fig EV3A), substantiating the occurrence of an elongation cessation.
Figure 3. Ribosome profiling analysis to capture the overview of IRD and its counteraction at a proteome scale.
-
ASchematic representation showing that the ribosome is released from the mRNA by IRD, leading to a decline in ribosome‐protected fragments (RPFs) after translation of IRD‐inducing sequences.
-
BIRD frequency around the N‐terminal region was evaluated from the ratio of RPF counts in the 12th–21st aa to those in the 2nd–11th aa including DE/P‐rich residues.
-
CDistribution of the RPFs on the ybeX mRNA in wild‐type cells. The region of the N‐terminal acidic‐rich sequence is highlighted.
-
DRatio of RPF counts in the 12th–21st aa to those in the 2nd–11th aa. The data are classified as follows: Total: all dataset (a), E. coli ORFs with DE/P‐rich and bulky (b) or small (c) radii residues in N‐terminal regions. A detailed representation of the classification is shown in Fig EV3B. The data from two biological replicates of wild‐type (panel 1) or IRD‐prone ∆bL31 (panel 2) cells are shown. Schematics at the bottom represent the nascent chain occupying the tunnel during the translation of the 2nd–11th aa. Meta gene plots for the data are shown in Appendix Fig S1.
-
ERatio of RPF counts in 10 amino acids with “small” and DE/P‐rich residues to those in the following 10 amino acids at different stages of elongation. RPF counts for 10 amino acids with “small” and DE/P‐rich residues were summed from the most N‐terminal (2nd–11th, (a)) to the subsequent every 10‐amino acid windows (b)‐(d). RPF counts for 10 amino acids with “small” and DE/P‐rich residues in (e) were summed from X‐30th to X‐21st, where X represents the last amino acid in the ORFs. The data from two biological replicates of wild‐type (panel 1) or IRD‐prone ∆bL31 (panel 2) cells are shown. The corresponding data for “bulky” and DE/P‐rich residues are shown in Fig EV3C.
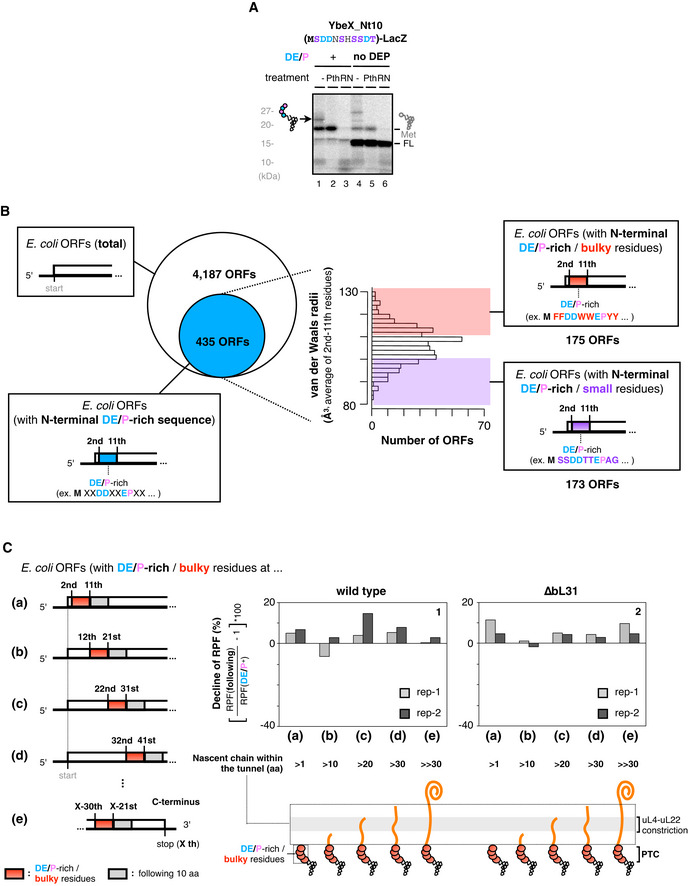
Figure EV3. Supplemental data for the ribosome profiling analysis.
-
APeptidyl‐tRNA accumulation during the ybeX translation. In vitro translation products of ybeX_Nt10‐lacZ or its “no DEP” variant were separated by SDS–PAGE with optional treatments as indicated.
-
BClassification of E. coli ORFs by the N‐terminal amino acid (aa) sequences in terms of the DE/P‐richness and the bulkiness. At first, ORFs carrying DE/P‐rich sequences (n = 435) at 2–11th aa were extracted from the entire ORFs [n = 4,187, (a) in Fig 3D]. They were further classified as DE/P‐rich/bulky [n = 175, (b) in Fig 3D] and DE/P‐rich/small [n = 173, (c) in Fig 3D] ORFs according to the average van der Waals radii of 2–11th aa.
-
CRatio of RPF counts in 10 amino acids with “bulky” and the DE/P‐rich residues to those in following 10 amino acids at the different stages of elongation. The RPF counts for 10 amino acids with the “bulky” and the DE/P‐rich residues were summed from the most N‐terminal (2nd–11th, (a)) to subsequent every 10‐amino acid windows, (b)–(d). The RPF counts for 10 amino acids with the “bulky” and the DE/P‐rich residues in (e) were summed from X‐30th to X‐21st, where X represents the last amino acids in the ORFs. The data from two biological replicates of wild‐type (panel 1) or the IRD‐prone ΔbL31 cells (panel 2) are shown.
We next analyzed the global Ribo‐seq data to compare the sum of the RPF counts at the first 10 codons with those at the following 10 codons in terms of bulkiness of the amino acids (Fig 3D, Appendix Fig S1). We extracted the ORFs enriched in DE/P residues in their N‐terminal regions and then classified them further into those enriched in small amino acids and those enriched in bulky amino acids (Fig EV3B). The analysis revealed that ˜16% of RPFs were lost after 10 codons (30 nucleotides) for ORFs with an abundance of small‐sized amino acids in their N‐terminal regions (Fig 3D, panel 1). The bL31‐deleted cells showed more pronounced decreases in RPFs after the 10th codon (Fig 3D, panel 2), further supporting our contention that the global low occupancy of the ribosomes after the risky sequences represents IRD. By contrast, we did not observe any decrease in the RPF for ORFs containing DE/P‐rich and large‐sized amino acids in the N‐terminal region (Fig 3D), consistent with our findings that bulky amino acids antagonize IRD.
We next undertook a systematic survey on the length dependency of the N‐terminal regions of E. coli proteins for the occurrence of IRD‐like phenomena. In this analysis, we searched for sequences enriched in DE/P and small‐sized residues in the successive 10‐amino acid windows moving from the N‐ to C‐terminal regions, as schematically shown in Fig 3E(a)‐(e). We then analyzed the numbers of RPFs across the putative IRD sequences. We found 15–20% declines when the windows starting at the second or the 12th codons were examined (Fig 3E, panel 1 (a), (b)). Such tendencies were gradually lost for windows moving further toward the 3' direction, in which the elongating nascent polypeptide should have possessed successively longer sequences preceding the IRD determinant. The results with the windows of 22–31 (c), 32–41 (d) and at the C terminus (e) indicated that the ribosomes essentially continue to translate these regions even when they encounter DE/P‐ and smaller residue‐enriched segments. The bL31 deletion uniformly enhanced the decline of RPFs, again supporting that the elongation discontinuations were due to the destabilization of translating ribosome (Fig 3E, panel 2). We did not observe equivalent decreases in RPFs when we extracted the DE/P‐enriched sequences that were associated with bulky amino acid residues (Fig EV3C), suggesting that the nascent chain “length”‐ and amino acids “bulkiness”‐dependent mechanisms synergistically affect the translation continuity. Collectively, our Ribo‐seq analysis revealed that DE/P‐enriched segments that also contained small‐sized residues generally induce IRD when they are present at N‐terminal regions.
Multifaceted nascent chain‐exit tunnel interactions contribute to the continuous translation elongation
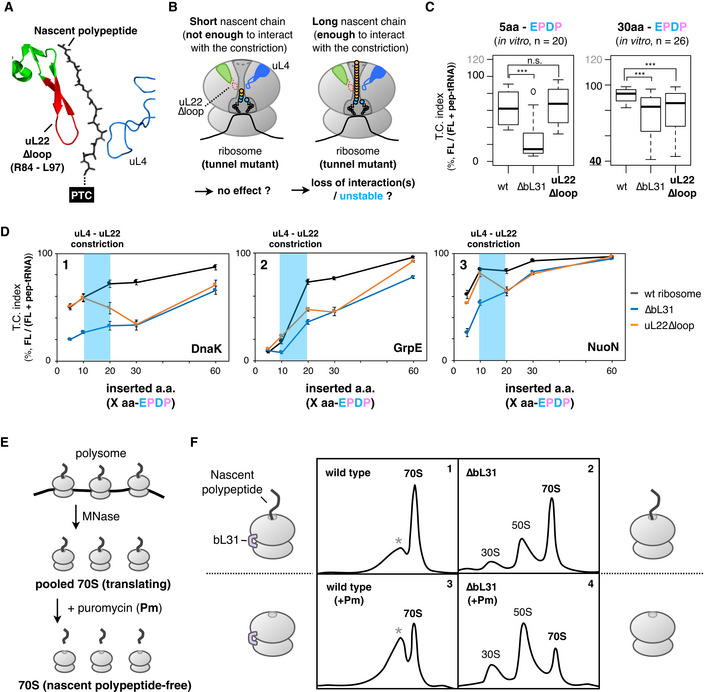
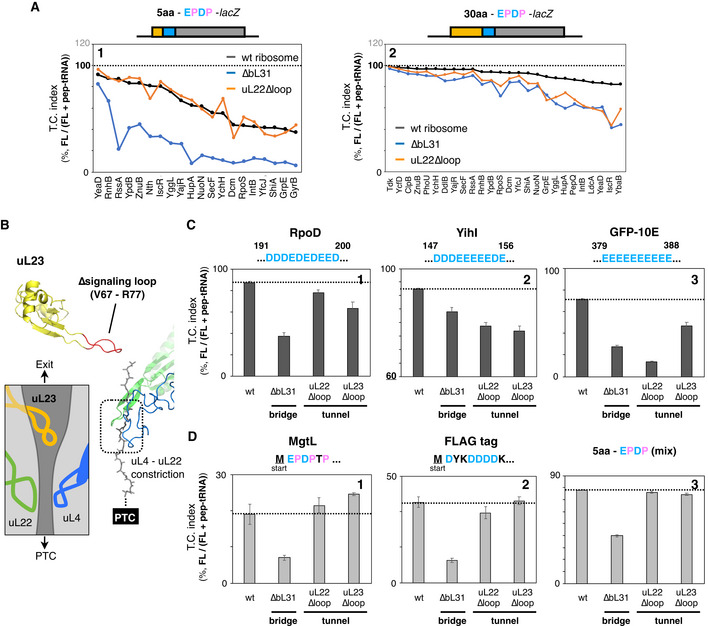
The above results imply that the occupancy of the exit tunnel with a nascent polypeptide plays a pivotal role in ensuring the translation continuation. Then, we tested whether the mutations of the tunnel wall components uL4 and uL22, which comprise the central constriction site (Ito & Chiba, 2013), affect the two modes of IRD antagonization studied above (Fig 4A). We first focused on the role played by the uL22 loop (Fig 4B) and constructed mutant ribosomes with the mutant form lacking the β‐hairpin loop (uL22∆loop; Fig 4B). We then developed a modified PUREfrex containing the mutant form of the ribosomes. The results revealed that the effects of the uL22∆loop mutation differ, depending on the preceding amino acid lengths. The uL22 mutated ribosome was almost indistinguishable from the wild‐type ribosome in the IRD profile of the EPDP constructs with the upstream five amino acids (Figs 4C and EV4A, orange line). In contrast, the absence of the loop enhanced IRD when the upstream region contained 30 amino acids (Figs 4C and EV4A).
Figure 4. Effect of mutations in the constriction site within the exit tunnel on the IRD counteraction.
-
AStructure of the uL4‐uL22 constriction site (PDB: 4V5H). Deleted residues in the uL22∆loop mutation are indicated in red.
-
BSchematic of the working hypothesis that stabilization of the translating ribosome depends on interactions between the ribosomal tunnel and the tunnel‐occupying nascent polypeptide.
-
CInserted amino acid length‐dependent IRD‐counteracting effects in the uL22∆loop‐ribosome. Messenger RNAs encoding a 5‐aa (left) or 30‐aa (right) insertion and EPDP (used in Fig 2) were individually translated by customized PUREfrex including the indicated ribosome variants. Evaluated downstream translation continuation indices of wild‐type, ∆bL31, or uL22∆loop ribosomes are represented by boxplots using R software. Box portion and central band are described according to the 25th percentile and the median, respectively. ***P‐value < 0.001, n.s.: no significant difference (Welch’s t test).
-
DPreceding nascent polypeptide‐length dependency on the IRD counteraction in the ribosome variants. The N‐terminal regions of E. coli DnaK (panel 1), GrpE (panel 2) or NuoN (panel 3) were utilized as preceding nascent polypeptides. The mean values ±SE estimated from three independent technical replicates are shown.
-
EExperimental design to evaluate the stability of the translating ribosomes. MNase: micrococcal nuclease.
-
FStability of the 70S ribosome complex with or without a tunnel‐occupying nascent polypeptide in wild‐type (panels 1 and 3) or ∆bL31 (panels 2 and 4) cells. Pooled 70S translating ribosomes with (panels 3 and 4) or without (panels 1 and 2) the puromycin treatment were fractionated by SDG in the presence of 10 mM magnesium ion. Recorded A254 values are shown. The peaks indicated as the asterisk (*) in panels 1 and 3 seem to be an aberrant form of the 70S ribosome due to the MNase treatment.
Figure EV4. Effect of the exit tunnel mutations on the IRD counteraction.
-
AInserted amino acid length‐dependent IRD‐counteracting effects in the uL22∆loop‐ribosome. Messenger RNA coding 5‐aa (panel 1) or 30‐aa (panel 2) insertion and the EPDP used in Fig 1G were individually translated by customized PURE systems, including ribosome variants as indicated. Individual translation continuation indices of wild type (black line), ∆bL31 (blue line), or uL22∆loop ribosomes (orange line) are shown.
-
BStructure of the uL23 signaling loop (PDB: 4V5H) and schematic drawing of the tunnel structure. Deleted residues in the uL23∆loop mutation are indicated in red.
-
CTranslation continuity of ORFs with the IRD‐inducing motif located in the middle of the ORFs. Messenger RNAs encoding rpoD (panel 1), yihI (panel 2), or GFP‐10E (panel 3) carrying 10 consecutive D/E residues were translated in vitro and the translation continuities were evaluated as described above. The mean values ± SE estimated from three independent technical replicates are shown.
-
DTranslation continuity of ORFs with an IRD sequence without a preceding nascent polypeptide occupying the tunnel. Messenger RNAs encoding mgtL (panel 1), FLAG tag (panel 2) or a mixture of 5 aa‐ EPDP constructs in Fig 1F and G (panel 3) were translated in vitro and analyzed as described above. The mean values ± SE estimated from three independent technical replicates are shown.
This substrate‐dependent effect of the uL22 mutation is in sharp contrast to our results with the ribosomes lacking bL31 (∆bL31). The lack of bL31 invariably enhanced IRD (lowered the translation continuity), with both templates having the 5 aa and 30 aa insertions (Fig 4C. For individual data, see also Fig EV4A, compare black and blue lines). These results indicate that the β‐hairpin loop of uL22 in the wild‐type ribosome plays a role in counteracting IRD, but only in the presence of the tunnel‐spanning upstream polypeptide region (Fig 4B).
We further explored the relationships between the exit tunnel occupation and the stability of the translating ribosome, using various lengths of the N‐terminal regions of DnaK, GrpE, and NuoN that were attached N terminally to the EPDP motif. The wild‐type and uL22∆loop ribosomes translated the EPDP constructs with the five amino acid‐ and the 10 amino acid‐upstream regions with almost the same degrees of IRD, which differed depending on the sequences (Fig 4D, compare black and orange lines). However, the uL22∆loop ribosomes showed an increased IRD when 20 or more amino acids were present before the EPDP (Fig 4D, orange lines).
Besides uL22, we found that the other ribosomal tunnel mutation, the deletion of the uL23 signaling loop, which otherwise extends into the PTC‐distal part of the exit tunnel (Bornemann et al, 2008, Fig EV4B), also affected the translation continuity of ORFs with the IRD‐inducing motifs located in the middle of the ORFs (Fig EV4C). Since previous studies suggested that the uL22 β‐hairpin loop and the uL23 signaling loop interact with the nascent polypeptides farther away from the PTC (Bornemann et al, 2008; Seidelt et al, 2009; Bhushan et al, 2011; Sohmen et al, 2015; Su et al, 2017; Shanmuganathan et al, 2019), our data suggest that nascent chains spanning the tunnel interact with the tunnel components to stabilize the translating ribosome and ensure translation continuity, irrespective of the amino acids being polymerized at the PTC. The involvement of multiple ribosomal proteins suggests that multiple interactions, which alone may be subtle, cumulatively contribute to the continuous translation.
Tunnel‐occupying nascent polypeptides stabilize the translating 70S ribosome by bridging the 50S and the 30S subunits
Our findings that the nascent polypeptide spanning the exit tunnel counteracts the IRD‐mediated translation discontinuation strongly suggested that the tunnel‐occupying nascent polypeptide functions as an inter‐subunit bridge, like the bL31‐supported B1b peripheral bridge.
To substantiate the concept that the nascent polypeptide stabilizes the association of the large and small ribosomal subunits more directly, we compared the stabilities of the 70S ribosomes with a tunnel‐traversing nascent polypeptidyl‐tRNA and those that had lost the intact polypeptidyl‐tRNA. We prepared the 70S ribosomes in the process of translation by treating polysomes with micrococcal nuclease, which cleaves unoccupied mRNA. The resulting nascent chain–ribosome complexes exhibited the ˜ 70S sedimentation speed. We then treated the 70S complexes with puromycin to prepare the “peptidyl‐tRNA‐free” 70S ribosomes and centrifuged the samples (Fig 4E). Wild‐type ribosomes sedimented at ˜70S irrespective of the puromycin treatment (Fig 4F, panels 1 and 3). By contrast, the bL31‐lacking ribosomes maintained the 70S complexes only without the puromycin treatment (Fig 4F, compare panel 2 and panel 4). Thus, the nascent polypeptides (polypeptidyl‐tRNAs) within the exit tunnel stabilize the translating 70S ribosome complex even in the absence of the bL31 bridge. It should be noted that the nascent polypeptides within the 70S ribosomes in this experiment consisted of a mixture of endogenous gene products, which do not necessarily contain IRD‐inducible DE/P‐enriched sequences near the PTC, implying that the tunnel‐occupying nascent polypeptides in general contribute to maintain the translating 70S complex.
Discussion
Recent advances in translation studies have revealed the widespread occurrence of noncanonical translation progression (Dever et al, 2020; Samatova et al, 2021). We showed that elongation pausing is frequent in E. coli, by capturing intermediate molecules with tRNAs at their ends (Chadani et al, 2016). More recently, we discovered the IRD phenomena (Chadani et al, 2017), in which the translation of certain acidic or proline‐intermitted acidic sequences lead to the destabilization of the 70S ribosome and translation cessation without involving a stop codon. In the present study, we addressed the generality, the position effects, and the sequence context dependence of potentially IRD‐inducing DE/P‐rich motifs in E. coli, featuring both model constructs and the native genes in the genome. Our results showed that nascent polypeptides in the interior of the translating ribosome have regulatory roles over the stability of the engaged ribosomes. In particular, we found that the nascent chain in the exit tunnel ensure elongation continuity by serving as a bridge and thus by protecting the large and small ribosomal subunits from dissociation. The role of tunnel‐occupying nascent chains compensates for the potential IRD induction by a risky DE/P‐rich part in the following region of the same polypeptide.
In this paper, we focus to IRD at early stages of translation, although IRD also takes place in the internal regions of ORFs, as exemplified by YagN, in which the IRD motif starts from 110th (110‐DPDPEPE) (Chadani et al, 2017). Therefore, we note that the IRD‐inducing sequences in the middle of ORF are not necessarily silenced by preceding nascent chain. On the other hand, the IRD‐mediated release of peptidyl‐tRNA at the early stages of translation reminds us of well‐known “peptidyl‐tRNA drop‐off” (Heurgué‐Hamard et al, 1998), since the consequences of both events, that is, the release of peptidyl‐tRNA, are the same. Although our previous study has shown that different dependence on translation factors and amino acid sequences between IRD and the peptidyl tRNA drop‐off (Chadani et al, 2017), IRD and the drop‐off could be indistinguishable in terms of molecular mechanisms. As shown in a supplementary data (Fig EV5A and B), we cannot currently distinguish between IRD at the early stages of translation and the peptidyl‐tRNA drop‐off from the mechanistic standpoint. Further study for both IRD and the drop‐off will be required to clearly distinguish these noncanonical ribosome dynamics.
Figure EV5. Supplemental data for the Discussion section.
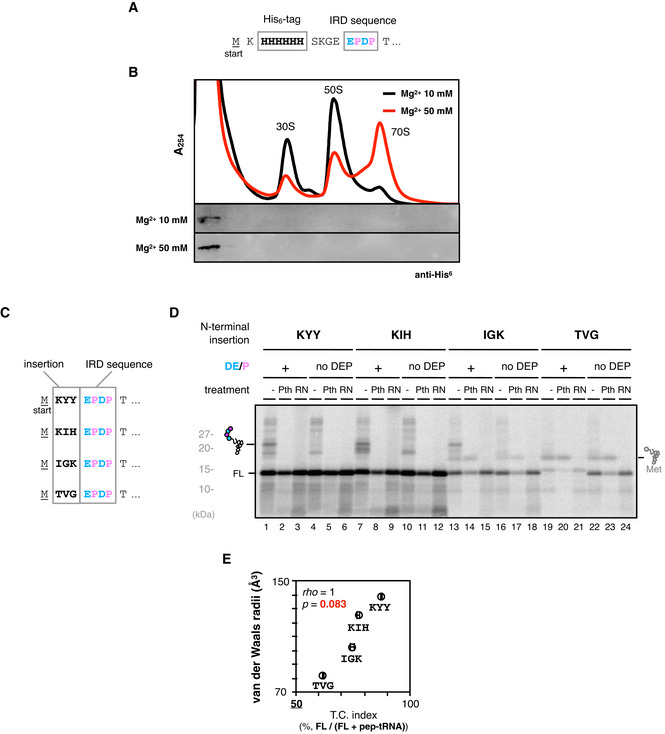
-
A, BDissociation of short peptidyl‐tRNA from the ribosome after IRD. N‐terminally His6‐tagged GFP(5aa)‐EPDP sequence (A) was translated by PURE system and mixed with an equal volume of HKM buffer (25 mM HEPES‐KOH pH 7.4, 100 mM KCl, 5 mM MgCl2) or HKM buffer supplemented with 100 mM MgCl2. Then the mixture was fractionated by SDG in the presence of 10 mM or 50 mM magnesium ion. Recorded A254 values (upper) and the distribution of aborted peptidyl‐tRNA detected by anti‐His6 Western blotting (bottom) are shown (B).
-
C–EInfluence of N‐terminal amino acid motifs that determine the initiation rate on IRD. Some of the 3 amino acid motifs reported by Verma et al, 2019 (KYY, KIH, IGK and TVG) were fused to N‐terminal EPDP motif (C) and in vivo translated to evaluate the TC index. Representative image of 35S‐Methionine‐labeled products separated by SDS–PAGE (D) and two‐dimensional plot of the TC indices of three independent technical replicates and averaged van der Waals radii of 3 aa motif (E) are shown with the result of Spearman's correlation test.
In the translocation step of translation, the large and small subunits undergo a relative rotation, which their association must withstand to produce a protein product (Horan & Noller, 2007; Voorhees & Ramakrishnan, 2013; Bock et al, 2015). Thus, the ribosome is equipped with multiple bridges, including bL31, that tether the large and small subunits in the functional monosome (Fischer et al, 2015; Chadani et al, 2017; Lilleorg et al, 2017; Ueta et al, 2017). In addition to the bridges, the nascent chain‐mediated IRD‐counteracting effects suggest that nascent polypeptidyl‐tRNA physically stabilizes the translating ribosome. A possible experiment is to compare the stability of the ribosome complex translating the IRD‐prone motif with various length/bulkiness of preceding nascent chain using sucrose density gradient (SDG) centrifugation. However, we are not currently able to optimize the experimental setup. Instead, we used cell lysates for the source of translating ribosomes (Fig 4F). The sedimentation analysis using cell lysates showed that endogenous sources of nascent polypeptidyl‐tRNA, not necessarily combined with a DE/P sequence, stabilize the 70S structure. Thus, we propose that the ribosome stabilization by the tunnel‐occupying nascent chain represents a general life principle.
Earlier studies reported the peptidyl‐tRNA‐dependent stabilization of the 70S structure under Mg2+‐depleted conditions (Schlessinger et al, 1967; Kohler et al, 1968; Ron et al, 1968; Duin et al, 1970). Other reports described the destabilization of a nascent chain‐free ribosome complex under high ionic conditions (Zylber & Penman, 1970; Blobel & Sabatini, 1971). Whereas these observations complement our findings, they were made before the discovery of the ribosomal exit tunnel. In addition, cryptic and apparently risky sequences like the IRD motifs were unknown, and thus, the physiological significance of the early findings remained unexplored. Our work can therefore be regarded as a revisitation of this problem with the cutting‐edge knowledge of translation dynamics. The role of nascent chain within the tunnel enables the ribosome to be a universal translation machine that can synthesize polypeptides of any sequence without discontinuation to produce the life‐supporting proteome of the species. The C‐terminal end of a nascent polypeptide is covalently tethered to a tRNA that interacts with the 30S component and the mRNA in the 30S decoding center. Meanwhile, its more N‐terminal region resides in the 50S subunit, entering into and occupying the exit tunnel. Thus, a nascent polypeptide, that is, a polypeptidyl‐tRNA, bridges the small and large subunits in collaboration with the tRNA moiety. This represents a self‐stabilization mechanism to prevent the dissociation of the translating ribosome.
Nascent polypeptides preceding the DE/P‐rich sequences antagonize IRD. In the “length”‐dependent but sequence‐independent mode of the stabilization, the nascent chain might act as an intersubunit scaffold. The deletion of either the uL22 β‐hairpin loop or the uL23 signaling loop weakens the effect but does not completely abolish it, suggesting that multiple weak interactions between the interior of the tunnel and the nascent polypeptide contribute to the stabilization. The diverse effects of the ribosomal mutations affecting different ribosomal proteins, such as uL22 and uL23, imply that the ribosome and the nascent chain communicate with each other by the local diversities, producing cumulative weak interactions to perform the biological role of the “peptidyl‐tRNA bridge”, although such interactions poorly inhibit the nascent chain movement through the tunnel.
The bulkiness, defined in this paper as the van der Waals radii, of the amino acids in the nascent chain adjacent to PTC also counteracted IRD. It is likely that the “bulkiness”‐dependent stabilization plays a more important role during the early stage of translation, at which the “length” works poorly. The “length”‐dependent mechanism apparently lacks a preference for amino acid sequences, but its effect would be insufficient until the nascent polypeptide fulfills the tunnel (no less than 20 aa). In contrast, the “bulkiness”‐dependent stabilization necessitating larger residues restricts the usage of amino acids, unlike the “length”‐dependent one. We assume that these two stabilization mechanisms complementarily compensate for their respective insufficiencies to avoid nonproductive premature termination. The strong biases on disfavored DE/P‐rich sequences and favored bulky amino acids in the N‐terminal regions of bacterial ORFs are in agreement with this assumption (Figs 2E and F, and EV2F and G). Many previous studies have pointed out that the sequence features just adjacent to the start codon significantly influence the efficiency of protein biosynthesis (Sprengart et al, 1996; Kudla et al, 2009; Salis et al, 2009; Allert et al, 2010; Goodman et al, 2013; Verma et al, 2019; Osterman et al, 2020). For example, some of such amino acid motifs (Verma et al, 2019) actually affect not only the rate of translation initiation but also the translation continuity (Fig EV5C–E). Considering the “bulkiness” of the nascent polypeptide when interpreting the datasets might disclose new landscapes from previous studies.
The “size (bulkiness)” dependence indicates the relationship between the geometry of the tunnel structure and the stabilization mechanism. Deutsch and her colleagues reported the “size”‐dependent rearrangement of the nascent polypeptide at the eukaryotic uL4‐uL22 constriction site and the regulation of elongation kinetics, indicating that larger residues have more interaction chances than smaller ones (Lu et al, 2011; Po et al, 2017). The translation of consecutive tryptophan residues, the largest natural amino acid, inhibits elongation by the eukaryotic ribosome, in agreement with this observation (Dimitrova et al, 2009). The prokaryotic uL4‐uL22 constriction site is slightly broader than that of eukaryotes (Dao Duc et al, 2019), and tryptophan repeats do not induce translational anomalies in E. coli (Chadani et al, 2017). However, the prokaryotic tunnel forms another constriction site at the entrance of the tunnel, which is as narrow as the eukaryotic uL4‐uL22 constriction site (tunnel radii are below 4 Å, Fischer et al, 2015; Dao Duc et al, 2019). Taken together with these facts, we assume that the squeezing of bulky residues by this “entry constriction site” represses the unwinding of the tRNA molecule within the P site of ribosome, thus stabilizing the 70S complex. Other bacterial ribosomes show similar architectural characteristics, supporting this assumption.
Finally, this study provides an answer to the naive question of why the ribosome has an exit tunnel for the nascent polypeptide. A tunnel may not be needed, if the essential function of the ribosome is simply to polymerize the polypeptides. Stabilization of the translating ribosome by preparing the tunnel for occupation with its own product would be a positive feedback system to continue the elongation. There might be a trade‐off between the stabilization effects and the obstacles of translation elongation. The tunnel architecture, which is less than 30 ˜ 40 aa in length, might represent a compromise for these characteristics.
Materials and Methods
E. coli strains, plasmids, and primers
E. coli strains, plasmids, and oligonucleotides used in this study are listed in Appendix Table S1, S2, and S3, respectively. The ASKA plasmid library (Kitagawa et al, 2006) was obtained from National Institute of Genetics (NIG). Plasmids were constructed using standard cloning procedures and Gibson assembly. Detailed schemes were summarized in Appendix Table S2, and sequences of constructed plasmids are available in the Mendeley repository (https://doi.org/10.17632/ftc93j5cj2.1).
PCR‐amplified DNA fragment from pDGT1 using primers PR0058 and PR0059 was electroporated into strain BW25113 harboring pKD46 (Datsenko & Wanner, 2000) and pCY927 (rpsJ‐rpsQ operon), selecting for a trimethoprim (20 µg/ml)‐resistant transformant. Genome‐introduced ∆rpsJ‐rpsQ::FRT‐DHFR‐FRT mutation was transferred by Phage P1 into MG1655 harboring pCY873 (rpsJ‐rpsQ operon), pCY920 (rpsJ‐rpsQ operon with uL22∆loop mutation), or pCY1457 (rpsJ‐rpsQ operon with uL23∆loop mutation), respectively.
In vitro translation and product analysis
A coupled transcription–translation reaction was performed using PUREfrex 1.0 (GeneFrontier) in the presence of 35S‐methionine at 37°C for 30 min. DNA templates were prepared by PCR, as summarized in dataset EV1. Mutant ribosomes (∆bL31, uL22∆loop, uL23∆loop) purified as described (Ohashi et al, 2007) were used when indicated. After 30‐min incubation, the reaction mixture was treated with 200 µg/ml of puromycin for 5 min at 37°C. Half of the mixture was further treated with 1 µM of purified Pth for 10 min at 37°C when indicated. The reaction was stopped by dilution into an excessive volume of 5% TCA. After standing on ice for 10 min or more, samples were centrifuged for 3 min at 4°C, and the supernatant was discarded by aspiration. Precipitates were then vortexed with 0.9 ml of acetone, centrifuged again, and dissolved in SDS sample buffer (62.5 mM Tris–HCl pH 6.8, 2% SDS, 10% glycerol, 50 mM DTT) that had been treated with RNasecure (Ambion). Finally, the sample without Pth treatment was divided into two portions, one of which was incubated with 50 µg /ml of RNase A (Roche) at 37°C for 30 min and separated by WIDE range SDS–PAGE system (Nakalai tesque).
Translation continuation (TC) index in vitro
The ratio of the translation‐completed chain (full length: FL) against Pth‐sensitive polypeptidyl‐tRNAs (pep‐tRNA), which signifies the occurrence of IRD‐induced abortion in the total in vitro translation products, was calculated as translation continuation (TC) index. In detail, the radioactivity (35S‐methionine) proportion of “FL” and “pep‐tRNA” among the sample with neither Pth nor RNase treatment were quantified by Multi Gauge (Fujifilm), and the TC value (in %) is obtained by the following formula.
The average of three independent experiments was presented as TC indices, except for Fig 4C (Fig EV4A).
β‐galactosidase assay
TC index in vivo
E. coli cells harboring lacZ reporter plasmid were grown overnight at 37°C in LB medium supplemented 100 µg/ml ampicillin. They were inoculated into fresh LB medium containing 2 × 10−4% arabinose and 100 µg/ml ampicillin and were grown at 37°C for ˜2.5 h (A660 = ˜0.4). Then, 20 µl portion was subjected to β‐galactosidase assay as described (Miller, 1972). Estimated miller unit (m.u.) were subjected to the following formula to calculate the TC index (in vivo).
TC indices were calculated from three independent experiments, and plasmids used in each experiment were summarized in dataset EV1.
Amino acids distribution analysis
We calculated the number of ORFs with more than three D, E, and P residues within 10‐amino acid moving windows in both N‐terminal and internal regions in entire E. coli ORFs. Datasets of protein sequences were obtained from UniProt (https://www.uniprot.org).
Ribosome profiling
Sequencing of ribosome‐protected fragment (RPF)
E. coli MG1655 (wild type) or ECY0228 (MG1655 ∆bL31) strain was grown overnight in LB medium at 37°C. They were inoculated into fresh LB medium and were grown at 37°C. When A660 reached 0.5, cells were treated with 100 µg/ml of chloramphenicol and collected by filtration (0.45 µm MF membrane). Filter‐trapped E. coli cells were scraped and dropped into liquid nitrogen dispensed to 50 ml corning tube in advance. In addition, RP buffer (20 mM Tris‐HCl pH 7.5, 150 mM NH4Cl, 10 mM MgCl2, 1 mM DTT, 5 mM CaCl2, 1% Triton X‐100, 100 µg/ml chloramphenicol) was added to form frozen droplets. The cell droplets were disrupted by Multi‐Beads Shocker [MB601 U(S), Yasui Kikai], thawed, and treated with 25 unit/µl of Turbo™ DNase (Thermo Fisher Scientific) for 10 min on ice. After centrifugation at 20,000 g for 10 min at 4°C, the supernatant was collected and 35 µg RNA per sample was treated with 150 unit of Micrococcal Nuclease (MNase, Roche) for 45 min at 25°C. RNA fragments ranging from 17 to 50 nt were gel‐excised as ribosome‐protected fragment (RPF). Following library preparation was performed as previously described (McGlincy & Ingolia, 2017). A Ribo‐Zero rRNA Removal Kit for Bacteria (Illumina) was used to deplete rRNA contamination. The libraries were sequenced on a HiSeq4000 (Illumina). After depleting the reads originating from noncoding RNAs, the remaining reads were mapped to the E. coli genome sequence (NC_000913.2). Empirically, we defined an A‐site position, which is essentially the same as the 3’ assignment as described previously (Woolstenhulme et al, 2015).
Classification of E. coli ORFs by sequential features
“DE/P‐rich” sequence
We defined two or more D, E, or P residues within continuous three codons as “DE/P+ cluster”. Furthermore, two or more “DE/P+ clusters” within the continuous ten codons were defined as “DE/P‐rich” sequences that potentially induce IRD and utilized for classification of E. coli ORFs.
“Van der Waals radii”
The averaged van der Waals radii of ten residues belonging to “DE/P‐rich” sequence at indicated region was calculated and utilized for classification of E. coli ORFs as follows.
“DE/P‐rich sequence” with bulky‐sized residues: 40% of the larger one
“DE/P‐rich sequence” with small‐sized residues: 40% of the smaller one
Calculation of normalized RPF
We utilized house‐made script to describe the distribution of normalized RPF counts among the subsets of E. coli ORFs classified as described above. Briefly, the relative RPF count in each nucleotide position was calculated for each ORF at first. Then, the obtained relative RPF count in each position was averaged for each subset. We excluded the ORFs with less than 150 bp length, poor expression (less than 400 RPF count/gene) or concentrated RPF count at specific site (b0621, b2094, b2218, b3709, and b2744) to convince the statistical analyses. The sum of normalized RPF counts among the indicated region was compared to that of following 10 amino acids residues to estimate the frequency of IRD‐dependent translation abortion.
Stability assay for 70S translating ribosome complex
E. coli cells were grown in LB medium overnight at 37°C. They were inoculated into 2x YTPG medium supplemented with Antifoam SI (Fujifilm‐WAKO) till A660 reaches to 3.0. Cell culture was mixed with crushed ice and centrifuged at 8,000 rpm for 3 min at 4°C. Pelleted cells were resuspended by polysome buffer [20 mM Tris‐acetate pH 7.5, 150 mM NH4Cl, 10 mM Mg(OAc)2, 1 mM DTT, (200 µg/ml chloramphenicol was added when indicated)] and dropped into liquid nitrogen dispensed to a 50‐ml corning tube in advance. Frozen cell droplets were disrupted by Multi‐Beads Shocker [MB601 U(S), Yasui Kikai] and treated with 10 unit of TurboTM DNase (Thermo Fisher Scientific) for 20 min on ice. Lysate was centrifuged at 20,000 g for 10 min at 4°C. The supernatant was fractionated by 10–50% sucrose gradient ultracentrifugation at 39,000 rpm for 2.5 h at 4°C (Beckman OptimaL‐90K, SW41‐Ti). The fractions containing polysome (larger than the 70S monosome) were collected and again centrifuged at 50,000 rpm for 3 h at 4°C (Beckmann OptimaTM TLX Ultracentrifuge, TLA100.3) to pellet down the polysome particles. The pellet was once rinsed by the polysome buffer and resolved by overnight‐standing within the polysome buffer supplemented with 5 mM CaCl2 at 4°C. Fifty µl of prepared polysome (A260 = ˜8.0) was treated with 300 unit of Micrococcal Nuclease (MNase, Roche) for 1 h at room temperature (24°C) with gentle mixing in the presence of 100 µg/ml of puromycin if indicated. Reaction mixture was mixed with 100 µl of polysome buffer and layered onto the top of 10–30% sucrose gradient containing the polysome buffer in Open Top Polyclear™ Centrifuge Tubes (14 × 89 mm, SETON) and centrifuged at 39,000 rpm for 3 h at 4°C (Beckman OptimaL‐90K, SW41‐Ti) to record A254 as described above.
Data analyses
Statistical analyses were conducted by using the software R (https://www.r‐project.org). Secondary structure of nascent polypeptide within the exit tunnel was predicted as by PSIPRED (http://bioinf.cs.ucl.ac.uk/psipred/).
Author contributions
YC and NS performed experiments; YC, NS, TN, YI, SI, and HT conceived the study, designed experiments, and analyzed the results; YC and HT supervised the entire project; YC and HT wrote the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Dataset EV1
Acknowledgements
We thank Koreaki Ito for critical reading and editing of the manuscript, Eri Uemura for technical support, the Bio‐support Center at Tokyo Tech for DNA sequencing, and The National BioResource Project, E. coli (National Institute of Genetics, Japan) for providing the ASKA library clones and Keio collection strains. This work was supported by MEXT Grants‐in‐Aid for Scientific Research (Grant Numbers JP26116002, JP18H03984, JP20H05925 to HT, JP17H05679, JP17H04998, JP19K22406, JP20H05784 to SI, JP17K15062, JP19K16038 to YC), and a grant from the Ohsumi Frontier Science Foundation to YC.
The EMBO Journal (2021) 40: e108299.
Contributor Information
Yuhei Chadani, Email: chadani.y.aa@m.titech.ac.jp.
Hideki Taguchi, Email: taguchi@bio.titech.ac.jp.
Data availability
Data related to this work are available in the Mendeley Data set public repository (https://doi.org/10.17632/ftc93j5cj2.1). Raw data for the ribosome profiling are available at NCBI's Gene Expression Omnibus (GEO) (accession number GSE184331; https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE184331).
References
- Allert M, Cox JC, Hellinga HW (2010) Multifactorial determinants of protein expression in prokaryotic open reading frames. J Mol Biol 402: 905–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan S, Hoffmann T, Seidelt B, Frauenfeld J, Mielke T, Berninghausen O, Wilson DN, Beckmann R (2011) SecM‐stalled ribosomes adopt an altered geometry at the Peptidyl Transferase Center. PLoS Biol 9: e1000581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G, Sabatini D (1971) Dissociation of mammalian polyribosomes into subunits by puromycin. Proc Natl Acad Sci USA 68: 390–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock LV, Blau C, Vaiana AC, Grubmüller H (2015) Dynamic contact network between ribosomal subunits enables rapid large‐scale rotation during spontaneous translocation. Nucleic Acids Res 43: 6747–6760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornemann T, Jöckel J, Rodnina MV, Wintermeyer W (2008) Signal sequence–independent membrane targeting of ribosomes containing short nascent peptides within the exit tunnel. Nat Struct Mol Biol 15: 494–499 [DOI] [PubMed] [Google Scholar]
- Buskirk AR, Green R (2017) Ribosome pausing, arrest and rescue in bacteria and eukaryotes. Phil Trans R Soc B 372: 20160183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadani Y, Niwa T, Chiba S, Taguchi H, Ito K (2016) Integrated in vivo and in vitro nascent chain profiling reveals widespread translational pausing. Proc Natl Acad Sci USA 113: E829–E838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadani Y, Niwa T, Izumi T, Sugata N, Nagao A, Suzuki T, Chiba S, Ito K, Taguchi H (2017) Intrinsic ribosome destabilization underlies translation and provides an organism with a strategy of environmental sensing. Mol Cell 68: 528–539.e5 [DOI] [PubMed] [Google Scholar]
- Chiba S, Lamsa A, Pogliano K (2009) A ribosome‐nascent chain sensor of membrane protein biogenesis in Bacillus subtilis . EMBO J 28: 3461–3475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao Duc K, Song YS (2018) The impact of ribosomal interference, codon usage, and exit tunnel interactions on translation elongation rate variation. PLoS Genet 14: e1007166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao Duc K, Batra SS, Bhattacharya N, Cate JHD, Song YS (2019) Differences in the path to exit the ribosome across the three domains of life. Nucleic Acids Res 47: 4198–4210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das G, Varshney U (2006) Peptidyl‐tRNA hydrolase and its critical role in protein biosynthesis. Microbiology 152: 2191–2195 [DOI] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL (2000) One‐step inactivation of chromosomal genes in Escherichia coli K‐12 using PCR products. Proc Natl Acad Sci USA 97: 6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dever TE, Ivanov IP, Sachs MS (2020) Conserved upstream open reading frame nascent peptides that control translation. Annu Rev Genet 54: 237–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrova LN, Kuroha K, Tatematsu T, Inada T (2009) Nascent peptide‐dependent translation arrest leads to Not4p‐mediated protein degradation by the proteasome. J Biol Chem 284: 10343–10352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duin J, Dieijen GV, Dieijen G, van Knippenberg PH, Bosch L (1970) Different species of 70S ribosomes of escherichia coli and their dissociation into subunits. Eur J Biochem 17: 433–440 [DOI] [PubMed] [Google Scholar]
- Fischer N, Neumann P, Konevega AL, Bock LV, Ficner R, Rodnina MV, Stark H (2015) Structure of the E. coli ribosome‐EF‐Tu complex at <3Å resolution by Cs‐corrected cryo‐EM. Nature 520: 567–570 [DOI] [PubMed] [Google Scholar]
- Gall AR, Datsenko KA, Figueroa‐Bossi N, Bossi L, Masuda I, Hou Y‐M, Csonka LN (2016) Mg2+ regulates transcription of mgtA in Salmonella Typhimurium via translation of proline codons during synthesis of the MgtL peptide. Proc Natl Acad Sci USA 113: 15096–15101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong F, Yanofsky C (2002) Instruction of translating ribosome by nascent peptide. Science 297: 1864–1867 [DOI] [PubMed] [Google Scholar]
- Goodman DB, Church GM, Kosuri S (2013) Causes and effects of N‐terminal codon bias in bacterial genes. Science 342: 475–479 [DOI] [PubMed] [Google Scholar]
- Guzman LM, Belin D, Carson MJ, Beckwith J (1995) Tight regulation, modulation, and high‐level expression by vectors containing the arabinose PBAD promoter. J Bacteriol 177: 4121–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P, Shichino Y, Schneider‐Poetsch T, Mito M, Hashimoto S, Udagawa T, Kohno K, Yoshida M, Mishima Y, Inada T et al (2020) Genome‐wide survey of ribosome collision. Cell Rep 31: 107610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heurgué‐Hamard V, Karimi R, Mora L, MacDougall J, Leboeuf C, Grentzmann G, Ehrenberg M, Buckingham RH (1998) Ribosome release factor RF4 and termination factor RF3 are involved in dissociation of peptidyl‐tRNA from the ribosome. EMBO J 17: 808–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan LH, Noller HF (2007) Intersubunit movement is required for ribosomal translocation. Proc Natl Acad Sci USA 104: 4881–4885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieong K‐W, Uzun Ü, Selmer M, Ehrenberg M (2016) Two proofreading steps amplify the accuracy of genetic code translation. Proc Natl Acad Sci USA 113: 13744–13749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada T (2017) The ribosome as a platform for mRNA and nascent polypeptide quality control. Trends Biochem Sci 42: 5–15 [DOI] [PubMed] [Google Scholar]
- Ingolia NT, Ghaemmaghami S, Newman JRS, Weissman JS (2009) Genome‐wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science 324: 218–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii E, Chiba S, Hashimoto N, Kojima S, Homma M, Ito K, Akiyama Y, Mori H (2015) Nascent chain‐monitored remodeling of the Sec machinery for salinity adaptation of marine bacteria. Proc Natl Acad Sci USA 112: E5513–E5522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Chiba S (2013) Arrest peptides: Cis‐acting modulators of translation. Annu Rev Biochem 82: 171–202 [DOI] [PubMed] [Google Scholar]
- Jenni S, Ban N (2003) The chemistry of protein synthesis and voyage through the ribosomal tunnel. Curr Opin Struc Biol 13: 212–219 [DOI] [PubMed] [Google Scholar]
- Keiler KC (2008) Biology of trans‐translation. Annu Rev . Microbiology 62: 133–151 [DOI] [PubMed] [Google Scholar]
- Kitagawa M, Ara T, Arifuzzaman M, Ioka‐Nakamichi T, Inamoto E, Toyonaga H, Mori H (2006) Complete set of ORF clones of Escherichia coli ASKA library (A Complete Set of E. coli K‐12 ORF Archive): unique resources for biological research. DNA Res 12: 291–299 [DOI] [PubMed] [Google Scholar]
- Kohler RE, Ron EZ, Davis BD (1968) Significance of the free 70 s ribosomes in Escherichia coli extracts. J Mol Biol 36: 71–82 [DOI] [PubMed] [Google Scholar]
- Kudla G, Murray AW, Tollervey D, Plotkin JB (2009) Coding‐sequence determinants of gene expression in Escherichia coli . Science 324: 255–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilleorg S, Reier K, Remme J, Liiv A (2017) The intersubunit bridge B1b of the bacterial ribosome facilitates initiation of protein synthesis and maintenance of translational fidelity. J Mol Biol 429: 1067–1080 [DOI] [PubMed] [Google Scholar]
- Lu J, Hua Z, Kobertz WR, Deutsch C (2011) Nascent peptide side chains induce rearrangements in distinct locations of the ribosomal tunnel. J Mol Biol 411: 499–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlincy NJ, Ingolia NT (2017) Transcriptome‐wide measurement of translation by ribosome profiling. Methods 126: 112–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JH (1972) Experiments in molecular genetics. New York, NY: Cold Spring Harbor Laboratory; [Google Scholar]
- Nakatogawa H, Ito K (2002) The ribosomal exit tunnel functions as a discriminating gate. Cell 108: 629–636 [DOI] [PubMed] [Google Scholar]
- Nissen P, Hansen J, Ban N, Moore PB, Steitz TA (2000) The structural basis of ribosome activity in peptide bond synthesis. Science 289: 920–930 [DOI] [PubMed] [Google Scholar]
- Noel JK, Whitford PC (2016) How EF‐Tu can contribute to efficient proofreading of aa‐tRNA by the ribosome. Nat Commun 7: 13314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi H, Shimizu Y, Ying B‐W, Ueda T (2007) Efficient protein selection based on ribosome display system with purified components. Biochem Biophys Res Commun 352: 270–276 [DOI] [PubMed] [Google Scholar]
- Onouchi H, Nagami Y, Haraguchi Y, Nakamoto M, Nishimura Y, Sakurai R, Nagao N, Kawasaki D, Kadokura Y, Naito S (2005) Nascent peptide‐mediated translation elongation arrest coupled with mRNA degradation in the CGS1 gene of Arabidopsis . Gene Dev 19: 1799–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterman IA, Chervontseva ZS, Evfratov SA, Sorokina AV, Rodin VA, Rubtsova MP, Komarova ES, Zatsepin TS, Kabilov MR, Bogdanov AA et al (2020) Translation at first sight: the influence of leading codons. Nucleic Acids Res 48: 6931–6942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S‐Y, Cromie MJ, Lee E‐J, Groisman EA (2010) A bacterial mRNA leader that employs different mechanisms to sense disparate intracellular signals. Cell 142: 737–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Po P, Delaney E, Gamper H, Szantai‐Kis DM, Speight L, Tu L, Kosolapov A, Petersson EJ, Hou Y‐M, Deutsch C (2017) Effect of nascent peptide steric bulk on elongation kinetics in the ribosome exit tunnel. J Mol Biol 429: 1873–1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requião RD, de Souza HJA, Rossetto S, Domitrovic T, Palhano FL (2016) Increased ribosome density associated to positively charged residues is evident in ribosome profiling experiments performed in the absence of translation inhibitors. RNA Biol 13: 561–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodnina MV, Wintermeyer W (2001) Fidelity of aminoacyl‐tRNA selection on the ribosome: kinetic and structural mechanisms. Annu Rev Biochem 70: 415–435 [DOI] [PubMed] [Google Scholar]
- Ron EZ, Kohler RE, Davis BD (1968) Magnesium ion dependence of free and polysomal ribosomes from Escherichia coli . J Mol Biol 36: 83–89 [DOI] [PubMed] [Google Scholar]
- Sabi R, Tuller T (2015) A comparative genomics study on the effect of individual amino acids on ribosome stalling. BMC Genom 16: S5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salis HM, Mirsky EA, Voigt CA (2009) Automated design of synthetic ribosome binding sites to control protein expression. Nat Biotechnol 27: 946–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samatova E, Daberger J, Liutkute M, Rodnina MV (2021) Translational control by ribosome pausing in bacteria: how a non‐uniform pace of translation affects protein production and folding. Front Microbiol 11: 619430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger D, Mangiarotti G, Apirion D (1967) The formation and stabilization of 30S and 50S ribosome couples in Escherichia coli . Proc Natl Acad Sci USA 58: 1782–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidelt B, Innis CA, Wilson DN, Gartmann M, Armache J‐P, Villa E, Trabuco LG, Becker T, Mielke T, Schulten K et al (2009) Structural insight into nascent polypeptide chain–mediated translational stalling. Science 326: 1412–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmuganathan V, Schiller N, Magoulopoulou A, Cheng J, Braunger K, Cymer F, Berninghausen O, Beatrix B, Kohno K, von Heijne G et al (2019) Structural and mutational analysis of the ribosome‐arresting human XBP1u. eLife 8: e46267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Kaushik S, Sinha M, Kushwaha GS, Singh A, Sikarwar J, Chaudhary A, Gupta A, Kaur P, Singh TP (2014) Structural and functional insights into peptidyl‐tRNA hydrolase. Biochim Biophys Acta 1844: 1279–1288 [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Inoue A, Tomari Y, Suzuki T, Yokogawa T, Nishikawa K, Ueda T (2001) Cell‐free translation reconstituted with purified components. Nat Biotechnol 19: 751–755 [DOI] [PubMed] [Google Scholar]
- Sohmen D, Chiba S, Shimokawa‐Chiba N, Innis CA, Berninghausen O, Beckmann R, Ito K, Wilson DN (2015) Structure of the Bacillus subtilis 70S ribosome reveals the basis for species‐specific stalling. Nat Commun 6: 6941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengart ML, Fuchs E, Porter AG (1996) The downstream box: an efficient and independent translation initiation signal in Escherichia coli . EMBO J 15: 665–674 [PMC free article] [PubMed] [Google Scholar]
- Su T, Cheng J, Sohmen D, Hedman R, Berninghausen O, von Heijne G, Wilson DN, Beckmann R (2017) The force‐sensing peptide VemP employs extreme compaction and secondary structure formation to induce ribosomal stalling. eLife 6: e25642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueta M, Wada C, Bessho Y, Maeda M, Wada A (2017) Ribosomal protein L31 in Escherichia coli contributes to ribosome subunit association and translation, whereas short L31 cleaved by protease 7 reduces both activities. Genes Cells 22: 452–471 [DOI] [PubMed] [Google Scholar]
- Verma M, Choi J, Cottrell KA, Lavagnino Z, Thomas EN, Pavlovic‐Djuranovic S, Szczesny P, Piston DW, Zaher HS, Puglisi JD et al (2019) A short translational ramp determines the efficiency of protein synthesis. Nat Commun 10: 5774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorhees RM, Ramakrishnan V (2013) Structural basis of the translational elongation cycle. Annu Rev Biochem 82: 203–236 [DOI] [PubMed] [Google Scholar]
- Voss NR, Gerstein M, Steitz TA, Moore PB (2006) The geometry of the ribosomal polypeptide exit tunnel. J Mol Biol 360: 893–906 [DOI] [PubMed] [Google Scholar]
- Wilson DN, Beckmann R (2011) The ribosomal tunnel as a functional environment for nascent polypeptide folding and translational stalling. Curr Opin Struct Biol 21: 274–282 [DOI] [PubMed] [Google Scholar]
- Woolstenhulme CJ, Guydosh NR, Green R, Buskirk AR (2015) High‐precision analysis of translational pausing by ribosome profiling in bacteria lacking EFP. Cell Rep 11: 13–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagitani K, Kimata Y, Kadokura H, Kohno K (2011) Translational pausing ensures membrane targeting and cytoplasmic splicing of XBP1u mRNA. Science 331: 586–589 [DOI] [PubMed] [Google Scholar]
- Zhao G, Kong W, Weatherspoon‐Griffin N, Clark‐Curtiss J, Shi Y (2011) Mg2+ facilitates leader peptide translation to induce riboswitch‐mediated transcription termination. EMBO J 30: 1485–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylber EA, Penman S (1970) The effect of high ionic strength on monomers, polyribosomes, and puromycin‐treated polyribosomes. Biochim Biophys Acta 204: 221–229 [DOI] [PubMed] [Google Scholar]


