Key Points
Question
Does administration of calcium during out-of-hospital cardiac arrest improve sustained return of spontaneous circulation?
Findings
In this randomized clinical trial that included 391 adults with out-of-hospital cardiac arrest, 19% had sustained return of spontaneous circulation after receiving treatment with intravenous or intraosseous calcium compared with 27% after receiving saline. This difference was not statistically significant, but the trial was terminated early due to concerns about harm in the calcium group.
Meaning
Treatment with intravenous or intraosseous calcium did not significantly improve sustained return of spontaneous circulation among adults with out-of-hospital cardiac arrest.
Abstract
Importance
It is unclear whether administration of calcium has a beneficial effect in patients with cardiac arrest.
Objective
To determine whether administration of calcium during out-of-hospital cardiac arrest improves return of spontaneous circulation in adults.
Design, Setting, and Participants
This double-blind, placebo-controlled randomized clinical trial included 397 adult patients with out-of-hospital cardiac arrest and was conducted in the Central Denmark Region between January 20, 2020, and April 15, 2021. The last 90-day follow-up was on July 15, 2021.
Interventions
The intervention consisted of up to 2 intravenous or intraosseous doses with 5 mmol of calcium chloride (n = 197) or saline (n = 200). The first dose was administered immediately after the first dose of epinephrine.
Main Outcomes and Measures
The primary outcome was sustained return of spontaneous circulation. The secondary outcomes included survival and a favorable neurological outcome (modified Rankin Scale score of 0-3) at 30 days and 90 days.
Results
Based on a planned interim analysis of 383 patients, the steering committee stopped the trial early due to concerns about harm in the calcium group. Of 397 adult patients randomized, 391 were included in the analyses (193 in the calcium group and 198 in the saline group; mean age, 68 [SD, 14] years; 114 [29%] were female). There was no loss to follow-up. There were 37 patients (19%) in the calcium group who had sustained return of spontaneous circulation compared with 53 patients (27%) in the saline group (risk ratio, 0.72 [95% CI, 0.49 to 1.03]; risk difference, −7.6% [95% CI, −16% to 0.8%]; P = .09). At 30 days, 10 patients (5.2%) in the calcium group and 18 patients (9.1%) in the saline group were alive (risk ratio, 0.57 [95% CI, 0.27 to 1.18]; risk difference, −3.9% [95% CI, −9.4% to 1.3%]; P = .17). A favorable neurological outcome at 30 days was observed in 7 patients (3.6%) in the calcium group and in 15 patients (7.6%) in the saline group (risk ratio, 0.48 [95% CI, 0.20 to 1.12]; risk difference, −4.0% [95% CI, −8.9% to 0.7%]; P = .12). Among the patients with calcium values measured who had return of spontaneous circulation, 26 (74%) in the calcium group and 1 (2%) in the saline group had hypercalcemia.
Conclusions and Relevance
Among adults with out-of-hospital cardiac arrest, treatment with intravenous or intraosseous calcium compared with saline did not significantly improve sustained return of spontaneous circulation. These results do not support the administration of calcium during out-of-hospital cardiac arrest in adults.
Trial Registration
ClinicalTrials.gov Identifier: NCT04153435
This placebo-controlled randomized clinical trial compares administration of calcium vs saline during out-of-hospital cardiac arrest for sustained return of spontaneous circulation in adults.
Introduction
In 2018, more than 5000 out-of-hospital cardiac arrests occurred in Denmark.1 Survival following out-of-hospital cardiac arrest is poor; only 16% of patients were still alive after 30 days based on data from 2018 for Denmark.1 Of those with a nonshockable rhythm, which accounts for approximately 80% of all cardiac arrests, less than 10% are alive after 30 days and, compared with those who had a shockable rhythm, survival has not improved substantially over the last decade.1 Pharmacological interventions for patients with cardiac arrest are limited and there is a need for evidence-based interventions to improve outcomes.2,3,4
Calcium plays an important role in cardiac muscle contraction and is generally acknowledged for its inotropic and vasopressor effects.5,6 These effects could be beneficial in the setting of cardiac arrest. Two small, randomized trials7,8 from 1985, including a total of 163 patients, found that administration of calcium did not result in a significant increase in return of spontaneous circulation for patients with out-of-hospital cardiac arrest and asystole or pulseless electrical activity. However, both trials7,8 had point estimates that favored calcium. Since then, to our knowledge, there have been no randomized clinical trials assessing the effect of administration of calcium during cardiac arrest. Observational studies with high risk of bias9,10 have found conflicting results.11,12,13,14 Although there are limited data to support the use of calcium during cardiac arrest, calcium is commonly administered during cardiac arrest in some settings.15,16
The Calcium for Out-of-Hospital Cardiac Arrest trial was designed to address the hypothesis that administration of calcium during out-of-hospital cardiac arrest would result in improved return of spontaneous circulation.
Methods
Trial Design and Oversight
This trial was an investigator-initiated, placebo-controlled, parallel group, double-blind, superiority, randomized clinical trial assessing administration of intravenous or intraosseous calcium during out-of-hospital cardiac arrest in adults. The trial protocol (Supplement 1) was written by the steering committee and was approved by the regional ethics committee and the Danish Medicines Agency. Discrepancies between the trial protocol and what is reported in this article appear in the eMethods in Supplement 2. Consent was temporarily obtained from a physician not involved in the trial. Oral and written consent were later obtained for all patients who survived. In accordance with Danish legislation, the patient consents were obtained after the patient regained capacity or when a surrogate became available (additional details appear in Supplement 1). An independent data and safety monitoring committee reviewed the trial data after inclusion of approximately 50, 200, and 400 patients. There were no predefined stopping criteria for harm, futility, or benefit.
Setting and Patients
The trial was conducted in the Central Denmark Region, which has approximately 1.3 million inhabitants. The 2-tiered emergency medical services system responds to all cardiac arrests with an ambulance and a physician-manned mobile emergency care unit.17 Almost all patients with return of spontaneous circulation or ongoing cardiopulmonary resuscitation during transfer are transported to a single university hospital capable of coronary catheterization and percutaneous coronary intervention, extracorporeal cardiopulmonary resuscitation, and care after cardiac arrest, including targeted temperature management. Treatment both during and after cardiac arrest generally adheres to European guidelines.18
Adult patients (aged ≥18 years) were eligible for the trial if they had an out-of-hospital cardiac arrest and received at least 1 dose of epinephrine during the cardiac arrest. The exclusion criteria were traumatic cardiac arrest (including strangulation and foreign body asphyxia), known or strongly suspected pregnancy, prior enrollment in the trial, receipt of epinephrine outside the trial (from a unit not participating in the trial), or a clinical indication (eg, suspected hypocalcemia or hyperkalemia) for calcium administration during the cardiac arrest.
Randomization
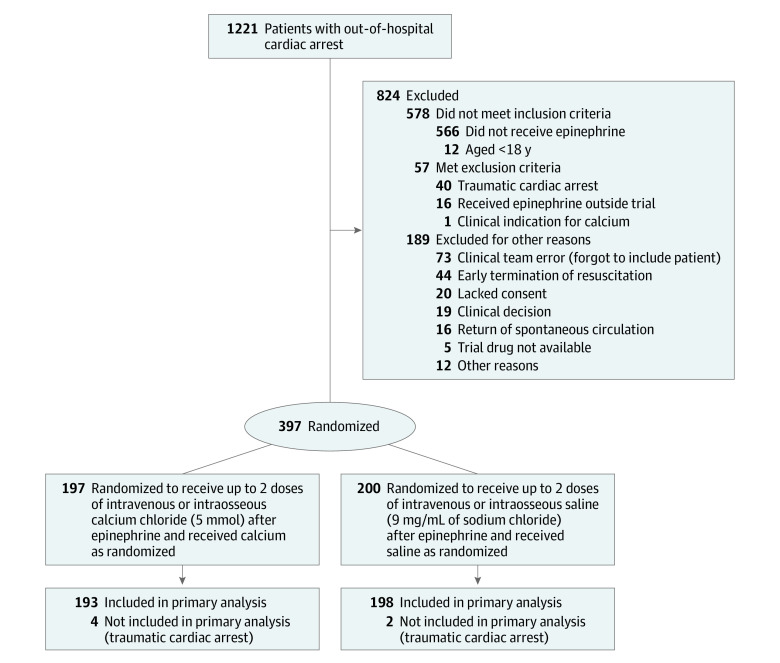
Patients were randomized in a 1:1 ratio to either calcium or saline in block sizes of 2, 4, or 6 (Figure 1). The randomization was generated using a random-number generator and stratified according to mobile emergency care unit stations.
Figure 1. Screening and Randomization of Patients in the Calcium for Out-of-Hospital Cardiac Arrest Trial.
Intervention
The trial drug consisted of 5 mmol of calcium chloride (corresponding to 200 mg of calcium or 735 mg of calcium chloride dihydrate) or 9 mg/mL of sodium chloride (saline control). The intravenous or intraosseous administration of the trial drug was performed immediately after the first dose of epinephrine. A second dose of the trial drug was administered after the second dose of epinephrine if the patient remained in cardiac arrest. The trial drug was administered as a rapid bolus.
The trial was double-blind with patients, investigators, and the clinical team being unaware of the allocated treatment.
Outcomes
The primary outcome was sustained return of spontaneous circulation, which was defined as spontaneous circulation with no further need for chest compressions for at least 20 minutes. Data also were collected on any return of spontaneous circulation and return of spontaneous circulation at hospital arrival.
The key secondary outcomes included survival at 30 days and survival at 30 days with a favorable neurological outcome, which was defined as a score of 0 to 3 on the modified Rankin Scale. Higher scores indicate worse outcomes on the 7-point modified Rankin Scale.19 Additional outcomes described below were considered tertiary.
At 30 days, health-related quality of life was assessed using the 5-dimensional, 5-level EuroQol score as a numeric value directly assessed by the patient and as an index value (based on Danish data20,21). The numeric value is reported on a scale from 0 to 100 with higher scores indicating a better health-related quality of life. The index value can be negative. Outcomes were assessed in person if the patient was still an inpatient at the hospital or by telephone interview if the patient had been discharged. If the patient was not able to participate, relatives of the patient or clinical personnel provided responses for the assessment. Similar outcomes were assessed at 90 days, 180 days, and 1 year. Results for 30-day and 90-day follow-up are provided in this article.
The Sequential Organ Failure Assessment score was collected at 2, 24, 48, and 72 hours after the cardiac arrest. Data were collected on vasopressor-free and ventilator-free days within the first 7 days. Predefined potential adverse events were collected. A full list of adverse events and definitions appears in the trial protocol (Supplement 1).
Sample Size Calculation
The sample size was based on the primary outcome of sustained return of spontaneous circulation. The original sample size (n = 430) was updated based on blinded review of event data after 270 patients were enrolled in the trial (additional details appear in Supplement 1). Based on this, it was assumed that 27% of patients in the calcium group and 18% in the saline group would achieve return of spontaneous circulation. With these estimates, an α level of .05, and the use of the χ2 test, a total of 674 patients were required to have 80% power to detect a statistically significant between-group difference.
Statistical Analysis
Patients were analyzed according to their randomized assignment. The analyses only included patients receiving the first dose of the trial drug and meeting all inclusion criteria and no exclusion criteria.22
Binary data are presented as counts and percentages and between-group differences are presented as both risk differences and risk ratios with 95% CIs. The 95% CIs were estimated using the method described by Miettinen and Nurmimen.23 Two-sided P values (obtained from the Fisher exact test) are reported for the primary outcome and only for key secondary outcomes. P<.05 was considered statistically significant. As a sensitivity analysis, the risk ratio for the primary outcome was estimated while adjusting for the stratification variable and strong prognostic factors (specifically age, whether the cardiac arrest was witnessed, whether bystander cardiopulmonary resuscitation was initiated, and the initial rhythm) were used as covariates.24 Log-binomial regression was used for this analysis. Continuous data are presented as means with SDs or medians with IQRs depending on the distribution of the data. Between-group differences for the continuous outcomes are presented as mean differences with 95% CIs obtained from a generalized linear model with robust errors.
Five predefined subgroup analyses were performed according to the initial rhythm, the timing of the drug administration, intravenous vs intraosseous administration, whether the cardiac arrest was witnessed, and whether bystander cardiopulmonary resuscitation was performed. Because the trial was not powered to detect subgroup differences, these analyses should be considered as exploratory and hypothesis-generating.
Bayesian analyses were conducted to supplement the primary frequentist analyses. Priors were specified to reflect a range of beliefs (the expected treatment effect expressed as a risk ratio) for the included outcomes. The priors included noninformative, skeptical (no effect), optimistic (beneficial effect), and pessimistic (harmful effect) beliefs.25 The strength of each informative belief (the variance of the expected treatment effect) was characterized as strong, moderate, or weak, allowing for harm or benefit of 5%, 15%, and 30%, respectively. All priors were prespecified using a standardized approach and assumed a normal distribution on a log-risk scale.25 Posterior probabilities were estimated using Markov chain Monte Carlo methods with 1 chain, 10 000 burn-ins, 1 000 000 iterations, and a thinning rate of 100 to reduce sample autocorrelation. The results are reported graphically and as mean risk ratios with equal-tailed 95% credible intervals and as the posterior probability of significant harm (risk ratio <1.0, <0.8, and <0.5) or benefit (risk ratio >1.0, >1.2, and >1.5).
All analyses were performed using SAS version 9.4 (SAS Institute Inc).
Results
Patient Characteristics
On April 15, 2021, the independent data and safety monitoring committee recommended that the trial be stopped due to a signal of harm in the calcium group (eTable 1 in Supplement 2). This was based on unblinded data from 383 patients included in the trial between January 20, 2020, and April 6, 2021. Based on this recommendation, the steering committee immediately stopped the trial.
From January 20, 2020, to April 15, 2021, a total of 1221 patients had an out-of-hospital cardiac arrest in the Central Denmark Region (Figure 1). Of these, 397 patients received the trial drug. Six patients with a traumatic cardiac arrest (an exclusion criterion) inadvertently received the trial drug and were excluded from the analyses, leaving 193 patients in the calcium group and 198 patients in the saline group. There was no loss to follow-up. The last 90-day follow-up was on July 15, 2021.
Baseline characteristics were similar in the 2 groups (Table 1 and eTable 2 in Supplement 2). The mean age was 68 years (SD, 14 years) and 114 (29%) were female. Most patients had the cardiac arrest at home (82%) and had an initial nonshockable rhythm (75%). There were data on fraction and frequency of chest compression for 65% of the patients and both were comparable between the groups (eFigures 1-2 in Supplement 2).
Table 1. Baseline Characteristics of Patients.
| Calcium (n = 193) |
Saline (n = 198) |
|
|---|---|---|
| Age, mean (SD), y | 67 (14) | 69 (14) |
| Sex, No. (%) | ||
| Male | 131 (68) | 146 (74) |
| Female | 62 (32) | 52 (26) |
| Medical history, No. (%) | ||
| Arterial hypertension | 76 (39) | 90 (45) |
| Pulmonary disease | 49 (25) | 56 (28) |
| Coronary artery disease | 46 (24) | 46 (23) |
| Diabetes | 38 (20) | 43 (22) |
| Kidney disease | 35 (18) | 44 (22) |
| Atrial fibrillation | 33 (17) | 50 (25) |
| Chronic heart failure | 32 (17) | 36 (18) |
| Stroke | 20 (10) | 24 (12) |
| Venous thromboembolism | 9 (5) | 10 (5) |
| Dementia | 7 (4) | 5 (3) |
| Cancer | 5 (3) | 9 (5) |
| Liver disease | 3 (2) | 5 (3) |
| Cardiac arrest characteristics | ||
| Location, No. (%) | ||
| Home | 160 (83) | 159 (80) |
| Public area | 33 (17) | 39 (20) |
| Witnessed status, No. (%) | ||
| Bystander | 101 (52) | 99 (50) |
| Emergency medical services | 16 (8) | 13 (7) |
| Not witnessed | 76 (39) | 86 (43) |
| Bystander response, No./total (%)a | ||
| Cardiopulmonary resuscitation | 146/177 (82) | 164/185 (89) |
| Automated external defibrillator shock | 14/177 (8) | 13/185 (7) |
| Initial manual rhythm analysis by emergency medical services, No. (%) | ||
| Asystole | 103 (53) | 96 (48) |
| Pulseless electrical activity | 47 (24) | 49 (25) |
| Ventricular fibrillation | 39 (20) | 49 (25) |
| Ventricular tachycardia | 4 (2) | 4 (2) |
| Administration and drug characteristics | ||
| Intravenous administration, No. (%) | 78 (40) | 79 (40) |
| Intraosseous administration, No. (%) | 115 (60) | 119 (60) |
| Tibial | 103 (90) | 103 (87) |
| Humeral | 12 (10) | 16 (13) |
| Time to administration, median (IQR), min | ||
| Epinephrine | 17 (12-22) | 17 (14-22) |
| Trial drug | 17 (13-23) | 18 (15-23) |
| No. of trial drug doses | ||
| 1 | 53 (27) | 53 (27) |
| 2 | 140 (73) | 145 (73) |
Not witnessed by emergency medical services (n = 362).
The median time from the cardiac arrest to administration of the trial drug was 18 minutes (IQR, 14- 23 minutes). The trial drug was most commonly administered through intraosseous access (60%) and 73% of patients received both doses of the trial drug. The only protocol deviations recorded (the second dose of the trial drug was not administered despite the patient still being in cardiac arrest) were in 9 patients (4.7%) in the calcium group and 9 patients (4.5%) in the saline group. Calcium chloride was administered outside the trial protocol to 4 patients (2.1%) in the calcium group and 2 patients (1.0%) in the saline group. Additional details on intracardiac arrest interventions appear in eTable 3 in Supplement 2. Details on interventions used after cardiac arrest appear in eTable 4 in Supplement 2.
Primary Outcome
The primary outcome of sustained return of spontaneous circulation occurred in 37 patients (19%) in the calcium group and 53 patients (27%) in the saline group (risk ratio, 0.72 [95% CI, 0.49-1.03], P = .09; Table 2). The results for any return of spontaneous circulation and return of spontaneous circulation at hospital arrival were similar (eTable 5 in Supplement 2). The results were attenuated in the adjusted analysis (risk ratio, 0.81 [95% CI, 0.56-1.17]). The results were generally consistent across predefined subgroups (Figure 2).
Table 2. Primary and Secondary Outcomes.
| Calcium (n = 193) |
Saline (n = 198) |
Risk ratio (95% CI) | Difference, % (95% CI)a |
P valueb | |
|---|---|---|---|---|---|
| Primary outcome | |||||
| Sustained return of spontaneous circulation | 37 (19) | 53 (27) | 0.72 (0.49 to 1.03) | −7.6 (−16 to 0.8) | .09 |
| Secondary outcomes | |||||
| Survival at 30 d | 10 (5.2) | 18 (9.1) | 0.57 (0.27 to 1.18) | −3.9 (−9.4 to 1.3) | .17 |
| Survival at 30 d with a favorable neurological outcomec | 7 (3.6) | 15 (7.6) | 0.48 (0.20 to 1.12) | −4.0 (−8.9 to 0.7) | .12 |
| 5-dimensional, 5-level EuroQol score at 30 d, mean (SD) | |||||
| Assessed by the patientd | 58 (25) | 66 (12) | −8 (−24 to 7) | ||
| Index valuee | 52 (23) | 62 (30) | −10 (−29 to 9) | ||
| Survival at 90 d | 10 (5.2) | 18 (9.1) | 0.57 (0.27 to 1.18) | −3.9 (−9.4 to 1.3) | |
| Survival at 90 d with a favorable neurological outcomec | 7 (3.6) | 18 (9.1) | 0.40 (0.17 to 0.91) | −5.5 (−11 to −0.7) | |
| 5-dimensional, 5-level EuroQol score at 90 d, mean (SD) | |||||
| Assessed by the patientd | 62 (33) | 79 (14) | −17 (−37 to 4) | ||
| Index valuee | 59 (35) | 85 (11) | −26 (−47 to −5) | ||
Risk difference for binary outcomes and mean difference for continuous outcomes.
Obtained from the Fisher exact test and are only provided for the primary and key secondary outcomes per the trial protocol.
Modified Rankin Scale score of 0 to 3 (7-point scale); higher scores indicate worse outcomes.
Reported on a scale from 0 to 100; higher scores indicate better health-related quality of life.
Indexed based on Danish data.21 Can be a negative value.
Figure 2. Subgroup Results for the Primary Outcome of Sustained Return of Spontaneous Circulation.
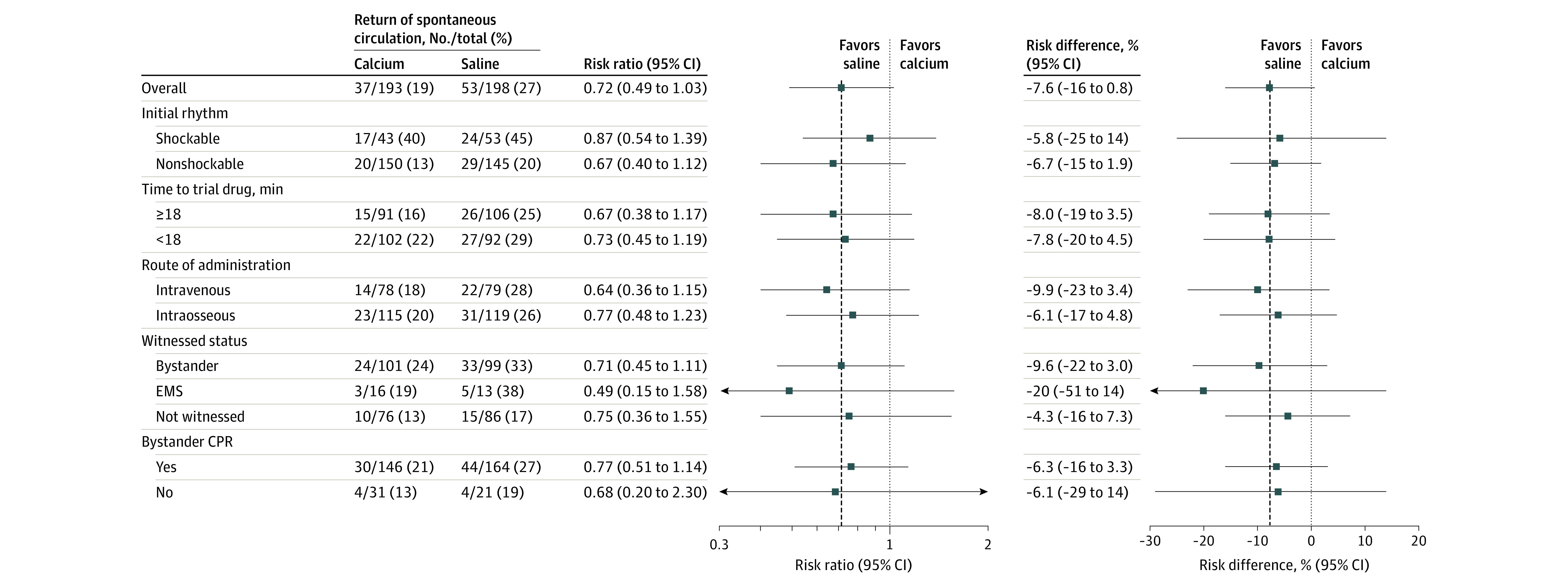
Results are presented for the 5 predefined subgroups. The time from cardiac arrest to trial drug administration was dichotomized at the median. Only cardiac arrests not witnessed by emergency medical services (EMS) were included in the bystander cardiopulmonary resuscitation (CPR) subgroup. The vertical dashed lines represent the estimated effect in the primary outcome analysis. The vertical dotted lines represent no difference between the calcium and saline groups.
Secondary Outcomes
Survival at 30 days occurred in 10 patients (5.2%) in the calcium group and 18 patients (9.1%) in the saline group (risk ratio, 0.57 [95% CI, 0.27-1.18], P = .17; Table 2). Survival at 30 days with a favorable neurological outcome occurred in 7 patients (3.6%) in the calcium group and 15 patients (7.6%) in the saline group (risk ratio, 0.48 [95% CI, 0.20-1.12], P = .12; Table 2). The results were generally consistent across predefined subgroups (eFigures 3-4 in Supplement 2).
Tertiary Outcomes
Survival at 90 days was identical to 30-day survival (Table 2). The Kaplan-Meier curve for 90-day survival appears in eFigure 5 in Supplement 2. Survival at 90 days with a favorable neurological outcome occurred in 7 patients (3.6%) in the calcium group and 18 patients (9.1%) in the saline group (risk ratio, 0.40 [95% CI, 0.17-0.91]). Quality-of-life scores in survivors were lower in the calcium group, although the 95% CIs were wide (Table 2).
The first ionized calcium level after return of spontaneous circulation was higher in the calcium group (1.41 mmol/L [SD, 0.15 mmol/L]) compared with the saline group (1.17 mmol/L [SD, 0.07 mmol/L]) and the mean between-group difference was 0.23 mmol/L (95% CI, 0.18-0.28 mmol/L), and remained higher for approximately 12 hours (eFigure 6 in Supplement 2). The first collected potassium level, pH level, and lactate level after return of spontaneous circulation appear in eTable 6 in Supplement 2. In addition, data on organ dysfunction after return of spontaneous circulation (assessed by the Sequential Organ Failure Assessment score and vasopressor-free and ventilator-free days) appear in eTable 6 in Supplement 2. Additional details on outcomes appear in eTables 7 through 9 in Supplement 2.
Adverse Events
Among patients with calcium values measured who had return of spontaneous circulation, 26 patients (74%) in the calcium group and 1 patient (2%) in the saline group had hypercalcemia. Additional adverse events appear in eTable 10 in Supplement 2.
Bayesian Analysis
The posterior probability distribution for return of spontaneous circulation, survival at 30 days, and survival at 30 days with a favorable neurological outcome based on noninformative priors appear in Figure 3. The probability that calcium has a beneficial effect (ie, a risk ratio >1.0) based on the data is 4% for return of spontaneous circulation, 6% for survival at 30 days, and 4% for survival with a favorable neurological outcome at 30 days. The corresponding probabilities for a risk ratio greater than 1.2 were 0%, 2%, and 1%. Additional results, including for all the informative priors, appear in eTables 11 through 13 and eFigures 7 through 9 in Supplement 2.
Figure 3. Posterior Probability Distributions Based on Noninformative Priors.
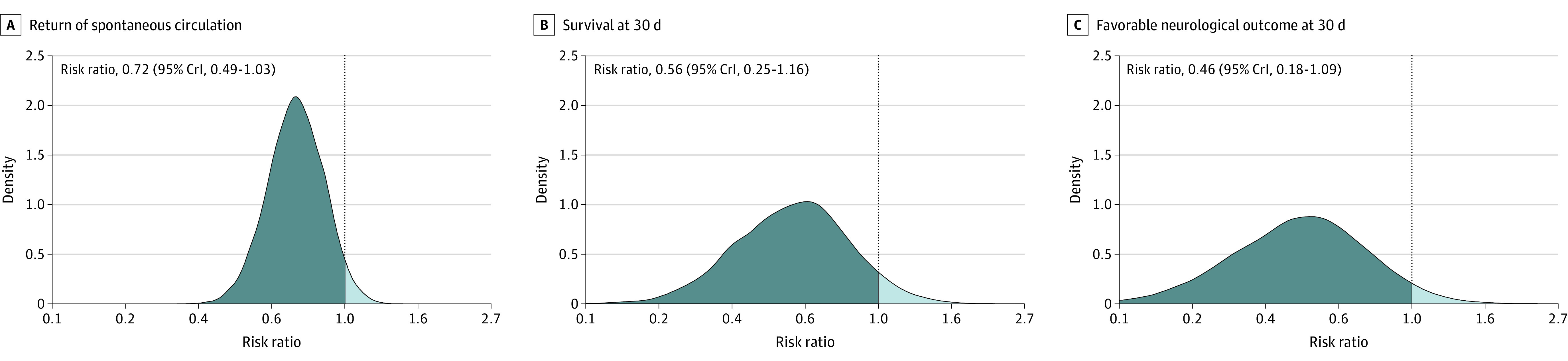
The results from the bayesian analyses are presented as posterior probability distributions based on noninformative priors. The x-axis is logarithmic. The vertical dotted lines represent no effect (ie, a risk ratio of 1). The dark blue shaded areas represent values below 1 (ie, a harmful effect of calcium) and the light blue shaded areas represent values above 1 (ie, a beneficial effect of calcium). CrI indicates credible interval. Additional results from the bayesian analysis appear in Supplement 2.
Discussion
In this randomized clinical trial, the administration of calcium, compared with saline, did not result in a statistically significant difference in sustained return of spontaneous circulation for patients with out-of-hospital cardiac arrest. In addition, there were no statistically significant differences in 30-day survival or 30-day survival with a favorable neurological outcome. Although not reaching statistical significance, patients receiving calcium had worse outcomes, including worse 30-day survival with a favorable neurological outcome. At 90 days, fewer patients in the calcium group had a favorable neurological outcome and quality of life was lower in survivors.
Given that the trial was stopped early, the results should be interpreted carefully. Trials that are stopped early based on knowledge of the accruing results tend to overestimate the effects.26 Furthermore, given the widths of the 95% CIs, it is possible that the point estimates suggesting harm are chance findings. In the adjusted analysis for the primary outcome, the effect estimate still suggested harm, but the size of the effect was attenuated. Supporting a true harmful effect of calcium administration during cardiac arrest is the consistent signal across multiple outcomes and time points.
The rationale for the current trial was the well-established inotropic effect of administered calcium, calcium’s role in maintaining vascular tone, and a nonsignificant increase in return of spontaneous circulation found in 2 previous small trials.7,8,27,28,29 Although contrary to the original hypothesis, there are theoretical mechanisms that could potentially explain a harmful effect of calcium during cardiac arrest. Due to adenosine triphosphate depletion during ischemia, sodium accumulates intracellularly, reducing the transmembrane sodium gradient and causing the sodium-calcium exchanger to operate in reverse mode.30,31 High levels of calcium immediately after administration of calcium may have caused cytosolic and mitochondrial calcium overload during the cardiac arrest. This may have caused cardiac hypercontraction, a phenomenon termed stone heart.30,32 In addition, because calcium is involved in multiple intracellular signaling pathways, cytosolic and mitochondrial calcium overload could have promoted oxidative stress, release of proapoptotic factors, and activation of calcium-dependent lipases, proteases, and nucleases.33,34
European and US cardiac arrest guidelines suggest that calcium should only be administered during cardiac arrest in special circumstances, such as during cardiac arrest caused by hyperkalemia or hypocalcemia or during an overdose of calcium channel blockers.2,35 Although limited data have been published on the actual use of calcium in the out-of-hospital cardiac arrest setting, calcium is often administered during in-hospital cardiac arrest.15,16 In a large, multicenter, US registry of in-hospital cardiac arrest, calcium was administered in approximately 25% to 30% of adult patients and 30% to 50% of pediatric patients, corresponding to approximately 90 000 patients receiving calcium during in-hospital cardiac arrest each year in the US alone.15,16,36 The rationale for administration of calcium in this setting is unclear but could reflect either a perceived etiology of the cardiac arrest in which calcium is currently recommended (eg, hyperkalemia) or based on a hypothesis that calcium would be beneficial in unselected patients with cardiac arrest. The findings from this trial suggest that the administration of calcium to an unselected cardiac arrest population is unlikely to result in improved outcomes and may in fact result in worse outcomes.
This trial has several strengths. Administration of the trial drug was blinded, delivered quickly after the administration of epinephrine, and there were few protocol deviations or use of calcium outside the protocol. The administration of calcium resulted in a clinically relevant increase in ionized calcium values at hospital arrival. The trial included patient-relevant outcomes, including quality of life, and there was no loss to follow-up.
Limitations
The trial also has several limitations. First, the trial was stopped early and did not reach its preplanned sample size. Even though continuing the trial would have resulted in more precise estimates of the treatment effect, it was not considered ethically justified to continue after the results of the interim analysis were evident. This decision was consistent with the recommendations from the independent data and safety monitoring committee.
Second, the trial only tested 1 dosing regime and timing and the trial results cannot necessarily be extrapolated to other doses or a different timing interval.
Third, the current trial was conducted in the out-of-hospital setting with a relatively long time to drug delivery. The generalizability to the in-hospital setting is therefore unclear.
Conclusions
Among adults with out-of-hospital cardiac arrest, treatment with intravenous or intraosseous calcium compared with saline did not significantly improve sustained return of spontaneous circulation. These results do not support the administration of calcium during out-of-hospital cardiac arrest in adults.
Trial protocol
eMethods
eTable 1. Results from the third independent data monitoring committee analysis
eTable 2. Additional baseline characteristics according to treatment assignment
eTable 3. Intra-cardiac arrest interventions
eTable 4. Post-cardiac arrest characteristics in those surviving at least 24 hours
eTable 5. Additional data on return of spontaneous circulation
eTable 6. Organ dysfunction and laboratory values after return of spontaneous circulation
eTable 7. Hospital disposition and cause of death
eTable 8. Neurological outcomes
eTable 9. EQ-5D-5L subcategories
eTable 10. Potential adverse events
eTable 11. Posterior estimates from Bayesian analyses
eTable 12. Posterior probabilities of benefit
eTable 13. Posterior probabilities of harm
eFigure 1. Chest compression fraction according to treatment assignment
eFigure 2. Chest compression frequency according to treatment assignment
eFigure 3. Subgroup results for 30-day survival
eFigure 4. Subgroup results for 30-day favorable neurological outcome
eFigure 5. Kaplan-Meier curve for 90-day survival according to treatment assignment
eFigure 6. Ionized calcium values in patients with return of spontaneous circulation
eFigure 7. Posterior probability distributions for return of spontaneous circulation
eFigure 8. Posterior probability distributions for survival at 30 days
eFigure 9. Posterior probability distributions for favorable neurological outcome at 30 days
Data sharing statement
References
- 1.Ringgren KB, Christensen HC, Schønau L, et al. Rapport for Dansk Hjertestopregister; 2018. Published in Danish. Accessed September 16, 2020. https://hjertestopregister.dk/wp-content/uploads/2019/11/Dansk-Hjertestopregister-2018-2.pdf
- 2.Soar J, Nolan JP, Böttiger BW, et al. ; Adult Advanced Life Support Section Collaborators . European Resuscitation Council guidelines for resuscitation 2015: section 3: adult advanced life support. Resuscitation. 2015;95:100-147. doi: 10.1016/j.resuscitation.2015.07.016 [DOI] [PubMed] [Google Scholar]
- 3.Holmberg MJ, Issa MS, Moskowitz A, et al. ; International Liaison Committee on Resuscitation Advanced Life Support Task Force Collaborators . Vasopressors during adult cardiac arrest: a systematic review and meta-analysis. Resuscitation. 2019;139:106-121. doi: 10.1016/j.resuscitation.2019.04.008 [DOI] [PubMed] [Google Scholar]
- 4.Ali MU, Fitzpatrick-Lewis D, Kenny M, et al. Effectiveness of antiarrhythmic drugs for shockable cardiac arrest: a systematic review. Resuscitation. 2018;132:63-72. doi: 10.1016/j.resuscitation.2018.08.025 [DOI] [PubMed] [Google Scholar]
- 5.Youngquist ST, Heyming T, Rosborough JP, Niemann JT. Hypocalcemia following resuscitation from cardiac arrest revisited. Resuscitation. 2010;81(1):117-122. doi: 10.1016/j.resuscitation.2009.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindqwister AL, Lampe JW, Gould JR, Kaufman CL, Moodie KL, Paradis NA. Intravenous calcium as a pressor in a swine model of hypoxic pseudo-pulseless electrical mechanical activity—a preliminary report. Intensive Care Med Exp. 2020;8(1):50. doi: 10.1186/s40635-020-00340-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stueven HA, Thompson B, Aprahamian C, Tonsfeldt DJ, Kastenson EH. The effectiveness of calcium chloride in refractory electromechanical dissociation. Ann Emerg Med. 1985;14(7):626-629. doi: 10.1016/S0196-0644(85)80874-X [DOI] [PubMed] [Google Scholar]
- 8.Stueven HA, Thompson B, Aprahamian C, Tonsfeldt DJ, Kastenson EH. Lack of effectiveness of calcium chloride in refractory asystole. Ann Emerg Med. 1985;14(7):630-632. doi: 10.1016/S0196-0644(85)80875-1 [DOI] [PubMed] [Google Scholar]
- 9.Andersen LW, Grossestreuer AV, Donnino MW. “Resuscitation time bias”—a unique challenge for observational cardiac arrest research. Resuscitation. 2018;125:79-82. doi: 10.1016/j.resuscitation.2018.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kette F, Ghuman J, Parr M. Calcium administration during cardiac arrest: a systematic review. Eur J Emerg Med. 2013;20(2):72-78. doi: 10.1097/MEJ.0b013e328358e336 [DOI] [PubMed] [Google Scholar]
- 11.Urban P, Scheidegger D, Buchmann B, Barth D. Cardiac arrest and blood ionized calcium levels. Ann Intern Med. 1988;109(2):110-113. doi: 10.7326/0003-4819-109-2-110 [DOI] [PubMed] [Google Scholar]
- 12.Gando S, Tedo I, Tujinaga H, Kubota M. Variation in serum ionized calcium on cardiopulmonary resuscitation. J Anesth. 1988;2(2):154-160. doi: 10.1007/s0054080020154 [DOI] [PubMed] [Google Scholar]
- 13.Stiell IG, Wells GA, Hebert PC, Laupacis A, Weitzman BN. Association of drug therapy with survival in cardiac arrest: limited role of advanced cardiac life support drugs. Acad Emerg Med. 1995;2(4):264-273. doi: 10.1111/j.1553-2712.1995.tb03220.x [DOI] [PubMed] [Google Scholar]
- 14.van Walraven C, Stiell IG, Wells GA, Hébert PC, Vandemheen K; OTAC Study Group . Do advanced cardiac life support drugs increase resuscitation rates from in-hospital cardiac arrest? Ann Emerg Med. 1998;32(5):544-553. doi: 10.1016/S0196-0644(98)70031-9 [DOI] [PubMed] [Google Scholar]
- 15.Moskowitz A, Ross CE, Andersen LW, Grossestreuer AV, Berg KM, Donnino MW; American Heart Association’s Get With The Guidelines–Resuscitation Investigators . Trends over time in drug administration during adult in-hospital cardiac arrest. Crit Care Med. 2019;47(2):194-200. doi: 10.1097/CCM.0000000000003506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross CE, Moskowitz A, Grossestreuer AV, et al. ; American Heart Association’s Get With The Guidelines–Resuscitation Investigators . Trends over time in drug administration during pediatric in-hospital cardiac arrest in the United States. Resuscitation. 2021;158:243-252. doi: 10.1016/j.resuscitation.2020.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindskou TA, Mikkelsen S, Christensen EF, et al. The Danish prehospital emergency healthcare system and research possibilities. Scand J Trauma Resusc Emerg Med. 2019;27(1):100. doi: 10.1186/s13049-019-0676-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soar J, Böttiger BW, Carli P, et al. European Resuscitation Council Guidelines 2021: adult advanced life support. Resuscitation. 2021;161:115-151. doi: 10.1016/j.resuscitation.2021.02.010 [DOI] [PubMed] [Google Scholar]
- 19.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604-607. doi: 10.1161/01.STR.19.5.604 [DOI] [PubMed] [Google Scholar]
- 20.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727-1736. doi: 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen CE, Sørensen SS, Gudex C, Jensen MB, Pedersen KM, Ehlers LH. The Danish EQ-5D-5L value set: a hybrid model using cTTO and DCE data. Appl Health Econ Health Policy. 2021;19(4):579-591. doi: 10.1007/s40258-021-00639-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fergusson D, Aaron SD, Guyatt G, Hébert P. Post-randomisation exclusions: the intention to treat principle and excluding patients from analysis. BMJ. 2002;325(7365):652-654. doi: 10.1136/bmj.325.7365.652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4(2):213-226. doi: 10.1002/sim.4780040211 [DOI] [PubMed] [Google Scholar]
- 24.Kahan BC. Accounting for centre-effects in multicentre trials with a binary outcome—when, why, and how? BMC Med Res Methodol. 2014;14:20. doi: 10.1186/1471-2288-14-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zampieri FG, Casey JD, Shankar-Hari M, Harrell FE Jr, Harhay MO. Using bayesian methods to augment the interpretation of critical care trials: an overview of theory and example reanalysis of the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial. Am J Respir Crit Care Med. 2021;203(5):543-552. doi: 10.1164/rccm.202006-2381CP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bassler D, Briel M, Montori VM, et al. ; STOPIT-2 Study Group . Stopping randomized trials early for benefit and estimation of treatment effects: systematic review and meta-regression analysis. JAMA. 2010;303(12):1180-1187. doi: 10.1001/jama.2010.310 [DOI] [PubMed] [Google Scholar]
- 27.Kass DA, Maughan WL, Guo ZM, Kono A, Sunagawa K, Sagawa K. Comparative influence of load versus inotropic states on indexes of ventricular contractility: experimental and theoretical analysis based on pressure-volume relationships. Circulation. 1987;76(6):1422-1436. doi: 10.1161/01.CIR.76.6.1422 [DOI] [PubMed] [Google Scholar]
- 28.Fabiato A, Fabiato F. Calcium and cardiac excitation-contraction coupling. Annu Rev Physiol. 1979;41:473-484. doi: 10.1146/annurev.ph.41.030179.002353 [DOI] [PubMed] [Google Scholar]
- 29.Jankowski S, Vincent JL. Calcium administration for cardiovascular support in critically ill patients: when is it indicated? J Intensive Care Med. 1995;10(2):91-100. doi: 10.1177/088506669501000205 [DOI] [PubMed] [Google Scholar]
- 30.Piper HM, Meuter K, Schäfer C. Cellular mechanisms of ischemia-reperfusion injury. Ann Thorac Surg. 2003;75(2):S644-S648. doi: 10.1016/S0003-4975(02)04686-6 [DOI] [PubMed] [Google Scholar]
- 31.Allen DG, Cairns SP, Turvey SE, Lee JA. Intracellular calcium and myocardial function during ischemia. In: Sideman S, Beyar R, eds. Interactive Phenomena in the Cardiac System. Springer US; 1993:19-29. doi: 10.1007/978-1-4615-2946-0_3 [DOI] [PubMed] [Google Scholar]
- 32.Lomivorotov VV, Leonova EA, Belletti A, Shmyrev VA, Landoni G. Calcium administration during weaning from cardiopulmonary bypass: a narrative literature review. J Cardiothorac Vasc Anesth. 2020;34(1):235-244. doi: 10.1053/j.jvca.2019.06.016 [DOI] [PubMed] [Google Scholar]
- 33.Clapham DE. Calcium signaling. Cell. 2007;131(6):1047-1058. doi: 10.1016/j.cell.2007.11.028 [DOI] [PubMed] [Google Scholar]
- 34.Cerella C, Diederich M, Ghibelli L. The dual role of calcium as messenger and stressor in cell damage, death, and survival. Int J Cell Biol. 2010;2010:546163. doi: 10.1155/2010/546163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panchal AR, Bartos JA, Cabanas JG, et al. Part 3: adult basic and advanced life support: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2020;142(16 suppl 2):S366-S468. [DOI] [PubMed] [Google Scholar]
- 36.Holmberg MJ, Ross CE, Fitzmaurice GM, et al. ; American Heart Association’s Get With The Guidelines–Resuscitation Investigators . Annual incidence of adult and pediatric in-hospital cardiac arrest in the United States. Circ Cardiovasc Qual Outcomes. 2019;12(7):e005580. doi: 10.1161/CIRCOUTCOMES.119.005580 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eMethods
eTable 1. Results from the third independent data monitoring committee analysis
eTable 2. Additional baseline characteristics according to treatment assignment
eTable 3. Intra-cardiac arrest interventions
eTable 4. Post-cardiac arrest characteristics in those surviving at least 24 hours
eTable 5. Additional data on return of spontaneous circulation
eTable 6. Organ dysfunction and laboratory values after return of spontaneous circulation
eTable 7. Hospital disposition and cause of death
eTable 8. Neurological outcomes
eTable 9. EQ-5D-5L subcategories
eTable 10. Potential adverse events
eTable 11. Posterior estimates from Bayesian analyses
eTable 12. Posterior probabilities of benefit
eTable 13. Posterior probabilities of harm
eFigure 1. Chest compression fraction according to treatment assignment
eFigure 2. Chest compression frequency according to treatment assignment
eFigure 3. Subgroup results for 30-day survival
eFigure 4. Subgroup results for 30-day favorable neurological outcome
eFigure 5. Kaplan-Meier curve for 90-day survival according to treatment assignment
eFigure 6. Ionized calcium values in patients with return of spontaneous circulation
eFigure 7. Posterior probability distributions for return of spontaneous circulation
eFigure 8. Posterior probability distributions for survival at 30 days
eFigure 9. Posterior probability distributions for favorable neurological outcome at 30 days
Data sharing statement