Abstract
Embryonic definitive hematopoiesis generates hematopoietic stem and progenitor cells (HSPCs) essential for establishment and maintenance of the adult blood system. This process requires the specification of a subset of vascular endothelial cells to become blood-forming, or hemogenic, and the subsequent endothelial-to-hematopoietic transition to generate HSPCs therefrom. The mechanisms that regulate these processes are under intensive investigation, as their recapitulation in vitro from human pluripotent stem cells has the potential to generate autologous HSPCs for clinical applications. In this review, we provide an overview of hemogenic endothelial cell development and highlight the molecular events that govern hemogenic specification of vascular endothelial cells and the generation of multilineage HSPCs from hemogenic endothelium. We also discuss the impact of hemogenic endothelial cell development on adult hematopoiesis.
Keywords: definitive hematopoiesis, hemogenic endothelial cell, endothelial cell specification, endothelial-to-hematopoietic transition, EHT, hematopoietic stem and progenitor cell, HSPC
INTRODUCTION
Vascular endothelial cells that line all blood vessels throughout the body are thought to emerge de novo from mesodermal progenitors to form primary vascular plexi that are then remodeled into arteries, veins, and capillaries that make up the blood circulatory system. During embryonic development, a small, specialized subset of vascular endothelial cells, termed hemogenic endothelial cells, acquires blood-forming potential and gives rise to the hematopoietic stem and progenitor cells (HSPCs) that function to generate all blood cells throughout embryonic and adult life. These multilineage HSPCs are generated within a narrow developmental window in distinct embryonic and extraembryonic tissues, wherein hemogenic endothelial cells are specified (outlined in Figure 1). The mechanisms that lead to hemogenic specification of vascular endothelial cells, as well as the mechanisms that lead to generation of HSPCs from hemogenic endothelium, are still under investigation. Further discovery of such underlying molecular mechanisms would greatly improve our understanding of vascular and hematopoietic development. Insights gained from continued developmental studies will also enable us to overcome current obstacles to mass produce human vascular cells and HSPCs from potentially autologous pluripotent human stem cells for the treatment of hematologic and cardiovascular disorders. In this review, we provide an overview of hemogenic endothelial cell development and highlight our current understanding of the molecular events that govern hemogenic specification of vascular endothelial cells and the generation of multilineage HSPCs therefrom. We also discuss the impact of embryonic hemogenic endothelial cell development on adult hematopoiesis.
Figure 1.
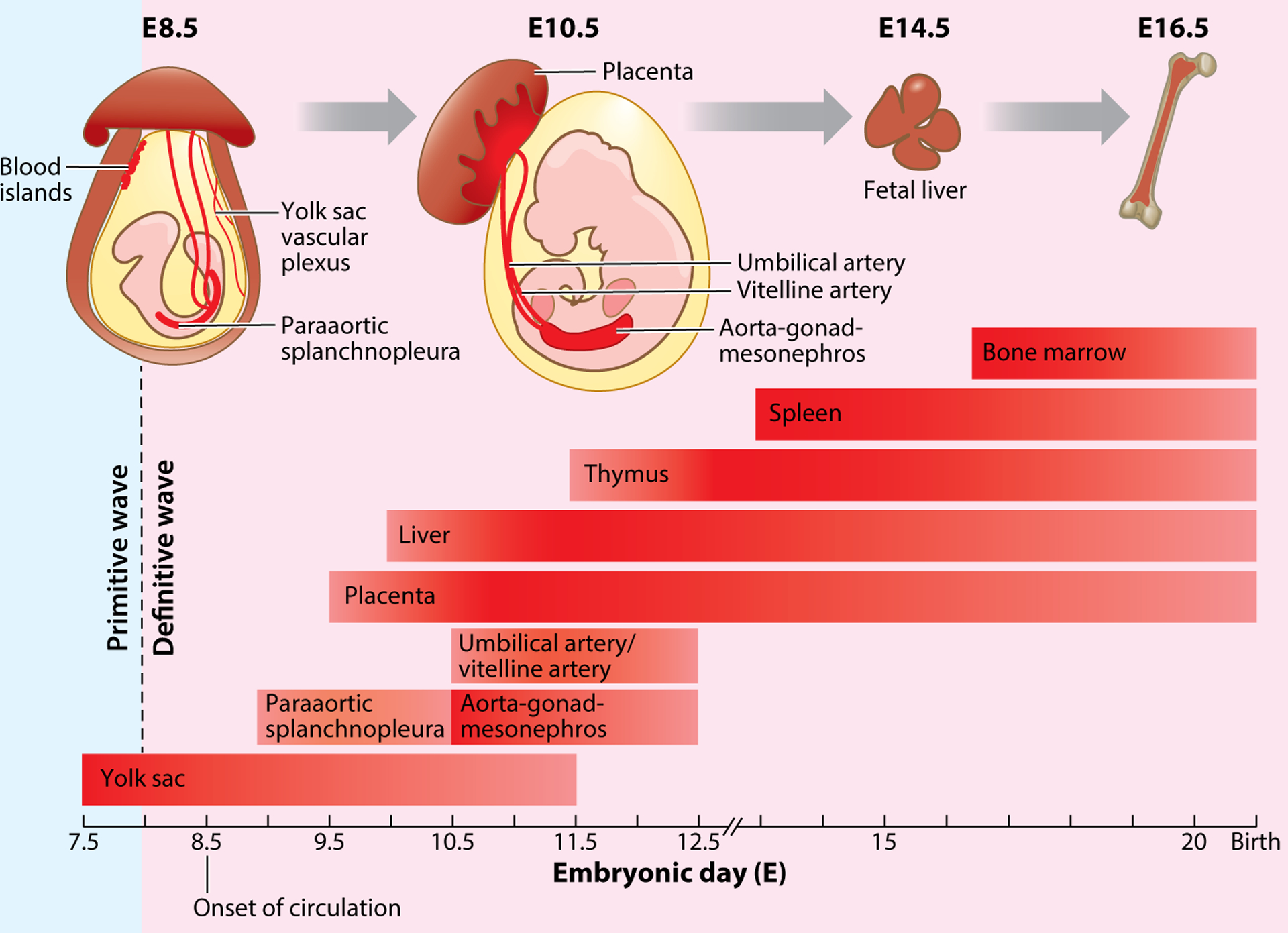
Time line and main sites of hematopoiesis in the mouse conceptus. Primitive hematopoiesis begins around E7.25 within yolk sac blood islands. Definitive hematopoiesis starts around E8 in the yolk sac, E9.5 in the placenta, and E10.5 in the aorta-gonad-mesonephros. The hematopoietic stem and progenitor cells generated from the yolk sac and aorta-gonad-mesonephros are thought to migrate to the fetal liver, thymus, and spleen and finally colonize the fetal bone marrow.
PRIMITIVE HEMATOPOIESIS
Primitive hematopoiesis is the first wave of blood production in the developing mammalian embryo and occurs exclusively in the extraembryonic yolk sac, beginning around mouse embryonic day (E)7.25 within yolk sac blood islands (Figure 1) (1). Hematopoietic cells generated within blood islands include primitive erythrocytes, macrophages, and megakaryocytes, but not self-renewing hematopoietic stem cells (HSCs) or some of the mature blood lineages that exist in adulthood (2, 3). Primitive erythrocytes differ from red blood cells generated later during definitive hematopoiesis in that they are larger in size and nucleated and express a distinct set of globin genes, whereas, primitive macrophages and megakaryocytes are not morphologically distinguishable from their mature counterparts (2). A second wave of hematopoiesis begins ~E8–8.25, when erythromyeloid progenitors (EMPs) emerge within the extraembryonic yolk sac, followed by the generation of erythrocytes and myeloid cells that become tissue-resident macrophages that remain throughout adulthood (4). Once the developing heart begins to beat at ~E8.5, these progenitors and their progeny enter the forming blood vascular circulatory network and are distributed throughout the embryo (5).
DEFINITIVE HEMATOPOIESIS
In mice, definitive hematopoiesis occurs in the yolk sac at E8.25, placenta at E9.5, and the intraembryonic aorta-gonad-mesonephros (AGM) region at approximately E10.5 (6). Definitive hematopoiesis has also been found to occur in the vitelline arteries, umbilical arteries, endocardium, and head arteries (6). During definitive hematopoiesis, the emergence of erythroid, myeloid, and lymphoid progenitors (7) and, most importantly, long-term multilineage repopulating HSCs (8), is observed. The HSPCs generated from the yolk sac and the AGM are thought to migrate to other sites of definitive hematopoiesis, including the fetal liver, thymus, and spleen, as these tissues develop and undergo massive self-expansion. The HSPCs then colonize fetal bone marrow by E16.5 and give rise to mature blood lineages throughout adulthood (9).
HEMOGENIC ENDOTHELIAL CELLS
Hemogenic endothelial cells represent a small (1–3% of endothelial cells in distinct tissues), transient population of specialized vascular endothelial cells that arise in the yolk sac, placenta, and AGM to initiate definitive hematopoiesis (6). Jaffredo and coworkers (10) demonstrated the existence of hemogenic endothelium using in vivo lineage-tracing studies in the avian embryo. In later studies, a Cre-recombinase system was used to trace mouse AGM endothelial cells that express VE-cadherin (VE-Cad) (11) and revealed that hematopoietic progeny derived from VE-Cad+ cells migrate to the fetal liver and bone marrow, where they expand and differentiate into multilineage hematopoietic cells (11). Via flow cytometry and in vitro clonal analysis, single hemogenic endothelial cells from E8.5 mouse yolk sac and E10.5 AGM were phenotypically characterized and shown to generate multilineage hematopoietic colonies (12, 13). Further evidence to support the endothelial origin of HSPCs comes from the use of dynamic time-lapse microscopy techniques in zebrafish (14, 15) and mouse (16), as well as from mouse and human pluripotent stem cell culture (17, 18). Collectively, these studies provide direct evidence that blood cells are generated from the endothelium during definitive hematopoiesis.
Interestingly, HSPCs generated from hemogenic endothelial cells within distinct sites, and at different developmental times, are functionally different (19). Cumano et al. (20) separated and performed explant culture of the yolk sac and intraembryonic paraaortic splanchnopleura (P-Sp)/AGM region before circulation begins. The cells derived from the yolk sac culture failed to give rise to multilineage hematopoietic progenitors when transplanted into the bone marrow of lethally irradiated adult mice; instead, they reconstituted only short-term myeloid lineages. In contrast, P-Sp/AGM-derived hematopoietic cells demonstrated multilineage potential and were able to rescue lethally irradiated neonatal and adult recipients (8, 20). These studies suggest that there might be different types of hemogenic endothelial cells that are functionally distinct or, alternatively, their microenvironment modulates their progeny.
Indeed, Chen and colleagues (21) demonstrated that Tie-2- and Ly-6A-expressing endothelial cells in the yolk sac and AGM exhibit different hematopoietic potential. CBFβ is the binding partner of Runx1, which is essential for the generation of EMPs and HSPCs (discussed below). Reexpression of CBFβ in Tie-2-expressing Cbfb−/− yolk sac endothelial cells rescues EMP generation but not HSPC formation. In contrast, CBFβ reexpression in Ly6a-expressing Cbfb−/− AGM endothelial cells restores HSPCs but not EMPs (21). These studies support the idea that yolk sac hemogenic endothelial cells may be different from their intraembryonic counterparts, raising the possibility that the potential of hemogenic endothelial cells and HSPCs is dependent on the developmental timing of their emergence, their phenotypes, and/or the cellular and extracellular composition of their microenvironment.
PHENOTYPE OF HEMOGENIC ENDOTHELIAL CELLS
Hemogenic endothelial cells comprise a small population of total endothelial cells within the murine yolk sac and AGM (13, 22); therefore, their cellular phenotype must be carefully delineated relative to nonblood-forming endothelial cells. No single marker has been identified as specific to hemogenic endothelial cells (6); however, Kabrun et al. (23) reported that fetal liver kinase 1 (Flk1), also called vascular endothelial growth factor receptor 2 (VEGFR2), is expressed in a small subset of endothelial cells that has hematopoietic potential. Nishikawa and colleagues (24) proposed that Flk1+VE-Cad+ endothelial cells represent cells transitioning to hematopoietic lineages. However, VE-Cad is not required for embryonic definitive hematopoiesis in the zebrafish and mouse, or for the blood-forming ability of hemogenic endothelial cells in the murine yolk sac (22, 25). To further distinguish hemogenic endothelial cells from HSPCs, mature hematopoietic lineages, and nonhemogenic endothelial cells, a combination of endothelial markers (Flk1, VE-Cad, CD31) and hematopoietic markers, including c-Kit, Runx1, CD45, CD41, and Ter119, has been applied (26–28). Furthermore, our group has reported a more precise identification and isolation strategy for hemogenic endothelial cells using flow cytometry (29). We found that hemogenic endothelial cells within the murine yolk sac and AGM exhibit Hoechst dye efflux (side population or SP) properties, exhibit a Flk1+c-Kit+CD45−SP phenotype, and give rise to Flk1−c-Kit+CD45+SP multilineage hematopoietic progenitors on a clonal level (13, 27).
DEFINITIVE HEMATOPOIESIS IS A DEVELOPMENTAL CONTINUUM
Definitive hematopoiesis requires the specification of hemogenic endothelial cells and their subsequent generation of HSPCs, which is referred to as the endothelial-to-hematopoietic transition (EHT) (14). Rybtsov et al. (30) recently identified two distinct cell types, type I and type II pre-HSCs, which represent a sequential transition from hemogenic endothelial cells to mature HSPCs. Type I and II pre-HSCs are characterized as VE-Cad+ CD41low CD45− and VE-Cad+ CD41low CD45+, respectively. Type I pre-HSCs are able to give rise to HSPCs via the type II pre-HSC intermediate, yet neither demonstrates endothelial cell properties in culture (30). It is also proposed that pre-HSCs have a precursor, termed pro-HSC, which is not functionally well characterized (21). Together, identification of intermediate stage cells defines a hierarchical cellular development of HSPCs from endothelium.
In contrast, recent single-cell transcriptome expression analysis suggests that definitive hematopoiesis represents a continuum of phenotypes from endothelial cells to fully determined HSPCs. It has been similarly proposed that differentiation of HSCs is not a progressive generation of a series of distinct hematopoietic progenitor types, but rather, HSCs undergo a continuous gain of specific lineage characteristics (31). This concept is supported by a recent study of cells generated during EHT. Swiers et al. (32) isolated Runx1-GFP+ hemogenic endothelial cells, nonhemogenic endothelial cells, emerging CD41+ hematopoietic progenitor cells (HPCs), and mature CD45+ HPCs from mouse E8.5 to E11.5 hematopoietic regions and performed single-cell Fluidigm qRT-PCR for hematopoietic and endothelial-related gene expression in these populations. Principal component analysis revealed that while nonhemogenic endothelial cells and CD45+ HPCs separate as two distinct populations, the CD41+ HPCs are scattered in between as a continuum (32). Moreover, Zhou et al. (33) isolated type I and II pre-HSCs from E11 mouse AGM and performed single-cell transcriptome analysis, which revealed heterogeneity in the pre-HSC population along with HSPC generation. Using similar single-cell RNA sequencing techniques, Baron and coworkers (34) found significant heterogeneity in intra-aortic hematopoietic clusters (IAHCs) in the E10.5 AGM. These studies also suggest transcriptional downregulation of the endothelial program and upregulation of the hematopoietic program in cells along the developmental axis of endothelium to HSPCs (32–34) between E10 and E11, the peak of AGM hematopoiesis (34). Thus, successive transcriptional alterations likely occur in endothelial cells and hemogenic endothelial cells along their differentiation into pre-HSCs and ultimately HSPCs.
HEMOGENIC ENDOTHELIAL CELL SPECIFICATION
Although the molecular mechanisms underlying hemogenic endothelial cell specification, as well as EHT, are still not completely understood and much more work is needed to define these complex processes, we next discuss the regulators that have thus far been revealed (Figure 2).
Figure 2.
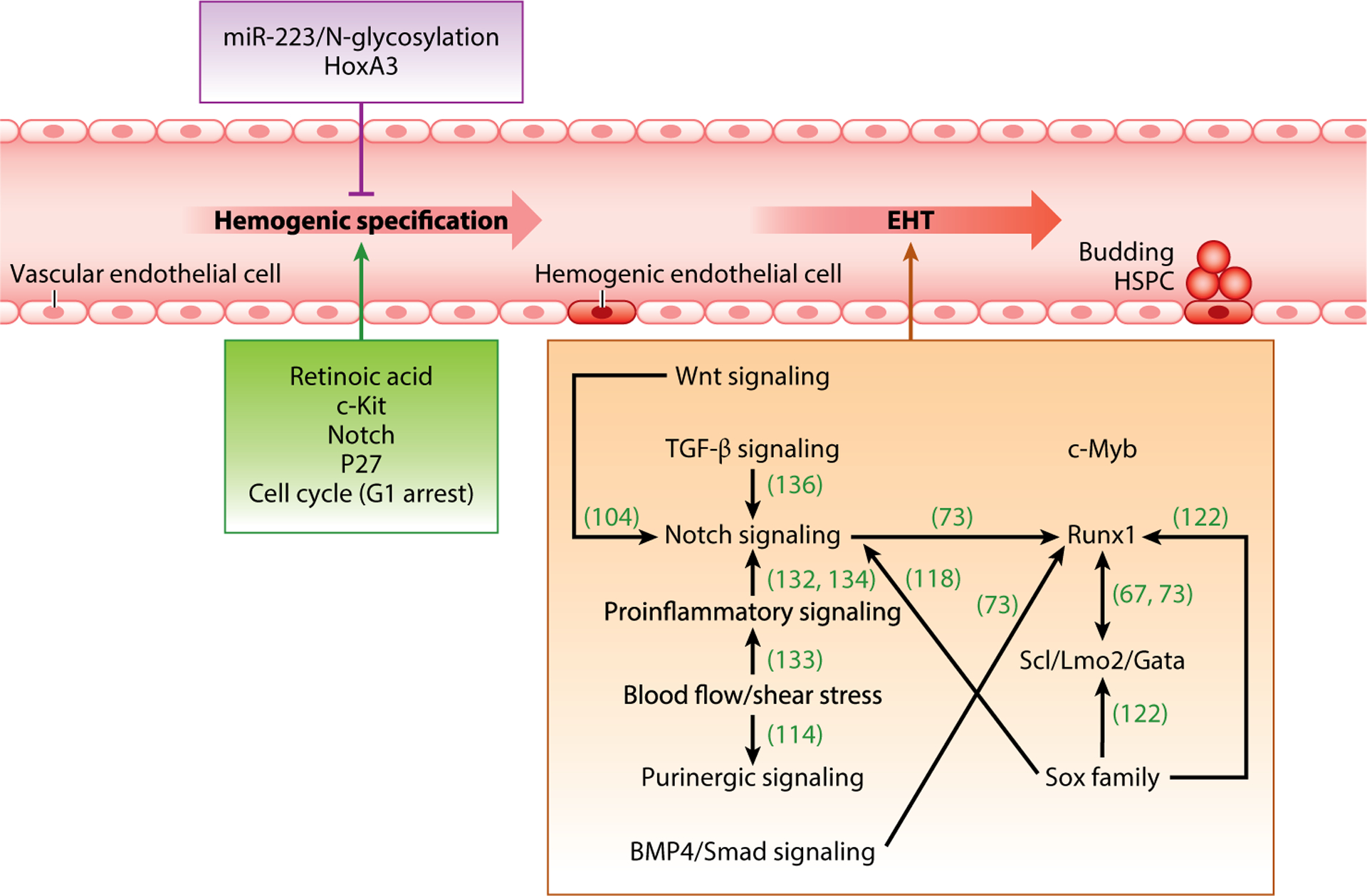
Summary of major molecular mechanisms governing hemogenic specification and EHT. Molecular events regulating hemogenic endothelial cell specification are listed in the green box (promoting) and purple box (inhibiting). Molecular events promoting EHT are listed in the orange box; interactions among them are highlighted. Abbreviations: EHT, endothelial-to-hematopoietic transition; HSPC, hematopoietic stem and progenitor cell; TGF-β, transforming growth factor-β.
Retinoic Acid Signaling
Retinoic acid (RA) is the oxidized derivative of dietary retinol (also known as vitamin A) that binds to a carrier protein, retinol-binding protein 4, in the circulation. When taken up by cells, retinol is first oxidized to retinaldehyde by either alcohol dehydrogenases or retinol dehydrogenases, followed by further oxidization to all-trans RA (ATRA) by retinaldehyde dehydrogenases (Raldh1-4). ATRA can function within the cell in which it was generated, or be released into circulation and taken up by surrounding cells, where it binds to nuclear localized retinoic acid receptors (RARα, β, and γ). Heterodimerization of RARα, β, and γ with rexinoid receptors (RXRα, β, and γ) forms an active transcription factor complex that binds to retinoic acid response elements within target genes to activate gene expression (35).
RA signaling is critical for vascular development and definitive hematopoiesis. During early murine yolk sac development, active RA is generated by Raldh2 that is expressed in the visceral endoderm (36). Raldh2−/− mice are RA deficient and die at ~E10.5 with abnormal vascular remodeling and impaired hemogenic endothelial cell specification resulting from endothelial cell hyperproliferation (37). Interestingly, hemogenic specification of endothelial cells can be rescued in Raldh2−/− embryos by provided exogenous ATRA in utero or ex vivo in embryo culture (22, 38). Among the RA signaling receptors, RARα1/2 specifically regulates hemogenic endothelial cell specification (13, 38). In fact, in both E8.5 yolk sac and E10.5 AGM, ~90% of endothelial cells with active RA signaling are hemogenic endothelial cells that highly express RARα1/2 (36, 38). In addition, overexpression of RARα dramatically increases multilineage potential of AGM-derived hemogenic endothelial cells in transplant assays (38). Moreover, enhanced RA signaling in AGM-derived hemogenic endothelial cells ex vivo promotes their hematopoietic potential (38).
c-Kit
c-Kit (CD117) is an important cell surface marker for hemogenic endothelial cells in both E8.5 yolk sac and E10.5 AGM tissues and, therein, is exclusively expressed in endothelial cells with hemogenic potential (35). c-Kit is a cytokine receptor that belongs to the receptor tyrosine kinase family. When binding its ligand, stem cell factor (SCF), c-Kit receptor forms a dimer that activates its intrinsic tyrosine kinase activity and initiates a series of downstream phosphorylation events and signal transduction. SCF/c-Kit signaling has been shown to regulate HSC development and maintenance. In Scf−/− mouse embryonic stem cells (ESCs), generation of hemogenic endothelial cells is undetectable (39), as are primitive or definitive hematopoietic lineages (40). In E8.5 Raldh2−/− mutant embryos, c-Kit expression is downregulated in yolk sac endothelial cells, and its reexpression to wild-type levels rescues hemogenic endothelial cell development and their multilineage hematopoietic potential, as well as endothelial cell cycle control (13). The presence of c-Kit in endothelium of E8.5 yolk sac also prevents hemogenic endothelial cell differentiation toward a cardiomyogenic fate (41).
Notch
Notch signaling is evolutionarily conserved and known to be involved in cell proliferation, differentiation, apoptosis, and fate decisions. Notch signaling allows communication between two cells in close contact, via binding of Notch ligands on the signal-sending cell to receptors on the signal-receiving cell. In mammals, there are five Notch ligands of the Delta (Dll1, Dll3, and Dll4) and Jagged (Jag1 and Jag2) families and four Notch receptors (Notch1-4) (6). The binding of ligands and receptors generates a physical force that alters the configuration of receptors, which consequently become vulnerable to sequential proteolysis, resulting in the release of the Notch intracellular domain (NICD). Subsequently, NICD translocates to the nucleus and forms a transcription activator complex with RBPj and coactivators such as mastermind-like (MAML), which activate target gene expression (6, 42).
It is well known that Notch signaling is essential for vascular and hematopoietic development (42), and evidence of its involvement in hemogenic endothelial cell specification has been accumulating. Inhibition of Notch signaling, using the γ-secretase inhibitor DAPT (N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester) in cultured wild-type E8.5 embryos results in endothelial cell hyperproliferation and impaired hemogenic endothelial cell development (13). Moreover, the expression of Notch1 and its downstream targets Hes1 and Hey1 are highly enriched in mouse hemogenic endothelial cells and downregulated in RA-deficient mutant embryos. In fact, Notch signaling has been shown to regulate hemogenic specification of endothelial cells, downstream of RA signaling, via control of endothelial cell cycle progression (13).
p27/Cell Cycle Control
In ESCs, fate decisions are molecularly linked to cell cycle state (reviewed in 43), and this appears to be similar for endothelial cells (13, 29). That is, in both RA-deficient and Notch-inhibited murine embryos, cell cycle inhibitor p27 is downregulated, and endothelial cells are hyperproliferative. This lack of endothelial cell cycle control is associated with impaired hemogenic specification (13). Interestingly, however, reexpression of p27 to wild-type levels in either RA or Notch mutants restores endothelial cell cycle control, which enables hemogenic specification (30). More specifically, cell cycle arrest in the G1 state is required for hemogenic specification (13). Although the molecular mechanism by which endothelial cell cycle state modulates hemogenic specification is still unclear, several hematopoietic-related genes are known to be upregulated in G1 phase, such as Hoxb4, notch1, notch4, Gata2, and Wnt (44). As p27 can also function as a transcriptional regulator, it is possible that it also directly regulates hematopoietic gene expression (45). Collectively, the studies discussed above support an RA/c-Kit/Notch/p27 regulatory axis that promotes hemogenic endothelial cell development.
miR-223/N-Glycosylation
MicroRNAs (miRNAs) are a class of short, noncoding RNAs that fine-tune gene expression by posttranscriptionally repressing complementary mRNA targets (46). Although specific miRNAs have been identified to be required for vascular and hematopoietic development (46, 47), the role for miRNA regulation in hemogenic endothelial cell specification and subsequent HSPC production has not been well addressed. Recently, miR-223 has been associated with embryonic hematopoiesis; that is, miR-223 is highly enriched in both zebrafish and mouse endothelial cells and hemogenic endothelial cells. miR-223-deficient zebrafish and mouse mutants exhibit increased numbers of hemogenic endothelial cells and HSPCs at the sites of definitive hematopoiesis (48), suggesting that miR-223 plays an inhibitory role in hemogenic specification and EHT.
Although still under investigation, miR-223 may repress embryonic hematopoiesis via control of the N-glycome (48). N-glycosylation refers to the attachment or modification of diverse sugar moieties (glycans) to the asparagine residue of proteins. The glycome regulates protein folding, stability, and activity, and particular glycan structures have been associated with stem cell differentiation (49). Several N-glycogenes were identified as potential miR-223 targets by comparative mRNA profiling of wild-type and miR-223-deficient endothelial cells in both mouse and zebrafish (48). Chemical treatment that alters N-glycan processing also promotes HSPC generation (48); thus, these studies suggest that miR-223 modulates hemogenic specification and HSPC production via regulation of endothelial cell N-glycosylation, although more work is needed to fully understand the underlying mechanisms.
Hox
The homeobox (Hox) gene family is a group of transcription factors that possesses a highly conserved homeobox domain that has been implicated in vertebrate patterning and tissue development, angiogenesis, and maintenance of cell identity (50). Notably, class I Hox genes have been associated with hematopoietic development (51–53). Among Hox genes, paralog group 3 (Hoxa3) specifically directs endothelial cell differentiation. In E8.25–8.5 yolk sac and E9.5–10.5 AGM, HoxA3 downregulation marks the site of hematopoiesis from the endothelium (51). The expression pattern of HoxA3 suggests it inhibits hemogenic specification and promotes arterial specification of endothelial cells, consistent with the fact that it is upregulated during angiogenesis (52). Indeed, in early mesodermal cells, HoxA3 restrains hematopoietic development and maintains endothelial identity (51). Surprisingly, overexpression of HoxA3 can revert committed hemogenic endothelial cells (CD41+CD45+) into CD41− endothelial cells (51). HoxA3 also represses the generation of HSCs by inhibiting the expression of key hematopoietic transcription factors, including Runx1, Gata1, Gfi1B, Ikaros, and PU.1, that promote hematopoietic development (51). HOXA3 also upregulates the Notch pathway ligand JAG1 in cultured human ESC-derived hemogenic endothelial cells, which leads to Notch ligand cis-inhibition and prevents blood cell commitment (53). These data suggest an important role for HoxA3 in maintenance of an endothelial state prior to hematopoietic transition.
Conversely, another Hox paralog, HoxB4, has been proposed to play a positive role in hemogenic specification. Genome-wide analysis in human ESC-derived HSPCs suggests that many hematopoietic genes are direct targets of HOXB4, including Runx1, Scl/Tal1, Gata2, and Gfi1 (54). HoxB4 ectopic expression increases the formation of hemogenic endothelial cell colonies from mouse ESCs, not by altering the frequencies of its progenitor cells, but by promoting the differentiation of progenitors toward a hematopoietic fate (55). Additionally, expression of HoxB4 ectopically in both human ESC-derived and mouse yolk sac–derived HPCs promotes long-term, multilineage hematopoiesis in transplant experiments (56, 57).
REGULATION OF HEMATOPOIETIC STEM AND PROGENITOR CELL GENERATION AND FUNCTION
Just as we need more ongoing work to better understand the molecular regulation of hemogenic endothelial cell specification, more work is also needed to define the complex regulation of HSPC generation from hemogenic endothelial cells. Nonetheless, we discuss below the regulators that have been revealed to date (Figure 2).
Runx1
Runx1 belongs to a transcription factor family called core-binding factors, which includes sequence-specific DNA-binding proteins Runx1-3 and their shared non-DNA binding protein CBFβ that helps Runx1-3 effectively bind DNA (58). Runx1 is an important regulator of, and marker for, the EHT process, and Runx1−/− and Cbfb−/− mice both die around E12.5 with hematopoietic defects and vascular hemorrhage (59, 60). In the mouse AGM, expression of Runx1 is observed in endothelial cells lining the ventral side of the dorsal aorta, in the underlying mesenchyme, and in IAHCs (58). Runx1 expression occurs prior to embryonic hematopoiesis and is conserved among vertebrate species (58). Runx1 is also functionally conserved; that is, Runx1 is expressed in multiple models, including mouse and zebrafish AGM and human ESCs, and its loss prevents HSPC formation and subsequent lineage-forming activities in Tek/Tie-2- or VE-Cad-expressing cells (39, 61–65). Importantly, the observed defects in embryonic definitive hematopoiesis in Runx1-deficient embryos are not the result of impaired dorsal aorta formation (61–65). Furthermore, the formation of VE-Cad+CD45−CD41+ precursors of definitive HSCs in the E10.5 mouse dorsal aorta form in the absence of Runx1. And deletion of Runx1 in mature blood lineages using fetal liver hematopoietic gene Vav1-Cre fails to block murine hemogenic endothelial cell development and HSPC formation (66). Thus, Runx1 appears to be required transiently during the formation of HSPCs from hemogenic endothelial cells.
Multiple proteins bind to the enhancer of Runx1, thus regulating its expression and function during EHT. In vivo, the Gata2/Ets and Scl/Lmo2/Ldb-1 complexes are recruited to a highly conserved Runx1 enhancer (67). In addition, deletion of Sox17 or Notch1 in mouse AGM during EHT upregulates expression of Runx1, as well as Gata2, and promotes HSPC generation (68). Runx1 expression also upregulates other hematopoietic regulators, including Scl, Lmo2, and Gata1-3 (58), which is discussed below. In addition, PU.1 is a known downstream target of Runx1 (69). A low-level transient expression of Runx1 in mouse ESC-derived HPCs is sufficient to result in chromatin remodeling and induction or repression of PU.1 expression, depending on the lineage type produced (69). Gfi1/Gfi1B are also thought to be direct targets of Runx1 (70). In the absence of Runx1, Gfi1 and Gfi1B inhibit the endothelial program and induce emergence of round cells similar in morphology to HSPCs (70). Mouse embryos lacking Gfi1 and Gfi1B fail to release HSPCs from hemogenic endothelial cells within the yolk sac (70) and AGM (71). It has been proposed that Gfi1 promotes EHT possibly by recruiting LSD1 protein to epigenetically repress endothelial genes and to allow hematopoietic gene expression (71). Another direct target of Runx1 is interleukin (IL)-3, which is expressed in the luminal layer of the dorsal aorta and in cells adhering to the aorta wall (72). Addition of IL-3 rescues HSPC levels in hemizygous Runx1 mouse AGM explants (72). An interlinked gene regulatory network study showed that Runx1, upregulated by Notch1 and BMP4 signaling, is able to regulate the triad of Scl (stem cell leukemia, also known as Tal1), Gata2, and Fli1, leading to increased HSPC formation (73). In addition, endothelial cell markers Flk1 and VE-Cad are downregulated by Runx1 (reviewed in 58). Collectively, these studies suggest that Runx1 represses endothelial gene expression and promotes hematopoietic differentiation.
Scl/Lmo2/Gata
As mentioned above, the regulatory regions of hematopoietic genes Scl/Tal1, Lmo2, and Gata1–3 can be bound and regulated by Runx1 (35). Among these factors, Lmo2 (also known as Rbtn2) may act as a bridge between Scl- and Gata-binding proteins, and the requirement for Lmo2 in zebrafish embryos is essentially the same as for Scl (74). Scl, a basic helix-loop-helix transcription factor, is highly expressed in endothelial and hematopoietic cells (75) and is a crucial regulator of early definitive hematopoiesis (76). Scl-null mouse embryos die around E9.5, with complete loss of yolk sac hematopoiesis and vascular remodeling, as well as impaired dorsal aorta formation (76). Two Scl isoforms were identified in zebrafish: the full-length Scl-α isoform and the N-terminal truncated Scl-β isoform, which are functionally redundant in primitive erythropoiesis and myelopoiesis (77). However, Scl-β is selectively expressed in hemogenic endothelial cells in the ventral wall of the zebrafish dorsal aorta and is required for HSPC emergence, whereas Scl-α is expressed in nascent HSPCs and required for their maintenance in the AGM (77, 78). SCL promotion of hematopoiesis has also been reported in human ESC; that is, impaired hemogenic specification and EHT are exhibited in myeloid ectopic viral integration site 2 homolog (MEIS2)-deficient cells and can be rescued by the expression of SCL (79).
Gata2 is expressed in most HPCs and all HSCs during hematopoiesis (80, 81), and embryos lacking Gata2 die at E10.5 with anemia (82). Gata2 deficiency leads to impaired EHT and HSPC repopulating ability in a dosage-dependent manner in the AGM, but not yolk sac or adult bone marrow (80, 83–85). Zebrafish possess two Gata2 genes: Gata2a and Gata2b, but only Gata2b functions within hemogenic endothelium to prevent HSPC emergence (86). Gata3 is expressed in Gata2-nonexpressing HSPCs, as well as some endothelial cells in mouse AGM, suggesting that Gata3 and Gata2 may have overlapping roles (87). Indeed, Gata3-deficient embryos show impaired hematopoiesis in mouse AGM and fetal liver, but not yolk sac (88, 89). Collectively, these findings demonstrate that these transcription factors play essential roles in EHT and definitive hematopoiesis.
Notch
In addition to the previous discussion about the role of Notch signaling in regulating hemogenic specification, Notch signaling has also been shown to regulate HSPC generation. Notch1 and 4 receptors are specifically expressed in mouse endothelial cells, whereas, Notch1, 2, and 4 are highly expressed in mouse HSPCs. Notch1 has been shown to be indispensable in regulating EHT in loss-of-function studies in various organisms, including mouse AGM and its explant culture (90–92), mouse fetal liver and fetal bone marrow, zebrafish AGM (62, 93), and human ESCs (94).
Although Notch1 signaling is required for both arterial specification and embryonic hematopoiesis, ligand specificity for each process has been suggested: Jag1 for definitive hematopoiesis (90, 95) and Dll4 for arterial specification (95, 96). Both are expressed in endothelial cells in mouse dorsal aorta (97, 98). Jag1-null mutant mouse embryos exhibit impaired HSPC generation in the AGM (90, 98), although Jag1-deficient AGM cells can be rescued by culturing on Jag1-expressing stromal cells or by transduction with the Gata2 gene (90, 98). In contrast, Dll4 is dispensable for hematopoiesis in mouse AGM (98). A model has been proposed that HSPC and arterial endothelial cells originate from cells with different Notch1 signaling strength (98). That is, high Dll4-activated Notch signaling enables the maintenance of endothelial cell identity, whereas, Jag1 induces low Notch activity, which represses the endothelial/arterial program and promotes EHT (98). These studies suggest antagonizing roles for Jag1 and Dll4 in the process of definitive hematopoiesis. However, studies in human pluripotent stem cell culture demonstrate that DLL4 may facilitate embryonic hematopoiesis (99). Human ESC-derived hemogenic endothelial cells expressing a low level of DLL4 can receive Notch-activating signals from neighboring cells with high DLL4 expression, thereby promoting EHT (100).
HSPC generation in AGM hematopoietic clusters has been shown to depend on Notch-induced Gata2 expression (86, 90, 101). Gata2 has two functional RBPj-binding sites, mutation of which results in loss of EHT in E10.5 mouse AGM (101). In zebrafish, dysregulated Gata2b expression is observed in Notch1 morpholino studies (86). Hes1 and Hes-related family genes are also direct targets of Notch signaling (97). Hes1 expression appears to antagonize Gata2, because mutation in RBPj-binding sites of Hes1 leads to increased Gata2 expression in mouse AGM HSPCs (101). The opposite role of Notch targets Gata2 and Hes family members in regulating embryonic hematopoiesis suggests that Notch ligands may have specific downstream transcriptional targets, which may explain why Notch signaling mediated by different Notch ligands leads to different effects. Additionally, downregulation of cell division cycle associated 7 (Cdca7) gene, a direct Notch target (102), impairs HSPC generation in zebrafish and is required for maintenance of the undifferentiated state of HPCs derived from cultured human ESCs (102). Notch signaling also regulates the expression of a number of hematopoietic genes, including c-myb, Runx1, and Foxc2, but whether they are direct or indirect targets of Notch is not known (90, 91, 103).
Notch signaling can function both cell-autonomously and nonautonomously in regulating embryonic definitive hematopoiesis. Hadland and colleagues (94) showed that Notch1 is required cell autonomously by injecting lacZ-tagged notch1-null mouse ESCs into wild-type blastocysts. β-Galactosidase-positive cells were subsequently isolated from yolk sac, fetal liver, and fetal bone marrow of the recipient embryos and found to lack blood cell colony–forming activity. Conversely, several Notch ligands have also been shown to function nonautonomously during definitive hematopoiesis (104–107). For example, in zebrafish, signaling via Notch ligands Dlc/Dld and Notch3 receptor were found to generate endothelial cell precursors that migrate to and colonize the dorsal aorta and induce definitive hematopoiesis (104–107). The somitic signaling by Dlc/Dld and Notch3 is spatially distinct from and earlier than the abovementioned cell-autonomous requirement for Notch signaling, in which Notch ligands that mediate hematopoiesis are thought to be expressed in the dorsal aorta endothelium and/or neighboring mesenchymal cells (90, 92, 104, 106, 107).
c-Myb
The evolutionary conserved nuclear proto-oncogene c-myb is thought to regulate definitive hematopoiesis, but not primitive hematopoiesis, in both mouse and zebrafish (108–110). In zebrafish, c-Myb expression is restricted to HSPCs in both larva and adult stages, and it is downregulated upon differentiation (111). C-Myb loss-of-function zebrafish fail to establish normal thymopoiesis and lack all blood cell lineages (110). Truncation of c-Myb to eliminate its transactivation domain impairs the budding of HSPCs from the ventral wall of dorsal aorta, partly via upregulation of chemokine stromal cell–derived factor 1a (SDF1a) (109). Mice lacking c-Myb die around E15.5 and lack fetal liver hematopoiesis (108); in addition, mouse c-myb−/− AGM explants yield fewer HSPCs than wild-type controls (112).
Purinergic Signaling
Purines (such as adenosine, ADP, and ATP) signal via cell surface receptors that regulate diverse cell functions, including proliferation and differentiation, as well as stem cell regeneration (113). Recently, purinergic signaling, especially via adenosine, has been reported to promote emergence of HSPCs in zebrafish and murine embryos (113). Zebrafish embryos treated with chemical enhancers of adenosine generate an increased number of HSPCs, whereas embryos treated with chemical adenosine antagonists exhibit impaired HSPC formation (113). Knockdown of adenosine receptor A2b in both zebrafish and mouse, and in ESC culture and AGM explants, leads to decreased formation of hemogenic endothelial cells, decreased HSPC production, and impaired blood-forming ability (113). Adenosine receptor A2b activation induces expression of CXCL8 (IL-8), which has been shown to trigger proliferation of HPCs, via the cAMP-protein kinase A (PKA) axis (113). Blockade of the cAMP–PKA pathway in mouse AGM also impairs hematopoietic potential of HSPCs (114); however, another study argues that PKA inhibition has no effect on HSPC generation in human pluripotent stem cell culture (115). Instead, it is suggested that inhibition of the exchange protein activated by cAMP (Epac)-cAMP axis impairs hemogenic endothelial formation and HSPC generation (115). In addition, induction of cAMP has been shown to regulate human definitive hematopoiesis by mitigating oxidative stress and enhancing expression of CXCR4, a chemokine receptor that maintains HSC quiescence in the bone marrow niche (115). Collectively, these findings demonstrate that purinergic signaling plays a pivotal role in HSPC generation.
Sox
Sox7, Sox17, and Sox18, members of the SoxF family of transcription factors, have been reported to regulate embryonic definitive, but not primitive, hematopoiesis (116–122). Sox17 is required for arterial and hemogenic specification and maintenance, making it of particular interest in the context of definitive hematopoiesis in the AGM (118, 120). Sox17 expression is highly restricted to fetal and neonatal stages in vivo and is enriched in hemogenic endothelial cells and emerging HSPCs derived from mouse AGM (118, 121). Endothelial-specific deletion of Sox17 in E9.5 mouse embryos ablates the generation of repopulating HSCs and hematopoietic lineages in the AGM (118). In mouse AGM explant culture and mouse ESC differentiation culture, Sox17 is required for the development and/or expansion of hemogenic endothelial cells (68, 118). Overexpression of Sox17 in endothelial cells derived from mouse and human ESCs results in increased hemogenic endothelial cell specification. In contrast, SOX17 overexpression in pre-HPCs and HPCs derived from human ESCs not only inhibits their hematopoietic differentiation ability but also reprograms them into hemogenic endothelial-like cells (122).
Many direct downstream targets of Sox17 in the EHT process have been reported, including Notch1, VE-Cad, Scl/Tal1, and Runx1 (118, 122). Endothelial-specific deletion of Sox17 also leads to compensatory overexpression of Sox7 and Sox18 (117). Transient expression of Sox7 and Sox18 has been seen in hemogenic endothelium and is decreased thereafter (116, 117, 119). Sox7- and Sox18-enhanced expression maintains endothelial cell identity while blocking their hematopoietic differentiation (116, 117, 119). Sox7 is known to bind and activate the promoter of VE-Cad, which is downregulated during EHT (116). Taken together, these findings suggest that Sox family members are key regulators of hemogenic endothelial cells and their hematopoietic activity, although they may have different roles.
Wnt
The transduction of Wnt factors through Frizzled receptors triggers a cascade of downstream signaling, including activation of β-catenin (canonical pathway) and/or JNK and PKC (noncanonical pathway), which translocate to the nucleus and regulate transcription of target genes (reviewed in 123). Several Wnt ligands have been shown to be required in definitive hematopoiesis (124–126). Somite-derived Wnt16 and endothelial overexpression of Wnt8 promote zebrafish EHT (104, 125). Conversely, loss of Wnt9a in the zebrafish somites or chemical inhibition of the secretion of all Wnt ligands impairs the generation of HSPCs but does not impact hemogenic specification (126). In human ESC differentiation studies, addition of WNT3A or WNT1 protein increases HSPC emergence (127, 128).
Wnt requirement for HSPC generation is further supported by the manipulation of its downstream signaling components. For example, β-catenin is expressed in the mouse dorsal aorta during definitive hematopoiesis and nuclear-localized only in cells at the base of IAHCs (129). Chemical interference with β-catenin or deletion of β-catenin in VE-Cad+ AGM endothelial cells leads to decreased HSPC generation (129). Similarly, inhibitors of GSK3 that promote phosphorylation and subsequent degradation of β-catenin cause increased HSPC formation in E10.5 mouse AGM explants, zebrafish, and human pluripotent stem cell culture (126, 129, 130).
Proinflammatory Signaling
Recently, inflammatory signaling mediated by interferons (IFNs) has been found to play an essential role in embryonic definitive hematopoiesis independent of its role in infection, termed sterile inflammation (131–135). Mice or zebrafish embryos lacking IFN-γ and IFN-α activity generate fewer HSPCs within the AGM (131, 133). More specifically, IFN-γ activated by Notch or blood flow inhibits the EHT process via receptor Crfb17 in a cell-autonomous manner (133). In contrast, tumor necrosis factors (TNFs) support HSPC emergence; that is, TNF-α, via receptor TNFR2, is required for HSPC generation from the dorsal aorta of zebrafish (132). Combinatorial knockdown of IFN-γ and TNF-α leads to decreased HSPC emergence, suggesting a cooperative role of the two inflammatory cytokines during hematopoiesis (131). In addition, granulocyte colony–stimulating factor (GCSF) signaling also promotes zebrafish HSPC formation and proliferation (135). Its receptor, GCSFR, as well as TNFR2, regulate EHT by activating Jag1a-mediated Notch signaling, as well as NF-κB signaling, via a conserved Toll-like receptor 4 (TLR4) (132, 134). Canonical NF-κB activation is also a regulator of HSPC emergence in both zebrafish and mouse; inhibiting translocation NF-κB to the nucleus leads to impaired generation of HSPCs and their progeny (132, 134). Interestingly, primitive myeloid cells have been implicated as a local source of multiple inflammatory signals, suggesting that definitive hematopoiesis is dependent on primitive hematopoiesis (131, 132).
TGF-β
The transforming growth factor-β (TGF-β) superfamily comprises secreted polypeptide growth factors in the TGF-β, bone morphogenetic protein (BMP), activin, and nodal families that regulate cell growth and differentiation. Recent studies found that signaling via TGF-β and BMP is required for HSPC emergence. Monteiro and coworkers (136) reported that three ligands of the TGF-β family act sequentially in zebrafish definitive hematopoiesis; that is, autocrine signaling via TGF-β1 and 2 within the endothelium promotes hemogenic specification, and subsequent paracrine signaling from notochord-derived TGF-β3 promotes EHT. All three ligands signal through the receptor TGF-βR2 and induce Jag1a expression, thus activating downstream Notch signaling (136). BMP4-mediated signaling is also required for definitive hematopoiesis in zebrafish and Xenopus (137, 138). In addition, the effectors of BMP signaling pathway, Smad1/5, and binding partner Smad4, were also shown to be necessary for hematopoiesis, especially for the EHT process (139, 140).
Blood Flow
Since the onset of heartbeat and establishment of mouse vascular circulation at E8.5 coincide with the hemogenic activity in the yolk sac and AGM, it is not surprising that hematopoietic development is impacted by biomechanical forces, such as blood flow–induced shear stress (141, 142). North and coworkers (142) found that zebrafish mutants that have no heartbeat or blood circulation show significantly reduced HSPC formation. In addition, Adamo et al. (141) found that cultured mouse embryos and ESC-derived cells exhibit increased HSPC formation and hematopoietic colony-forming potential under flow-induced shear stress. Several signaling pathways responsible for flow-dependent hematopoiesis have been identified, including nitric oxide, the proinflammatory cytokine IFN-γ and its receptor crfb17, the PKA-cAMP pathway, and calcium efflux (114, 133, 141, 142). However, further studies are needed to precisely describe the mechanisms that coordinate blood flow–induced shear stress with these and other signaling pathways in the regulation of definitive hematopoiesis.
IMPACT OF HEMOGENIC ENDOTHELIAL CELL DEVELOPMENT ON ADULT HEMATOPOIESIS
It has been suggested that the hematopoietic system is maintained by a pool of HSC subtypes, which are lineage biased (143). Myeloid-biased HSCs and lymphoid-biased HSCs display stable phenotypes during aging or serial transplantation and can be identified and purified (143). However, it is not clear whether adult lineage-biased HSCs derive from a uniform pool of hemogenic endothelial cells, or whether the different lineage potentials of HSCs result from their generation from different subtypes of hemogenic endothelial cells. In support of the latter possibility, recent in vitro studies using human ESCs suggest that defects in, or stimulation of, hemogenic endothelial cells can change their blood lineage generation. For example, GATA2 deletion in human ESC-derived hemogenic endothelial cells leads to a decreased number of HPCs and granulocytes, although the generation of erythrocytes and macrophages is unaffected (144). Interestingly, the granulocyte potential of Gata2−/− human ESC-derived cells can be rescued by activating Notch signaling. Other work demonstrates that stimulation of human ESC-derived HSPCs with DLL4 reduces their ability to generate erythrocytes and megakaryocytes yet increases myeloid lineage generation (18). Thus, it is possible that defects in hemogenic endothelial cell development lead to alterations in adult hematopoiesis, which needs to be further explored.
SUMMARY
The specification of hemogenic endothelial cells and their subsequent generation of HSPCs are complex processes that involve interplay between developmental timing and molecular and mechanical signals from the microenvironment. This interplay, which is yet to be fully elucidated, results in a gradual loss of endothelial cell characteristics with concomitant acquisition of hematopoietic characteristics. Although molecular mechanisms underlying the generation of hemogenic endothelial cells and subsequent generation and differentiation of HSPCs have been accumulating, important questions remain unanswered. It is still unclear why the ability of embryonic HSPCs to repopulate the adult blood system differs depending on where and when these cells were generated. It is also unknown whether hemogenic endothelial cells exist in fetal, neonatal, or adult bone marrow and whether they continue to generate HSPCs postnatally. Although this is not a well-accepted concept, it has been demonstrated by lineage-tracing studies that HSCs generated during embryogenesis do not account for all hematopoietic cells generated after birth (11). It has also been shown in vitro that human cord blood HPCs are able to differentiate into adherent proliferating endothelial precursors capable of functioning as hemogenic endothelial cells under the instruction of hematopoietic growth factors (145). These observations support the possibility of the existence of hemogenic endothelium after birth, but this requires further investigation. In addition, given that embryonic hematopoiesis serves as the foundation of the adult hematopoietic system, we need to better understand how dysregulation of hemogenic endothelial cell specification and HSPC production during development impacts the development of hematologic malignancies and other postnatal hematopoietic disorders. Thus, further elucidating the molecular regulation of hemogenic endothelial cell specification and their subsequent generation of HSPCs not only advances our understanding of embryonic development, but also our understanding of adult HSCs and potentially lifelong hematopoiesis. Insights gained can also be applied toward the treatment of hematopoietic disorders and the in vitro generation of human HSCs for clinical therapies.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Ferkowicz MJ, Yoder MC. 2005. Blood island formation: longstanding observations and modern interpretations. Exp. Hematol 33:1041–47 [DOI] [PubMed] [Google Scholar]
- 2.Palis J, Robertson S, Kennedy M, Wall C, Keller G. 1999. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development 126:5073–84 [DOI] [PubMed] [Google Scholar]
- 3.Tober J, Koniski A, McGrath KE, Vemishetti R, Emerson R, et al. 2007. The megakaryocyte lineage originates from hemangioblast precursors and is an integral component both of primitive and of definitive hematopoiesis. Blood 109:1433–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perdiguero EG, Klapproth K, Schulz C, Busch K, Azzoni E, et al. 2015. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 518:547–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lucitti JL, Jones EA, Huang C, Chen J, Fraser SE, Dickinson ME. 2007. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development 134:3317–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gritz E, Hirschi KK. 2016. Specification and function of hemogenic endothelium during embryogenesis. Cell. Mol. Life Sci 73:1547–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshimoto M, Porayette P, Glosson NL, Conway SJ, Carlesso N, et al. 2012. Autonomous murine T-cell progenitor production in the extra-embryonic yolk sac before HSC emergence. Blood 119:5706–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medvinsky A, Dzierzak E. 1996. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell 86:897–906 [DOI] [PubMed] [Google Scholar]
- 9.Coskun S, Chao H, Vasavada H, Heydari K, Gonzales N, et al. 2014. Development of the fetal bone marrow niche and regulation of HSC quiescence and homing ability by emerging osteolineage cells. Cell Rep. 9:581–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaffredo T, Gautier R, Brajeul V, Dieterlen-Lievre F. 2000. Tracing the progeny of the aortic hemangioblast in the avian embryo. Dev. Biol 224:204–14 [DOI] [PubMed] [Google Scholar]
- 11.Zovein AC, Hofmann JJ, Lynch M, French WJ, Turlo KA, et al. 2008. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell 3:625–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldie LC, Lucitti JL, Dickinson ME, Hirschi KK. 2008. Cell signaling directing the formation and function of hemogenic endothelium during murine embryogenesis. Blood 112:3194–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcelo KL, Sills TM, Coskun S, Vasavada H, Sanglikar S, et al. 2013. Hemogenic endothelial cell specification requires c-Kit, Notch signaling, and p27-mediated cell-cycle control. Dev. Cell 27:504–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kissa K, Herbomel P. 2010. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 464:112–15 [DOI] [PubMed] [Google Scholar]
- 15.Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY, Traver D. 2010. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 464:108–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boisset JC, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, Robin C. 2010. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature 464:116–20 [DOI] [PubMed] [Google Scholar]
- 17.Eilken HM, Nishikawa S, Schroeder T. 2009. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature 457:896–900 [DOI] [PubMed] [Google Scholar]
- 18.Rafii S, Kloss CC, Butler JM, Ginsberg M, Gars E, et al. 2013. Human ESC-derived hemogenic endothelial cells undergo distinct waves of endothelial to hematopoietic transition. Blood 121:770–80 [DOI] [PubMed] [Google Scholar]
- 19.Hirschi KK. 2012. Hemogenic endothelium during development and beyond. Blood 119:4823–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cumano A, Ferraz JC, Klaine M, Di Santo JP, Godin I. 2001. Intraembryonic, but not yolk sac hematopoietic precursors, isolated before circulation, provide long-term multilineage reconstitution. Immunity 15:477–85 [DOI] [PubMed] [Google Scholar]
- 21.Chen MJ, Li Y, De Obaldia ME, Yang Q, Yzaguirre AD, et al. 2011. Erythroid/myeloid progenitors and hematopoietic stem cells originate from distinct populations of endothelial cells. Cell Stem Cell 9:541–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldie LC, Lucitti JL, Dickinson ME, Hirschi KK. 2008. Cell signaling directing the formation and function of hemogenic endothelium during murine embryogenesis. Blood 112:3194–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kabrun N, Buhring HJ, Choi K, Ullrich A, Risau W, Keller G. 1997. Flk-1 expression defines a population of early embryonic hematopoietic precursors. Development 124:2039–48 [DOI] [PubMed] [Google Scholar]
- 24.Nishikawa SI, Nishikawa S, Hirashima M, Matsuyoshi N, Kodama H. 1998. Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development 125:1747–57 [DOI] [PubMed] [Google Scholar]
- 25.Anderson H, Patch TC, Reddy PN, Hagedorn EJ, Kim PG, et al. 2015. Hematopoietic stem cells develop in the absence of endothelial cadherin 5 expression. Blood 126:2811–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swiers G, Rode C, Azzoni E, de Bruijn MF. 2013. A short history of hemogenic endothelium. Blood Cells Mol. Dis 51:206–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nadin BM, Goodell MA, Hirschi KK. 2003. Phenotype and hematopoietic potential of side population cells throughout embryonic development. Blood 102:2436–43 [DOI] [PubMed] [Google Scholar]
- 28.Nishikawa SI, Nishikawa S, Kawamoto H, Yoshida H, Kizumoto M, et al. 1998. In vitro generation of lymphohematopoietic cells from endothelial cells purified from murine embryos. Immunity 8:761–69 [DOI] [PubMed] [Google Scholar]
- 29.Fang JS, Gritz EC, Marcelo KL, Hirschi KK. 2016. Isolation of murine embryonic hemogenic endothelial cells. J. Vis. Exp 112:e54150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rybtsov S, Sobiesiak M, Taoudi S, Souilhol C, Senserrich J, et al. 2011. Hierarchical organization and early hematopoietic specification of the developing HSC lineage in the AGM region. J. Exp. Med 208:1305–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Velten L, Haas SF, Raffel S, Blaszkiewicz S, Islam S, et al. 2017. Human haematopoietic stem cell lineage commitment is a continuous process. Nat. Cell Biol 19:271–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swiers G, Baumann C, O’Rourke J, Giannoulatou E, Taylor S, et al. 2013. Early dynamic fate changes in haemogenic endothelium characterized at the single-cell level. Nat. Commun 4:2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou F, Li X, Wang W, Zhu P, Zhou J, et al. 2016. Tracing haematopoietic stem cell formation at single-cell resolution. Nature 533:487–92 [DOI] [PubMed] [Google Scholar]
- 34.Baron CS, Kester L, Klaus A, Boisset JC, Thambyrajah R, et al. 2018. Single-cell transcriptomics reveal the dynamic of haematopoietic stem cell production in the aorta. Nat. Commun 9:2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marcelo KL, Goldie LC, Hirschi KK. 2013. Regulation of endothelial cell differentiation and specification. Circ. Res 112:1272–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bohnsack BL, Lai L, Dolle P, Hirschi KK. 2004. Signaling hierarchy downstream of retinoic acid that independently regulates vascular remodeling and endothelial cell proliferation. Genes Dev. 18:1345–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lai L, Bohnsack BL, Niederreither K, Hirschi KK. 2003. Retinoic acid regulates endothelial cell proliferation during vasculogenesis. Development 130:6465–74 [DOI] [PubMed] [Google Scholar]
- 38.Chanda B, Ditadi A, Iscove NN, Keller G. 2013. Retinoic acid signaling is essential for embryonic hematopoietic stem cell development. Cell 155:215–27 [DOI] [PubMed] [Google Scholar]
- 39.Lancrin C, Sroczynska P, Stephenson C, Allen T, Kouskoff V, Lacaud G. 2009. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature 457:892–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Porcher C, Swat W, Rockwell K, Fujiwara Y, Alt FW, Orkin SH. 1996. The T cell leukemia oncoprotein SCL/tal-1 is essential for development of all hematopoietic lineages. Cell 86:47–57 [DOI] [PubMed] [Google Scholar]
- 41.Van Handel B, Montel-Hagen A, Sasidharan R, Nakano H, Ferrari R, et al. 2012. Scl represses cardiomyogenesis in prospective hemogenic endothelium and endocardium. Cell 150:590–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gering M, Patient R. 2010. Notch signalling and haematopoietic stem cell formation during embryogenesis. J. Cell. Physiol 222:11–16 [DOI] [PubMed] [Google Scholar]
- 43.Chen X, Hartman A, Guo S. 2015. Choosing cell fate through a dynamic cell cycle. Curr. Stem Cell Rep 1:129–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aggarwal R, Lu J, Pompili VJ, Das H. 2012. Hematopoietic stem cells: transcriptional regulation, ex vivo expansion and clinical application. Curr. Mol. Med 12:34–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bachs O, Gallastegui E, Orlando S, Bigas A, Morante-Redolat JM, et al. 2018. Role of p27Kip1 as a transcriptional regulator. Oncotarget 9:26259–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montagner S, Dehó L, Monticelli S. 2014. MicroRNAs in hematopoietic development. BMC Immunol. 15:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Small EM, Olson EN. 2011. Pervasive roles of microRNAs in cardiovascular biology. Nature 469:336–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kasper DM, Hintzen J, Wu Y, Ghersi J, Mandl HK, et al. 2020. The N-Glycome regulates the endothelial-to-hematopoietic transition. bioRxiv 602912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berger RP, Dookwah M, Steet R, Dalton S. 2016. Glycosylation and stem cells: regulatory roles and application of iPSCs in the study of glycosylation-related disorders. Bioessays 38:1255–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McGinnis W, Krumlauf R. 1992. Homeobox genes and axial patterning. Cell 68:283–302 [DOI] [PubMed] [Google Scholar]
- 51.Iacovino M, Chong D, Szatmari I, Hartweck L, Rux D, et al. 2011. HoxA3 is an apical regulator of haemogenic endothelium. Nat. Cell Biol 13:72–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bahrami SB, Veiseh M, Dunn AA, Boudreau NJ. 2011. Temporal changes in Hox gene expression accompany endothelial cell differentiation of embryonic stem cells. Cell Adhes. Migr 5:133–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanghez V, Luzzi A, Clarke D, Kee D, Beuder S, et al. 2017. Notch activation is required for downregulation of HoxA3-dependent endothelial cell phenotype during blood formation. PLOS ONE 12:e0186818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oshima M, Endoh M, Endo TA, Toyoda T, Nakajima-Takagi Y, et al. 2011. Genome-wide analysis of target genes regulated by HoxB4 in hematopoietic stem and progenitor cells developing from embryonic stem cells. Blood 117:e142–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Teichweyde N, Kasperidus L, Carotta S, Kouskoff V, Lacaud G, et al. 2018. HOXB4 promotes hemogenic endothelium formation without perturbing endothelial cell development. Stem Cell Rep. 10:875–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kyba M, Perlingeiro RC, Daley GQ. 2002. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell 109:29–37 [DOI] [PubMed] [Google Scholar]
- 57.Chan KM, Bonde S, Klump H, Zavazava N. 2008. Hematopoiesis and immunity of HOXB4-transduced embryonic stem cell-derived hematopoietic progenitor cells. Blood 111:2953–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Swiers G, de Bruijn M, Speck NA. 2010. Hematopoietic stem cell emergence in the conceptus and the role of Runx1. Int. J. Dev. Biol 54:1151–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Q, Stacy T, Binder M, Marin-Padilla M, Sharpe AH, Speck NA. 1996. Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. PNAS 93:3444–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Okuda T, van Deursen J, Hiebert SW, Grosveld G, Downing JR. 1996. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell 84:321–30 [DOI] [PubMed] [Google Scholar]
- 61.North T, Gu TL, Stacy T, Wang Q, Howard L, et al. 1999. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development 126:2563–75 [DOI] [PubMed] [Google Scholar]
- 62.Burns CE, Traver D, Mayhall E, Shepard JL, Zon LI. 2005. Hematopoietic stem cell fate is established by the Notch-Runx pathway. Genes Dev. 19:2331–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kalev-Zylinska ML, Horsfield JA, Flores MV, Postlethwait JH, Vitas MR, et al. 2002. Runx1 is required for zebrafish blood and vessel development and expression of a human RUNX1-CBF2T1 transgene advances a model for studies of leukemogenesis. Development 129:2015–30 [DOI] [PubMed] [Google Scholar]
- 64.Li Z, Chen MJ, Stacy T, Speck NA. 2006. Runx1 function in hematopoiesis is required in cells that express Tek. Blood 107:106–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lam EY, Hall CJ, Crosier PS, Crosier KE, Flores MV. 2010. Live imaging of Runx1 expression in the dorsal aorta tracks the emergence of blood progenitors from endothelial cells. Blood 116:909–14 [DOI] [PubMed] [Google Scholar]
- 66.Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. 2009. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature 457:887–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nottingham WT, Jarratt A, Burgess M, Speck CL, Cheng JF, et al. 2007. Runx1-mediated hematopoietic stem-cell emergence is controlled by a Gata/Ets/SCL-regulated enhancer. Blood 110:4188–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lizama CO, Hawkins JS, Schmitt CE, Bos FL, Zape JP, et al. 2015. Repression of arterial genes in hemogenic endothelium is sufficient for haematopoietic fate acquisition. Nat. Commun 6:7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoogenkamp M, Lichtinger M, Krysinska H, Lancrin C, Clarke D, et al. 2009. Early chromatin unfolding by RUNX1: a molecular explanation for differential requirements during specification versus maintenance of the hematopoietic gene expression program. Blood 114:299–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lancrin C, Mazan M, Stefanska M, Patel R, Lichtinger M, et al. 2012. GFI1 and GFI1B control the loss of endothelial identity of hemogenic endothelium during hematopoietic commitment. Blood 120:314–22 [DOI] [PubMed] [Google Scholar]
- 71.Thambyrajah R, Mazan M, Patel R, Moignard V, Stefanska M, et al. 2016. GFI1 proteins orchestrate the emergence of haematopoietic stem cells through recruitment of LSD1. Nat. Cell Biol 18:21–32 [DOI] [PubMed] [Google Scholar]
- 72.Robin C, Ottersbach K, Durand C, Peeters M, Vanes L, et al. 2006. An unexpected role for IL-3 in the embryonic development of hematopoietic stem cells. Dev. Cell 11:171–80 [DOI] [PubMed] [Google Scholar]
- 73.Narula J, Williams CJ, Tiwari A, Marks-Bluth J, Pimanda JE, Igoshin OA. 2013. Mathematical model of a gene regulatory network reconciles effects of genetic perturbations on hematopoietic stem cell emergence. Dev. Biol 379:258–69 [DOI] [PubMed] [Google Scholar]
- 74.Patterson LJ, Gering M, Eckfeldt CE, Green AR, Verfaillie CM, et al. 2007. The transcription factors Scl and Lmo2 act together during development of the hemangioblast in zebrafish. Blood 109:2389–98 [DOI] [PubMed] [Google Scholar]
- 75.Kim PG, Albacker CE, Lu YF, Jang IH, Lim Y, et al. 2013. Signaling axis involving Hedgehog, Notch, and Scl promotes the embryonic endothelial-to-hematopoietic transition. PNAS 110:E141–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Robb L, Lyons I, Li R, Hartley L, Kontgen F, et al. 1995. Absence of yolk sac hematopoiesis from mice with a targeted disruption of the scl gene. PNAS 92:7075–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Qian F, Zhen F, Xu J, Huang M, Li W, Wen Z. 2007. Distinct functions for different scl isoforms in zebrafish primitive and definitive hematopoiesis. PLOS Biol. 5:e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ren X, Gomez GA, Zhang B, Lin S. 2010. Scl isoforms act downstream of etsrp to specify angioblasts and definitive hematopoietic stem cells. Blood 115:5338–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang M, Wang H, Wen Y, Chen X, Liu X, et al. 2018. MEIS2 regulates endothelial to hematopoietic transition of human embryonic stem cells by targeting TAL1. Stem Cell Res. Ther 9:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ling KW, Ottersbach K, van Hamburg JP, Oziemlak A, Tsai FY, et al. 2004. GATA-2 plays two functionally distinct roles during the ontogeny of hematopoietic stem cells. J. Exp. Med 200:871–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang K, Gao J, Du J, Ma N, Zhu Y, et al. 2016. Generation and analysis of GATA2w/eGFP human ESCs reveal ITGB3/CD61 as a reliable marker for defining hemogenic endothelial cells during hematopoiesis. Stem Cell Rep. 7:854–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsai FY, Keller G, Kuo FC, Weiss M, Chen J, et al. 1994. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature 371:221–26 [DOI] [PubMed] [Google Scholar]
- 83.de Pater E, Kaimakis P, Vink CS, Yokomizo T, Yamada-Inagawa T, et al. 2013. Gata2 is required for HSC generation and survival. J. Exp. Med 210:2843–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gao X, Johnson KD, Chang YI, Boyer ME, Dewey CN, et al. 2013. Gata2 cis-element is required for hematopoietic stem cell generation in the mammalian embryo. J. Exp. Med 210:2833–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eich C, Arlt J, Vink CS, Kartalaei PS, Kaimakis P, et al. 2018. In vivo single cell analysis reveals Gata2 dynamics in cells transitioning to hematopoietic fate. J. Exp. Med 215:233–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Butko E, Distel M, Pouget C, Weijts B, Kobayashi I, et al. 2015. Gata2b is a restricted early regulator of hemogenic endothelium in the zebrafish embryo. Development 142:1050–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kaimakis P, de Pater E, Eich C, Kartalaei PS, Kauts ML, et al. 2016. Functional and molecular characterization of mouse Gata2-independent hematopoietic progenitors. Blood 127:1426–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pandolfi PP, Roth ME, Karis A, Leonard MW, Dzierzak E, et al. 1995. Targeted disruption of the GATA3 gene causes severe abnormalities in the nervous system and in fetal liver haematopoiesis. Nat. Genet 11:40–44 [DOI] [PubMed] [Google Scholar]
- 89.Fitch SR, Kimber GM, Wilson NK, Parker A, Mirshekar-Syahkal B, et al. 2012. Signaling from the sympathetic nervous system regulates hematopoietic stem cell emergence during embryogenesis. Cell Stem Cell 11:554–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Robert-Moreno A, Guiu J, Ruiz-Herguido C, Lopez ME, Ingles-Esteve J, et al. 2008. Impaired embryonic haematopoiesis yet normal arterial development in the absence of the Notch ligand Jagged1. EMBO J. 27:1886–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jang IH, Lu YF, Zhao L, Wenzel PL, Kume T, et al. 2015. Notch1 acts via Foxc2 to promote definitive hematopoiesis via effects on hemogenic endothelium. Blood 125:1418–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kumano K, Chiba S, Kunisato A, Sata M, Saito T, et al. 2003. Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity 18:699–711 [DOI] [PubMed] [Google Scholar]
- 93.Gerri C, Marass M, Rossi A, Stainier DYR. 2018. Hif-1α and Hif-2α regulate hemogenic endothelium and hematopoietic stem cell formation in zebrafish. Blood 131:963–73 [DOI] [PubMed] [Google Scholar]
- 94.Hadland BK, Huppert SS, Kanungo J, Xue Y, Jiang R, et al. 2004. A requirement for Notch1 distinguishes 2 phases of definitive hematopoiesis during development. Blood 104:3097–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Krebs LT, Shutter JR, Tanigaki K, Honjo T, Stark KL, Gridley T. 2004. Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev. 18:2469–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Duarte A, Hirashima M, Benedito R, Trindade A, Diniz P, et al. 2004. Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev. 18:2474–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Robert-Moreno A, Espinosa L, de la Pompa JL, Bigas A. 2005. RBPjκ-dependent Notch function regulates Gata2 and is essential for the formation of intra-embryonic hematopoietic cells. Development 132:1117–26 [DOI] [PubMed] [Google Scholar]
- 98.Gama-Norton L, Ferrando E, Ruiz-Herguido C, Liu Z, Guiu J, et al. 2015. Notch signal strength controls cell fate in the haemogenic endothelium. Nat. Commun 6:8510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Uenishi GI, Jung HS, Kumar A, Park MA, Hadland BK, et al. 2018. NOTCH signaling specifies arterial-type definitive hemogenic endothelium from human pluripotent stem cells. Nat. Commun 9:1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ayllon V, Bueno C, Ramos-Mejia V, Navarro-Montero O, Prieto C, et al. 2015. The Notch ligand DLL4 specifically marks human hematoendothelial progenitors and regulates their hematopoietic fate. Leukemia 29:1741–53 [DOI] [PubMed] [Google Scholar]
- 101.Guiu J, Shimizu R, D’Altri T, Fraser ST, Hatakeyama J, et al. 2013. Hes repressors are essential regulators of hematopoietic stem cell development downstream of Notch signaling. J. Exp. Med 210:71–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guiu J, Bergen DJ, De Pater E, Islam AB, Ayllon V, et al. 2014. Identification of Cdca7 as a novel Notch transcriptional target involved in hematopoietic stem cell emergence. J. Exp. Med 211:2411–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nakagawa M, Ichikawa M, Kumano K, Goyama S, Kawazu M, et al. 2006. AML1/Runx1 rescues Notch1-null mutation-induced deficiency of para-aortic splanchnopleural hematopoiesis. Blood 108:3329–34 [DOI] [PubMed] [Google Scholar]
- 104.Clements WK, Kim AD, Ong KG, Moore JC, Lawson ND, Traver D. 2011. A somitic Wnt16/Notch pathway specifies haematopoietic stem cells. Nature 474:220–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kobayashi I, Kobayashi-Sun J, Kim AD, Pouget C, Fujita N, et al. 2014. Jam1a–Jam2a interactions regulate haematopoietic stem cell fate through Notch signalling. Nature 512:319–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee Y, Manegold JE, Kim AD, Pouget C, Stachura DL, et al. 2014. FGF signalling specifies haematopoietic stem cells through its regulation of somitic Notch signalling. Nat. Commun 5:5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nguyen PD, Hollway GE, Sonntag C, Miles LB, Hall TE, et al. 2014. Haematopoietic stem cell induction by somite-derived endothelial cells controlled by meox1. Nature 512:314–18 [DOI] [PubMed] [Google Scholar]
- 108.Mucenski ML, McLain K, Kier AB, Swerdlow SH, Schreiner CM, et al. 1991. A functional c-myb gene is required for normal murine fetal hepatic hematopoiesis. Cell 65:677–89 [DOI] [PubMed] [Google Scholar]
- 109.Zhang Y, Jin H, Li L, Qin FX, Wen Z. 2011. cMyb regulates hematopoietic stem/progenitor cell mobilization during zebrafish hematopoiesis. Blood 118:4093–101 [DOI] [PubMed] [Google Scholar]
- 110.Soza-Ried C, Hess I, Netuschil N, Schorpp M, Boehm T. 2010. Essential role of c-myb in definitive hematopoiesis is evolutionarily conserved. PNAS 107:17304–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mukai HY, Motohashi H, Ohneda O, Suzuki N, Nagano M, Yamamoto M. 2006. Transgene insertion in proximity to the c-myb gene disrupts erythroid-megakaryocytic lineage bifurcation. Mol. Cell. Biol 26:7953–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Takakura N, Watanabe T, Suenobu S, Yamada Y, Noda T, et al. 2000. A role for hematopoietic stem cells in promoting angiogenesis. Cell 102:199–209 [DOI] [PubMed] [Google Scholar]
- 113.Jing L, Tamplin OJ, Chen MJ, Deng Q, Patterson S, et al. 2015. Adenosine signaling promotes hematopoietic stem and progenitor cell emergence. J. Exp. Med 212:649–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Diaz MF, Li N, Lee HJ, Adamo L, Evans SM, et al. 2015. Biomechanical forces promote blood development through prostaglandin E2 and the cAMP–PKA signaling axis. J. Exp. Med 212:665–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Saxena S, Ronn RE, Guibentif C, Moraghebi R, Woods NB. 2016. Cyclic AMP signaling through Epac axis modulates human hemogenic endothelium and enhances hematopoietic cell generation. Stem Cell Rep. 6:692–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Costa G, Mazan A, Gandillet A, Pearson S, Lacaud G, Kouskoff V. 2012. SOX7 regulates the expression of VE-cadherin in the haemogenic endothelium at the onset of haematopoietic development. Development 139:1587–98 [DOI] [PubMed] [Google Scholar]
- 117.Gandillet A, Serrano AG, Pearson S, Lie ALM, Lacaud G, Kouskoff V. 2009. Sox7-sustained expression alters the balance between proliferation and differentiation of hematopoietic progenitors at the onset of blood specification. Blood 114:4813–22 [DOI] [PubMed] [Google Scholar]
- 118.Clarke RL, Yzaguirre AD, Yashiro-Ohtani Y, Bondue A, Blanpain C, et al. 2013. The expression of Sox17 identifies and regulates haemogenic endothelium. Nat. Cell Biol 15:502–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Serrano AG, Gandillet A, Pearson S, Lacaud G, Kouskoff V. 2010. Contrasting effects of Sox17- and Sox18-sustained expression at the onset of blood specification. Blood 115:3895–8 [DOI] [PubMed] [Google Scholar]
- 120.Corada M, Orsenigo F, Morini MF, Pitulescu ME, Bhat G, et al. 2013. Sox17 is indispensable for acquisition and maintenance of arterial identity. Nat. Commun 4:2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim I, Saunders TL, Morrison SJ. 2007. Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell 130:470–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nakajima-Takagi Y, Osawa M, Oshima M, Takagi H, Miyagi S, et al. 2013. Role of SOX17 in hematopoietic development from human embryonic stem cells. Blood 121:447–58 [DOI] [PubMed] [Google Scholar]
- 123.Richter J, Traver D, Willert K. 2017. The role of Wnt signaling in hematopoietic stem cell development. Crit. Rev. Biochem. Mol. Biol 52:414–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen B, Dodge ME, Tang W, Lu J, Ma Z, et al. 2009. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat. Chem. Biol 5:100–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Goessling W, North TE, Loewer S, Lord AM, Lee S, et al. 2009. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell 136:1136–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Grainger S, Richter J, Palazon RE, Pouget C, Lonquich B, et al. 2016. Wnt9a is required for the aortic amplification of nascent hematopoietic stem cells. Cell Rep. 17:1595–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Woll PS, Morris JK, Painschab MS, Marcus RK, Kohn AD, et al. 2008. Wnt signaling promotes hematoendothelial cell development from human embryonic stem cells. Blood 111:122–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gertow K, Hirst CE, Yu QC, Ng ES, Pereira LA, et al. 2013. WNT3A promotes hematopoietic or mesenchymal differentiation from hESCs depending on the time of exposure. Stem Cell Rep. 1:53–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ruiz-Herguido C, Guiu J, D’Altri T, Ingles-Esteve J, Dzierzak E, et al. 2012. Hematopoietic stem cell development requires transient Wnt/β-catenin activity. J. Exp. Med 209:1457–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sturgeon CM, Ditadi A, Awong G, Kennedy M, Keller G. 2014. Wnt signaling controls the specification of definitive and primitive hematopoiesis from human pluripotent stem cells. Nat. Biotechnol 32:554–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li Y, Esain V, Teng L, Xu J, Kwan W, et al. 2014. Inflammatory signaling regulates embryonic hematopoietic stem and progenitor cell production. Genes Dev. 28:2597–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Espin-Palazon R, Stachura DL, Campbell CA, Garcia-Moreno D, Del Cid N, et al. 2014. Proinflammatory signaling regulates hematopoietic stem cell emergence. Cell 159:1070–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sawamiphak S, Kontarakis Z, Stainier DY. 2014. Interferon gamma signaling positively regulates hematopoietic stem cell emergence. Dev. Cell 31:640–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.He Q, Zhang C, Wang L, Zhang P, Ma D, et al. 2015. Inflammatory signaling regulates hematopoietic stem and progenitor cell emergence in vertebrates. Blood 125:1098–106 [DOI] [PubMed] [Google Scholar]
- 135.Stachura DL, Svoboda O, Campbell CA, Espin-Palazon R, Lau RP, et al. 2013. The zebrafish granulocyte colony-stimulating factors (Gcsfs): 2 paralogous cytokines and their roles in hematopoietic development and maintenance. Blood 122:3918–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Monteiro R, Pinheiro P, Joseph N, Peterkin T, Koth J, et al. 2016. Transforming growth factor β drives hemogenic endothelium programming and the transition to hematopoietic stem cells. Dev. Cell 38:358–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wilkinson RN, Pouget C, Gering M, Russell AJ, Davies SG, et al. 2009. Hedgehog and Bmp polarize hematopoietic stem cell emergence in the zebrafish dorsal aorta. Dev. Cell 16:909–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kirmizitas A, Meiklejohn S, Ciau-Uitz A, Stephenson R, Patient R. 2017. Dissecting BMP signaling input into the gene regulatory networks driving specification of the blood stem cell lineage. PNAS 114:5814–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McReynolds LJ, Gupta S, Figueroa ME, Mullins MC, Evans T. 2007. Smad1 and Smad5 differentially regulate embryonic hematopoiesis. Blood 110:3881–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lan Y, He W, Li Z, Wang Y, Wang J, et al. 2014. Endothelial Smad4 restrains the transition to hematopoietic progenitors via suppression of ERK activation. Blood 123:2161–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Adamo L, Naveiras O, Wenzel PL, McKinney-Freeman S, Mack PJ, et al. 2009. Biomechanical forces promote embryonic haematopoiesis. Nature 459:1131–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.North TE, Goessling W, Peeters M, Li P, Ceol C, et al. 2009. Hematopoietic stem cell development is dependent on blood flow. Cell 137:736–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Challen GA, Boles NC, Chambers SM, Goodell MA. 2010. Distinct hematopoietic stem cell subtypes are differentially regulated by TGF-β1. Cell Stem Cell 6:265–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Huang K, Du J, Ma N, Liu J, Wu P, et al. 2015. GATA2−/− human ESCs undergo attenuated endothelial to hematopoietic transition and thereafter granulocyte commitment. Cell Regen. 4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pelosi E, Castelli G, Martin-Padura I, Bordoni V, Santoro S, et al. 2012. Human haemato-endothelial precursors: cord blood CD34+ cells produce haemogenic endothelium. PLOS ONE 7:e51109. [DOI] [PMC free article] [PubMed] [Google Scholar]


