Abstract
Selinexor is a selective inhibitor of nuclear export with anti-cancer properties. We performed a phase I study to determine the safety and maximum tolerated dose of selinexor when combined with high-dose dexamethasone, ifosfamide, carboplatin and etoposide (DICE) in relapsed/refractory T-cell lymphoma (TCL) and natural-killer/T-cell lymphoma (NKTL). Patients with relapsed/refractory TCL and NKTL were treated with standard dose ICE, dexamethasone 20 mg on days 3 to 7, and escalating doses of oral selinexor on days 3, 5 and 7 in a 3+3 design. Dose levels (DL) 1, 2 and 3 were 40, 60 and 80 mg, respectively. Eleven patients with a median age of 60 years were enrolled; six at DL1 and five at DL2. Patients had received a median of two (range, 1-4) prior lines of treatment and seven had primary refractory disease at entry into the study. Patients received a median of three cycles (range, 1-6) of selinexor-DICE. The most common grade 1 or 2 toxicities included nausea (64%), fatigue (55%), and anorexia (45%) and the most common grade 3 or 4 toxicities included thrombocytopenia (82%), anemia (82%), neutropenia (73%), and hyponatremia (73%). Two patients developed dose-limiting toxicities at DL2 and one at DL1. Five patients discontinued treatment for reasons other than disease progression or lack of response. Of the ten evaluable patients, the overall and complete response rates were 91% and 82%, respectively. The maximum tolerated dose of selinexor was 40 mg when combined with DICE. The combination showed promising complete response rates in patients with relapsed/refractory TCL and NKTL but was poorly tolerated. (clinicaltrials. gov identifier: NCT03212937).
Introduction
T-cell lymphoma (TCL) is a heterogeneous group of non-Hodgkin lymphomas seen more commonly in Asia than in the West.1,2 The 5-year overall survival rates are approximately 30% for the most common subtypes of TCL, including peripheral- T cell lymphoma (PTCL)-not otherwise specified (NOS), angioimmunoblastic T-cell lymphoma (AITL) and natural-killer/T-cell lymphoma (NKTL).2 Patients with PTCL who relapse or progress after initial therapy have poor survival outcomes with median progression-free and overall survival of 3.1 and 5.5 months, respectively. 3 However, patients who achieve a complete response to salvage therapy have better median progression-free and overall survival (12.2 and 18 months, respectively).3 Some patients who achieve a complete response to salvage therapy may be considered for high-dose chemotherapy (HDC) and autologous stem cell transplantation (SCT) consolidation with curative intent.4 Thus there is a need to improve complete response rates for salvage regimens.
Exportin 1 (XPO1/CRM1) is a nuclear export protein that is responsible for the nuclear to cytoplasmic translocation of tumor suppressor proteins (TSP) and growth regulator proteins (GRP) such as TP53, p21, p27, FOXO3 and nucleophosmin 1 (NPM1), leading to their inactivation.5 XPO1 is overexpressed in many malignancies including TCL and increased XPO1 expression is associated with poor survival.6-10 XPO1 also transports topoisomerase II enzymes to the cytoplasm and cytoplasmic localization of topoisomerase II enzymes has been identified as a mechanism of cancer resistance. Therefore, when topoisomerase IIα enzymes are not in contact with DNA, topoisomerase II inhibitors, such as doxorubicin, are unable to induce cell death.11 Selinexor® is an oral, first-in-class, potent selective inhibitor of nuclear export, which binds to XPO1, leading to nuclear retention of the TSP, GRP, and topoisomerase IIα enzymes, restoring their function.
Selinexor has received Food and Drug Administration approval for relapsed or refractory multiple myeloma and diffuse large B-cell lymphoma, and has shown significant anticancer activity across a range of preclinical models of cancer, including T-cell acute lymphoblastic leukemia.12 There were also preclinical studies demonstrating the ability of selinexor to sensitize cancer cells to topoisomerase inhibitors,13 alkylating agents5 and steroids.14 A phase I study of selinexor in relapsed/refractory non-Hodgkin lymphomas showed overall response rates of about 30%.15 We hypothesized that selinexor could synergize with ifosfamide (an alkylating agent) and etoposide (a topoisomerase II inhibitor) in the ifosfamide, carboplatin and etoposide (ICE) regimen and we added high-dose dexamethasone to this regimen to improve the efficacy of ICE as a salvage regimen for TCL. We conducted a phase I study to identify the dose of selinexor that could be combined safely with standard-dose ICE and high-dose dexamethasone (DICE) in relapsed or refractory TCL (clinicaltrials. gov identifier: NCT03212937).
Methods
Patients
Recruited patients had histologically confirmed relapsed or refractory PTCL or NKTL. Patients with CD30+ anaplastic large cell lymphoma (ALCL) had to have failed treatment with brentuximab vedotin. The study was conducted at the National Cancer Center Singapore and the Singapore General Hospital after approval by the Singhealth Institutional Review Board and in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines of the International Conference on Harmonization. Written consent was obtained from all patients prior to their entry into the study.
Study design
This was an open-label, phase I study in which eligible patients were treated with DICE plus escalating doses of oral selinexor in a 3+3 design. The primary objective was to assess the safety and determine the maximum tolerated dose of selinexor that could be combined with DICE.
The first dose level (DL) of selinexor was chosen as 40 mg because at the time of developing the study the recommended phase II dose of selinexor from phase I studies was 60 mg (fixed dose)15,16 and there was concern that Asian patients tolerated selinexor less well than Caucasian patients. Hence DL -1, 1, 2 and 3 were 20, 40, 60 and 80 mg, respectively.
All patients received intravenous doses of ICE in a 21-day cycle: ifosfamide 5 g/m2 over days 1-3, carboplatin (area under the curve 5) on day 1 and etoposide 100 mg/m2 on days 1-3. Selinexor was administered on days 3, 5 and 7. Additionally, all patients received oral dexamethasone 20 mg/day for 5 days on days 3-7 for anticipated anticancer synergy of steroids with selinexor. Anti-emetics included oral aprepitant and granisetron 3 mg on days 1-3 and dexamethasone 8 mg on days 1-2. Oral olanzapine 5 mg was recommended with each dose of selinexor.
Eligible patients could undergo HDC and SCT after at least two cycles of study treatment. Patients who were not eligible for SCT could receive up to six cycles of the study treatment. Patients could also receive maintenance selinexor (60 mg weekly) if they had not progressed upon completion of selinexor-DICE.
Assessment of adverse events and dose-limiting toxicities
Dose-limiting toxicities were defined as any of the following treatment-related toxicities occurring during the first cycle of treatment: failure to resolve any grade 3 or higher non-hematologic toxicities, platelet count of less than 75x109/L or absolute neutrophil count of less than 1x109/L by day 29, a platelet count of less than 25x109/L or an absolute neutrophil count of less than 0.5x109/L lasting more than 14 days, a platelet nadir of 10x109/L or less, or any grade 5 toxicities.
Response assessment
Responses were assessed using the revised International Working Group Criteria for non-Hodgkin lymphoma.17 Tumor measurements with positron emission tomography and computed tomography scans were performed at baseline, after two cycles of selinexor-DICE, and 6-8 weeks after the last cycles of selinexor- DICE or after HDC/SCT.
Statistical analysis
Any patient who received one dose of selinexor was included in the safety population and only the patients who completed two cycles of treatment and the first response assessment were included in the efficacy analysis.
Results
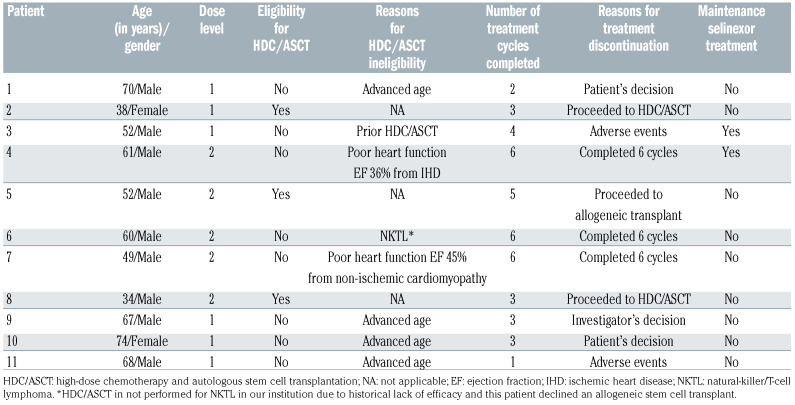
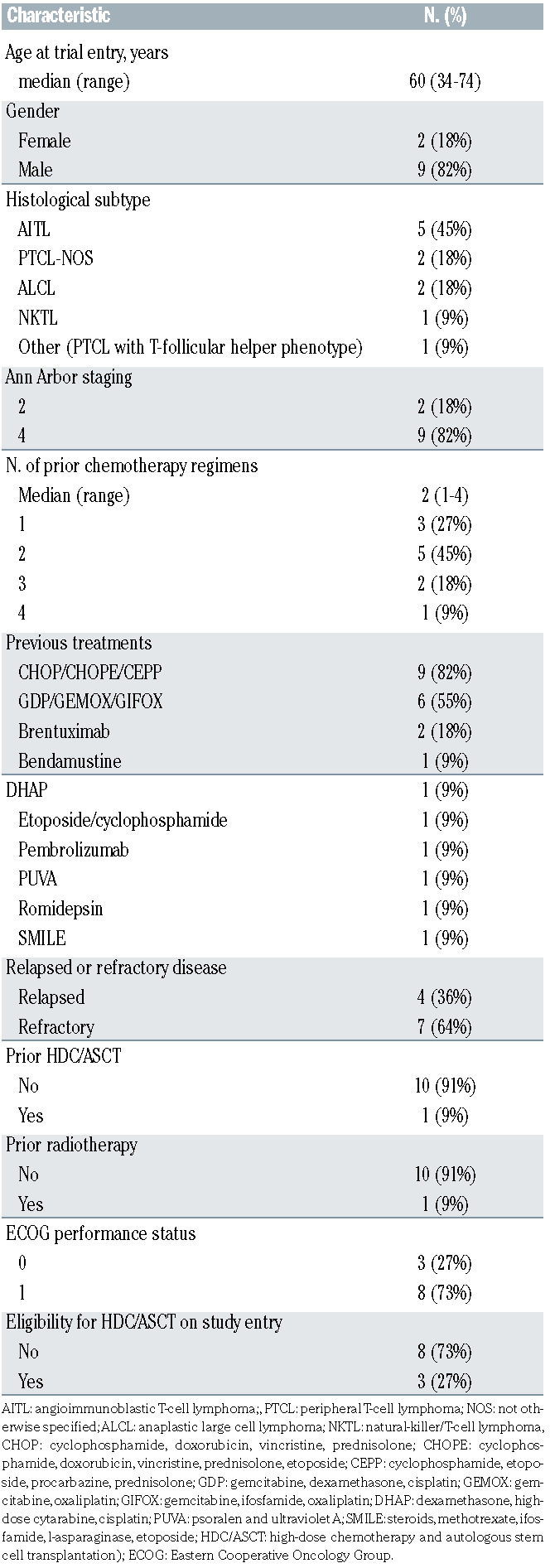
Eleven patients were recruited into the study. The median age was 60 years (range, 34-74), and nine were male. All patients were Asian; seven (64%) were Chinese, two (18%) were Malay, one (9%) was Indian and another (9%) was Myanmarese. The most common histological subtype in this study was AITL (n=5), followed by PTCL-NOS (n=2). There were one of each of the following histological subtypes; ALK-negative ALCL, ALK-positive ALCL, NKTL and PTCL with T-follicular helper phenotype. Two patients had stage II disease and the rest had stage IV disease at study entry. The patients had received a median of two prior lines of treatment, one had received prior HDC and autologous SCT, and one had been previously administered radiotherapy. Seven patients (64%) had primary refractory disease, defined as disease that had not responded to any prior chemotherapy, or disease that progressed within 8 weeks from the end of treatment response assessment. All patients had an Eastern Cooperative Oncology Group performance status of 0 or 1. (Table 1)
Patients received a median number of three cycles (range, 1-6) of selinexor-DICE. Three patients were eligible for HDC/SCT. (Table 2) Two patients underwent SCT, one autologous and the other allogeneic SCT. The patient who underwent autologous SCT had 8.52x106/L CD34 cells collected prior to the transplant: engraftment of neutrophils and platelets occurred on days 9 and 8, respectively. Engraftment of neutrophils and platelets in the patient who underwent allogeneic SCT occurred on days 10 and 14, respectively. The third patient eligible for HDC experienced disease progression just before autologous SCT and he was given alectinib before proceeding to allogeneic SCT. Eight patients were not eligible for HDC/autologous SCT and these patients received a median of 3.5 of the planned six cycles of study treatment (Table 2). Three patients completed all six cycles of study treatment, two patients discontinued treatment because of adverse events, another two refused to continue treatment, and one patient’s treatment was discontinued as a result of the investigator’s decision. Two patients received maintenance selinexor upon completing six cycles of selinexor-ICE.
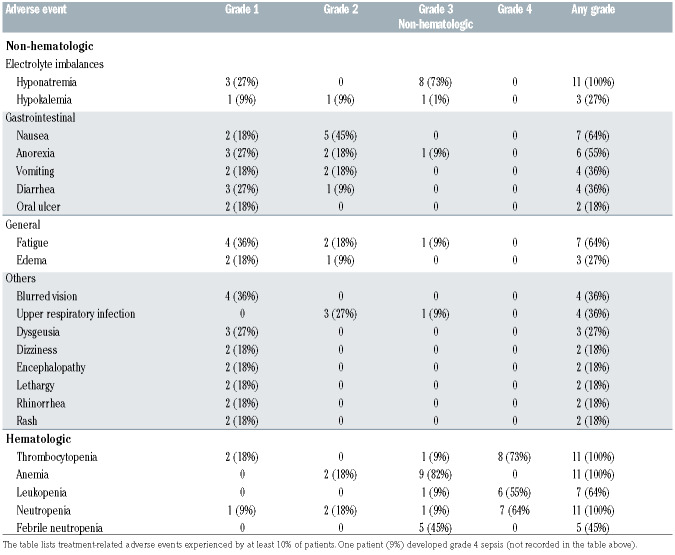
The most common grade 1 and 2 toxicities included nausea (64%), fatigue (55%), and anorexia (45%). Grade 3 or 4 toxicities occurring in at least one patient included thrombocytopenia (82%), anemia (82%), neutropenia (73%), hyponatremia (73%), leukopenia (64%), febrile neutropenia (45%), and one patient each who developed fatigue, anorexia, fever, hypokalemia, sepsis, and an upper respiratory tract infection (Table 3). There were no treatment- related deaths. All patients had hyponatremia (any grade), which took a median of 7 days (range, 1 to 21) to resolve. Considering all the cycles of treatment, dose reductions for selinexor, ifosfamide, carboplatin and etoposide were required in five (45%), ten (91%), eight (73%), and nine (82%) of patients, respectively. At DL1 and DL2, the median dose intensities (range) for selinexor were 94.2% (range, 66.7-100%) and 77.8% (range, 72.2-88.9%), respectively. The median dose intensities for ifosfamide, carboplatin and etoposide were 79.8% (range, 74.8-99.4%), 87.7% (range, 76.9-105.7%), and 73.0% (range, 26.0-98.7%), respectively.
Six and five patients received selinexor at the dose of 40 mg and 60 mg, respectively. Patient 11 developed a doselimiting toxicity at DL1. He developed a platelet nadir of less than 10x109/L and failed to recover his platelet count to at least 75x109/L by day 28. Two patients developed doselimiting toxicities at DL2 (patients 5 and 8): one had a platelet nadir of less than 10x109/L on day 11 and in another the platelet count failed to recover to at least 75x109/L by day 28 but did recover by day 32. For these two patients, the platelet counts recovered to 75x109/L within 11 days of the dose-limiting toxicity and they both remained on study. All three patients who developed dose-limiting toxicities had a baseline platelet count of more than 100x109/L. Thus, 40 mg of selinexor was the maximum tolerated dose that could be combined with DICE in this study.
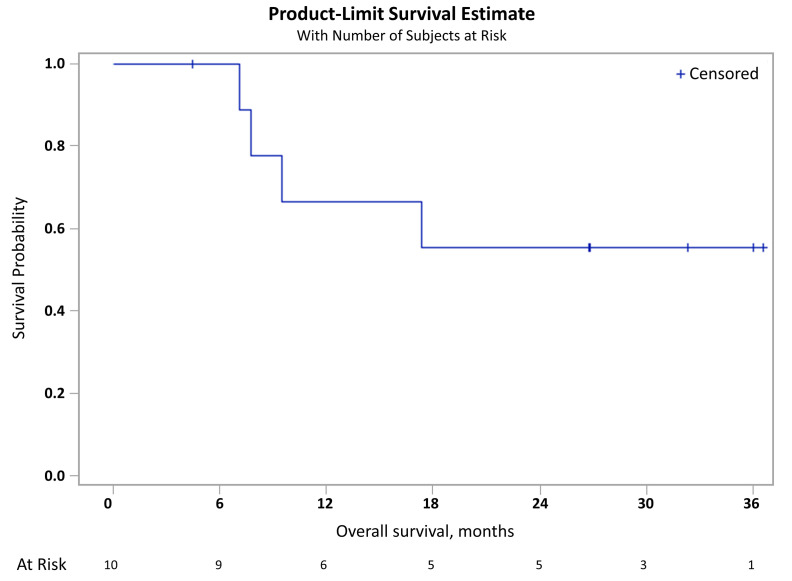
Ten patients were evaluable for response after two cycles of treatment. One patient (patient 11) progressed before he could be evaluated by positron emission tomography. Of the ten evaluable patients, all responded; nine (82% [95% confidence interval: 48-98]) achieved a complete response and one (10%) achieved a partial response (Table 3). The median follow-up of the study was 32.3 months (range, 4.4-36.6). During this period, seven patients experienced disease progression and five patients died. The median overall survival was not reached and the 1-year overall survival rate was 66.7% (95% confidence interval: 28.2-87.8%) (Figure 1).
Table 1.
Baseline demographic data.
Discussion
In this study of Asian patients with relapsed or refractory TCL, we found that the maximum tolerated dose of selinexor that could be combined with high-dose dexamethasone and standard dose ICE i.e., DICE, in a 21-day cycle, was 40 mg on days 3, 5 and 7. The combination was highly active with a response rate of 100% among the evaluable patients. However, toxicities were significant. Patients ineligible for HDC/autologous SCT underwent a median of 3.5 cycles of this treatment, and five of 11 (45.5%) discontinued treatment for reasons other than disease progression or a lack of response.
Hyponatremia is a known and well-established adverse event associated with selinexor and was the most common non-hematologic adverse event that occurred in this study. All-grade hyponatremia occurred after selinexor was administered, was transient and resolved after a median of 7 days from the onset. Patients who developed hyponatremia were generally asymptomatic and managed with oral rehydration salts, sodium tablets, intravenous saline infusions and, from mid-way through the study, patients were also encouraged to prophylactically hydrate with electrolyte-rich salt drinks during the period they were on selinexor. Our clinical trial together with currently available data suggest that there may be a higher incidence of grade 3 or more selinexor-induced hyponatremia among Asian patients16,18-20 and further studies will be required to substantiate and understand this phenomenon better. Although hyponatremia can occur with high-dose ifosfamide, the rates of hyponatremia that occurred in this study were much higher than those based on experiences with ICE therapy alone.
Figure 1.
Kaplan-Meier plot of overall survival in the efficacy population.
Table 2.
Disposition of the patients.
Table 3.
Treatment-related adverse events.
The incidence of grade 3 or 4 thrombocytopenia in this study was high (82%) and both dose-limiting toxicities were related to low platelet counts. This can be expected given the overlapping toxicities of selinexor and ICE (especially carboplatin). However, there were no bleeding complications associated with the thrombocytopenia. In addition, the thrombocytopenia was transient and resolved within 11 days, with platelet counts reaching 75x109/L. In phase I studies of single-agent selinexor, the rates of grade 3 or 4 thrombocytopenia were between 14-50%,15,16,21 with the higher rates seen in the phase I study of selinexor in relapsed or refractory non-Hodgkin lymphoma.15 In the phase II study of selinexor in multiply relapsed multiple myeloma, the rates of grade 3 or 4 thrombocytopenia were about 58%.22 Preclinical studies suggest that the mechanism of selinexor-induced thrombocytopenia are related to selinexor inhibiting megakaryocyte maturation from progenitor cells.23 Thrombocytopenia, which is a well-established side effect of selinexor, appears dose- and schedule-dependent and can be managed with dose interruptions and modifications.24 It may be that patients who had received more myelotoxic chemotherapy, prior to receiving selinexor, were more prone to severe thrombocytopenia and this will be an important consideration for future clinical trial development.
The most common grade 1 and 2 adverse events were nausea, anorexia and fatigue and the rates of these adverse events were not very different from those in phase I studies of single-agent selinexor in solid tumors and hematologic malignancies.15,16,21 It is likely that these adverse events, which overlap with the adverse events seen with ICE, were not much more frequent than those seen in the single-agent studies because selinexor was administered with an aggressive anti-emetic strategy and only in the first week of each cycle so that patients had 2 weeks to recover before the next cycle was due.
Although not powered to assess response, we found the high complete response rate of 90% striking among this group of patients, the majority of whom had primary refractory disease on entering the study, including a patient with NKTL who progressed on SMILE. Although not directly comparable, in a retrospective multicenter study of 76 PTCL and NKTL patients treated with ICE chemotherapy in Singapore, we found that the overall and complete response rates were 57% and 34%, respectively, among this group of patients who were treated in the relapsed or refractory setting. The median progressionfree survival for this group of patients was 4.5 months.25 Zelenetz et al. previously also reported that PTCL patients treated with ICE had an overall response rate of about 54% and a complete response rate of 31%.26 The high response rates seen in this clinical trial may warrant further investigation of the role of selinexor with DICE in patients with relapsed and refractory PTCL and NKTL.
Funding Statement
Funding: The authors would like to thank the National Medical Research Council (Singapore) Clinical Trials Grants (NMRC/CTGICT/0001/2017) and Karyopharm Therapeutics for funding this study. The protocol development was supported by the American Society of Hematology, Clinical Research Training Institute (2015).
References
- 1.Anderson JR, Armitage JO, Weisenburger DD. Epidemiology of the non-Hodgkin's lymphomas: distributions of the major subtypes differ by geographic locations. Non- Hodgkin's Lymphoma Classification Project. Ann Oncol. 1998;9(7):717-720. [DOI] [PubMed] [Google Scholar]
- 2.Vose J, Armitage J, Weisenburger D, International TCLP. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124-4130. [DOI] [PubMed] [Google Scholar]
- 3.Mak V, Hamm J, Chhanabhai M, et al. Survival of patients with peripheral T-cell lymphoma after first relapse or progression: spectrum of disease and rare longterm survivors. J Clin Oncol. 2013;31(16):1970-1976. [DOI] [PubMed] [Google Scholar]
- 4.Hwang WY, Koh LP, Lim ST, et al. Multicenter study of comparative outcomes of hematopoietic stem cell transplant for peripheral T cell lymphoma and natural killer/T-cell lymphoma. Leuk Lymphoma. 2011;52(7):1382-1386. [DOI] [PubMed] [Google Scholar]
- 5.Turner JG, Dawson J, Cubitt CL, Baz R, Sullivan DM. Inhibition of CRM1-dependent nuclear export sensitizes malignant cells to cytotoxic and targeted agents. Semin Cancer Biol. 2014;27:62-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang WY, Yue L, Qiu WS, Wang LW, Zhou XH, Sun YJ. Prognostic value of CRM1 in pancreas cancer. Clin Invest Med. 2009;32(6):e315. [PubMed] [Google Scholar]
- 7.Noske A, Weichert W, Niesporek S, et al. Expression of the nuclear export protein chromosomal region maintenance/exportin 1/Xpo1 is a prognostic factor in human ovarian cancer. Cancer. 2008;112(8):1733-1743. [DOI] [PubMed] [Google Scholar]
- 8.Shen A, Wang Y, Zhao Y, Zou L, Sun L, Cheng C. Expression of CRM1 in human gliomas and its significance in p27 expression and clinical prognosis. Neurosurgery. 2009;65(1):153-159; discussion 159-160. [DOI] [PubMed] [Google Scholar]
- 9.van der Watt PJ, Maske CP, Hendricks DT, et al. The karyopherin proteins, Crm1 and karyopherin beta1, are overexpressed in cervical cancer and are critical for cancer cell survival and proliferation. Int J Cancer. 2009;124(8):1829-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kojima K, Kornblau SM, Ruvolo V, et al. Prognostic impact and targeting of CRM1 in acute myeloid leukemia. Blood. 2013;121(20):4166-4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engel R, Valkov NI, Gump JL, Hazlehurst L, Dalton WS, Sullivan DM. The cytoplasmic trafficking of DNA topoisomerase IIalpha correlates with etoposide resistance in human myeloma cells. Exp Cell Res. 2004;295(2):421-431. [DOI] [PubMed] [Google Scholar]
- 12.Etchin J, Sanda T, Mansour MR, et al. KPT- 330 inhibitor of CRM1 (XPO1)-mediated nuclear export has selective anti-leukaemic activity in preclinical models of T-cell acute lymphoblastic leukaemia and acute myeloid leukaemia. Br J Haematol. 2013;161(1):117-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turner JG, Marchion DC, Dawson JL, et al. Human multiple myeloma cells are sensitized to topoisomerase II inhibitors by CRM1 inhibition. Cancer Res. 2009; 69(17):6899-6905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muqbil I, Aboukameel A, Elloul S, et al. Anti-tumor activity of selective inhibitor of nuclear export (SINE) compounds, is enhanced in non-Hodgkin lymphoma through combination with mTOR inhibitor and dexamethasone. Cancer Lett. 2016;383(2):309-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuruvilla J, Savona M, Baz R, et al. Selective inhibition of nuclear export with selinexor in patients with non-Hodgkin's lymphoma. Blood. 2017;129(24):3175-3183. [DOI] [PubMed] [Google Scholar]
- 16.Abdul Razak AR, Mau-Soerensen M, Gabrail NY, et al. First-in-class, first-inhuman phase I study of selinexor, a selective inhibitor of nuclear export, in patients with advanced solid tumors. J Clin Oncol. 2016;34(34):4142-4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juweid ME, Wiseman GA, Vose JM, et al. Response assessment of aggressive non- Hodgkin's lymphoma by integrated International Workshop Criteria and fluorine- 18-fluorodeoxyglucose positron emission tomography. J Clin Oncol. 2005; 23(21):4652-4661. [DOI] [PubMed] [Google Scholar]
- 18.Kuruvilla J, Gutierrez M, Shah BD, et al. Preliminary evidence of anti tumor activity of selinexor (KPT-330) in a phase I trial of a first-in-class oral selective inhibitor of nuclear export (SINE) in patients with relapsed/refractory non-Hodgkin's lymphoma and chronic lymphocytic leukemia. Blood. 2013;122(21):90. [Google Scholar]
- 19.Tan DSP, Pang MY, Yong WP, et al. Phase I study of the safety and tolerability of the Exportin 1 (XPO1) inhibitor selinexor (SXR) in Asian patients (pts) with advanced solid cancers. J Clin Oncol. 2015;22(15 suppl):2542. [Google Scholar]
- 20.Sweet K, Komrokji R, Padron E, et al. Phase I clinical trial of selinexor in combination with daunorubicin and cytarabine in previously untreated poor-risk acute myeloid leukemia. Clin Cancer Res. 2020;26(1):54-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garzon R, Savona M, Baz R, et al. A phase 1 clinical trial of single-agent selinexor in acute myeloid leukemia. Blood. 2017;129(24):3165-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chari A, Vogl DT, Gavriatopoulou M, et al. Oral selinexor-dexamethasone for tripleclass refractory multiple myeloma. N Engl J Med. 2019;381(8):727-738. [DOI] [PubMed] [Google Scholar]
- 23.Machlus KR, Wu SK, Vijey P, et al. Selinexor-induced thrombocytopenia results from inhibition of thrombopoietin signaling in early megakaryopoiesis. Blood. 2017;130(9):1132-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang AY, Weiner H, Green M, et al. A phase I study of selinexor in combination with high-dose cytarabine and mitoxantrone for remission induction in patients with acute myeloid leukemia. J Hematol Oncol. 2018;11(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tay T, Somasundaram N, Khoo LP, et al. Treatment outcomes of T and naturalkiller/ T-cell lymphoma with ifosfamide, carboplatin, etoposide (ICE) chemotherapy. Br J Haematol. 2019;185(Suppl. 1):3-202.30916380 [Google Scholar]
- 26.Zelenetz AD, Hamlin P, Kewalramani T, Yahalom J, Nimer S, Moskowitz CH. Ifosfamide, carboplatin, etoposide (ICE)- based second-line chemotherapy for the management of relapsed and refractory aggressive non-Hodgkin's lymphoma. Ann Oncol. 2003;14(Suppl 1):i5-10. [DOI] [PubMed] [Google Scholar]