Abstract
Paroxysmal nocturnal hemoglobinuria (PNH) is characterized by complement-mediated intravascular hemolysis due to the absence of complement regulators CD55 and CD59 on affected erythrocytes. Danicopan is a first-in-class oral proximal, complement alternative pathway factor D inhibitor. Therapeutic factor D inhibition was designed to control intravascular hemolysis and prevent C3-mediated extravascular hemolysis. In this open-label, phase II, dose-finding trial, ten untreated PNH patients with hemolysis received danicopan monotherapy (100-200 mg thrice daily). Endpoints included changes in the concentrations of lactate dehydrogenase (LDH) at day 28 (primary endpoint), of LDH at day 84, and of hemoglobin. Safety, pharmacokinetics/ pharmacodynamics, and patient-reported outcomes were assessed. Ten patients reached the primary endpoint; two later discontinued treatment: one because of a serious adverse event (elevated aspartate aminotransferase/ alanine aminotransferase coincident with breakthrough hemolysis, resolving without sequelae) and one for personal reasons unrelated to safety. Eight patients completed treatment. Intravascular hemolysis was inhibited, as demonstrated by a mean decrease of LDH (5.7 times upper limit of normal [ULN] at baseline vs. 1.8 times ULN at day 28 and 2.2 times ULN at day 84; both P<0.001). Mean baseline hemoglobin, 9.8 g/dL, increased by 1.1 (day 28) and 1.7 (day 84) g/dL (both P<0.005). No significant C3 fragment deposition occurred on glycosylphosphatidylinositol- deficient erythrocytes. Mean baseline Functional Assessment of Chronic Illness Therapy–Fatigue score, 34, increased by 9 (day 28) and 13 (day 84) points. The most common adverse events were headache and upper respiratory tract infection. These phase II, monotherapy data show that proximal inhibition with danicopan provides clinically meaningful inhibition of intravascular hemolysis and increases hemoglobin concentration in untreated PNH patients, without evidence of C3-mediated extravascular hemolysis. This trial was registered at www.clinicaltrials.gov (#NCT03053102).
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare hematologic disease characterized by chronic intravascular hemolysis, severe thrombophilia, and bone marrow failure.1 PNH is due to somatic mutations of the phosphatidylinositol N-acetylglucosaminyltransferase subunit A (PIGA) gene in hematopoietic stem cells that impair biosynthesis of glycosylphosphatidylinositol (GPI) anchors and surface expression of GPI-linked proteins.2-4 While bone marrow failure is secondary to T-cell-mediated immune attack that spares PIGA-mutated hematopoietic stem cells,5,6 both hemolysis and thromboembolism are complement-mediated. GPI-deficient erythrocytes (and platelets) lack GPI-linked complement regulators CD557 and CD598 and are exquisitely vulnerable to complement activation, which occurs continuously and spontaneously due to “C3 tick-over”9 and acutely with specific triggers. PNH treatment was revolutionized by introduction of the terminal complement C5 inhibitor eculizumab, which has proven effective in addressing intravascular hemolysis10,11 and thromboembolism,12 with a significant impact on long-term survival.13,14 Recently, a new long-acting C5 inhibitor dosed every 8 weeks, ravulizumab, has demonstrated non-inferiority to eculizumab in controlling intravascular hemolysis.15,16 Although the significant benefits of C5 inhibition in the treatment of PNH patients have been clearly demonstrated, the hematologic benefit may be hampered by the emergence of C3-mediated extravascular hemolysis from early phases of complement activation, 17,18 which C5 inhibition cannot address.19-21 Thus, alternative strategies of complement inhibition are required to improve PNH treatment, and agents are in development to address this and other unmet patients’ needs, including improved convenience.22
The complement cascade has three activating pathways (alternative, classical, and lectin-mannose) that merge at the key complement component C3; from this level (which is amplified by alternative pathway [AP] proteins), the effector pathway starts, with generation of anaphylatoxins and the membrane attack complex (MAC).23 Novel strategies of therapeutic complement inhibition in development aim to intercept the complement cascade upstream of C5, some targeting upstream at pathway-specific initiating events.22 During AP initiation, the serine protease complement factor D cleaves factor B, leading to AP C3 convertase generation. Danicopan (ACH-4471, ACH-044471, ALXN2040) is a first-in-class, oral, smallmolecule factor D inhibitor that prevents new AP C3 convertase formation.24 Consequently, proximal inhibition at the level of factor D blocks AP-initiated upstream events and up to 80% of classical or lectin pathway-initiated downstream events via amplification-loop inhibition.25 In vitro, danicopan inhibited both AP-mediated hemolysis and C3 fragment deposition on red blood cells from PNH patients.24 Phase I data from healthy human volunteers in single and multiple ascending-dose trials showed that danicopan was generally well tolerated and could achieve inhibition of AP complement activity.26 This work identified danicopan 200 mg thrice daily (tid) as a safe and effective dose/regimen.26 For PNH, targeting factor D inhibition with a small molecule represents a potentially important treatment advancement because proximal AP inhibition may disable both terminal complement activation (inhibiting MAC–mediated intravascular hemolysis) and C3 fragment opsonization (preventing extravascular hemolysis), with additional convenience of oral administration.
We investigated the factor D inhibitor danicopan as single- agent treatment for untreated PNH, aiming to control intravascular hemolysis while preventing C3-mediated extravascular hemolysis.
Methods
Study design
This international, open-label, single-arm, dose-finding, phase II study investigated danicopan in patients with hemolytic PNH not receiving complement inhibitor treatment. This trial was approved by regulatory agencies/local ethics committees and conducted according to International Conference on Harmonisation and Good Clinical Practice Standards. Achillion, Inc., a subsidiary of Alexion Pharmaceuticals, Inc., designed and sponsored the study, with advice from the investigators. All participants provided written informed consent. The trial is registered at www.clinicaltrials.gov as #NCT03053102.
Patients
This study was conducted from March 2017 to November 2018 and involved adults with untreated PNH. To be enrolled, patients had to have hemoglobin <12 g/dL (and adequate reticulocytosis according to the investigator), GPI-deficient granulocytes or type III erythrocyte clone size ≥10%, lactate dehydrogenase (LDH) ≥1.5 times upper limit of normal (ULN), platelet counts ≥50x109/L, and willingness to be vaccinated for N. meningitidis, H. influenzae, and S. pneumoniae. Investigators used their clinical judgement to assess whether patients had enough bone marrow capacity to derive potential benefit by comparing the level of pre-entry hemoglobin to the absolute reticulocyte count. None of the subjects was receiving eculizumab, because of lack of availability and/or the patients’ willingness.
Treatment
Patients received oral danicopan at a starting dose of 100 mg or 150 mg tid. The starting dose was based on phase I data showing that danicopan doses of 200 to 600 mg reached peak plasma levels within 1 to 2 h and were well-tolerated, and that 200 mg tid was effective on PNH red blood cells.22,26 Dose escalations were permitted based on hemolysis control, assessed by LDH, per investigator assessment in stepwise increments up to 200 mg tid. Dose escalation criteria for the first 28 days were specified in the study protocol with potential dose escalation points occurring at days 7 and 14 (Online Supplementary Appendix). Dose escalation was permitted thereafter and was done in consultation between the investigator and sponsor based on the hemoglobin response; absolute reticulocyte count, LDH, and indirect bilirubin were reviewed to evaluate evidence for possible additional clinical benefit from dose escalation. Because this study was the first treatment experience with danicopan in PNH patients, dose escalations were approached cautiously, especially when moving to the higher doses in the study (from 150 to 175 mg and 175 to 200 mg) or if alanine aminotransferase levels had fluctuated relative to the baseline value. Patients were instructed to take their medication approximately every 8 h. On study center days, when blood for laboratory tests was drawn, the morning dose was to be administered in the study center by the site personnel following safety and pharmacokinetic assessments. Additionally, patients were instructed to take each dose with food, including prior to intensive sampling for pharmacokinetic studies on days 1, 13, and 28. The planned duration of treatment was 84 days; patients completing treatment with clinical benefit entered a long-term extension study (ClinicalTrials.gov; NCT03181633).
Endpoints
The primary efficacy endpoint was change in LDH concentration from baseline at day 28. Secondary efficacy parameters were change of hemoglobin concentration from baseline at days 28 and 84 and LDH change from baseline at day 84. Safety, tolerability, pharmacokinetics, and pharmacodynamics were also investigated. Laboratory assessments comprised hematology, chemistry and urinalysis. Patient-reported outcomes were measured at baseline and during the study via the validated Functional Assessment of Chronic Illness Therapy (FACIT)–Fatigue scale. Haptoglobin, bilirubin, reticulocytes, GPI-deficient clone size of erythrocytes (type III) and granulocytes (type II and III unless type III values provided), and percentage of C3 fragment-coated erythrocytes were also monitored. Soluble C5b-9 was evaluated as an exploratory endpoint (non-GLP); C5a was not monitored. Transfusion history up to 3 years prior to and during the study was collected.
Assay methods
Plasma danicopan concentrations were determined by liquid chromatography. Pharmacodynamics were determined by measuring serum AP activity with an AP Wieslab assay. Plasma Bb concentration, serum factor D, C3, and C4 concentrations were also monitored. All aforementioned complement tests were conducted in a central laboratory using commercial kits. C3 fragment deposition on erythrocytes was measured using flow cytometry with FITC-conjugated anti-human C3d antibody (see the Online Supplement for details).
Statistical analysis
This was a proof-of-concept, first-in-patients, exploratory, phase II study. The sample size was determined based on the very limited number of untreated PNH patients and the exploratory nature of this study to evaluate effectiveness of danicopan. Given the small sample size, only descriptive and exploratory statistics were utilized to present results for continuous biochemical and quality-of-life measurements. Patients who discontinued treatment during the trial were not replaced. Missing values were not imputed. To summarize categorical data, frequency counts and percentages are presented. The Pearson correlation coefficient (Pearson r) was used to examine the relationship between two variables. The quantitative analysis between pharmacokinetics (plasma danicopan concentration) and pharmacodynamics (AP inhibition) was conducted with nonlinear regression using the simple Emax dose-response equation (Prism 5.02, GraphPad Software, La Jolla, CA, USA).
Results
Patients’ characteristics
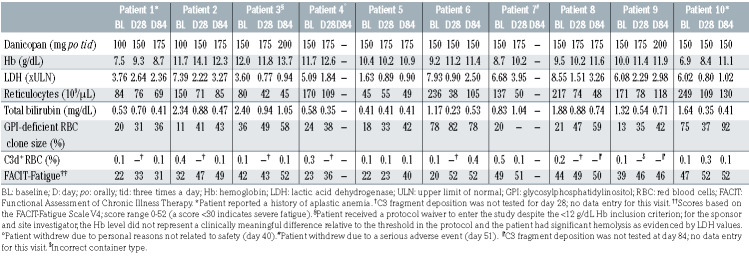
Eleven patients were screened. Ten untreated patients with hemolytic PNH were enrolled and received danicopan. These patients’ baseline characteristics are presented in Table 1 and Online Supplementary Table S1. Their median age was 33 years (range, 17-63 years) and median disease duration was 5.9 years (range, 0-14 years). The mean GPI-deficient clone size was 79% for granulocytes and 32% for erythrocytes. Overt hemolysis was demonstrated by elevated LDH levels (1416±540 U/L, corresponding to 5.7±2.17 times ULN), increased reticulocyte count (154±69×109/L), increased total bilirubin (1.3±0.74 mg/dL), and reduced haptoglobin (5.8±2.9 mg/dL). The baseline hemoglobin concentration was heterogeneous among patients (9.8±1.8 g/dL); two patients received transfusions in the 12 weeks preceding study entry, with one of these patients also having a medical history of aplastic anemia.
Study disposition and safety
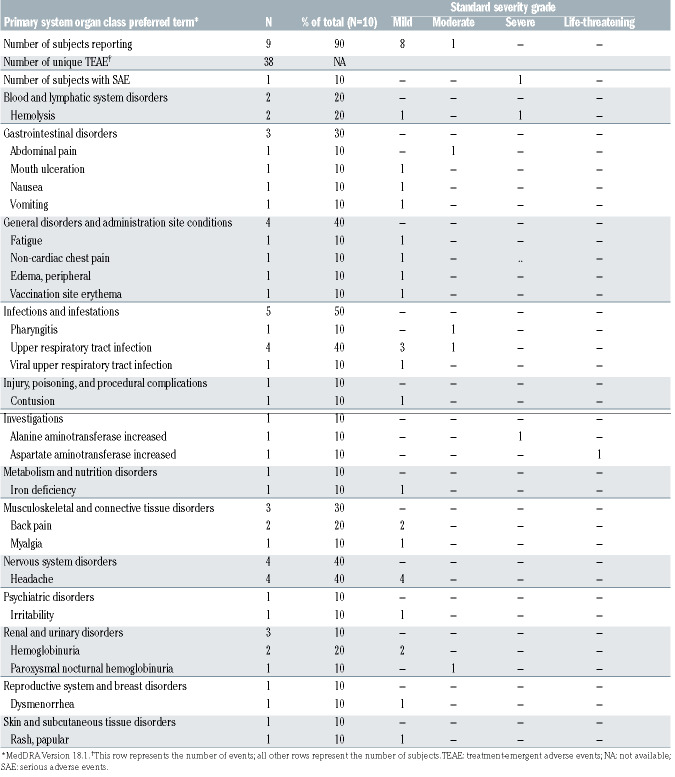
The disposition of patients in the study is shown in Online Supplementary Figure S1. Two patients started danicopan at 100 mg tid and increased to 150 mg tid, and eight started at 150 mg tid. Increases to 175 mg and 200 mg tid were performed in eight and four patients, respectively. All ten patients reached day 28 and are included in the primary endpoint evaluation. Two discontinued before day 84: one because of a serious adverse event, elevated aspartate aminotransferase/alanine aminotransferase coincident with breakthrough hemolysis, which resolved without sequelae; the other withdrew for personal reasons unrelated to safety. All patients were evaluated until they left the study or reached day 84 (n=8). Nine patients (90%) developed at least one adverse event during treatment; only one was serious (described above). In total, 38 unique treatment-emergent adverse events were recorded, of which four were considered possibly related and two probably related to danicopan. The most frequent events were PNH-related (hemolysis and its signs or symptoms) and infections, usually of the upper respiratory tract (Table 2). With few exceptions, adverse events were mild and resolved during the study. There were no clinically significant changes in other key laboratory parameters during treatment (Online Supplementary Table S2).
Pharmacokinetics and pharmacodynamics
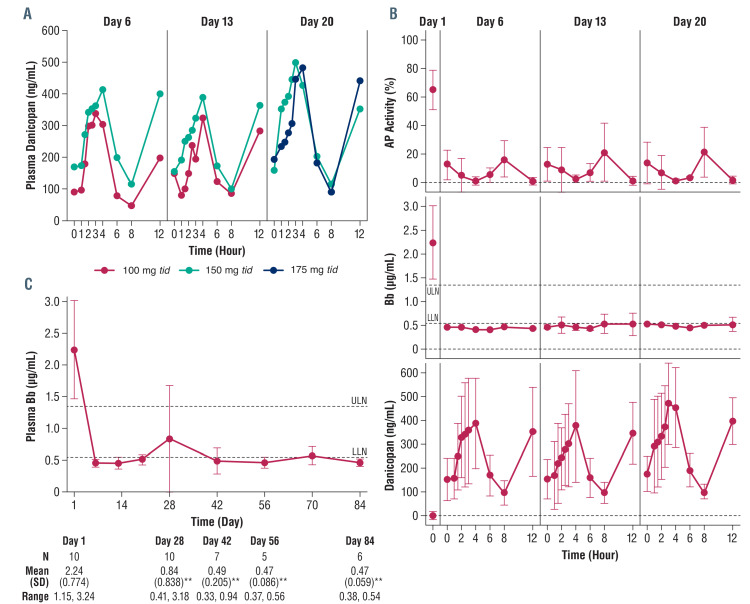
Danicopan was bioavailable with dose-proportional exposure (peak serum concentration [Cmax] and area under the curve [AUC]) at 100, 150, and 175 mg tid doses, demonstrated by intensive pharmacokinetic and pharmacodynamic profiling on days 6, 13, and 20 (Figure 1A; Online Supplementary Table S3), whereas trough concentrations assessed biweekly at single time points from days 28 to 84 were variable (Online Supplementary Table S4). Trough drug concentration was much more variable than AUC and Cmax. For example, at the 175 mg tid dose, the concentrations varied from 62.6 to 223.1 ng/mL. There was appreciable inter-patient variability, as anticipated for a study with a small number of patients. One of two patients who received 200 mg tid was not included in day 56 analyses because the sample was not available (missed study visit). Per protocol, no patients were receiving 200 mg tid by day 20 (pharmacokinetic sampling was performed on days 6, 13, and 20). Plasma factor D concentration did not change during treatment (Online Supplementary Figure S2A). As anticipated from its mechanism of action, treatment resulted in selective AP inhibition (Figure 1B, upper panel; Online Supplementary Table S4) with no effect on classical pathway activity (Online Supplementary Figure S2B). Notably, AP activity ≤10% was observed at all time points except at 0 and 8 h when danicopan concentration was lowest (Figure 1B) indicating that the AP activity in patients may not be fully inhibited at the end of the 8 h dosing period. Pharmacokinetic/pharmacodynamic analysis showed a dose-response relationship between danicopan and AP inhibition (Online Supplementary Figure S2D).
Table 1.
Baseline characteristics and clinical results.
Efficacy
Primary endpoint
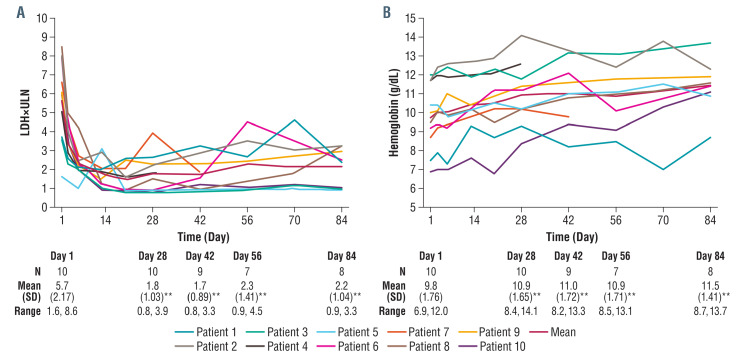
Change in LDH concentration from baseline to day 28 was the primary efficacy endpoint. A significant reduction was observed in all ten patients from a mean value of 5.7±2.17 times ULN at baseline to 1.8±1.03 times ULN at day 28 (P<0.001) (Figure 2A, Table 1), demonstrating achievement of the primary endpoint. The percentages of patients showing LDH <3 times ULN, <2 times ULN, and <1 time the ULN were 90%, 60%, and 40%, respectively.
Secondary endpoints
Significant reductions in LDH from baseline were sustained throughout the study, with values being 2.3±1.41 times ULN at day 56 (P<0.005) and 2.2±1.04 times ULN (P<0.001) at day 84 (Figure 2A). The percentages of patients with LDH <3 times ULN, <2 times ULN, and <1 time ULN were 71%, 43%, and 43% at day 56, and 75%, 37.5%, and 25% at day 84, respectively. Danicopan is a highly permeable drug and even in patients with high body mass index, plasma concentrations do not appear to cause a clinically significant change in effect. Fluctuations in LDH indicated that low-level residual intravascular hemolysis remained in most patients, with possible transient exacerbations; this was due to transient weaker AP inhibition around predose periods, as described above, in addition to an increase of susceptible GPI-deficient erythrocytes during treatment (see below). Nevertheless, only two breakthrough hemolytic events were recorded by the investigator as adverse events (Table 2); a third patient experienced recurrent subclinical breakthrough episodes as a consequence of inadequate control of complement activation.
Treatment with danicopan translated into an improvement of anemia: mean hemoglobin increased from 9.8 g/dL at baseline (range, 6.9 to 12.0 g/dL) to 10.9 g/dL at day 28 (range, 8.4 to 14.1 g/dL; P<0.005), 10.9 g/dL at day 56 (range, 8.5 to 13.1 g/dL; P<0.005), and 11.5 g/dL at day 84 (range, 8.7 to 13.7 g/dL; P<0.005) (Figure 2B, Table 1). The mean increase from baseline was 0.9 g/dL at day 28 and 1.7 g/dL at day 84. In the 12 weeks preceding study entry, two patients received transfusions (Figure 3A). One of these patients, who had aplastic anemia (not receiving immunosuppressive therapy), had received five transfusions totaling ten units. During treatment, this patient received three transfusions totaling seven units. The second patient had one transfusion (two units) during breakthrough hemolysis in the setting of a viral infection; in this case, danicopan treatment was not detrimental to the course of the infection, but (irrespective of its possible effect on other complement pathways) did not effectively counteract the transient acute complement activation (possibly due to pharmacokinetic/pharmacodynamic reasons). The remaining patients in the trial were transfusion independent through the 84 days of treatment. Accompanying the control of intravascular hemolysis and improvement of anemia, patient-reported outcomes were assessed. The mean FACIT–Fatigue score at baseline was 34 and increased by 9 and 13 points at days 28 and day 84, respectively (P<0.05) (Figure 3B, Table 1).
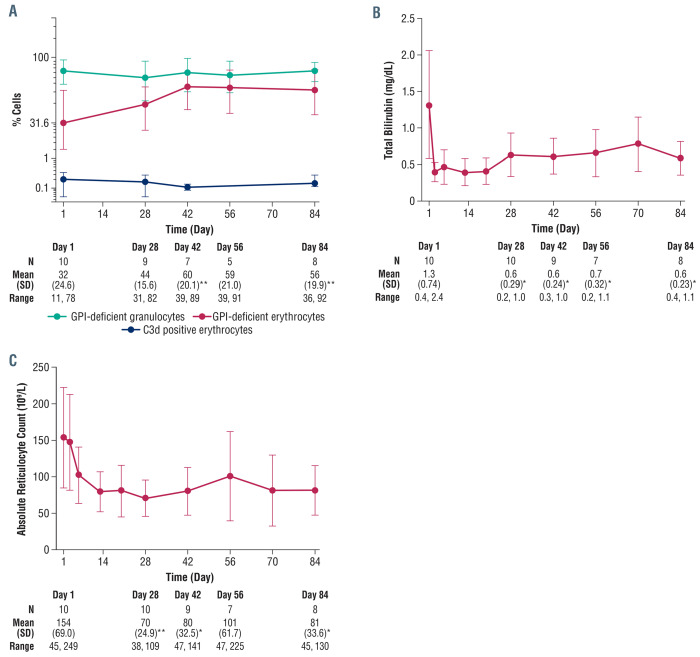
Control of intravascular hemolysis by danicopan was further confirmed by changes in other laboratory parameters. Danicopan significantly increased the percentage of GPI-deficient erythrocytes (56±19.9% at day 84 vs. 32±24.6% at baseline; P=0.001), whereas no change was observed for GPI-deficient granulocytes (Figure 4A). Total bilirubin decreased after danicopan treatment (0.6±0.23 mg/dL at day 84 vs. 1.3±0·74 mg/dL at baseline, P<0.05) (Figure 4B). A sustained but not statistically increase in haptoglobin was observed (15.3±16.08 mg/dL at day 84 vs. 5.8±2.89 mg/dL at baseline; P=0.15) (Online Supplementary Figure S3A), as was a transient decrease in free hemoglobin (83±51 mg/dL at day 28 vs. 138±132 mg/dL at baseline; P=0.26) (Online Supplementary Figure S3B). Absolute reticulocyte count decreased quickly and was sustained with treatment (81±33.6×109/L at day 84 vs. 154±69×109/L at baseline; P<0.05) (Figure 4C). Additional laboratory results can be found in Online Supplementary Table S6.
Exploratory endpoints
To investigate the mechanistic effect of proximal complement inhibition by danicopan on PNH, complement biomarkers were monitored. Bb fragment, an activation product of factor B, tracks complement AP activation in vivo. Plasma Bb level was significantly elevated at baseline (2.24±0.77 mg/mL) compared with that in healthy volunteers (0.84±0.212 mg/mL; P<0.05). After danicopan, the Bb levels were significantly reduced: 0.84±0.84 mg/mL at day 28 (P<0.005); 0.47±0.09 mg/mL at day 56 (P<0.005); and 0.47±0.06 μg/mL at day 84 (P<0.005) (Figure 1C). In contrast to residual AP activity, Bb level remained consistently low irrespective of subtherapeutic plasma danicopan levels in predose periods (Figure 1B, middle panel). A strong positive correlation was found between Bb and LDH (Pearson r=0.80, P<0.0001) (Online Supplementary Figure S2E), supporting Bb as a reliable biomarker of in vivo AP activation in PNH and, therefore, its value for monitoring efficacy. Danicopan also showed strong linear correlations with Bb and LDH (negative), as did AP with Bb and LDH (positive); there was no correlation of classical pathway activity with any of these parameters (Online Supplementary Table S5), validating the role of danicopan in AP inhibition and subsequent in vivo changes of Bb and LDH. Additional laboratory results can be found in Online Supplementary Table S6.
There was a slight increase in serum C3 (114.2±17.3 mg/dL at day 84 vs. 102.2±20.2 mg/dL at baseline, P=0.08) (Online Supplementary Figure S2C), likely from reduced C3 consumption because of upstream complement blockade. Importantly, C3 fragment deposition on erythrocytes was very low (<0.5% of erythrocytes) throughout treatment (Figure 4A).
sC5b-9 was normal at baseline and remained relatively constant over time (data not shown).
Discussion
Current PNH treatments target C5 inhibition.10-14 Novel complement inhibitors in development aim to address unmet needs of PNH patients.22 Here, we describe a novel approach to PNH treatment, which aims to change the current paradigm of PNH therapy by improving hemoglobin levels in addition to reducing hemolysis, with the added advantage and convenience of oral administration. We investigated danicopan, a first-in-class oral factor D inhibitor, which blocks the proximal complement cascade upstream of C5 at the level of AP initiation and amplification. In untreated PNH patients, danicopan monotherapy resulted in inhibition of intravascular hemolysis, with significant reductions of LDH at day 28 (primary endpoint) and throughout treatment duration. In contrast with standard C5 inhibitor therapies, the inhibition of intravascular hemolysis observed during danicopan treatment was not associated with persistently increased bilirubin and reticulocyte count, or with the emergence of C3 deposition on surviving GPI-deficient erythrocytes, in agreement with its anticipated effect on extravascular hemolysis. Concomitant inhibition of intravascular hemolysis and prevention of C3-mediated extravascular hemolysis resulted in improvement of anemia, with a mean hemoglobin gain of 1.7 g/dL after 12 weeks of treatment. Consistent with these findings, all patients exhibited significant increases in the percentage of GPI-deficient erythrocytes, confirming the extended half-life of these cells in vivo. Furthermore, all patients had improvements in FACIT-Fatigue quality-of-life measurements; FACITFatigue scores were used in this proof-of-concept study due to the lack of validated instruments for patient-reported outcomes in PNH. Additional patient-reported outcome instruments will be utilized in a phase III trial. With the caveat of limited sample size and exposure, no safety concerns emerged during the study other than those described; in particular, infectious events were rare, clinically mild, and self-limiting, irrespective of unchanged danicopan treatment. Although the short study duration through the primary endpoint limits the ability to draw robust conclusions, the data do not appear to support the postulated increased risk of infectious complications (due to upstream inhibition of the complement cascade),27 in agreement with in vitro data showing that killing of encapsulated and unencapsulated meningococci was nearly unaffected relative to that occurring with eculizumab.28,29
Figure 1.
Pharmacokinetic-pharmacodynamic evaluation of danicopan. (A) The mean plasma danicopan concentration by dose at hours 0 (predosing), 1, 1.5, 2, 2.5, 3, 4, 6, 8 (predosing) and 12 of days 6, 13, and 20. The number of patients is, respectively, two (100 mg tid) and eight (150 mg tid) at day 6 and day 13, and five (150 mg tid) and five (175 mg tid) at day 20. (B) The mean ± standard deviation of ex vivo serum alternative pathway (AP) activity, plasma Bb concentration, and plasma danicopan concentration, combining all dosing groups together. Serum AP activity and plasma Bb concentration were determined for a subset of the aforementioned time points by the AP Wieslab assay (Euro Diagnostica) and Bb enzyme-linked immunosorbent assay, respectively. (C) Plasma Bb concentration (mean ± standard deviation) at baseline (day 1) through the end of the study (day 84) with descriptive statistics. The dashed lines represent the upper and lower limits of normal, which were derived from phase I studies in healthy volunteers (see Assay Methods in the Online Supplement). N values of <10 for plasma Bb on days 42, 56, and 84 reflect the two early discontinuations and additional samples not collected. **P<0.005. tid: thrice daily; ULN: upper limit of normal; LLN: lower limit of normal; SD: standard deviation.
Table 2.
Adverse events.
Danicopan is the first oral complement inhibitor treatment demonstrating efficacy and safety in PNH patients as monotherapy in a phase II study. The clinical effects observed were achieved irrespective of low-level, residual intravascular hemolysis, which remained detectable in some patients. This residual intravascular hemolysis is likely the consequence of the increase of GPI-deficient erythrocytes susceptible to complement-mediated hemolysis and the pharmacokinetic/pharmacodynamic characteristics of danicopan. Danicopan plasma level and AP activity also correlated with in vivo biomarkers (LDH and Bb), and Bb seems a reliable indicator of complement activation in vivo during danicopan treatment. Based on the results of the phase I single-dose and multiple ascendingdose trials in healthy human volunteers, 200 mg tid was shown to be both safe and efficacious.26 However, in this cohort of patients, full blockade of AP activity was not consistently achieved in all patients, irrespective of individual dose adjustment and the broad dose ranges used during the study. These observations suggest that residual intravascular hemolysis is due to low residual AP activity observed in some patients, which may be better inhibited by a more potent second-generation factor D inhibitor analog that will be assessed for safety and efficacy in a phase II trial (Clinicaltrials.gov, NCT04170023). In a concurrent phase II trial, patients (n=12) on a stable regimen of eculizumab with hemoglobin <10 g/dL and who were transfusion dependent (≥1 red blood cell transfusion within 12 weeks of screening) received oral danicopan 100–150 mg tid, with possible response-based dose escalation to 200 mg tid at predefined time points. The addition of danicopan led to clinically and statistically significant reductions in frequency of red blood cell transfusions and in the number of transfused units in patients compared to those in patients with a history of eculizumab treatment alone.30
Figure 2.
Effect of danicopan on lactate dehydrogenase and hemoglobin levels. (A) Change in lactate dehydrogenase (LDH) concentration from baseline (day 1) to day 28 was the primary efficacy endpoint. LDH reduction per patient is shown here including the reduction from a mean value of 5.7±2.17 times upper limit of normal (ULN) at baseline to 1.8±1.03 times ULN at day 28 (P<0.001) demonstrating achievement of the primary endpoint. A significant mean LDH reduction from baseline was sustained throughout the study up to day 84 (2.2±1.04 times ULN; P<0.001). (B) Per patient effects on hemoglobin with a mean group increase from 9.8 g/dL at baseline (day 1) (range, 6.9 to 12.0 g/dL) to 10.9 g/dL at day 28 (range, 8.4 to 14.1 g/dL; P<0.005), and 11.5 g/dL at day 84 (range, 8.7 to 13.7 g/dL, P<0.005). Note, patient 3 received a protocol waiver to enter the study despite the <12 g/dL hemoglobin inclusion criterion. For the sponsor and site investigator, the hemoglobin level did not represent a clinically meaningful difference relative to the threshold in the protocol and the patient had significant hemolysis, as evidenced by LDH values. **P<0.005; SD:standard deviation.
Figure 3.
Effect of danicopan on blood transfusions and Functional Assessment of Chronic Illness Therapy-Fatigue score. (A) Two patients required transfusions during the trial, for a total of nine units on four occasions over 84 days. The transfusion history (84 days prior to screening through to the end of the study; sum for all patients) is provided. (B) Mean Functional Assessment of Chronic Illness Therapy–Fatigue score values (± standard deviation) at baseline (day 1) through to the end of the study (day 84) with descriptive statistics. The range of scores was 0 to 52; a score of <30 indicates severe fatigue. *P<0.05; **P<0.005. Note, data were not obtained for one patient at day 56. FACIT: Functional Assessment of Chronic Illness Therapy; SD: standard deviation.
Clinical development of proximal complement inhibitors has been motivated by the description of C3- mediated extravascular hemolysis as a mechanism driving significant anemia and limiting hematologic benefit in some PNH patients on eculizumab and other C5 inhibitors.17,31 Indeed, it is conceivable that, in combination with C5 inhibitors, proximal inhibitors can address C3-mediated extravascular hemolysis by preventing the generation of C3 convertase, eventually leading to better hematologic response in PNH patients.17,31 Moreover, it has been hypothesized that proximal inhibitors, by preventing generation of downstream C5 convertases, could be effective even in the absence of terminal inhibitors (i.e., eculizumab or other C5 inhibitors). Danicopan is the first compound demonstrating that proximal complement inhibitors can be used safely and effectively as monotherapy in PNH. By disabling the initiating event of complement activation, danicopan prevents generation of C5 convertases, obviating the need for downstream C5 inhibition. Additionally, specific targeting of AP can preserve classical and lectin pathway-mediated antimicrobial activity. The landscape of complement inhibition in the treatment of PNH will continue to evolve with the availability of proximal inhibitors. In addition to danicopan, other oral agents targeting the AP, such as factor B inhibitors, are under investigation for the treatment of PNH.22,31,32 Proximal complement inhibitors also include subcutaneously administered agents targeting C3; one of these agents was reported to be effective in a recent phase III trial in patients with PNH.33 All of these approaches look promising for the treatment of PNH and clinical data should tell us very soon what are the viable treatment options in terms of safety and efficacy, and how we can best utilize them in the appropriate patients (i.e., monotherapy vs. add-on treatment). Preclinical data seem to suggest that AP inhibitors may be as effective as terminal complement blockade with clinical differences mostly due to the specific pharmacokinetic/pharmacodynamic profile of the individual inhibitor, rather than to its target in the complement cascade.22,31 Indeed targeting the AP, as PNH is a disease due to AP dysregulation (i.e., continuous, spontaneous C3 tick-over, eventually exacerbated at times of additional complement activation), possible applications in other diseases will require an understanding of their pathogenic mechanisms.34
Figure 4.
Additional clinical efficacy evaluation of danicopan. (A) The mean (± standard deviation) clone size percentages displayed for GPI-deficient erythrocytes and granulocytes at baseline (day 1) through to the end of the study (day 84) and mean (± standard deviation) percentage of erythrocytes with C3 fragment deposition with descriptive statistics for GPI-deficient erythrocytes. Descriptive statistics for GPI-deficient granulocytes and erythrocytes with C3 fragment deposition are listed in Online Supplementary Table S6. Erythrocytes with C3 fragment deposition are defined as erythrocytes that stain positive for anti-C3d antibody. (B) Total bilirubin at baseline (day 1) through to the end of the study (day 84) with descriptive statistics. Data for one patient were not obtained at day 56. (C) Absolute reticulocyte count at baseline (day 1) through to the end of the study (day 84) with descriptive statistics. Data for one patient were not obtained at day 56. *P<0.05; **P<0.005. SD: standard deviation; GPI: glycosylphosphatidylinositol.
In conclusion, in this study danicopan appeared to be well-tolerated and showed clinically meaningful inhibition of intravascular hemolysis and hemoglobin improvement in untreated PNH patients. This is the first evidence that a proximal complement inhibitor, used as monotherapy, can have a clinical impact on PNH by inhibiting intravascular hemolysis and preventing extravascular hemolysis. While second-generation compounds with improved pharmacokinetics and pharmacodynamics are in development, this study paves the way to improved hematologic response and novel standards of care, with an easier mode of administration, for hemolytic PNH patients.
Supplementary Material
Acknowledgments
We thank the patients and investigators, as well as their staff, who participated in this trial: Serena Marotta, Luana Marano and Fabiana Cacace (Naples), Petra Muus and Shreyans Gandhi (London), Federica Barone (Florence), Sung-Soo Park (Seoul), and Paul Hamilton (Auckland).
Funding Statement
Funding: The study was sponsored and entirely supported by Achillion Inc., a subsidiary of Alexion Pharmaceuticals, Inc. We thank Heather Robison (an employee of Achillion Inc., a subsidiary of Alexion Pharmaceuticals, Inc.) and Steven Podos, Danny Shin and Julia Catini (Alexion employees and former employees of Achillion Inc., a subsidiary of Alexion Pharmaceuticals, Inc.) for their assistance in writing this manuscript and The Curry Rockefeller Group (funded by Achillion Inc., a subsidiary of Alexion Pharmaceuticals, Inc.) for their editorial assistance.
References
- 1.Luzzatto L, Notaro R. Paroxysmal nocturnal hemoglobinuria. In: Handin RI, Lux SE, Stossel TP, eds. Blood, Principles and Practice of Hematology. 2nd edition. Philadelphia: Lippincott Williams & Wilkins; 2003:319-334. [Google Scholar]
- 2.Takeda J, Miyata T, Kawagoe K, et al. Deficiency of the GPI anchor caused by a somatic mutation of the PIG-A gene in paroxysmal nocturnal hemoglobinuria. Cell. 1993;73(4):703-711. [DOI] [PubMed] [Google Scholar]
- 3.Miyata T, Yamada N, Iida Y, et al. Abnormalities of PIG-A transcripts in granulocytes from patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1994;330(4):249-255. [DOI] [PubMed] [Google Scholar]
- 4.Medof ME, Gottlieb A, Kinoshita T, et al. Relationship between decay accelerating factor deficiency, diminished acetylcholinesterase activity, and defective terminal complement pathway restriction in paroxysmal nocturnal hemoglobinuria erythrocytes. J Clin Invest. 1987;80(1):165-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rotoli B, Luzzatto L. Paroxysmal nocturnal haemoglobinuria. Baillieres Clin Haematol. 1989;2(1):113-138. [DOI] [PubMed] [Google Scholar]
- 6.Luzzatto L, Risitano AM. Advances in understanding the pathogenesis of acquired aplastic anaemia. Br J Haematol. 2018; 182(6):758-776. [DOI] [PubMed] [Google Scholar]
- 7.Nicholson-Weller A, March JP, Rosenfeld SI, Austen KF. Affected erythrocytes of patients with paroxysmal nocturnal hemoglobinuria are deficient in the complement regulatory protein, decay accelerating factor. Proc Natl Acad Sci U S A. 1983;80(16):5066-5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holguin MH, Fredrick LR, Bernshaw NJ, Wilcox LA, Parker CJ. Isolation and characterization of a membrane protein from normal human erythrocytes that inhibits reactive lysis of the erythrocytes of paroxysmal nocturnal hemoglobinuria. J Clin Invest. 1989;84(1):7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lachmann PJ, Halbwachs L. The influence of C3b inactivator (KAF) concentration on the ability of serum to support complement activation. Clin Exp Immunol. 1975; 21(1):109-114. [PMC free article] [PubMed] [Google Scholar]
- 10.Hillmen P, Young NS, Schubert J, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006;355(12):1233-1243. [DOI] [PubMed] [Google Scholar]
- 11.Brodsky RA, Young NS, Antonioli E, et al. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood. 2008; 111(4): 1840-1847. [DOI] [PubMed] [Google Scholar]
- 12.Hillmen P, Muus P, Dührsen U, et al. Effect of the complement inhibitor eculizumab on thromboembolism in patients with paroxysmal nocturnal hemoglobinuria. Blood. 2007;110(12):4123-4128. [DOI] [PubMed] [Google Scholar]
- 13.Kelly RJ, Hill A, Arnold LM, et al. Longterm treatment with eculizumab in paroxysmal nocturnal hemoglobinuria: sustained efficacy and improved survival. Blood. 2011;117(25):6786-6792. [DOI] [PubMed] [Google Scholar]
- 14.Loschi M, Porcher R, Barraco F, et al. Impact of eculizumab treatment on paroxysmal nocturnal hemoglobinuria: a treatment versus no-treatment study. Am J Hematol. 2016;91(4):366-370. [DOI] [PubMed] [Google Scholar]
- 15.Lee JW, Sicre de Fontbrune F, Wong Lee Lee L, et al. Ravulizumab (ALXN1210) vs eculizumab in adult patients with PNH naive to complement inhibitors: the 301 study. Blood. 2019;133(6):530-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kulasekararaj AG, Hill A, Rottinghaus ST, et al. Ravulizumab (ALXN1210) vs eculizumab in C5-inhibitor-experienced adult patients with PNH: the 302 study. Blood. 2019;133(6):540-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Risitano AM, Notaro R, Marando L, et al. Complement fraction 3 binding on erythrocytes as additional mechanism of disease in paroxysmal nocturnal hemoglobinuria patients treated by eculizumab. Blood. 2009;113(17):4094-4100. [DOI] [PubMed] [Google Scholar]
- 18.Hill A, Rother RP, Arnold L, et al. Eculizumab prevents intravascular hemolysis in patients with paroxysmal nocturnal hemoglobinuria and unmasks low-level extravascular hemolysis occurring through C3 opsonization. Haematologica. 2010; 95(4):567-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luzzatto L, Risitano AM, Notaro R. Paroxysmal nocturnal hemoglobinuria and eculizumab. Haematologica. 2010; 95(4): 523-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Risitano AM, Notaro R, Luzzatto L, Hill A, Kelly R, Hillmen P. Paroxysmal nocturnal hemoglobinuria--hemolysis before and after eculizumab. N Engl J Med. 2010; 363(23):2270-2272. [DOI] [PubMed] [Google Scholar]
- 21.Notaro R, Sica M. C3-mediated extravascular hemolysis in PNH on eculizumab: mechanism and clinical implications. Semin Hematol. 2018;55(3):130-135. [DOI] [PubMed] [Google Scholar]
- 22.Risitano AM, Marotta S. Toward complement inhibition 2.0: Next generation anticomplement agents for paroxysmal nocturnal hemoglobinuria. Am J Hematol. 2018; 93(4):564-577. [DOI] [PubMed] [Google Scholar]
- 23.Taylor RP, Lindorfer MA. Mechanisms of complement-mediated damage in hematological disorders. Semin Hematol. 2018; 55(3):118-123. [DOI] [PubMed] [Google Scholar]
- 24.Yuan X, Gavriilaki E, Thanassi JA, et al. Small-molecule factor D inhibitors selectively block the alternative pathway of complement in paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome. Haematologica. 2017; 102(3):466-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harboe M, Ulvund G, Vien L, Fung M, Mollnes TE. The quantitative role of alternative pathway amplification in classical pathway induced terminal complement activation. Clin Exp Immunol. 2004; 138(3): 439-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiles JA, Galvan MD, Podos SD, Geffner M, Huang M. Discovery and development of the oral complement factor D inhibitor danicopan (ACH-4471). Curr Med Chem. 2020;27(25):4165-4180. [DOI] [PubMed] [Google Scholar]
- 27.Sprong T, Roos D, Weemaes C, et al. Deficient alternative complement pathway activation due to factor D deficiency by 2 novel mutations in the complement factor D gene in a family with meningococcal infections. Blood. 2006;107(12):4865-4870. [DOI] [PubMed] [Google Scholar]
- 28.Granoff DM, Kim H, Topaz N, MacNeil J, Wang X, McNamara LA. Differential effects of therapeutic complement inhibitors on serum bactericidal activity against non-groupable meningococcal isolates recovered from patients treated with eculizumab. Haematologica. 2019; 104(8): e340-e344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konar M, Granoff DM. Eculizumab treatment and impaired opsonophagocytic killing of meningococci by whole blood from immunized adults. Blood. 2017; 130(7):891-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kulasekararaj A, Risitano AM, Maciejewski JP, et al. A phase 2 open-label study of danicopan (ACH-0144471) in patients with paroxysmal nocturnal hemoglobinuria (PNH) who have an inadequate response to eculizumab monotherapy. Blood. 2019; 134(Suppl_1):3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Risitano AM, Marotta S, Ricci P, et al. Anticomplement treatment for paroxysmal nocturnal hemoglobinuria: time for proximal complement inhibition? A position paper from the SAAWP of the EBMT. Front Immunol. 2019;10:1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Risitano AM, Roth A, Soret J, et al. LNP023 - a new oral complement factor-B inhibitor normalizes hemoglobin in paroxysmal nocturnal hemoglobinuria patients with poor response to eculizumab, both as add-on and monotherapy. The 46th Annual Meeting of the European Society for Blood and Marrow Transplantation; August 29 - September 1, 2020; O019. [Google Scholar]
- 33.Hillmen P, Szer J, Weitz I, et al. Results of the Pegasus phase III randomized trial demonstrating superiority of the C3 inhibitor, pegcetacoplan, compared to eculizumab in patients with paroxysmal nocturnal hemoglobinuria. The 25th European Hematology Association Annual Congress; June 12, 2020; 295012; S192. [Google Scholar]
- 34.Holers VM. The complement system as a therapeutic target in autoimmunity. Clin Immunol. 2003;107(3):140-151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.