Abstract
The COVID-19 pandemic has had a heavy impact on global health and economy and vaccination remains the primary way of controlling the infection. During the ongoing vaccination campaign some unexpected thrombotic events have emerged in subjects who had recently received the AstraZeneca (Vaxzevria) vaccine or the Johnson&Johnson (Janssen) vaccine, two adenovirus vector-based vaccines. Epidemiological studies confirm that the observed/expected ratio of these unusual thromboses is abnormally increased, especially in women in fertile age. The characteristics of this complication, with venous thromboses at unusual sites, most frequently in the cerebral vein sinuses but also in splanchnic vessels, often with multiple associated thromboses, thrombocytopenia, and sometimes disseminated intravascular coagulation, are unique and the time course and tumultuous evolution are suggestive of an acute immunological reaction. Indeed, plateletactivating anti-PF4 antibodies have been detected in a large proportion of the affected patients. Several data suggest that adenoviruses may interact with platelets, the endothelium and the blood coagulation system. Here we review interactions between adenoviral vectors and the hemostatic system that are of possible relevance in vaccine-associated thrombotic thrombocytopenia syndrome. We systematically analyze the clinical data on the reported thrombotic complications of adenovirus-based therapeutics and discuss all the current hypotheses on the mechanisms triggering this novel syndrome. Although, considering current evidence, the benefit of vaccination clearly outweighs the potential risks, it is of paramount importance to fully unravel the mechanisms leading to vaccineassociated thrombotic thrombocytopenia syndrome and to identify prognostic factors through further research.
Introduction
The coronavirus disease 19 (COVID-19) pandemic has prompted an unprecedented effort to develop highly effective vaccines to prevent further spreading of the infection, the associated mortality and the enormous strain on healthcare systems. Indeed, in a previously unimaginable short time, many vaccines have been developed. Several of them underwent controlled randomized phase III clinical trials and, as of 22 June, 2021, 13 have been licensed globally for clinical use. By July 18, 2021 they had been administered to more than 1.9 billion subjects worldwide (923 million of whom are fully vaccinated; 3.66 billion doses have been administered globally; 26.3% of the world’s population has received at least one dose of a COVID-19 vaccine). This represents the most massive vaccination campaign ever undertaken (https://www.who.int/emergencies/diseases/novel-coronavirus- 2019/covid-19-vaccines; https://ourworldindata.org/covid-vaccinations).
Although careful scrutiny of vaccine safety in controlled randomized phase III clinical trials did not highlight significant thrombotic risks, exceedingly rare events may have been missed and indeed during the vaccination campaign several cases of thrombosis, in particular thrombotic events at unusual sites associated with thrombocytopenia, were reported. Most events occurred in subjects who had received the ChAdOx1 (Vaxzevria) vaccine in the preceding weeks, but more recently several cases have also been reported following the Ad26-CoV2S Johnson&Johnson (Janssen) vaccine.1-9 Not only was the observed/expected ratio of these thromboses abnormally high in subjects receiving the Vaxzevria vaccine, but the clinical characteristics of the events were unique, associating unusual site venous thromboses, mainly cerebral vein sinus thrombosis (CVST), with thrombocytopenia and sometimes disseminated intravascular coagulation (DIC). In contrast, no thromboses were reported in about 90 million subjects who had received the messenger RNA (mRNA)-based Pfizer BioNTech and only very few in those who had received the Moderna vaccine (Spikevax), although the latter had characteristics apparently dissimilar from those observed in Vaxzevria recipients, with one exception.10
These findings suggest that the reported thrombotic complications, which have variously been called vaccineinduced prothrombotic immune thrombocytopenia (VIPIT), vaccine-induced immune thrombotic thrombocytopenia (VITT), thrombotic thrombocytopenia syndrome (TTS) and vaccine-associated thrombotic thrombocytopenia syndrome (VATTS),2,11-13 are peculiar to adenoviral (Ad) vector-based vaccines and have led to limitations and/or temporary suspensions of the use of such vaccines in several countries.
From the most recently available UK pharmacovigilance data (July 7, 2021), CVST and other major thromboembolic events with concurrent thrombocytopenia had been reported in 147 (average age, 54 years) and 258 subjects (average age, 54 years), respectively, among an estimated 24.6 million recipients of a first dose and an estimated 22.3 million recipients of a second dose of the Vaxzevria vaccine. Thus, the overall incidence after first or unknown doses was 14.8 cases per million doses in the UK (https://www.gov.uk/government/publications/coronaviruscovid- 19-vaccine-adverse-reactions/coronavirus-vaccine-summary- of-yellow-card-reporting). Concerning Europe, as of June 27, 2021, there were spontaneous reports to EudraVigilance of 479 suspected cases, 100 of which had had a fatal outcome, among recipients of about 51.4 million doses of Vaxzevria, i.e. 19.3 cases per million doses (https://www.ema.europa.eu/en/documents/covid-19-vaccinesafety- update/covid-19-vaccine-safety-update-vaxzevria-previously- covid-19-vaccine-astrazeneca-14-july-2021_en.pdf), and 21 cases of suspected TTS associated with the Janssen COVID-19 vaccine, four of which were fatal, among recipients of about 7 million doses of this vaccine, i.e. 3 cases per million doses (https://www.ema.europa.eu/en/documents/ covid-19-vaccine-safety-update/covid-19-vaccine-safetyupdate- covid-19-vaccine-janssen-14-july-2021_en.pdf).
This review aims to discuss the interactions between Ad vectors and Ad-based vaccines and the hemostatic system and the hypotheses on the mechanisms triggering VITT.
Adenoviruses, platelets and the blood coagulation system
Based on available data and given that VITT has been associated with Ad-vector-based vaccines, hypotheses on a direct role of the interaction between Ad and blood components can be made.
Ad are non-enveloped DNA viruses with a nucleoprotein core encapsulated by an icosahedral protein capsid from which proteinaceous fibers protrude. The C-terminal knob domain at the distal end of these fibers is responsible for virus binding to its primary cellular receptor, a 46-kDa transmembrane protein14-16 which also functions as a receptor for Coxsackie B virus and is, therefore, called coxsackie and Ad receptor (CAR).15-17 The high affinity binding of Ad to CAR starts receptor-mediated endocytosis.18 Moreover, Ad have evolved other mechanisms to facilitate cell entry via recognition of the arginine- glycine-aspartate (RGD) sequence on cell surface integrins. Molecules expressed on host cell surfaces involved in cell infection include the vitronectin-binding integrins αvb3 and αvb5,19 the fibronectin-binding integrin α5b1 20 and others, such as αVb1,21 all characterized by a common RGD peptide sequence which is recognized by the RGD ligand in the HI fiber knob loop of the Ad penton base protein. Although the CAR is expressed in almost all tissues, including the adult nervous system and cerebral vasculature,22,23 muscle,24 heart25 and the hematopoietic system,26 its presence in platelets is debated. Othman et al. identified CAR (by flow cytometry) and its mRNA (by reverse transcriptase polymerase chain reaction) in human platelets27 while Shimony et al. did not confirm the presence of the receptor and proposed that binding of Ad to platelets is mediated by an interaction between RGD-binding motifs of Ad and platelet αVb3 28 (Figure 1). Indeed, human megakaryocytes either do not express mRNA for CAR or express it at extremely low levels (J. Rowley and A.S. Weyrich, University of Utah, personal communication). After intravenous inoculation in mice, Ad rapidly bind circulating platelets causing their activation and subsequent entrapment in liver sinusoids where virus-platelet aggregates are taken up by Küpffer cells and degraded. Platelet activation is followed by activation of blood coagulation, leading to DIC.29 Activated platelets also release cytokines promoting endothelial cell activation with secretion of von Willebrand factor, binding of platelets to endothelial cells and the formation of platelet/leukocyte aggregates, eventually triggering the development of microthrombi in liver sinusoids.21,29 There is also a complex interplay between Ad and the coagulation system. In fact, the distribution and activity of Ad in blood is affected by interactions with plasma proteins, including complement and vitamin K-dependent coagulation factors, which act as opsonizing agents. Our knowledge of these interactions derives mainly from in vitro observations and it is unknown whether the interplay of Ad with coagulation proteins affects the activity of the latter. Vitamin K-dependent coagulation factors, including the anticoagulant protein C, interact with Ad-5, the most widely used Ad vector. Activated protein C is generated on endothelial cells via the interaction of protein C with the thrombin-thrombomodulin complex and the endothelial protein C receptor (EPCR). Activated protein C requires protein S to express anticoagulant activity.30 Protein S circulates either free or associated with C4BP, a regulatory protein of the complement system.31 C4BP binds to activated platelets through mechanisms involving chondroitin sulfate expressed on activated platelets32 and to membrane-associated protein S on platelets.33 Interestingly, the protein C anticoagulant pathway plays a peculiar pathophysiological role in CVST.34
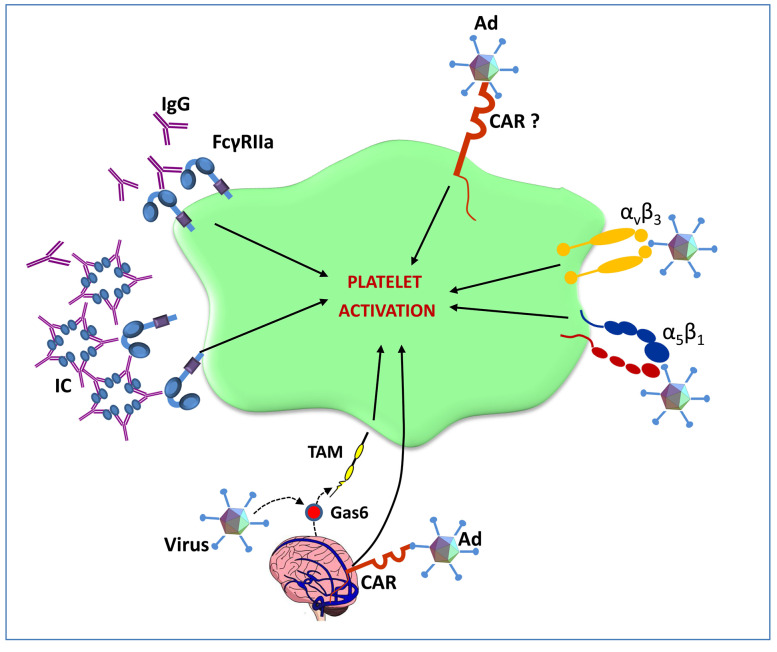
Figure 1.
Hypothesized interactions between platelets and adenoviruses. Adenoviruses (Ad) induce platelet activation either by binding to platelet coxsackie and adeno - virus receptor (CAR) or to plateletsurface integrins, such as αvb3 or α5b1. Moreover, circulating Ad-elicited IgG or immune complexes may directly activate platelets through FcgRIIa. Gas6 exposed by cerebral vein endothelial cells may bind Ad and activate platelets acting on Tyro3, Axl and Mer (TAM) receptors. Ad may also bind CAR expressed by cerebral vein vessels in this way activating endothelial cells which in turn may elicit platelet activation.
Small but measurable amounts of EPCR are also found in plasma. Soluble EPCR binds both protein C and activated protein C with an affinity similar to that of membrane- bound EPCR35 but, in contrast to the latter, it inhibits activated protein C anticoagulant activity thus limiting its ability to inactivate activated factor V, and also binds protein C impeding its activation by thrombinthrombomodulin complexes.32,36 An increase in soluble EPCR was observed in CVST, possibly leading to a procoagulant condition and enhanced risk of thrombosis.37
Finally, Gas6 (encoded by the growth arrest-specific 6 gene), a vitamin K-dependent protein with 44% sequence homology with protein S but devoid of anticoagulant activity, is widely expressed in the cerebral nervous system where it is found on resting endothelial cells. Gas6 potentiates platelet activation acting on Tyro3, Axl and Mer (TAM) receptors leading to thrombus formation38 and in vitro studies have shown that Gas6 binds to Ad enhancing their gene expression.39
The affinity of different Ad for coagulation factors is variable, with a considerable number of Ad types unable to bind them. Ad-5, Ad-2 and Ad-16 bind strongly to factor X.40 Moreover, the ability of Ad to bind coagulation factors is species-specific, e.g., Ad-5 binds human and mouse factor X with similar affinity, but Ad-2 binds human factor X with 10-fold lower affinity than mouse factor X.41
Vitamin K-dependent coagulation factors VII, IX, X, and protein C mediate the binding of Ad to hepatocytes. 42-44 For instance, for Ad-5 hepatotropism is critically dependent on the ability of the Ad-5 hexon to bind factor X. In contrast, non-factor X-binding Ad, such as Ad-48 and Ad-26, do not show hepatocyte tropism.45 The primary reason why factor X is required for Ad-5 transduction to the liver is that it protects Ad-5 from attack by complement.46
It has been previously ascertained that components of intramuscularly-injected vaccines, including the Ad vector, are disseminated in the circulation47 and it is thus conceivable that some of the above described activating interactions between Ad and platelets, endothelium and the blood clotting system can occur in recipients of Advector- based vaccines. However, so far no experimental evidence that this may have a role in VITT is available and actually it seems unlikely that sufficiently high circulating levels of a non-replicating Ad vector may be reached to trigger platelet activation or blood coagulation changes. In fact, it should be considered that around 2,500 billion virions/kg are required to trigger this reaction in mice and non-human primates,29,48 and even if all the Vaxzevria viral content were to spill-over into the blood after intramuscular administration, a concentration of 0.7 billion/kg Ad viral vectors would be reached, which is probably insufficient to activate platelets/coagulation.49
Antibody-dependent enhancement and vaccine-associated adverse events
Antibody-dependent enhancement (ADE) is an immunological form of a more general phenomenon called enhanced respiratory disease, leading to the clinical worsening of respiratory viral infections. ADE can occur either through an antibody-mediated increase of virus uptake by Fcg receptor IIa (FcgRIIa)-expressing phagocytic cells, thus facilitating viral infection and replication, or by boosting immune activation through excessive Fc-mediated immunological cell effector functions or immune complex formation with consequent increase of inflammation and immunopathology.50 Both ADE pathways can occur when non-neutralizing antibodies or antibodies at sub-neutralizing levels bind to viral antigens without blocking or clearing the infection. ADE has been reported for vaccines against both severe acute respiratory syndrome corona virus (SARS-CoV) and Middle East respiratory syndrome corona virus (MERS-CoV) in vitro and in animal models.50 The cytoplasmic tail of FcgRIIa activates the protein-tyrosine kinases Src 51,52 and Syk.53-55 Srcdependent signaling has been shown to be crucial for ADE triggered by Ebola virus, enhancing viral uptake into cells and thus worsening the infection.56
Circulating antibodies activating platelet IgG FcgRIIa may be key determinants of a host response leading to uncontrolled platelet aggregation and thrombosis. Studies in transgenic mice expressing human FcgRIIa on platelets showed that the administration of anti-CD9 antibodies caused thrombosis accompanied by platelet consumption, a response that was absent in mice lacking the receptor.57 The clinical relevance of this pathway for thrombotic disorders in humans is confirmed by the observation that FcgRIIa expression is higher in patients with stroke58 and that relatively common FcgRIIa polymorphisms are associated with increased risk of thrombosis in patients with heparin-induced thrombocytopenia (HIT).59 Immuno-complex formation, complement deposition and local immune activation are likely mechanisms triggered by SARS-CoV-2 vaccine. Furthermore, preexisting antibodies to coronavirus strains endemic in humans could mediate ADE by facilitating cross-reactive recognition of SARS-CoV-2 in the absence of viral neutralization. 60
Interestingly, compared to Ad-5 and Ad-6, chimpanzee adenoviruses (ChAd) are much less frequently neutralized by pre-existing antibodies present in humans. The prevalence of vector-neutralizing antibodies against Y25, now renamed ChAdOx1, the vector of the Vaxzevria vaccine, in human sera from British and Gambian adults was found to be 0% (n=100) and 9% (n=57), respectively.61 The presence of these antibodies in rare patients in Europe might theoretically represent one potential mechanism triggering ADE, and possibly VITT, in vaccine recipients but no data on this are available yet.
Despite the above hypotheses, preliminary in vitro evidence suggests that serum from convalescent COVID-19 patients does not induce either enhancement of SARSCoV- 2 infection or innate immunity responses in human macrophages, indicating that ADE may not be involved in the immune-pathological processes associated with COVID-19 infection or immunization.62
Use of adenovirus vectors and thrombotic events
Adenovirus vectors for gene therapy
Ad vectors have been used therapeutically for their ability to transduce and deliver transgenes to different cell types. However, for these indications the clinical use of Ad vectors has been limited to a few tens of patients and the main concerns have been the development of humoral and cellular immunity occurring upon repeated administration and/or the possible neutralization of the vector by pre-existing immunity against the virus, while little attention had been paid to the possible interactions of Ad vectors with platelets and the blood clotting system.
The first use of Ad vectors for gene therapy of inherited disorders or to treat neoplasia dates back to the 1990s. An analysis of the risks associated with the use of Ad-vectored gene therapies among 90 individuals who received 140 administrations for various diseases (cystic fibrosis, metastatic colorectal cancer, cardiovascular disease), showed that 13 deaths were recorded. The authors concluded that none was linked to the Ad vector.63 The reported hematologic abnormalities were decreased hemoglobin, leukocytosis, thrombocytopenia, and prolongation of the activated partial thromboplastin time (aPTT), with no cases of DIC.63
It is, however, puzzling that a recently European Medicines Agency-licensed Ad-vectored gene therapy for spinal muscular atrophy received a warning about the possible risk of thrombotic microangiopathy based on the reporting of five cases in treated infants (https://www.ema.europa.eu/en/medicines/human/EPAR/zolgensma).
Adenovirus-vectored vaccines
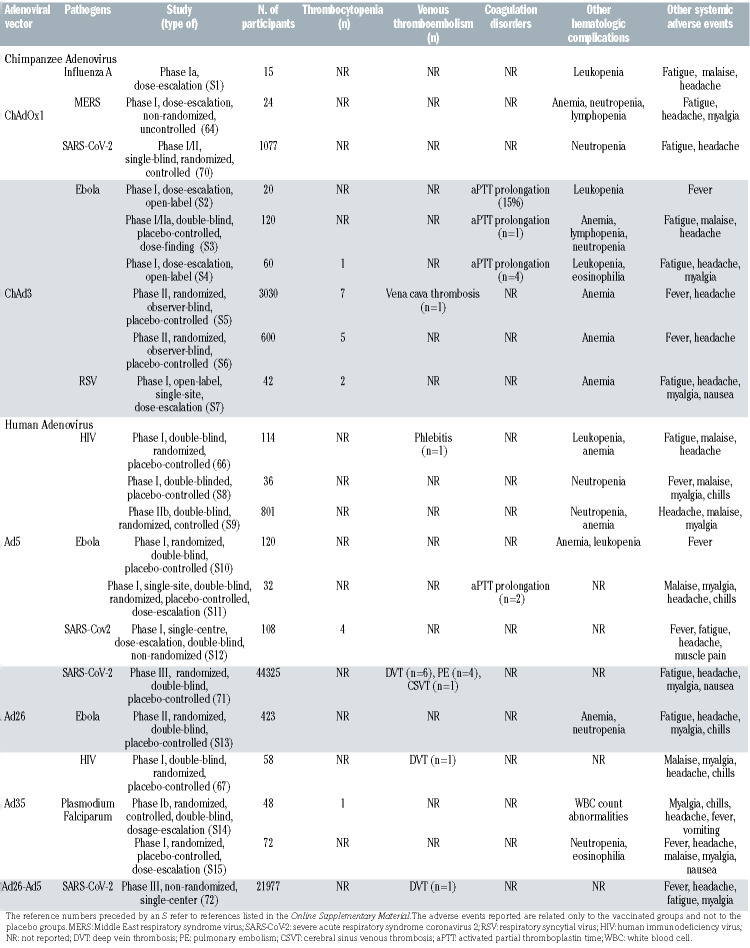
Beside SARS-CoV-2, Ad vectors have been used for the preparation of other vaccines, including the ChAdOx1- vectored vaccines for MERS-CoV and Chikungunya; with only a few hundred volunteers having received these vaccines up to June 2020,64 no excess of thrombotic events had been noted.65 Even for the Ebola vaccination campaign, the largest previous example of large-scale vaccination using an Ad vector, a maximum of around 200,000 volunteers were treated, with only one vena cava thrombosis reported (Table 1). However, it may be extremely difficult to prove that adverse events following immunization are caused by the vaccine itself when their occurrence is extremely rare (https://www.nature.com/articles/d41586-021-00880-9. Accessed on April 9, 2021).
Except for common mild/moderate reactome reactions, the most frequently recorded adverse events in clinical trials were hematologic (e.g., mild hemoglobin decrease, thrombocytopenia, leukopenia) the majority of which recovered a few days or weeks after vaccination. The extent and rate of hematologic adverse events associated with Ad-vectored vaccines are summarized in Table 1. Occasional abnormalities of coagulation were reported, with prolongation of the aPTT, possibly due to the development of transient antiphospholipid antibodies. Thrombotic events were rare both for human and nonhuman Ad-vectored vaccines. One case of phlebitis was observed among 114 volunteers who received a recombinant, replication-defective Ad-5-vectored vaccine expressing human immunodeficiency virus (HIV)-1 antigenic proteins.66 Another case of deep vein thrombosis was observed among 58 volunteers after administration of a recombinant, replication-defective Ad-35-vectored vaccine expressing HIV-1 antigens.67 Both events were considered unrelated to the vaccine.
A systematic review identified 200 clinical studies on active immunization against SARS-CoV-2. The second most used vaccine platform, after mRNA-based vaccines, was represented by Ad vectors (24%).68 Concerning chimpanzee Ad-vectored vaccines (ChAdOx1 nCoV-19), neutropenia was the most common hematologic abnormality (Table 1). Across all studies, vaccines had a good safety profile with no difference in severe reactions between study arms.69,70 In a phase III trial with a recombinant, replication-incompetent human Ad-26 vector encoding the SARS-CoV-2 spike protein, with 43,783 participants, 11 venous thromboembolic events were observed in the vaccine group compared to three in the placebo group (Table 1); however, most subjects had underlying medical conditions that might have contributed to these events. In the vaccine group there were six cases of lower leg deep venous thrombosis and four cases of pulmonary embolism. Interestingly, however, a CVST, with cerebral hemorrhage and thrombocytopenia, occurred 21 days after vaccination in a 25-year-old male who had multiple predisposing factors, including preexisting cerebral sigmoid sinus stenosis and infection from an unknown pathogen. Subsequent testing identified anti-PF4 antibodies at the time of the event. The patient recovered.7
Table 1.
Studies with adenovirus-vectored vaccines reporting hematologic adverse effects.
In a phase III controlled randomized clinical trial with a recombinant Ad-26-vectored and a recombinant Ad-5- vectored vaccine (Sputnik V) among 16,501 participants, ten vascular events (0.061%) were observed including: one deep vein thrombosis (0.006%), one transient ischemic attack (0.006%), one cerebral circulation failure (0.006%), one vascular encephalopathy (0.00659%) and two acute myocardial infarctions (0.012%) (4 additional events were non thrombotic)72 (Table 1).
The vaccine-induced immune thrombotic thrombocytopenia syndrome
When the anti-SARS-CoV-2 vaccination campaign was well underway worldwide a few cases of spontaneous, severe thromboembolic events in otherwise healthy subjects began to be reported, leading to a pause in the administration of the Vaxzevria vaccine in several European countries (https://www.ema.europa.eu/en/news/emas-safetycommittee- continues-investigation-covid-19-vaccine-astrazenecathromboembolic- events). Soon after several case reports were published, mainly concerning young females, with new ones continuing to accrue, although many were not subject to rigorous central review and with anti-PF4 antibodies measured using disparate methods, not allowing to conclude that all were typical VITT cases
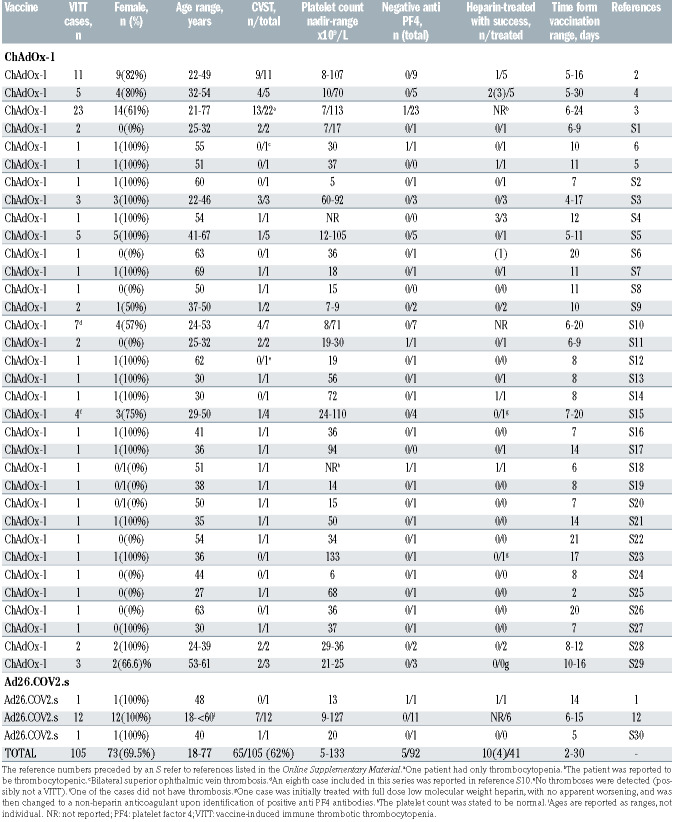
Up to July 17, 2021, 105 such cases with two Ad-vectored vaccines had been published (Table 2) with some common clinical features characterizing a new syndrome, including thrombocytopenia, often severe, venous thrombosis at unusual sites, in particular of the cerebral sinuses but also of the splanchnic veins, frequently associated with thromboses in multiple sites, both venous and arterial, and sometimes DIC combined with hemorrhage. A comparative evaluation of the clinical characteristics of the published ChAdOx1 or Ad26.CoV2.S VITT cases suggests that while clinical symptoms are comparable, Ad26.CoV2.S-associated cases show more thrombosis and intracerebral hemorrhage, lower D-dimer and less altered aPTT, but a similar mortality.9 In a recent, large nationwide healthcare register-based study in Denmark and Norway involving 281,264 ChAdOx1-S-vaccinated subjects aged 18-65 and as controls the entire agematched populations of the two countries studied in the period 2016-2019, the standardized morbidity ratio for CVST was 20.25 (8.14-41.7), with an excess of 2.5 events per 100,000 vaccinations, particularly evident in women 18-44 years old,73 confirming the crucial relationship between Vaxzevria administration and occurrence of VITT. The catastrophic syndrome, burdened by a 20-50% mortality rate, has the time course and tumultuous evolution of an acute immunological reaction and indeed three groups of investigators identified, in several of their patients, circulating antibodies to PF4/heparin complexes using an enzyme-linked immunosorbent assay (ELISA) and a heparin-induced platelet activation assay,2-4 and thus proposed that this disorder is a peculiar form of autoimmune HIT.
The autoimmune heparin-induced thrombocytopenia hypothesis
HIT is a rare immune-mediated adverse drug reaction that may occur after exposure to heparin. Circulating heparin binds to PF4, a positively charged platelet protein released in plasma upon activation. PF4 normally binds to negatively charged glycosaminoglycans on the endothelium, displacing antithrombin and thus activating coagulation. However, PF4 binds with greater affinity to heparin, forming heparin/PF4 complexes which become neoantigens inducing the formation of autoantibodies. Heparin- PF4-IgG immune complexes in turn bind to platelet FcgRIIA receptors causing activation, aggregation, and additional release of PF4, with ignition of a positive feedback loop leading to further platelet activation and consumption. Moreover, these complexes also activate monocytes, which release tissue factor, thus promoting concomitant activation of coagulation.
HIT is a potentially fatal condition, associated with the development of arterial or venous thrombosis.74 Thrombocytopenia occurs in more than 85% of HIT patients and is usually of moderate severity, with median platelet counts of approximately 50-60×109/L, although values <20×109/L can be found in approximately 10% of cases. Typically, the platelet count starts to decrease 5-10 days after initiation of heparin, but early-onset thrombocytopenia (rapid-onset), within 24 h of exposure, can develop in 25-30% of cases if patients have been treated with heparin in the preceding 3 months.75 Thromboembolic complications occur in 35-75% of HIT patients and are usually severe. They can be venous (i.e., deep vein thrombosis and pulmonary embolism, but rarely also CVST or splanchnic thrombosis), arterial (ischemic stroke, myocardial infarction, acute occlusion of limb arteries) or microvascular (digital infarction).76
Recently, another clinical picture not triggered by exposure to heparin has been recognized and defined as autoimmune HIT.77 The main characteristic of this condition is the presence of circulating antibodies able to activate platelets also in the absence of heparin. Polyanion molecules potentially involved in the development of autoimmune HIT are typically bacteria and virus components, hypersulfated chondroitin sulfate, DNA and RNA and polyphosphates.78 Patients with this syndrome show slightly different clinical features from those with classical HIT, including severe thrombocytopenia (<20x109/L), sometimes in combination with DIC, microvascular thrombosis and CVST in up to 40% of cases.78 From a therapeutic standpoint, besides the indication for an alternative anticoagulant, valid also for HIT, the intravenous administration of high doses of IgG in combination with steroids has been proposed for autoimmune HIT.77
Table 2.
Cases of vaccine-induced immune thrombotic thrombocytopenia reported in the literature as of July 17, 2021.
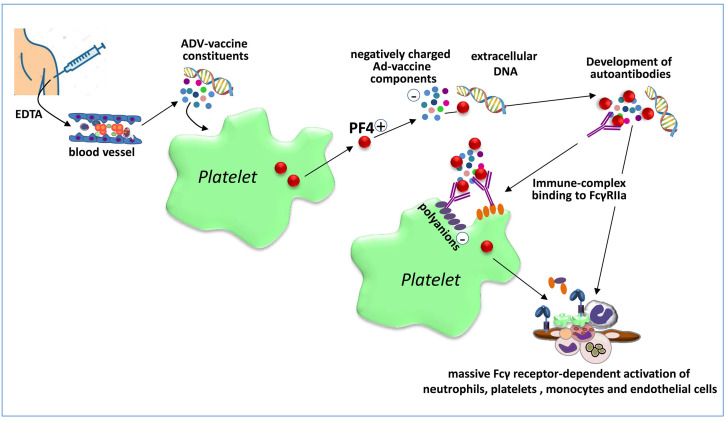
Very early after the first reports of unusual types of thrombosis associated with the Vaxzevria vaccine, a German group suggested a tentative pathogenic mechanism underlying these rare events, which they named VIPIT, based on findings in nine cases of previously vaccinated subjects.11 Indeed, in sera from four of these subjects the investigators detected antibodies to PF4/heparin complexes using an enzyme-immunoassay; these antibodies were inhibited by the addition of high concentrations of heparin (i.e., 100 U/mL), and the sera were able to activate washed control platelets when either PF4 or the Vaxzevria vaccine was added in vitro to the samples.11 The investigators showed that platelet activation was triggered by FcgRIIA stimulation, because an FcgRIIAblocking antibody prevented this phenomenon. Given the absence of previous exposure to heparin, the authors suggested a condition resembling autoimmune HIT. More recently, in a preliminary report published in a non-peerreviewed internet repository, the German group went on to suggest that the Ad vector and/or some protein components of the Vaxzevria vaccine activate platelets to release PF4 which then forms complexes with virus proteins and other anionic constituents of the vaccine, generating neoantigens against which antibodies develop and induce strong platelet activation via FcgRIIa stimulating granulocyte activation with NETosis and ultimately catastrophic thrombosis.47 The presence of EDTA in the vaccine would favor vascular leakage at the inoculation site, facilitating dissemination of the vaccine components in blood (Figure 2).
Figure 2.
The autoimmune heparin-induced thrombocytopenia hypothesis. Vaccine components leaking into the bloodstream from the vaccination site (facilitated by ethylenediaminetetraacetic acid present in the vaccine) activate platelets to release platelet factor 4 (PF4). Vaccine constituents, likely polyanions or viral DNA, form complexes with positively-charged PF4 which are recognized as neoantigens by B cells that then produce antibodies against these complexes. The resulting immune complexes activate platelets through FcgRIIa, triggering the release of additional PF4 and polyphosphates thereby initiating a positive feedback loop that leads to further platelet activation and consumption. Extracellular DNA in neutrophil extracellular traps binds PF4 and the resulting DNA/PF4 complexes further recruit anti-PF4 antibodies inducing massive Fcg receptor-dependent activation of neutrophils, platelets, monocytes and endothelial cells leading to massive activation of coagulation and thrombosis. EDTA: ethylenediaminetetraacetic acid.
Very recently a study using alanine scanning mutagenesis explored the binding sites on PF4 of antibodies isolated from patients with VITT or with classical HIT. While the binding of VITT anti-PF4 antibodies was restricted to eight surface amino acids, all located within the heparinbinding site of PF4, HIT anti-PF4 antibodies bound amino acids corresponding to two different sites on PF4; moreover, VITT antibodies had a stronger binding response than HIT antibodies. The authors concluded that VITT antibodies mimic the effect of heparin by binding to a similar site on PF4, allowing PF4 tetramers to cluster with the formation of immuno-complexes which, in turn, cause FcγRIIa-dependent platelet activation.79
These peculiar characteristics explain why the identification of VITT requires different tests from those needed for the identification of classical HIT.80 In suspected VITT anti-PF4 antibodies can be identified by an ELISA, but not by other rapid immunological assays typically positive in HIT, such as the STic® Expert HIT kit, latex immunoassays and chemiluminescence-based assays,4,80 and it is better characterized by a heparin-induced platelet aggregation (HIPA) or PF4-induced platelet activation (PIPA) test.2,4,81 It should be noted that no single ELISA method detected all possible/probable VITT cases.82
While the autoimmune HIT hypothesis is important and provides the basis for understanding this novel catastrophic autoimmune thrombotic syndrome, several aspects do not fit completely with the clinical presentation of VITT and various issues remain unclarified.
First, among the reported VITT cases in which anti-PF4 antibodies were measured, in a few they were negative (Table 2).4,5 Second, it is expected that treatment with heparin would worsen the clinical evolution of these patients, and indeed it is generally cautiously recommended not to use this drug.2,4,13 However, in around one fourth of the heparin-treated cases, the use of this anticoagulant was successful (Table 2). Third, while VITT resembles autoimmune HIT in several respects, the latter is not as frequently associated with CVST and rarely with DIC, and the above summarized mechanistic hypothesis does not explain the preferential localization of the venous thrombotic events in the cerebral and splanchnic circulations.
Other unclear aspects are its relatively precocious onset, as early as 4 days after vaccination, which seems too soon to generate high-titer, class-switched, high-affinity anti-PF4 antibodies. Furthermore, there is no evidence that the anti-PF4 antibodies isolated from patients with VITT cause thrombosis and thrombocytopenia in animal models.49,83 Finally, recent observations show that 1.2% to 8.0% of subjects receiving a first dose of Vaxzevria develop circulating anti-PF4 antibodies while the prevalence of VITT ranges from 0.0006% to 0.00125%.84,85
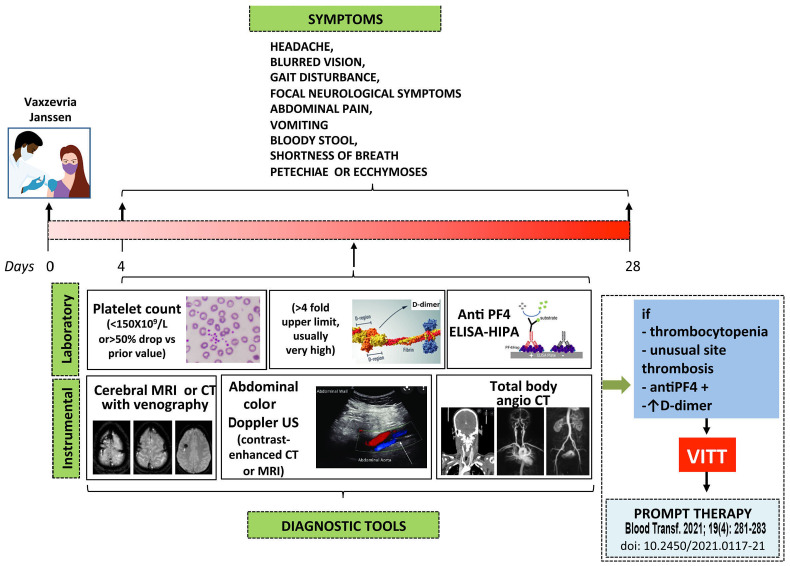
Figure 3.
A suggested clinical surveillance and diagnostic approach to suspected vaccine-induced immune thrombotic thrombocytopenia. Subjects receiving the Vaxzevria and the Janssen vaccines who develop new onset headache, especially if severe or with unprecedented characteristics, and/or associated with other clinical manifestations (blurred vision, gait disturbance, focal neurological symptoms and/or abdominal pain, vomiting, bloody stool, shortness of breath, petechiae or ecchymoses) should be referred for immediate laboratory evaluation (platelet count and D-dimer measurement). If thrombocytopenia is detected they should undergo anti-PF4 antibody testing, imaging and evaluation for cerebral vein sinus thrombosis, splanchnic vein thrombosis or pulmonary embolism. If confirmed, therapy for vaccine-induced immune thrombocytopenic thrombosis (VITT) should be immediately started according to the statement from the Italian Society for the Study of Haemostasis and Thrombosis (SISET)13 Although almost one quarter of the reported patients with VITT in whom unfractionated or low-molecular weight heparin was used apparently responded well to treatment (Table 2), subjects positive for anti-PF4 antibodies, as determined by a heparin-induced platelet aggregation (HIPA) test and/or enzyme-linked immunosorbent assay, or who have not been tested should, for prudence, be treated with alternative anticoagulants, until new information becomes available. In subjects in whom these anti-PF4 antibodies do not cross-react with heparin, as shown by a HIPA test in the presence of a low concentration of heparin, the use of heparin as anticoagulant may be allowed. ELISA: enzyme-linked immunosorbent assay; MRI: magnetic resonance imaging; CT: computed tomography; US: ultrasound.
Other possible pathogenic mechanisms of vaccine-induced immune thrombotic thrombocytopenia
Very recently a preliminary report, published in a nonpeer- reviewed repository, provided an interesting alternative potential pathogenic mechanism of VITT.86 COVID- 19 is caused by SARS-CoV-2 which is a single-strand RNA virus that is translated and replicates only in the cytosol of infected cells in the absence of processes which are necessary when nuclear-encoded genes are transcribed, and in particular of mRNA splicing. Nuclear encoded genes have intronic sequences, thus their transcripts require splice reactions at consensus RNA sequences to eliminate them. When an Ad-vectored viral RNA sequence is administered the vector infects host cells, adenoviral DNA enters the nucleus and is then transcribed by the host transcription machinery. However, the viral piece of DNA deriving from the SARS-CoV-2 virus is not optimized to be transcribed into the nucleus and its open reading frame may thus be disrupted by arbitrary splice events. These splice events would produce shorter spike protein variants, including forms missing the C-terminal membrane anchor, thus leading to soluble circulating spike protein molecules. The soluble spike protein may cause a strong activation of endothelial cells expressing ACE2.87 Moreover, when the host immune system starts to produce antibodies against the spike protein, endothelial cells binding soluble spike would also be decorated by these antibodies, triggering a strong inflammatory reaction through antibody-dependent or complement- dependent cytotoxicity, thus eliciting VITT. With this hypothesis, the preferential involvement of cerebral veins could be explained by the non-unidirectional blood flow in these vessels due to the lack of venous valves, with prolonged residence time of the soluble spike protein in this district depending on body posture or when sleeping. The immunological part of this hypothesis is also in agreement with the apparent higher prevalence of VITT in young women, because they have stronger immune reactions than men and older people. To explain the rarity of VITT the authors hypothesized that only some individuals, due to specific major histocompatibility complex combinations, are not able to produce neutralizing anti-spike antibodies which would instead prevent the binding of soluble spike to endothelial ACE2 and its ominous consequences in most vaccine recipients. This hypothesis was partly validated by the identification through in silico analysis of potential splice sites in the AstraZeneca and Johnson&Johnson codon-optimized spike opening frames and by in vitro studies with HeLa cells showing that vaccine-transduced cells generate transcripts smaller than the full spike protein. It would also explain why VITT has not been reported with mRNA vaccines, which release their cargo mRNA directly into the host cells’ cytosol where it is translated into spike protein without undergoing splicing reactions. Finally, it would account for why the incidence of VITT seems to be lower with the Johnson&Johnson vaccine than with the AstraZeneca one, given that the latter carries more splice donor sequences than the former.86
Additional hypotheses on the mechanisms triggering VITT include a genetically determined enhanced expression of FcgRIIa in susceptible subjects, an altered glycosylation state of IgG produced in response to vaccination in some individuals making these antibodies more reactive to platelet FcgRIIa,49 the leakage of the Ad vector into the circulation and/or the prior presence of cross-reactive antibodies to other coronaviruses forming immune complexes activating platelets.88 However, the hypothesis that VITT develops in subjects with previous, not apparent SARS-CoV-2 infection with prior circulating IgG antibodies against the spike protein able to activate platelet FcgRIIa89 should be excluded by the observation that most VITT subjects tested for previous or recent COVID-19 infections were negative. Excessive transcription of the spike protein, which would then activate platelets binding to ACE2,90 and vaccine-induced expression of the spike by megakaryocytes and platelets, leading to a thrombo-inflammatory storm,49,91 have also been proposed. Another hypothesis starts from the observation that both the ChAdOx1 and the Ad26.CoV2.S vaccines use polysorbate 80 as an excipient. Polysorbate 80 is a non-anionic surfactant that crosses the blood-brain barrier and enhances microparticle uptake by endothelial cells. Therefore leakage of Ad vector and polysorbate into the circulation and the spike protein produced by vaccination could preferentially localize in brain vessels triggering endothelial activation.92 However, considering that VITT usually develops at least 1 week after vaccination, it is very unlikely that circulating Ad vector or vaccine excipients would still be present in blood, making the alternative explanations, and in particular an immunological reaction, more likely.
Conclusive remarks
At least two Ad vector-based vaccines against SARSCoV- 2 have been associated with an excess rate of a special form of catastrophic thrombotic syndrome associated with thrombocytopenia of likely autoimmune origin, not observed so far with mRNA-based vaccines, suggesting that the vectors may play a role in eliciting it. Several elements of the Ad vectors and/or of vaccine composition may theoretically interact with platelets, the endothelium and the blood clotting system precipitating this rare complication. However, the exact sequence of events leading to the development of this syndrome and, most importantly, the reason why it evolves in only very few subjects without apparent predisposing factors remain to be clarified.
It is clear that our understanding of the pathogenesis of VITT is far from complete and that more mechanistic studies are required to clarify it and, it is hoped, to identify risk factors predictive of its development. What is quite likely is that the Ad vector-based vaccine triggers an immunological reaction which, for unknown reasons, in some rare subjects involves especially blood platelets, and possibly some peculiar vascular endothelial districts such as those of the cerebral and splanchnic veins, precipitating the catastrophic vaccine-induced autoimmune thrombocytopenia thrombosis syndrome. Awareness of this condition and the prompt identification/evaluation of affected patients may lead to successful treatment and recovery (Figure 3).13,84
COVID-19 continues to be a serious global health problem and vaccination against SARS-CoV-2 is the most effective way of limiting illness and death due to the pandemic. Based on the current available information, and in light of the relative rarity of VITT, the benefits of vaccination clearly outweigh the potential risks (https://www.ema.europa.eu/en/documents/dhpc/direct-healthcare- professional-communication-dhpc-zolgensma-onasemnogene- abeparvovec-risk-thrombotic_en.pdf). However, once the global pandemic begins to retreat, the relative importance of even small risks will increase,65 making it critically important to understand the mechanisms leading to this ominous thrombotic syndrome, to identify the prognostic factors for its development and to define the best management strategies.13
Acknowledgments
This work was supported in part by grants from Fondazione Cassa di Risparmio di Perugia (#19663 (2020.0508) and FISR 2020 (# 1049) to PG.
References
- 1.Muir KL, Kallam A, Koepsell SA, Gundabolu K. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N Engl J Med. 2021;384(20):1964-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(22):2124-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021,384(23):2202-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bayas A, Menacher M, Christ M, Behrens L, Rank A, Naumann M. Bilateral superior ophthalmic vein thrombosis, ischaemic stroke, and immune thrombocytopenia after ChAdOx1 nCoV-19 vaccination. Lancet. 2021;397(10285):e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muster V, Gary T, Raggam RB, WÖlfler A, Brodmann M. Pulmonary embolism and thrombocytopenia following ChAdOx1 vaccination. Lancet. 2021;397(10287):1842. [DOI] [PubMed] [Google Scholar]
- 7.Shay DK, Gee J, Su JR, et al. Safety monitoring of the Janssen (Johnson & Johnson) COVID-19 vaccine - United States, March- April 2021. MMWR Morb Mortal Wkly Rep. 2021;70(18):680-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodeghiero F, Balduini CL. A new enemy is emerging in the fight against the SARSCoV- 2 pandemic. Haematologica. 2021;106(8):2040-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwang J, Lee SB, Lee SW. Comparison of vaccine-induced thrombotic events between ChAdOx1 nCoV-19 and Ad26.COV.2.S vaccines. J Autoimmun. 2021;122:102681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sangli S, Virani A, Cheronis N. Thrombosis with thrombocytopenia after the messenger RNA-1273 vaccine. Ann Intern Med. 2021;L21-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle P, Eichinger S. A prothrombotic thrombocytopenic disorder resembling heparin-induced thrombocytopenia following coronavirus vaccination. Blood Transfus. 2021; 19(4): 281-28333871350 [Google Scholar]
- 12.See I, Su JR, Lale A, Woo EJ, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325(24):2448-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gresele P, Marietta M, Ageno W, et al. Management of cerebral and splanchnic vein thrombosis associated with thrombocytopenia in subjects previously vaccinated with Vaxzevria (AstraZeneca): a position statement from the Italian Society for the Study of Haemostasis and Thrombosis (SISET). Blood Transfus. 2021;19(4):281-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu KH, Lonberg-Holm K, Alstein B, Crowell RL. A monoclonal antibody specific for the cellular receptor for the group B coxsackieviruses. J Virol. 1988;62(5):1647-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergelson JM, Cunningham JA, Droguett G, et al. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275(5304):1320-1323. [DOI] [PubMed] [Google Scholar]
- 16.Bewley MC, Springer K, Zhang YB, Freimuth P, Flanagan JM. Structural analysis of the mechanism of adenovirus binding to its human cellular receptor CAR. Science. 1999;286(5444):1579-1583. [DOI] [PubMed] [Google Scholar]
- 17.Roelvink PW, Mi Lee G, Einfeld DA, Kovesdi I, Wicham TJ. Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing Adenoviridae. Science. 1999;286(5444):1568-1571. [DOI] [PubMed] [Google Scholar]
- 18.Greber U, Willets M, Webster P, Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993;75(3):477-486. [DOI] [PubMed] [Google Scholar]
- 19.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73(2):309-319. [DOI] [PubMed] [Google Scholar]
- 20.Davison E, Diaz RM, Hart IR, Santis G, Marshall JF. Integrin a5b1-mediated adenovirus infection is enhanced by the integrin- activating antibody TS2/16. J Virol. 1997;71(8):6204-6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Bergelson JM. AD receptors. J Virol. 2005;79(19):12125-12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Honda T, Saitoh H, Masuko M, et al. The coxsackievirus-adenovirus receptor protein as a cell adhesion molecule in the developing mouse brain. Brain Res Mol Brain Res. 2000;77(1):19-28. [DOI] [PubMed] [Google Scholar]
- 23.Patzke C, Max KEA, Behlke J, et al. The coxsackievirus-adenovirus receptor reveals complex homophilic and heterophilic interactions on neural cells. J Neurosci. 2010;30(8):2897-2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nalbantoglu J, Pari G, Karpati G, et al. Expression of the primary coxsackie and adenovirus receptor is downregulated during skeletal muscle maturation and limits the efficacy of adenovirus-mediated gene delivery to muscle cells. Hum Gene Ther. 1999;10(6):1009-1019. [DOI] [PubMed] [Google Scholar]
- 25.Noutsias M, Fechner H, de Jonge H, et al. Human coxsackie-adenovirus receptor is colocalized with integrins alpha(v)beta(3) and alpha(v)beta(5) on the cardiomyocyte sarcolemma and upregulated in dilated cardiomyopathy: implications for cardiotropic viral infections. Circulation. 2001;104(3): 275-280. [DOI] [PubMed] [Google Scholar]
- 26.Rebel V, Hartnett S, Denham J, et al. Maturation and lineage-specific expression of the coxsackie and adenovirus receptor in hematopoietic cells. Stem Cells. 2000;18(13):176-182. [DOI] [PubMed] [Google Scholar]
- 27.Othman M, Labelle A, Mazzetti I, Elbatarny HS, Lillicrap D. Adenovirusinduced thrombocytopenia: the role of von Willebrand factor and P-selectin in mediating accelerated platelet clearance. Blood. 2007;109(7):2832-2839. [DOI] [PubMed] [Google Scholar]
- 28.Shimony N, Elkin G, Kolodkin-Gal D, Krasny L, Urieli-Shoval S, Haviv YS. Analysis of adenoviral attachment to human platelets. Virol J. 2009;6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stone D, Liu Y, Shayakhmetov D, Li ZY, Ni S, Lieber A. Adenovirus-platelet interaction in blood causes virus sequestration to the reticuloendothelial system of the liver. J Virol. 2007;81(9):4866-4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griffin JH, Zlokovic BV, Mosnier LO. Activated protein C: biased for translation. Blood. 2015;125(19):2898-2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Esmon CT. Protein C. Prog Hemost Thromb. 1984;7:25-54. [PubMed] [Google Scholar]
- 32.Hamad OA, Nilsson PH, Lasaosa M, et al. Contribution of chondroitin sulfate A to the binding of complement proteins to activated platelets. PLoS One. 2010;5(9): e12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dahlbäck B, Wiedmer T, Sims PJ. Binding of anticoagulant vitamin K-dependent protein S to platelet-derived microparticles. Biochemistry. 1992;31(51):12769-12777. [DOI] [PubMed] [Google Scholar]
- 34.Ferro JM, Canhão P, Stam J, Bousser MG, Barinagarrementeria F; ISCVT Investigators. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 2004;35(3):664-670. [DOI] [PubMed] [Google Scholar]
- 35.Kurosawa S, Stearns-Kurosawa DJ, Hidar N, Esmon CT. Identification of functional endothelial protein C receptor in human plasma. J Clin Invest. 1997;100(2):411-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gandrille S. Endothelial cell protein C receptor and the risk of venous thrombosis. Haematologica. 2008;93(6):812-816. [DOI] [PubMed] [Google Scholar]
- 37.Javanmard SH, Shahsavarzadeh T, Saadatnia M. Soluble thrombomodulin and endothelial cell protein C receptor levels in patients with cerebral venous and sinus thrombosis. Eur Neurol. 2013;70(3-4):156-158. [DOI] [PubMed] [Google Scholar]
- 38.Gould WR, Baxi SM, Schroeder R, et al. Gas6 receptors Axl, Sky and Mer enhance platelet activation and regulate thrombotic responses. J Thromb Haemost. 2005;3(4):733-741. [DOI] [PubMed] [Google Scholar]
- 39.Nidetz NF, Gallagher TM, Wiethoff CM. Inhibition of type I interferon responses by adenovirus serotype-dependent Gas6 binding. Virology. 2018;515:150-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allen RJ, Byrnes AP. Interaction of adenovirus with antibodies, complement, and coagulation factors. FEBS Letters. 2019;593(24):3449-3460. [DOI] [PubMed] [Google Scholar]
- 41.Lenman A, Muller S, Nygren MI, Frangsmyr L, Stehle T, Arnberg N. Coagulation factor IX mediates serotypespecific binding of species A adenoviruses to host cells. J Virol. 2011;85(24):13420-13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parker AL, McVey JH, Doctor JH, et al. Influence of coagulation factor zymogens on the infectivity of adenoviruses pseudotyped with fibers from subgroup D. J Virol. 2007;81(7):3627-3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parker AL, Waddington SN, Nicol CG, et al. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood. 2006;108(8):2554-2561. [DOI] [PubMed] [Google Scholar]
- 44.Shayakhmetov DM, Gaggar A, Ni S, Li Lieber A. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J Virol. 2005;79(12):7478-7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalyuzhniy O, Di Paolo NC, Silvestry M, et al. Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc Natl Acad Sci U S A. 2008;105(14): 5483-5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duffy MR, Doszpoly A, Turner G, Nicklin SA, Baker AH. The relevance of coagulation factor X protection of adenoviruses in human sera. Gene Ther. 2016;23(7):592-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greinacher A, Selleng K, Wesche J, et al. Towards understanding ChAdOx1 nCov- 19 vaccine-induced immune thrombotic thrombocytopenia (VITT). Research Square. 2021. Apr 20. [Epub ahead of print] [Google Scholar]
- 48.Brunetti-Pierri N, Palmer DJ, Beaudet AL, Carey KD, Finegold M, Ng P. Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into nonhuman primates. Hum Gene Ther. 2004;15(1):35-46. [DOI] [PubMed] [Google Scholar]
- 49.Kadkhoda K. Post-adenoviral-based COVID-19 vaccines thrombosis: a proposed mechanism. J Thromb Haemost. 2021;19(7):1831-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee WS, Wheatley AK, Kent SJ, DeKosky BJ. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat Microbiol. 2020;5(10):1185-1191. [DOI] [PubMed] [Google Scholar]
- 51.Hamada F, Aoki M, Akiyama T, Toyoshima K. Association of immunoglobulin G Fc receptor II with Src like protein-tyrosine kinase Fgr in neutrophils. Proc Natl Acad Sci U S A. 1993;90(13):6305-6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghazizadeh S, Bolen JB, Fleit HB. Physical and functional association of Src-related protein tyrosine kinases with Fc gamma RII in monocytic THP-1 cells. J Biol Chem. 1994;269(12):8878-8884. [PubMed] [Google Scholar]
- 53.Kiener PA, Rankin BM, Burkhardt AL, et al. Cross-linking of Fc gamma receptor I (Fc gamma RI) and receptor II (Fc gamma RII) on monocytic cells activates a signal transduction pathway common to both Fc receptors that involves the stimulation of p72 Syk protein tyrosine kinase. J Biol Chem. 1993;268(32):24442-24448. [PubMed] [Google Scholar]
- 54.Nimmerjahn F, Ravetch JV. Fcγ receptors as regulators of immune responses. Nat Rev Immunol. 2008;8(1):34-47. [DOI] [PubMed] [Google Scholar]
- 55.Joshi T, Butchar JP, Tridandapani S. Fcg receptor signaling in phagocytes. Int J Hematol. 2006;84(3):210-216. [DOI] [PubMed] [Google Scholar]
- 56.Furuyama W, Marzi A, Carmody AB, et al. Fcg-receptor IIa-mediated Src signaling pathway is essential for the antibodydependent enhancement of Ebola virus infection. PLoS Pathog. 2016;12(12): e1006139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taylor SM, Reilly MP, Schreiber AD, Chien P, Tuckosh JR, McKenzie SE. Thrombosis and shock induced by activating antiplatelet antibodies in human Fc gamma RIIA transgenic mice: the interplay among antibody, spleen, and Fc receptor. Blood. 2000;96(13):4254-4260. [PubMed] [Google Scholar]
- 58.Calverley DC, Brass E, Hacker MR, et al. Potential role of platelet FcgammaRIIA in collagen- mediated platelet activation associated with atherothrombosis. Atherosclerosis. 2002;164(2):261-267. [DOI] [PubMed] [Google Scholar]
- 59.Pamela S, Anna Maria L, Elena D, et al. Heparin-induced thrombocytopenia: the role of platelets genetic polymorphisms. Platelets. 2013;24(5):362-368. [DOI] [PubMed] [Google Scholar]
- 60.Tetro JA. Is COVID-19 receiving ADE from other coronaviruses? Microbes Infect. 2020;22(2):72-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Collocca S, Barnes E, Folgori A, et al. Vaccine vectors derived from a large collection of simian adenoviruses induce potent cellular immunity across multiple species. Sci Transl Med. 2012;4(115):115ra2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Obdulio GN, V'kovski V, Zettl F, Zimmer G, Thiel V, Summerfield A. No evidence for human monocyte-derived macrophage infection and antibody-mediated enhancement of SARS-CoV-2 Infection. Front Cell Infect Microbiol. 2021;11:644574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crystal RG, Harvey BG, Wisnivesky JP, et al. Analysis of risk factors for local delivery of low- and intermediate-dose adenovirus gene transfer vectors to individuals with a spectrum of comorbid conditions. Hum Gene Ther. 2002;13(1):65-100. [DOI] [PubMed] [Google Scholar]
- 64.Folegatti PM, Bittaye M, Flaxman A, et al. Safety and immunogenicity of a candidate Middle East respiratory syndrome coronavirus viral-vectored vaccine: a dose-escalation, open-label, non-randomised, uncontrolled, phase 1 trial. Lancet Infect Dis. 2020;20(7):816-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kupferschmidt K, Vogel G. What’s the future of vaccines linked to rare clotting disorders? Science breaks down the latest. 2021. May 3. [Epub ahead of print] [Google Scholar]
- 66.Jaoko W, Karita E, Kayitenkore K, et al. Safety and immunogenicity study of multiclade HIV-1 Adenoviral vector vaccine alone or as boost following a multiclade HIV-1 DNA vaccine in Africa. PLoS One. 2010;5(9):e12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Keefer MC, Gilmour J, Hayes P, Gill D, et al. A phase I double blind, placebo-controlled, randomized study of a multigenic HIV-1 adenovirus subtype 35 vector vaccine in healthy uninfected adults. PLoS One. 2012;7(8):e41936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rego GNA, Nucci MP, Alves AH, et al. Current clinical trials protocols and the global effort for immunization against SARS-CoV-2. Vaccines (Basel). 2020;8(3):474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van Doremalen N, Lambe T, Spencer A, et al. ChAdOx1 nCoV-19 vaccination prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020;586(7830):578-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Folegatti PM, Ewer KJ, Aley PK, et al. Oxford COVID Vaccine Trial Group. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARSCoV- 2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sandoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384(23):2187-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous primeboost COVID-19 vaccine: an interim analysis of a randomized controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pottegård A, Lund LC, Karlstad Ø, et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ. 2021;May 5;373:n1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marcucci R, Berteotti M, Gori AM, et al. Heparin induced thrombocytopenia: position paper from the Italian Society on Thrombosis and Haemostasis (SISET). Blood Transfus. 2021;19(1):14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2(22): 3360-3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Greinacher A, Farner B, Kroll H, et al. Clinical features of heparin-induced thrombocytopenia including risk factors for thrombosis. A retrospective of 408 patients. Thromb Haemost. 2005;94(1): 132-135. [DOI] [PubMed] [Google Scholar]
- 77.Greinacher A, Selleng K, Warkentin TE. Autoimmune heparin-induced thrombocytopenia. J Thromb Haemost. 2017;15(11): 2099-2114. [DOI] [PubMed] [Google Scholar]
- 78.Warkentin TE, Greinacher A. Spontaneous HIT syndrome: knee replacement, infection, and parallels with vaccine-induced immune thrombotic thrombocytopenia. Thromb Res. 2021;204:40-51. [DOI] [PubMed] [Google Scholar]
- 79.Huynh A, Kelton JG, Arnold DM, Daka M, Nazy I. Antibody epitopes in vaccineinduced immune thrombotic thrombocytopenia. Nature. 2021;596(7873):565-569. [DOI] [PubMed] [Google Scholar]
- 80.Favaloro EJ. Laboratory testing for suspected COVID-19 vaccine-induced (immune) thrombotic thrombocytopenia. Int J Lab Hematol. 2021;43(4): 559-570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vayne C, Guery EA, Kizlik-Masson C, et al. Beneficial effect of exogenous platelet factor 4 for detecting pathogenic heparininduced thrombocytopenia antibodies. Br J Haematol. 2017;179(5):811-819. [DOI] [PubMed] [Google Scholar]
- 82.Platton S, Bartlett A, MacCallum P, et al. Evaluation of laboratory assays for antiplatelet factor 4 antibodies after ChAdOx1 nCOV-19 vaccination. J Thromb Haemost. 2021;19(8):2007-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cines DB, Bussel JB. SARS-CoV-2 Vaccineinduced immune thrombotic thrombocytopenia. N Engl J Med. 2021;384(23):2254-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sørvoll IH, Horvei KD, Ernstsen SL, et al. An observational study to identify the prevalence of thrombocytopenia and anti- PF4/polyanion antibodies in Norwegian health care workers after COVID-19 vaccination. J Thromb Haemost. 2021;19(7): 1813-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thiele T, Ulm L, Holtfreter S, et al. Frequency of positive anti-PF4/polyanion antibody tests after COVID-19 vaccination with ChAdOx1 nCoV-19 and BNT162b2. Blood. 2021;138(4):299-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kowarz E, Krutzke L, Reis J, Bracharz S, Kochanek S, Marschalek R. Vaccineinduced Covid-19 spike open reading frame results in spike protein variant that may cause thromboembolic events in patients immunized with vector-based vaccine. Research Square. 2021. May 26. [Epub ahead of print] [Google Scholar]
- 87.Lei Y, Zhang J, Schiavon CR, et al. SARSCoV- 2 spike protein impairs endothelial function via downregulation of ACE 2. Circ Res. 2021;128(9):1323-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chakraborty S, Gonzalez J, Edwards K. Proinflammatory IgG Fc structures in patients with severe COVID-19. Nat Immunol. 2021;22(1):67-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Douxfils J, Favresse J, Dogné JM. Hypotheses behind the very rare cases of thrombosis with thrombocytopenia syndrome after SARS-CoV-2 vaccination. Thromb Res. 2021;203:163-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shen S, Zhang J, Fang Y, et al. SARS-CoV-2 interacts with platelets and megakaryocytes via ACE2-independent mechanism. J Hematol Oncol. 2021;29;14(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Millington-Burgess SL, Harper MT. A double- edged sword: antibody-mediated procoagulant platelets in COVID-19. Platelets. 2021; 32(5):579-581. [DOI] [PubMed] [Google Scholar]
- 92.Choi PHI. Thrombotic thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;385(3):e11. [DOI] [PubMed] [Google Scholar]